Abstract
The definition of Generalized Anxiety Disorder (GAD) has been narrowed in successive editions of DSM by emphasizing intrusive worry and deemphasizing somatic symptoms of hyperarousal. We tried to determine the clinical characteristics of more broadly defined chronically anxious patients, and whether they would show physiological signs of sympathetic activation. A group whose chief complaint was frequent, unpleasant tension over at least the last six weeks for which they desired treatment, was compared with a group who described themselves as calm. Participants were assessed with structured interviews and questionnaires. Finger skin conductance, motor activity, and ambient temperature were measured for 24 hours. Results show that during waking and in bed at night, runs of continuous minute-by-minute skin conductance level (SCL) declines were skewed towards being shorter in the tense group than in the calm group. In addition, during waking, distributions of minute SCLs were skewed towards higher levels in the tense group, although overall mean SCL did not differ. Thus, the tense group showed a failure to periodically reduce sympathetic tone, presumably a corollary of failure to relax. We conclude that broader GAD criteria include a substantial number of chronically anxious and hyperaroused patients who do not fall within standard criteria. Such patients deserve attention by clinicians and researchers.
Keywords: tension, anxiety, Generalized Anxiety Disorder, sympathetic activation, skin conductance, autonomic nervous system, sleep
1. Introduction
Whether psychiatric diagnostic categories are distinct biological entities or the artificial product of classificatory logic has long been a matter of debate. The absence of firm biological foundations for most psychiatric diagnoses has weakened arguments for biological categories and encouraged logical ones. From a logical point of view, anxiety disorders should be assigned to categories on the basis of the presence or absence of sets of features. To qualify for a disorder, the anxiety should be excessive, more than the anxiety of the average person under similar circumstances and severe enough to impair functioning. Essential for categorization as an anxiety disorder is that anxiety be a primary aspect of the diagnosis and not secondary to other diagnoses such as psychosis or depression. After that, further classification is attempted on the basis of further qualitative or quantitative descriptors of the anxiety. Features usually considered are whether the anxiety is acute or chronic, whether it is in response to identifiable external stimuli, whether there was a history of traumatic events, and what behavior or thinking accompanies the anxiety.
Based on these considerations, diagnostic systems have usually identified a category of chronic anxiety where external stimuli, traumatic events, and psychotic thought processes have not played a major role. In the current diagnostic system, this category is Generalized Anxiety Disorder (GAD), the evolution of which through DSM editions is instructive of classificatory logic. Patients diagnosed with DSM-III GAD were often given other diagnoses by clinicians ostensibly following the same diagnostic definitions, which challenged the distinctness and thereby the legitimacy of this category. To improve separation from other mood and anxiety disorders, DSM-III-R made worry that included topics different from those typical of other anxiety disorders, a required symptom (reviewed by Barlow, Blanchard, Vermilyea, Vermilyea, & DiNardo, 1986). Extending the requirement in DSM-IV to be that the worry had to be difficult to control, further improved this separation. At the same time, many of the symptoms of autonomic hyperactivity were dropped because DSM-III-R defined GAD patients endorsed these symptoms infrequently and inconsistently (Marten et al., 1993).
We suspected as have others that the changes in the definition of GAD might have left undiagnosed a substantial number of patients with general persistent anxiety who do not have intrusive worry as a prominent symptom (e.g., Rickels & Rynn, 2001). If this less worrying group properly belongs to a valid category of chronic anxiety, it should show signs of sympathetic activation, perhaps even more so than in currently defined GAD, since worriers as a subgroup of chronic anxiety patients may be anomalous in having little physiological arousal. Evidence for this comes from experiments in which worry was manipulated: heart rate increases to phobic imagery were diminished in the high worry condition (e.g., Borkovec & Hu, 1990). That may be a reason why GAD patients are often found to show a narrowed range of physiological activation, “diminished physiological flexibility” (Hoehn-Saric, McLeod, & Zimmerli, 1989), rather than increased activation. A recent ambulatory study confirmed this by showing that DSM-IV defined GAD patients had neither higher heart rates nor skin conductance than controls (Hoehn-Saric, McLeod, Funderburk, & Kowalski, 2004). On the other hand, a number of studies have found autonomic changes associated with GAD and worry. In one, heart rate was elevated and respiratory sinus arrhythmia (a measure of vagal tone) was reduced in a GAD sample at baseline (Thayer, Friedman, & Borkovec, 1996). Others reported a similar pattern of heart rate changes (reviewed by Brosschot, Gerin, & Thayer, 2006).
