Abstract
The recent discovery of leptin receptors in peripheral tissue raises questions about which of leptin’s biological actions arise from direct effects of the hormone on extraneural tissues and what intracellular mechanisms are responsible for leptin’s effects on carbohydrate and lipid metabolism. The present study is focused on the action of leptin on hepatic metabolism. Nondestructive 13C NMR methodology was used to follow the kinetics of intermediary metabolism by monitoring flux of 13C-labeled substrate through several multistep pathways. In perfused liver from either ob/ob or lean mice, we found that acute treatment with leptin in vitro modulates pathways controlling carbohydrate flux into 13C-labeled glycogen, thereby rapidly enhancing synthesis by an insulin-independent mechanism. Acute treatment of ob/ob liver also caused a rapid stimulation of long-chain fatty acid synthesis from 13C-labeled acetyl-CoA by the de novo synthesis route. Chronic leptin treatment in vivo induced homeostatic changes that resulted in a tripling of the rate of glycogen synthesis via the gluconeogenic pathway from [2-13C]pyruvate in ob/ob mouse liver perfused in the absence of the hormone. Consistent with the 13C NMR results, leptin treatment of the ob/ob mouse in vivo resulted in significantly increased hepatic glycogen synthase activity. Chronic treatment with leptin in vivo exerted the opposite effect of acute treatment in vitro and markedly decreased hepatic de novo synthesis of fatty acids in ob/ob mouse liver. In agreement with the 13C NMR findings, activities of hepatic acetyl-CoA carboxylase and fatty acid synthase were significantly reduced by chronic treatment of the ob/ob mouse with leptin. Our data represent a demonstration of direct effects of leptin in the regulation of metabolism in the intact functioning liver.
Leptin’s ability to normalize food intake is mediated largely by receptors in the hypothalamus (1). The ob/ob mouse model of obesity and insulin resistance has an intact leptin receptor, but is leptin deficient owing to mutation of the ob gene (2). In the ob/ob mouse the lipid-depletion effects of chronic leptin treatment exceed that explained by reduced food intake and weight loss alone (3, 4). Discovery of leptin receptors in peripheral tissue (1, 5) raises questions about which of leptin’s biological actions arise from direct effects of the hormone on extraneural tissues, and what intracellular mechanisms are responsible for leptin’s effects on carbohydrate and lipid metabolism. Using nondestructive 13C NMR methodology to follow the kinetics of intermediary metabolism, we define the action of leptin directly on liver. By monitoring flux through several multistep pathways simultaneously in the intact hepatocyte, 13C NMR spectroscopy helped identify the metabolic pathways controlled; assays of enzymic activities supported the NMR findings. We found that leptin up-regulates hepatic glycogen synthesis in ob/ob mouse liver with both acute and chronic treatment. Simultaneously, we found that acute treatment of ob/ob liver with leptin up-regulated fatty acid synthesis. Chronic treatment of the ob/ob mouse in vivo, however, produced the opposite effect of a sharp decrease in the hepatic rate of lipogenesis.
MATERIALS AND METHODS
Perfused Mouse Liver for NMR Studies.
The isolated perfused liver technique and apparatus designed for liver perfusion in the NMR magnet were as described previously (6, 7). C57BL/6J ob/ob or lean C57BL/6J mice, 8 weeks old, of either sex received chow (Purina Diet 5010) and water ad libitum. Perfusion experiments were started during the middle of the dark part of the 12-h/12-h lighting cycle. Four ob/ob mouse liver groups were studied: (i) The ob/ob mouse group treated chronically with leptin in vivo received leptin twice daily, s.c., 0.5 mg/kg body weight, for the 5 days before excision and perfusion of the liver. No leptin was added during perfusion of these livers. (ii) The ob/ob mouse control group was sham-treated for 5 days but received no exposure to leptin, either in vivo or in vitro. (iii) To test the effects of acute leptin treatment in vitro, livers from sham-treated ob/ob mice received leptin added directly to the perfusion medium every 30 min throughout the perfusion at a level sufficient to match the serum levels measured on day 5 of the leptin in vivo protocol. (iv) The effects of inactivated leptin (trypsinization) were studied under the leptin in vitro conditions; ob/ob mouse livers treated with inactivated leptin behaved the same as the ob/ob control group that received no leptin. Two lean mouse perfused liver groups were studied: livers in the lean mouse control group had no exposure to exogenous leptin, and the effects of acute leptin treatment in vitro were tested in perfused lean mouse liver by the direct addition of leptin to the perfusion medium as described for the corresponding ob/ob group. All livers were perfused in the absence of added insulin. Either 176 min (ob/ob liver) or 150 min (lean liver) after initial addition of 13C-labeled substrate to the perfused liver, glucagon (0.1 nM) was added to initiate breakdown of glycogen to glucose for quantitation purposes. Several assessments of hepatic viability were made. Only preparations with ATP levels of ≈3 μmol/g liver wet weight (glww), stable over the first 30 min of 31P NMR, were used. After 4–4.5 h of perfusion, ATP levels were either unchanged or decreased by <15%. Lactate dehydrogenase (LDH) release was monitored. Perfusate LDH levels were 0.01 units/ml at t = 0 and about 0.10–0.12 units/ml at t = 4 h. A rigorous test of liver function is the ability to synthesize glucose at acceptable, linear rates from substrates that enter the pathway at the level of pyruvate. Livers from the ob/ob mouse control, leptin in vitro, and leptin in vivo groups metabolized [2-13C]pyruvate to produce 13C-glucose over 3 h at rates of 0.56, 0.75, and 1.18 μmol/glww per min, respectively.
