Abstract
Leptin is a fat-derived hormone that exerts pleiotropic effects on energy balance and neuroendocrine functions. Mice defective in leptin or its receptor [leptin receptor, isoform b (LepRb)] exhibit profound obesity, infertility, and reduced linear growth. Leptin binding to its receptor triggers multiple signaling pathways, including signal transducer and activator of transcription 3 (Stat 3), phosphatidylinositol-3-kinase, and ERK. A considerable amount of effort has been focused on how these signaling pathways mediate diverse leptin functions. Mice containing a mutant LepRb incapable of Stat3 signaling are obese but remain fertile with enhanced linear growth. In contrast, deletion of Stat3 in the whole brain with Nestin-Cre results in infertility and decreased linear growth, in addition to obesity. The additional phenotypes of the Nestin-mediated deletion could reflect Stat3 action in non-LepRb neurons or leptin-independent Stat3 actions in LepRb neurons. To resolve this discrepancy and to gain more insight into the metabolic actions of Stat3, we have generated mice in which Stat3 is disrupted specifically in LepRb neurons after the onset of leptin receptor expression. We show that mutant mice exhibit profound obesity with increased linear growth and normal fertility. In addition, impaired glycemic control in these animals correlates with their degree of obesity. These results demonstrate that Stat3 in LepRb neurons does not regulate linear growth or fertility. These results further suggest that leptin’s effects on growth and reproduction are mediated by other signaling pathways, and that Stat3-mediated control of these functions is mediated independently of leptin and LepRb neurons.
LEPTIN, A 16-kDa adipose-derived hormone, plays a crucial role in multiple physiological processes such as energy balance, glucose homeostasis, growth, and reproduction. Leptin is secreted at a level that is proportional to the body’s fat mass and acts in the brain to relay the body’s energy reserve (1). Leptin deficiency signals a state of energy deficit to the brain, which triggers complex neuroendocrine changes resulting in increased appetite, decreased energy expenditure, and reduced fertility and body growth. Thus, being a gauge of the body’s energy reserves, leptin functions not only as a master regulator of energy balance, but also acts to coordinate other physiological processes such as growth and reproduction, all of which heavily rely on the body’s energy reserves. Indeed, mice that are deficient in leptin (ob/ob) or its functional receptor (db/db) show profound obesity, hyperinsulinemia, and hyperglycemia. In addition, these mice have reduced linear growth and are infertile (2).
Leptin receptor exists in multiple isoforms, and only the long form (isoform b, LepRb) is able to mediate leptin signaling (3). LepRb expression is enriched in the hypothalamus, and its expression is found on multiple neuronal populations including those expressing Agouti-related protein (Agrp), neuropeptide Y (Npy), and proopiomelanocortin (Pomc), molecules that are essential for energy homeostasis. Leptin administration rapidly and robustly stimulates phosphorylation of signal transducer and activator of transcription 3 (Stat 3) in multiple regions of the hypothalamus. Leptin binding to its receptor activates Jak 2, leading to phosphorylation and dimerization of Stat3, which then acts as a transcription factor to regulate transcription of leptin target genes (4). Leptin has also been shown to signal through other pathways, namely the phosphatidylinositol 3-kinase and ERK pathways (4,5,6). However, the relative role of each individual signaling pathway in the regulation of leptin’s diverse functions is still poorly understood.
The importance of Stat3 in mediating leptin signaling has been recently investigated. In one study, tyrosine 1138 on the leptin receptor long form was mutated to a serine residue such that Stat3 binding to the leptin receptor was abolished (7). Mice harboring such mutations are severely obese but are otherwise fertile and exhibit increased linear growth (7). However, in a different study, Stat3 was deleted from the whole brain in mice carrying a Nestin-Cre allele and a floxed Stat3 allele (8). These mice exhibit severe obesity and reduced linear growth, and are sterile, recapitulating the ob/ob and db/db phenotype (8). The additional phenotypes of the Nestin-mediated deletion could reflect Stat3 action in non-LepRb neurons or leptin-independent Stat3 actions in LepRb neurons. To resolve this discrepancy and to better understand the metabolic actions of Stat3, we have crossed the LepRb-Cre mice with a flox allele of Stat3, such that Stat3 function is specifically removed in the LepRb neurons after the onset of leptin receptor expression. We show that LepRb-Stat3 mutant mice are severely obese and hyperglycemic, but they exhibit increased linear growth and normal fertility. Impaired glycemic control correlates with the degree of obesity. These results demonstrate that the major function of Stat3 in leptin signaling is to mediate leptin’s effect on body weight, and that Stat3 is not required for leptin’s effects on growth and reproduction. These results further suggest that leptin’s effects on growth and reproduction are mediated by other leptin-signaling pathways, and that Stat3-mediated control of these functions are mediated independently of leptin and LepRb neurons.