With the study reported here we attempted to determine what kinds of chronically anxious people are being left out by the current DSM-IV GAD criteria, and whether a more broadly defined group would show physiological signs of sympathetic activation, a cardinal biological characteristic of fear. In rats, for example, the neural pathways of fear have been traced from the central amygdaloid nucleus to the lateral hypothalamic area and from there down the sympathetic branch of the autonomic nervous system (LeDoux, Iwata, Cicchetti, & Reis, 1988). We recruited people whose chief complaint was frequent, unpleasant tension over at least the last six weeks for which they desired treatment. After casting this wider net, we examined our catch for symptoms and signs of anxiety, and established where they fell among DSM-IV categories. Unlike most diagnostic endeavors, we supplemented the participants' verbal report of anxiety and its symptoms with a psychophysiological assessment of anxiety repeated over a 24-hour period. Using finger skin conductance, we measured sympathetic autonomic activation less inferentially than from reports of activation symptoms. Since skin conductance is affected by temperature and physical activity, we measured these variables along with it.
2. Methods
Participants
Participants who might be suffering from persistent anxiety were recruited and selected with broader and simpler criteria than DSM-IV defined GAD. We posted advertisements in newspapers, flyers, and the internet for the following: “Have you recently been suffering from so much tension that it is distressing you or interfering in your daily life? Has this been going on for 6 weeks or more? Are you between 18 and 65 years old? Researchers at Stanford University and the Palo Alto VA Health Care System are offering free physiological evaluations of your tension and a seminar in ways of reducing it.” To be included, applicants had to answer “yes” to the following questions at phone screening:
“Do you often feel tense?” The word “tense” was chosen rather than “anxious” or “afraid” as being a more general description of negatively felt hyperarousal. In factor analytic studies of moods as self-reported in terms of adjectives, a bipolar dimension emerges that loads on the words “tense,” “jittery,” “fearful” vs. “calm,” “quiet,” “placid,” showing that tension and fear are closely associated semantically and in life, and that tension is not at all restricted to muscle sensations (reviewed by Thayer, 1989).
“Does the tension interfere with your life or is it greater than with other people?”
“Have you felt this way more than half the days in the last 6 weeks?” Questions 2 and 3 were meant to exclude applicants whose tension did not rise above that occasionally experienced in daily life by ordinary people. In DSM-III GAD symptoms had to last only 1 month, while in DSM-III-R this was increased to 6 months to reduce comorbidity, with the intention of making the disorder less overlapping with other disorders and less like a situational stress reaction. However, a recent analysis of a US household sample of almost 10,000 people has shown that GAD symptom episodes of 1 to 5 months do not differ from > 6 month episodes in onset, persistence, impairment, comorbidity, parental GAD, or sociodemographic correlates (Kessler et al., 2005). Thus, we chose 6 weeks as a broader criterion than DSM-IV's 6 months.
“Do you find these feelings unpleasant?”
“Are you interested in learning how to be less tense and to relax?” Questions 4 and 5 were meant to establish that the feeling had a negative valence. Psychophysiological studies show that heightened arousal accompanies both positive and negative emotions (Lang, 1995), although ordinarily it is only the latter that are sources of subjective distress.
In addition to answering yes to these questions in the telephone screening, tense participants had to confirm on a questionnaire when they came in for evaluation that they had felt tense for at least half the days in the last two months (rather than 6 weeks, because the questionnaire was given after the screening).
Exclusion criteria for the study were a history of, or current, psychosis, cognitive impairment, or substance abuse or dependence in the past year. Participants were allowed to continue on stable doses of medicines prescribed by physicians, but were excluded if they were taking drugs with substantial anticholinergic effects, such as tricyclic antidepressants, which have direct effects on skin conductance. They were also told not to take benzodiazepines prescribed “as needed” on the day of testing.
Calm comparison subjects were recruited with the following advertisement: “Researchers at Stanford University and the Palo Alto VA Health Care System are looking for healthy men and women ages 18-65, who are not anxious or depressed. Volunteers are needed as a comparison group in an evaluation of physiological reactions, emotions, and thoughts. Those who qualify will be paid for their participation.” Inclusion criteria were answering “yes” to these two questions during the phone screening: (1) “Do you generally feel calm?” and (2) “Have you felt this way most days in the last 6 weeks?”
