Abstract
Bioprosthetic heart valves (BHVs) derived from glutaraldehyde crosslinked porcine aortic valves are frequently used in heart valve replacement surgeries. However, BHVs have limited durability and fail either due to degeneration or calcification. Glycosaminoglycans (GAGs), one of the integral components of heart valve cuspal tissue, are not stabilized by conventional glutaraldehyde crosslinking. Previously we have shown that valvular GAGs could be chemically fixed with GAG-targeted chemistry. However, chemically stabilized GAGs were only partially stable to enzymatic degradation. In the present study an enzyme inhibitor was incorporated in the cusps to effectively prevent enzymatic degradation. Thus, neomycin trisulfate, a known hyaluronidase inhibitor, was incorporated in cusps via 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/ N-hydroxysuccinimide (EDC/NHS) chemistry followed by glutaraldehyde crosslinking (NEG). Controls included cusps crosslinked with either EDC/NHS followed by glutaraldehyde (ENG) or only with glutaraldehyde (GLUT). NEG group showed improved resistance to in vitro enzymatic degradation as compared to GLUT and ENG groups. All groups showed similar collagen stability, measured as a thermal denaturation temperature by differential scanning calorimetry (DSC). The cusps were implanted subdermally in rats to study in vivo degradation of GAGs. NEG group preserved significantly more GAGs compared to ENG and GLUT. NEG and ENG groups showed reduced calcification than GLUT.
Introduction
Cryopreserved aortic heart valve transplants obtained from human cadavers (homografts) have been used since early 1960s [1]. Due to limited availability of cadaver valves, xenograft heart valves fabricated from glutaraldehyde crosslinked porcine aortic valves or from bovine pericardium are frequently used. In 2001 approximately 300,000 patients worldwide underwent heart valve transplantation surgeries and nearly 40% of those patients received tissue based valves [2, 3]. New generation of tissue valves are considerably improved in terms of valve design. However, glutaraldehyde (GLUT) is still the only tissue fixative used for valve fixation. GLUT is known to be an excellent fixative for the collagenous components of the BHVs, however; it does not stabilize other important extracellular matrix components such as glycosaminoglycans (GAGs) and elastin [4, 5]. We have shown previously that GAGs are lost from GLUT crosslinked porcine aortic tissue valves during fixation, storage, in vitro fatigue testing, and after in vivo implantation [5–8]. We believe that cuspal GAGs are important components for valvular biomechanics and loss of GAGs may in part be responsible for degenerative failure of BHVs. We are pursuing additional fixatives that would allow GAG fixation. Such GAG fixation strategies would improve tissue hydration resulting in improved biomechanics such as better shear resistance and shock absorption, and eventually would lead to improved durability. We studied two crosslinkers in the past, viz. sodium meta periodate and EDC for this purpose [7, 9]. Both crosslinkers were effective in preservation of GAGs in the cuspal tissue after crosslinking as compared to GLUT [7, 9]. However, such chemical fixation strategies were only partially effective in preventing enzyme-mediated GAG degradation [7]. We have shown previously that GAG-degrading enzymes invade cuspal tissue after in vivo implantation [6, 7, 10]. Thus, a new strategy is required to prevent enzyme-mediated GAG degradation.
In the present study, we propose a new GAG-targeted fixation method where a hyaluronidase inhibitor, neomycin trisulfate, is linked to the cusps with carbodiimide fixation chemistry. Chemically linked neomycin inhibited enzymatic degradation of cuspal GAGs and thereby resulted in improved stabilization of GAGs in the BHV cusps.
Materials and Methods
Glutaraldehyde (GLUT) – 50 wt% in water, Ammonium acetate, neomycin trisulfate salt hydrate, (D+)-glucosamine HCl, hyaluronidase type VI-s from bovine testes (3000 units), Chondroitinase ABC from Proteus Vulgaris affinity purified (10 units), 1-9-dimethylmethylene blue (DMMB) were all obtained from Sigma Aldrich Corporation (St. Louis, MO). 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), n-hydroxysulfosuccinimide (NHS), and bicinchoninic assay (BCA) protein kits were purchased from Pierce Biotech (Rockford, IL). P-dimethyl aminobenzaldehyde, acetyl acetone, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were purchased from Fisher Scientific (Fair Lawn, NJ), 4-morpholinoethanesulfonic acid hydrate (MES) hydrate and papain were obtained from Acros Organics, NJ. Rabbit anti-neomycin antibody (29.5 mg/ml) was obtained from Biogenesis Inc, NH. Rabbit IgG Vectastain Elite ABC kit (PK6101) was obtained from Vector Laboratories, Burlingame, CA.
Fixation of porcine aortic valve cusps
Porcine aortic heart valves were collected at the time of slaughter from a local abattoir. They were immediately dissected along the cuspal commissures. The cusps were left attached to the aortic sinus at the basal insertion to minimize GAG loss from the cusps. Unbuffered saline was used to rinse the cusps and the cusps were transported to the laboratory in saline on ice. Cusps were chemically crosslinked within 3–4 h of harvesting in three different fixation groups.
Group I
Cusps were fixed in 0.6% GLUT in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) at pH 7.4 and at room temperature for 24 h. This was followed by fixation with 0.2% GLUT in HEPES buffer at pH 7.4 for additional 6 days. This group was designated as GLUT.
