Abstract
Metabolic dysregulation is associated with reproductive disorders, but the underlying mechanisms are not clearly understood. Adiponectin is an adipocyte-derived secretory factor that improves insulin sensitivity. Results from animal models indicate that overexpression of adiponectin impairs female fertility. We hypothesized that adiponectin regulates reproduction by altering the hypothalamic-pituitary axis. Mouse LβT2 immortalized gonadotrope cells express both adiponectin receptors 1 and 2. Adiponectin increases phosphorylation of AMP-activated protein kinase (AMPK), a downstream target of adiponectin receptors, and reduces basal and GnRH-stimulated LH secretion, acutely. The repression of LH secretion can be mimicked by 5-aminoimidazole-4-carboxamide-1-β-riboside, an AMP analog, suggesting the involvement of AMPK. A dominant-negative AMPK mutant or compound C, a selective AMPK inhibitor, potentiates basal LH secretion and abolishes the inhibitory effect of adiponectin. Chronic activation of AMPK by 5-aminoimidazole-4-carboxamide-1-β-riboside decreases cellular LH levels, and expression of dominant-negative AMPK increases cellular LH levels, suggesting a second effect of AMPK to regulate LH synthesis. Lastly, intravenous injection of an adenovirus expressing adiponectin into male mice reduces serum LH levels without changing FSH levels. In conclusion, our results suggest that adiponectin decreases LH secretion in pituitary gonadotropes in an AMPK-dependent manner.
THE HYPOTHALAMIC/PITUITARY/GONADAL (HPG) axis is central to the mammalian reproductive system (1). Pulsatile release of GnRH from neurons in the hypothalamus stimulates the secretion of LH and FSH from gonadotropes in the anterior pituitary. LH regulates estrogen synthesis and ovulation in females and androgen synthesis in males. FSH promotes follicle maturation and estrogen release in females and spermatogonia in males. Androgen and estrogen production from the gonads also exerts feedback regulation on GnRH, LH, and FSH synthesis and secretion. It has long been recognized that reproductive function is closely associated with energy balance, and metabolic dysregulation is linked with reproductive abnormalities. Obesity may cause anovulation, reduced fertility, and high risk of miscarriage (2,3). In addition, caloric deprivation can cause amenorrhea in women (1,2). Therefore, both the deficiency and surplus of nutrients can result in reproductive disorders. However, the underlying mechanisms are not known.
Adipose tissue participates in energy homeostasis not only as a lipid storage organ but also as an endocrine organ by secreting bioactive peptides, namely adipokines (4). These adipokines, such as leptin (5,6), resistin (7), and TNFα (8,9), regulate satiety, insulin sensitivity, and inflammation (4). Adiponectin, also known as adipocyte complement-related protein of 30 kDa, is an adipocyte-derived secretory factor that improves insulin sensitivity (10,11,12,13). Adiponectin belongs to the complement 1q protein family and has been shown to form homomultimers in circulation, including trimer, hexamer, and high-molecular weight structures (14,15). A smaller globular fragment of adiponectin has been detected in human plasma, although its level is much lower than full-length adiponectin (16). Serum adiponectin levels in humans and mice are inversely correlated with insulin resistance and metabolic syndrome (17). That is to say, adiponectin levels are low in subjects with obesity, diabetes, hypertension, cardiovascular diseases, or polycystic ovary syndrome (PCOS) (18) and are elevated in insulin-sensitive subjects. Gain-of-function and loss-of-function studies (19,20,21,22) illustrated that adiponectin increases glucose uptake and fatty acid β-oxidation and decreases gluconeogenesis and triglyceride synthesis in the liver and skeletal muscle. Recent reports also suggest that adiponectin may act on central nervous system to modulate feeding and energy expenditure (23).
Two receptors, namely AdipoR1 and AdipoR2, have been identified for adiponectin. These receptors share homology with G protein-coupled receptors, yet they do not appear to signal through canonical G proteins (24). Instead, adiponectin receptors, upon ligand binding, activate downstream targets such as AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-α (25). AMPK, a heterotrimeric complex of αβγ-subunits, is a pivotal cellular energy monitor (26). AMPK is activated by the cellular AMP/ATP ratio when ATP levels drop (27,28). AMPK inhibits anabolic pathways and stimulates glucose uptake and fatty acid β-oxidation to restore cellular ATP levels (26). In addition, several adipokines, including adiponectin and leptin, activate AMPK in peripheral tissues, although the underlying mechanisms are not clear (29,30,31). It has been established that AMPK mediates the glucose-lowering effect of adiponectin in various tissues (30,32).
How the reproductive system senses energy status is not understood. It is conceivable that adipokines function as energy storage signals from adipose tissue. For example, leptin plays a permissive role for reproductive function by regulating the HPG axis depending on fuel availability (2,33,34). However, little is known about the effect of adiponectin on reproduction. Results from animal studies indicate that the level of adiponectin is tightly controlled during puberty, sexual differentiation, gestation, and lactation (35). In addition, overexpression of adiponectin impairs female fertility in mice (21), but loss of adiponectin has no effect. Because the interaction between gluco-regulatory hormones, metabolic status, and the HPG reproductive system has proven to be remarkably complex with multiple overlapping regulatory pathways, we sought to dissect the effect of adiponectin on an individual component of the HPG axis. We hypothesized that adiponectin regulates reproduction by altering gonadotrope function. In the present study, we show that LβT2 pituitary gonadotrope cells express adiponectin receptors and respond to adiponectin by phosphorylating AMPK. The resultant AMPK activation diminishes LH secretion. Adenoviral expression of adiponectin in male mice results in reduced serum LH. This study provides direct evidence that adiponectin regulates pituitary gonadotrope function in vitro and in vivo.
RESULTS
LβT2 Gonadotropes Express Adiponectin Receptors and Respond to 5-Aminoimidazole-4-Carboxamide-1-β-Riboside (AICAR) Treatment
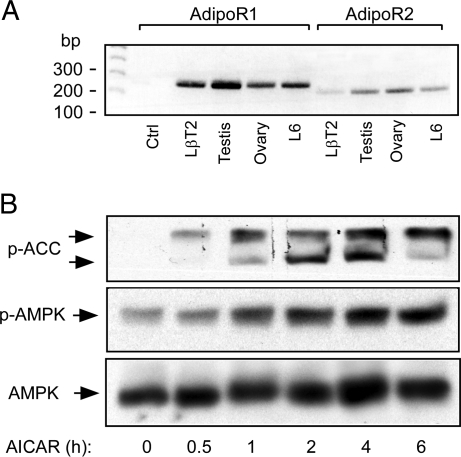
We first investigated the expression of adiponectin receptors in LβT2 immortalized mouse gonadotropes cells using RT-PCR. PCR products of the appropriate size for adiponectin receptor 1 (270 bp) and adiponectin receptor 2 (220 bp) were detected (Fig. 1A). Interestingly, both receptors are expressed in mouse ovary and testis, which indicates other potential sites of adiponectin action on the reproductive system. We used rat L6 myoblast as a positive control, as this cell is known to express both receptors (36) and the primer sets were designed to match both rat and mouse sequences. In addition, a recently identified adaptor molecule for adiponectin receptor signaling, APPL1 (37), was also detected in LβT2 cells (data not shown). We then determined whether LβT2 cells possess a functional AMPK pathway. Artificial AMPK activation by AICAR, an AMP analog, dramatically enhanced the phosphorylation of AMPK (Thr172) and ACC (acetyl coenzyme A carboxylase) (Ser79), a downstream target of AMPK (Fig. 1B) (26). This shows that LβT2 cells express AMPK and are responsive to changes in the AMP/ATP ratio.