Leptin.
Human leptin was expressed in Escherichia coli, purified from inclusion bodies, and refolded (8). Larger-scale refolding was achieved by using an Amicon spiral-wound membrane cartridge for buffer exchange. Perfusate leptin levels were determined by RIA. Perfusate leptin levels were sampled every 15 min and were maintained in a range of 4.0–5.0 ng/ml.
NMR Conditions and Analysis.
13C and 31P NMR spectra of perfused liver at 37°C were measured at 8.46 T as previously described (7). Four 31P spectra were accumulated, each 200 scans over 8 min. One natural abundance 13C spectrum of 800 scans was accumulated over 11 min. Initial addition of 13C-labeled substrate was followed by accumulation of 19–22 13C spectra under isotopic steady-state conditions. All 13C spectra were measured under the conditions used for the natural abundance spectrum. At 246–279 min after measurement of the initial 31P spectrum, two final 31P spectra were measured. Peak areas in 13C NMR spectra of perfused liver were determined by fitting spectra to appropriate line shapes with subsequent integration using the CF module of NMR1 software; appropriate guidelines (9) were used for the glycogen resonance. Areas of 13C NMR signals of metabolites in each perfused liver were converted from absolute integral units to μmol of 13C enrichment at individual carbons by standard approaches (6, 7, 10) using information from three assays of perfusate glucose. Total glucose (12C + 13C enriched) was measured by the glucose oxidase method in perfusate aliquots sampled first at baseline before substrate addition, then at the time of peak 13C-glycogen formation, and again when the 13C spectra indicated complete breakdown of glycogen to glucose. Ratios of 13C/12C at C-1 of glucose produced by gluconeogenesis from labeled substrate and endogenous substrates were obtained by 1H NMR at 400 MHz for perfusate samples prepared by precipitating proteins with 4% perchloric acid, followed by lyophilization of the supernatant and reconstitution in D2O. Relative 13C enrichments at the individual carbon atoms of glucose produced by gluconeogenesis were measured in perfusate samples by 13C NMR at 11.75 T.
Enzyme and mRNA Assays.
Livers used for enzyme and mRNA assays were from ob/ob mice that were dosed with leptin in vivo under the same regimen used for the NMR studies or from control ob/ob mice that were sham-treated in the same way. Livers were excised in the middle of the dark part of the 12-h/12-h lighting cycle and immediately were freeze-clamped without perfusion. Standard procedures were used to assay the activities of glycogen synthase (total and active a-form, GST and GSa, respectively) (EC 2.4.1.11) (11), glycogen phosphorylase a-form (EC 2.4.1.1) (12), fatty acid synthase (FAS) (EC 2.3.1.85) (13), and acetyl-CoA carboxylase (ACC) (EC 6.4.1.2) (14). Total RNA was prepared by using Trizol reagent (GIBCO/BRL). mRNAs were probed with a digoxigenin (DIG)-labeled specific antisense probe (rat ACC gene sequence, nucleotides 994-2420 (15) (GenBank/EMBL accession no. J03808) with bands detected according to the DIG protocol using chemiluminescence (Boehringer Mannheim). Northern blot was reprobed with a DIG-labeled mouse β-actin cDNA probe as control.
Statistical Analysis.
ANOVA was performed on each response to assess differences among the groups. Follow-up comparisons were performed where indicated by using least-squares means. P-values of 0.05 or lower were deemed significant for all tests and comparisons. All values are given as mean ± SEM.
RESULTS AND DISCUSSION
In this study, rates of gluconeogenesis, glycogen synthesis, and synthesis of long-chain fatty acids via the de novo synthesis pathway are measured in liver from ob/ob mice treated chronically with leptin in vivo, but perfused without addition of leptin, and are compared with the rates measured in liver from untreated control ob/ob mice, or to rates observed for ob/ob liver treated with leptin in vitro during the perfusion period. In this way, a direct comparison was made of homeostatic changes, which were induced in liver by chronic treatment of the animal with leptin, with changes in metabolism that occurred in liver from untreated animals in response to direct addition of the hormone to the perfusion medium during the NMR study. The spectra shown in Fig. 1 are of a liver perfused under approximately metabolic and isotopic steady-state conditions with the gluconeogenic substrate [2-13C]pyruvate (6.7 mM) + NH4Cl (2.7 mM), which is interconverted to [2-13C]alanine in the liver. The natural abundance of 13C is only 1%, permitting our use of specifically labeled pyruvate that was highly (≥99%) enriched with 13C at carbon 2. In the Krebs cycle, the 13C-label is randomized; the 13C-distribution at specific carbons in metabolites synthesized from this substrate gives information on fluxes through metabolic pathways (6, 10, 16, 17). By accumulating a spectrum of the 13C natural abundance of each liver before addition of 13C-labeled substrate and then subtracting this spectrum from each spectrum accumulated after the addition of 13C-labeled substrate, we selectively quantitated only those metabolites that could be traced back directly to [2-13C]pyruvate, such as glutamate, glutamine, glucose, and glycogen (Figs. 1 and 2).
