Abstract
In C. elegans, insulin signaling affects development, lifespan and stress resistance. Several studies have shown that insulin signaling affects lifespan in an endocrine-like manner from different cells, while the major downstream target of insulin, the FOXO transcription factor encoded by daf-16, may act preferentially in intestinal cells to prolong lifespan. This discrepancy raised the possibility that insulin may have both endocrine and cell-intrinsic outputs. Here, we further investigated the types of cells capable of producing endocrine outputs of insulin and also identified a new cell-intrinsic insulin output. We found that insulin signaling within groups of neurons promoted wildtype lifespan, showing that the endocrine outputs of insulin were not restricted to specific cells. In contrast, DAF-16 appeared to have a greater effect on lifespan when expressed in a combination of tissues. These results suggest that insulin signaling may regulate DAF-16 through cell-intrinsic and endocrine pathways. We also found that an insulin-dependent response to fasting in intestinal cells was preferentially regulated by intestinal insulin signaling and was less responsive to insulin signaling from non-intestinal cells. Together, these results show that C. elegans insulin signaling has endocrine as well as tissue-specific outputs which could influence lifespan in a combinatorial fashion.
Keywords: Insulin, Phosphoinositide 3-kinase, FOXO, Caenorhabditis elegans, Aging, Lifespan, Dauer, Diapause
Introduction
In C. elegans, a conserved insulin-like signaling pathway promotes wildtype lifespan, stress resistance and reproductive development (Kenyon, 2005). Adult lifespan and stress resistance are increased in animals with mutations in the DAF-2/insulin receptor-like protein, AGE-1, a homolog of the p110 catalytic subunit of phosphoinositide 3-kinase (PI3K) or in AKT-1, PDK-1 or SGK-1, three downstream targets of DAF-2 (Hertweck et al, 2004; Johnson, 1990; Kenyon et al., 1993; Morris et al., 1996; Kimura et al., 1997; Paradis et al., 1999; Paradis and Ruvkun, 1998). In addition, signaling through the DAF-2 pathway is necessary to bypass arrest at the dauer larval stage, an alternative third stage larval form that is optimized for long-term survival under harsh environmental conditions (Riddle et al., 1981). The major target of insulin-like signaling is the FOXO transcription factor, DAF-16, whose mammalian orthologs are FOXO1, FOXO3a and FOXO4 (Lin et al., 1997; Ogg et al., 1997). DAF-2/IR signaling antagonizes DAF-16/FOXO via phosphorylation by AKT-1, AKT-2 and SGK-1, and promoting DAF-16’s cytoplasmic retention (Henderson and Johnson, 2001; Hertweck et al., 2004; Lee et al., 2001; Lin et al., 2001; Paradis and Ruvkun, 1998). Impairment of signaling downstream of DAF-2 relieves inhibition of DAF-16 and frees DAF-16 to enter the nucleus where it may induce or repress target gene expression.
Previous studies to identify cell types where insulin signaling promotes wildtype lifespan and reproductive development in C. elegans showed that daf-2 and age-1 could function non-cell autonomously, from at least the nervous system and intestine, to promote wildtype lifespan and reproductive development (Apfeld and Kenyon, 1998; Wolkow et al., 2000). In addition, daf-18, a negative regulator of insulin signaling, could also promote dauer arrest and longevity from a variety of cells (Masse et al., 2005). All these studies supported the existence of endocrine outputs of insulin signaling that coordinate dauer arrest and lifespan. It was therefore unexpected to find that daf-16 activity was apparently required in specific tissues for dauer arrest and longevity. Intestinal daf-16 activity was necessary for extended lifespan in the daf-2(e1370) background, while daf-16 activity in the nervous system was necessary for dauer larval arrest (Libina et al., 2003).
The discrepancies between the sites of action for daf-16 and the upstream insulin pathway components necessitated further characterization of tissue-restricted and endocrine effects of this pathway. Here, we report that age-1 has clear endocrine-like effects on dauer arrest and longevity. The endocrine effects of age-1 were not restricted to specific cells, and could be produced by a variety of cells within the nervous system. We also examined the effects of cell-restricted daf-16 activity. Although neuron- or intestine-specific daf-16 expression had little effect on lifespan, combined expression in both tissues could lengthen lifespan of daf-16 mutant animals. This suggests that insulin signaling may coordinate lifespan incrementally by regulating daf-16 activity in multiple tissues, possibly through a combination of endocrine and cell-intrinsic effects. In addition, we found that insulin signaling also regulates a tissue-restricted response to fasting in intestinal cells. This suggests that not all effects of insulin signaling are mediated by endocrine outputs. Together these results support the existence of endocrine-like outputs of insulin signaling that control lifespan and dauer arrest, as well as cell-intrinsic outputs that are less responsive to endocrine regulation. These findings constitute an important step forward for understanding the complex roles of insulin in development and aging.
Materials and methods
Strains and general methods
The C. elegans strains used in this work were N2 Bristol (wildtype), CB1370 (daf-2(e1370)), GR1307 (daf-16(mgDf50)), WCAW196 (age-1 (mg305)), SP75 (sqt-1(sc13) age-1(mg44)/mnC1); SGP296 (sqt-1(sc13) age-1(mg109)/mnC1); WCAW118 (daf-16(mg242); age-1 (mg109)). The daf-16(mg242) mutation was isolated in a screen for suppressors of the dauer-constitutive phenotype of age-1 (mg109) animals and contains a nonsense mutation at tryptophan 220 that affects both the daf-16a and daf-16b splice forms. daf-16(mg242) completely suppresses the lifespan, stress tolerance and dauer constitutive phenotypes of age-1(mg109) animals. Strains were provided by the Caenorhabditis Genetics Center at the University of Minnesota. All strains were cultivated at 15°C on NGM agar plates seeded with the E. coli strain OP50 following standard protocols (Brenner, 1974).
To express a rescuing age-1 cDNA from cell-specific promoters, a cassette strategy was used to clone each promoter as cassettes into GFP-and age-1 cDNA-containing vectors. Promoters were amplified from C. elegans genomic DNA and cloned into a GFP reporter vector (pPD95.75). The promoters were also inserted into a plasmid containing the age-1 cDNA with an unc-54 3′UTR, using unique restriction sites. The subcloning junctions, along with some or all of each promoter sequence, were confirmed by direct DNA sequencing. Promoters were functionally analyzed by examining the expression of GFP from the GFP reporters (Fig. S1). Primer sequences and promoter sizes are presented in Table S1.
The gfpdaf-16 cDNA fusion was constructed by inserting gfp in-frame at the amino terminus of the daf-16a full-length cDNA yk1006c10 to create a daf-16 construct identical to that used previously (Libina et al., 2003). Prior to subcloning, the entire daf-16 cDNA insert in yk1006c10 was sequenced to identify any mutations. The cDNA contained one base pair that differed with the published daf-16 sequence (t1245c), but this change is not predicted to affect the amino acid sequence of DAF-16. For insertion of gfp, a unique SmaI site was inserted at the daf-16 amino terminus using PCR and used to insert gfp from pPD95.02. Promoters for gfpdaf-16 expression were inserted upstream of gfp into unique SphI and KpnI sites. Cloning junctions and PCR-amplified sequences were confirmed by sequence analysis.