RESULTS
Loss of Stat3 Function from Leptin Receptor Neurons Abolishes Leptin-Induced Stat3 Phosphorylation
To generate LepRb-specific Stat3-deficient mice, we crossed two validated transgenic lines, mice carrying Stat3flox/flox (9) and mice carrying LeprCre (10). Stat3flox/flox contains targeted loxP sites flanking exon 22 of the Stat3 gene, which encodes the critical tyrosine residue the phosphorylation of which is essential for Stat3 dimerization and nuclear translocation (9,11,12). These mice were crossed with LeprCre mice in which Cre recombinase is specifically expressed in cells expressing leptin receptor long form (LepRb) (10). In subsequent breeding, LeprCre/+, Stat3flox/flox mice were crossed with +/+, Stat3flox/flox mice to generate LepRb-Stat3 mutants (LeprCre/+, Stat3flox/flox) and control animals (+/+, Stat3flox/flox) that were used for following analyses. Control animals (+/+, Stat3flox/flox) do not show any noticeable phenotypes compared with to wild-type animals (+/+, Stat3+/+) as previously demonstrated (9,11,12).
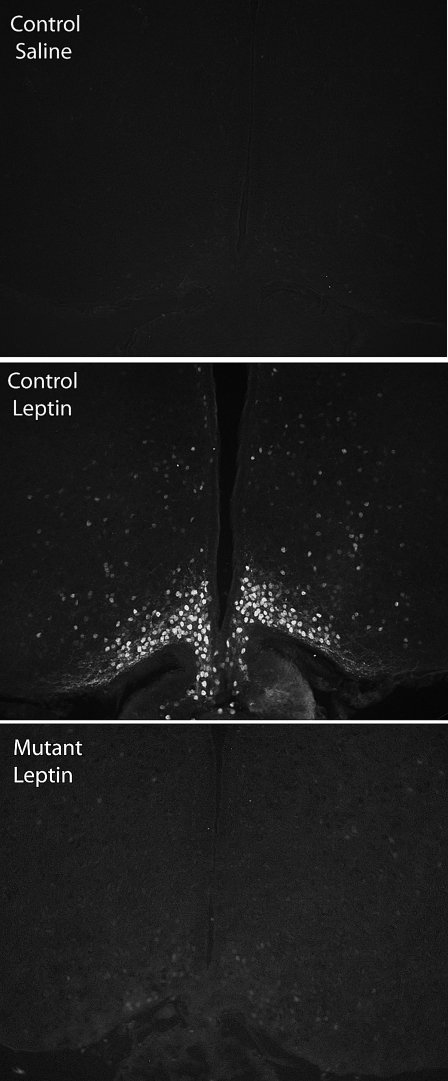
Mutant animals were born in Mendelian ratio with no gross morphological defects. To determine whether these mice have lost their ability to activate Stat3 in response to leptin administration, adult LepRb-Stat3 mutants and controls were given ip injection of mouse recombinant leptin (5 mg/kg). Animals were perfused with fixative 30 min after leptin injection, and frozen hypothalamic sections were prepared. Immunofluorescence experiments were carried out to examine immunoreactivity for phospho-Stat3 (pStat3). Whereas leptin administration robustly stimulated pStat3 in the control hypothalamus, it failed to do so in the mutants (Fig. 1), demonstrating that leptin is not able to activate Stat3 in the mutant hypothalamus.
Figure 1.
Leptin Administration Fails to Induce pStat3 in the Hypothalamus of LepRb-Stat3 Mutant Mice
Panels show coronal sections of control and LepRb-Stat3 mutant mice 30 min after ip injection of saline or leptin (5 mg/kg), as indicated (control saline, n = 3; control leptin, n = 5; mutant leptin, n = 3).