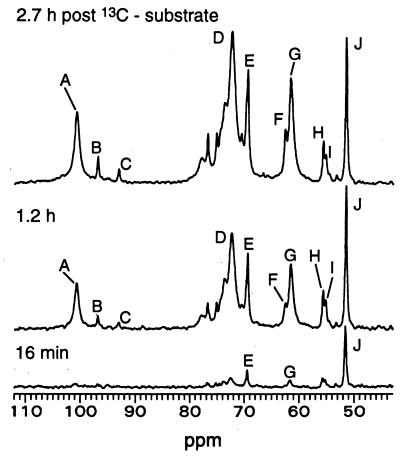
Figure 1.
13C NMR spectra of a representative perfused liver from an ob/ob mouse treated chronically with leptin in vivo. No leptin was added during perfusion of the liver. Three spectra measured at the indicated times postsubstrate are shown. The 105–60 ppm region contains signals of newly synthesized 13C-labeled glucose and glycogen, which increased monotonically as the level of substrate, seen as [2-13C]alanine, was held approximately constant. The 13C natural abundance background spectrum of the liver, measured under identical conditions before addition of substrate, was subtracted from each of the spectra shown. (A) C-1 of glucosyl units in glycogen. (B and C) C-1 of β and α anomers of glucose, respectively. (D) C-2 to C-5 of glucosyl units in glycogen. (E) Lactate C-2 and C-2 of the glycerol backbone of triacylglycerols (TG). (F) C-1 + C-3 of TG glycerol. (G) C-6 of glucose and of glucosyl units of glycogen. (H) Glutamate C-2. (I) Glutamine C-2. (J) Alanine C-2. Label in TG glycerol arises from both exchange into endogenous pools and synthesis.
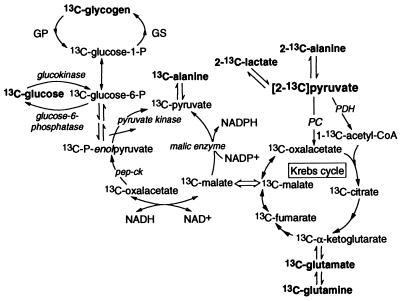
Figure 2.
Simplified gluconeogenic pathway from [2-13C]pyruvate. GS, glycogen synthase; GP, glycogen phosphorylase. 13C-metabolite denotes the presence of more than one isotopomer owing to randomization of the original 13C label in the Krebs cycle. Randomized 13C-pyruvate can reenter the Krebs cycle (not shown).
Effects of Leptin in Vivo and in Vitro on Glycogen Synthesis.
Our 13C spectra follow the gluconeogenic pathway from [2-13C]pyruvate to the branch point at glucose-6-P, and thereby either directly to 13C-labeled glucose via glucose-6-phosphatase activity, or to 13C-labeled glycogen by the glycogen synthase route (Fig. 2). The kinetics of net glycogen synthesis (as a gluconeogenic process) can be monitored by measurement of 13C enrichment at C-1 of the glucosyl units in glycogen by 13C NMR. As seen in Fig. 1, glycogen C-1 gives rise to 13C NMR signals free of overlapping peaks. The rate of glycogen synthesis (Fig. 3A) by ob/ob mouse liver was 3-fold greater after chronic administration of leptin in vivo as compared with the ob/ob control liver rate (P < 0.001) and 1.9-fold greater as compared with the rate in ob/ob liver treated with leptin in vitro (P < 0.01) (Table 1). When leptin was administered directly to ob/ob perfused liver in vitro, glycogen synthesis (Fig. 3A) was 1.6-fold greater compared with untreated control ob/ob liver (P < 0.05) (Table 1). Leptin treatment in vitro also markedly enhanced glycogen synthesis in liver from normal lean mice (Fig. 3B). This enhancement is reflected in a 2-fold increase in the rate of glycogen synthesis with direct administration of leptin to perfused lean mouse liver (Table 1). Our NMR analysis methodology, which is based on 13C NMR of perfused liver and both 13C (Table 2) and 1H NMR of perfusate samples, allowed quantitation of the effects of leptin treatment on contributions of both endogenous and 13C-labeled carbon sources to the total glucose output (Table 1). There was no significant difference in the contribution of endogenous substrates to total hepatic glucose output among the three ob/ob treatment groups. However, gluconeogenesis from the 13C-labeled substrate was increased significantly in livers from ob/ob mice chronically treated with leptin in vivo as compared with either ob/ob control livers or to ob/ob livers treated with leptin in vitro (Table 1). These findings are consistent with increased flux through phosphoenolpyruvate carboxykinase (PEPCK) in ob/ob liver after leptin treatment in vivo. PEPCK is rate limiting for gluconeogenesis and is unusual in that regulation of its activity is exerted only at the level of gene expression.