Transgenic animals were created by standard microinjection-mediated transformation of plasmid DNA at a final concentration of 25–100 μg/mL with coinjection markers expressing GFP from either the mec-7 or gcy-7 promoters. Transmitting lines were isolated from second generation (G2) progeny of the injected animals and the transgenic extrachromosomal arrays were crossed into the age-1(mg44) and age-1(mg305) backgrounds for phenotypic characterization. The mg44 allele is a nonsense mutation in age-1 that deletes the lipid kinase domain and causes a presumptive null phenotype (Morris et al., 1996). The age-1(mg305) allele contains a 45-nucleotide insertion in exon 3 after nucleotide 809 which results in a 15 amino acid insertion in the AGE-1 ras-binding domain, causing a reduction-of-function phenotype (Wang and Ruvkun, 2004). Transgenic animals expressing gfpdaf-16 were constructed as for age-1, except that DNA was injected directly into daf-16(mg242);age-1(mg109) animals. Phenotypic analyses were performed for multiple transgenic lines for each transgenic construct.
Lifespan analysis
Lifespan assays were performed at 20°C or 25°C on NGM plates supplemented with 50 μg/mL FUDR (5-fluorodeoxyuracil, Sigma) to prevent progeny overgrowth. Synchronized populations were obtained by allowing gravid adults to lay eggs at 15°C for 5–6 hours. The embryos were allowed to complete larval development at 15°C and were transferred onto FUDR-containing medium and shifted to 20°C or 25°C on the first day of adulthood, as indicated. The number of animals alive was scored every 2–3 days until death, which was defined as the failure to respond to gentle prodding on the head and tail with a platinum wire.
Statistical analysis of lifespan data was performed using the JMP software package (version 5.1). For all tables, transgenic rescue of lifespan was determined by comparing transgenic survival data against the control survival data from single experiments. Table 1 shows all survival data for control strains by experiment. Lifespan experiments for age-1(mg44) and age-1(mg109) controls were performed using homozygous progeny from age-1 heterozygous hermaphrodites, which would be maternally rescued for the dauer-arrest phenotype. These animals are referred to as m+z− (maternal+, zygotic−) in the text. Transgenic age-1 expression rescued dauer arrest in the mg44 background, so it was not necessary to obtain first generation progeny from heterozygous hermaphrodites for analysis of transgenic mg44 strains.
Table 1.
| Genotype | Expt. | Mean (days) | Std error | (n) | % wildtype lifespan |
|---|---|---|---|---|---|
| age-1(+) | 1 | 15.6 | 0.28 | 51 | |
| age-1(+) | 3 | 23.1 | 0.56 | 88 | |
| age-1(+) | 6 | 18.2 | 0.59 | 70 | |
| age-1(+) | 8 | 21.7 | 0.66 | 63 | |
| 9 | 18.9 | 0.30 | 140 | ||
| 10 | 17.7 | 0.42 | 30 | ||
| age-1(+) | 11 | 14.9 | 0.44 | 106 | |
| age-1(+) | 12 | 18.4 | 0.24 | 50 | |
| age-1(+) | 13 | 16.8 | 0.30 | 49 | |
| age-1(+) | 15 | 15.8 | 0.72 | 37 | |
| age-1(mg44)a | 1 | 22.6 | 1.76 | 22 | 145% |
| age-1(mg44)a | 2 | 25.1 | 0.79 | 63 | |
| age-1(mg44)a | 6 | 24.3 | 1.36 | 39 | 133% |
| age-1(mg44)a | 7 | 22.1 | 0.93 | 73 | |
| age-1(mg44)a | 8 | 26.3 | 0.90 | 92 | 121% |
| 9 | 22.1 | 0.91 | 46 | 117% | |
| 10 | 23.9 | 0.65 | 45 | 135% | |
| 11 | 19.9 | 0.63 | 98 | 134% | |
| 13 | 19.8 | 1.33 | 26 | 118% | |
| age-1(mg44)a | 15 | 20.6 | 1.11 | 39 | 130% |
| age-1(mg305) | 1 | 29.8 | 2.00 | 31 | 191% |
| 2 | 21.9 | 3.25 | 15 | ||
| age-1(mg305) | 3 | 32.4 | 0.51 | 57 | 140% |
| age-1(mg305) | 4 | 28.8 | 1.76 | 20 | |
| 6 | 25.1 | 1.31 | 77 | 138% | |
| age-1(mg305) | 7 | 23.5 | 1.98 | 31 | |
| age-1(mg305) | 13 | 36.5 | 0.84 | 52 | 218% |
age-1(mg44) animals were progeny of mg44/+ hermaphrodites, were maternally-rescued for the constitutive dauer-arrest phenotype of age-1(mg44).
Dauer assays
Gravid adult hermaphrodites were allowed to lay eggs at 22°C for 4 hours and the embryos were then shifted to 25°C. Development was scored after 72 and 96 hours and the number of dauer larvae, sterile adults and fertile adults was counted.
Histochemical staining for esterase activity
Bulk esterase activity was detected in fixed animals following the protocol of Karnovsky and Roots, with slight modifications (Karnovsky and Roots, 1964). Young adult worms at the first day of egg-laying were fixed in −20°C methanol for 10 minutes and stained overnight at room temperature in a solution of 144 mM sodium acetate, 5.6 mM sodium citrate, 3.3 mM copper sulfate, 0.54 mM K3(Fe(CN)6), and 2 mM acetylthiocholine (Sigma-Aldrich, Inc., St. Louis, MO). Stained specimens were mounted on a 2% agarose pad, and viewed on a Nikon E800 microscope. Images were collected using a Hamamatsu ORCA-ER CCD camera using OpenLab software (Improvision, Lexington, MA). For fasting, animals were first cleaned of bacterial food either by washing in M9 buffer or transfer to clean NGM plates. Bacteria-free animals were transferred to NGM agar with 100 μg/mL ampicillin (to prevent bacterial growth) and incubated at 25°C for 6 hours before fixation.
Results
age-1 can act in multiple tissues to promote wildtype lifespan
Our previous study examined the ability of cell-type restricted expression of age-1 and daf-2 to rescue the long-lifespan phenotypes of age-1(mg44) and daf-2(e1370) mutants (Wolkow et al., 2000). In the previous study, age-1 cDNA expression from the unc-14 promoter rescued the Age phenotype of age-1(mg44) animals, while expression from the intestine-specific ges-1 or muscle-specific unc-54 promoters did not. However, we wished to further investigate a requirement for age-1 expression in the intestine and muscles. To do this, we integrated the Pges-1:age-1 and Punc-54: age-1 transgenes by UV-irradiation, backcrossed them 4 times into the wildtype background and then crossed them into the age-1 mutants. The integrated transgenes were able to rescue long lifespan of both age-1(mg305) and age-1(mg44) strains (Table 2). These positive results negate the previously published negative results for age-1, and they are consistent with the previous finding that intestine-restricted daf-2 expression shortened daf-2(e1370) lifespan.
Table 2.
