Abstract
Prostate cancer invariably recurs after androgen deprivation therapy. Growth of this recurrent/androgen-independent form of prostate cancer may be due to increased androgen receptor (AR) transcriptional activity in the absence of androgen. This ligand-independent AR activation is promoted by some growth factors but the mechanism is not well understood. Vav3, a Rho guanosine triphosphatase guanine nucleotide exchange factor, which is activated by growth factors, is up-regulated in human prostate cancer. We show here that Vav3 levels increase during in vivo progression of prostate cancer to androgen independence. Vav3 strikingly enhanced growth factor activation of AR in the absence of androgen. Because Vav3 may be chronically activated in prostate cancer by growth factor receptors, we examined the effects of a constitutively active (Ca) form of Vav3 on AR transcriptional activity. Ca Vav3 caused nuclear localization and ligand-independent activation of AR via the Rho guanosine triphosphatase, Rac1. Ca Rac1 activation of AR occurred, in part, through MAPK/ERK signaling. Expression of active Rac1 conferred androgen-independent growth of prostate cancer cells in culture, soft agar, and mice. These findings suggest that Vav3/Rac 1 signaling is an important modulator of ligand-independent AR transcriptional activity in prostate cancer progression.
THE ANDROGEN RECEPTOR (AR) is a ligand-activated transcription factor that belongs to the nuclear receptor superfamily. Signaling by AR is required for normal prostate development and cell differentiation, yet the AR exerts growth-promoting effects on prostate tumors (1,2,3,4,5,6,7). Androgen deprivation therapy is typically used for non-organ-confined prostate cancer. Unfortunately, the majority of patients undergoing this treatment eventually relapse. Prostate cancer that progresses after androgen deprivation therapy is termed either androgen independent, hormone refractory, or recurrent (8). The expression of functional AR and AR target genes is a common feature of androgen-independent prostate cancer (1,9,10,11). In fact, several studies suggest that AR is essential for tumor progression (3,5,12). We and others recently reported that levels of Vav3 protein, a Rho guanosine triphosphatase (GTPase) guanine nucleotide exchange factor (GEF), are elevated in human prostate cancer specimens and during in vitro progression of prostate cancer cells to androgen independence (13,14). Vav3 is also overexpressed in androgen-independent prostate cancer in the Nkx3.1;Pten mutant mouse model (15). We previously demonstrated that Vav3 enhances AR transcriptional activity in a ligand-dependent manner (13). Vav3 is a broadly expressed, multidomain protein and the most recently identified member of the Vav subfamily of diffuse B-cell lymphoma (DBL) proteins (16). Like other DBL family GEFs, Vav proteins contain the canonical DBL homology (DH) domain, where guanine nucleotide exchange factor (GEF) activity resides, and a pleckstrin homology (PH) domain linked in tandem (17). Vav3 supports the exchange of GDP for GTP on the Rho GTPases: Rho A, Rho G, and Rac1 (18). Surprisingly, enhancement of AR activity by Vav3 in the presence of androgen does not require Vav3 GEF activity (13).
A key mechanism of AR signaling under conditions of androgen deprivation is through ligand-independent activation of the AR. This atypical form of receptor activation has been demonstrated for several steroid hormone receptors (reviewed in Ref. 19). In the case of the AR, IGF, epidermal growth factor (EGF), keratinocyte growth factor, Her2/Neu, and IL-6 activate AR in prostate cancer cells; however, the mechanisms for ligand-independent receptor activation are not well understood (20,21,22,23,24,25). Signaling by EGF, IGF, and IL-6 is up-regulated in androgen-independent prostate cancer and is associated with disease progression (24,26,27,28,29). Interestingly, Vav3 is coupled to EGF and IGF receptor signaling pathways, and the closely related Vav1 is activated in response to IL-6 (18,30). Upon growth factor binding, both EGF and IGF receptors recruit Vav3 via the C-terminal SH2 domain. Activated growth factor receptors phosphorylate Vav3 at tyrosine 173, thereby relieving autoinhibition imposed by the amino-terminal domains (16). IL-6 utilizes similar mechanisms to activate Vav1 (30). Activation of Vav3 can also be achieved by deleting this autoinhibitory amino-terminal region (16,18).
We show that Vav3 greatly enhanced ligand-independent activation of AR by growth factors (EGF+IGF). We chose to investigate further the effects of a constitutively active (Ca) form of Vav3 on AR in prostate cancer cells because Vav3 is overexpressed in prostate cancer, and Vav3 may be hyperactive due to activation by growth factor receptors (EGF receptor and IGF receptor) that are themselves highly active in prostate cancer. Ca Vav3 activated AR in the absence of ligand and promoted nuclear localization of AR. The ligand-independent activation of AR by Ca Vav3 required Ca Vav3 GEF activity. Consistent with this observation, Ca Vav3 enhanced Rac1 activity and Rac1 signaling was essential for ligand-independent activation of AR by Ca Vav3. Ca Rac1 conferred androgen-independent growth of prostate cancer cells both in vitro (culture and soft agar) and in vivo (tumor xenografts), suggesting that Rac1 plays a key role in prostate cancer progression.
RESULTS
Vav3 mRNA Is Elevated in Androgen-Independent LNCaP Xenograft Tumors
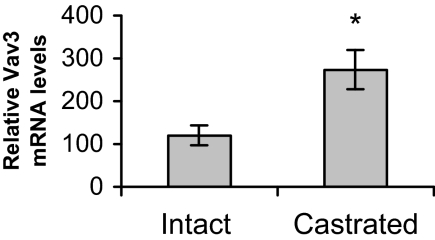
We used an LNCaP xenograft model to examine whether Vav3 up-regulation occurs during progression of androgen-dependent prostate cancer cells to androgen independence in vivo. LNCaP is an androgen-dependent human prostate cancer cell line. LNCaP cells were injected into the flanks of male nude mice, and half the animals were castrated after small tumors formed. Although, as expected, LNCaP tumors did not grow or grew very slowly after castration, almost 70% of tumors eventually progressed (defined as ≥33% increase in tumor volume after castration). These tumors were considered androgen independent. We compared Vav3 mRNA levels in tumors from intact mice (androgen dependent) with androgen-independent tumors from castrated mice. Vav3 mRNA levels were significantly up-regulated in androgen-independent tumors (Fig. 1). Thus, Vav3 is up-regulated after transition to androgen-independence in vivo.
Figure 1.
Vav3 mRNA Is Elevated in Androgen-Independent LNCaP Xenografts
LNCaP cells and equal volumes of Matrigel were injected sc into male nude mice (n = 13). Mice were left intact or castrated after tumor formation (∼5 wk after inoculation of tumor cells). Approximately 7–10 wk after castration or mock surgeries (intact mice), animals were killed, and tumor tissue was excised and flash frozen. Total RNA was extracted from tumor tissue and LNCaP cells and subjected to semiquantitative RT-PCR analysis using Vav3- and actin-specific primers. Data are plotted as percent of Vav3 mRNA in androgen-dependent (intact) and androgen-independent (castrated) tumor xenografts compared with LNCaP cells ± sem. Graph shows results from four independent RT-PCRs performed in triplicate. Significance was determined using a two-tailed Student’s t test (*, P < 0.05).