Figure 1.
Detection of AdipoR1 and -2 and Downstream Signaling Pathways in LβT2 Gonadotropes
A, LβT2 cells express AdipoR1 and R2. Total RNA extracted from LβT2 gonadotropes, L6 rat skeletal muscle cells, mouse testis, and ovary were subjected to RT-PCR for mouse and rat AdipoR1 (270 bp) or -R2 (220 bp). B, AICAR stimulates AMPK and ACC phosphorylation. LβT2 cells were treated with 1 mm AICAR for the times indicated. Total cell lysates were extracted and subjected to immunoblotting. Phospho-AMPKα (Thr172), phospho-ACC (Ser79), and total AMPKα were detected by immunoblotting. Ctrl, Control.
Adiponectin Induces AMPK Activation in LβT2 Cells
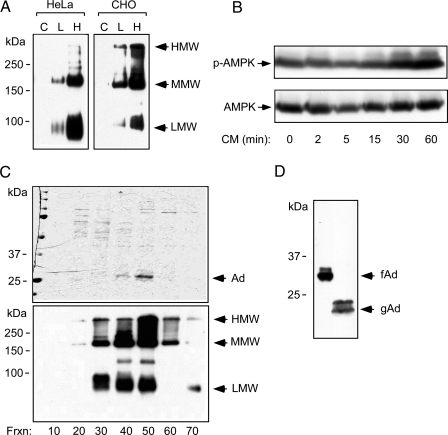
To test whether adiponectin stimulates AMPK in LβT2 cells, we chose to prepare recombinant adiponectin from mammalian cells because posttranslational modification and multimerization are critical for its function (38,39). Adiponectin was expressed in HeLa and Chinese hamster ovary (CHO) cells by adenoviral infection. Because analysis of the multimeric forms on native acrylamide gels showed that CHO cells gave a greater proportion of high-molecular weight adiponectin, these cells were used for large-scale purification (Fig. 2A). We verified that the adiponectin was functional by demonstrating that the conditioned medium could stimulate phosphorylation of AMPK (Fig. 2B). Full-length adiponectin was purified by ammonium sulfate precipitation followed by fast protein liquid chromatography, and fractions containing adiponectin were combined and concentrated (Fig. 2C). We confirmed that the purified adiponectin retains its high-order structure as all molecular-weight forms of adiponectin could be detected on a nonreducing gel (Fig. 2C). Recombinant globular adiponectin is approximately 7 kDa smaller than monomeric full-length adiponectin (Fig. 2D). Acute treatment of LβT2 cells with full-length adiponectin at a physiological concentration (20 μg/ml) induces phosphorylation of AMPK and ACC (Fig. 3A). The ability of adiponectin to activate AMPK decreases after 60 min, which is suggestive of desensitization, and this time course is similar to published data from other cell types (30,40). Globular adiponectin also induces ACC phosphorylation acutely albeit less strongly than AICAR (Fig. 3B). ACC phosphorylation is stimulated in a dose-dependent manner by full-length adiponectin at physiological concentrations, but globular adiponectin did not show a dose dependence with the two concentrations tested (Fig. 3C). These experiments demonstrated that recombinant full-length and globular adiponectin stimulate AMPK activity and ACC phosphorylation in LβT2 cells.
Figure 2.
Purification of Recombinant Adiponectin from Mammalian Cells
A, Adiponectin expression in HeLa or CHO cells. Cells infected with an adenovirus- expressing mouse adiponectin were used to produce recombinant full-length adiponectin (C, L, and H indicate control, low dose, and high virus dose, respectively). Conditioned media (48 h) were subjected to nondenaturing, nonreducing SDS-PAGE. Multimerization of adiponectin was detected by immunoblotting. Arrows indicate the high-, medium (hexamer)-, and low-molecular weight (trimer) multimeric forms. B, Infected cells secrete adiponectin. LβT2 cells were serum starved and treated with conditioned media (CM) for the times indicated. Phosphorylation of AMPK and total AMPK α-subunit were detected by immunoblotting as before. C, Purifcation of recombinant full-length adiponectin from adenovirally infected CHO cells. Recombinant proteins were separated by fast protein liquid chromatography. Protein samples from different fractions, as indicated, were subjected to SDS-PAGE and Coommassie blue staining to determine the purity (top panel). The same samples were subjected to a nonreducing and nondenaturing gel followed by immunoblotting to determine the higher-order structure of adiponectin (bottom panel). Arrows indicate the high (HMW), the medium (MMW), and the low molecular (LMW) form of the recombinant adiponectin. D, Recombinant mouse full-length adiponectin (fAd) and human globular adiponectin (gAd) (a kind gift from Xencor, Inc.) were subjected to SDS-PAGE and detected by immunoblotting.
Figure 3.
Purified Recombinant Adiponectin Activates AMPK in LβT2 Cells
A, Full-length adiponectin stimulates AMPK. LβT2 cells were serum starved overnight and then treated with 20 μg/ml recombinant mouse full-length adiponectin (fAd) for the times indicated. Phospho-AMPKα, phospho-ACC, and total AMPKα were detected by immunoblotting. B, Globular adiponectin stimulates ACC phosphorylation. LβT2 cells were serum starved overnight followed by treatments with 3 μg/ml of human globular adiponectin (gAd) for 5 min. Treatment with 1 mm AICAR for 1 h served as a positive control. For inhibition of AICAR-induced AMPK activation, compound C (20 μm) was added for 30 min before AICAR treatment. C, Dose-dependent phosphorylation of ACC by fAd (10 and 20 μg/ml) and gAd (3 and 10 μg/ml). Data shown are the fold induction of ACC phosphorylation (mean ± sd) from three experiments. Asterisk indicates statistical significance (P < 0.05). Cpd C, Compound C.
Adiponectin Inhibits LH Secretion from LβT2 Gonadotrope Cells
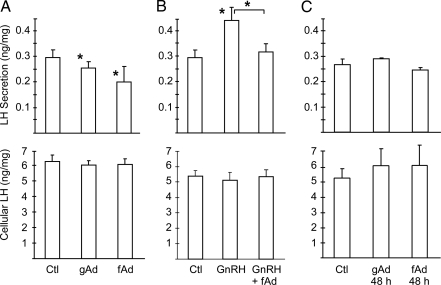
To address whether adiponectin alters gonadotrope function, we investigated the regulation of LH secretion. Acute (30 min) treatment with either globular or full-length adiponectin inhibits basal LH secretion from LβT2 cells without changing the cellular content of LH (Fig. 4A). In contrast, acute treatment (30 min) with GnRH increases LH secretion, but this stimulation was abolished by acute cotreatment with adiponectin (Fig. 4B). Chronic treatment (48 h) with adiponectin had no effect on LH secretion or cellular content consistent with the down-regulation of signaling (Fig. 4C).