Loss of Stat3 Function from LepRb Neurons Results in Severe Obesity, Hyperinsulinemia, and Hyperglycemia
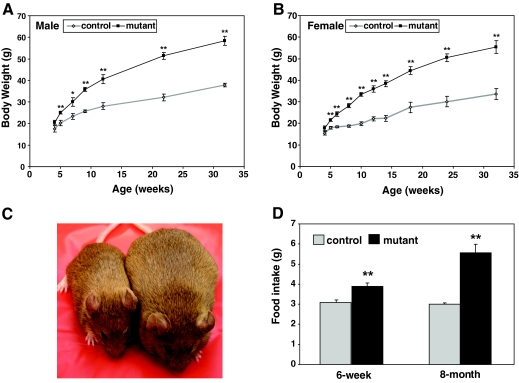
Upon weaning, body weight of LepRb-Stat3 mutant mice accelerated. At 5 wk of age, the mutants were already significantly heavier than their control littermates, and at 5–6 months of age, the mutant males were almost twice as heavy as the controls (Fig. 2A). Body weight of female mutants followed the same trend as the males (Fig. 2B). Food intake was significantly elevated in the mutants (Fig. 2D), indicating that Stat3 function is required for leptin’s effect on feeding.
Figure 2.
Body Weight and Food Intake Are Significantly Increased in LepRb-Stat3 Mutant Mice
A, Body weight for males (n for each time point: controls 7, 14, 8, 11, 7, 6, 4; mutants 7, 13, 5, 8, 4, 8, 5). Last two time points represent ages ± 4 wk. B, Body weight for females (n for each time point: controls, 15, 13, 9, 13, 9, 11, 7, 7, 8, 5; mutants 10, 8, 6, 7, 10, 5, 5, 8, 9, 7). Last two time points represent ages ± 4 wk. C, Representative control (left) and mutant (right) littermates, age 8 months. D, Average daily food intake for male mice at 6 wk and 8 months of age (controls n = 4–5; mutants n = 5). *, P < 0.05; **, P < 0.01 between controls and mutants as determined by Student’s t test. Error bars represent sem.
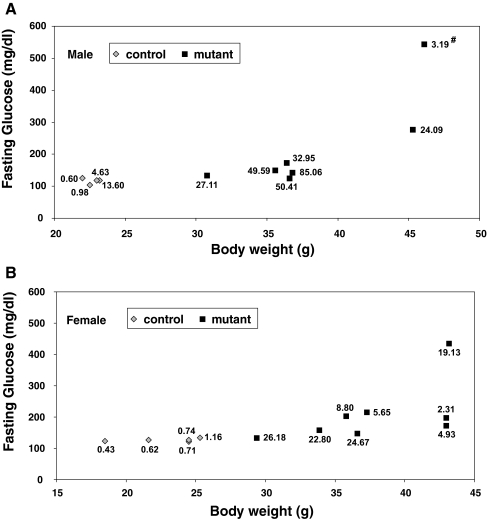
We next measured insulin and glucose levels in adult LepRb-Stat3 mutant mice. Fasted insulin levels in male mutants were almost 10-fold higher than the controls, whereas fasted insulin levels in female mutants were more than 20-fold higher than the controls (Table 1). Consistent with being hyperinsulinemic, both male and female mutants exhibited significant increase in fasted blood glucose levels (Table 1). Despite the fact that insulin and glucose levels were higher in the mutants than in the controls, there was considerable variation in the degree of severity of hyperglycemia among the different mutant mice. We therefore correlated fasted blood glucose levels with body weight, and a strong and significant correlation was observed in the mutants (Fig. 3; male: r = 0.717, P = 0.01; female: r = 0.645, P = 0.02) but not in the controls. This result suggests that impaired glycemic control is likely the consequence of obesity in the mutants.
Table 1.
Glucose and Insulin Levels in Control and Mutant Mice
| Control Male | Mutant Male | Control Female | Mutant Female | |
|---|---|---|---|---|
| Fed glucose (mg/dl) | 140 ± 6 | 256 ± 41a | 135 ± 11 | 196 ± 49 |
| Fast glucose (mg/dl) | 116 ± 5 | 220 ± 57b | 126 ± 2 | 207 ± 34b |
| Fed insulin (μg/liter) | 5.74 ± 3.18 | 19.70 ± 10.13 | 0.37 ± 0.18 | 20.59 ± 16.64 |
| Fast insulin (μg/liter) | 4.95 ± 3.02 | 44.87 ± 6.87b | 0.73 ± 0.12 | 16.33 ± 3.62b |
Control n = 4–5; mutant n = 7–8. Glucose was measured at 10–14 wk of age, and insulin was measured at 20–24 wk of age. Values are given as mean ± sem.
