Abstract
Macrocyclic hexaoxazoles having one or two lysinyl side chains in which the terminal nitrogen is either a primary amine, N,N-dimethylamine, or an acetamide have been synthesized. Sodium ion has been found to be beneficial to the macrocyclization step by acting as a template around which the linear polyoxazole can organize. Each of the targeted compounds selectivity stabilizes G-quadruplex vs. duplex DNA. Compounds with one valine and one lysine residue display the best combination of G-quadruplex stabilizing ability with no detectable stabilization of duplex DNA.
The stabilization of G-quadruplex DNA represents a new strategy for the treatment of cancer.1 G-Quadruplex structures have been identified in the G-rich tails of telomeres as well as in the promoter regions of certain oncogenes, including c-myc and KRAS.2 Telomerase cannot access the telomere while it is in the G-quadruplex conformation. Compounds that stabilize telomeric G-quadruplexes therefore indirectly inhibit telomerase and allow the telomere to become truncated until a critical length is reached, at which point DNA damage responses are initiated, including cell cycle arrest, senescence, and apoptosis.3 Stabilization of G-quadruplex conformations formed within the promoter region of oncogenes is associated with the decreased expression of such oncogenes.2e,f,4
A large number of compounds are reported to stabilize G-quadruplex DNA although most lack selectivity over duplex and triplex DNA. Telomestatin, a 24-membered macrocycle comprised of seven oxazoles and one thiazoline, is a natural product isolated from Streptomyces anulatus 3533-SV4.5 Until recently, telomestatin was the most potent and selective G-quadruplex stabilizer identified. Computational studies suggest that the exceptional activity of telomestatin may be associated with π-stacking interactions and hydrogen bonding with the G-quadruplex, with which it is comparable in size.6 Recently a 24-membered macrocycle containing six oxazole moieties and two valine residues (HXDV) was synthesized and found to stabilize G-quadruplex DNA with no detectable levels of duplex or triplex DNA stabilization.7 It has been determined that HXDV binds with a stoichiometry of 2:1 to G-quadruplex DNA by capping at both ends as opposed to intercalation between G-tetrads.8 HXDV is cytotoxic towards human lymphoblastoma RPMI 8402 cells with an IC50 value of 0.4 µM.7
One disadvantage of HXDV is its limited solubility in aqueous solution. It was reasoned that the replacement of one or both isopropyl groups of the macrocycle with basic side chains would allow for the preparation of more water-soluble derivatives. These basic side chains might also exhibit increased G-quadruplex stabilizing activity by providing an additional point(s) of interaction with the quadruplex, namely the formation of salt bridges with the phosphate backbone. In this report the synthesis of macrocyclic hexaoxazoles having one or two 4- aminobutyl (lysinyl) side chains is described. For the monolysinyl compound 1a the N,N-dimethylamino and acetamide derivatives were also prepared (Figure 1). The effect of these structural modifications on selectivity for G-quadruplex DNA and on the degree of stabilization of the quadruplex has been determined.
Figure 1.
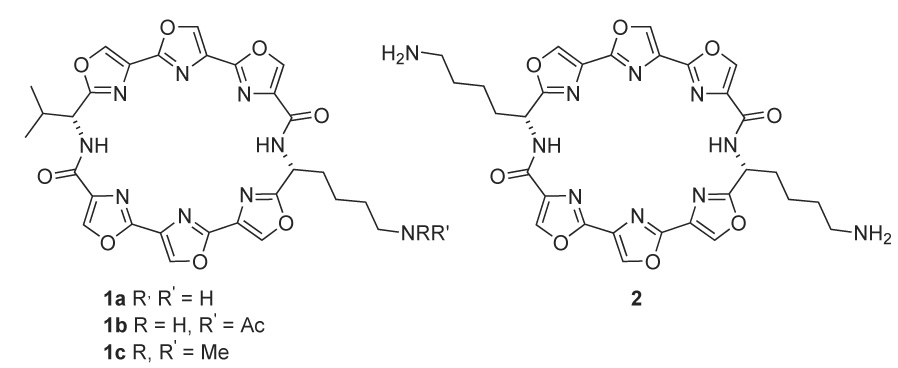
Structures of target molecules.