Procedure
Individuals who passed the phone screening were invited to an appointment, where after complete description of the study, they gave written informed consent for further assessment. This began with a Structured Clinical Interview for DSM-IV-TR Axis I Disorders (First, Spitzer, Gibbon, & Williams, 2002). If no grounds for exclusion were found, they were asked to wash their hands with soap in preparation for the application of skin conductance electrodes. Next they underwent testing in a laboratory room where numerous autonomic and respiratory measures were taken while they were trying to relax and to change their breathing according to several different instructions. The results of this testing will be reported separately.
After the laboratory session, an ambulatory monitoring device was connected and participants performed the first of four identical ambulatory relaxation tests (ARTs). For each test they first walked at a normal pace for 3 minutes and then sat quietly, without talking or moving for 8 minutes, during which they were instructed to relax. Test instructions and timing had been recorded on a portable cassette recorder, which subjects played at the prescribed times of each test: the first in the hospital at a mean time of 13:40 hours by the 24-hour clock, the second in the afternoon after leaving the hospital (mean 19:00), the third the next morning (mean 9:30) before returning to the hospital, and the fourth back at the hospital (mean 12:30). Subjects filled out a short questionnaire each time before and after they completed the 8 minutes relaxation part of the ART. This questionnaire assessed their current emotional state before and during the last minute of relaxation on a subjective units of distress scale from 0, “not at all”, to 10, “extremely”. Of most interest for this report are the adjectives “anxious” and “tense.”
In addition, participants were given a packet of questionnaires to return completed the next day. The packet included the Penn State Worry Questionnaire (Meyer, Miller, Metzger, & Borkovec, 1990) asking “how typical these statements are for you,” the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983) to be answered for the past week, the short version of the Beck Depression Inventory (Abdel-Khalek, 2001) to be answered for the past week, the Pittsburgh Sleep Quality Index (Buysse, Reynolds III, Monk, Berman, & Kupfer, 1989) to be answered for the past month, the Liebowitz Social Anxiety Scale (Liebowitz, 1987) to be answered for the past week, and the Anxiety Sensitivity Index Revised (Peterson & Reiss, 1993) without a specific time frame.
Equipment
Physiological data were recorded with a 3-channel ambulatory digital recorder (3991x/3 BioLog, UFI, Moro Bay, CA, USA) worn in a handbag or waist pack. The device measures 3.3 × 7.1 × 12.7 cm and weighs 230 g with its battery. Channels were (1) skin conductance measured by applying 0.5 V DC to electrodes on the middle or lower phalanges of digits 2 and 3 of the non-dominant hand. Skin conductance in the range 0.01 to 39.95 μS(iemens) was sampled with +/− 0.01 μS resolution at 10 Hz. Disposable electrodes with a circular contact area of 1 cm diameter pre-filled with isotonic gel were used (EL507, Biopac Systems, Inc., Goleta, CA, USA). In order to maintain contact for 24 hours, additional Biopac isotonic gel (Gel 101) was applied to the center of the electrode. (2) Bodily activity was sampled at 1 Hz from a UFI 1110 Jitterbug Actigraph attached to the same side as the dominant hand. This device responds to motion in all directions and axes. (3) Ambient temperature was sampled with an accuracy of 0.1 degrees C at 0.1 Hz from a sensor in the pack. The device had an event-marker button, with which participants could indicate periods of emotional arousal, time of getting into bed and turning out the lights, getting up in the morning, or unusual events. The nature of the event was recorded in a paper log along with the time the button was pressed.
The selection of channels was based on the following considerations: Skin conductance and heart rate are the two most common experimental indices of autonomic arousal, but skin conductance has the advantage of increasing with tension and arousal, while heart rate can either increase or decrease depending, among other things, on whether cardiovascular preparation will be beneficial in coping with the arousing events (Obrist, 1981). Interpretation of heart rate is complicated by the dual sympathetic-parasympathetic innervation of the heart with opposing effects, whereas skin conductance depends only on the effects of firing of sympathetic fibers that raise it. Since sweating is affected by temperature, it is necessary to monitor ambient temperature to avoid confusing effects of temperature with effects of emotion. Since physical activity may have some of the same activating effects on skin conductance that it has on heart rate, activity should also be monitored. Of course, no single autonomic measure such as skin conductance can be presumed to represent all sympathetic activity or general activation, insofar as the last concept is even valid.
Data analysis
Physiological recordings were examined visually for artifacts, particularly in the skin conductance channel, where they were sometimes caused by loose electrodes. Segments containing artifacts were excluded from further analysis. The record was segmented into waking and sleeping (in bed with the intention to sleep) parts by locating two points: the time of getting into bed and turning out the lights and the time of getting up, both on the basis of event markers and of an examination of the activity channel for cessation or resumption of activity. The results presented below for the two periods are based on one-minute epochs of measurements from each channel, excluding the periods when the ART was performed.