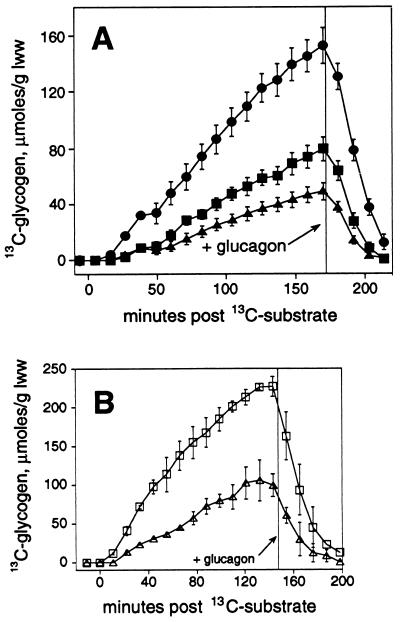
Figure 3.
Quantitation of the effects of leptin treatment on kinetics of glycogen synthesis in ob/ob mouse liver (A) and lean mouse liver (B). 13C enrichments in the glucosyl units (μmol/glww) are shown over time after initial addition of [2-13C]pyruvate and NH4Cl. For ob/ob mouse liver (A), the effects of leptin treatment in vivo (•) (n = 7) or in vitro (▪) (n = 5) are compared with control (▴) (n = 9). For lean mouse liver (B), the effect of leptin treatment in vitro (□) is compared with control (▵), n = 2–3 each. Where not visible, error bar is smaller than symbol.
Table 1.
Effect of leptin treatment on carbon source contribution to total hepatic glucose output in ob/ob mouse liver and rate of glycogen synthesis in ob/ob and lean mouse liver
| Group | From endogenous sources | From 13C-pyruvate μmol glucose/glww | Total glucose output | Net rate of glycogen synthesis* μmol per glww/min |
|---|---|---|---|---|
| ob/ob control (7) | 151 ± 12 | 101 ± 8 | 252 ± 11 | 0.36 ± 0.04 |
| ob/ob leptin in vitro (5) | 134 ± 7 | 136 ± 14 | 271 ± 10 | 0.57 ± 0.06† |
| ob/ob leptin in vivo (7) | 115 ± 16 | 216 ± 20‡§ | 331 ± 28¶ | 1.07 ± 0.10‡§ |
| Lean control (3) | 0.84 ± 0.17 | |||
| Lean leptin in vitro (2) | 1.71 ± 0.03 |
Leptin in vivo group received leptin, s.c., 0.5 mg/kg body weight, twice a day for 5 days before liver excision, with no leptin added during perfusion. Livers from the the leptin in vitro group were from untreated mice and received leptin added to the perfusate at a level that matched day 5 serum levels of the leptin in vivo protocol. Control livers received no leptin treatment. Number of experiments is in parentheses.
Rate of incorporation of 13C-labeled glucosyl units/glww per min.
P < 0.05 versus control group.
P < 0.001 versus control group.
P < 0.01 versus leptin in vitro group.
P < 0.01 versus control group.
Table 2.
Isotopic distribution in carbons of glucose produced from [2-13C]pyruvate substrate by perfused liver from ob/ob and lean mice
| Relative 13C enrichment of glucose | ob/ob liver (20)* | Lean liver (5)† | |
|---|---|---|---|
| C-1 | 73.6 ± 1.4 | 78.5 ± 0.6 | |
| C-2 | 89.7 ± 1.5 | 96.0 ± 0.6 | |
| C-3 | 34.6 ± 1.3 | 48.0 ± 3.0 | |
| C-4 | 38.4 ± 1.3 | 51.0 ± 3.0 | |
| C-5 | 100.0 | 100.0 | |
| C-6 | 79.0 ± 1.0 | 81.0 ± 3.0 | |
|
0.91 ± 0.01 | 0.96 ± 0.02 |
Although distributions for the individual livers were used in calculations, mean values are given to indicate the liver-to-liver variation in this distribution.
Mean values for 20 ob/ob livers of which eight were untreated controls, five were treated with leptin in vitro only, and seven were perfused in absence of hormone but were from mice treated with leptin in vivo. Leptin treatment as given in Table 1.
Mean values for two untreated lean mouse livers and three lean livers treated with leptin in vitro.
Our results establish that leptin up-regulates hepatic glycogen synthesis by two mechanisms. The larger increase in glycogen synthesis measured in livers from ob/ob mice chronically treated with leptin suggests expression level changes affecting regulation of the activities of one or more of the pathway enzymes beyond the glucose-6-phosphate branchpoint because these changes persisted throughout several hours of subsequent perfusion postexcision without addition of either leptin or insulin to the perfusion medium. The finding of enhanced hepatic glycogen synthesis after short-term leptin treatment in vitro is intriguing as this rapid response is consistent with leptin modulating signal transduction pathways controlling hepatic glycogen synthesis in an insulin-independent manner. As shown in Table 3, leptin treatment of the ob/ob mouse in vivo resulted in significantly increased hepatic glycogen synthase activity as well as in significant activation of the enzyme as indicated by an increase in the ratio of GSa to GST. Thus, leptin treatment in vivo results in long-term remodeling of hepatic glycogen synthase activity as well as activation of the enzyme (Table 3). These findings are consistent with our 13C NMR results that demonstrated increased glycogen synthesis with leptin treatment of ob/ob mouse liver in vitro and an even larger increase in glycogen synthesis in liver from ob/ob mice treated chronically with leptin. Hepatic glycogen phosphorylase activity was unchanged by leptin treatment in vivo (Table 3).