The synthetic plan for these target molecules requires the coupling of two teroxazoles followed by macrocyclization. In each instance a teroxazole having a lysinyl moiety at the 2-position is required. It was reasoned that protection of the C6-amino group of lysine as a tert-butyl carbamate (Boc) would ensure that amidation reactions occur selectively at the C2-amine. N6-Boc-N2-Cbz-L-lysine was prepared from N-Cbz lysine using Boc2O (Scheme 1). This was coupled with serine methyl ester hydrochloride using BOP to give amide 3 in high yield. Treatment with DAST afforded the oxazoline which was aromatized by treatment with BrCCl3 and DBU.9,10 Hydrolysis of the ester with LiOH afforded acid 4 in 73% overall yield (5 steps) from N2-Cbz-L-lysine.
Scheme 1.
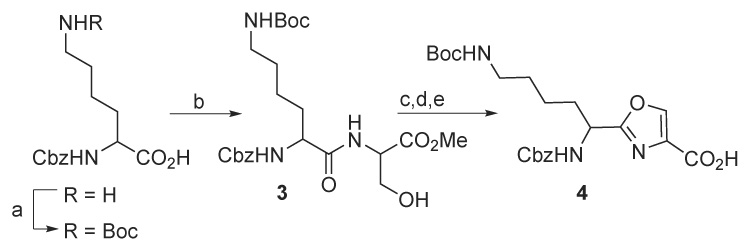
(a) Boc2O, NaHCO3 (b) serine Me ester HCl, BOP, Et3N, CH3CN (c) DAST; K2CO3 (d) BrCCl3, DBU (e) LiOH, THF/H2O.
Acid 4 was coupled with O-TIPS serine-derived amino oxazole 57 using EDC and HOBt to afford a bis(oxazolyl)amide. This was subjected to desilylation using HF/pyridine and then cyclization/ aromatization using the DAST/BrCCl3 protocol to give teroxazole 6 in 45% yield for the four steps (Scheme 2).
Scheme 2.
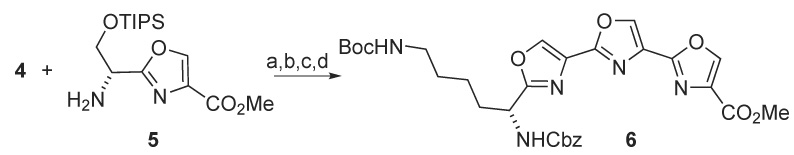
(a)EDC, HOBt, 2,6-lutidine, CH2Cl2 (b) HF/pyridine, THF (c) DAST; K2CO3, CH2Cl2 (d) BrCCl3, DBU, CH2Cl2.
Ester 6 was hydrolyzed with LiOH to afford acid 7 in quantitative yield and this was then coupled with valine-derived amino teroxazole 87 to yield a linear hexaoxazole in 85% yield (Scheme 3). The Cbz group was removed by transfer hydrogenolysis and the remaining ester was then hydrolyzed with LiOH to give an amino acid. When macrocyclization of this hexaoxazole was affected using HATU with N-methylmorpholine in DMF at high dilution, cyclic hexaoxazole 9 was formed in 54% yield, accompanied by a small amount of a by-product tentatively identified as imidate 10. The imidate was likely formed by deprotonation of the amide and intramolecular attack on the tert-butyl carbamate moiety of the side chain, with displacement of tert-butanol. Attempts to hydrolyze the imidate under acidic or basic conditions led to decomposition. Treatment of 9 with TFA removed the Boc group to afford amine 1a (HXLV) in quantitative yield. A portion of 1a was treated with Ac2O in pyridine to give HXLV-AC, 1b in 72% yield. Treatment of another portion of 1a with aqueous formaldehyde and sodium borohydride gave HXLV-DM, 1c in 67 % yield.11
Scheme 3.