The skin conductance channel yielded two measurements, mean skin conductance level and skin conductance fluctuation. The latter was calculated as within-minute standard deviations of skin conductance level after it had been digitally filtered with a bandpass of 0.1 to 4 Hz to reduce low frequency tonic activity in favor of the higher frequency phasic activity. The temperature channel needed no filtering. Signals from the activity channels were oscillations around zero volts. The activity measure was calculated as the average absolute deviation from zero of these oscillations over the one-minute epochs.
Physiological measures were analyzed separately for the waking and sleeping periods in two ways. First, within-subject distribution parameters of the run lengths of continuous minute-by-minute SCL decline (based on one-min SCL averages) were calculated for each measure and each subject. The n+1st minute was considered a continuation of a descending run if its value was less or equal than the value of the nth minute. The distribution parameters included the mean, minimum, maximum, standard deviation, and skew (like the standard deviation but based on the cube rather than the square of the differences from the mean). Second, within-subject distribution parameters of the one-minute averages for each measure and participant were calculated, and these were compared between tense and calm subjects.
In a separate analysis the means of all the physiological measures and the run lengths of SCL decline were calculated for the 8-min sitting periods of the ART combined over the 4 repetitions for each subject. Similarly, the mean ratings on the current emotional state questionnaire for the adjectives “anxiety” and “tension” were calculated by combining scores for each subject and each adjective from before and during the last minute of each of the 4 repetitions of the ART.
In addition, two measures of sleep disruption in the sleeping period were calculated based on the number of 30-minute periods during which above threshold maxima SCL or above threshold maxima activity levels occurred. Bursts of physical activity in arms and legs have been documented as a useful albeit imperfect indicators of sleep disruption (reviewed by Ancoli-Israel et al., 2003), but whether bursts of SCL activity are also indicators was unknown.
Physiological, clinical, and questionnaire measures were tested for group differences using independent-sample two-tailed t-tests corrected for unequal group variance when necessary using the Levene test. Data were generally log transformed to make their distributions more normal. Rather than adjusting our statistics for multiple comparisons because of the high ratio of number of measures to number of subjects, we selected some measures as primary for testing the hypothesis of sympathetic group differences: mean waking SCL, mean ART SCL, mean run length of SCL decline, skew of waking SCL, and skew of run length of SCL decline. For these measures we predicted that tense subjects would show higher SCLs and shorter SCL declines than the calm, and that skews of SCLs would be more positive in the tense and skew of run length of SCL decline would be more negative in the calm. We considered maxima and minima to be secondary, descriptive measures since they are easily affected by singular events during the recording period. Standard deviations were likely to depend on events and activities more than group. All skin conductance fluctuations measures were considered secondary since they usually give much the same information as SCL, with which they tend to be highly correlated.
3. Results
The sample comprised 18 tense participants, 42.3 ± 12.4 (mean ± standard deviation) years old, and 18 calm participants, 39.7 ± 12.4 years old. The tense group had 9 women, the calm group, 11.
Diagnoses and medications
Only 5 of the tense group met DSM-IV criteria for GAD. In the structured interview, 14 of the tense group definitely endorsed the initial question of being particularly nervous or anxious in the last 6 months, and of these, 4 were diagnosed with GAD. The 10 of this 14 who failed to meet GAD criteria did so for various reasons: for example, 5 did not report much worry; one did not find it hard to stop worrying; 3 could not affirm that “anxiety, worry, or physical symptoms” caused them significant distress or impairment. One member of the tense group whose answer to the initial question was rated as unsure was ultimately diagnosed as having GAD.
Other current diagnoses and the number of people diagnosed with them were Major Depressive Disorder (3), PTSD (3), Adjustment Disorder (2), Specific Phobia (2), Social Anxiety Disorder (1), Panic Disorder (1), and Somatoform Disorder (1). One participant suffered from clinically significant anxiety that did not meet the criteria of any of the DSM-IV anxiety disorders and was therefore diagnosed with Anxiety Disorder NOS. Two participants had three diagnoses, two had two, 10 had one, and four had no diagnosis. None of the calm group met criteria for a current DSM-IV diagnosis. The tense patients with anxiety disorders in which an object or situation triggers their anxiety did not encounter that object or situation during the day tested.