Figure 4.
Secretion of LH from LβT2 Cells Is Reduced by Adiponectin
A, Adiponectin acutely inhibits LH secretion. LβT2 cells were serum starved, washed, and then treated with vehicle control (Ctl), 20 μg/ml full-length adiponectin (fAd,), or 10 μg/ml globular adiponectin (gAd) for 30 min. Secreted LH and cellular LH were assayed in conditioned media or cell lysates. LH levels were normalized to total cellular protein concentrations, and LH secretion (ng) per mg of cell lysate is shown. Experiments were performed in triplicate. Data are shown as mean ± sd from three experiments. B, Adiponectin inhibits GnRH-stimulated secretion. LβT2 cells were serum starved, washed, and then treated with 20 μg/ml full-length adiponectin in the presence or absence of 100 nm GnRH for 30 min. Secreted LH and cellular LH were assayed in conditioned media and cell lysates as above. C, Chronic adiponectin treatment has no effect on LH secretion. LβT2 cells were cultured and serum starved in the presence of 10 μg/ml globular adiponectin or 20 μg/ml full-length adiponectin for 48 h. Secreted LH and cellular LH were assayed as above. Asterisks indicate statistical significance (P < 0.05) vs. control or between conditions connected by bars.
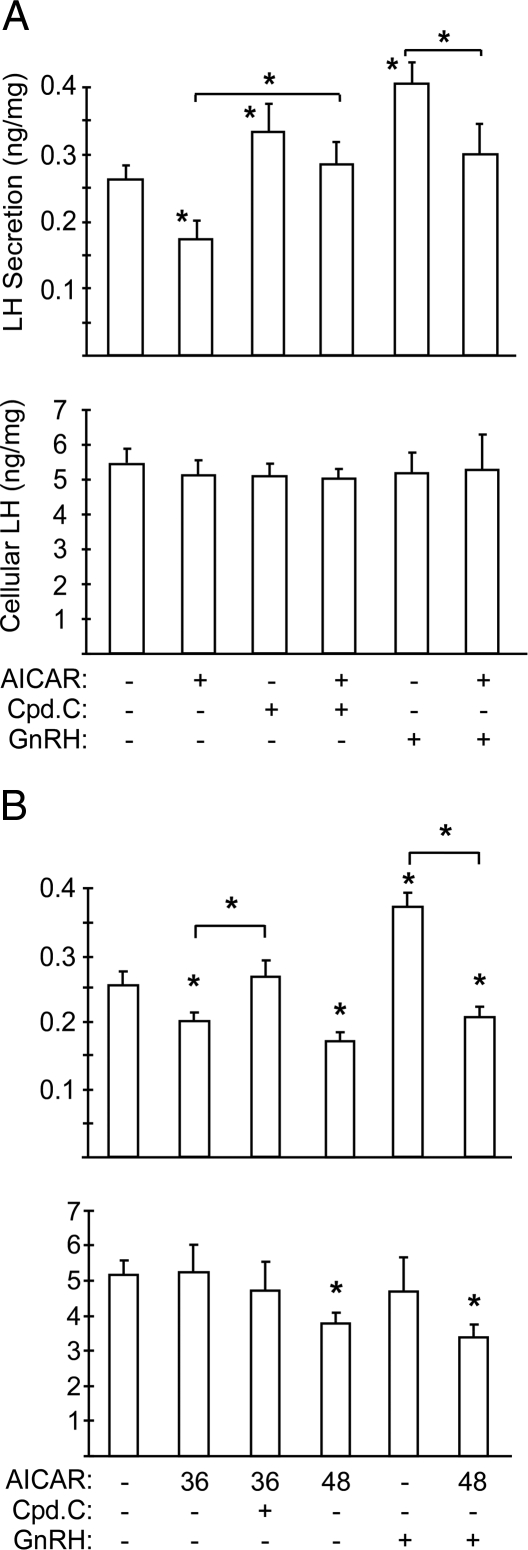
Because AMPK is the major cellular target of adiponectin and is activated in LβT2 cells, we tested whether AMPK modulates gonadotrope function. Artificial AMPK activation by AICAR (1 mm) resulted in an acute decrease in basal LH secretion with no effect on cellular LH content similar to adiponectin (Fig. 5A). Conversely, inhibition of AMPK by compound C, a relatively specific AMPK inhibitor, augmented basal LH secretion and blocked the AICAR effect (Fig. 5A). Acute AICAR treatment also blocked GnRH-stimulated LH secretion without changing cellular LH content (Fig. 5A). To explore the effect of chronic AMPK activation on LH secretion, we used a lower dose of AICAR (200 μm) that mirrors the activation of AMPK by adiponectin, because the higher dose (1 mm) impairs cell viability upon prolonged exposure (data not shown). We found that activation of AMPK for 36 h decreased LH secretion without changing the cellular level of LH (Fig. 5B). The reduced LH secretion could be rescued by a 1-h inhibition of AMPK by compound C, suggesting that the secretory apparatus is intact. Interestingly, AMPK activation for longer periods (48 h) led to both decreased LH release and cellular content (Fig. 5B). The cell morphology was unchanged, and there was no decrease in total cellular protein; therefore, the decline of LH level in the cells was not due to nonspecific inhibition of protein synthesis. These results suggest that AMPK contributes to LH regulation at two steps: acutely at the level of secretion, and chronically at the level of synthesis.
Figure 5.
AMPK Stimulation Decreases LH Secretion in LβT2 Cells
A, Artificial activation of AMPK inhibits LH secretion. LβT2 cells were serum starved and then treated with 1 mm AICAR for 2 h with or without 20 μm compound C (Cpd.C) or 100 nm GnRH for 30 min. LH levels were normalized to cellular protein concentrations, and LH secretion (ng) per mg of cell lysates is shown. B, Chronic activation of AMPK inhibits LH secretion and synthesis. LβT2 cells were cultured and serum starved in the presence of 200 μm AICAR for 36 or 48 h. Compound C (20 μm) was added 1 h before the secretion assay and 100 nm GnRH was added for the final 30 min. Secreted LH and cellular LH were assayed as above. Experiments were performed in triplicate. Data are shown as mean ± sd from three experiments. Asterisks indicate statistical significance (P < 0.05) vs. control or between conditions connected by bars.