Group II
Carbodiimide based fixation chemistry was used for fixation of GAGs in the valve cusps. Cusps were fixed in 30 mM EDC + 6 mM NHS solution buffered with 50 mM 4-morpholinoethanesulfonic acid hydrate (MES) at a pH of 5.5 for 24h at room temperature. This was followed by GLUT crosslinking as per group I. This group was designated as ENG.
Group III
Neomycin trisulfate, a hyaluronidase inhibitor was coupled to the cusps with carbodiimide fixation. Cusps were incubated in 1 mM neomycin trisulfate solution at a pH of 7.4 for 1hr under constant shaking. The cusps were then rinsed with deionized water and subsequently fixed as per ENG fixation shown in group II. This group was designated as NEG.
Cusps in all groups were stored in 0.2% GLUT for at least three weeks prior to further analysis.
Immunohistochemistry (IHC)
Immunoperoxidase staining was performed on cuspal sections in order to visualize the effectiveness of specific binding of neomycin to the cusps. Vectastain elite ABC kit for rabbit IgG and Diaminobenzidine tetrahydrochloride (DAB) peroxidase substrate kit were used to visualize the specific staining (Vector Laboratories, Burlingame, CA). The sections were incubated overnight in rabbit anti-neomycin primary antibody (1:500 dilution). To minimize cross reactivity, rat-adsorbed biotinylated anti-rabbit IgG was used in place of biotinylated secondary antibody provided with the staining kit. Negative staining control was performed with the omission of the primary antibody. The sections were finally counter stained using hematoxylin.
Determination of cusp stability to GAG degrading enzymes
The cusps, cut from their respective sinuses, were cut symmetrically along the radial direction and washed thoroughly in 100 mM ammonium acetate buffer at a pH of 7.0 for 3 x 5 minutes. One half of the cusp was incubated in 1.2 ml of 5 U/ml of hyaluronidase and 0.1 U/ml of chondroitinase ABC (HAase + CSase) in 100 mM ammonium acetate buffer at 37°C and was vigorously shaken at 650 rpm for 24 h. The other half was incubated in ammonium acetate buffer alone as a control. The optimum concentration of these enzymes was chosen as described previously [6]. For papain digestion studies the cusps were digested in 1ml of Papain digestion buffer per cusp (5mM L-cysteine, 100 mM Na2HPO4, 5 mM EDTA, pH 7.5) containing 125 μg/ml of Papain at 60°C for 24 h. The enzyme-digested cusp samples were thoroughly washed in deionized water, lyophilized, and used for GAG quantification using hexosamines assay.
GAG quantification by hexosamine assay
Total tissue hexosamine contents were determined by previously published methods [6, 7]. In brief, lyophilized cusps were hydrolyzed in 2N HCl for 20 h at 95°C in a vacuum dessicator and further dried under nitrogen gas. The residues were dissolved in 2 ml of 1M sodium chloride solution and then reacted with 2 ml of 3% acetyl acetone in 1.25M sodium carbonate. Then 4 ml of 100% ethanol and 2ml of Ehrlich’s reagent (0.18M p-dimethylaminobenzaldehyde in 50% ethanol containing 3N HCl) were added and solutions were left at room temperature for 45 min. The pinkish-red color product was indicative of the tissue hexosamine quantities and the absorbance was read at 540nm [11]. A set of D(+)-glucosamine solutions (0 – 200 μg) were used as a standard curve.
GAG quantification by DMMB assay
The GAGs released into the enzyme solutions (HAase + CSase, or papain) were quantified by DMMB assay as described previously [12–14] with minor modifications. In a 96 well-plate, 20 μl of enzyme solution along with 30 μl of PBE buffer (100 mM Na2HPO4, 5 mM EDTA, pH 7.5) and 200 μl of DMMB Reagent Solution (40 mM NaCl, 40 mM Glycine, 46 μM DMMB, pH 3.0) were added into each well and absorbances were read at 525 nm. Chondroitin sulfate (0 – 1.25 μg) standards were used. As additional controls, the chondroitin sulfate standards were also treated with 20 μl of the enzyme mixture (HAase + CSase).
Determination of collagen stability
Differential Scanning Calorimetry (DSC) (Model DSC 7, Perkin-Elmer, Boston, MA) was used to determine the thermal denaturation temperature (Td), which is an indicator of collagen crosslinking stability [6, 7, 15]. Td, the temperature at the endothermic peak, was measured for cuspal tissue fixed with GLUT, ENG and NEG groups (n=6 per group) by heating the samples at a rate of 10°C/min from 20°C to 110°C in hermetically sealed aluminum pans.
Subdermal implantation studies
Cusps fixed with GLUT, ENG and NEG (n=10 per group) were rinsed in sterile saline prior to implantation. Male juvenile Sprague-Dawley rats (35–40g, Harlan Laboratories, Indianapolis, IN) were anesthetized by inhalation of 3% isoflurane gas and two subdermal pockets were created in the dorsal side. Cusps were implanted into the pocket (one cusp per pocket, 2 cusps per rat) and the incision was closed using surgical staples. Cusps and the capsule tissue surrounding the cusps were explanted after three weeks. All animals received humane care in compliance with protocols approved by Clemson University Animal Research Committee as formulated by NIH (Publication No. 86-23, revised 1996). Cuspal explants were further used for hexosamine, calcium, and histological analysis. Cuspal explants for GAG quantification were immediately frozen on dry ice and lyophilized.