Five of the tense group took psychotropic medications: two took two medications, and three, one. Three took selective serotonin reuptake inhibitors, two took buproprion, and two took benzodiazepines irregularly, but not on the day of testing. None of the calm group took psychoactive drugs.
Self-report and physiological measures
As shown in Table 1, the tense group rated themselves as more worried, under more stress, more depressed, and having poorer sleep than the calm group. Their worry on the Penn State Worry Questionnaire was less than expected for people meeting GAD criteria, who, for example, in another study (Molina & Borkovec, 1994) had a mean of 67.7. Only 5 of the tense group had scores above 65, the optimal score for discriminating GAD from Social Anxiety Disorder (Fresco, Mennin, Heimberg, & Turk, 2003).
TABLE 1.
Questionnaire Scores
| Measure | Tense (mean ± SD) |
Calm (mean ± SD) |
t | df | p |
|---|---|---|---|---|---|
| PSWQ | 57.9 ± 12.4 | 32.9 ± 9.9 | 6.46 | 32 | <.001 |
| PSS10 | 22.0 ± 7.5 | 8.1 ± 6.8 | 5.52 | 31 | <.001 |
| BDIsv | 44.3 ± 14.2 | 21.4 ± 13.1 | 5.02 | 34 | <.001 |
| PSQI | 6.7 ± 3.4 | 3.9 ± 2.0 | 2.97 | 27.5 | <.01 |
| LSAS-F | 21.1 ± 12.3 | 10.0 ± 8.3 | 2.98 | 31 | <.01 |
| ASI | 21.4 ± 10.2 | 12.9 ± 12.7 | 2.22 | 34 | <.04 |
Abbreviations: SD=standard deviation. t=unpaired t-test. df=degrees of freedom (these vary because of missing data. They are corrected to lower fractional values by the Levene test if variances are significantly unequal.) p=two-tailed probability. PSWQ=Penn State Worry Questionnaire. PSS10=Perceived Stress Scale, 10 item version. BDIsv=Beck Depression Inventory Short Version. PSQI=Pittsburgh Sleep Quality Index (higher scores indicate worse sleep.). LSAS-F= Liebowitz Social Anxiety Scale-Fear. ASI=Anxiety Sensitivity Inventory. The time frames for the PSS10, BDI, and LSAS-F are the past week, and for the PSQI, the past 4 weeks. The PSWQ and ASI ask how typical the statements are for the person.
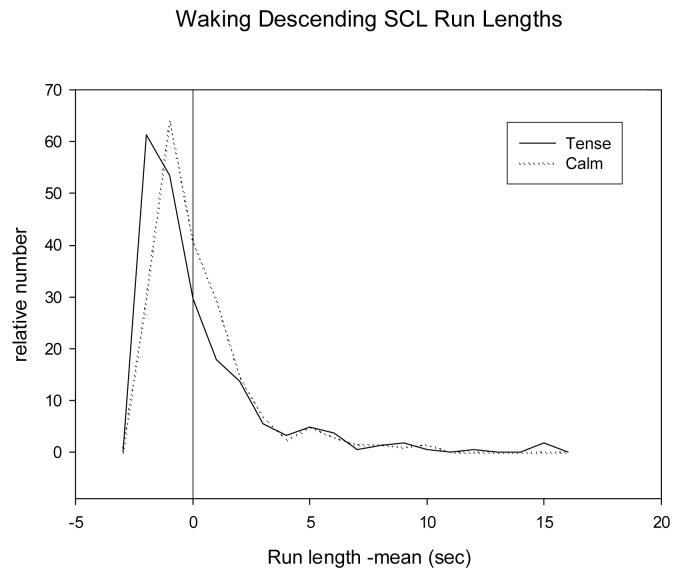
Table 2 gives distribution parameters of waking descending SCL run lengths, a measure of length of periods of sympathetic deactivation, and by inference relaxation. There is a more positive skew in the calm group and a greater maximum (tense= 0.13 ± 0.25, calm= 0.00± 0.00, t= 2.21, df= 17, p<.05). Figure 1 plots the distributions of the descending run lengths for the two groups. These distributions, pooled within groups, are consistent with the statistics based on individual distributions in that the curves are skewed towards longer run lengths for calm individuals. Because the means of individual distributions are subtracted from the run lengths, negative values occur even though skews are always positive.
TABLE 2.