Growth Factor Activation of Vav3 Results in Ligand-Independent AR Transactivation
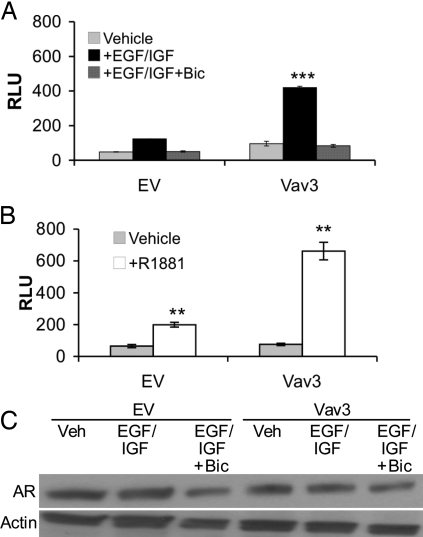
Because Vav3 is activated by EGF and IGF receptors and is overexpressed in prostate cancer, we explored a possible link between Vav3 and growth factor receptor pathways that activate AR in prostate cancer cells. We chose to use the PC3 prostate cancer cell line because these cells express relatively low levels of Vav3 (13). We observed modest enhancement of AR transcriptional activity in the presence of growth factors (EGF plus IGF) but no androgen as shown previously (14,20,31) (Fig. 2A). However, a striking increase in ligand-independent activation of AR was evident in cells transfected with wild-type Vav3 and treated with growth factors (Fig. 2A). Bicalutamide, an AR antagonist, blocked this ligand-independent AR activation consistent with AR regulation of the prostate-specific antigen (PSA) promoter/enhancer (Fig. 2A). The extent of growth factor-mediated induction of AR activity in cells expressing Vav3 was comparable to that observed in cells treated with androgen (Fig. 2, A and B). Neither expression of Vav3 nor treatment with growth factors significantly altered AR levels (Fig. 2C). These results suggest that Vav3 participates in growth factor signaling pathways leading to ligand-independent activation of AR.
Figure 2.
Growth Factor Activation of Wild-Type Vav3 Results in Ligand-Independent AR Transactivation
A and B, PC3 cells were transfected with AR, the reporter plasmid PSA promoter/enhancer-luc, and wild-type Vav3, or equimolar amounts of the empty vector (EV). Cells were treated with either vehicle, a combination of EGF (100 ng/ml) plus IGF (10 ng/ml) or EGF+IGF and bicalutamide (5 μm) (A) or with vehicle or R1881 (5 nm) (B). Luciferase activity was assayed 48 h after transfection. Data are representative of two experiments performed in triplicate (A) or more than six experiments performed in triplicate (B). The means of triplicate determinations are plotted (RLU ± sem). Significance was determined using a two-tailed Student’s t test and represents differences from vehicle vs. growth factor or hormone treatment (**, P < 0.01; ***, P < 0.001). C, Lysates from PC3 cells treated as in A were immunoblotted to assess AR levels. Actin is shown as a loading control. Bic, Bicalutamide; RLU, relative light units; Veh, vehicle.
Ca Vav3 Mediates Ligand-Independent Transcriptional Activation and Promotes Nuclear Localization of AR
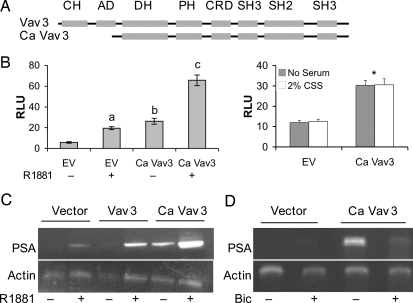
Because Vav3 enhances EGF plus IGF-mediated ligand-independent AR activation, we asked whether Ca Vav3 would be sufficient to activate AR in the absence of both androgen and growth factors. Ca Vav3 lacks the N-terminal calponin homology and acidic domains (Fig. 3A) and has constitutive Vav3 GEF activity (16). Ca Vav3 expression resulted in a substantial ligand-independent increase in PSA enhancer/promoter-directed luciferase activity (Fig. 3B). This effect was blocked (87± 9.7%) by the AR antagonist, bicalutamide (5 um) (data not shown), confirming the involvement of AR. Addition of androgen resulted in a further increase in reporter gene activity consistent with our previous findings that Vav3 enhances androgen-induced AR activity (13). Ca Vav3 activation of AR in the absence of hormone did not require AR overexpression because Ca Vav3 promoted ligand-independent activation of endogenous AR in LNCaP cells (Fig. 3B, right panel). Similar activation of AR by Ca Vav3 occurred in the absence of serum or in the presence of 2% charcoal-stripped serum, suggesting that serum did not contribute residual amounts of androgen (Fig. 3B, right panel). As we observed previously with wild-type Vav3 (13), Ca Vav3 did not significantly alter AR levels (data not shown). We also examined native PSA mRNA levels in LNCaP cells using semiquantitative RT-PCR. Ca Vav3 up-regulated PSA mRNA in the absence of androgen; however, wild-type Vav3 had no effect on PSA mRNA levels in the absence of androgen, consistent with our previous findings (Fig. 3C and Ref. 13). Ligand-independent activation of endogenous AR by Ca Vav3 was comparable to that observed after hormone treatment (Fig. 3, B and C, and data not shown). Also consistent with our previous findings with Vav3, Ca Vav3 further enhanced PSA mRNA production after androgen treatment (Fig. 3C). The AR antagonist, bicalutamide, blocked ligand-independent Ca Vav3-mediated induction of PSA mRNA (Fig. 3D).
Figure 3.
Ca Vav3 Mediates Ligand-Independent Transcriptional Activation of AR
A, Schematic identifying regions of wild-type and Ca Vav3. Domains are as follows: CH, calponin homology; AD, acidic domain; DH, DBL homology (GEF) domain; CRD, cysteine-rich domain; SH3, src homology 3 domain; SH2, src homology 2 domain. B, PC3 cells (left panel) were transfected with AR, the reporter plasmid PSA-luc, and either Ca Vav3, or equimolar amounts of the empty vector (EV). Cells were treated with R1881 (+) or vehicle (−). Data are representative of six experiments performed in triplicate. Significance was determined using a one-way ANOVA. Samples a and b were compared with EV (−) (P < 0.01); c was compared with b (P < 0.01). LNCaP cells (right panel) were transfected with PSA-luc, and either Ca Vav3 or equimolar amounts of the empty vector (EV) in the presence or absence of 2% CSS. Luciferase activity was determined 48 h after transfection. The means of triplicate determinations are plotted (RLU ± sem). Ca Vav3 transfected cells were significantly different from EV transfected cells (*, P < 0.05). C, LNCaP cells were transfected with the indicated plasmids and treated with the synthetic androgen R1881 (5 nm), the AR antagonist, bicalutamide (Bic, 5 μm), or vehicle. Cells were harvested 48 h after transfection, and RNA was isolated. RT-PCR was conducted during the linear range of amplification using PSA- and actin-specific primers (PSA 26 cycles, actin 18 cycles) (13). The image is representative of four independent experiments (left panel) and two independent experiments (right panel).
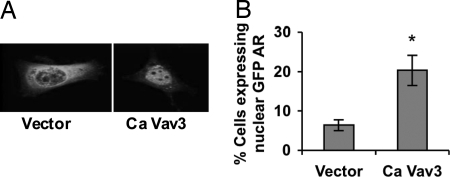
Because Ca Vav3 caused ligand-independent AR activation, we examined the effects of Ca Vav3 on the subcellular localization of a green fluorescent protein (GFP)-AR fusion protein. Introduction of Ca Vav3 resulted in increased GFP-AR nuclear localization in cells not exposed to androgen (Fig. 4, A and B). As expected, treatment with androgen resulted in nuclear localization of GFP-AR (data not shown). We verified by reporter gene assay that Ca Vav3 activates the GFP-AR fusion protein similar to wild type AR (data not shown).
Figure 4.