Adult lifespan of animals with age-1 expression restricted to neurons, intestine or muscle
| Genotype | Mean (days) | Std error | (n) | Expt. | % shortened | P vs control a |
|---|---|---|---|---|---|---|
| Pan-neuronal (Pric-19: age-1) | ||||||
| age-1(+); bvIs2 | 13.3 | 0.48 | 73 | 13 | 20 | <0.0001, <0.0001 |
| age-1(+); bvIs2 | 14.4 | 0.65 | 56 | 13 | 14 | 0.2209, 0.0603 |
| age-1(mg44); bvIs2 | 19.9 | 0.77 | 74 | 7 | 10 | 0.0010, 0.0319 |
| age-1(mg44); bvIs2 | 24.3 | 1.37 | 45 | 8 | 8 | 0.1131, 0.2136 |
| age-1(mg305); bvIs2 | 19.7 | 0.77 | 122 | 7 | 16 | 0.0003, 0.0961 |
| age-1(mg305); bvIs2 | 21.3 | 1.02 | 44 | 13 | 58 | <0.0001, <0.0001 |
| age-1(+); bvEx125 | 15.5 | 0.62 | 42 | 13 | 7 | 0.5718, 0.0699 |
| age-1(+); bvEx128 | 16.6 | 0.78 | 49 | 13 | 1 | 0.0335, 0.5328 |
| age-1(mg44); bvEx11 | 17.8 | 0.88 | 45 | 1 | 21 | 0.0004, 0.0192 |
| age-1(mg44); bvEx12 | 14.3 | 0.29 | 78 | 1 | 37 | <0.0001, <0.0001 |
| age-1(mg44); bvEx12 | 16.7 | 0.64 | 81 | 11 | 16 | <0.0001, <0.0001 |
| age-1(mg44); bvEx12 | 16.7 | 0.64 | 81 | 11 | 16 | <0.0001, <0.0001 |
| age-1(mg44); bvEx120 | 21.1 | 0.67 | 119 | 9 | 5 | 0.8562, 0.5176 |
| age-1(mg44); bvEx121 | 21.9 | 0.43 | 85 | 9 | 1 | 0.0133, 0.1121 |
| age-1(mg44); bvEx122 | 20.2 | 0.45 | 135 | 9 | 9 | 0.0001, 0.0028 |
| age-1(mg44); bvEx123 | 19.3 | 0.56 | 92 | 9 | 13 | <.0001, 0.0004 |
| age-1(mg44); bvEx123 | 19.6 | 0.69 | 41 | 10 | 18 | <0.0001, <0.0001 |
| age-1(mg44); bvEx125 | 19.3 | 0.88 | 50 | 9 | 13 | 0.0091, 0.0095 |
| age-1(mg44); bvEx125 | 17.4 | 0.90 | 49 | 10 | 27 | <0.0001, <0.0001 |
| age-1(mg44); bvEx125 | 19.0 | 0.54 | 61 | 13 | 4 | 0.0248, 0.1169 |
| age-1(mg44); bvEx128 | 22.0 | 1.01 | 26 | 9 | 0 | 0.5992, 0.4764 |
| age-1(mg44); bvEx128 | 16.6 | 0.78 | 49 | 13 | 16 | 0.0002, 0.0102 |
| age-1(mg44); bvEx128 | 18.1 | 0.56 | 36 | 15 | 12 | <0.0001, 0.0030 |
| age-1(mg305);bvEx(ric-19: age-1) | 26.0 | 1.14 | 79 | 3 | 20 | <0.0001, <0.0001 |
| Intestine(Pges-1: age-1) | ||||||
| age-1(mg44); bvIs1 | 20.5 | 0.72 | 50 | 8 | 22 | <0.0001, <0.0001 |
| age-1(mg305); bvIs1 | 21.3 | 1.05 | 47 | 1 | 6 | <0.0001, <0.0001 |
| age-1(mg305); bvIs1 | 27.0 | 1.06 | 77 | 3 | 17 | <0.0001, <0.0001 |
| Body muscle(Punc-54: age-1) | ||||||
| age-1(mg44); mgIs37 | 21.4 | 1.41 | 44 | 8 | 19 | 0.0057, 0.0067 |
| age-1(mg305); mgIs37 | 19.7 | 0.85 | 45 | 1 | 34 | <0.0001,<0.0001 |
| age-1(mg305); mgIs37 | 23.6 | 0.99 | 75 | 3 | 27 | <0.0001, <0.0001 |
Log-Rank, Wilcoxan, versus non-transgenic control.
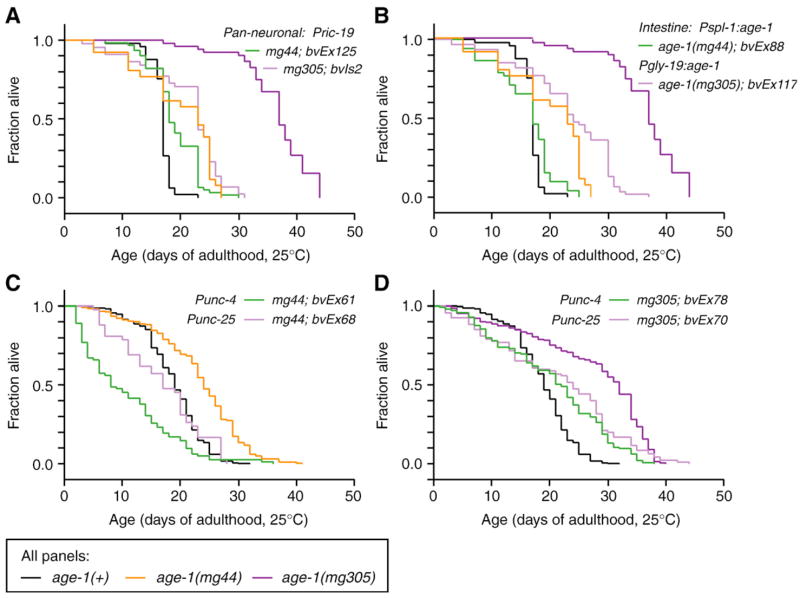
We also re-examined whether neural-specific age-1 activity rescued lifespan in age-1(−) strains by driving age-1 expression from the neural-specific promoter Pric-19 (Pilon et al., 2000). Lifespan was rescued in animals carrying Pric-19:age-1 transgenes (Fig. 1A, Table 2). In most experiments, age-1(−) animals with extrachromosomal Pric-19: age-1 transgenes lived 12–37% shorter than nontransgenic controls, while these transgenes only shortened wildtype lifespan by 1–7%. An integrated Pric-19: age-1 transgene also shortened lifespan of age-1(mg305) animals (16–58% shorter), while reducing wildtype lifespan approximately 20% (Fig. 1A, bvIs2). The negative effect of the integrated array in the wildtype strain could be due to the presence of second site mutations possibly incurred during UV-irradiation. Since transgenic age-1 expression had more significant effects on lifespan of age-1(−) animals than wildtype animals, we conclude that the transgenes specifically rescued the long lifespan phenotype of age-1(−) rather than nonspecifically shortening lifespan.
Fig. 1.
Rescue of long lifespan by neuron- or intestine-specific age-1 expression. (A, B) Results from one representative experiment; (C, D) cumulative survivorship. Data for individual trails presented in Tables 1–4. In all panels, black designates wildtype age-1(+), orange designates age-1(mg44) adults maternally rescued for dauer arrest, dark purple designates age-1(mg305) adults. Transgenic lines as follows: (A) Pan-neuronal age-1(Pric-19: age-1); (B) intestine-specific age-1(Pspl-1: age-1 or Pgly-19: age-1); (C, D) green, cholinergic motor neuron-specific age-1(Punc-4: age-1); light purple, GABAergic motorneuron-specific age-1(Punc-25: age-1).