Parameters of Within-Subject Distributions of Run Lengths of One-minute Skin Conductance Level Declines
|
Measure (minutes) |
Tense (mean ± SD) |
Calm (mean ± SD) |
t | df | p |
|---|---|---|---|---|---|
| Waking Mean | 3.90 ± 0.48 | 3.98 ± 0.38 | 0.54 | 34 | NS |
| SD | 2.52 ± 0.75 | 2.92 ± 0.99 | 1.32 | 34 | NS |
| Skew | 2.46 ± 0.74 | 3.69 ± 2.14 | 2.22 | 22.3 | <.037 |
| Sleeping Mean | 5.05 ± 1.39 | 5.34 ± 1.37 | 0.66 | 34 | NS |
| SD | 4.89 ± 2.31 | 6.05 ± 2.53 | 1.49 | 34 | NS |
| Skew | 2.69 ± 0.84 | 3.51 ± 1.38 | 2.14 | 29.6 | <.041 |
Abbreviations: SD=standard deviation. t=unpaired t-test on logarithmically transformed data. df=degrees of freedom (these vary because of missing data. They are corrected to lower fractional values if variances are significantly unequal.) p=two-tailed probability.
FIGURE 1.
Distributions of mean deviations of waking run lengths of one-minute skin conductance level declines pooled across all subjects within each group. The mean run length for each subject was subtracted from each of that subject's run lengths. The relative numbers are normalized as the fraction of the total number for each subject. Bins are 1 minute. No smoothing was applied.
Table 3 documents a similar positive skew for sleep period descending run lengths in the calm group, which in addition, had longer maximum run length (tense= 27.4 ± 10.6, calm= 37.4± 15.6, t= 2.14, df= 32.4, p<.04). Sleep disruption was assessed by the number of 30-minute periods during which above threshold maxima SCL or activity levels occurred. For SCL, the number of such periods did not differ between groups (tense=14.4 ± 3.6, calm=13.7 ± 4.1, t= 0.61, df= 34, NS), but for activity, the tense group had significantly more disruptions (tense=3.8 ± 3.1, calm= 1.6 ± 2.1: t= 2.53, df= 29.9, p<.02).
TABLE 3.
Parameters of Within-Subject Distributions of One-minute Waking Skin Conductance Levels
|
Measure (μSiemens) |
Tense (mean ± SD) |
Calm (mean ± SD) |
t | df | p |
|---|---|---|---|---|---|
| Mean | 5.91 ± 3.01 | 5.79 ± 2.76 | 0.07 | 34 | NS |
| SD | 2.20 ± 0.96 | 2.56 ± 1.39 | 0.83 | 34 | NS |
| Skew | 1.21 ± 1.22 | 0.58 ± 0.44 | 2.07 | 23.0 | <.05 |
Abbreviations: Same as Table 2.
Table 4 gives the distribution parameters of one-minute SCL levels during the waking period. Means and standard deviations of two groups do not differ, but skews for the tense group are significantly more positive than skews for the calm group, which is congruent with the results for descending run length, since more sustained runs are likely to result in lower SCLs as the run continues. The two physical variables that could have a direct effect on SCL, namely temperature and activity did not differ in distributions between groups, except for the skew of waking temperature (tense= −0.27 ± 0.76, calm= 0.37 ± 0.68, t= 2.45, df= 32.2, p<.02). Note that the difference in temperature skew between the groups is in the wrong direction to account for the difference in SCL levels. During the sleeping period, distribution parameters of SCL levels did not differ between groups. However, one parameter of the sleep period skin conductance variable, minimum one-minute skin conductance fluctuation, was less in the calm group (tense= 0.13 ± 0.25, calm= 0.00± 0.00, t= 2.21, df= 17, p<.05).
In comparisons of ART physiological measures between the two groups, none approached significance. However, on the ART questionnaire assessing current emotional state, the tense group rated itself as more anxious (tense= 2.0 ± 0.76, calm= 0.2 ± 0.68, t= 2.90, df= 34, p<.01) and more tense (tense= 2.4 ± 4.5, calm= 0.3 ± 0.8, t= 3.0, df= 34, p<.01) than the calm group.
4. Discussion
Considering that most of our 18 tense participants were chronically anxious by self-report and desirous of treatment, reported elevated anxiety and tension on a test day, and exhibited evidence of less relaxation in terms of shorter periods of sympathetic deactivation, it is surprising that only four met DSM-IV criteria for GAD. They did fulfill criteria for other DSM anxiety disorders and depression as is common in GAD patients, but to regard their tension and anxiety as secondary to another primary anxiety diagnosis would be a dubious inference. Participants with comorbid anxiety disorders did not regard their anxiety as focused on particular external events like phobics or the internal event of an anticipated panic attack as do Panic Disorder patients. Three met the criteria for Major Depressive Disorder, but as usual whether to assign the depression or anxiety precedence was uncertain.
