Abstract
Microtubule-associated protein tau is abnormally hyperphosphorylated and aggregated into neurofibrillary tangles in brains with Alzheimer’s disease. The phosphorylation sites of tau are mainly localized in the proline-rich (residues 172–251) and C-terminal tail (residues 368–441) regions, which flank the microtubule-binding repeats. Here, we investigated the effects of tau phosphorylation at these distinct sites/regions on its activity of stimulating microtubule assembly and its self-aggregation. We found that tau phosphorylation at the proline-rich region by dual-specificity tyrosine-phosphorylated and -regulated kinase 1A inhibited its microtubule assembly activity moderately and promoted its self-aggregation slightly. Tau phosphorylation at the C-terminal tail region by glycogen synthase kinase-3β increased its activity and promoted its self-aggregation markedly. Tau phosphorylation at both regions plus the microtubule-binding region by cAMP-dependent protein kinase diminished its activity (~70% inhibition) and disrupted microtubules. These studies reveal the differential regulation of tau’s biological activity and self-aggregation by phosphorylation at various sites/regions.
Keywords: Alzheimer’s disease, cAMP-dependent protein kinase, dual-specificity tyrosine-phosphorylated and -regulated kinase 1A, glycogen synthase kinase-3β
Introduction
Tau is a major neuronal microtubule (MT)-associated protein. The main known biological function of tau is to stimulate MT assembly and to stabilize the structure of MTs. Tau is a phosphoprotein and its phosphorylation state is developmentally regulated. In normal adult human brain, tau contains two to three phosphate groups per molecule (Ksiezak-Reding et al., 1992; Kopke et al., 1993). However, in Alzheimer’s disease (AD), tau is abnormally hyperphosphorylated, containing 7–8 mol phosphates (Pi)/mol tau (Kopke et al., 1993; Kenessey & Yen, 1993). Hyperphosphorylated tau is the major component of neurofibrillary tangles, which are one of the histopathological hallmarks in AD brain. Many studies have demonstrated that hyperphosphorylation of tau is responsible for its loss of biological activity of stimulating MT assembly and stabilizing MT structure, for its gain of a toxic activity to sequester MT-associated proteins, and for its self-aggregation into neurofibrillary tangles (Iqbal et al., 1986; Bramblett et al., 1993; Yoshida & Ihara, 1993; Alonso et al., 1994, 2001a,b, 2004; Lucas et al., 2001; Fath et al., 2002; Jackson et al., 2002; Perez et al., 2002).
Adult human brain expresses six tau isoforms that are generated from a single gene by alternative mRNA splicing. The largest isoform consists of 441 amino acids (tau441) and includes 80 serine (Ser) or threonine (Thr) residues and five tyrosine residues (Goedert et al., 1989). Thus, almost 20% of the residues of tau are potential acceptors for phosphorylation. To date, phosphorylation of tau at over 30 Ser/Thr residues has been identified in AD brain (reviewed in Gong et al., 2005; Liu et al., 2006a). These phosphorylation sites are localized mainly in the proline-rich region (P-region) (residues 172–251) and the C-terminal tail region (C-region) (residues 368–441), which flank the MT-binding repeats of tau (Fig. 1A). By using iron-chelated affinity chromatography to isolate tau with various levels of phosphorylation, de Ancos et al. (2003) found that neither under-phosphorylated nor highly phosphorylated tau isoforms are associated with MT, suggesting that phosphorylation at certain sites is needed for tau binding to MTs, whereas phosphorylation at some other sites may prevent the association.
Fig. 1.
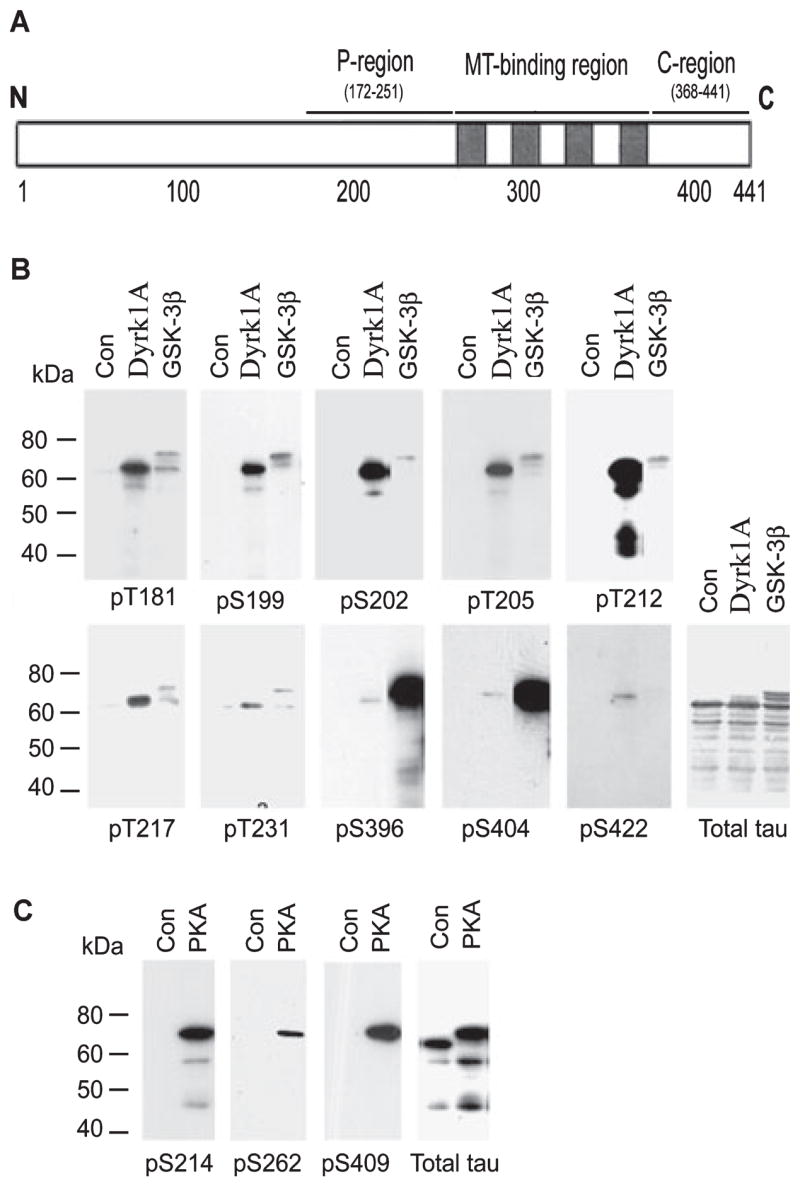
Site-specific phosphorylation of tau by dual-specificity tyrosine-phosphorylated and -regulated kinase 1A (Dyrk1A), glycogen synthase kinase-3β (GSK-3β) and cAMP-dependent protein kinase (PKA). (A) Schematic diagram of tau441, showing the proline-rich region (P-region), microtubule (MT)-binding region and C-terminal tail region (C-region), where the majority of the serine and threonine residues are phosphorylated in Alzheimer’s disease brain. (B and C) Recombinant tau441 was first phosphorylated in vitro to ~2.0 mol phosphates/mol tau by catalysis with Dyrk1A, GSK-3β or PKA. The phosphorylated tau and the control-treated tau (Con) were then subjected to western blots developed with antibodies recognizing total tau or tau phosphorylated at specific sites, as indicated under each blot.