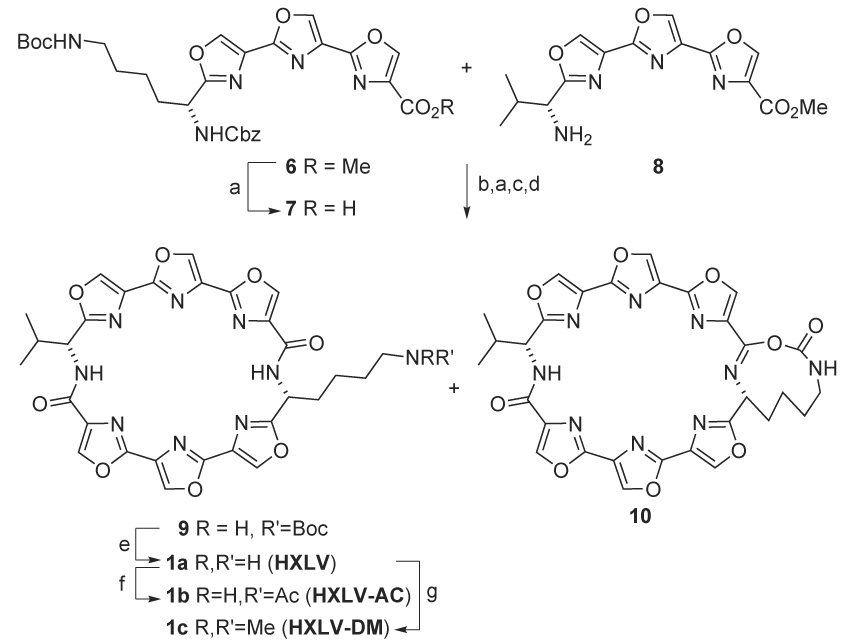
(a) LiOH, THF/H2O (b) EDC, HOBt, 2,6-lutidine, DMF (c) 1,4-cyclohexadiene, 20% Pd(OH)2/C, THF/EtOH, Δ, 2d (d) HATU, NMM, DMF (e) TFA, CH2Cl2 (f) Ac2O, pyridine (g) MeOH, HCl, HCHO/H2O; NaBH4.
The synthesis of dilysinyl analog 2 is outlined in Scheme 4. A portion of teroxazole 6 was subjected to hydrogenolysis and the resulting amine was coupled with acid 7 to give amide 13. After sequential deprotection of the ester and Cbz groups, macrocyclization was achieved using HATU to give the desired product 14 in low yield (7%). A significant amount of a by-product, similar to cyclic imidate 10, was also obtained from this reaction. This by-product could not be converted into 14. Changing the reagent from HATU to BOP led to an improvement in the yield of 14 without forming the by-product. A chance observation in which a linear hexaoxazole was found by high resolution mass spectrometry to be tightly bound to a sodium ion suggested that organization of the cyclization precursor around a metal cation template might enhance the rate and yield of the macrocyclization process. The template effect of sodium and potassium ions on the formation of 14 was evaluated. These results are summarized in Table 1. The addition of 5 equivalents of NaI resulted in a 60% increase in the yield of 14. Additional NaI did not significantly improve the yield. Potassium ions actually had a deleterious effect on the macrocyclization. The Boc groups were removed from 14 in quantitative yield upon treatment with TFA in CH2Cl2 to give 2 (HXDL).11
Scheme 4.
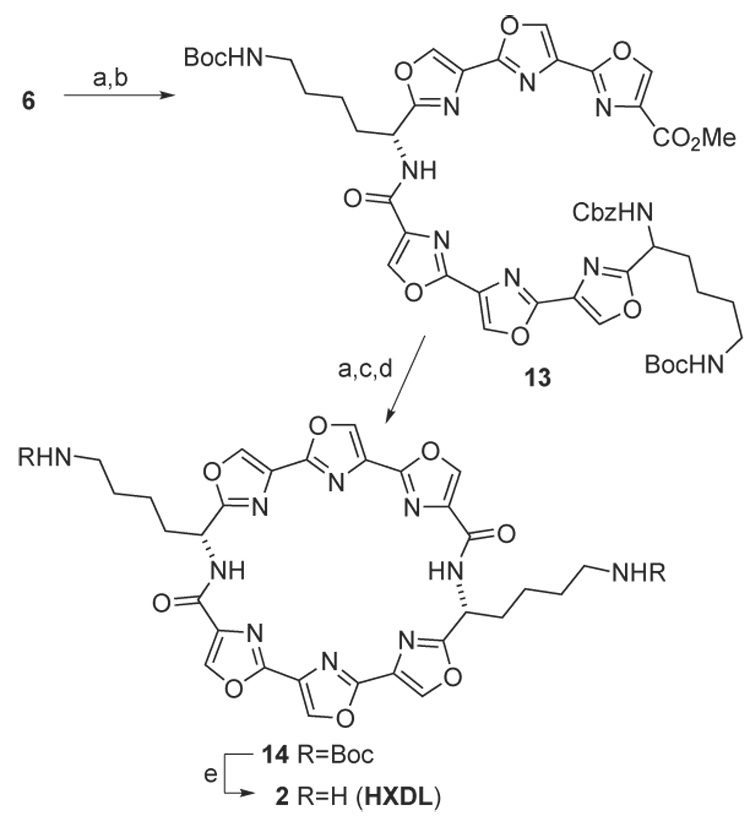
(a) 20% Pd(OH)2/C, cyclohexadiene, Δ, 3d (b) 7, EDC, HOBt, 2,6-lutidine, DMF (c) LiOH, THF/H2O (d) HATU, NMM, DMF/CH2Cl2 (e) TFA, CH2Cl2.