P < 0.05; b P < 0.01 between controls and mutants as determined by Mann-Whitney rank sum test.
Figure 3.
Correlation between Body Weight and Fasted Glucose Levels in Control and LepRb-Stat3 Mutant Mice
Mice were fasted for 6 h, and blood glucose was measured in males (A), and females (B) age 20–24 wk. Serum insulin level was measured 4 wk later under the same conditions. Each data point represents a single mouse with its corresponding insulin level. Both males and females show significant correlation between body weight and glucose levels as determined by linear regression analysis using Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL) (male: r = 0.717, P = 0.01; female: r = 0.645, P = 0.02). #, Low insulin levels of the indicated animal most likely reflects pancreatic β-cell failure.
Loss of Stat3 Function from LepRb Neurons Results in Increased Linear Growth
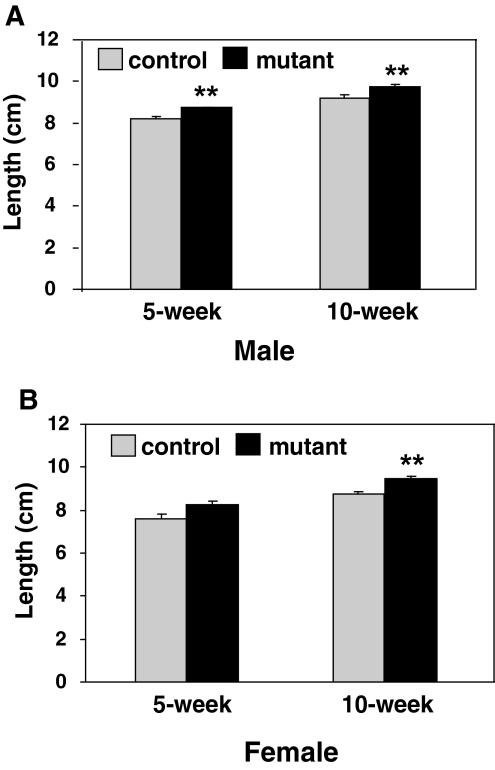
ob/ob, db/db, and Nestin-Stat3 mutant mice have reduced body length, suggestive of decreased linear growth. To this end, linear growth was determined by measuring the snout-anus distance in the LepRb-Stat3 mutants and control littermates. At 5 wk of age, mutant males showed a significant increase in length (Fig. 4). The mutant females showed the same trend although it was not significant. At 10 wk of age, both male and female mutants were significantly longer than the controls (Fig. 4). The lack of a growth defect of LepRb-Stat3 mutant mice suggests that leptin’s effect on growth is not mediated by Stat3 signaling.
Figure 4.
LepRb-Stat3 Mutant Mice Exhibit Increased Linear Growth
A, Length of males as measured by snout to anus distance at 5 and 10 wk of age (5 wk: controls, n = 8; mutants, n = 10; 10 wk: controls, n = 8; mutants, n = 10). B, Length of females at 5 and 10 wk of age (5 wk: controls, n = 3; mutants, n = 3; 10 wk: controls, n = 10; mutants, n = 4). **, P < 0.01 between controls and mutants as determined by Student’s t test. Error bars represent sem.
Loss of Stat3 Function from LepRb Neurons Has No Effect on Fertility
Because ob/ob, db/db, and Nestin-Stat3 mutant mice are infertile, we next determined whether loss of Stat3 function in leptin receptor-expressing neurons affected reproduction of these mice. No difference in fertility was detected between LepRb-Stat3 mutants and controls, as measured by number of litters produced, size of the litters, and reproductive success rate (Table 2). Thus, Stat3 function is dispensable for leptin’s effect on fertility.
Table 2.