Several protein kinases are involved in catalysis of tau phosphorylation. Each kinase has its own favorable phosphorylation sites or regions on the tau molecule. For example, cAMP-dependent protein kinase (PKA) mainly phosphorylates tau at Ser214, Ser262, Ser356 and Ser409 in vitro (Liu et al., 2006b). Therefore, PKA phosphorylates the sites spanning the P-region, the MT-binding repeats and the C-region of tau. Glycogen synthase kinase (GSK)-3β prefers to phosphorylate Ser/Thr residues followed by a proline (Ser/Thr-proline motif) and phosphorylates tau at many Ser/Thr-proline motifs with variable efficiencies. The most favorable phosphorylation sites of GSK-3β are Ser396 and Ser404 in the C-region of tau (Liu et al., 2006b). In contrast to GSK-3β, dual-specificity tyrosine-phosphorylated and -regulated kinase 1A (Dyrk1A) phosphorylates tau at Thr212 of the P-region with the highest efficiency (Woods et al., 2001). In this study, we phosphorylated tau in vitro at different sites/regions by using these three kinases and studied the effects of the site-specific phosphorylation on its biological activity and self-aggregation.
Materials and methods
Materials
The longest isoform of human brain tau (tau441) and of rat Dyrk1A were expressed and purified as described previously (Alonso et al., 2001b; Chen-Hwang et al., 2002). Recombinant GSK-3β and the catalytic subunit of PKA were purchased from Calbiochem (San Diego, CA, USA) and Sigma (St Louis, MO, USA), respectively. Tubulin was purchased from Cytoskeleton, Inc. (Dover, CO, USA). The monoclonal antibody 43D against total tau in a phosphorylation-independent manner and the polyclonal antibody R145 against the phosphorylated Ser422 of tau were raised in Dr Grundke-Iqbal’s laboratory (Pei et al., 1998; Liu et al., 2004). Phosphorylation-dependent and site-specific tau antibodies pT181, pS199, pS202, pT205, pT212, pS214, pT217, pT231, pS262, pS396, pS400, pS404 and pS409 were purchased from Biosource International (Camarillo, CA, USA). Peroxidase-conjugated anti-mouse and anti-rabbit IgGs were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The ECL kit was from Amersham Pharmacia (Piscataway, NJ, USA) and [γ-32P]ATP was from ICN Biomedicals (Costa Mesa, CA, USA).
Preparation of tau phosphorylated by dual-specificity tyrosine-phosphorylated and -regulated kinase 1A, glycogen synthase kinase-3β or cAMP-dependent protein kinase
Recombinant tau441 was phosphorylated with Dyrk1A, GSK-3β or PKA by incubating tau441 with the kinase in the following reaction mixtures (20–250 μL) at 30 °C for certain periods of time (see below). Tau phosphorylation by Dyrk1A was carried out by incubating 1.0 mg/mL tau441 with 50 μg/mL Dyrk1A in a reaction mixture consisting of 40 mm Tris-HCl (pH 7.4), 0.1 mm EGTA, 10 mm MgCl2 and 0.2 mm ATP. For GSK-3β, 1.0 mg/mL tau441 was incubated with 2.2 μg/mL GSK-3β in a buffer containing 40 mm Tris-HCl (pH 7.4), 10 mm MgCl2, 10 mm β-mercaptoethanol and 0.2 mm ATP. For PKA, 1.0 mg/mL of tau441 was incubated with the catalytic subunit of PKA (10 μg/mL) in the buffer consisting of 40 mm HEPES (pH 6.8), 10 mm β-mercaptoethanol, 10 mm MgCl2, 1.0 mm EGTA and 0.2 mm ATP. Parallel experiments using [γ-32P]ATP (500 cpm/pmol) to replace ATP were carried out to determine the stoichiometry of the phosphorylation. When the stoichiometry of tau phosphorylation reached the desired levels, the reaction was terminated by adding trichloroacetic acid to the reaction mixture to a concentration of 8%. The precipitated tau was collected by centrifugation and washed with ethanol. The dried tau pellets were then reconstituted in 5.0 mm 2-(N-morpholino) ethanesulfonic acid monohydrate (pH 6.8) containing 0.1 mm EGTA. The control tau was treated the same way in parallel except that kinase was added to the mixture after the addition of trichloroacetic acid.
Quantification of total tau and its phosphorylation at specific sites
Tau phosphorylation at individual phosphorylation sites was determined by western blots developed with various phosphorylation-dependent and site-specific tau antibodies, as described previously (Liu et al., 2002a). A phosphorylation-independent monoclonal tau antibody, 43D, was also used to detect the total tau levels. In some experiments, tau levels and phosphorylation at individual phosphorylation sites were also measured by using an immuno-dot-blot assay, as described by Liu et al. (2002b).
Microtubule assembly assays
The MT assembly-stimulating activity of tau was determined as described previously (Alonso et al., 1994). Briefly, the MT assembly reaction was carried out by adding tau (0.07 mg/mL) to the MT assembly mixture consisting of 100 mm 2-(N-morpholino) ethanesulfonic acid monohydrate (pH 6.8), 1.0 mm EGTA, 1.0 mm MgCl2 and 2.0 mg/mL tubulin. The assembly mixture (70 μL) was incubated in quartz microcuvettes at 35 °C in a thermostatically controlled Cary 1 recording spectrophotometer; MT assembly was initiated by the addition of tau and recorded continuously at 350 nm.