For histological evaluation using paraffin sections, samples were placed in 10% alcoholic acid formalin at room temperature for 24h prior to infiltration and embedding. GAGs were visualized in the samples using an Alcian Blue staining with Brazilliant!® nuclear fast red as a counter stain [6].
Calcium analysis from explanted samples
For calcium analysis cusps were hydrolyzed using 6N Ultrex II pure hydrochloric acid (J. T. Baker, Philipsburg, NJ) for 8h in a boiling water bath. This was followed by evaporating the HCl under nitrogen gas and reconstitution of the residue in 1 ml of 0.001 N Ultrex II HCl. Atomic absorption spectroscopy (Perkin-Elmer 3030 Atomic Absorption Spectrophotometer, Norwalk, CT) was used to measure the calcium content of the samples [16].
Gel electrophoresis (zymography)
To determine the presence of GAG degrading enzymes in vivo and the inhibitory effect of neomycin trisulfate on hyaluronidase, a substrate gel electrophoresis (zymography) was performed as previously described [7, 10] on tissue capsules surrounding the cusps after 3 weeks of implantation. Briefly, 10% polyacrylamide gels containing 170 μg/ml of hyaluronic acid were prepared. The capsules were homogenized and 20 μg of soluble protein (quantified by BCA assay) were loaded into each lane. The gels were then run at 90 V for 150 min in running buffer (25 mM Tris, 2 mM Glycine, 1% SDS and pH 8.3). Protein molecular weights standards (10 – 150 kDa) were used to identify the molecular weight. The gels were then incubated in a developing buffer (50 mM citric acid, 50 mM dibasic sodium phosphate, 8 g/l sodium chloride, 0.02% sodium azide, pH 3.75) for 20 h at 37°C. For studying inhibitory effect of neomycin trisulfate, 1 mM of neomycin trisulfate was added to the developing buffer. After developing, the gels were stained with 0.5% Alcian blue in 20% methanol and 10% acetic acid. GAG-degrading enzyme activities appeared as clear bands on a blue background of undigested GAGs. Images were taken with a digital camera and the lanes were analyzed using densitometry.
Statistical Analysis
The results were expressed as a mean ± standard error of the mean (SEM) and statistical analysis was performed using single-factor analysis of variance (ANOVA). Subsequently, differences between means were determined using the least significant difference (LSD) with an alpha value of 0.05.
Results
Neomycin binding to the cusps
Immunohistochemical staining showed neomycin staining throughout the cusp tissue (Figure 1A) suggesting that neomycin was bound uniformly throughout the cusp tissue. The staining was negative in control ENG group where neomycin was not linked to the tissue (Figure 1B). Negative controls with omission of primary antibodies were also negative (data not shown).
Figure 1.
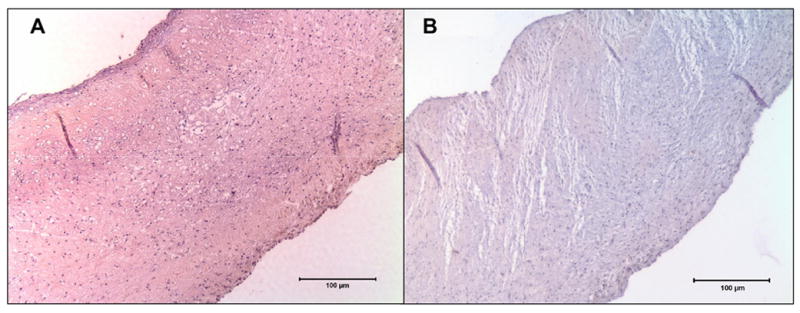
Immunoperoxidase staining for Neomycin sulfate; A. Cusps in the NEG group showed uniform brown stain for neomycin, B. Cusps in the ENG group where neomycin was not added showed no staining.
Histological GAG staining
Qualitative amounts of GAGs present in the cusps in the three groups were determined by Alcian Blue staining with Brazilliant!® nuclear fast red as counter stain. Cusps crosslinked with GLUT retained the least amount of GAGs as seen by the least amount of blue stain, while cusps in ENG group and in NEG group retained more GAGs as seen by the higher intensity of blue stain (Figure 2).
Figure 2.
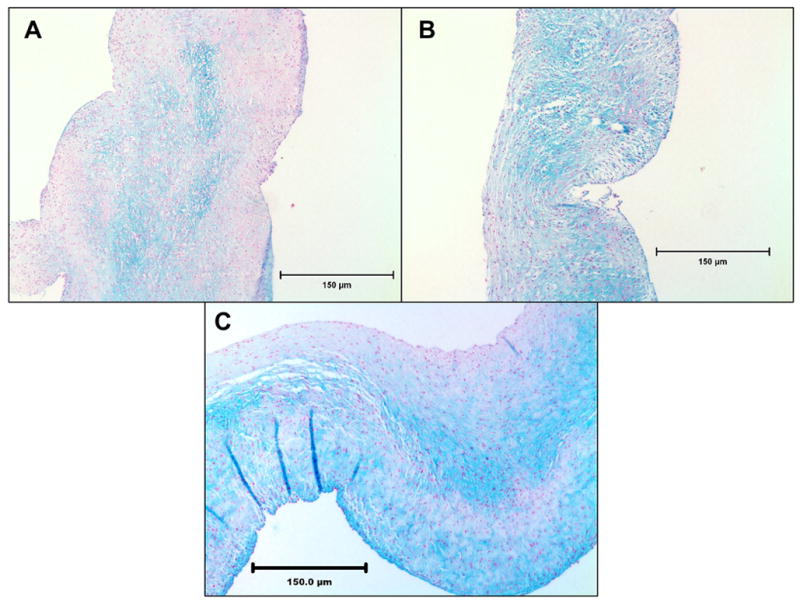
Alcian blue staining of cusps for GAGs after one week of fixation and four weeks of storage in 0.2% GLUT. (A) GLUT (B) ENG and (C) NEG. GLUT group showed least staining while NEG group showed intense staining for GAGs. Most of the GAGs were in the middle spongiosa layer.