Ca Vav3 Mediates Nuclear Translocation of AR in the Absence of Androgen
A, PC3 cells grown on coverslips were transfected with GFP-AR, and either Ca Vav3 or empty vector. Cells were fixed 48 h after transfection, and GFP-AR localization was assessed by confocal immunofluorescence microscopy. Twenty percent of Ca Vav3-transfected cells vs. 8% of vector-transfected cells exhibited predominantly nuclear GFP-AR as shown in the right hand image. A representative cell transfected with empty vector exhibiting cytoplasmic localization of GFP-AR is shown on the left. B, Cells were visualized and scored for GFP-AR localization. The percentage of GFP-AR expressing cells that exhibited predominantly nuclear GFP-AR in each group is plotted. Data are representative of three independent experiments, and significance was determined using a two-tailed Student’s t test (*, P < 0.05).
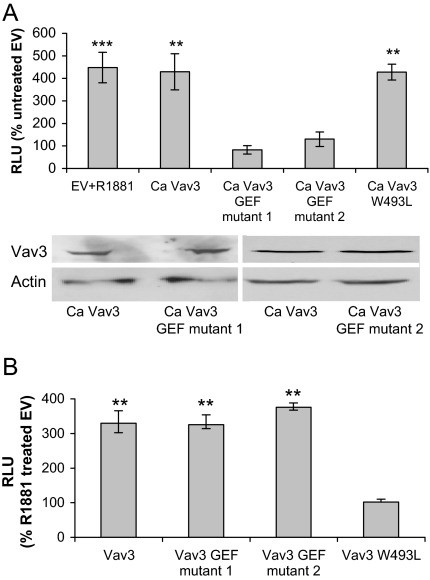
The GEF Function of Ca Vav3 Is Required for Ligand-Independent Activation of AR
To examine the contribution of Vav3 GEF activity to ligand-independent AR activation, we generated two distinct Ca Vav3 GEF mutant proteins and examined their effects on AR-mediated gene expression. Neither of the CaVav3 GEF (DBL homology) domain mutants had the capacity to activate AR in a ligand-independent manner as shown by reporter gene assay (Fig. 5A) and PSA mRNA analysis (data not shown). Ca Vav3 and Ca Vav3 GEF mutants were expressed to similar levels (Fig. 5B). To complement these findings, we generated a Ca Vav3 truncation mutant, consisting of the DBL homology, pleckstrin homology (PH), and cysteine-rich domains, which is the minimal Vav3 unit that retains GEF activity (16). Like Ca Vav3, this truncated Vav3 mutant promoted ligand-independent activation of AR (data not shown).
Figure 5.
The GEF Function of Ca Vav3 Is Required for Ligand-Independent Activation of AR
A, PC3 cells were transfected with AR, PSA-Luc, and either Ca Vav3, Ca Vav3 mutants lacking GEF activity [Ca Vav3 ISO III (mutant 1), Ca Vav3 QIF (mutant 2)], Ca Vav3 containing a PH domain mutation (Ca Vav3 W493L), or equimolar amounts of the empty vector. Cells were treated with vehicle or R1881 (1 nm). Luciferase activity was determined 48 h after transfection. The means of triplicate determinations from four independent experiments are plotted as a percent of vector control (RLU ± sem). Lysates from A were immunoblotted to assess relative Ca Vav3 and mutant expression levels. Actin is shown as a loading control. B. PC3 cells were transfected with AR, PSA-luc, Vav3 (full length), or Vav3 harboring analogous mutations as in panel A in the DH (GEF) or PH domains (W493L). Luciferase activity was determined as described in panel A. Significance was determined using a one-way ANOVA and represents differences from untreated, empty vector transfected cells (A) or from androgen-treated empty vector transfected cells (B) (**, P < 0.01; ***, P < 0.001). RLU, Relative light units.
Our previous findings revealed that the Vav3 PH domain, but not the GEF domain, was required for ligand-dependent enhancement of AR activity (Ref. 13 and Fig. 5B). To examine the role of the Vav3 PH domain on Ca Vav3-mediated ligand-independent activation of AR, we mutated a conserved residue in the Ca Vav3 PH domain (W493L) that disrupts the function of this domain (32). Ca Vav3 W493L activated AR in a ligand-independent manner (Fig. 5A). Thus, ligand-independent activation of AR transcriptional activity by Ca Vav3 requires GEF activity, but not the Vav3 PH domain, demonstrating that Ca Vav3 activation of AR is distinct mechanistically from Vav3 coactivation of liganded AR.
The Rho GTPase Rac1 Mediates Ligand-Independent Activation of AR by Ca Vav3
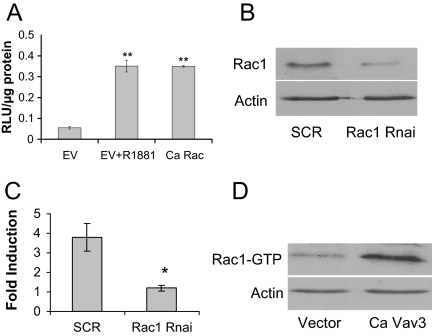
Our findings demonstrate that ligand-independent activation of AR by Ca Vav3 requires Vav3 GEF activity. We therefore examined the possible role of the Rho GTPases, Rac1 and RhoA, which are activated by Vav3 (16,18). A Ca Rac1 mutant, but not Ca RhoA (data not shown), activated AR independently of ligand (Fig. 6A).
Figure 6.
The Rho GTPase Rac1 Mediates Ligand-Independent Activation of AR by Ca Vav3
A, PC3 cells were transfected with AR, PSA-luc, and Ca Rac 1 or equimolar amounts of the empty vector. Samples were assayed for luciferase activity as described. The means of triplicate samples are plotted as RLU per μg protein ± sem. Significance was determined using a two-tailed Student’s t test and represents differences from empty vector samples (**, P < 0.01). B, PC3 cells were transfected with Rac1-specific siRNA (Rac1 RNAi) or a scrambled (SCR) control siRNA. Lysates were immunoblotted 48 h after transfection for Rac1 and actin. C, PC3 cells were transfected with AR, PSA-luc, Ca Vav3 (or empty vector), and either Rac1-specific siRNA or a scrambled siRNA control. Samples were assayed for luciferase activity as described. Data are plotted as fold induction compared with empty vector ±sem. Significance was determined using a two-tailed Student’s t test (*, P < 0.05). D, PC3 cells were transfected with the empty vector or Ca Vav3. Rac1 activity (Rac1-GTP) was determined by pull down assays. GTP-bound Rac1 was separated from GDP-Rac1 using the binding domain from the Rac1/Cdc42 effector protein p21-activated kinase fused to GST as described in Materials and Methods. EV, Empty vector; RLU, relative light units.
To test further the role of Rac1 in Ca Vav3 activation of AR, we used small interfering RNA (siRNA) directed against Rac1 to specifically knock down levels of Rac1 in PC3 cells (Fig. 6B). Knockdown of Rac1 blocked Ca Vav3 activation of AR (Fig. 6C). These studies demonstrate that ligand-independent activation of AR by Ca Vav3 requires Rac1 signaling. In addition, we verified that Ca Vav3 activated Rac1 in prostate cancer cells (Fig. 6D).