Expression of a Dominant-Negative AMPK Stimulates LH Secretion and Blocks the Effect of Adiponectin
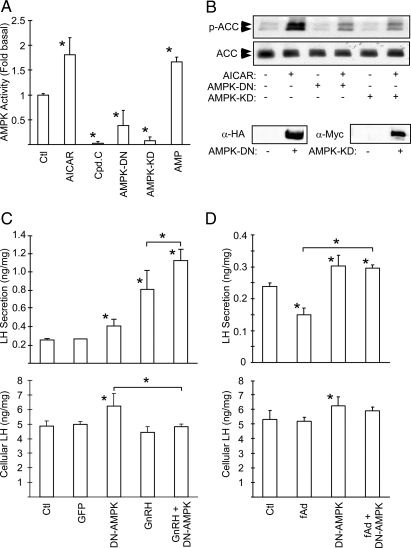
To determine whether the adiponectin effect on LH secretion is mediated by AMPK, we created adenoviruses expressing either a dominant-negative human AMPKα1 (D159A) or kinase-dead rat AMPKα2 (K45R). These mutants inhibit endogenous AMPK activity by replacing the endogenous AMPKα subunit with the inactive form in the αβγ holoenzyme (41,42). The ability of these mutants to inhibit AMPK was verified by measuring AMPK activity in LβT2 cells infected with either of the two mutants. Both mutants reduced AMPK activity, similar to the selective AMPK inhibitor compound C (Fig. 6A), whereas AICAR and AMP stimulated AMPK activity as expected (43). To verify the inhibitory effect, we measured ACC phosphorylation in cells infected with either of the two mutant AMPKs. Both the basal and AICAR-stimulated phosphorylations of ACC were dramatically reduced in LβT2 cells infected with these adenoviruses, confirming the inhibition of endogenous AMPK activity (Fig. 6B).
Figure 6.
AMPK Mediates the Inhibitory Effect of Adiponectin on LH Secretion
A, In vitro kinase activity of mutant AMPKs. LβT2 cells were infected with adenoviruses expressing either a dominant-negative form of AMPKα1 (AMPK-DN) or a kinase-dead form of AMPKα2 (AMPK-KD) for 48 h. Cells were also treated with 20 μm compound C (Cpd.C) or 1 mm AICAR for 4 h. AMPK activity was determined by kinase assay using SAMS peptide. A kinase reaction in the presence of 300 μm AMP serves as a positive control. B, Mutant AMPKs block endogenous AMPK activity in cells. CHO cells were infected with AMPK-DN or AMPK-KD. Cells were treated with 1 mm AICAR for 2 h, and phospho-ACC was detected by immunoblotting whole-cell extracts. Extracts of uninfected and infected cells were immunoblotted for the HA or c-Myc tags to demonstrate mutant protein expression (lower panels). C, A dominant-negative AMPK mutant increases LH secretion and synthesis. LβT2 cells were infected with dominant-negative AMPK adenovirus (AMPK-DN) or control virus (GFP). Cells were serum starved 32 h later for an additional 16 h. Cells were then washed and subjected to secretion in a 30-min period with or without 100 nm GnRH. Secreted LH and cellular LH were determined, and LH levels (ng) per mg of cell lysates are shown. D, A dominant-negative AMPK mutant blocks the acute effect of adiponectin. Infected cells were treated with 20 μg/ml adiponectin (fAd) for 30 min. Secreted and cellular LH levels were determined. Experiments were performed in triplicate. Data are shown as mean ± sd from three experiments. Asterisks indicate statistical significance (P < 0.05) vs. control or between conditions connected by bars. HA, Hemagglutinin; Ctl, control.
When gonadotrope function was assessed, dominant-negative AMPK expression increased both basal LH secretion and cellular LH content (Fig. 6C). The increase in cellular LH content was also observed in cells treated for 2 h with the AMPK inhibitor (data not shown). Inhibition of endogenous AMPK also potentiated the GnRH-induced secretion of LH (Fig. 6C) and blocked the ability of full-length adiponectin to reduce LH secretion (Fig. 6D). Similar results were obtained with the kinase-dead AMPK mutant (data not shown). These results confirm that adiponectin represses LH secretion via activation of AMPK and that AMPK regulates LH protein synthesis.
Overexpression of Adiponectin Reduces Circulating LH in Vivo
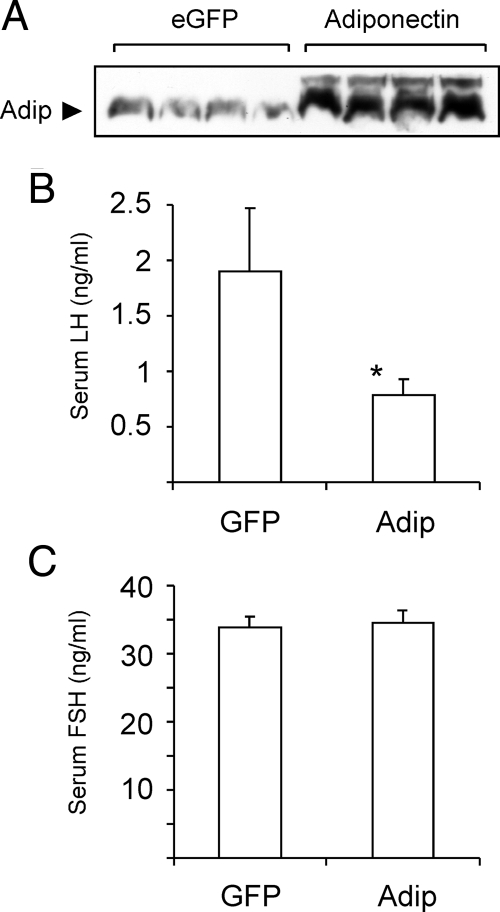
Given the demonstrated role of adiponectin in gonadotrope function in vitro, we studied the regulation of gonadotropin production in vivo. It has been shown that transgenic overexpression of wild-type adiponectin in adipose tissue interferes with the secretion of endogenous adiponectin and does not increase the level of circulating adiponectin (21). Therefore, we chose to overexpress adiponectin in the liver by in vivo adenovirus injection. This approach causes a transient increase of circulating adiponectin in rats for up to 10 d (44). Because of the transient expression of exogenous adiponectin, it would be difficult to study the effect of adiponectin on cyclicity in female mice. Therefore, we chose to restrict our study to male mice. Mice were injected with an adenovirus expressing full-length adiponectin or a control virus expressing enhanced green fluorescent protein (eGFP). Seven days after injection, serum adiponectin levels were markedly elevated in Ad-adiponectin-injected animals (Fig. 7A). Furthermore, we found that serum LH was significantly lower in these mice than in Ad-eGFP-injected mice (Fig. 7B). Serum levels of FSH, on the other hand, were not altered by adiponectin (Fig. 7C). This in vivo finding is in concert with the in vitro results supporting the notion that LH release from the pituitary is inhibited by adiponectin.
Figure 7.
Adiponectin Regulates Serum LH In Vivo
A, Adiponectin expression in infected mice. Male C57BL/6J mice (8 wk old) were injected iv with either GFP adenovirus (eGFP) (n = 16) or adiponectin adenovirus (adiponectin) (n = 17). All mice were killed 7 d later, and serum samples were subjected to immunoblotting to determine adiponectin levels. B, Adiponectin expression reduces serum LH. C, Adiponectin expression has no effect on serum FSH. Graphs show serum gonadotropin levels (mean ± sem). Asterisk indicates statistical significance (P < 0.05) vs. GFP-infected mice. Adip, Adiponectin.