Stability of cuspal GAGs towards GAG-degrading enzymes
To assess the effectiveness of bound neomycin in preventing enzyme digestion of cuspal GAGs, crosslinked cusps were treated with a chondroitinase and hyaluronidase mixture. The hexosamine data showed that GAGs were lost from GLUT group (Figure 3 A). NEG group retained more GAGs after digestion as compared to the other groups (p<0.05). There was no significant difference (p<0.05) between the digested and undigested values in NEG and ENG groups. It is to be noted that there were significantly lower GAGs in cusps in GLUT and ENG group before enzyme digestion suggesting that GAGs were unstable. The enzyme solutions were analyzed for released GAGs by DMMB assay (Figure 3 B). Insignificant amounts (p<0.05) of GAGs were released in to the enzyme solution from cusps in NEG group while significantly higher (p<0.05) GAG contents were seen in enzyme solutions from GLUT and ENG groups, clearly suggesting that binding of neomycin sulfate to the cusps lead to almost complete resistance to GAG-degrading enzymes.
Figure 3.
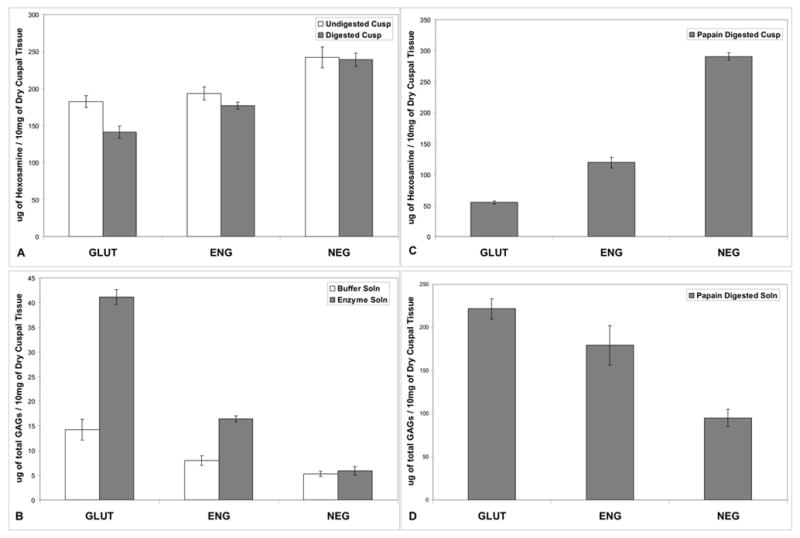
A. Cuspal tissue hexosamine content after one week of fixation and four weeks of storage in 0.2% GLUT showed higher GAG content in the NEG group than the ENG and GLUT groups (p<0.05). NEG group also retained more GAGs after hyaluronidase and chondroitinase treatment (p<0.05); B. GAGs released from the cusps into the enzyme solution was measured by DMMB assay. Significantly fewer amounts of GAGs were released in NEG group showing better stabilization efficiency (p<0.05). C. Hexosamine assay performed on the cusps after papain digestion showed highest GAG retention in NEG group. Some retention was also seen in ENG group (p<0.05); D. DMMB assay on papain solution showed least amount of GAGs released (p<0.05) into papain solution in the NEG group confirming that GAGs were stabilized efficiently in this group.
To further test the stability of crosslinked GAGs against enzymes, cusps were treated with papain enzyme. This is harsher GAG digestion method and it has been used to remove GAGs from tissues for GAG analysis [12–14]. The cusps after papain digestion were used for hexosamine assay. The cusps in NEG group retained most GAGs after papain digestion followed by ENG (p<0.05). GLUT showed the least resistance to papain digestion and retained the least amount of GAGs (Figure 3C). The amount of degraded GAGs released into the papain solution was analyzed using DMMB assay. Cusps from NEG group released least amount of GAGs into papain digestion buffer (Figure 3D). These results were complementary to the hexosamine assays on the cusps.
Thermal Denaturation Temperature (Td)
Thermal denaturation temperatures (Td) of the fixed aortic valve cusps from the three groups were measured to ensure that the targeted GAG-fixation chemistry does not alter the collagen stability. Td values were similar for all groups (NEG: 92.26 ± 0.43, ENG: 91.94 ± 0.36, GLUT: 91.37 ± 0.35, °C, p<0.05) suggesting that the targeted GAG-fixation chemistry did not alter collagen stability in the valve cusps.