To further verify that intestinal age-1 expression could rescue long lifespan of age-1(−) animals, we constructed animals expressing the age-1 cDNA from the promoters for the intestinal genes, gly-19 and spl-1 (Mendel et al., 2003; Warren et al., 2001). Both the gly-19 and spl-1 promoters directed robust expression of GFP reporters through late life (not shown). Expression of age-1 from gly-19 and spl-1 promoters also robustly rescued long lifespan in both the age-1(mg44) and age-1(mg305) backgrounds (Fig. 1B, Table 3). In age-1(mg44) animals carrying age-1 expressed from either the gly-19 or spl-1 promoters, lifespan was reduced between 16–46% (Table 2). The same transgenes only slightly reduced lifespan of age-1(+) animals (0–14% reduction). These results confirm the findings with the integrated Pges-1: age-1 array.
Table 3.
Adult lifespan of animals with intestine-restricted age-1
| Genotype | Mean (days) | Std error | (n) | Expt. | % Shortened | P vs control a |
|---|---|---|---|---|---|---|
| age-1(+); bvEx86 | 16.3 | 0.54 | 48 | 13 | 3 | 0.3217, 0.6986 |
| age-1(+); bvEx87 | 14.4 | 0.79 | 26 | 13 | 14 | 0.2814, 0.0504 |
| age-1(+); bvEx87 | 18.2 | 0.66 | 38 | 15 | +15 | 0.0072, 0.0136 |
| age-1(+); bvEx88 | 16.3 | 0.30 | 56 | 13 | 3 | 0.5703, 0.4297 |
| age-1(+); bvEx89 | 15.9 | 0.36 | 67 | 13 | 5 | 0.7575, 0.3576 |
| age-1(+); bvEx90 | 16.7 | 0.42 | 30 | 13 | 0 | 0.3238, 0.3575 |
| age-1(+); bvEx90 | 19.4 | 0.53 | 33 | 15 | 22 | 0.0006, 0.0011 |
| age-1(+); bvEx117 | 16.7 | 0.76 | 44 | 13 | 1 | 0.0053, 0.0662 |
| age-1(mg44); bvEx86 | 17.9 | 1.21 | 44 | 8 | 32 | <0.0001, <0.0001 |
| age-1(mg44); bvEx87 | 11.9 | 1.23 | 24 | 7 | 46 | <0.0001, <0.0001 |
| age-1(mg44); bvEx87 | 21.3 | 1.94 | 29 | 8 | 19 | 0.1057, 0.0160 |
| age-1(mg44); bvEx87 | 17.4 | 0.66 | 64 | 9 | 21 | <0.0001, <0.0001 |
| age-1(mg44); bvEx87 | 25.2 | 1.36 | 19 | 15 | +22 | 0.0366, 0.0868 |
| age-1(mg44); bvEx88 | 13.2 | 1.18 | 38 | 7 | 40 | <0.0001, <0.0001 |
| age-1(mg44); bvEx88 | 15.9 | 0.71 | 52 | 13 | 20 | 0.0001, 0.0056 |
| age-1(mg44); bvEx89 | 17.8 | 1.81 | 31 | 8 | 32 | 0.0009, <0.0001 |
| age-1(mg44); bvEx89 | 16.1 | 1.16 | 16 | 15 | 22 | 0.0011, 0.0051 |
| age-1(mg44); bvEx90 | 12.8 | 1.04 | 51 | 7 | 42 | <0.0001, <0.0001 |
| age-1(mg44); bvEx90 | 16.6 | 0.93 | 60 | 13 | 16 | 0.0166, 0.0438 |
| age-1(mg305); bvEx117 | 23.1 | 1.03 | 61 | 13 | 63 | <0.0001, <0.0001 |
Log-Rank, Wilcoxan, versus non-transgenic control.
We further analyzed this survival data to determine whether insulin signaling regulates lifespan in a binary “all-or-none” fashion or promoted fractional changes in lifespan. If insulin were to act in a binary fashion, then transgenic populations should consist of a mixture of rescued and non-rescued subpopulations, resulting in survival curves with features of both subpopulations. Such a pattern has been observed in heterozygous populations of animals with single-gene mutations that affect lifespan (Friedman and Johnson, 1988). Similar binary effects on lifespan have also been observed in genetic mutants that promote long lifespan only in a fraction of the total population, without altering lifespan of all animals (Gerisch and Antebi, 2004; Nanji et al., 2005). Alternatively, if insulin regulates lifespan through fractional effects, then survival data for partially rescued populations should show features that are intermediate between the wildtype and non-transgenic mutant controls. Such fractional effects on lifespan are observed for calorie restriction and temperature in C. elegans, where a range of dietary and environmental conditions causes incremental changes in lifespan (Houthoofd et al., 2003; Johnson et al., 1984; Klass, 1977).
To address this question, we created exponential plots of the survival data for Pric-19: age-1 and Pgly-19: age-1 or Pspl-1: age-1 transgenic animals, and compared these curves to wildtype and non-transgenic controls. These transgenes appeared to promote incremental rescue of age-1(−) lifespan. In both the case of neuron- and intestine-restricted age-1 expression, mortality increased at ages that were intermediate between wildtype and non-transgenic controls (Fig. 2). This incremental behavior was most pronounced for transgenes in the age-1(mg305) background, which had a stronger effect on lifespan. Transgenic rescue of lifespan in age-1(mg44) animals was closer to that of wildtype. This analysis suggests that endocrine-like outputs of insulin signaling affect lifespan in a graded fashion from several different tissues.
Fig. 2.
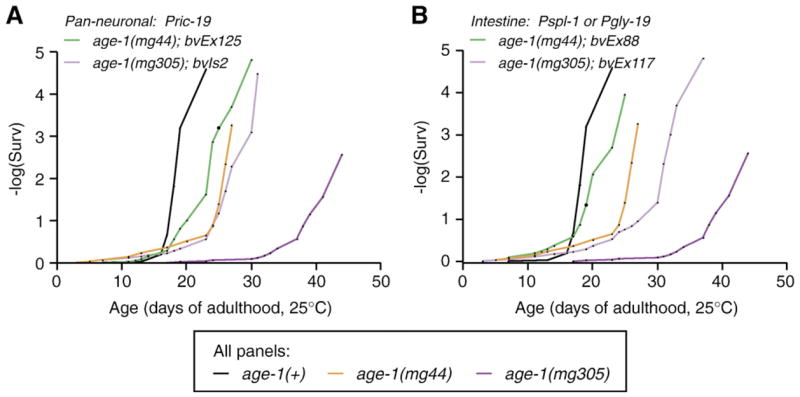
Exponential plot of survival data shown in Figs. 1A and B. Neuron- or intestine-restricted age-1 expression was correlated with mortality increases that were intermediate between wildtype and non-transgenic control animals.
age-1 can act in several types of neurons to promote wildtype lifespan and reproductive development
To determine whether specific neurons could regulate lifespan, or if insulin signaling were required throughout the nervous system, we used a similar transgenic approach to express age-1 in groups of neurons. The rescuing age-1 cDNA was restricted to subsets of neurons as follows: 32 motor neurons using the unc-4 promoter, 26 inhibitory motor neurons using the unc-25 promoter, or to 8–11 interneurons using the flp-1 promoter (McIntire et al., 1993; Miller et al., 1992; Nelson et al., 1998; White et al., 1992). Restricted age-1 expression to these subsets of neurons partially rescued lifespan age-1(−) animals (Figs. 1C, D, Table 4). Interneuron-restricted age-1 expression shortened lifespan in age-1(−) animals by 25–45%, but had a negligible effect on lifespan of age-1(+) animals (0–7% shorter). Motor-neuron-restricted age-1 expression also significantly shortened lifespan of age-1(mg44) animals (Punc-4: age-1, 39–60% shorter than controls; Punc-25: age-1, 18–56% shorter). Motor-neuron-restricted age-1 expression had weaker effects on age-1(mg305) lifespan (Punc-4:age-1, 11–18% shorter; Punc-25:age-1, 0–31% shorter). In control experiments with wildtype animals, the Punc-4: age-1 transgene shortened lifespan by 5–14%, although the Punc-25: age-1 transgene had negligible effects (0–4% shorter). These results suggest that insulin signaling in groups of neurons can contribute, at least partially, to normal lifespan. An exponential plot of the survival data confirmed that mortality increased at ages intermediate between wildtype and non-transgenic controls (not shown). This supports the hypothesis that insulin signaling in different groups of neurons can affect lifespan in a graded fashion.