Table 3.
Effects of leptin treatment in vivo on the activities of glycogen synthase, glycogen phosphorylase, and ACC in ob/ob mouse liver
| Enzyme | Control, units/glww | Leptin, units/glww | |
|---|---|---|---|
| GSa | 0.19 ± 0.05 (7) | 0.58 ± 0.11 (8) | P = 0.01 |
| GST | 0.62 ± 0.10 (7) | 1.15 ± 0.18 (8) | P < 0.05 |
| GSa/GST | 0.30 ± 0.05 (7) | 0.50 ± 0.05 (8) | P < 0.05 |
| GPa* | 12.5 ± 2.0 (7) | 11.3 ± 1.5 (8) | NS |
| ACC(−cit)† | 0.37 ± 0.02 (8) | 0.27 ± 0.04 (8) | P < 0.05 |
| ACC(+cit)† | 0.72 ± 0.03 (8) | 0.51 ± 0.05 (8) | P < 0.005 |
| ACC(−/+)† | 0.52 ± 0.02 (8) | 0.53 ± 0.02 (8) | NS |
| FAS‡ | 0.52 ± 0.04 (10) | 0.29 ± 0.03 (10) | P < 0.001 |
Leptin treatment as given in Table 1. Units/glww is defined as the conversion of 1 μmol of substrate per min at 30°C per glww. Number of experiments is in parentheses. GPa, the a-form of glycogen phosphorylase. NS, not significant.
For comparison, GPa for lean mouse liver (N = 4) was 7.2 ± 4.2 units/glww.
ACC activity was measured in the absence (−cit) or presence (+cit) of 4 mM citrate. ACC(−/+) is the ratio of activity at 0 and 4 mM citrate.
For FAS activity is defined as the synthesis of 1 nmol of palmitic acid per min at 30°C per mg of liver protein.
Effects of Leptin in Vivo and in Vitro on Long-Chain Fatty Acid Synthesis.
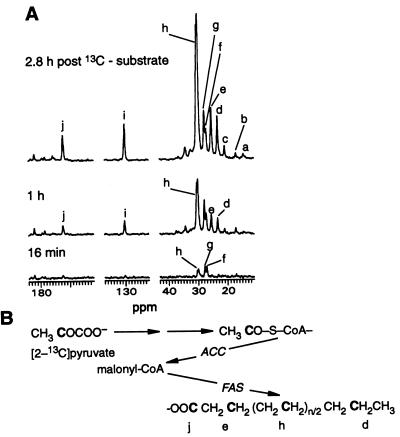
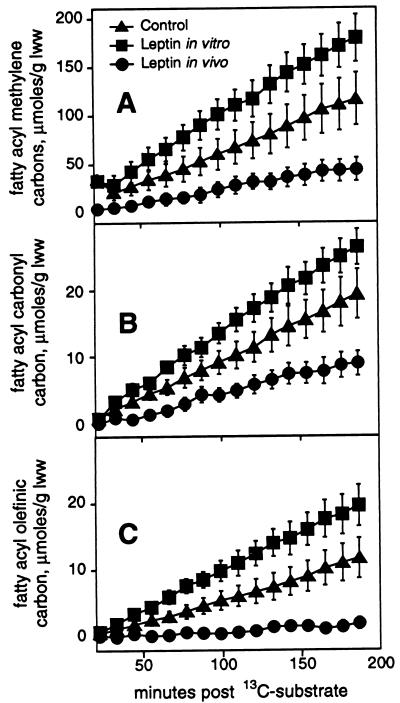
Our 13C spectra also followed the kinetics of long-chain fatty acid synthesis (Fig. 4A). In perfused liver, the substrate [2-13C]pyruvate is converted to [1-13C]acetyl-CoA, which in the de novo synthesis pathway labels alternate carbons in fatty acids (Fig. 4B). With the exception of the repeating methylene groups, the labeled carbons gave rise to signals distinguishable on the basis of chemical shift from signals of the adjacent fatty acyl carbons that should not show 13C enrichment under these conditions (10). Thus, measurement of 13C enrichment at several specific sites in fatty acyl chains in time-resolved spectra of liver perfused under these steady-state conditions offers a way to follow the effects of leptin treatment on the kinetics of fatty acid synthesis (Fig. 5 A–C). Hepatic fatty acid synthesis, which is best measured at the repeating methylene carbons (Fig. 5A), was decreased 2.6-fold in perfused liver from ob/ob mice treated with leptin in vivo as compared with the ob/ob control group (P < 0.05). Leptin was not added during perfusion of livers from mice treated with leptin in vivo. When leptin was administered directly in vitro to ob/ob liver from untreated mice, stimulation of fatty acid synthesis rather than reduction was observed (Fig. 5A). Fatty acid synthesis was 1.6-fold greater with short-term leptin treatment of the perfused ob/ob mouse liver compared with untreated control ob/ob liver (P < 0.05) and 4-fold greater than fatty acid synthesis in liver from ob/ob mice treated with leptin in vivo (P < 0.005). From the data shown in Fig. 5A, the rates of long-chain fatty acid synthesis from [1-13C]acetyl-CoA were 0.24 ± 0.06. 1.01 ± 0.12, and 0.64 ± 0.16 μmol/glww per min for the leptin in vivo, leptin in vitro, and control ob/ob liver groups, respectively. Fatty acid synthesis in lean mouse liver was barely measurable.