In vivo cuspal GAG stability and cusp calcification
The cusps in the NEG group retained significantly more GAGs as compared to ENG and GLUT groups after three weeks of subdermal implantation (p<0.05), (Figure 4A). Calcium assay showed that cusps from NEG and ENG groups had significantly (p<0.05) lower calcification compared to GLUT (Figure 4B). However the GAG stabilization did not lead to inhibition of calcification. As all groups were terminally fixed with glutaraldehyde, which is known to exacerbate calcification [1, 3, 17], it is possible that glutaraldehyde effect was dominant in the calcification process.
Figure 4.
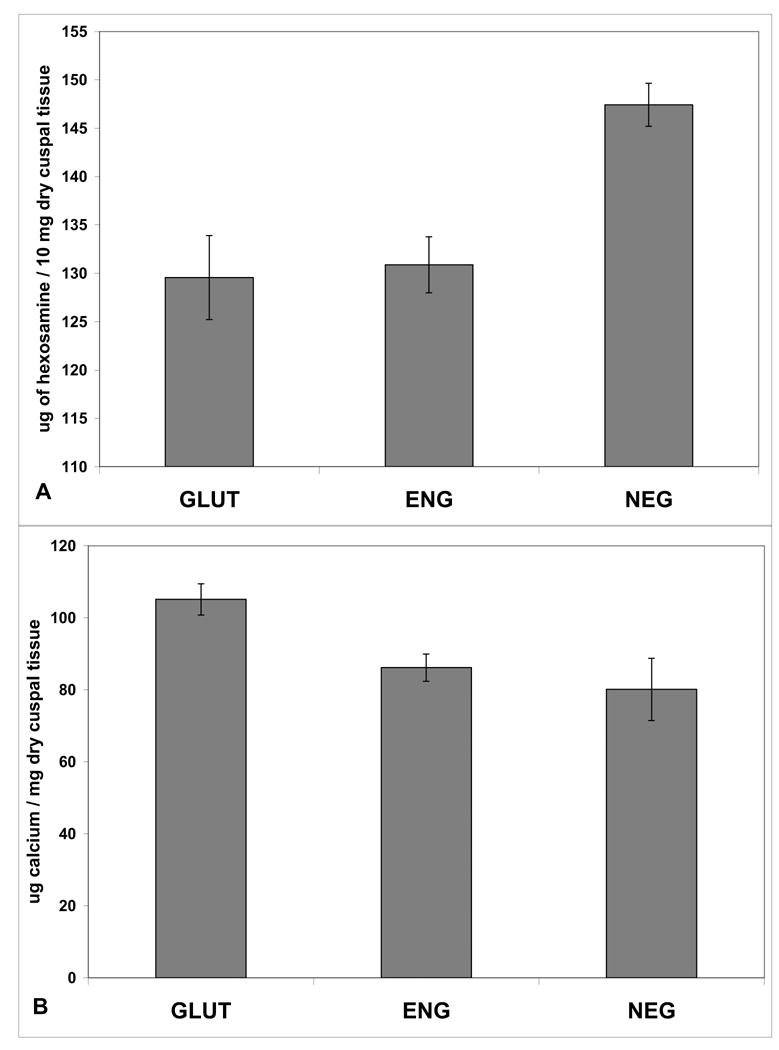
A. Hexosamine assay on cusps explanted after three weeks. The NEG group has significantly greater GAGs retained as compared to the other two groups (p<0.05). B. Calcium assay on three weeks explanted cusp samples showed lower calcification in NEG and ENG groups as compared to GLUT (p<0.05).
Alcian Blue staining for tissue sections from the three different groups confirmed quantitative GAG data. The cusps in NEG group retained more GAGs as seen by the higher intensity of blue stain and GLUT retained the least amount of GAGs as seen by the least intensity of blue stain (Figure 5). These results confirmed that NEG treatment was more effective for GAG stabilization than ENG and GLUT crosslinking. Tissue capsules surrounding cusps were analyzed for HA-degrading enzymes by HA zymography. This assay showed similar band intensities for active hyaluronidases in all groups (Figure 6). Thus, presence of more GAGs in the NEG group was not due to lower enzyme activity in this group as compared to other groups. When neomycin trisulfate was added to the development buffer it inhibited enzyme activity (Figure 6). This suggests that incorporation of neomycin trisulfate into cuspal structures could prevent enzyme-mediated degradation of GAGs in BHVs.
Figure 5.
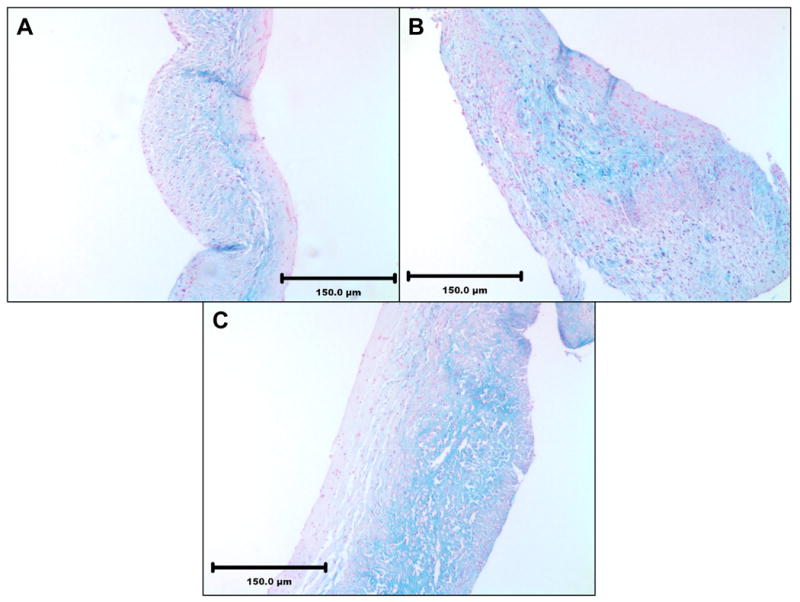
Alcian blue staining of cusps after three weeks explantation showing more GAGs retained in the NEG group. (A) GLUT (B) ENG (C) NEG
Figure 6.