Some of our tense sample failed to qualify for GAD because they felt their worries were controllable. For some, worry was not a salient symptom, which is understandable considering that human emotions such as anxiety are expressed in three general ways: motoric-behavioral (e.g., avoidance), verbal-cognitive (e.g., worrying), and physiological (e.g., sympathetic activation) (Lang, 1988). Individual and situational differences between individuals result in different proportions of these expressions. Not all individuals express their fear and anxiety in cognitive terms of worry, any more than all exhibit avoidance or less sympathetic deactivation. However, the average levels of these expressions can be expected to be higher in an anxious group than in a calm group.
Evidence for sympathetic abnormalities in the tense group was not found in mean SCL levels but in the skews of run lengths of SCL decline and in waking SCLs. Skew represents the degree of asymmetry of the distribution because one of the tails is higher than the other. It depends on the cube of the deviation from the mean, so extreme values have more weight. Our results are most straightforwardly interpreted as indicating fewer and less prolonged periods of relaxation in tense subjects. In a typical active day, many cognitive and emotional challenges accompanied by sympathetic activation are likely to occur both in tense and in calm subjects. What characterized the tense group, however, was the relative shortness and infrequency of periods of sympathetic deactivation. This did not occur enough for the mean SCL in the tense group to be higher than in the calm group, since periods of relaxation in a typical day were relatively short and infrequent even in the calm. Sleep disruption in the tense group resulted in fewer long runs of SCL decline in while in bed. Another reason that run lengths and the distributional metric skew had larger effect sizes in cross-sectional comparisons than mean SCL is that SCL varies widely across subjects due to idiosyncratic skin differences unrelated to emotion (Levey, 1980).
Lack of group differences in mean SCL and runs of SCL decline in our Ambulatory Relaxation Test may indicate that the tense subjects could relax as much as calm subjects if they took time to sit quietly and temporarily disengage from their current activities. Whether the instruction to relax made a difference cannot be known without a contrast condition in which subjects sat without trying to relax. Without that we cannot conclude that the tense group equaled the calm group in the ability to relax voluntarily.
To compare our results to those of previous studies of the psychophysiology of GAD is difficult because recording was usually confined to the special environment of the laboratory and was not repeated throughout the day. However, in one recent study patients with DSM-IV GAD were monitored intermittently during their daily activities for heart rate, skin conductance, respiration rate, ambient temperature, and activity (Hoehn-Saric et al., 2004). As in the current study, those patients did not have higher mean skin conductance levels than controls, but sympathetic deactivation was not calculated using run lengths of SCL decline or skew. Some of our tense group had other anxiety disorders in which objects or situations would trigger anxiety, but since our group did not encounter these situations during the test day, sympathetic activation in our sample cannot be attributed to them. However, the tense group did experience themselves as encountering more stressors, so it is possible that their shorter relaxation periods were a result of relaxation being more frequently interrupted by external activating events.
A strength of our study is its 24-hour outside-the-laboratory assessment of an autonomic variable, skin conductance, in parallel with physical activity and temperature, which could spuriously affect it. Most tests of autonomic activation have been more restricted in time and space. A weakness, however, is that only one sympathetic activation variable was assessed: cardiovascular measures and other autonomic and hormonal variables associated with emotional arousal could contribute important information. It is uncertain whether skin conductance would validly reflect activation in colder environments where marked hand vasoconstriction might occur. The minimum temperature recorded by our monitor for these participants was 13.8° C. Furthermore, the anxious group tested was small and was recruited by a specific advertisement. Samples recruited in others ways might lead to different conclusions. Another sample is also desirable for trying to replicate the current findings: only a few of our measures showed group differences, and multiple measures increase the probability of Type 2 errors.
In summary, we suspect that current DSM-IV GAD criteria define a subtype of chronic anxiety that is atypical for the disorder as a whole. The majority of chronic anxiety patients have become “orphans”, who fall outside the purview of most psychological and pharmaceutical research, and consequently whose treatment with medication is prejudiced as “off label.” On a biological level, this GAD subtype may be atypical in having greater activation of regions of the frontal cortex reflecting worried thinking and less activation of the amygdala and ANS as a result of cortical inhibition. Perhaps the diagnostic categories of DSM-V can be reconfigured in a way that will give disenfranchised chronic anxiety patients an appropriate diagnosis and give researchers appropriate subjects for investigating how the mechanisms that regulate anxiety and arousal can go awry. However, exactly how such categories should be reconfigured is not clear. We could simply broaden the current category of GAD, although one implication of deemphasizing worry is that the focus of current psychological therapies for the disorder might have to change. We could create new categories or subcategories such as “Generalized Worry Disorder” and “Generalized Tension Disorder,” although the boundaries between categories like these might be difficult to set. Finally, we could change from a categorical to a dimensional system where worry and tension are two dimensions, although dimensional descriptions have so far not been as successful as categories in communicating the nature and degree of a mental disorder within the mental health system and between that system and the public.