Figure 4.
The kinetics of de novo synthesis of long-chain fatty acid from [1-13C]acetyl-CoA in ob/ob mouse liver. 13C NMR spectra (A) of a representative ob/ob mouse liver exposed to leptin in vitro. The regions containing fatty acyl carbon signals are shown for spectra measured at the indicated times after initial addition of [2-13C]pyruvate and NH4Cl substrate. Only signals of metabolites that can be traced back to [2-13C]pyruvate are seen. NMR conditions are as given for Fig. 1. (a) Fatty acyl terminal methyl carbon. (b) Alanine C-3. (c) Lactate C-3. Fatty acyl carbons include: (d) CH313CH2CH2−; (e) −13CH2CH2CO−; (h), the repeating methylene carbons; (i) −CH = 13CHCH2CH2−; and (j) −CH2CH213CO−. (f) Glutamine C-3. (g) Glutamate C-3. Randomization of 13C label is seen in slight labeling of a. (B) Simplified de novo synthesis pathway showing incorporation of original 13C label of pyruvate into specific sites in fatty acids. C represents 13C.
Figure 5.
Quantitation of effect of leptin treatment in vivo (•) (n = 7) or in vitro (▪) (n = 5), compared with control (▴) (n = 7), on kinetics of 13C enrichment at specific carbons of fatty acid synthesized via the de novo route in ob/ob mouse liver. Leptin was not added during perfusion of livers from mice treated with leptin in vivo. 13C enrichments (μmol/glww) are shown over time after initial addition of [2-13C]pyruvate for the repeating methylene carbons (A), for the fatty acyl carbonyl carbon (B), and for the monounsaturated olefinic carbon (C). Where not visible, error bar is smaller than symbol.
Our finding of enhanced hepatic long-chain fatty acid synthesis in ob/ob mouse liver after short-term leptin treatment in vitro is consistent with leptin modulating signaling pathways involved in stimulation of fatty acid synthesis from acetyl-CoA by ACC, the key regulatory enzyme of de novo synthesis of fatty acids (18), possibly by activation of phosphatidylinositol 3-kinase (19). The decrease in lipogenesis induced by chronic treatment of ob/ob mice with leptin is consistent with decreases in the total activities of lipogenic enzymes (18). We tested this last hypothesis by measuring the effect of leptin treatment in vivo on the activities of FAS and ACC and on the activation state of ACC. As shown in Table 3, hepatic FAS and ACC activities both were significantly reduced by leptin treatment of the ob/ob mouse in vivo. However, the activation state of ACC as estimated by assaying the ratio of activity at 0 and 4 mM citrate (14, 20) was unchanged by chronic leptin treatment. Within the limits of detection there was no significant difference in mRNA levels for hepatic ACC in control and chronically treated ob/ob mice (data not shown), implying posttranslational regulation of this enzyme by chronic treatment. Our findings on the effects of leptin treatment in vivo on hepatic FAS and ACC activities support the 13C NMR results showing decreased de novo synthesis of fatty acids in perfused liver from ob/ob mice treated chronically with leptin.
Glutamate Labeling and Ketone Body Levels.