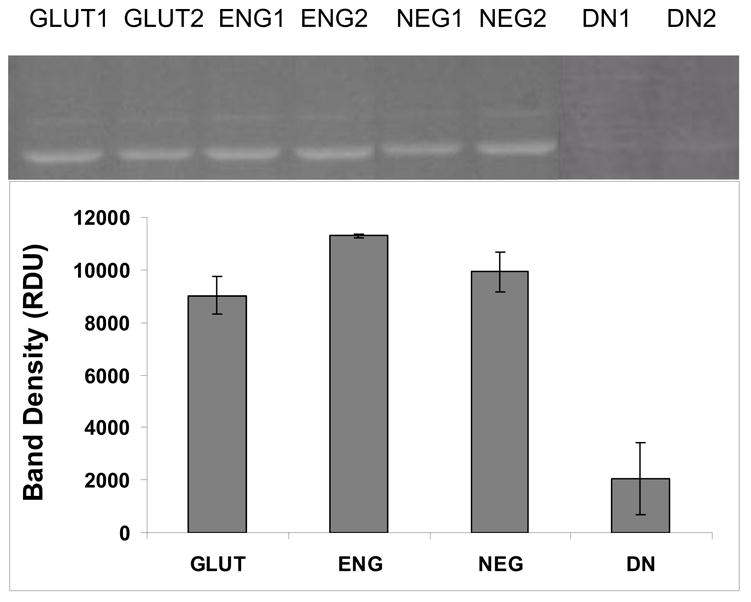
HA gel zymography on soluble proteins extracted from the capsule surrounding the three weeks explanted cusps revealed equal amounts of hyaluronidase activity (migrating at ~ 55 kD) in all the groups. Addition of 1mM neomycin trisulfate to the developing buffer inhibited enzyme activity (lanes DN1 and DN2).
4. Discussion
Glycosaminoglycans are high molecular weight, hydrophilic, and anionic polysaccharides that are able to support large compressive loads [18]. These molecules are present extensively in cartilage, heart valve cusps and other cardiovascular connective tissues. It has been shown previously that GAGs play an important role in cartilage lubrication and biomechanical function [19]. Also it has been shown that GAGs are responsible for the water storage capacity that gives the articular cartilage its unique property of hydration and compressibility [20]. GAGs are present in the spongiosa layer of heart valve cusps and prevent local compressive buckling during valve function. Glutaraldehyde crosslinking of porcine aortic valves does not stabilize GAGs present in the cusps and these GAGs leach out during storage, in vitro cyclic fatigue, and in vivo implantation [6, 7, 10]. Such loss of GAGs may be partly responsible for valve deterioration [3].
Our previous attempts to chemically stabilize GAGs were only partially effective in preventing GAG loss. Enzyme mediated GAG degradation could not be abolished by chemical GAG stabilization [6, 7, 10]. The purpose of the present study was to evaluate the effectiveness of a hyaluronidase inhibitor coupled with carbodiimide based crosslinking chemistry as a targeted GAG stabilization strategy for improved valve function.
We chose neomycin trisulfate as a GAG enzyme inhibitor for several reasons. It has been shown that sulphated neomycin possess comparable inhibition for all hyaluronidases and much more than the widely accepted hyaluronidase inhibitor apigenin [21]. Our HA zymography studies also showed that neomycin trisulfate has an inhibitory effect on hyaluronidase. Neomycin trisulfate also has amine functionalities that could be utilized to chemically attach it to the cusps with carbodiimide chemistry. Thus, binding of neomycin to the cusps was achieved by this chemistry. IHC studies clearly showed that such binding of neomycin was effective and it was uniformly bound throughout the cusp tissue.
Chemically bound neomycin significantly inhibited enzyme-mediated GAG degradation in the cuspal tissue. This, to our knowledge, is the first time that bound neomycin was shown to be effective in preventing GAG degradation in BHVs. Sulphated oligosaccharides such as neomycin trisulfate have a combination of hydrophilic moiety with an affinity-conferring lipophilic residues that bind to hyaluronidase thereby blocking the enzymatic degradation [21]. It has been shown previously [15] that EDC activates the carboxyl group of the GAGs and collagen and this further forms a stable intermediate with NHS. This intermediate forms an amide crosslinks with the free amine groups of collagen thereby crosslinking the GAGs effectively. Carboxyl groups of GAGs are the active sites for enzyme-mediated degradation [22, 23]. Thus, ENG crosslinking was partially effective in preventing GAG degradation as some of the carboxyl groups were utilized in GAG crosslinking. However, EDC crosslinking does not crosslink all carboxyls present in the structure of GAGs and some of the carboxyl residues are still prone to enzyme-mediated degradation. Additional binding of neomycin trisulfate along with ENG crosslinking (NEG group) inhibited enzyme-mediated degradation and thus further prevented GAG loss. Our data showed that binding of neomycin inhibited GAGases as well as papain mediated degradation. Hexosamine values in the cusps in GLUT and ENG groups after papain digestion were much lower than after GAGase digestion. This may be because papain digests non-GAG related hexosamines from the tissue. Binding of neomycin also significantly prevented degradation of non-GAG related hexosamines. Whether this has any clinical implications is uncertain at this time.