Table 4.
Lifespan of animals with transgenic age-1 in groups of neurons
| Genotype | Mean (days) | Std error | (n) | Expt. | % Shortened | P vs control a |
|---|---|---|---|---|---|---|
| Interneurons | ||||||
| Pflp-1: age-1 | ||||||
| age-1(+); bvEx141 | 18.7 | 0.30 | 46 | 12 | +2 | 0.5305, 0.7701 |
| age-1(+); bvEx142 | 17.0 | 0.34 | 47 | 12 | 7 | 0.0028, 0.0017 |
| age-1(+); bvEx140 | 18.0 | 0.32 | 47 | 12 | 2 | 0.4283, 0.3017 |
| age-1(mg44); bvEx47 | 14.4 | 2.34 | 13 | 8 | 45 | <0.0001, <0.0001 |
| age-1(mg305); bvEx47 | 23.1 | 0.82 | 105 | 4 | 20 | 0.0006, 0.0050 |
| Motor neurons | ||||||
| Punc-4: age-1 | ||||||
| age-1(+); bvEx131 | 17.1 | 0.45 | 36 | 12 | 7 | 0.0243, 0.0106 |
| age-1(+); bvEx132 | 16.6 | 0.49 | 49 | 12 | 10 | 0.0077, 0.0017 |
| age-1(+); bvEx133 | 15.9 | 0.28 | 28 | 12 | 14 | <0.0001, <0.0001 |
| age-1(+); bvEx134 | 16.0 | 0.64 | 25 | 12 | 13 | <0.0001, <0.0001 |
| age-1(+); bvEx135 | 16.7 | 0.43 | 53 | 12 | 9 | 0.0084, 0.0026 |
| age-1(+); bvEx136 | 17.4 | 0.32 | 24 | 12 | 5 | 0.0242, 0.0246 |
| age-1(mg44); bvEx61 | 12.7 | 1.48 | 29 | 2 | 49 | <0.0001, <0.0001 |
| age-1(mg44); bvEx61 | 9.6 | 1.06 | 53 | 6 | 60 | <0.0001, <0.0001 |
| age-1(mg44); bvEx62 | 15.2 | 0.86 | 44 | 2 | 39 | <0.0001, <0.0001 |
| age-1(mg305); bvEx62 | 19.6 | 1.20 | 77 | 2 | 11 | 0.1209, 0.4104 |
| age-1(mg305); bvEx75 | 21.8 | 1.11 | 116 | 6 | 13 | 0.1071, 0.0474 |
| age-1(mg305); bvEx78 | 20.5 | 0.95 | 102 | 6 | 18 | <0.0001, 0.0021 |
| Punc-25: age-1 | ||||||
| age-1(+); bvEx137 | 18.2 | 0.25 | 58 | 12 | 1 | 0.5807, 0.4141 |
| age-1(+); bvEx138 | 17.7 | 0.28 | 40 | 12 | 4 | 0.0659, 0.0537 |
| age-1(+); bvEx139 | 18.4 | 0.32 | 42 | 12 | 0 | 0.9592, 0.7579 |
| age-1(mg44); bvEx67 | 20.6 | 1.33 | 15 | 2 | 18 | 0.0010, 0.0010 |
| age-1(mg44); bvEx68 | 16.7 | 1.11 | 42 | 2 | 33 | <0.0001, <0.0001 |
| age-1(mg44); bvEx69 | 12.7 | 0.87 | 56 | 2 | 49 | <0.0001, <0.0001 |
| age-1(mg44); bvEx70 | 11.1 | 0.85 | 34 | 2 | 56 | <0.0001, <0.0001 |
| age-1(mg305); bvEx67 | 15.1 | 0.99 | 24 | 2 | 31 | 0.0064, 0.1304 |
| age-1(mg305); bvEx67 | 24.0 | 1.35 | 70 | 6 | 4 | 0.1681, 0.3491 |
| age-1(mg305); bvEx68 | 21.3 | 2.36 | 12 | 2 | 3 | 0.8320, 0.9221 |
| age-1(mg305); bvEx68 | 22.8 | 1.07 | 112 | 6 | 9 | 0.1136, 0.1237 |
| age-1(mg305); bvEx69 | 23.7 | 1.72 | 27 | 2 | +8 | 0.7893, 0.9043 |
| age-1(mg305); bvEx69 | 21.4 | 1.39 | 94 | 6 | 15 | 0.8705, 0.0665 |
| age-1(mg305); bvEx70 | 21.6 | 1.14 | 95 | 6 | 14 | 0.0364, 0.0225 |
Log-Rank, Wilcoxan, versus non-transgenic control.
In addition to rescuing lifespan, each promoter tested provided sufficient age-1 activity to rescue the dauer arrest phenotype of age-1(mg44) and age-1(mg305) animals (Fig. 3, Table 5). These findings are consistent with earlier studies showing that daf-2, age-1 and daf-16 promoted reproductive development in an endocrine-like manner primarily from neurons (Apfeld and Kenyon, 1998; Libina et al., 2003; Wolkow et al., 2000). However, the level of rescue varied between transgenic lines. In some lines, such as bvEx122, reproductive development was fully restored. Full rescue was usually correlated with age-1 expression from the pan-neuronal ric-19 promoter. In other cases, such as bvEx47, dauer arrest was partially rescued and a majority of the transgenic larvae developed into sterile adults. Weak or partial rescue of dauer arrest was observed primarily when age-1 was expressed from subsets of neurons or in the intestine. Partial rescue of dauer arrest by age-1 expression in groups of neurons is also consistent with a graded effect of insulin signaling upon development, as observed for lifespan.
Fig. 3.