Fertility in Control and Mutant Mice
| Genotype | No. of Animals Tested | Age Range When Fertility Was Measured (wk) | Average Litter Size | No. Fertile Mice/No. Mice Assayed |
|---|---|---|---|---|
| Control male | 6 | 8–17 | 6.55 ± 0.45 | 6/6 |
| Mutant male | 5 | 9–19 | 6.33 ± 0.87 | 5/5 |
| Control female | 11 | 6–19 | 7.06 ± 0.53 | 11/11 |
| Mutant female | 7 | 8–16 | 6.67 ± 0.78 | 7/7 |
Melanocortin Gene Expression in LepRb-Stat3 Mutant Mice
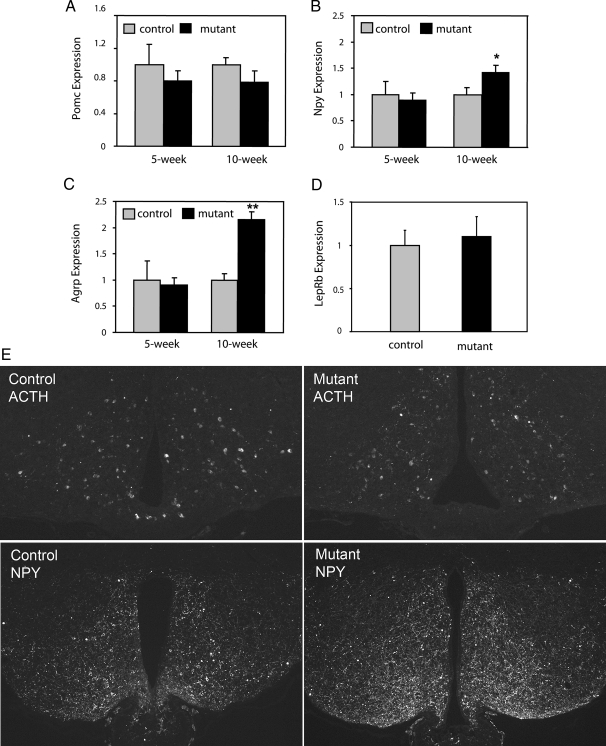
It is known that leptin positively regulates Pomc expression, whereas it negatively regulates Agrp and Npy expression. To determine whether loss of Stat3 function in LepRb neurons resulted in altered neuropeptide expression, we analyzed expression of Pomc, Npy, and Agrp in LepRb-Stat3 mutants and control littermates by semiquantitative RT-PCR. At 5 wk of age, no significant difference in Pomc, Npy, and Agrp expression was detected. However, at 10 wk of age, Npy and Agrp expression was significantly elevated, whereas Pomc expression was slightly reduced (Fig. 5, A–C). No difference in leptin receptor long-form mRNA expression was detected (Fig. 5D). In addition, we examined ACTH and NPY immunoreactivity by immunofluorescence experiments. ACTH and NPY signals were comparable in 10-wk-old controls and mutants (data not shown). However, a dramatic increase in NPY immunoreactivity in the hypothalamus was detected in 6-month-old mutant animals, and ACTH immunoreactivity was slightly lower (Fig. 5E). Our ability to detect a significant increase in Npy expression by real-time RT-PCR in younger animals is likely due to the higher sensitivity of this approach. Alternatively, it could represent a difference in Npy mRNA and protein levels. The implication of such a finding will be discussed below.
Figure 5.
Neuropeptide Expression in Control and LepRb-Stat3 Mutant Mice
Hypothalamic total RNA was extracted from control and LepRb-Stat3 mutant mice that were fed ad libitum. Pomc (A), Npy (B), Agrp (C), and LepRb (D) expression was analyzed by semiquantitative real-time PCR. β-Actin was used as an internal control to normalize expression levels. Control values were set to 1 in the y-axis for easy comparison. (5 wk: controls, n = 7; mutants, n = 8; 10 wk: controls, n = 6; mutants, n = 6). LepRb expression was measured at 10 wk of age. E, Expression of ACTH and NPY immunoreactivity in 6-month-old controls and mutants by immunofluorescence (controls, n = 3; mutants, n = 3). *, P < 0.05; **, P < 0.01 between controls and mutants as determined by Student’s t test. Error bars represent sem.
DISCUSSION
The aim of this study was to clarify the role of Stat3 in mediating leptin function, especially leptin’s function on growth and reproduction. We used a genetic strategy to disrupt Stat3 function specifically in neurons expressing leptin receptor long form such that Stat3 signaling was abolished only in relevant cell types and only after the onset of LepRb expression. Our study shows that LepRb-Stat3 mutant mice are obese, hyperglycemic, long, and fertile. This study demonstrates that Stat3, although essential for leptin’s effect for energy balance, is not required for leptin’s function on growth and reproduction. It further suggests that other leptin signaling pathways, such as ERK and phosphatidylinositol 3-kinase, are required for leptin’s effect on growth and reproduction.