Table 1.
Effect of salts on the formation of 14a
| Additive | Equivalents | Yield of 14 (%) |
|---|---|---|
| None | - | 30 |
| NaI | 5 | 48 |
| 10 | 50 | |
| KI | 5 | 20 |
| 10 | 27 |
Reactions were performed by slow addition (syringe pump, 1 h) of a solution of deprotected 13 (27 µmol) in DMF (10 mL) to a solution of BOP (1.5 equiv.), HOBt (1.5 equiv.), the salt, and iPr2NEt (3 equiv.) in 20 mL of DMF at 0 °C.
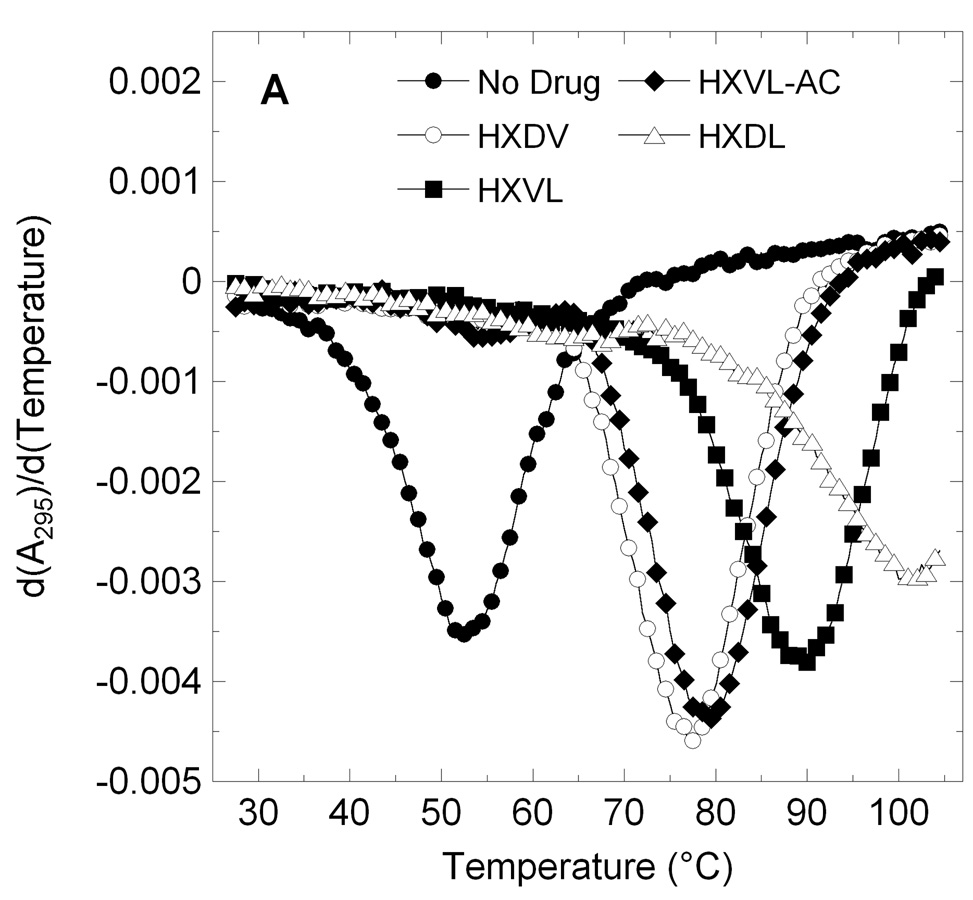
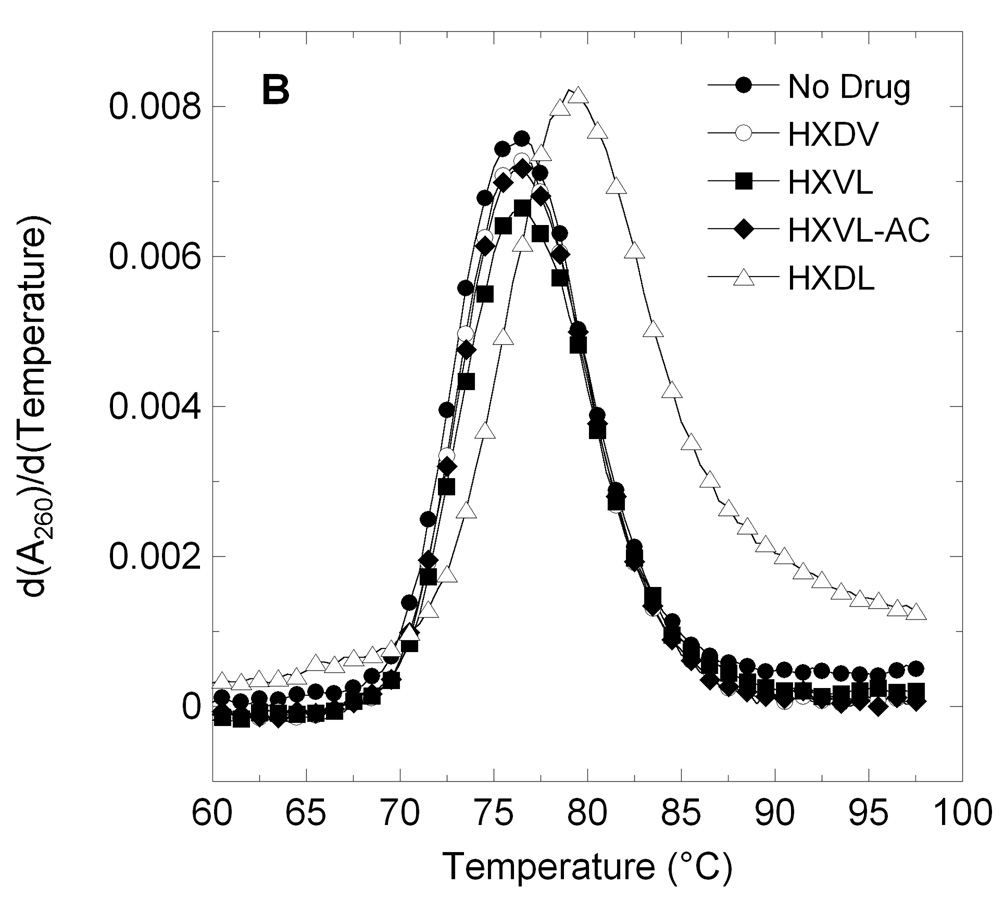
HXDV, HXLV (1a), HXLV-AC (1b), HXLV-DM (1c), and HXDL (2) were evaluated for their abilities to bind and thermally stabilize G-quadruplex and duplex DNA in the presence of K+ ions. We used salmon testes DNA (ST-DNA) and d[T2G3(T2AG3)3A] as representative models for duplex and G-quadruplex DNA, respectively. Patel and coworkers have shown that the structure adopted by d[T2G3(T2AG3)3A] in K+ solution is an intramolecular (3 + 1) G-quadruplex in which three strands are oriented in one direction and the fourth strand is oriented in the opposite direction.12 Figure 2 shows the UV melting profiles (depicted in their first-derivative forms) of d[T2G3(T2AG3)3A] and ST-DNA in the absence and presence of HXDV, HXLV, HXLV-AC, and HXDL. The transition temperatures (Ttran) corresponding to the maxima or minima of these first-derivative melting profiles are listed in Table 2, as are the corresponding Ttran values derived from the melting profile (not shown) conducted in the presence of HXDV, HXLV-DM. The presence of HXDV, HXLV, HXLV-DM, or HXLV-AC does not alter the thermal stability of ST-DNA, with any observed changes in Ttran being within the experimental uncertainty. This observation is consistent with little or no duplex DNA binding on the part of these four compounds. With regard to HXDV, the observed behavior confirms that which we have previously reported.7,8 The presence of HXDL increases the Ttran value of ST-DNA by 2.5 °C. Thus, unlike the monolysinyl compounds, the dilysinyl compound appears to exhibit some degree of duplex DNA binding under the solution conditions employed.