Collagen fibers impart the framework and provide mechanical strength to the heart valve cusps [17]. To minimize valve degeneration, it is essential to maintain the stability of collagen [3, 6, 7]. Thermal denaturation temperature Td, also called as shrinkage temperature is used as a measure of collagen crosslinking [2]. Results from our studies for Td values showed that the denaturation temperature of tissues treated with NEG, ENG and GLUT are in the comparable range as reported previously by our group [6, 7, 9]. Thus, additional binding of neomycin did not affect collagen crosslinking in the cusps.
The ultimate test of any fixative is to prevent in vivo enzyme mediated GAG degradation. We have shown earlier that chemical crosslinking of GAGs was only partially effective in preventing GAG loss in vivo when the cusps were implanted subdermally in rats [6, 7, 10]. In the present study, neomycin binding to the cusps significantly abolished in vivo enzyme mediated GAG degradation. Chemical linking of GAGs with carbodiimide chemistry alone was not effective in preventing GAG loss and GAG loss in ENG group was comparable to glutaraldehyde crosslinked cusps. The overall values for hexosamines normalized to dry weight of the tissue were lower after implantation as the cusps gain little weight after implantation probably due insudation of proteins.
It has been hypothesized that GAGs might act as inhibitors of calcification in cartilage [10]. However, role of GAGs in preventing bioprosthetic heart valve calcification is still unclear. Recently, Herrero and colleagues have shown increases in calcification of bovine pericardium when GAGs were selectively removed from the tissue [24]. Additionally, others have demonstrated the ability of utilizing added exogenous GAGs to successfully mitigate calcification of BHV tissue [25]. Furthermore, distinct losses in GAGs have been observed in clinically explanted calcified BHVs [26, 27]. In present study, cusps from GAG-fixation groups NEG and ENG, showed lower calcification than GLUT fixed cusps, but the calcification was not completely inhibited despite the fact that NEG group had considerable amounts of GAGs. Initial calcification deposits in BHVs occur in devitalized cells. As cusps from all groups were crosslinked with glutaraldehyde, it may be possible that GAG stabilization had very little effect in preventing cell-mediated calcification. Thus, GAG stabilization chemistries need to be combined with well known anti-calcification strategies to prevent calcification and degeneration of BHVs.
5. Conclusions
The present studies indicate that binding of neomycin trisulfate coupled with GAG-targeted carbodiimide chemistry could be effectively used to inhibit the action of hyaluronidase and prevent GAG loss from BHVs. The GAG-targeted chemistry does not alter collagen stability, which is comparable to that of commercially used fixative GLUT. Whether such GAG stabilization would lead to better heart valve mechanics and function is currently under investigation. Such GAG stabilization strategy could be combined with anti-calcification agents to improve long-term durability of bioprosthetic heart valves.
Acknowledgments
This work was supported by a grant from NIH (HL70969). The authors would like to thank Dr. Agneta Simionescu for help with gel zymography and immunohistochemistry. The authors would also like to thank Jeremy Mercuri and Dr. Jason Isenburg for their help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005 Mar;79(3):1072–80. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Everaerts F, Torrianni M, van Luyn M, van Wachem P, Feijen J, Hendriks M. Reduced calcification of bioprostheses, cross-linked via an improved carbodiimide based method. Biomaterials. 2004 Nov;25(24):5523–30. doi: 10.1016/j.biomaterials.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 3.Schoen FJ, Levy RJ. Founder's Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28-May 2, 1999. Tissue heart valves: current challenges and future research perspectives. J Biomed Mater Res. 1999 Dec 15;47(4):439–65. doi: 10.1002/(sici)1097-4636(19991215)47:4<439::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Simionescu DT, Lovekamp JJ, Vyavahare NR. Degeneration of bioprosthetic heart valve cusp and wall tissues is initiated during tissue preparation: an ultrastructural study. J Heart Valve Dis. 2003 Mar;12(2):226–34. [PubMed] [Google Scholar]