Rebuilding of the microtubule network in detergent-extracted 3T3 cells
Fibroblast 3T3 cells were cultured on poly-D-lysine-coated eight-well culture slides and treated with 10 μM nocodazole for 4 h to destroy endogenous MTs, followed by extraction of cytosolic proteins with 0.2% Triton X-100 for 1 min, as described previously (Li et al., 2007). The extracted cells were then incubated with freshly prepared rat brain cytosol (15%) containing 1.0 mm GTP at 37 °C for 1 h to allow the rebuilding of MT networks from tubulin and MT-associated proteins of the rat brain cytosol. Tau441 (0.1 mg/mL) with or without phosphorylation was added to the rat brain cytosol to study its effect on the formation of the MT network. Finally, the cells were fixed with 0.3% glutaraldehyde/0.5% Nonidet P-40 for 10 min and double-labeled with mouse antibody DM1A to α-tubulin and rabbit antibody R134d to tau. The bound antibodies were detected with a mixture of Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 594-conjugated goat anti-rabbit IgG. The slides were mounted in antifade reagent (Invitrogen, Carlsbad, CA, USA) and examined with an Eclipse E800 epifluorescent microscope (Nikon). Images were captured and processed with a PCM 2000 Confocal Microscope.
Tau self-aggregation assays
The control-treated tau and the tau phosphorylated by various kinases to a stoichiometry of ~2 mol Pi/mol tau, as described above, were incubated at 0.5 mg/mL in 50 mm Tris-HCl (pH 7.2) at 37 °C for 12 h. The mixtures were divided into two parts. One part was centrifuged at 30 000 g at 4 °C for 20 min to sediment the self-aggregated tau. Levels of tau in the mixtures and in the resulting supernatants were quantified by using both quantitative western blots and immuno-dot-blots developed with tau antibody 43D, as described above. The other part of the mixture was examined by negatively stained electron microscopy, as described by Alonso et al. (1994).
Results
Dual-specificity tyrosine-phosphorylated and -regulated kinase 1A, glycogen synthase kinase-3β and cAMP-dependent protein kinase phosphorylate tau at different phosphorylation sites
We first phosphorylated recombinant human tau441 to a similar phosphorylation level (~2.0 stoichiometry) by using Dyrk1A, GSK-3β or PKA. At this stoichiometric level, these three kinases phosphorylated tau at their preferred sites. The phosphorylation sites of tau were examined by western blots developed with phosphorylation-dependent and site-specific tau antibodies. As expected, Dyrk1A and GSK-3β phosphorylated tau at all of the proline-directed sites examined with different efficiencies (Fig. 1B) but not at Ser214, Ser262 or Ser409 that are not followed by proline (data not shown). Comparing with GSK-3β, Dyrk1A mainly phosphorylated Ser/Thr at the P-region, whereas GSK-3β mainly phosphorylated Ser/Thr at the C-region (i.e. Ser396 and Ser404) under these conditions. Among these phosphorylation sites, Thr181, Ser199, Ser202, Thr205, Thr212 and Thr217 were the major phosphorylation sites by Dyrk1A, and Ser396/Ser404 were the predominant sites by GSK-3β. In contrast to Dyrk1A and GSK-3β, PKA did not phosphorylate tau at the above proline-directed sites (data not shown) but instead phosphorylated tau at Ser214, Ser262 and Ser409, which span over the P-region, the MT-binding repeats and the C-region of tau (Fig. 1C).
Tau phosphorylation at various sites affects its activity differently
The main known biological function of tau is to stimulate MT assembly. To investigate the effects of tau phosphorylation at different phosphorylation sites or regions on its biological activity, we measured the MT assembly activity of unphosphorylated tau441 and tau441 phosphorylated to ~2.0 mol Pi/mol tau in vitro by using Dyrk1A, GSK-3β or PKA under the same conditions as described above. At this stoichiometric level, these three kinases phosphorylated tau at distinct sites/regions (Fig. 1). MT assembly was monitored by measuring the assembly mixture turbidity (O.D.350 nm) at real times. Without tau, the turbidity of the tubulin solution did not increase during incubation, indicating that no MT was formed from tubulin subunits under the conditions used (Fig. 2A). When control-treated tau was added to the tubulin solution, the turbidity increased dramatically, indicating the assembly of MTs. When Dyrk1A-phosphorylated tau was used, the rate of MT assembly was much slower and ~30% less MT assembly was seen with Dyrk1A-phosphorylated tau as compared with the control-treated tau (Fig. 2A). In contrast, GSK-3β-phosphorylated tau had ~10% higher activity in stimulating MT assembly than the control-treated tau (Fig. 2B). When PKA-phosphorylated tau was used, ~70% less MT assembly activity was observed as compared with control-treated tau (Fig. 2C).