DISCUSSION
Reproduction is an energetically costly process and should only be undertaken when nutrition is sufficient. Food restriction, increased energy demand, or a catabolic state have profound influences on the HPG axis (1). A potential link between adiponectin and reproduction is suggested by the observations that women with anorexia and female endurance runners have high levels of plasma adiponectin and are infertile or subinfertile (1,2,45,46,47). Adiponectin polymorphisms are associated with obesity, type 2 diabetes, and coronary artery diseases in various populations (48,49). Acute exercise does not alter adiponectin expression in lean healthy individuals, but caloric restriction or exercise increases adiponectin levels in obese individuals (50,51,52,53,54,55,56,57). Circulating adiponectin is higher in women than men, with the high molecular weight form showing the greatest change (14,58), and pregnancy causes a decrease that is related to the increased adiposity and insulin resistance in both rats and humans (35,59,60,61). Adiponectin levels are also under hormonal control because prolactin inhibits the production and secretion of adiponectin by primary adipocytes (62,63). Expression is negatively regulated by androgens and is elevated in androgen receptor null mice, which likely explains the sexual dimorphism (35,64,65,66).
In women with PCOS, adiponectin is an independent predictor of insulin resistance (18,67,68). Rosiglitazone treatment increases plasma adiponectin in PCOS and promotes ovulation (69,70). Despite these observations, the precise function of adiponectin on the reproductive axis has yet to be uncovered. Adiponectin is produced primarily by the adipocyte, but low-level expression has been demonstrated in other tissues including liver, placenta, heart, the anterior pituitary, and the brain (71,72,73,74,75,76). Nonetheless, no adiponectin protein expression could be detected in LβT2 cells (data not shown). Adiponectin receptors are expressed widely, including in the hypothalamus and paraventricular nucleus, raising the possibility of auto- or paracrine regulation (76). Recent studies indicate that adiponectin receptors are expressed in porcine ovary (77), porcine uterus (77), and rat pituitary (73). Adiponectin is present in follicular fluid and induces cyclooxygenase 2, prostaglandin E synthase, vascular endothelial growth factor, and steroidogenic acute regulatory protein mRNAs and inhibits aromatase mRNA in cultured granulosa cells (78).
Our current study demonstrates that adiponectin receptors are expressed in mouse pituitary, ovary, and testis. We demonstrate that adiponectin decreases basal and GnRH-stimulated LH secretion via activation of AMPK in LβT2 cells in culture and that adiponectin overexpression decreases plasma LH in mice. This finding confirms and extends a previous report on primary pituitary cultures (73). In that study, recombinant globular adiponectin acutely (4 h) repressed basal and GnRH-stimulated LH secretion from primary rat pituitary cultures. A longer treatment (24 h) had no effect on LH secretion, similar to our findings in the LβT2 immortalized gonadotrope cells. The GnRH-R mRNA was decreased by 50% with acute (4 h) adiponectin treatment and more profoundly by 80% after 24-h treatment. Interestingly, adiponectin and both receptors, AdipoR1 and AdipoR2, were expressed in the primary cultures. Adiponectin and AdipoR2 mRNA levels increased with adiponectin treatment, but AdipoR1 mRNA levels decreased. This may represent expression in other anterior pituitary cell types because adiponectin also had effects on GH secretion. Our results confirm these observations and extend them by showing that the adiponectin effect is mediated by AMPK, that adiponectin reduces LH levels in male mice, and that adiponectin may have effects on LH synthesis as well as secretion. Future studies involving long-term adiponectin overexpression in female mice would be useful to understand the role of adiponectin on female fertility.
The down-regulation of serum LH by adiponectin may have physiological implications for PCOS, because circulating adiponectin correlates with insulin resistance in women with PCOS, a disease associated with elevated LH. Because testosterone can inhibit adiponectin (66), it is possible that the hyperandrogenism in PCOS may directly reduce the circulating level of adiponectin, which would decrease insulin sensitivity and increase LH synthesis and secretion. Hence, adiponectin could be a potential therapeutic target in PCOS. Indeed, insulin-sensitizing thiazolidinedione drugs, which are used to induce ovulation in PCOS, may work by increasing adiponectin levels because the adiponectin gene is a direct target for peroxisome proliferator-activated receptor-γ (79,80,81,82). Metformin, another insulin-sensitizing drug used in PCOS patients, may use a similar mechanism but bypasses adiponectin and activates AMPK directly by reducing ATP levels (43,83).
Adiponectin has been proposed as a starvation signal, because its level is increased by food restriction and/or physical training. Undernutrition is associated with a switch in metabolic fuel utilization from carbohydrate to fat and protein. This substrate switch is accomplished by the AMPK inhibition of ACC, thus reducing malonyl CoA and activating fatty acid transport into mitochondria for oxidation. At the cellular level, AMPK is the intrinsic energy sensor and is activated by a fall in ATP, to restore ATP levels. This is achieved by inhibition of energy-consuming processes such as protein, glycogen and lipid synthesis, and stimulation of ATP-generating processes such as glycolysis, fatty acid β-oxidation, and glucose uptake (26). Hence, AMPK is a sensor of metabolic status at both the cellular level via allosteric activation by AMP and at the organismal level via activation by circulating adipokines. In LβT2 cells, basal LH secretion is not reduced by long-term adiponectin treatment in agreement with the transient activation of AMPK by adiponectin, which suggests receptor desensitization. Adiponectin has been shown to down-regulate the expression of AdipoR2 in muscle cells but, paradoxically, AdipoR2 is increased whereas AdipoR1 is decreased in primary pituitary cultures by prolonged adiponectin treatment. We do not know which receptor isoform is mediating the adiponectin effect on LH secretion, but we suspect AdipoR1, because this isoform couples preferentially to AMPK (25).
Chronic activation of AMPK revealed another potential role in gonadotrope function, because not only LH secretion but also cellular LH content was suppressed. Expression of a dominant-negative AMPK mutant increased cellular LH content confirming that AMPK regulates LH synthesis. The acute secretion of LH may be controlled at multiple levels. A decrease in cellular ATP activates AMPK that, in turn, inhibits energy-requiring processes and stimulates ATP production by increasing glucose uptake and fatty acid oxidation. Trafficking and fusion of secretory vesicles is ATP dependent, so may be directly inhibited by AMPK. Alternatively, the calcium increases essential for stimulating secretion may be blocked. The synthesis of LH may also be regulated at many levels because both transcription of the LHβ gene and translation of the LHβ mRNA are subject to hormonal regulation. Based on our results, it is tempting to propose that pituitary AMPK serves as an energy sensor to monitor cellular ATP levels and to sense the nutritional status of the whole body and accordingly determine the release of gonadotropin to control reproduction. Future studies involving modulation of pituitary AMPK activity in vivo will be necessary to test this hypothesis.
MATERIALS AND METHODS
Materials and Cell Culture
AICAR was purchased from Sigma-Aldrich (St. Louis, MO). Compound C was obtained from Calbiochem (La Jolla, CA). Human globular adiponectin was a kind gift from Xencor (Monrovia, CA). Antiadiponectin antibodies were purchased from Affinity BioReagent (Golden, CO). All other antibodies were purchased from Cell Signaling Technology (Danvers, MA). Mouse gonadotrope LβT2 cells and human embryonic kidney 293 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). CHO cells were cultured in DMEM/F12 (50:50) supplemented with 10% FBS.