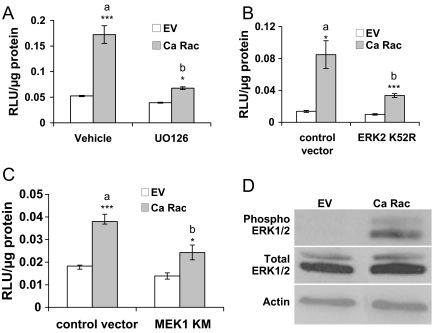
To define the signaling pathway(s) by which Ca Rac1 activates AR in the absence of ligand, we tested a panel of protein kinase inhibitors including UO126 [MAPK kinase (MEK) inhibitor], SP600125 (c-Jun N-terminal kinase inhibitor), SB203580 (p38 kinase inhibitor), LY290042 (phosphatidylinositol 3-kinase inhibitor), and SU6656 (Src inhibitor). For each inhibitor, control experiments were conducted in parallel to show that the drugs inhibited their molecular targets (data not shown). Of the compounds examined, only the MEK inhibitor, UO126, substantially blocked Ca Rac1 activation of AR in LNCaP cells (Fig. 7A). The same results were obtained in PC3 cells transfected with AR and Ca Rac1 (data not shown). In addition, UO126 significantly inhibited Ca Vav3 activation of AR (data not shown) consistent with our observation that Ca Vav3 activation of AR is mediated by Rac 1. Also in agreement with a role for MAPK/ERK signaling in ligand-independent activation of AR by Ca Rac1, dominant-negative MEK and ERK mutants significantly inhibited Ca Rac1-stimulated AR activity (Fig. 7, B and C). Transfection of Ca Rac1 into LNCaP cells resulted in increased levels of phosphor-ERK1/2 but did not affect total ERK levels (Fig. 7D).
Figure 7.
Ligand-Independent Activation of AR by Ca Rac1 Is Attenuated by MAPK/ERK Inhibition
A, LNCaP cells transfected with PSA-luc, and either Ca Rac or equal amounts of the empty vector (EV) were treated with vehicle or 10 μm UO126. Luciferase activity was determined 24 h after treatment. B and C, LNCaP cells were transfected with PSA-luc, Ca Rac, or equal amounts of the empty vector (EV), kinase dead form of ERK2 (K52R), or MEK1 (K97M), or equal amounts of control vector. Luciferase activity was determined 48 h after transfection. Luciferase data are representative of two to four experiments performed in triplicate. The means of triplicate determinations are plotted (RLU/μg protein ±sem). Significance was determined using a two-tailed Student’s t test and represents differences from vehicle or control vectors (*, P < 0.05; ***, P < 0.001). In panel A, a is significantly different from b (P < 0.01). In panels B and C, a is significantly different from b (P < 0.05). D, A representative immunoblot of LNCaP cells transfected as described in panel A, 48 h after transfection is shown. RLU, Relative light units.
Ca Rac1 Confers Androgen-Independent Growth to LNCaP Cells in Culture and in Vivo
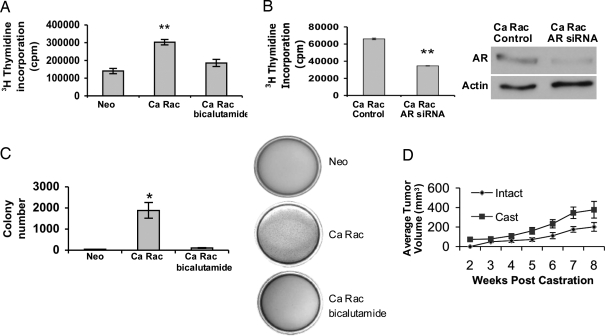
Our findings demonstrate a role for Rac1 in transducing Ca Vav3 signaling to AR in the absence of androgen. To examine the pathological significance of Rac1 activation in androgen-independent prostate cancer progression, we stably introduced Ca Rac1 into LNCaP cells, which exhibit low Rac1 activity (33). Stable expression of Ca Rac1 conferred androgen-independent growth to LNCaP cells both in culture and in soft agar as compared with the LNCaP/Neo control cells (Fig. 8, A and C). Androgen-independent growth exhibited by LNCaP/Ca Rac1 cells in culture and soft agar was blocked by bicalutamide or by knockdown of AR using siRNAs (Fig. 8, A and B). These results suggest that Ca Rac1 caused androgen-independent growth by activating AR. As expected, LNCaP/Neo cells do not exhibit significant growth in soft agar lacking androgen (Fig. 8C).
Figure 8.
Ca Rac1 Confers Androgen-Independent Growth to LNCaP Cells
A, LNCaP cells stably expressing either the neomycin vector (LNCaP/Neo) or Ca Rac1 (LNCaP/Ca Rac1) were seeded onto 60-mm culture dishes at equal densities and maintained in androgen-depleted media with or without bicalutamide. Tritiated thymidine (1 μCi/ml) was added to cells 48 h after plating, and cells were incubated for an additional 18 h. Cells were then harvested and tritiated-thymidine uptake was monitored as described in Materials and Methods. Data are plotted as average counts per minute (cpm) ± sem. B, LNCaP/Ca Rac1 cells were transfected with AR siRNA or control siRNA using Lipofectamine 2000 for 24 h. Tritiated thymidine (1 μCi/ml) was added to cells 72 h after transfection, and cells were incubated for an additional 18 h. Data are representative of three independent experiments performed in triplicate. Cell lysates were immunoblotted with AR and actin antibodies. C, LNCaP/Neo or LNCaP/Ca Rac1 growth in soft agar was assessed as described in MATERIALS AND METHODS. Average colony number ± sem is plotted. Significance was determined using a two-tailed Student’s t test (*, P < 0.05; **, P < 0.01). D, LNCaP/Ca Rac1 cells (5 × 106) were injected sc into male nude mice. Mice were left intact (n = 8) or castrated (n = 14) 10–14 d after injections (before tumor formation). Tumor volumes were monitored weekly and calculated using the formula L × W × H × 0.53. Data are plotted as average tumor volume ± sem. Cast, Castrated.
To examine the tumorigenic effects of Ca Rac1 expression in vivo, we inoculated LNCaP/Ca Rac1 cells into the flanks of nude mice and castrated half the mice before tumor formation. Whereas LNCaP cells fail to form tumors in castrated mice (34,35), LNCaP/Ca Rac1 cells formed tumors equally readily in both castrated and intact nude mice (57% tumor formation in intact mice vs. 62% in castrated mice) (Fig. 8D). Furthermore, tumor growth was rapid in both intact and castrated animals (Fig. 8D).
DISCUSSION
Because Vav3 is overexpressed in prostate cancer and is coupled to growth factor receptor pathways that are up-regulated in androgen-independent disease, we speculated that Vav3 may participate in promiscuous, ligand-independent activation of AR. We found that Vav3 strikingly enhanced growth factor activation of AR. We investigated a possible role for oncogenic/Ca Vav3 in ligand-independent AR activation because Vav3 is likely to be chronically activated in advanced prostate cancer by growth factor receptors (36,37,38,39). Indeed, Ca Vav3 mediated ligand-independent nuclear translocation and activation of AR. Ca Vav3 activation of AR did not require overexpression of AR because endogenous AR was also activated in LNCaP cells. Ligand-independent activation of AR required GEF activity of Vav3 and signaling of the Rho GTPase, Rac1. We found that Ca Rac1 activation of AR occurred through MAPK/ERK signaling and resulted in androgen-independent prostate cancer cell growth in vitro and in vivo.