Evidence consistent with decreased generation of NADPH by malic enzyme, an important lipogenic enzyme, after leptin treatment of the ob/ob mouse in vivo was provided by methodology based on distribution of 13C label in key metabolites in liver (data not shown). However, flux through malic enzyme is largely driven by the rate of utilization of NADPH (18). Decreased demand for NADPH reducing equivalents is consistent with the decreased activity of ACC with chronic leptin treatment given in Table 3. Leptin has been shown to lower triacylglycerol content in rat pancreatic islets by increasing oxidation of fatty acids (21). We first tested this hypothesis in liver by examining the 13C enrichments in hepatic glutamate. Pyruvate can enter the Krebs cycle (Fig. 2) by two routes: by activity of pyruvate carboxylase (PC) or via flux through pyruvate dehydrogenase (PDH). The metabolic changes that occur during β-oxidation of fatty acids are known to inactivate PDH (22). The relative proportion of [2-13C]pyruvate entering the Krebs cycle via PDH is reflected in the 13C enrichment at glutamate C-5, whereas flux through PC is reflected in labeling at glutamate C-2 and C-3 (10). The ratio of 13C-labeling at glutamate C-5 to that at (C-2+C-3) was 1.15 ± 0.12 in ob/ob control liver and 0.94 ± 0.15 in ob/ob liver treated with leptin in vitro, indicating about equal flux through PC and PDH in both groups. In liver from ob/ob mice treated with leptin in vivo, the corresponding ratio was 1.64 ± 0.09, which is significantly greater than the ratio in either of the other two ob/ob groups (P < 0.05); the glutamate ratio measured after chronic leptin treatment indicates 60% greater flux through PDH relative to PC posttreatment. Thus, glutamate labeling suggests that leptin treatment in vivo did not inactivate PDH as would be anticipated if hepatic fatty acid oxidation had increased. We then tested the hypothesis by measuring perfusate concentrations of an important product of β-oxidation, β-hydroxybutyrate (β-HB), that might accumulate if leptin increased fatty acid oxidation. However, [3-13C]β-HB was not different in ob/ob mouse liver control, leptin in vitro, and leptin in vivo groups, with perfusate levels of 0.41 ± 0.06, 0.36 ± 0.15, and 0.41 ± 0.09 mM, respectively. Acetoacetate (AA) also was not different in the three groups: [3-13C]AA accumulated to 0.12 ± 0.04, 0.14 ± 0.02, and 0.09 ± 0.04 mM in perfusate of the ob/ob control, leptin in vitro, and leptin in vivo groups, respectively. The ratios of perfusate AA to β-HB, an indicator of the mitochondrial-free NAD/NADH ratio, were also about the same, 0.29 ± 0.10, 0.36 ± 0.11, and 0.25 ± 0.10, respectively, in the same ob/ob mouse liver treatment groups.
Effects on Fatty Acid Chain Length and Unsaturation.
Fatty acid synthesis measured at the other carbons labeled in the de novo synthesis pathway [i.e., the carbonyl carbon (Fig. 5B) and the chemically unique methylene carbons, d and e in Fig. 4 A and B] was decreased 2.2- to 3-fold in liver from ob/ob mice treated with leptin in vivo as compared with either the ob/ob controls (P < 0.05) or the ob/ob leptin in vitro group (P < 0.001). Quantitation of the kinetics of 13C enrichment at fatty acyl methylene carbons d and e (data not shown) closely followed the enrichment pattern shown for the carbonyl carbon (Fig. 5B). With our 13C method, contributions of simple chain elongation are distinguishable from de novo synthesis as 13C labeling of the carbonyl carbon occurs by both routes, whereas 13C labeling of carbon d in the terminal acetyl unit occurs only by the de novo synthesis pathway (10). By this method, the average chain lengths of fatty acids synthesized by ob/ob mouse livers in the control, leptin in vitro, and leptin in vivo groups were 18.8 ± 0.1, 18.8 ± 0.1, and 18.0 ± 0.4, respectively, indicating elongation of the initial pathway product, palmitate. Decreases in 13C enrichment at the monounsaturated olefinic carbon (Fig. 5C) in the ob/ob leptin in vivo group were even more pronounced, being 5.6-fold lower than in the ob/ob control group (P < 0.01) and 10-fold lower compared with ob/ob mouse livers treated directly with leptin (P < 0.001). Comparison of 13C enrichment at the monounsaturated olefinic carbon (i) with the 13C enrichment at the methylene carbon (d) in the terminal acetyl unit indicated a sharp decrease in Δ9 desaturase activity in ob/ob mouse liver after treatment with leptin in vivo. Whereas 66% of fatty acids synthesized by control ob/ob mouse livers and 75% of fatty acids synthesized by ob/ob livers exposed to leptin in vitro were monounsaturated, only 24% of fatty acids synthesized by livers from mice chronically treated with leptin were monounsaturated. Hormonal regulation of Δ9 desaturase activity is incompletely defined, although insulin appears to be an inducer of the enzyme in vivo. Consequently, these data on the control of Δ9 desaturase by leptin treatment in vivo focus attention on understanding regulation of this enzyme. Our results on chain length and percent monounsaturation are consistent with biosynthesis of oleic, stearic, 9-eicosenoic, and arachidic acids, in addition to palmitic acid, all of which are products of the major pathways of fatty acid biosynthesis (23).
Taken together, our 13C NMR data demonstrate two direct effects of leptin on hepatic metabolism: up-regulation of glycogen synthesis in liver from ob/ob and lean mice and up-regulation of long-chain fatty acid synthesis by the de novo synthesis pathway in ob/ob liver. Up-regulation of fatty acid synthesis was demonstrated only for acute treatment. Livers from ob/ob mice chronically treated with leptin exhibited down-regulation of fatty acid synthesis. Chronic administration of leptin to ob/ob mice induced homeostatic changes that persisted throughout several hours of subsequent liver perfusion postexcision without addition of leptin and resulted in greater enhancement of hepatic glycogen synthesis than was observed with acute treatment. Consistent with our 13C NMR results, leptin treatment of the ob/ob mouse in vivo resulted in significantly increased hepatic glycogen synthase activity as well as activation of the enzyme. Analysis of 13C and 1H NMR results for ob/ob mouse liver showed a significant increase in flux of 13C-labeled metabolites through the gluconeogenic pathway after chronic, but not acute, administration of leptin. Chronic treatment of the ob/ob mouse with leptin in vivo markedly decreased hepatic de novo synthesis of long-chain fatty acids during perfusion without addition of leptin. In agreement with our 13C NMR results, the activities of hepatic ACC and FAS were significantly reduced by chronic treatment of the ob/ob mouse with leptin. Liver’s pivotal position in lipid metabolism suggests that our finding that leptin treatment in vivo blocks hepatic de novo synthesis of long-chain fatty acids from acetyl-CoA, without increasing β-oxidation of fatty acids, is an additional mechanism through which leptin might reduce adiposity in the ob/ob mouse. Our data represent a demonstration of direct effects of leptin in the regulation of metabolism in the intact functioning liver and show the 13C NMR method to be useful in investigations of leptin’s role as a regulator of metabolism.