Fig. 2.
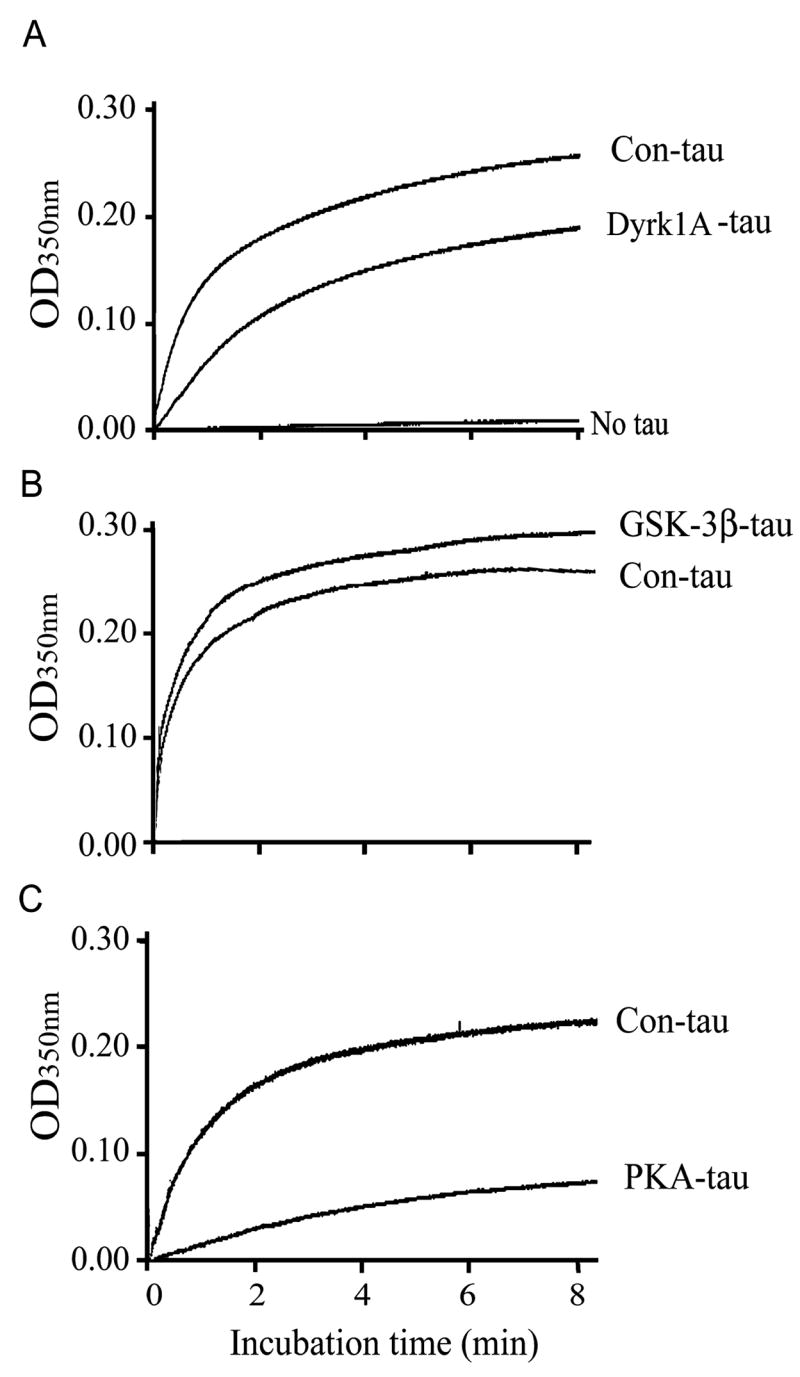
Differential effects of tau phosphorylation by different protein kinases on its ability to stimulate microtubule (MT) assembly. Real-time MT assembly was monitored by measuring the turbidity (O.D.350 nm) of tubulin (2.0 mg/mL) at 35 °C in the presence of 0.07 mg/mL control-treated tau441 (Con-tau) or tau441 phosphorylated by dual-specificity tyrosine-phosphorylated and -regulated kinase 1A (Dyrk1A) (A), glycogen synthase kinase-3β (GSK-3β) (B) or cAMP-dependent protein kinase (PKA) (C). Representative real-time turbidimetric changes of MT assembly of three independent experiments with the same results are shown.
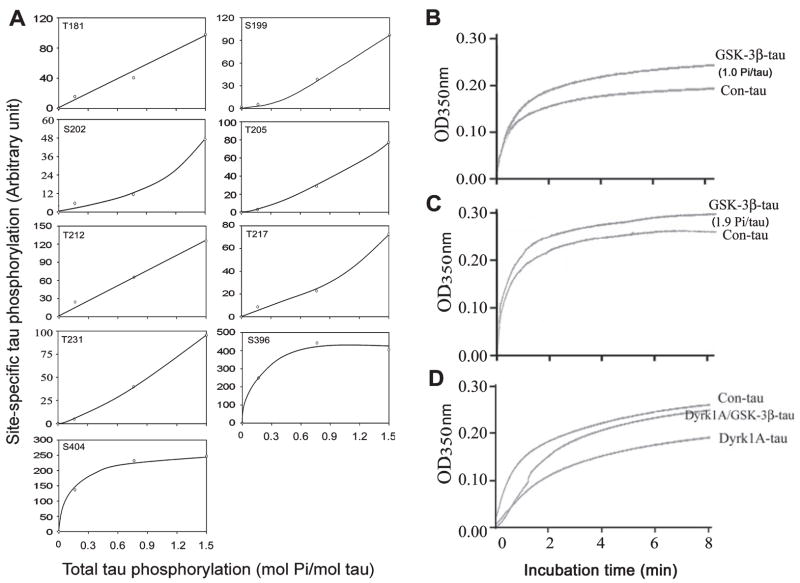
It is generally believed that phosphorylation of tau inhibits its biological activity. To confirm our finding that GSK-3β-catalysed phosphorylation increases tau’s activity of stimulating MT assembly, we phosphorylated tau to different phosphorylation levels with GSK-3β and studied its activity. We found that when tau was phosphorylated to a level of 1.0 mol Pi/mol tau, the phosphorylation at Ser396 and Ser404 had already reached saturation, whereas phosphorylation at other sites in the P-region, including Thr181, Ser199, Ser202, Thr205, Thr212, Thr217 and Thr231, was very little and was just at the initial stages of phosphorylation (Fig. 3A). This tau was also found to have a significantly higher activity in stimulating MT assembly than the control-treated tau (Fig. 3B). Further phosphorylation of tau with GSK-3β occurred mainly at the sites of the P-region but not at Ser396/Ser404 (C-region) (Fig. 3A). When we compared the MT assembly activity between the less phosphorylated tau (1.0 mol Pi/mol tau) and the more phosphorylated tau (1.9 mol Pi/mol tau), we found that the more phosphorylated tau had slightly less activity in stimulating MT assembly (compare Fig. 3B with 3C). These results are consistent with the above observation that tau phosphorylation at the C-region increased its activity but tau phosphorylation at the P-region inhibited its activity of stimulating MT assembly. To further confirm this phenomenon, we compared tau activities between tau phosphorylated with Dyrk1A alone (mainly at the P-region) and tau phosphorylated by both Dyrk1A and GSK-3β (at both the P-region and C-region). As expected, the inhibition of MT assembly activity of tau due to Dyrk1A-induced phosphorylation at the P-region was partially reversed by further phosphorylation at the C-region of tau with GSK-3β (Fig. 3D). These results together indicate that phosphorylation of tau at the C-region (Ser396 and Ser404) increases its MT assembly activity, whereas phosphorylation at the P-region inhibits its activity.
Fig. 3.
Kinetics of glycogen synthase kinase-3β (GSK-3β)-catalysed tau phosphorylation at individual sites and its impact on microtubule (MT) assembly activity. (A) Tau441 was incubated with GSK-3β for various periods of time and the aliquots of the reaction mixture were examined for both the stoichiometry of total tau phosphorylation and tau phosphorylation at individual sites. The site-specific phosphorylation was then plotted against the total tau phosphorylation. (B–D) Real-time MT assembly was monitored by measuring the turbidity (O.D.350 nm) of tubulin (2.0 mg/mL) at 35 °C in the presence of 0.07 mg/mL control-treated tau441 (Con-tau) or tau441 phosphorylated by GSK-3β to a stoichiometry of 1.0 mol phosphates (Pi)/mol tau (B) or 1.9 mol Pi/mol tau (C), or first phosphorylated by dual-specificity tyrosine-phosphorylated and -regulated kinase 1A (Dyrk1A) to 2.0 mol Pi/mol tau and then further phosphorylated by GSK-3β to a total stoichiometry of 4.3 mol Pi/mol tau (D). Representative data of three independent experiments with the same results are shown.