Figure 2.
First derivatives of the UV melting profiles of d[T2G3(T2AG3)3A] (profile A) and ST-DNA (profile B) in the absence and presence of macrocyclic lysinyl-containing hexaoxazoles. Profiles were acquired on an AVIV model 14NT-UV-VIS spectrophotometer using quartz cuvettes with a 1 cm pathlength. The temperature was raised in 0.5 °C increments and the samples were allowed to equilibrate for 1.5 minutes at each temperature setting, whereupon absorbances were recorded over a period of 5 seconds and averaged. When present, ST-DNA was used at a base pair concentration of 15 µM, while d[T2G3(T2AG3)3A] was used at a strand concentration of 5 µM. Macrocyclic ligands were used at a concentration of 15 µM in the ST-DNA experiments and 20 µM in the d[T2G3(T2AG3)3A] experiments. The solution conditions in the ST-DNA experiments were 10 mM EPPS (pH 7.5) and sufficient KCl to bring the total K+ concentration to 50 mM. The solution conditions in the d[T2G3(T2AG3)3A] experiments were 10 mM potassium phosphate (pH 7.5) and sufficient KCl to bring the total K+ concentration to 150 mM. In the ST-DNA experiments, the acquisition wavelength was 260 nm, while being 295 nm in the d[T2G3(T2AG3)3A] experiments.
Table 2.
Transition temperatures for the melting of ST-DNA and d[T2G3(T2AG3)3A] in the absence and presence of macrocyclic lysinyl-containing hexaoxazoles
| Sample | Ttran (°C)a |
|---|---|
| ST-DNA | 76.5 |
| ST-DNA + HXDV | 76.5 |
| ST-DNA + HXLV | 76.5 |
| ST-DNA + HXLV-DM | 76.0 |
| ST-DNA + HXLV-AC | 76.5 |
| ST-DNA + HXDL | 79.0 |
| d[T2G3(T2AG3)3A] | 52.5 |
| d[T2G3(T2AG3)3A] + HXDV | 77.0 |
| d[T2G3(T2AG3)3A] + HXLV | 90.0 |
| d[T2G3(T2AG3)3A] + HXLV-DM | 81.0 |
| d[T2G3(T2AG3)3A] + HXLV-AC | 79.0 |
| d[T2G3(T2AG3)3A] + HXDL | 101.5 |
DNA transition temperatures (Ttran) reflect the maxima or minima of the first derivatives of UV melting profiles acquired as described in the legend to Figure 2. The uncertainty in the Ttran values is ±0.5 °C.
In marked contrast to their negligible impacts on ST-DNA thermal stability, HXDV, HXLV, HXLV-DM, and HXLV-AC, increase the Ttran value of d[T2G3(T2AG3)3A] by 24.5, 37.5, 28.5, and 26.5 °C, respectively. Although HXDL does increase the thermal stability of ST-DNA (ΔTtran = 2.5 °C), the extent to which it increases the thermal stability of d[T2G3(T2AG3)3A] is significantly greater (ΔTtran = 49.0 °C). Taken together, our UV melting data indicate that the divalinyl hexaoxazole HXDV, the monolysinyl and dilysinyl hexaoxazoles bind to G-quadruplex DNA with a high degree of specificity.
In summary, macrocyclic hexaoxazoles having one or two lysinyl side chains in which the terminal nitrogen is present as either a primary amine, N,N-dimethylamine, or an acetamide have been synthesized. Sodium ion has been found to be beneficial to the macrocyclization step by acting as a template around which the linear polyoxazole can organize. All four targeted compounds selectively stabilize G-quadruplex DNA. HXLV provides the greatest degree of G-quadruplex stabilization with no detectable stabilizing effect on duplex DNA.