The difference in growth and reproduction phenotypes observed in LepRb-Stat3 and Nestin-Stat3 mutants could be explained by several potential mechanisms. First, Nestin expression begins from embryonic d 10.5 in neural progenitor cells throughout the brain (13). As a result, Stat3 function is knocked out very early during embryogenesis in the central nervous system (CNS), which could potentially affect the proliferation, differentiation, and migration of neurons and glial cells. Alternatively, Stat3 signaling in non-LepRb neurons could play an important role in regulating energy balance. This view is supported by a recent finding that estrogen has tonic effects on the rewiring of melanocortin neurons, and it reduces feeding and body weight gain in a leptin-independent, but Stat3-dependent manner (14).
It should be acknowledged that the Nestin-Stat3 mice were on a C57BL6 background, and our LepRb-Stat3 mice were maintained on a mixed genetic background. On a C57BL6/J background, about 20% of ob/ob male mice show transient fertility, but female ob/ob mice are never fertile (2). On a mixed genetic background, male ob/ob mice show improved fertility in that about 41% of the ob/ob males became fertile. However, none of the ob/ob females on a mixed background was fertile (15). In contrast, all of the LepRb-Stat3 mutant males and females were fertile. Thus, whereas genetic background can influence reproduction, genetic background alone may not fully explain the discrepant phenotypes exhibited by the Nestin-Stat3 and LepRb-Stat3 mice.
It has been recently suggested that Stat3 signaling is required for leptin’s acute effect on glucose homeostasis (16), although other non-Stat3 signaling pathways have also been implicated in this process (17). Our result shows a significant correlation between body weight and fasting glucose levels in both sexes, suggesting that Stat3 plays a dominant role in body weight regulation and that impaired glucose homeostasis in the mutant mice is likely a secondary effect of obesity.
Leptin receptor long form has been reported to express in several peripheral tissues. However, several lines of evidence suggest that the phenotypes observed in our LepRb-Stat3 mice are mediated by the CNS. First, mice with deletion of leptin receptor in the brain are obese and insulin resistant, whereas mice with deletion of leptin receptor in the liver are normal (18), suggesting that direct leptin signaling in the liver alone does not affect energy balance. Furthermore, a recent study shows that removal of signaling domain of leptin receptor in most peripheral tissues including liver, adipose tissues, and small intestine, but not in the brain, causes no changes in energy balance or glucose homeostasis (19). Second, a neuron-specific LepRb transgene completely rescues the obesity, diabetes, and infertility in db/db mice, indicating that leptin’s effects on energy balance, glucose homeostasis, and fertility are centrally mediated (20). Third, in Nestin-Stat3 knockout mice, Stat3 is removed from the CNS only, and these mice exhibit obesity and diabetes phenotypes. Taken together, the above evidence strongly suggests that the phenotypes observed in the LepRb-Stat3 mutant mice are largely due to lack of Stat3 signaling in leptin receptor neurons in the CNS, but not in leptin receptor-expressing cells in the periphery.
Pomc and Agrp/Npy neurons have long been considered to be the primary first-order neurons regulated by leptin. Pomc expression is reduced, whereas Agrp and Npy expression is increased in ob/ob and db/db mice. Whereas Stat3 signaling is clearly required for leptin’s effect on feeding and body weight, the neuronal subgroups in which Stat3 is required for leptin function have not been elucidated. As a transcription factor, Stat3 is thought to mediate transcription of leptin target genes. Thus, Stat3 was hypothesized to play an important role in mediating leptin-regulated transcription of Pomc, Agrp, and Npy. However, we have recently shown that removal of Stat3 function from the Pomc neurons does not alter Pomc expression in males whereas it slightly reduces Pomc expression in females (12). Similarly, deletion of Stat3 from the Agrp neurons does not affect Agrp expression in either the fed or fasted states (21). Consistent with these results, we have shown in this study that removal of Stat3 function from LepRb neurons does not result in significant changes in expression of Pomc, Agrp, or Npy in young mice (5 wk of age), although body weights in the mutants are significantly elevated at this age. However, a significant increase in Agrp and Npy expression is detected in older mice (10 wk of age) when the mice are severely obese. Thus, increases in Agrp and Npy expression could be the result of leptin resistance secondary to obesity. Leptin receptor long form is expressed in many extraarcuate regions, including ventromedial hypothalamus, dorsomedial hypothalamus, lateral hypothalamus, ventral tegmental area, and the nucleus of solitary tract. The fact that Agrp and Pomc expression did not change in the mutants at an early age when they are already obese suggests that Stat3 function in other leptin target neurons may play an important role in mediating leptin’s effect on body weight. Consistent with this view, recent studies have shown that leptin signals through Stat3 in ventral tegmental area neurons to influence food intake and body weight (22,23).