Ligand-independent activation of AR may account for the maintenance of AR signaling in patients undergoing androgen ablation therapy (4,31,40,41,42). The involvement of the EGF receptor, IGF receptor, and IL-6 signaling pathways in ligand-independent AR activation is well documented (20,43,44). We demonstrate that wild-type Vav3 enhanced growth factor (EGF plus IGF) activation of AR transcriptional activity. This finding suggests that EGF and IGF receptor pathways activate Vav3 in prostate cancer cells and, further, that Vav3 promotes AR activation under conditions of androgen deprivation. Although it is not known whether IL-6 activates Vav3, IL-6 stimulates the closely related Vav1 as well as Rac1 (45). Thus, Vav3 appears to be commonly situated downstream from EGF, IGF, and IL6 signaling pathways. With regard to the mechanism of ligand-independent activation of AR, a recent report showed that growth factor stimulation results in tyrosine phosphorylation and transcriptional activation of AR via Src kinase (46). However, CaVav3/Rac1 activation of AR is unlikely to involve Src kinase because the Src kinase inhibitor SU6656 had comparatively minimal effects on Ca Rac1 activation of AR (data not shown). In contrast, the MEK1 inhibitor UO126, as well as MEK and ERK dominant-negative mutants, resulted in a substantial decrease in Ca Rac1-mediated AR activation. MAPK/ERK signaling is implicated in ligand-independent AR activation initiated by the diverse signals IL-6 and vasoactive intestinal peptide (43,47). These signaling cascades converge on MEK/ERK1/2, resulting in AR activation. In the case of IL6, AR activation in the absence of ligand depends on MAPK signaling and is enhanced by MAPK-directed phosphorylation of the steroid receptor coactivator SRC1 (43). Active mutants of both MEKK1 and MEK have been shown to activate AR in the absence of ligand; however, the specific mechanisms are unknown (47,48). Although MAPK/ERK participates in Ca Rac1 activation of AR, it is not known whether this involves phosphorylation or modification of AR and/or coactivators.
Consistent with our findings, Dong et al. (14) reported that a Vav3 mutant lacking the entire DH domain loses the ability to activate AR in the absence of ligand in HeLa cells. Our findings demonstrate that GEF activity specifically, and not simply the physical lack of the DH domain, is essential for Ca Vav3 activation of AR in prostate cancer cells. Further, together with our previous report, we demonstrate two distinct mechanisms by which Vav3 enhances AR activity. Our finding that ligand-independent activation of AR by Ca Vav3 required Rac 1 supports the importance of the Vav3 DH (GEF) domain. Similar to what was observed for Ca Vav3, introduction of Ca Rac1 into prostate cancer cells was sufficient to drive GFP-AR into the nucleus in the absence of ligand (data not shown). These studies suggest that Ca Rac1 promotes AR activity under conditions of androgen deprivation. In addition, the importance of Rac1 signaling in prostate cancer is supported by our earlier work demonstrating that several androgen-independent prostate cancer cell lines exhibit elevated levels of Rac1 activity compared with androgen-dependent cells (33).
A role for modulation of steroid hormone receptor activity by Rho GTPases has been documented in several studies (50,51,52,53). The Dbl family GEF, BRX, interacts with the estrogen receptor (ER) and mediates increased ER transcriptional activity through a mechanism that involves the Rho GTPase, Cdc42 (50). Additionally, BRX enhances glucocorticoid receptor activity and colocalizes with Rho A at glucocorticoid response elements in the nucleus (53). Muller et al. (52) show that nuclear translocation of the AR coactivator FHL2 is stimulated by Rho-GTPase signaling. These studies did not address a role for Rho signaling in ligand-independent activation of these steroid receptors.
Our earlier report defined a role for wild-type Vav3 in androgen action in prostate cancer. We showed that wild-type Vav3 enhances AR transcriptional activity solely in the presence of androgen and that this effect does not require Vav3 GEF activity (13). This ligand-dependent coactivation of AR by wild-type Vav3 requires the Vav3 PH domain. The present study reveals that Ca Vav3 activates AR in the absence of ligand. This promiscuous activation of AR is GEF dependent and occurs via Rac 1. In contrast to the ligand-dependent coactivation of AR by wild-type Vav3, the effects of Ca Vav3 on AR do not require a functional Vav3 PH domain (Fig. 5A). Because the Vav3 PH domain appears to be dispensable for activation of Rac1, the ability of CaVav3 W493L (PH domain mutant) to activate AR in the absence of androgen is consistent with a requirement for Vav3 GEF activity (32). Thus, our findings indicate that Vav3 activates AR in the presence or absence of ligand through two distinct mechanisms. Together these data support a versatile and critical role for Vav3 modulation of AR signaling in androgen-dependent and -independent prostate cancer.
Our current findings hold particular relevance for androgen-independent prostate cancer in which we demonstrated up-regulation of Vav3 and a striking enhancement of growth factor activation of AR by wild-type Vav3. Further, Vav3 is likely to be chronically activated by growth factors and thereby able to stimulate AR activity via Rac1 under conditions of androgen deprivation. The work presented here therefore provides a plausible mechanism for growth factor-mediated, ligand-independent activation of AR by oncogenic Vav3/Rac1 signaling, leading to androgen-independent prostate cancer.
MATERIALS AND METHODS
Experimental Animals
All experiments involving animals were conducted in a manner approved by the University of Miami Animal Care and Use Committee (Miami, FL).
Cell Culture and Chemical Reagents
The human prostate cancer cell lines LNCaP.FGC [American Type Culture Collection (ATCC) catalog no. CRL 1740; batch F-11701] and PC-3 (ATCC catalog no. CRL 1435; batch F-11154) were obtained from ATCC (Manassas, VA). Cell culture media (RPMI-1640 and DMEM) were obtained from Life Science Technologies (Gaithersburg, MD). Fetal bovine serum (FBS) was obtained from Hyclone Laboratories, Inc. (Logan, UT). LNCaP and PC-3 cell lines were cultured in RPMI supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine (Life Science Technologies) and 10% FBS. UO126 was purchased from Promega Corp. (Madison, WI); LY290042, SP600125, SB203580, and SU6656 were purchased from Calbiochem.