Acknowledgements
This study was supported by NIH Grants CA098127 (EJL) and CA097123 (DSP). Mass spectrometry was provided by the Washington University Mass Spectrometry Resource with support from the NIH National Center for Research Resources (Grant No. P41RR0954).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.(a) Hurley LH, Wheelhouse RT, Sun D, Kerwin SM, Salazar M, Federoff OY, Han FX, Han H, Izbicka E, Von Hoff DD. Pharmacol. Ther. 2000;85:141. doi: 10.1016/s0163-7258(99)00068-6. [DOI] [PubMed] [Google Scholar]; (b) Han H, Hurley LH. Trends Pharmacol. Sci. 2000;21:136. doi: 10.1016/s0165-6147(00)01457-7. [DOI] [PubMed] [Google Scholar]; (c) Neidle S, Read MA. Biopolymers. 2001;56:195. doi: 10.1002/1097-0282(2000)56:3<195::AID-BIP10009>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (d) Perry PJ, Jenkins TC. Mini-Rev. Med. Chem. 2001;1:31. doi: 10.2174/1389557013407304. [DOI] [PubMed] [Google Scholar]; (e) Hurley LH. Biochem. Soc. Trans. 2001;29:692. doi: 10.1042/0300-5127:0290692. [DOI] [PubMed] [Google Scholar]
- 2.(a) Wang Y, Patel DJ. Biochemistry. 1992;31:8112. doi: 10.1021/bi00150a002. [DOI] [PubMed] [Google Scholar]; (b) Parkinson GN, Lee MP, Neidle S. Nature. 2002;417:876. doi: 10.1038/nature755. [DOI] [PubMed] [Google Scholar]; (c) Rankin S, Reska AP, Huppert J, Zloh M, Parkinson G, Todd A, Ladame S, Balasubramanian S, Neidle S. J. Am. Chem. Soc. 2006;127:10584. doi: 10.1021/ja050823u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dai J, Dexheimer TS, Chen D, Carver M, Ambrus A, Jones RA, Yang D. J. Am. Chem. Soc. 2006;128:1096. doi: 10.1021/ja055636a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ambrus A, Ding D, Jones RA, Yang D. Biochemistry. 2005;44:2048. doi: 10.1021/bi048242p. [DOI] [PubMed] [Google Scholar]; (f) Cogoi S, Xodo LE. Nucleic Acids Res. 2006;34:2536. doi: 10.1093/nar/gkl286. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Thibault G, Dennis P, Rajaa M, Eliane M, Mailliet P, Riou J-F. Biochem. Biophys. Res. Commun. 2004;323:802. doi: 10.1016/j.bbrc.2004.08.150. [DOI] [PubMed] [Google Scholar]
- 3.(a) Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10114. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Garvik B, Carson M, Hartwell L. Mol. Cell Biol. 1995;15:6128. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Harley CB, Futcher AB, Greider CW. Nature. 1990;345:458. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]; (d) Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Nature. 1990;346:866. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]; (e) Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. Science. 1999;283:1321. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]; (f) Karlseder J, Smogorzewska A, de Lange T. Science. 2002;295:2446. doi: 10.1126/science.1069523. [DOI] [PubMed] [Google Scholar]; (g) Qi H, Li TK, Kuo D, Nur-E-Kamal, Liu LF. J. Biol. Chem. 2003;178:15136. doi: 10.1074/jbc.M212808200. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11593. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin-ya K, Wierzba K, Matsuo K-I, Ohtani T, Yamada Y, Furihata K, Hayakawa Y, Seto H. J. Am. Chem. Soc. 2001;123:1262. doi: 10.1021/ja005780q. [DOI] [PubMed] [Google Scholar]