Animal Experiments
Male C57BL/6J mice (8 wk old) were purchased from the Jackson Laboratory (Bar Harbor, ME) and adapted to a 12-h light, 12-h dark cycle for 2 wk. Adenoviruses expressing mouse adiponectin or eGFP were described previously (44). Recombinant viruses were purified using CsCl gradient. Mice were injected with adenoviruses (1 × 109 plaque-forming units) via tail vein. The mice were killed 7 d after injection. Sera were extracted and subjected to hormone assays or Western blotting. All animal experiments were approved by the institutional animal care and use committee. Mouse LH and FSH were measured by the Ligand Core of the Center for Reproductive Research of the University of Virginia.
Adenovirus Preparation and Cell Infection
Human AMPKα1 and rat AMPKα2 cDNA were cloned as previously described (41,83). The dominant-negative AMPKα1 (D159A) and the kinase-dead AMPKα2 (K45R) mutants were constructed by site-directed mutagenesis. Recombinant adenoviruses expressing the AMPKα mutants were created as previously described (84) by use of the Adeno-X system from CLONTECH Laboratories, Inc. (Palo Alto, CA). Briefly, cDNA encoding hemagglutinin-tagged dominant-negative AMPKα1 (AMPKα1-DN), Myc-tagged kinase-dead AMPKα2 (AMPKα2-KD) was inserted into a viral shuttle vector. The I-CeuI/PI-SceI-digested shuttle vector was then ligated into Adeno-X viral DNA. The authenticity of all the plasmids was verified by sequencing. The PacI-digested viral DNA was transfected into human embryonic kidney 293 cells for virus amplification. The titer of the viruses was determined by agarose plaque assay. LβT2 cells cultured in poly-l-lysine-coated plates were incubated with various adenoviruses at indicated multiplicities of infection for 6 h in DMEM containing 2% FBS. The medium was then replaced by fresh DMEM supplemented with 2% FBS.
RT-PCR
Total RNA from LβT2 cells, rat L6 myoblast cells, mouse testis, and mouse ovary were extracted using RNA Bee reagent (Tel-Test, Friendswood, TX). RNA samples were subjected to reverse transcription (RT) in the presence of oligo-dT primer. For the control experiment, the RNA sample was subjected to RT without the oligo-dT primer but with an antisense primer for the PCR described below. The adiponectin receptor 1 cDNA was amplified using the following primer set: 5′-gggcttgcttcaagagcatcttc-3′ and 5′-caatccctgaatagtccagtttgg-3′. The adiponectin receptor 2 cDNA was amplified using the following primer set: 5′-gtattcttcctgtgcctggggat-3′ and 5′-aaagccaaggaacaaaacttccc-3′. The PCRs were carried out with the following thermal cycles: 95 C for 5 min; 22 × (95 C for 1 min, 60 C for 1 min, 72 C for 1 min); 72 C for 10 min. PCR products were subjected to 2% agarose gel.
In Vitro AMPK Activity Assay
The AMPK activity assay was performed as previously described (85). In brief, LβT2 cells were treated with AICAR (1 mm), Compound C (20 μm) for 2 h, or infected with various adenoviruses for 48 h. After treatments, cells were lysed in buffer A [50 mm HEPES (pH 7.4), 1% Triton, 1 mm EDTA, 1 mm EGTA, 10% glycerol, 1 mm dithiothreitol, phosphatase inhibitor cocktail (Sigma) and protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN)]. The cell debris was removed by centrifugation at 13,000 rpm for 5 min at 4 C. The supernatant was then adjusted to 10% polyethylene glycol 8000 and incubated for 45 min at 4 C on a rotator. Precipitated proteins were pelleted by centrifugation at 18000 × g for 15 min and were resuspended in buffer A. Aliquots were assayed for AMPK activity using SAMS peptide corresponding to amino acids 74-88 of acetyl-coenzyme A carboxylase in the presence of 100 μm AMP as described previously (86).
Western Blotting
LβT2 cells were serum starved overnight before treatments with agonists. Cells were treated with full-length adiponectin (20 μg/ml), globular adiponectin (3 μg/ml), AICAR (1 mm), compound C (20 μm) for the indicated times, and then were lysed in radioimmunoprecipitation buffer containing protease inhibitors and phosphatase inhibitors. Total cell lysates were separated by 10% SDS-PAGE. The blots were incubated first with blocking buffer (Tris-buffered saline containing 0.05% Tween 20 and 5.5% nonfat milk) followed by primary antibodies in blocking buffer or Tris-buffered saline containing 1% BSA. The bound antibodies were detected by horseradish peroxidase-conjugated secondary antibodies (1:4000, vol/vol) in blocking buffer and visualized by enhanced chemiluminescence. For detection of multimer species of adiponectin under non-heat-denaturing and nonreducing conditions, conditioned media or mouse sera were diluted in Laemmli loading buffer without dithiothreitol or β-mercaptoethanol as previously described (58). Samples were kept at room temperature for 1 h and subjected to SDS-PAGE.
LH Secretion Assay
LβT2 cells were seeded in poly-l-lysine-coated 12-well dishes. Cells were serum starved for overnight in DMEM containing 0.2% BSA. After agonist treatment, cells were extensively washed with DMEM containing 0.2% BSA three times and incubated in the same media (300 μl/well) to allow secretion. In some cases, the secretion media applied to the cells contained treatments such as adiponectin or GnRH. The conditioned medium was collected 30 min later and centrifuged to remove the residual cell debris. Cells were lysed in radioimmunoprecipitation buffer (150 μl/well), and cellular protein concentrations were measured. Mouse LH in both conditioned media and cell lysates was measured by sandwich ELISA by the Ligand Core of the Center for Research in Reproduction at the University of Virginia. All values were normalized to total cellular protein concentrations.
Purification of Recombinant Adiponectin
Mouse full-length adiponectin was purified as described previously (87). Briefly, mouse adiponectin was overexpressed from adenovirally infected CHO cells. Cells were allowed to recover in DMEM/F12 supplemented with 2% FBS. When cells reached confluence, they were washed with PBS and maintained in serum-free medium with 0.1 g/liter ascorbic acid for 48 h. Medium was then collected and filtered through a 0.2-μm filter (Millipore Corp., Bedford, MA). Recombinant adiponectin was precipitated by 40% ammonium sulfate, resuspended in low-salt buffer (10 mm HEPES, pH 8; 50 mm NaCl, 1 mm CaCl2) and dialyzed against the same buffer at 4 C overnight. Recombinant proteins were loaded onto a 5-ml EconoPac High Q anion exchange cartridge (Bio-Rad Laboratories, Inc., Hercules, CA) followed by extensive washing with low-salt buffer before elution with a 100–500 mm NaCl salt gradient. Adiponectin-containing fractions were determined by Dot Blot, and purity was determined by Coomassie staining. Adiponectin-positive fractions were concentrated.
Statistical Analysis
Unless otherwise noted, data were analyzed by ANOVA followed by Tukey post hoc tests. Individual pair-wise comparisons were performed using two-tailed t test. Analysis was performed using Excel (Microsoft, Redmond, WA) or Prizm (GraphPad Software, Inc., San Diego, CA).