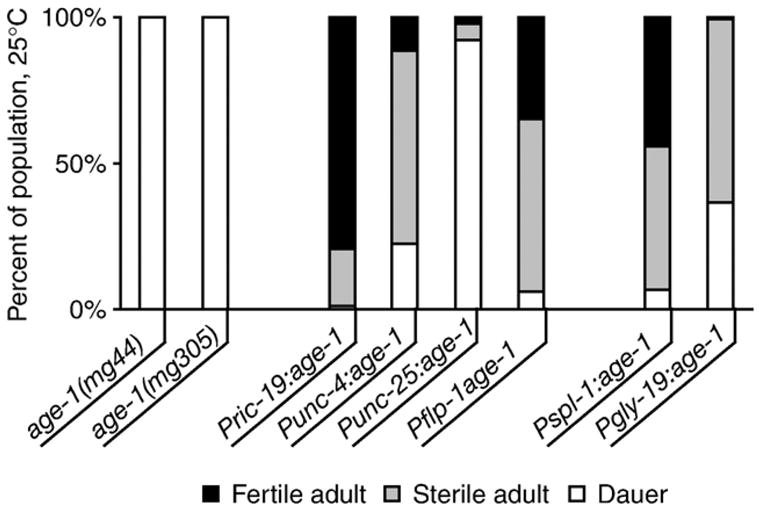
Rescue of dauer larval arrest by neuron- or intestine-specific age-1 expression. Rescue of dauer larval arrest was assayed at 25°C as the fraction of animals arrested as dauer larvae (white), or who had completed development to fertile (black) or sterile (grey) adults after 72 hours. Fertile and sterile adults were differentiated by the presence or absence of eggs in the uterus in fertile adults. Graph shows cumulative data for all independent lines from each transgene; 1–5 lines were tested for each transgene. Results for individual lines are presented in Table 5.
Table 5.
Developmental phenotype of age-1(−) animals with cell-type restricted age-1 expression
| Promoter | Parental genotype | Developmental phenotype (25°C)
|
Rescue development (Y/P/N)* | |||
|---|---|---|---|---|---|---|
| Dauers (%) | Adults (%)
|
n | ||||
| Fertile | Sterile | |||||
| age-1(mg44) m+z− | 100.0 | 0.0 | 0.0 | 133 | ||
| age-1(mg305) | 100.0 | 0.0 | 0.0 | 183 | ||
| ric-19 | mg44; bvEx120 | 1.3 | 79.6 | 19.1 | 152 | Y |
| mg44; bvEx122 | 0.0 | 93.4 | 6.6 | 198 | Y | |
| mg44; bvEx123 | 1.9 | 87.5 | 10.6 | 104 | Y | |
| mg44; bvEx125 | 0.9 | 50.5 | 48.6 | 111 | Y/P | |
| mg44; bvEx128 | 0.9 | 86.0 | 13.1 | 107 | Y | |
| unc-4 | mg305; bvEx78 | 22.4 | 11.4 | 66.2 | 210 | P |
| unc-25 | mg305; bvEx68 | 92.2 | 2.2 | 5.6 | 90 | N/P |
| flp-1 | mg44; bvEx47 | 10.1 | 9.6 | 80.3 | 208 | P |
| mg305; bvEx47 | 2.0 | 60.1 | 37.9 | 198 | Y | |
| spl-1 | mg44;bvEx87 | 0.0 | 28.6 | 71.4 | 28 | P |
| mg305;bvEx88 | 25.9 | 0.9 | 73.2 | 112 | P | |
| mg44; bvEx90 | 0.0 | 75.0 | 25.0 | 52 | Y | |
| mg305;bvEx90 | 1.3 | 72.7 | 26.0 | 231 | Y | |
| gly-19 | mg305;bvEx117 | 36.6 | 0.7 | 62.7 | 279 | P |
Rescue: Yes/Partial/None.
daf-16 may act in combinations of tissues to incrementally increase lifespan
The FOXO transcription factor, DAF-16, is the ultimate output of signaling by DAF-2 and AGE-1 and is required for extended lifespan in animals with defective DAF-2 pathway signaling (Ogg et al., 1997). Previous analysis showed that daf-16 activity in the intestine was necessary to prolong lifespan, while neuron-restricted daf-16 activity promoted dauer arrest but had no effect on lifespan (Libina et al., 2003). Given the endocrine-like effect of age-1 on lifespan, we asked whether daf-16 function in multiple tissues could have additive effects on lifespan. If this were the case, then lifespan could reflect the regulation of daf-16 through a combination of endocrine and tissue-restricted outputs of insulin.
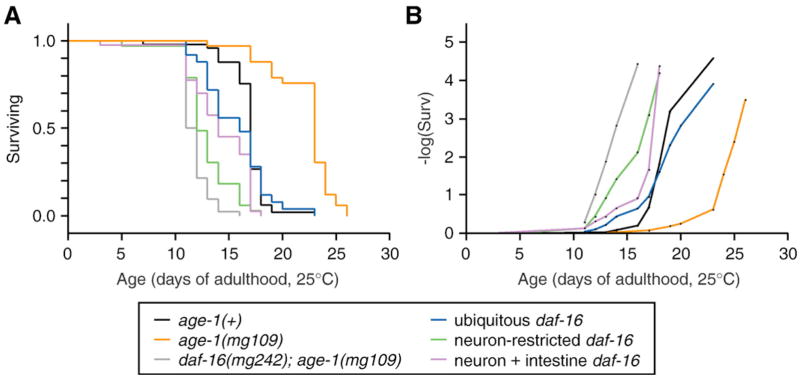
We constructed a GFP-tagged daf-16 cDNA construct identical to that used previously, which was expressed either from the native daf-16a promoter, the ubiquitous dpy-30 promoter, the neuronal ric-19 promoter or the intestinal gly-19 or spl-1 promoters. We then examined whether transgenic daf-16 expression in these tissues could rescue the dauer-defective and shortened lifespan phenotypes in a daf-16(mg242); age-1(mg109) double mutant background. Expression of gfp:daf-16 from either the ubiquitous dpy-30 promoter or the endogenous daf-16a promoter weakly rescued dauer arrest in daf-16(mg242); age-1(mg109) animals, although these transgenes were associated with high rates of embryonic lethality. In particular, 87% of transgenic progeny died as embryos, 6% arrested as dauer or dauer-like larvae and the remaining 7% grew to adulthood, among the first generation progeny of hermaphrodites injected with the Pdpy-30:gfp:daf-16 construct (not shown). Due to the high level of embryonic lethality, we were only able to isolate one line which could transmit the Pdpy-30:gfp:daf-16 transgene. Dauer arrest was not observed in this line, but lifespan was lengthened by 25% (not shown, Fig. 4A, Table 6).
Fig. 4.
Lifespan in animals with tissue-restricted daf-16 expression. (A) Survival plot or (B) exponential plot of survival data for wildtype (black) or daf-16(mg242); age-1(mg109) (grey) fertile adults at 25°C. Transgenic daf-16 activity was provided by expressing gfpdaf-16 in the daf-16(mg242); age-1(mg109) background, as follows: ubiquitous gfpdaf-16(Pdpy-30:gfpdaf-16, blue), neuron-specific gfpdaf-16(Pric-19:gfpdaf-16, green), or gfpdaf-16 in both neurons and intestine (Pgly-19: gfpdaf-16 + Pric-19:gfpdaf-16, purple). Results from one representative trial are presented. Complete lifespan statistics are presented in Table 6.
Table 6.