- 6.Kim M-Y, Vankayalapati H, Shin-ya K, Wierzba K, Hurley LH. J. Am. Chem. Soc. 2002;124:2098. doi: 10.1021/ja017308q. [DOI] [PubMed] [Google Scholar]
- 7.Minhas GS, Pilch DS, Kerrigan JE, LaVoie EJ, Rice JE. Bioorg. Med. Chem. Lett. 2006;16:3891. doi: 10.1016/j.bmcl.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Barbieri CM, Srinivasan AR, Rzuczek SG, Rice JE, LaVoie EJ, Pilch DS. Nucleic Acids Res. 2007;35:3272. doi: 10.1093/nar/gkm188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips AJ, Uto Y, Wipf P, Reno MJ, Williams DR. Org. Lett. 2000;2:1165. doi: 10.1021/ol005777b. [DOI] [PubMed] [Google Scholar]
- 10.Williams DR, Lowder PD, Gu Y-G, Brooks DA. Tetrahedron Lett. 1997;38:331. [Google Scholar]
- 11.The structures of all compounds were determined using 1H (200 MHz) and 13C-NMR (50 MHz) in CDCl3 unless otherwise noted. Results are reported as ppm downfield from internal TMS: HXLV, 1a: mp 297–300 °C (TFA salt); [α]d= −20.6° (c=0.18, 10% MeOH/ CHCl3); 1H NMR (CDCl3) δ 8.31 (m, 8H), 5.37 (m, 2H), 2.85 (m, 2H), 2.44 (m, 1H), 1.93 (m, 2H), 1.29 (m, 2H), 0.98 (m, 8H); 13C NMR (CDCl3/CD3OD) δ 164.9, 164.0, 159.3, 155.6, 153.8, 140.4, 139.8, 139.2, 138.5, 138.3, 136.0, 135.6, 129.5, 128.2, 128.1, 63.0 52.6, 39.3, 32.5, 32.0, 26.2, 20.1, 17.5, 17.3; HRMS (FAB) m/z calcd for C29H27N9O8Li (M + Li) 636.2137; found, 636.2139. HXLV-AC, 1b: mp 222–224 °C; [α]d = −37.4° (c=0.605, 10% MeOH/CHCl3); 1H NMR (CDCl3) δ 8.60 (d, 1H, J=8), 8.48 (d, 1H, J=8), 8.23 (m, 6H), 5.78 (br s, 1H), 5.43 (dt, 1H, J=6, 7), 5.33 (dd, 1H, J=5, 8), 3.22 (m, 2H), 2.40 (m, 1H), 1.75 (m, 5H), 1.44 (m, 4H), 1.05 (d, 3H, J=7), 0.99 (d, 3H, J=7); 13C NMR (CDCl3) δ 169.3, 164.0, 163.7, 159.1, 159.0, 155.4, 155.2, 153.9, 153.8, 140.0, 139.4, 138.3, 138.1, 137.5, 136.2, 136.1, 130.24, 130.16, 128.8, 52.2, 46.8, 38.5, 33.8, 33.1, 27.8, 22.4, 21.2, 17.6, 17.3; HRMS (ESI) m/z calcd for C31H29N9O9 (M + H) 672.2161; found 672.2199. HXLV-DM, 1c: mp 308–310 °C (dec); [α]d = +19° (c = 0.5, 20% CHCl3/MeOH); 1H NMR (CDCl3) δ 8.76 (d, 1H, J=8), 8.41 (d, 1H, J=8), 8.23 (m, 6H), 5.48 (dt, 1H, J=6, 7), 5.34 (dd, 1H, J=5, 8), 2.96 (m, 2H), 2.77 (s, 6H), 2.44 (m, 1H), 2.11 (m, 2H), 1.90 (m, 2H), 1.06 (d,3H, J=7), 1.00 (d, 3H, J=7); HRMS (FAB) m/z calcd for C31H31N9O8Li (M + Li) 658.2368; found, 658.2373. HXDL, 2: mp 305 °C (TFA salt); [α]d = +79.1° (c= 1.05, MeOH); 1H NMR (CDCl3/CD3OD) δ 8.47 (s, 4H), 8.35 (s, 2H), 5.40 (m, 2H), 3.40-2.90 (m, 4H), 2.08 (m, 4H), 1.82 (m, 4H), 1.60-1.20 (m, 6H); 13C NMR (CD3OD) δ 164.0, 159.9, 155.9, 154.4, 141.4, 141.3, 139.9, 135.8, 129.3, 127.8, 39.0, 37.4, 26.7, 21.2; HRMS (ESI) m/z calcd for C30H31N10O8 (M + H) 659.2326; found, 659.2311.
- 12.Luu KN, Phan AT, Kuryavyi V, Lacroix L, Patel DJJ. Am. Chem. Soc. 2006;128:9963. doi: 10.1021/ja062791w. [DOI] [PMC free article] [PubMed] [Google Scholar]