Tau phosphorylation at various sites affects rebuilding of the microtubule network in situ differently
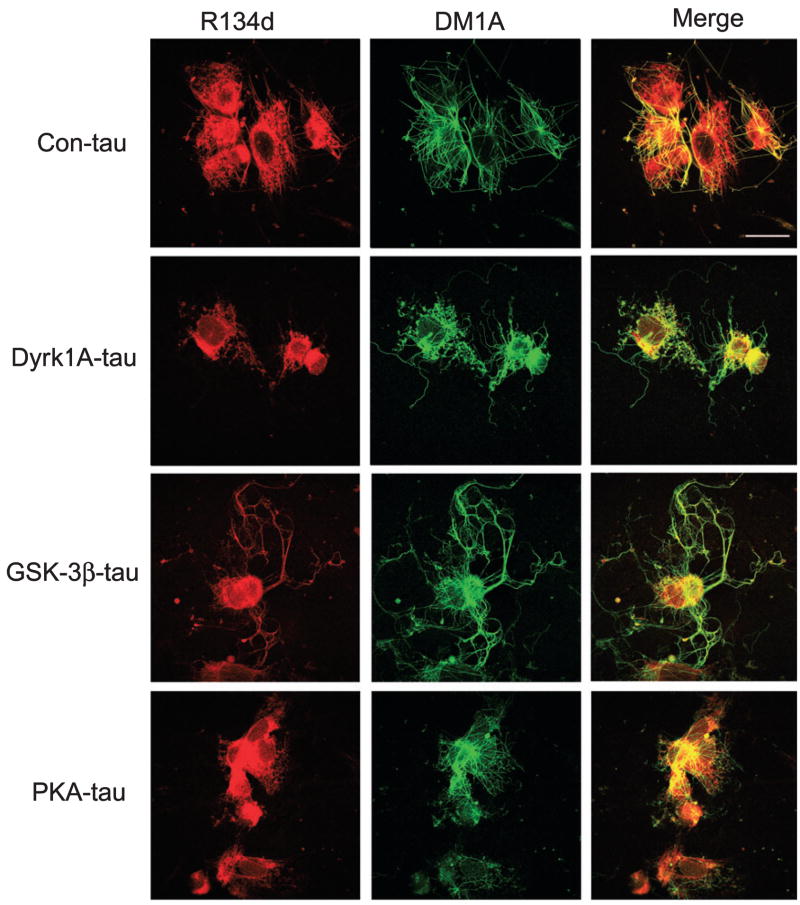
It is known that hyperphosphorylated tau can sequester normal tau and other MT-associated proteins and disrupt MT assembly (Alonso et al., 1994, 1996, 1997; Li et al., 2007). We thus studied the effect of tau phosphorylation at various sites/regions on its ability to interfere with the rebuilding of the MT network in 3T3 cells. When the nocodozole-treated and detergent-extracted 3T3 cells are incubated with fresh rat brain cytosol, the MT network can be formed from tubulin and MT-associated proteins present in the rat brain extract (Li et al., 2007). A similar MT network was seen when the control-treated tau was added to the rat brain extracts (Fig. 4). When Dyrk1A-phosphorylated tau was added instead, we observed slightly less MT network as compared with the control-treated tau. However, when GSK-3β-phosphorylated tau was added to the rat brain cytosol, a clearly larger and longer MT network was formed, and tau was better colocalized with MTs compared with when control tau was used. In contrast, much fewer MTs were formed and tau was not largely colocalized with MTs when PKA-phosphorylated tau was added to the cytosol for MT rebuilding. These results suggest that the PKA-induced phosphorylated tau at the P-region, the MT-binding repeats and the C-region decreases MT assembly, whereas the GSK-3β-induced phosphorylated tau at the C-region facilitates MT assembly. These results are consistent with the above results obtained by turbidimetric studies.
Fig. 4.
Effect of tau phosphorylation at various sites on rebuilding of the microtubule (MT) network in situ. Nocodazole-treated and Triton X-100-extracted 3T3 cells were incubated at 37 °C for 1 h with 15% fresh rat brain cytosol in MT assembly buffer containing 0.5 mg/mL control-treated tau (Con-tau) or tau phosphorylated with dual-specificity tyrosine-phosphorylated and -regulated kinase 1A (Dyrk1A), glycogen synthase kinase-3β (GSK-3β) or cAMP-dependent protein kinase (PKA). The cells were then doubly stained with monoclonal antibody DM1A against tubulin (green) and polyclonal antibody R134d against tau (red), and visualized using a confocal microscope. Bar, 25 μm.
Tau phosphorylation at various sites promotes its self-aggregation differently
Tau hyperphosphorylation has been shown to promote its self-aggregation into filaments. To investigate the impact of tau phosphorylation at various sites by the three kinases on promoting its self-aggregation, we phosphorylated tau with Dyrk1A, GSK-3β or PKA to a stoichiometry of ~2 mol Pi/mol tau, which allowed tau phosphorylation at distinct sites/regions by these kinases (see Fig. 1). We then analysed the total tau and the non-aggregated tau after centrifugation. We found that almost all of the control-treated tau (without phosphorylation) stayed in the supernatant, suggesting no significant self-aggregation (Fig. 5A). However, the amounts of the phosphorylated tau, especially of the GSK-3β-phosphorylated tau, in the supernatants were smaller than those in the samples before centrifugation, suggesting that significant amounts of tau were aggregated and spun down by centrifugation. We further quantified tau self-aggregation by measuring the non-aggregated tau levels in the supernatants by immuno-dot-blots. We found that Dyrk1A-, GSK-3β-and PKA-induced tau phosphorylation reduced the amounts of the non-aggregated tau in the supernatants by 30, 58 and 40%, respectively (Fig. 5B and C). These results suggest that tau phosphorylation at the C-region by GSK-3β promotes its self-aggregation more efficiently than tau phosphorylation at the P-region and the MT-binding repeats.
Fig. 5.