Acknowledgments
We thank G. J. Kaczorowski, L. Van der Ploeg, and D. E. Moller for helpful discussions; E. Frazier for the RIA assays; T. Smith and J. P. Varnerin for leptin production, refolding, and leptin quality control; R. A. Reamer for the 1H NMR spectroscopy; and Markus Hanner for Northern blot analysis of mRNA.
ABBREVIATIONS
- glww
gram liver wet weight
- GST
total glycogen synthase
- GSa
a-form of glycogen synthase
- FAS
fatty acid synthase
- ACC
acetyl-CoA carboxylase
- PDH
pyruvate dehydrogenase
References
- 1.Lee G H, Proenca R, Montez J M, Carroll K M, Darvishzadeh J G, Lee J I, Friedman J M. Nature (London) 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 2.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 3.Levin N, Nelson C, Gurney A, Vandlen R, de Sauvage F. Proc Natl Acad Sci USA. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz M W, Baskin D G, Bukowski T R, Kuijper J L, Foster D, Lasser G, Prunkard D E, Porte D, Jr, Woods S C, Seeley R J, Weigle D S. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 5.Fei H, Okano H J, Li C, Lee G H, Zhao C, Darnell R, Friedman J M. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen S M. Biochemistry. 1987;26:563–572. doi: 10.1021/bi00376a031. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S M. In: Research in Perfused Liver. Ballet F, Thurman R G, editors. London: Libbey; 1991. pp. 43–68. [Google Scholar]
- 8.Rosenblum C I, Tota M, Cully D, Smith T, Colum R, Qureshi S, Hess J F, Phillips M S, Hey P J, Vongs A, et al. Endocrinology. 1996;137:5178–5181. doi: 10.1210/endo.137.11.8895396. [DOI] [PubMed] [Google Scholar]
- 9.Overloop K, Vanstapel F, Van Hecke P. Magn Reson Med. 1996;36:45–51. doi: 10.1002/mrm.1910360109. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S M. Biochemistry. 1987;26:581–589. doi: 10.1021/bi00376a033. [DOI] [PubMed] [Google Scholar]
- 11.Nuttall F Q, Gannon M C. Anal Biochem. 1989;178:311–319. doi: 10.1016/0003-2697(89)90644-1. [DOI] [PubMed] [Google Scholar]
- 12.Hems D A, Rodrigues L M, Whitton P D. Biochem J. 1976;160:367–374. doi: 10.1042/bj1600367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nepokroeff C M, Lakshmanan M, R, Porter J W. Methods Enzymol. 1975;35:37–44. doi: 10.1016/0076-6879(75)35136-7. [DOI] [PubMed] [Google Scholar]
- 14.Jamil H, Madsen N B. J Biol Chem. 1987;262:638–642. [PubMed] [Google Scholar]
- 15.Lopez-Casillas F, Bai D-H, Luo X, Kong I-S, Hermodson M A, Kim K-H. Proc Natl Acad Sci USA. 1988;85:5784–5788. doi: 10.1073/pnas.85.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S M. J Biol Chem. 1983;258:14294–14308. [PubMed] [Google Scholar]
- 17.Cohen S M. Biochemistry. 1987;26:573–580. doi: 10.1021/bi00376a032. [DOI] [PubMed] [Google Scholar]
- 18.Goodridge A G. In: Biochemistry of Lipids, Lipoproteins, and Membranes. Vance D E, Vance J, editors. Vol. 20. New York: Elsevier; 1991. pp. 111–139. [Google Scholar]
- 19.Moule S K, Edgell N J, Welsh G I, Diggle T A, Foulstone E J, Heesom K J, Proud C G, Denton R M. Biochem J. 1995;311:595–601. doi: 10.1042/bj3110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunfeld C, Soued M, Adi S, Moser A H, Dinarello C A, Feingold K R. Endocrinology. 1990;127:46–54. doi: 10.1210/endo-127-1-46. [DOI] [PubMed] [Google Scholar]
- 21.Shimabukuro M, Koyama K, Chen G, Wang M Y, Trieu F, Lee Y, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson M S. In: Textbook of Biochemistry with Clinical Correlations. Devlin T M, editor. New York: Wiley; 1992. pp. 237–289. [Google Scholar]
- 23.Cook H W. In: Biochemistry of Lipids, Lipoproteins, and Membranes. Vance D E, Vance J E, editors. Vol. 31. New York: Elsevier; 1996. pp. 129–152. [Google Scholar]