In summary, we have provided evidence that Stat3 function, although required for leptin’s effect on energy balance, is dispensable for leptin’s effect on growth and reproduction. This has clarified the role of Stat3 signaling in leptin regulation of growth and fertility. It further suggests that Stat3 signaling acts in neurons other than the Pomc and Agrp/Npy neurons to regulate food intake and body weight.
MATERIALS AND METHODS
Mouse Genetics
Generation and characterization of the LeprCre mice have been reported (10). These mice were intercrossed with mice carrying a conditional allele of Stat3 (Stat3flox/flox), which has been described previously (9). Briefly, loxP sites were inserted into the Stat3 gene flanking exon 22, which encodes the critical tyrosine phosphorylation site that is required for Stat3 function. Animals with LepRb-specific Stat3 deletion were generated by crossing LeprCre/+, Stat3flox/flox mice with Stat3flox/flox mice. Whereas LepRb-Cre mice were on C57BL6 background, the Stat3flox/flox mice were maintained on a mixed genetic background with contribution from C57BL/6, FVB, and 129 strains. Thus, we have compared the LepRb-Stat3 mutants with their control littermates to minimize genetic variation. All mice were housed in the University of California, San Francisco’s mouse barrier facility in a room with a 0600–1800 h light-dark cycle. Mice were fed standard mouse chow and were given access to water ad libitum. All experiments were carried out under a protocol approved by the University of California, San Francisco Institutional Animal Care and Use Committee.
Blood Glucose and Insulin
Blood was collected from LepRb-Stat3 mutant and control mice via mandibular vein collection under either fed or fasting (6 h) conditions. Plasma insulin was measured with an insulin ELISA kit (ALPCO Diagnostics, Cypress, CA) according to manufacturers’ instructions. A glucometer (LifeScan, Inc., Milpitas, CA) was used to determine glucose levels from tail blood.
Fertility
For analysis of fertility, pregnancy or delivery of pups within 6 wk of being housed with an animal of the opposite sex was scored as reproductive success and is expressed as fertile animals per animals assayed per genotype. Average litter size was determined by averaging the total number of pups born within the 6-wk time frame after birth of the first litter.
Molecular Biology
Measurements of mRNA levels were carried out by quantitative RT-PCR on RNA extracted from dissected hypothalamic tissue. Total RNA was purified using TRIzol reagent (Invitrogen, Carlsbad, CA) and an RNeasy kit (QIAGEN, Valencia, CA), and quantified by NanoDrop spectrophotometry. From each total RNA sample 1 μg was reverse transcribed and then PCR-amplified using the 7900HT Fast Real-Time PCR System (Applied Biosystems, Inc., Foster City, CA) and SYBR green to measure relative cDNA levels. β-Actin was used as an internal control to normalize expression. Agrp primers were TGCTACTGCCGCTTCTTCAA and CTTTGCCCAAACAACATCCA; Npy primers were TAACAAGCGAATGGGGCTGT and ATCTGGCCATGTCCTCTGCT; Pomc primers were AGGCCTGACACGTGGAAGAT and AGGCACCAGCTCCACACAT; leptin receptor long form-specific primers were: TGTTTTGGGACGATGTTCCA and GCTTGGTAAAAAGATGCTCAAATG; and β-actin primers were CTGCGTTTTACACCCTTTCTTTG and GCCATGCCAATGTTGTCTCTTAT.