Adult lifespan of animals with tissue-restricted transgenic expression of gfp:daf-16
| Genotype | Mean (days) | Std error | (n) | Expt. | % Increase | P vs control a |
|---|---|---|---|---|---|---|
| daf-16(+);age-1(+) | 15.3 | 0.74 | 38 | 1 | 31 | |
| daf-16(+);age-1(+) | 19.2 | 0.48 | 85 | 2 | 41 | |
| daf-16(+);age-1(+) | 16.8 | 0.30 | 49 | 3 | 29 | |
| daf-16(+);age-1(+) | 19.7 | 1.12 | 36 | 4b | 25 | |
| age-1(mg109) m+z− | 22.2 | 0.51 | 33 | 3 | 46 | |
| age-1(mg109) m+z− | 35.5 | 1.86 | 23 | 4b | 59 | |
| daf-16(mg242); age-1(mg109) | 10.6 | 0.31 | 42 | 1 | ||
| daf-16(mg242); age-1(mg109) | 11.3 | 0.67 | 21 | 2 | ||
| daf-16(mg242); age-1(mg109) | 11.9 | 0.18 | 42 | 3 | ||
| daf-16(mg242); age-1(mg109) | 14.7 | 0.19 | 61 | 4b | ||
| Ubiquitous daf-16 (Pdpy-30:daf-16) | ||||||
| daf-16(−);age-1(−); bvEx130 | 15.7 | 0.59 | 25 | 3 | 25 | <0.0001, <0.0001 |
| daf-16(−);age-1(−); bvEx130 | 19.5 | 0.90 | 29 | 4b | 25 | <0.0001, <0.0001 |
| Neuronal daf-16 (Pric-19:daf-16) | ||||||
| daf-16(−);age-1(−); bvEx94 | 13.0 | 0.41 | 46 | 1 | 18 | <0.0001, <0.0001 |
| daf-16(−);age-1(−); bvEx94 | 12.8 | 0.41 | 33 | 3 | 7 | 0.0023, 0.0037 |
| daf-16(−);age-1(−); bvEx94 | 16.0 | 0.71 | 28 | 4b | 8 | 0.0043, 0.0883 |
| daf-16(−);age-1(−); bvEx92 | 11.1 | 0.32 | 35 | 1 | 5 | 0.2902, 0.2660 |
| daf-16(−);age-1(−); bvEx93 | 12.8 | 0.36 | 46 | 1 | 17 | <0.0001, <0.0001 |
| daf-16(−);age-1(−); bvEx96 | 11.3 | 0.48 | 40 | 1 | 6 | 0.0578, 0.2336 |
| daf-16(−);age-1(−); bvEx98 | 11.8 | 0.50 | 24 | 1 | 10 | 0.0744, 0.0499 |
| Intestinal daf-16 (Pgly-19:daf-16, Pspl-1:daf-16) | ||||||
| daf-16(+); bvEx103 | 18.5 | 0.36 | 76 | 2 | −4 | 0.0160, 0.0256 |
| daf-16(+); bvEx105 | 19.3 | 0.43 | 79 | 2 | 0 | 0.6309, 0.5742 |
| daf-16(−);age-1(−); bvEx#13 | 11.9 | 0.88 | 7 | 1 | 11 | 0.1244, 0.2050 |
| daf-16(−);age-1(−); bvEx#P1 | 12.3 | 0.65 | 19 | 1 | 14 | 0.0278, 0.0300 |
| Neuronal + intestinal daf-16(Pric-19: daf-16 + Pgly-19:daf-16) | ||||||
| daf-16(−);age-1(−); bvEx113 | 14.5 | 0.45 | 62 | 2 | 22 | <0.0001, <0.0001 |
| daf-16(−);age-1(−); bvEx113 | 14.6 | 0.46 | 39 | 3 | 18 | <0.0001, <0.0001 |
| daf-16(−);age-1(−); bvEx113 | 18.0 | 0.61 | 53 | 4b | 18 | <0.0001, <0.0001 |
| daf-16(−);age-1(−); bvEx114 | 14.9 | 0.61 | 31 | 2 | 24 | <0.0001, <0.0001 |
| daf-16(−);age-1(−); bvEx114 | 14.1 | 0.48 | 40 | 3 | 16 | <0.0001, <0.0001 |
| daf-16(−);age-1(−); bvEx114 | 19.3 | 0.98 | 28 | 4b | 24 | <0.0001, <0.0001 |
Log-Rank, Wilcoxan, versus non-transgenic control.
Experiment 4 was performed at 20°C; Expts. 1–3 were performed at 25°C.
Expression of gfpdaf-16 from either the neuronal ric-19 or the intestinal gly-19 or spl-1 promoters in daf-16(mg242); age-1(mg109) animals did not cause embryonic lethality or dauer arrest (not shown). In addition, neuronal or intestinal daf-16 expression had weak effects on lifespan (Pric-19: gfpdaf-16: 8–18% increased lifespan; Pgly-19:gfpdaf-16 0–14% increased lifespan, Fig. 4A, Table 6). We also examined the effect of expressing daf-16 in both neurons and intestine together. Co-transformation of daf-16(mg242); age-1(mg109) animals with both the Pric-19:gfp:daf-16 and Pgly-19:gfp:daf-16 transgenes also did not affect dauer arrest, but did lengthen lifespan more than daf-16 expression in either tissue alone (Fig. 4A, Table 6). An exponential plot of the survival data for transgenic daf-16 expression suggests an incremental effect of increased daf-16 expression on lifespan, with the least lifespan-lengthening activity in animals with only neuronal daf-16, slightly more in animals with neuron + intestine daf-16 and the greatest in animals with ubiquitous daf-16 activity (Fig. 4B).
Insulin signaling affects a cellular response to fasting
The transgenic studies suggested that insulin signaling has graded effects on development and lifespan, possibly through endocrine-like outputs. We therefore wished to investigate whether insulin also had cell-intrinsic outputs. To address this question, we developed an assay for monitoring fasting response at the cellular level in the intact organism. Esterase enzymes are highly abundant in the C. elegans intestine where they function to assist digestion and detoxification of injested material. In situ staining for esterase activity in wild type young adult animals revealed cytoplasmic distribution of esterase activity in the intestines of well-fed animals (Fig. 5A). We found that fasting caused a dramatic alteration in the subcellular distribution of esterase activity. When wild-type young adult animals were kept without food for six hours, intestinal esterase activity redistributed from the cytoplasm to the nucleus (Fig. 5B). We refer to this as the FIRE response (Fasting-Induced Redistribution of Esterase activity). Insulin signaling was required for a normal FIRE response to fasting. When daf-2(e1370) or age-1(mg305) animals were fasted for 6 hours, intestinal esterase activity remained cytoplasmic. (Figs. 5C, D). The altered FIRE response in insulin pathway mutants required daf-16 activity, as daf-16(mgDf50); age-1(mg305) animals displayed the wildtype FIRE response to fasting (Fig. 5E). The basis for the defective FIRE response to fasting in insulin pathway mutant strains may be due to altered metabolism in these animals, which have been shown to accumulate high levels of fat and may be hypometabolic (Halaschek-Wiener et al., 2005; Holt and Riddle, 2003; Kimura et al., 1997).
Fig. 5.