Footnotes
This work was supported by National Institutes of Child Health and Human Development/National Institutes of Health through a cooperative agreement (Grant U54 HD012303) (to N.J.G.W., J.M.O., P.L.M.) as part of the Specialized Cooperative Centers Program in Reproduction Research. The gonadotropin measurements were subsidized by a U54 (HD28934) grant to the Center for Research in Reproduction Ligand Core at the University of Virginia.
Disclosure Statement: The authors have nothing to disclose
First Published Online November 15, 2007
Abbreviations: ACC, Acetyl coenzyme A carboxylase; AdipoR, adiponectin receptor; AICAR, 5-aminoimidazole-4-carboxamide-1-β-riboside; AMPK, AMP-activated protein kinase; CHO, Chinese hamster ovary cell; FBS, fetal bovine serum; eGFP, enhanced green fluorescent protein; HPG, hypothalamus/pituitary/gonad; PCOS, polycystic ovary syndrome; RT, reverse transcription.
References
- Schneider JE 2004 Energy balance and reproduction. Physiol Behav 81:289–317 [DOI] [PubMed] [Google Scholar]
- Wade GN, Jones JE 2004 Neuroendocrinology of nutritional infertility. Am J Physiol 287:R1277–R1296 [DOI] [PubMed] [Google Scholar]
- Mitchell M, Armstrong DT, Robker RL, Norman RJ 2005 Adipokines: implications for female fertility and obesity. Reproduction (Cambridge, England) 130:583–597 [DOI] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE 2006 Adipose tissue-derived factors: impact on health and disease. Endocr Rev 27:762–778 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM 1995 Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546 [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA 2001 The hormone resistin links obesity to diabetes. Nature 409:307–312 [DOI] [PubMed] [Google Scholar]
- Hofmann C, Lorenz K, Braithwaite SS, Colca JR, Palazuk BJ, Hotamisligil GS, Spiegelman BM 1994 Altered gene expression for tumor necrosis factor-α and its receptors during drug and dietary modulation of insulin resistance. Endocrinology 134:264–270 [DOI] [PubMed] [Google Scholar]
- Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB 1995 The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest 95:2111–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF 1995 A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749 [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM 1996 AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M 1996 Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem 120:803–812 [DOI] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K 1996 cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1). Biochem Biophys Res Commun 221:286–289 [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE 2003 Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem 278:9073–9085 [DOI] [PubMed] [Google Scholar]
- Shapiro L, Scherer PE 1998 The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol 8:335–338 [DOI] [PubMed] [Google Scholar]
- Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF 2001 Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98:2005–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T 2005 Adiponectin and adiponectin receptors. Endocr Rev 26:439–451 [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Alvarez-Blasco F, Sanchon R, Luque-Ramirez M, San Millan JL 2006 Adiponectin and resistin in PCOS: a clinical, biochemical and molecular genetic study. Hum Reprod 21:2257–2265 [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T 2002 Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277:25863–25866 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T 2003 Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 278:2461–2468 [DOI] [PubMed] [Google Scholar]
- Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE 2004 A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145:367–383 [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y 2002 Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8:731–737 [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS 2004 Adiponectin acts in the brain to decrease body weight. Nat Med 10:524–529 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, et al. 2003 Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, et al. 2007 Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13:332–339 [DOI] [PubMed] [Google Scholar]
- Towler MC, Hardie DG 2007 AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100:328–341 [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hardie DG 1994 Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol 4:315–324 [DOI] [PubMed] [Google Scholar]
- Weekes J, Hawley SA, Corton J, Shugar D, Hardie DG 1994 Activation of rat liver AMP-activated protein kinase by kinase kinase in a purified, reconstituted system. Effects of AMP and AMP analogues. Eur J Biochem 219:751–757 [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB 2002 Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415:339–343 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T 2002 Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295 [DOI] [PubMed] [Google Scholar]
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB 2002 Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99:16309–16313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B 2006 Liver adenosine monophosphate-activated kinase-α2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology 147:2432–2441 [DOI] [PubMed] [Google Scholar]
- Chehab FF, Lim ME, Lu R 1996 Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12:318–320 [DOI] [PubMed] [Google Scholar]
- Mounzih K, Lu R, Chehab FF 1997 Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology 138:1190–1193 [DOI] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE 2003 Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 52:268–276 [DOI] [PubMed] [Google Scholar]
- Fang X, Palanivel R, Zhou X, Liu Y, Xu A, Wang Y, Sweeney G 2005 Hyperglycemia- and hyperinsulinemia-induced alteration of adiponectin receptor expression and adiponectin effects in L6 myoblasts. J Mol Endocrinol 35:465–476 [DOI] [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ 2006 APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8:516–523 [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, Hoo RC, Mak WW, Cooper GJ, Xu A 2006 Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem 281:16391–16400 [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu A, Knight C, Xu LY, Cooper GJ 2002 Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem 277:19521–19529 [DOI] [PubMed] [Google Scholar]
- Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A 2007 Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56:1387–1394 [DOI] [PubMed] [Google Scholar]
- Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D 2000 Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol 20:6704–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Brozinick Jr JT, Valladares O, Bucan M, Birnbaum MJ 2001 A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7:1085–1094 [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE 2001 Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Nguyen MT, Trujillo M, Imamura T, Usui I, Scherer PE, Olefsky JM 2005 Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes 54:1304–1313 [DOI] [PubMed] [Google Scholar]
- Manore MM 2002 Dietary recommendations and athletic menstrual dysfunction. Sports Med 32:887–901 [DOI] [PubMed] [Google Scholar]
- Prather H, Hunt D 2005 Issues unique to the female runner. Phys Med Rehabil Clin N Am 16:691–709 [DOI] [PubMed] [Google Scholar]
- Starkey TA, Lee RA 1969 Menstruation and fertility in anorexia nervosa. Am J Obstet Gynecol 105:374–379 [DOI] [PubMed] [Google Scholar]