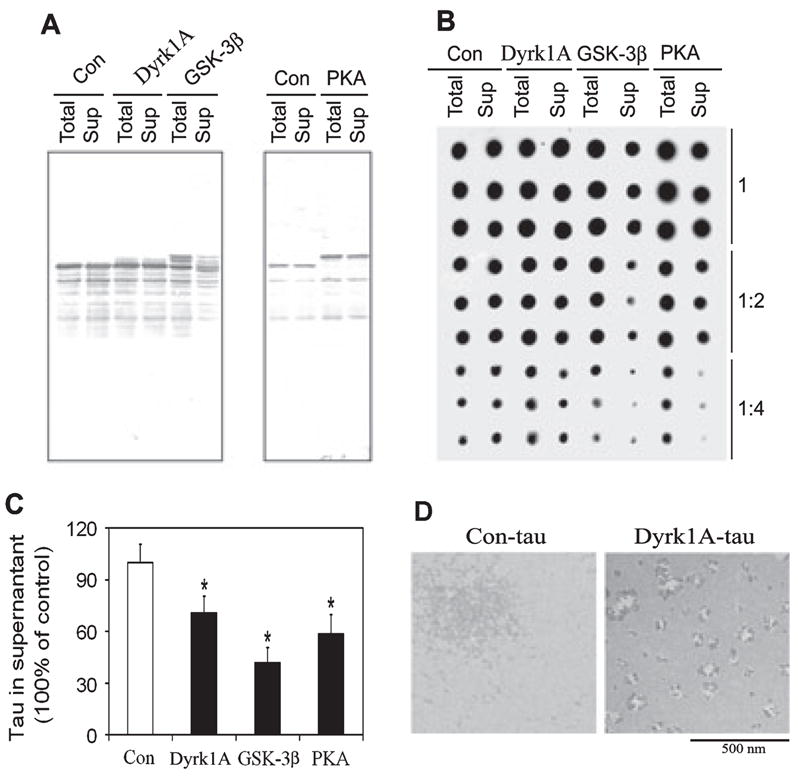
Effect of phosphorylation by dual-specificity tyrosine-phosphorylated and -regulated kinase 1A (Dyrk1A), glycogen synthase kinase-3β (GSK-3β) and cAMP-dependent protein kinase (PKA) on self-aggregation of tau. Tau441 was phosphorylated by Dyrk1A, GSK-3β or PKA to a stoichiometry of ~2.0 mol phosphates/mol tau and then incubated at 37 °C for 12 h, followed by centrifugation at 30 000 g for 20 min. The equivalent amounts of the supernatants (Sup) and the samples before centrifugation (Total) were assayed for tau levels by both western blots (A) and immuno-dot-blots (B and C). In B, all samples were analysed in three different dilutions and in triplicate, and the quantification of the blots is shown in C. Tau aggregates in the preparations were also examined by negatively stained electron microscopy (D). Con, control; Con-tau, control-treated tau. *P < 0.05 as compared with control group.
We noticed that incubation for phosphorylation of tau did not induce any further proteolysis of tau, which otherwise might have promoted tau aggregation (Wang et al., 2007b). We also examined the tau aggregates by negatively stained electron microscopy and found that these aggregates were mainly non-filamentous aggregates (Fig. 5D). Occasionally, some thin filaments were seen in the pellets of GSK-3β-phosphorylated tau (data not shown).
Discussion
The biological activity of tau is regulated by its phosphorylation level. Neither unphosphorylated nor hyperphosphorylated tau has the optimal activity to bind to MTs, suggesting that phosphorylation at certain sites is beneficial whereas at other sites it is inhibitory to its binding to MTs (de Ancos et al., 2003). Hyperphosphorylation of tau has been demonstrated to inhibit its biological activity to stimulate MT assembly and promote its self-aggregation into filaments (reviewed in Iqbal et al., 2005; Avila, 2006). Hyperphosphorylated tau isolated from AD brain contains more than 30 phosphorylation sites (reviewed in Gong et al., 2005). It is not fully understood how tau phosphorylation at various sites differentially affects its biological activity and self-aggregation. In this study, we phosphorylated recombinant tau in vitro with Dyrk1A, GSK-3β and PKA to a stoichiometry of ~2 mol Pi/mol tau, which allowed phosphorylation at different preferred regions/sites of the tau molecule by these kinases. We found that tau phosphorylation at the sites of the P-region with Dyrk1A inhibited its activity of stimulating MT assembly. This inhibition became much stronger when tau was phosphorylated with PKA at the MT-binding repeats and the C-region in addition to the P-region. On the contrary, tau phosphorylation by GSK-3β only at the C-region increased its activity of stimulating MT assembly. Because the overall phosphorylation levels of tau with these three kinases were the same (~2 mol Pi/mol tau), the differences were due to site/region-specific phosphorylation, rather than the total phosphorylation levels. Our findings are in agreement with previous reports showing that phosphorylation of tau at Ser262/Ser356 in the MT-binding repeats inhibits its activity to stimulate MT assembly (Biernat et al., 1993; Drewes et al., 1995; Singh et al., 1996; Biernat & Mandelkow, 1999).
It was very interesting to observe that phosphorylation at Ser396/Ser404 in the C-region of tau with GSK-3β increases its activity of stimulating MT assembly. To confirm these unexpected observations, we studied the activity of tau after phosphorylation to different levels and thus at different sites with GSK-3β alone and also together with Dyrk1A. These experiments further indicated that tau phosphorylation at Ser396/Ser404 indeed stimulates its activity and this activation can be weakened by additional phosphorylation at sites located in the P-region. It has been shown that GSK-3β can phosphorylate tau at both unprimed (e.g. Ser396 and Ser404) and primed (e.g. Thr181 and Thr231) sites (Cho & Johnson, 2003; Liu et al., 2006b), which affects the activity of tau differently. Phosphorylation of tau at the primed sites decreases its binding to MTs but that at the unprimed sites does not (Cho & Johnson, 2003). Ser396/Ser404-phosphorylated tau is found exclusively in the MT-bound fraction, as determined by monoclonal antibody PHF-1 (Cho & Johnson, 2003), also suggesting that it has a strong ability to bind to MTs.
By using a mixture of GSK-3α and GSK-3β, it was previously observed that tau phosphorylation by GSK-3 inhibits its ability to stimulate MT assembly (Wang et al., 1998). The apparent contradiction of the present study by that study may be because GSK-3α also phosphorylates tau in the P-region and the MT-binding region, such as at Ser235 and Ser262 (Wang et al., 1998; Sengupta et al., 2006). Tau phosphorylation at these sites strongly inhibits its MT assembly-stimulating activity (Biernat et al., 1993; Biernat & Mandelkow, 1999; Drewes et al., 1995; Wang et al., 1998, 2007a) and could have overridden the activation caused by GSK-3β-induced phosphorylation at Ser396/Ser404. This analysis is supported by our observations that further phosphorylation at the P-region reduced the MT assembly-stimulating activity of tau caused by phosphorylation only at Ser396/Ser404. When tau is phosphorylated by GSK-3β to a stoichiometry of 6 mol Pi/mol tau, which highly phosphorylates several phosphorylation sites in the P-region in addition to the C-region, an overall inhibition of MT assembly activity was also observed (Wang et al., 2007a). Taken together, tau phosphorylation in the C-region stimulates its activity but phosphorylation in the P-region and the MT-binding repeats inhibits its activity with a much stronger potency. Phosphorylation in all of these regions has an overall strong inhibitory effect on the activity of tau in stimulating MT assembly.