Plasmids
The PSA luciferase (PSA-Luc) reporter plasmid (kindly provided by Dr. Carlos Perez-Stable, University of Miami) consists of the PSA promoter and 5′-flanking region, which contain both the distal (−5325 to −4023) and proximal (−542 to +12) ARE-containing enhancer regions but lacks the intervening sequences (54). The following DNA constructs were generously provided: GFP-AR (Dr. Karen Knudsen, University of Cincinnati); Vav3 pIRES2 enhanced GFP (EGFP) (Dr. Michael McClelland, Sidney Kimmel Cancer Center); Ca Rac1, RhoA, and Cdc42 (Dr. Martin Schwartz, University of Virginia, Charlottesville, VA); Erk2K52R (Dr. Melanie Cobb, University of Texas, Southwestern Medical Center); MEK1K97M (Dr. Lynn Heasley, University of Colorado Health Sciences Center, Denver, CO). All PCR-based approaches were performed using the Expand Hi Fidelity PCR system (Roche Applied Bioscience, Indianapolis, IN). For subcloning purposes, the Vav3 cDNA was transferred into pCMV HA (CLONTECH Laboratories, Inc., division of BD Biosciences, Mountain View, CA) by digesting pIRES2 EGFP Vav3 with EcoRI and SalI and cloning the Vav3 cDNA fragment into the same sites of pCMV hemagglutinin (HA) to generate pCMV HA Vav3. Ca Vav3 was constructed by removal of the N-terminal calponin homology and acidic domains using a PCR-based approach. Briefly, the Vav3 region corresponding to nucleotides 529-1872 was amplified by PCR using the following primers: F: 5′-TGA ATT CGA GGT ACC ATG AAG GCA GAG GAA GC-3′ R: 5′-TGT TTA GGA GTT CTT CGC AGT CC-3′; the forward Ca Vav3 primer contains an EcoRI site. The PCR-amplified fragment was digested with EcoR1 and Nsi1 (Vav3 internal site 1598) and inserted into the same sites of PCMV HA Vav3 (CLONTECH) to generate pCMV HA Ca Vav3. pCMVHA Ca Vav3 was then digested with EcoRI and SalI to free the inserted Vav3 cDNA that was cloned into the same sites of pIRES2 EGFP to generate PIRES2 EGFP Ca Vav3. The Ca Vav3 QIF and ISOIII GEF mutants were constructed using the Stratagene QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. Three conserved leucines (336–338) were changed to glutamine, isoleucine, and phenylalanine to generate Ca Vav3 QIF. Mutation of these conserved amino acids eradicates the GEF activity of the closely related Vav2 protein (55). Residues 336–342 were mutated from LLLQELV to IIIQDAA to generate Ca Vav3 ISOIII. These alterations eliminate Vav3 GEF activity (56). Mutagenic primers for Ca Vav3 QIF were as follows: Forward (F), 5′-GTG TTT TAA AGT ACC ACC AGA TCT TCC AGG AAC TGG TCA AAC ATA CC-3′; reverse (R): 5′-GGT ATG TTT GAC CAG TTC CTG GAA AAT CTG GTG GTA CTT TAA AAC AC-3′; and for Ca Vav3 ISO III F, 5′-CGT GTT TTA AAG TAC CAC ATC ATC ATC CAG GAC GCG GCC AAA CAT ACC ACT GAT CCG-3′; R, 5′-CGG ATC AGT GGT ATG TTT GGC CGC ATC CTG GAT GAT GAT GTG GTA CTT TAA AAC ACG-3′. The CaVav3 W493L mutant was created using site-directed mutagenesis on the Ca Vav3 template using the following mutagenic primers: F, 5′-GCA AAA CAA AAG ATT TAA AGA AGA AAT TGC TAG AAC AGT TTG AAA TGG CTT TGT C-3′; R, 5′-GAC AAA GCC ATT TCA AAC TGT TCT AGC AAT TTC TTc TTT AAA TCT TTT GTT TTG C-3′.
Reporter Gene Assays and Transfections
All transfections except those for RNA interference (RNAi) experiments were carried out using the cationic lipid reagent Lipofectamine (Invitrogen Life Technologies, Grand Island, NY) according to the manufacturer’s instructions. Transfections for RNAi experiments were conducted using Lipofectamine Plus or Lipofectamine 2000 (Invitrogen Life Technologies) according to the manufacturer’s protocol. For luciferase assays, RNAi experiments and analysis of Vav3 mutant expression, cells were plated at a density of 2 × 106 cells in 60-mm dishes 16–20 h before transfection. Immediately before transfection, media were replaced with unsupplemented DMEM. For luciferase assays and Vav3 mutant expression studies, cells were transfected with: 5 μg reporter PSA luc, 250 ng CMVhAR or no AR (LNCaP cells), 250 ng of either vector or Vav3 cDNA (Ca, wild type, or mutant) or Ca Rac1. After a 4- to 5-h incubation with DNA/lipid complexes, cells were refed with serum-free RPM1. Studies using the AR antagonist bicalutamide were carried out as described above with the exception that after transfection, cells were allowed to recover for 18 h and then treated with bicalutamide (5 μm). Cells were harvested 48 h after transfection, lysed, and assessed for protein expression by Western blotting and luciferase activity using the Promega luciferase assay kit. For luciferase assays using RNAi, cells were transfected with 10 nm control or Rac1-specific RNAi.
RT-PCR
Total RNA was harvested using the TRIzol method according to the manufacturer’s protocol (Invitrogen Life Technologies). Total RNA (5 μg) was reverse transcribed using Superscript (Invitrogen Life Technologies) reverse transcriptase. For amplification of PSA mRNA, RT reactions were subjected to PCR using the following F and R primers: F, 5′-GAT GAC TCC AGC CAC GAC CT-3; R, 5′-CAC AGA CAC CCC ATC CTA TC-3′. These primers result in the amplification of a 780-bp fragment. To control for cDNA input in PCRs, a 420-bp fragment of β-actin was amplified using the following primers: F, 5′-GTG GGG CGC CCC AGG CAC CA-3′; R, 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′. For semiquantitiative RT-PCR, the linear range of amplification for PSA and actin was determined by removing aliquots at various intervals during the PCR. Once this range was determined, experiments were performed by removing aliquots at two- to three-cycle intervals during the linear range. Bands were quantified using the NIH Image J analysis program.
Glutathione-S-transferase (GST) Protein Purification
GST and p21-binding domain (PBD)-GST were from Dr. Martin Schwartz (University of Virginia, Charlottesville, VA). GST and GST-PBD proteins were purified using the bulk GST purification module according to the manufacturer’s protocol (Amersham Pharmacia Biotech, Piscataway, NJ). BL-21 competent cells were transformed with expression plasmids encoding GST proteins and grown to log phase. Gene expression was induced by the addition of 100 mm isopropyl β-d-thiogalactosidase for 90 min. Cell lysates were added to glutathione columns, and then eluted with reduced glutathione. Purified protein concentrations were determined using Bio-Rad DC protein assay (Bio-Rad Laboratories, Inc., Hercules, CA), and purified proteins were visualized by SDS-PAGE and subsequent Coomassie blue staining.
Rac GTPase Activity Pull-Down Assays
Rac1 activity was assessed by pull-down assays using the Rac/Cdc42 binding domain (PBD) of p21-activated kinase coupled to GST (PBD-GST). These assays take advantage of the selective ability of active (GTP-bound) GTPases to bind to their respective effector proteins. Approximately 8 × 106 cells were plated in 100-mm tissue culture dishes and transfected as described with 10 μg of either the pIRES vector or Ca Vav3 constructs. Cells were harvested 48 h after transfections and lysed in Mg2+ lysis buffer (12.5 mm HEPES, 75 mm NaCl, 5 mm MgCl2, 0.5% Igepal CA-630, 0.5 mm EDTA, 10% glycerol, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 50 mm NaF, and 0.1 mm NaVO4) containing 200 mg/ml PBD-GST. After protein assays, cell lysates containing 800-1000 μg total protein were immediately added to 50 μl glutathione sepharose beads and rotated gently for 45 min at 4 C. Sepharose beads were pelleted by centrifugation, and complexes were washed three times with 1× lysis buffer not containing PBD-GST. GTP-bound Rac1/Cdc42 was eluted with sodium dodecyl sulfate sample buffer and separated by SDS-PAGE. Proteins were transferred to nitrocellulose membranes and analyzed by standard Western blotting using a Rac1 antibody (Upstate Biotechnology, Inc., Lake Placid, NY) at a concentration of 1 μg/ml. Immunoblots were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech).
Soft Agar and Tritiated Thymidine Uptake Assays
LNCaP cells were stably transfected with Ca Rac1 GFP (LNCaP/Ca Rac1) or the GFP Neo vector (LNCaP/Neo) control using a similar approach as described previously (57). Cells were maintained in androgen-depleted media (RPMI supplemented with penicillin, streptomycin, and l-glutamine as described and 350 μg/ml geneticin and 5% charcoal-stripped FBS) for 2 d before experiments. LNCaP/Neo or LNCaP/Ca Rac1 cells were counted, resuspended in RPMI charcoal stripped serum (CSS), and treated with either vehicle or 5 μm bicalutamide. Cells were then mixed with an equal volume of 0.7% noble agar and seeded at a density of 4000 cells in 35-mm Petri plates containing a base layer of 0.5% noble agar. Plates were incubated for 2 wk. Colonies were stained with 0.001% crystal violet and counted using the Bio-Rad Geldoc system.