Immunofluorescence
Sex-matched and age-matched LepRb-Stat3 mutant and control mice were injected ip with leptin (5 mg/kg) or saline and were perfused with 4% paraformaldehyde 30 min later. Mice for neuropeptide expression analysis were not injected. Brains were removed, placed in 4% paraformaldehyde at 4 C overnight, and then cryoprotected with 30% sucrose in PBS overnight at 4 C. Frozen sections (6 μm) were prepared and mounted on Superfrost/Plus slides. Polyclonal anti-phospho (Tyr-705)-Stat3 (Cell Signaling Technology, Danvers, MA) was used at a 1:200 dilution according to the manufacturer’s protocol. Polyclonal antirat ACTH (National Hormone and Peptide Program, University of California, Los Angeles, CA) was used at a 1:200 dilution according to the protocol above. Polyclonal anti-NPY (Peninsula Laboratories, LLC, San Carlos, CA) was used at a 1:500 dilution according to the protocol above. Goat-antirabbit Alexa488 (Molecular Probes, Inc., Eugene, OR) was used at a 1:200 dilution for secondary antibody detection. Sections were mounted using Vectashield with 4′,6-diamidino-2-phenylindole, hard mount (Vector Laboratories, Burlingame, CA). Fluorescence images were captured using a Zeiss Axioscope2 system (Carl Zeiss, Thornwood, NY) equipped with a Hamamatsu C4742–95 digital camera (Hamamatsu Photonic Systems Corp., Bridgewater, NJ).
Acknowledgments
We thank Dr. Kiyoshi Takeda and Dr. Shizuo Akira (Osaka University, Osaka, Japan) for providing the flox-Stat3 mice.
Footnotes
This work was supported in part by grants from the Hurlbut Johnson Foundation and the Sandler Family Foundation (to A.W.X.), and by National Institutes of Health Grant DK67768 (to M.G.M.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 20, 2007
Abbreviations: Agrp, Agouti-related protein; CNS, central nervous system; LepRb, leptin receptor, isoform b; Npy, Neuropeptide Y; Pomc, proopiomelanocortin; pStat3, phospho-Stat3; Stat, signal transducer and activator of transcription 3.
References
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- Charlton HM 1984 Mouse mutants as models in endocrine research. Q J Exp Physiol 69:655–676 [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP 1996 Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–495 [DOI] [PubMed] [Google Scholar]
- Munzberg H, Bjornholm M, Bates SH, Myers Jr MG 2005 Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci 62:642–652 [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers Jr MG, Schwartz MW 2001 Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413:794–795 [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS 2005 PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers Jr MG 2003 STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421:856–859 [DOI] [PubMed] [Google Scholar]
- Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY 2004 Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA 101:4661–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S 1998 Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol 161:4652–4660 [PubMed] [Google Scholar]
- Leshan RL, Bjornholm M, Munzberg H, Myers Jr MG 2006 Leptin receptor signaling and action in the central nervous system. Obesity 14(Suppl 5):208S–212S [DOI] [PubMed] [Google Scholar]
- Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, Oz OK, Pourbahrami T, Lu CY, Richardson JA, Karsenty G, Li C 2004 Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol 24:258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Ste-Marie L, Kaelin CB, Barsh GS 2007 Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology 148:72–80 [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U 2001 β1-Class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31:367–379 [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL 2007 Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13:89–94 [DOI] [PubMed] [Google Scholar]
- Ewart-Toland A, Mounzih K, Qiu J, Chehab FF 1999 Effect of the genetic background on the reproduction of leptin-deficient obese mice. Endocrinology 140:732–738 [DOI] [PubMed] [Google Scholar]
- Buettner C, Pocai A, Muse ED, Etgen AM, Myers Jr MG, Rossetti L 2006 Critical role of STAT3 in leptin’s metabolic actions. Cell Metab 4:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Kulkarni RN, Seifert M, Myers Jr MG 2005 Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab 1:169–178 [DOI] [PubMed] [Google Scholar]
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM 2001 Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108:1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, Loh D, Li C, Chua Jr S, Zhang Y 2007 Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology 148:3987–3997 [DOI] [PubMed] [Google Scholar]
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua Jr SC 2005 Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115:3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin CB, Gong L, Xu AW, Yao F, Hockman K, Morton GJ, Schwartz MW, Barsh GS, Mackenzie RG 2006 Stat binding sites but not Stat3 are required for fasting-induced transcription of Agouti-related protein mRNA. Mol Endocrinol 20:2591–2602 [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ 2006 Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810 [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS 2006 Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51:811–822 [DOI] [PubMed] [Google Scholar]