- 5.Vyavahare N, Ogle M, Schoen FJ, Zand R, Gloeckner DC, Sacks M, et al. Mechanisms of bioprosthetic heart valve failure: fatigue causes collagen denaturation and glycosaminoglycan loss. J Biomed Mater Res. 1999 Jul;46(1):44–50. doi: 10.1002/(sici)1097-4636(199907)46:1<44::aid-jbm5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Lovekamp JJ, Simionescu DT, Mercuri JJ, Zubiate B, Sacks MS, Vyavahare NR. Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials. 2006 Mar;27(8):1507–18. doi: 10.1016/j.biomaterials.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercuri JJ, Lovekamp JJ, Simionescu DT, Vyavahare NR. Glycosaminoglycan-targeted fixation for improved bioprosthetic heart valve stabilization. Biomaterials. 2007 Jan;28(3):496–503. doi: 10.1016/j.biomaterials.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Simionescu DT, Lovekamp JJ, Vyavahare NR. Extracellular matrix degrading enzymes are active in porcine stentless aortic bioprosthetic heart valves. J Biomed Mater Res A. 2003 Sep 15;66(4):755–63. doi: 10.1002/jbm.a.10066. [DOI] [PubMed] [Google Scholar]
- 9.Lovekamp J, Vyavahare N. Periodate-mediated glycosaminoglycan stabilization in bioprosthetic heart valves. J Biomed Mater Res. 2001 Sep 15;56(4):478–86. doi: 10.1002/1097-4636(20010915)56:4<478::aid-jbm1119>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Simionescu DT, Lovekamp JJ, Vyavahare NR. Glycosaminoglycan-degrading enzymes in porcine aortic heart valves: implications for bioprosthetic heart valve degeneration. J Heart Valve Dis. 2003 Mar;12(2):217–25. [PubMed] [Google Scholar]
- 11.Elson LA, Morgan WT. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–8. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–8. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 13.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 14.Hoemann CD, Sun J, Chrzanowski V, Buschmann MD. A multivalent assay to detect glycosaminoglycan, protein, collagen, RNA, and DNA content in milligram samples of cartilage or hydrogel-based repair cartilage. Anal Biochem. 2002 Jan 1;300(1):1–10. doi: 10.1006/abio.2001.5436. [DOI] [PubMed] [Google Scholar]
- 15.Pieper JS, Oosterhof A, Dijkstra PJ, Veerkamp JH, van Kuppevelt TH. Preparation and characterization of porous crosslinked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials. 1999 May;20(9):847–58. doi: 10.1016/s0142-9612(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 16.Vyavahare N, Hirsch D, Lerner E, Baskin JZ, Schoen FJ, Bianco R, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Efficacy and mechanisms. Circulation. 1997 Jan 21;95(2):479–88. doi: 10.1161/01.cir.95.2.479. [DOI] [PubMed] [Google Scholar]
- 17.Simionescu DT. Prevention of calcification in bioprosthetic heart valves: challenges and perspectives. Expert Opin Biol Ther. 2004 Dec;4(12):1971–85. doi: 10.1517/14712598.4.12.1971. [DOI] [PubMed] [Google Scholar]
- 18.Neame PJ, Barry FP. The link proteins. Exs. 1994;70:53–72. doi: 10.1007/978-3-0348-7545-5_5. [DOI] [PubMed] [Google Scholar]
- 19.Quinn TM, Dierickx P, Grodzinsky AJ. Glycosaminoglycan network geometry may contribute to anisotropic hydraulic permeability in cartilage under compression. J Biomech. 2001 Nov;34(11):1483–90. doi: 10.1016/s0021-9290(01)00103-8. [DOI] [PubMed] [Google Scholar]
- 20.Claassen H, Cellarius C, Scholz-Ahrens KE, Schrezenmeir J, Gluer CC, Schunke M, et al. Extracellular matrix changes in knee joint cartilage following bone-active drug treatment. Cell Tissue Res. 2006 Jan;28:1–11. doi: 10.1007/s00441-005-0131-y. [DOI] [PubMed] [Google Scholar]
- 21.Salmen S, Hoechstetter J, Kasbauer C, Paper DH, Bernhardt G, Buschauer A. Sulphated oligosaccharides as inhibitors of hyaluronidases from bovine testis, bee venom and Streptococcus agalactiae. Planta Med. 2005 Aug;71(8):727–32. doi: 10.1055/s-2005-871255. [DOI] [PubMed] [Google Scholar]
- 22.Zhong SP, Campoccia D, Doherty PJ, Williams RL, Benedetti L, Williams DF. Biodegradation of hyaluronic acid derivatives by hyaluronidase. Biomaterials. 1994 Apr;15(5):359–65. doi: 10.1016/0142-9612(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 23.Menzel EJ, Farr C. Hyaluronidase and its substrate hyaluronan: biochemistry, biological activities and therapeutic uses. Cancer Lett. 1998 Sep 11;131(1):3–11. doi: 10.1016/s0304-3835(98)00195-5. [DOI] [PubMed] [Google Scholar]
- 24.Jorge-Herrero E, Fernandez P, Gutierrez M, Castillo-Olivares JL. Study of the calcification of bovine pericardium: analysis of the implication of lipids and proteoglycans. Biomaterials. 1991 Sep;12(7):683–9. doi: 10.1016/0142-9612(91)90117-s. [DOI] [PubMed] [Google Scholar]
- 25.Ohri R, Hahn SK, Hoffman AS, Stayton PS, Giachelli CM. Hyaluronic acid grafting mitigates calcification of glutaraldehyde-fixed bovine pericardium. J Biomed Mater Res A. 2004 Aug 1;70(2):328–34. doi: 10.1002/jbm.a.30088. [DOI] [PubMed] [Google Scholar]
- 26.Grande-Allen KJ, Mako WJ, Calabro A, Shi Y, Ratliff NB, Vesely I. Loss of chondroitin 6-sulfate and hyaluronan from failed porcine bioprosthetic valves. J Biomed Mater Res A. 2003 May 1;65(2):251–9. doi: 10.1002/jbm.a.10475. [DOI] [PubMed] [Google Scholar]
- 27.Mako WJea. Loss of Glycosaminoglycans (GAGs) from Implanted Bioprosthetic Heart Valves. Circulation. 1997;(155):863. [Google Scholar]