Acknowledgments
This study was supported by the Department of Veterans Affairs and NIH grant RO1 MH-66953 (Dr. Roth, principal investigator). We thank William O. Faustman, Ph.D., and Paul M. Insel, Ph.D., for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Khalek AM. A short version of the Beck Depression Inventory without omission of clinical indicators. European Journal of Psychological Assessment. 2001;17:233–240. [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Blanchard EB, Vermilyea JA, Vermilyea BB, DiNardo PA. Generalized anxiety and generalized anxiety disorder: Description and reconceptualization. American Journal of Psychiatry. 1986;143:40–44. doi: 10.1176/ajp.143.1.40. [DOI] [PubMed] [Google Scholar]
- Borkovec T, Hu S. The effect of worry on cardiovascular response to phobic imagery. Behaviour Research and Therapy. 1990;28:126–138. doi: 10.1016/0005-7967(90)90056-o. [DOI] [PubMed] [Google Scholar]
- Brosschot J, Gerin W, Thayer J. The perseverative cognition hypothesis: A review of wory, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/ PSY SCREEN) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Fresco D, Mennin D, Heimberg R, Turk C. Using the Penn State Worry Qeustionnaire to identify individuals with generalized anxiety disorder: a receiver operating characteristic analysis. Journal of Behavior Therapy and Experimental Psychiatry. 2003;34:283–291. doi: 10.1016/j.jbtep.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod D, Funderburk F, Kowalski P. Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder. An ambulatory monitor study. Archives of General Psychiatry. 2004;61:913–921. doi: 10.1001/archpsyc.61.9.913. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR, Zimmerli WD. Somatic manifestations in women with generalized anxiety disorder. Archives of General Psychiatry. 1989;46:1113–1119. doi: 10.1001/archpsyc.1989.01810120055009. [DOI] [PubMed] [Google Scholar]
- Kessler R, Brandenburg N, Lane M, Roy-Byrne P, Stang P, Stein D, Wittchen H-U. Rethinking the duration requirement for generalized anxiety disorder: evidence from the National Comorbidity Survey Replication. Psychological Medicine. 2005;35:1073–1082. doi: 10.1017/s0033291705004538. [DOI] [PubMed] [Google Scholar]
- Lang P. What are the data of emotion? In: Hamilton V, Bower GH, Frijda N, editors. Cognitive perspectives on emotion and motivation. Marinus Nijhoff; Boston: 1988. pp. 173–191. [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- LeDoux J, Iwata J, Cicchetti P, Reis D. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AB. Measurement units in psychophysiology. In: Martin I, Venables PH, editors. Techniques in psychophysiology. Wiley & Sons; New York: 1980. pp. 597–628. [Google Scholar]
- Liebowitz M. Social phobia. Modern Problems in Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Marten P, Brown T, Barlow D, Borkovec TD, Shear M, Lydiard R. Evaluation of the ratings comprising the associated symptom criterion of DSMIII-R generalized anxiety disorder. Journal of Nervous and Mental Disease. 1993;181:676–682. doi: 10.1097/00005053-199311000-00005. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Molina S, Borkovec TD. The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In: Graham FTE, Davey CL, editors. Worrying: Perspectives on theory, assessment and treatment. Wiley series in clinical psychology. John Wiley & Sons; Chichester, England: 1994. pp. 265–283. [Google Scholar]
- Obrist P. Cardiovascular Psychophysiology. Plenum Press; New York, NY: 1981. [Google Scholar]
- Peterson RA, Reiss S. Anxiety Sensitivity Index Revised test manual. IDS Publishing Corporation; Worthington, OH: 1993. [Google Scholar]
- Rickels K, Rynn M. Overview and Clinical Presentation of Generalized Anxiety Disorder. Psychiatric Clinics of North America. 2001;24:1–17. doi: 10.1016/s0193-953x(05)70203-3. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–66. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Thayer RE. The Biopsychology of Mood and Arousal. Oxford University Press; New York: 1989. [Google Scholar]