LNCaP/Ca Rac1 and LNCaP/Neo cells were seeded at a density of 500,000 cells per 60-mm tissue culture dish and maintained in RPMI CSS supplemented with 350 μg/ml geneticin and either 5 μm bicalutamide or vehicle. Approximately 48 h after seeding, tritiated thymidine (1 μCi/ml) was added, and cells were allowed to incubate for approximately 18 h. After this incubation, thymidine incorporation was determined as previously described (58). For experiments using siRNAs (Santa Cruz), LNCaP/Ca Rac1 cells were transfected with either AR siRNA or control siRNA (35 nm). Media were changed 24 h later to RPMI supplemented with 2% CSS, and 72 h later, tritiated thymidine was added. AR levels were determined in parallel cultures by Western blot analysis.
Xenograft Tumor Model
All experiments involving animals were conducted in a manner approved by the University of Miami Animal Care and Use Committee (Miami, FL). LNCaP cells (5 × 106) suspended in an equal volume of Matrigel (BD Biosciences, Franklin Lakes, NJ) were injected sc in flanks of intact male nude mice (Charles River Laboratories, Wilmington, MA). Animals were observed weekly for the presence of tumors. Tumors were measured weekly using calipers, and volume was calculated using the following formula: volume = length × width × height × 0.52 (49). After a period of 4–7 wk after injections (to allow tumor formation), mice were either castrated or subjected to mock surgeries (intact mice). Tumor growth was monitored for approximately 8 wk after castration or mock surgery. Androgen-independent tumors were defined as those tumors that exhibited a 33% or greater increase in tumor volume compared with tumor size at castration. Based on these criteria, eight of 12 total tumors progressed to androgen independence. Typically, several weeks (3,4,5,6) elapsed before tumors resumed growth in castrated mice. Experiments analyzing tumor formation by LNCaP/Ca Rac1 cells were performed in a similar manner except castrations were performed 10 d after injections, which was before tumor formation.
For castrations, nude (nu/nu) male mice were anesthetized, and a small midline incision was made in the abdominal wall through which the testes were exposed. The vas deferens and accompanying vessels were cauterized and the testes dissected away. The body wall and skin were closed with suture and staples, respectively, and the animals were allowed to recover. Animals that were not castrated were subjected to sham operations in which all procedures except cautery and dissection were performed.
Acknowledgments
We thank Drs. Karen Knudsen, Dan Gioeli, and David Helfman for insightful comments and suggestions on the manuscript. Drs. Melanie Cobb, Lynn Heasley, Carlos Perez-Stable, Karen Knudsen, Michael McClelland, and Martin Schwartz generously provided reagents.
Footnotes
This work was supported by grants from the National Institutes of Health (DK065281) and U.S. Department of Defense (DAMD 17–02-1–0094) (to K.L.B.). L.S.L. was supported by fellowships from the PhRMA Foundation and U.S. Department of Defense (PC060504). S.R. is the recipient of an American Heart Association predoctoral fellowship.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 13, 2007
Abbreviations: AR, Androgen receptor; Ca, constitutively active; CSS, charcoal stripped serum; DBL, diffuse B-cell lymphoma; DH, DBL homology; EGF, epidermal growth factor; EGFP, enhanced GFP; F, forward; FBS, fetal bovine serum; GEF, guanine nucleotide exchange factor; GFP, green fluorescent protein; GST, glutathione-S-transferase; GTPase, guanosine triphosphatase; HA, hemagglutinin; MEK, MAPK kinase; PBD, p21-binding domain; PH, pleckstrin homology; PSA, prostate-specific antigen; R, reverse; RNAi, RNA interference; siRNA, small interfering RNA.
References
- Grossmann ME, Huang H, Tindall DJ 2001 Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst 93:1687–1697 [DOI] [PubMed] [Google Scholar]
- Huang H, Tindall DJ 2002 The role of the androgen receptor in prostate cancer. Crit Rev Eukaryot Gene Expr 12:193–207 [DOI] [PubMed] [Google Scholar]
- Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ 2002 Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res 62:1008–1013 [PubMed] [Google Scholar]
- Taplin ME, Balk SP 2004 Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem 91:483–490 [DOI] [PubMed] [Google Scholar]
- Haag P, Bektic J, Bartsch G, Klocker H, Eder IE 2005 Androgen receptor down regulation by small interference RNA induces cell growth inhibition in androgen sensitive as well as in androgen independent prostate cancer cells. J Steroid Biochem Mol Biol 96:251–258 [DOI] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D 2001 The development of androgen-independent prostate cancer. Nat Rev Cancer 1:34–45 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2004 Androgen receptor in prostate cancer. Endocr Rev 25:276–308 [DOI] [PubMed] [Google Scholar]
- Roy-Burman P, Tindall DJ, Robins DM, Greenberg NM, Hendrix MJ, Mohla S, Getzenberg RH, Isaacs JT, Pienta KJ 2005 Androgens and prostate cancer: are the descriptors valid? Cancer Biol Ther 4:4–5 [DOI] [PubMed] [Google Scholar]
- Gelmann EP 2002 Molecular biology of the androgen receptor. J Clin Oncol 20:3001–3015 [DOI] [PubMed] [Google Scholar]
- Balk SP 2002 Androgen receptor as a target in androgen-independent prostate cancer. Urology 60:132–138 [DOI] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL 2004 Molecular determinants of resistance to antiandrogen therapy. Nat Med 10:33–39 [DOI] [PubMed] [Google Scholar]
- Cheng H, Snoek R, Ghaidi F, Cox ME, Rennie PS 2006 Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res 66:10613–10620 [DOI] [PubMed] [Google Scholar]
- Lyons LS, Burnstein KL 2006 Vav3, a Rho GTPase guanine nucleotide exchange factor, increases during progression to androgen independence in prostate cancer cells and potentiates androgen receptor transcriptional activity. Mol Endocrinol 20:1061–1072 [DOI] [PubMed] [Google Scholar]
- Dong Z, Liu Y, Lu S, Wang A, Lee K, Wang LH, Revelo M, Lu S 2006 Vav3 oncogene is overexpressed and regulates cell growth and androgen receptor activity in human prostate cancer. Mol Endocrinol 20:2315–2325 [DOI] [PubMed] [Google Scholar]
- Banach-Petrosky W, Jessen WJ, Ouyang X, Gao H, Rao J, Quinn J, Aronow BJ, Abate-Shen C 2007 Prolonged exposure to reduced levels of androgen accelerates prostate cancer progression in Nkx3.1; Pten mutant mice. Cancer Res 67:9089–9096 [DOI] [PubMed] [Google Scholar]
- Movilla N, Bustelo XR 1999 Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol Cell Biol 19:7870–7885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaza JL, Lopez-Lago MA, Caloca MJ, Dosil M, Movilla N, Bustelo XR 2002 Structural determinants for the biological activity of Vav proteins. J Biol Chem 277:45377–45392 [DOI] [PubMed] [Google Scholar]
- Zeng L, Sachdev P, Yan L, Chan JL, Trenkle T, McClelland M, Welsh J, Wang LH 2000 Vav3 mediates receptor protein tyrosine kinase signaling, regulates GTPase activity, modulates cell morphology, and induces cell transformation. Mol Cell Biol 20:9212–9224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel NL, Zhang Y 1998 Ligand-independent activation of steroid hormone receptors. J Mol Med 76:469–479 [DOI] [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H 1994 Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res 54:5474–5478 [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Bartsch G, Klocker H 2000 Androgen receptor–an update of mechanisms of action in prostate cancer. Urol Res 28:211–219 [DOI] [PubMed] [Google Scholar]