The aggregation of abnormally hyperphosphorylated tau into neurofibrillary tangles is a hallmark brain lesion of AD (reviewed in Avila, 2006). Previous studies have demonstrated that hyperphosphorylation is critical to convert soluble tau into neurofibrillary tangles (reviewed in Iqbal et al., 2005; Gong et al., 2006). However, tau phosphorylation at various sites may play different roles in promoting its self-aggregation into tangles. On the basis of several in-vitro studies, Alonso et al. (2004) proposed a model explaining the mechanism by which hyperphosphorylation promotes its self-aggregation into filaments. This hypothesis suggests that tau aggregates via its MT-binding repeats but normally the regions flanking the MT-binding repeats are inhibitory to the self-aggregation. Phosphorylation in these two regions neutralizes these basic domains and thus enables tau–tau interaction. In the case of the C-region, the highly acidic segment normally masks the MT-binding repeats. Phosphorylation in this region opens this segment and allows the self-aggregation of tau. Our findings that phosphorylation of tau in both the P-region and the C-region promotes its self-aggregation and that phosphorylation in the C-region by GSK-3β has a larger effect further support the above hypothesis. Consistent with our findings, mutation of Ser396, Ser404 and Ser422 of the C-region of tau into glutamate to mimic phosphorylation makes it more fibrillogenic than the wild-type tau (Abraha et al., 2000; Haase et al., 2004) and mutation of Ser422 to Ala prevents β-amyloid-induced tau aggregation (Ferrari et al., 2003). It should be noted that the phosphorylated tau aggregates observed under our conditions were mainly non-filamentous aggregates. This might be because only 2 mol Pi/mol tau were incorporated and a relatively short time was used for aggregation in the absence of polyanions. It has been reported recently that tau first forms non-filamentous aggregates before forming typical filaments (Maeda et al., 2007; Sahara et al., 2007).
A recent structural study demonstrated an important role of the C-region of tau in promoting both MT assembly and self-aggregation (Scaramozzino et al., 2006). This study is consistent with our observations that modifications of tau by phosphorylation at the C-region markedly affect its MT-binding activity and self-aggregation.
In conclusion, by phosphorylation of tau at sites in the selective regions of tau, we demonstrate that tau phosphorylation at the P-region inhibited its MT assembly-stimulating activity and promoted its self-aggregation. Tau phosphorylation at the C-region sites increased its MT assembly-stimulating activity and promoted its self-aggregation markedly. Tau phosphorylation at all of the P-region, MT-binding region and C-region diminished its activity and disrupted the MT network. These studies reveal the differential regulation of the biological activity and self-aggregation of tau by phosphorylation at various sites/regions and may provide more specific information for designing therapies aimed to inhibit hyperphosphorylation of tau.
Acknowledgments
We thank Dr E. El-Akkad for the preparation of recombinant tau441, Dr V. Chauhan for critical reading of the manuscript and Ms J. Murphy for secretarial assistance. This work was supported in part by the New York State Office of Mental Retardation and Developmental Disabilities, National Institute of Health Grants (AG 027429 and AG 019158), Alzheimer Association Grant (IIRG-05-13095), Nantong University, National Natural Science Foundation of China Grant (30572076), Natural Science Foundation of Jiangsu Province (BK2004047, China) and Li Foundation, NY.
Abbreviations
- AD
Alzheimer’s disease
- C-region
C-terminal tail region
- Dyrk1A
dual-specificity tyrosine-phosphorylated and -regulated kinase 1A
- GSK
glycogen synthase kinase
- MT
microtubule
- Pi
phosphates
- PKA
cAMP-dependent protein kinase
- P-region
proline-rich region
- Ser
serine
- Thr
threonine
References
- Abraha A, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, Binder LI. C-terminal inhibition of tau assembly in vitro and in Alzheimer’s disease. J Cell Sci. 2000;113:3737–3745. doi: 10.1242/jcs.113.21.3737. [DOI] [PubMed] [Google Scholar]
- Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated τ in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, del C, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- Alonso A, del C, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, del C, Zaidi T, Novak M, Barra HS, Grundke-Iqbal I, Iqbal K. Interaction of tau isoforms with Alzheimer disease abnormally hyperphosphorylated tau and in vitro phosphorylation into disease like protein. J Biol Chem. 2001a;276:37 967–37 973. doi: 10.1074/jbc.M105365200. [DOI] [PubMed] [Google Scholar]
- Alonso A, del C, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001b;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, del C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004;279:34 873–34 881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- de Ancos J, Correas I, Avila J. Differences in microtubule binding and self association abilities of bovine brain tau isoforms. J Biol Chem. 2003;268:7976–7982. [PubMed] [Google Scholar]
- Avila J. Tau phosphorylation and aggregation in Alzheimer’s disease pathology. FEBS Lett. 2006;580:2922–2927. doi: 10.1016/j.febslet.2006.02.067. [DOI] [PubMed] [Google Scholar]
- Biernat J, Mandelkow EM. The development of cell processes induced by tau protein requires phosphorylation of serine 262 and 356 in the repeat domain and is inhibited by phosphorylation in the proline-rich domains. Mol Biol Cell. 1999;10:727–740. doi: 10.1091/mbc.10.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM. Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Chen-Hwang MC, Chen HR, Elzinga M, Hwang YW. Dynamin is a minibrain kinase/dual specificity Yak1-related kinase 1A substrate. J Biol Chem. 2002;277:17 597–17 604. doi: 10.1074/jbc.M111101200. [DOI] [PubMed] [Google Scholar]
- Cho JH, Johnson GV. Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J Biol Chem. 2003;278:187–193. doi: 10.1074/jbc.M206236200. [DOI] [PubMed] [Google Scholar]
- Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt-Ulms G, Meyer HE, Mandelkow EM, Mandelkow E. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau–microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem. 1995;270:7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- Fath T, Eidenmuller J, Brandt R. Tau-mediated cytotoxicity in a pseudohyperphosphorylation model of Alzheimer’s disease. J Neurosci. 2002;22:9733–9741. doi: 10.1523/JNEUROSCI.22-22-09733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A, Hoerndli F, Baechi T, Nitsch RM, Gotz J. beta-Amyloid induces paired helical filament-like tau filaments in tissue culture. J Biol Chem. 2003;278:40 162–40 168. doi: 10.1074/jbc.M308243200. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989;8:393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Post-translational modifications of tau protein in Alzheimer’s disease. J Neural Transm. 2005;112:813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- Gong C-X, Liu F, Grundke-Iqbal I, Iqbal K. Dysregulation of protein phosphorylation/dephosphorylation in Alzheimer’s disease: a therapeutic target. J Biomed Biotechnol. 2006;3:31 825. doi: 10.1155/JBB/2006/31825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase C, Stieler JT, Arendt T, Holzer M. Pseudophosphorylation of tau protein alters its ability for self-aggregation. J Neurochem. 2004;88:1509–1520. doi: 10.1046/j.1471-4159.2003.02287.x. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, Wisniewski HM, Alafuzoff I, Winblad B. Defective brain microtubule assembly in Alzheimer’s disease. Lancet. 1986;2:421–426. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- Iqbal K, del Alonso AC, Chen S, Chohan MO, El-Akkad E, Gong C-X, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Wiedau-Pazos M, Sang TK, Wagle N, Brown CA, Massachi S, Geschwind DH. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron. 2002;34:509–519. doi: 10.1016/s0896-6273(02)00706-7. [DOI] [PubMed] [Google Scholar]
- Kenessey A, Yen SH. The extent of phosphorylation of fetal tau is comparable to that of PHF-tau from Alzheimer paired helical filaments. Brain Res. 1993;629:40–46. doi: 10.1016/0006-8993(93)90478-6. [DOI] [PubMed] [Google Scholar]
- Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I. Microtubule associated protein tau: abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem. 1993;268:24 374–24 384. [PubMed] [Google Scholar]
- Ksiezak-Reding H, Liu WK, Yen SH. Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res. 1992;597:209–219. doi: 10.1016/0006-8993(92)91476-u. [DOI] [PubMed] [Google Scholar]
- Li B, Chohan MO, Grundke-Iqbal I, Iqbal K. Disruption of microtubule network by Alzheimer abnormally hyperphosphorylated tau. Acta Neuropathol. 2007;113:501–511. doi: 10.1007/s00401-007-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and GSK-3β. FEBS Lett. 2002a;530:209–214. doi: 10.1016/s0014-5793(02)03487-7. [DOI] [PubMed] [Google Scholar]
- Liu F, Zaidi T, Iqbal K, Grundke-Iqbal I, Gong CX. Aberrant glycosylation modulates phosphorylation of tau by protein kinase A and dephosphorylation of tau by protein phosphatase 2A and 5. Neuroscience. 2002b;115:829–837. doi: 10.1016/s0306-4522(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:10 804–10 809. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Liang ZH, Gong CX. Hyperphosphorylation of tau and protein phosphatases in Alzheimer disease. Panminerva Med. 2006a;48:97–108. [PubMed] [Google Scholar]
- Liu F, Liang ZH, Shi JH, Yin DM, El-Akkad E, Grundke-Iqbal I, Iqbal K, Gong CX. PKA modulates GSK-3β- and cdk5-catalyzed phosphorylation of tau in site- and kinase-specific manners. FEBS Lett. 2006b;580:6269–6274. doi: 10.1016/j.febslet.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Sahara N, Saito Y, Murayama M, Yoshiike Y, Kim H, Miyasaka T, Murayama S, Ikai A, Takashima A. Granular tau oligomers as intermediates of tau filaments. Biochemistry. 2007;46:3856–3861. doi: 10.1021/bi061359o. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Gong CX, Iqbal K, Grundke-Iqbal I, Wu QL, Winblad B, Cowburn RF. Subcellular distribution of protein phosphatases and abnormally phosphorylated tau in the temporal cortex from Alzheimer’s disease and control brains. J Neural Transm. 1998;105:69–83. doi: 10.1007/s007020050039. [DOI] [PubMed] [Google Scholar]
- Perez M, Hernandez F, Gomez-Ramos A, Smith M, Perry G, Avila J. Formation of aberrant phosphotau fibrillar polymers in neural cultured cells. Eur J Biochem. 2002;269:1484–1489. doi: 10.1046/j.1432-1033.2002.02794.x. [DOI] [PubMed] [Google Scholar]
- Sahara N, Maeda S, Murayama M, Suzuki T, Dohmae N, Yen SH, Takashima A. Assembly of two distinct dimers and higher-order oligomers from full-length tau. Eur J Neurosci. 2007;25:3020–3029. doi: 10.1111/j.1460-9568.2007.05555.x. [DOI] [PubMed] [Google Scholar]
- Scaramozzino F, Peterson DW, Farmer P, Gerig JT, Graves DJ, Lew J. TMAO promotes fibrillization and microtubule assembly activity in the C-terminal repeat region of tau. Biochemistry. 2006;45:3684–3691. doi: 10.1021/bi052167g. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Novak M, Grundke-Iqbal I, Iqbal K. Regulation of phosphorylation of tau by cyclin-dependent kinase 5 and glycogen synthase kinase-3 at substrate level. FEBS Lett. 2006;580:5925–5933. doi: 10.1016/j.febslet.2006.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh TJ, Wang JZ, Novak M, Kontzekova E, Grundke-Iqbal I, Iqbal K. Calcium/calmodulin-dependent protein kinase II phosphorylates tau at Ser-262 but only partially inhibits its binding to microtubules. FEBS Lett. 1996;387:145–148. doi: 10.1016/0014-5793(96)00485-1. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Wu Q, Smith A, Grundke-Iqbal I, Iqbal K. Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett. 1998;436:28–34. doi: 10.1016/s0014-5793(98)01090-4. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007a;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YP, Biernat J, Pickhardt M, Mandelkow E, Mandelkow EM. Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer-like aggregation of full-length tau in a neuronal cell model. Proc Natl Acad Sci USA. 2007b;104:10 252–10 257. doi: 10.1073/pnas.0703676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods YL, Cohen P, Becker W, Jakes R, Goedert M, Wang X, Proud CG. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem J. 2001;355:609–615. doi: 10.1042/bj3550609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Ihara Y. τ in paired helical filaments is functionally distinct from fetal τ: Assembly incompetence of paired helical filament- τ. J Neurochem. 1993;61:1183–1186. doi: 10.1111/j.1471-4159.1993.tb03642.x. [DOI] [PubMed] [Google Scholar]