- Qi L, Li T, Rimm E, Zhang C, Rifai N, Hunter D, Doria A, Hu FB 2005 The +276 polymorphism of the APM1 gene, plasma adiponectin concentration, and cardiovascular risk in diabetic men. Diabetes 54:1607–1610 [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Tschritter O, Fritsche A, Staiger H, Renn W, Weisser M, Machicao F, Haring H 2002 Association of the T-G polymorphism in adiponectin (exon 2) with obesity and insulin sensitivity: interaction with family history of type 2 diabetes. Diabetes 51:37–41 [DOI] [PubMed] [Google Scholar]
- Bluher M, Bullen Jr JW, Lee JH, Kralisch S, Fasshauer M, Kloting N, Niebauer J, Schon MR, Williams CJ, Mantzoros CS 2006 Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. J Clin Endocrinol Metab 91:2310–2316 [DOI] [PubMed] [Google Scholar]
- Ferguson MA, White LJ, McCoy S, Kim HW, Petty T, Wilsey J 2004 Plasma adiponectin response to acute exercise in healthy subjects. Eur J Appl Physiol 91:324–329 [DOI] [PubMed] [Google Scholar]
- Kimura M, Shinozaki T, Tateishi N, Yoda E, Yamauchi H, Suzuki M, Hosoyamada M, Shibasaki T 2006 Adiponectin is regulated differently by chronic exercise than by weight-matched food restriction in hyperphagic and obese OLETF rats. Life Sci 79:2105–2111 [DOI] [PubMed] [Google Scholar]
- Kraemer RR, Castracane VD 2007 Exercise and humoral mediators of peripheral energy balance: ghrelin and adiponectin. Exp Biol Med 232:184–194 [PubMed] [Google Scholar]
- Kriketos AD, Gan SK, Poynten AM, Furler SM, Chisholm DJ, Campbell LV 2004 Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care 27:629–630 [DOI] [PubMed] [Google Scholar]
- Brichard SM, Delporte ML, Lambert M 2003 Adipocytokines in anorexia nervosa: a review focusing on leptin and adiponectin. Horm Metabolic Res 35:337–342 [DOI] [PubMed] [Google Scholar]
- Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, Roth GS, Ingram DK 2004 Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol 39:1049–1059 [DOI] [PubMed] [Google Scholar]
- Oberbach A, Tonjes A, Kloting N, Fasshauer M, Kratzsch J, Busse MW, Paschke R, Stumvoll M, Bluher M 2006 Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol 154:577–585 [DOI] [PubMed] [Google Scholar]
- Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T 2003 Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 278:40352–40363 [DOI] [PubMed] [Google Scholar]
- Caminos JE, Nogueiras R, Gallego R, Bravo S, Tovar S, Garcia-Caballero T, Casanueva FF, Dieguez C 2005 Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab 90:4276–4286 [DOI] [PubMed] [Google Scholar]
- O’Sullivan AJ, Kriketos AD, Martin A, Brown MA 2006 Serum adiponectin levels in normal and hypertensive pregnancy. Hypertens Pregnancy 25:193–203 [DOI] [PubMed] [Google Scholar]
- Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, Hauguel-De Mouzon S 2006 Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia 49:1677–1685 [DOI] [PubMed] [Google Scholar]
- Asai-Sato M, Okamoto M, Endo M, Yoshida H, Murase M, Ikeda M, Sakakibara H, Takahashi T, Hirahara F 2006 Hypoadiponectinemia in lean lactating women: prolactin inhibits adiponectin secretion from human adipocytes. Endocrine J 53:555–562 [DOI] [PubMed] [Google Scholar]
- Nilsson L, Binart N, Bohlooly YM, Bramnert M, Egecioglu E, Kindblom J, Kelly PA, Kopchick JJ, Ormandy CJ, Ling C, Billig H 2005 Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem Biophys Res Commun 331:1120–1126 [DOI] [PubMed] [Google Scholar]
- Bottner A, Kratzsch J, Muller G, Kapellen TM, Bluher S, Keller E, Bluher M, Kiess W 2004 Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab 89:4053–4061 [DOI] [PubMed] [Google Scholar]
- Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H 2005 Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes 54:1000–1008 [DOI] [PubMed] [Google Scholar]
- Xu A, Chan KW, Hoo RL, Wang Y, Tan KC, Zhang J, Chen B, Lam MC, Tse C, Cooper GJ, Lam KS 2005 Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem 280:18073–18080 [DOI] [PubMed] [Google Scholar]
- Glintborg D, Andersen M, Hagen C, Frystyk J, Hulstrom V, Flyvbjerg A, Hermann AP 2006 Evaluation of metabolic risk markers in polycystic ovary syndrome (PCOS). Adiponectin, ghrelin, leptin and body composition in hirsute PCOS patients and controls. Eur J Endocrinol 155:337–345 [DOI] [PubMed] [Google Scholar]
- Gulcelik NE, Aral Y, Serter R, Demir Y, Culha C 2006 Adiponectin is an independent determinant of insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol 22:511–515 [DOI] [PubMed] [Google Scholar]
- Majuri A, Santaniemi M, Rautio K, Kunnari A, Vartiainen J, Ruokonen A, Kesaniemi YA, Tapanainen JS, Ukkola O, Morin-Papunen L 2007 Rosiglitazone treatment increases plasma levels of adiponectin and decreases levels of resistin in overweight women with PCOS: a randomized placebo-controlled study. Eur J Endocrinol 156:263–269 [DOI] [PubMed] [Google Scholar]
- Stout DL, Fugate SE 2005 Thiazolidinediones for treatment of polycystic ovary syndrome. Pharmacotherapy 25:244–252 [DOI] [PubMed] [Google Scholar]
- Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Dieguez C, Gualillo O, Gonzalez-Juanatey JR, Lago F 2005 Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett 579:5163–5169 [DOI] [PubMed] [Google Scholar]
- Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM 2004 Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology 145:5589–5597 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, Castano JP, Malagon MM 2007 Regulation of pituitary cell function by adiponectin. Endocrinology 148:401–410 [DOI] [PubMed] [Google Scholar]
- Wolf AM, Wolf D, Avila MA, Moschen AR, Berasain C, Enrich B, Rumpold H, Tilg H 2006 Up-regulation of the anti-inflammatory adipokine adiponectin in acute liver failure in mice. J Hepatol 44:537–543 [DOI] [PubMed] [Google Scholar]
- Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, Vatish M, Randeva HS 2006 Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia 49:1292–1302 [DOI] [PubMed] [Google Scholar]
- Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, Snead DR, Hoggart B, O’Hare JP, McTernan PG, Kumar S 2007 Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab 92:1129–1136 [DOI] [PubMed] [Google Scholar]
- Lord E, Ledoux S, Murphy BD, Beaudry D, Palin MF 2005 Expression of adiponectin and its receptors in swine. J Anim Sci 83:565–578 [DOI] [PubMed] [Google Scholar]
- Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD 2006 Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology 147:5178–5186 [DOI] [PubMed] [Google Scholar]
- Sharabi Y, Oron-Herman M, Kamari Y, Avni I, Peleg E, Shabtay Z, Grossman E, Shamiss A 2007 Effect of PPAR-γ agonist on adiponectin levels in the metabolic syndrome: lessons from the high fructose fed rat model. Am J Hypertens 20:206–210 [DOI] [PubMed] [Google Scholar]
- Yilmaz MI, Sonmez A, Caglar K, Gok DE, Eyileten T, Yenicesu M, Acikel C, Bingol N, Kilic S, Oguz Y, Vural A 2004 Peroxisome proliferator-activated receptor γ (PPAR-γ) agonist increases plasma adiponectin levels in type 2 diabetic patients with proteinuria. Endocrine 25:207–214 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T 2001 The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946 [DOI] [PubMed] [Google Scholar]
- LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E 2006 Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab 291:E175–E181 [DOI] [PubMed] [Google Scholar]
- Hardie DG 2006 Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology 131:973; author reply 974–975 [DOI] [PubMed] [Google Scholar]
- Lu M, Shyy JY 2006 Sterol regulatory element-binding protein 1 is negatively modulated by PKA phosphorylation. Am J Physiol Cell Physiol 290:C1477–C1486 [DOI] [PubMed] [Google Scholar]
- Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F 2005 Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem 280:25250–25257 [DOI] [PubMed] [Google Scholar]
- Davies SP, Carling D, Hardie DG 1989 Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem 186:123–128 [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE 2001 The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953 [DOI] [PubMed] [Google Scholar]