- Culig Z 2003 Role of the androgen receptor axis in prostate cancer. Urology 62:21–26 [DOI] [PubMed] [Google Scholar]
- Gregory CW, Fei X, Ponguta LA, He B, Bill HM, French FS, Wilson EM 2004 Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem 279:7119–7130 [DOI] [PubMed] [Google Scholar]
- Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z 1998 Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res 58:4640–4645 [PubMed] [Google Scholar]
- Craft N, Shostak Y, Carey M, Sawyers CL 1999 A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med 5:280–285 [DOI] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci E, Ma J, Pollak M 2000 Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and prostate cancer risk: epidemiological studies. Growth Horm IGF Res 10(Suppl A):S32–S33 [DOI] [PubMed] [Google Scholar]
- Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L, Hahnfeldt P, Kantoff P, Loda M 2000 Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst 92:1918–1925 [DOI] [PubMed] [Google Scholar]
- Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM 2001 Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 58:1008–1015 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo G, Tortora G, D’Armiento FP, De Rosa G, Staibano S, Autorino R, D’Armiento M, De Laurentiis M, De Placido S, Catalano G, Bianco AR, Ciardiello F 2002 Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res 8:3438–3444 [PubMed] [Google Scholar]
- Lee IS, Liu Y, Narazaki M, Hibi M, Kishimoto T, Taga T 1997 Vav is associated with signal transducing molecules gp130, Grb2 and Erk2, and is tyrosine phosphorylated in response to interleukin-6. FEBS Lett 401:133–137 [DOI] [PubMed] [Google Scholar]
- Jenster G 2000 Ligand-independent activation of the androgen receptor in prostate cancer by growth factors and cytokines. J Pathol 191:227–228 [DOI] [PubMed] [Google Scholar]
- Booden MA, Campbell SL, Der CJ 2002 Critical but distinct roles for the pleckstrin homology and cysteine-rich domains as positive modulators of Vav2 signaling and transformation. Mol Cell Biol 22:2487–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight-Krajewski S, Welsh CF, Liu Y, Lyons LS, Faysal JM, Yang ES, Burnstein KL 2004 Deregulation of the Rho GTPase, Rac1, suppresses cyclin-dependent kinase inhibitor p21(CIP1) levels in androgen-independent human prostate cancer cells. Oncogene 23:5513–5522 [DOI] [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP 1983 LNCaP model of human prostatic carcinoma. Cancer Res 43:1809–1818 [PubMed] [Google Scholar]
- Lim DJ, Liu XL, Sutkowski DM, Braun EJ, Lee C, Kozlowski JM 1993 Growth of an androgen-sensitive human prostate cancer cell line, LNCaP, in nude mice. Prostate 22:109–118 [DOI] [PubMed] [Google Scholar]
- Chen T, Wang LH, Farrar WL 2000 Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res 60:2132–2135 [PubMed] [Google Scholar]
- Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M 2001 In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR). Cancer Res 61:6276–6280 [PubMed] [Google Scholar]
- Hobisch A, Fiechtl M, Sandahl-Sorensen B, Godoy-Tundidor S, Artner-Dworzak E, Ramoner R, Bartsch G, Culig Z 2004 Prostate cancer cells generated during intermittent androgen ablation acquire a growth advantage and exhibit changes in epidermal growth factor receptor expression. Prostate 59:401–408 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo G, Autorino R, De Laurentiis M, Cindolo L, D’Armiento M, Bianco AR, De Placido S 2004 HER-2/neu receptor in prostate cancer development and progression to androgen independence. Tumori 90:163–170 [DOI] [PubMed] [Google Scholar]
- Jenster G 1999 The role of the androgen receptor in the development and progression of prostate cancer. Semin Oncol 26:407–421 [PubMed] [Google Scholar]
- Culig Z 2004 Androgen receptor cross-talk with cell signalling pathways. Growth Factors 22:179–184 [DOI] [PubMed] [Google Scholar]
- Dehm SM, Tindall DJ 2006 Ligand-independent androgen receptor activity is activation function-2-independent and resistant to antiandrogens in androgen refractory prostate cancer cells. J Biol Chem 281:27882–27893 [DOI] [PubMed] [Google Scholar]
- Ueda T, Mawji NR, Bruchovsky N, Sadar MD 2002 Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem 277:38087–38094 [DOI] [PubMed] [Google Scholar]
- Ueda T, Bruchovsky N, Sadar MD 2002 Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem 277:7076–7085 [DOI] [PubMed] [Google Scholar]
- Faruqi TR, Gomez D, Bustelo XR, Bar-Sagi D, Reich NC 2001 Rac1 mediates STAT3 activation by autocrine IL-6. Proc Natl Acad Sci USA 98:9014–9019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, Nesheiwat I, Kong X, Melamed J, Handratta VD, Njar VC, Brodie AM, Yu LR, Veenstra TD, Chen H, Qiu Y 2006 Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell 10:309–319 [DOI] [PubMed] [Google Scholar]
- Xie Y, Wolff DW, Lin MF, Tu Y 2007 Vasoactive intestinal peptide transactivates the androgen receptor through a protein kinase A-dependent extracellular signal-regulated kinase pathway in prostate cancer LNCaP cells. Mol Pharmacol 72:73–85 [DOI] [PubMed] [Google Scholar]
- Abreu-Martin MT, Chari A, Palladino AA, Craft NA, Sawyers CL 1999 Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol 19:5143–5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umekita Y, Hiipakka RA, Kokontis JM, Liao S 1996 Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc Natl Acad Sci USA 93:11802–11807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino D, Driggers P, Arbit D, Kemp L, Miller B, Coso O, Pagliai K, Gray K, Gutkind S, Segars J 1998 Characterization of Brx, a novel Dbl family member that modulates estrogen receptor action. Oncogene 16:2513–2526 [DOI] [PubMed] [Google Scholar]
- Su LF, Knoblauch R, Garabedian MJ 2001 Rho GTPases as modulators of the estrogen receptor transcriptional response. J Biol Chem 276:3231–3237 [DOI] [PubMed] [Google Scholar]
- Muller JM, Metzger E, Greschik H, Bosserhoff AK, Mercep L, Buettner R, Schule R 2002 The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J 21:736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Souvatzoglou E, Charmandari E, Ichijo T, Driggers P, Mayers C, Alatsatianos A, Manoli I, Westphal H, Chrousos GP, Segars JH 2006 Rho family guanine nucleotide exchange factor Brx couples extracellular signals to the glucocorticoid signaling system. J Biol Chem 281:9118–9126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Stable CM, Pozas A, Roos BA 2000 A role for GATA transcription factors in the androgen regulation of the prostate-specific antigen gene enhancer. Mol Cell Endocrinol 167:43–53 [DOI] [PubMed] [Google Scholar]
- Doody GM, Billadeau DD, Clayton E, Hutchings A, Berland R, McAdam S, Leibson PJ, Turner M 2000 Vav-2 controls NFAT-dependent transcription in B- but not T-lymphocytes. EMBO J 19:6173–6184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inabe K, Ishiai M, Scharenberg AM, Freshney N, Downward J, Kurosaki T 2002 Vav3 modulates B cell receptor responses by regulating phosphoinositide 3-kinase activation. J Exp Med 195:189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JL, Maiorino CA, Gkonos PJ, Burnstein KL 1996 Androgenic up-regulation of androgen receptor cDNA expression in androgen-independent prostate cancer cells. Steroids 61:531–539 [DOI] [PubMed] [Google Scholar]
- Yang ES, Burnstein KL 2003 Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J Biol Chem 278:46862–46868 [DOI] [PubMed] [Google Scholar]