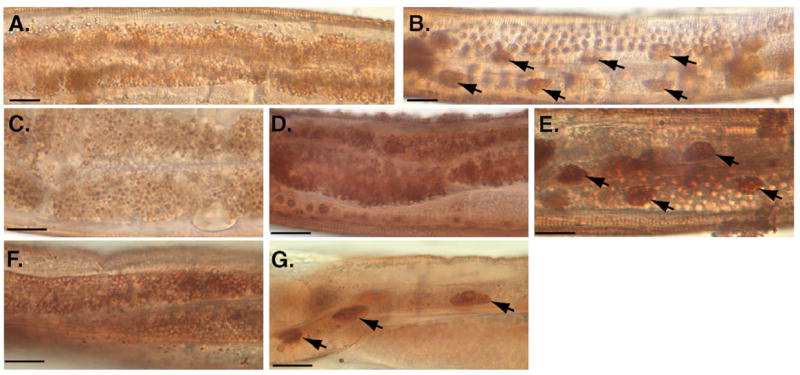
Altered FIRE response to fasting in insulin pathway mutants. (A) In situ detection of cytoplasmic esterase activity in intestinal cells of well-fed young adult animals. (B) Intestinal esterase activity in a young adult after 6-hour fast displayed FIRE response by redistribution of esterase activity to intestinal nuclei (arrows). (C–E), Insulin signaling modulates the FIRE response to fasting. Intestinal esterase activity remained cytoplasmic in (C) daf-2(e1370) or (D) age-1(mg305) young adult animals after 6-hour fast. (E) daf-16 was required for fasting resistance of daf-2 pathway mutants; the wildtype nuclear pattern for esterase localization was restored in daf-16(mgDf50); age-1(mg305) young adults after fasting. (F) Pan-neuronal age-1 expression did not rescue altered FIRE response of age-1(mg44) animals (age-1(mg44); bvEx123); (G) Intestine-specific age-1 expression rescued FIRE response, (age-1(mg44); bvEx87). Scale bars, 20 nm. Complete data for FIRE response phenotype is presented in Table 7.
We next asked whether the endocrine-like outputs of insulin regulated the FIRE response by examining this response in the transgenic strains with tissue-restricted age-1 and daf-16 expression. In most cases, neuron-restricted age-1 expression did not rescue the altered FIRE response. In particular, age-1 expression from the pan-neuronal ric-19 or the motorneuron-specific unc-25 promoters failed to rescue the altered FIRE response of age-1(−) animals, although these transgenes could rescue lifespan and dauer arrest (Figs. 5F, 6, Table 7). Intestinal age-1 expression from the spl-1 promoter restored the normal FIRE response in about 40% of transgenic animals, indicating partial rescue of this phenotype (Fig. 5G, Table 7). Consistent results were obtained for daf-16. Ubiquitous or intestinal daf-16 expression had the strongest effect on promoting an altered FIRE response in daf-16(mg242); age-1(mg109) animals (Table 7). Neuron-restricted daf-16 expression had a much weaker effect on this response. The observation that the FIRE response was better restored by intestinal insulin signaling than by neuronal insulin signaling suggests that this response reflects an intestine-intrinsic insulin output.
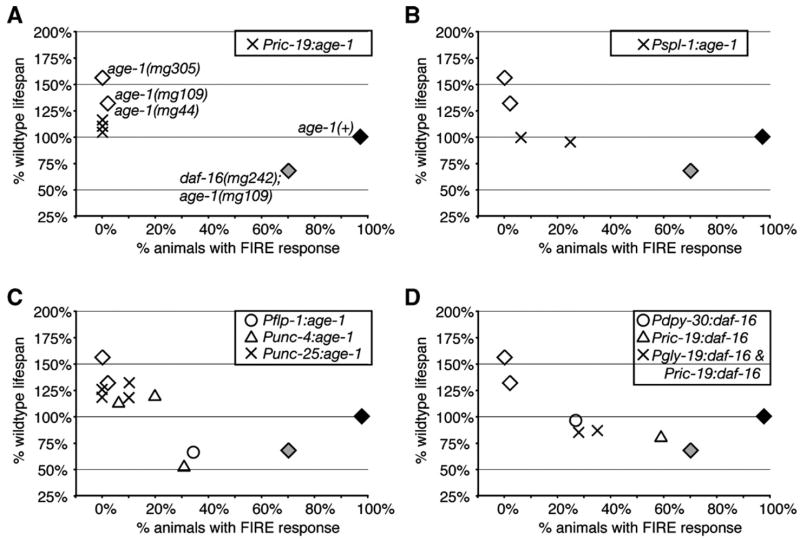
Fig. 6.
Effects of tissue-specific age-1 expression on lifespan and FIRE response. Adult lifespan and FIRE response were plotted to examine the correlation between these phenotypes. In all panels, black diamond, wildtype; white diamonds are age-1(lf) alleles, as indicated in (A); grey diamond, daf-16(mg242); age-1(mg109). For age-1(mg44) and age-1(mg109), fertile adults were age-1(mg44/mg44) progency of age-1(mg44/+) hermaphrodites. Crosses, circles and triangle shapes represent tissue-specific age-1 or daf-16 expression, as indicated.
Table 7.
FIRE response in animals with tissue-restricted age-1 and gfpdaf-16 expression
| Genotype | Intestinal esterase distribution (6 hour fast)
|
(n) | Rescue (Y/P/N) | ||
|---|---|---|---|---|---|
| Nuclear | Intermediate a | Cytoplasm | |||
| age-1(+) | 100% | 0% | 0% | 44 | |
| age-1(mg44) m+z− | 3% | 41% | 56% | 24 | |
| age-1(mg305) | 0% | 6% | 94% | 18 | |
| age-1(mg109) m+z− | 2% | 30% | 68% | 59 | |
| daf-16(mg242); age-1(m109) | 70% | 21% | 9% | 70 | |
| Pric-19: age-1 | |||||
| mg44; bvEx12 | 0% | 0% | 100% | 11 | N |
| mg44; bvIs2 | 0% | 18% | 82% | 11 | N |
| mg44; bvEx123 | 0% | 12% | 88% | 26 | N |
| mg44; bvEx121 | 0% | 7% | 93% | 15 | N |
| mg44; bvEx125 | 0% | 60% | 40% | 15 | P |
| mg44; bvEx128 | 0% | 8% | 92% | 12 | N |
| Pflp-1: age-1 | |||||
| mg44;bvEx47 | 34% | 9% | 56% | 32 | P |
| Punc-4: age-1 | |||||
| mg44;bvEx61 | 31% | 31% | 38% | 13 | P |
| mg305;bvEx75 | 20% | 30% | 50% | 10 | P |
| mg305;bvEx78 | 6% | 13% | 81% | 16 | N |
| Punc-25: age-1 | |||||
| mg305; bvEx67 | 10% | 10% | 80% | 10 | N |
| mg305; bvEx68 | 0% | 6% | 94% | 16 | N |
| mg305; bvEx69 | 10% | 50% | 40% | 10 | P |
| mg305; bvEx70 | 0% | 80% | 20% | 5 | P |
| Pspl-1: age-1 | |||||
| mg44;bvEx87 | 25% | 38% | 38% | 8 | P |
| mg44; bvEx88 | 25% | 50% | 25% | 4 | P |
| mg44; bvEx90 | 6% | 19% | 75% | 16 | P |
| Pdpy-30:daf-16 | |||||
| daf-16(−); age-1(−); bvEx130 | 27% | 73% | 0% | 15 | P |
| Pric-19:daf-16 | |||||
| daf-16(−); age-1(−); bvEx94 | 59% | 41% | 0% | 17 | N |
| Pric-19:daf-16 + Pgly-19:daf-16 | |||||
| daf-16(−); age-1(−); bvEx113 | 28% | 72% | 0% | 18 | P |
| daf-16(−); age-1(−); bvEx114 | 35% | 60% | 4% | 23 | P |
Both nuclear and cytoplasmic esterase activity detected throughout intestine.
Discussion
Insulin signaling in C. elegans controls dauer developmental arrest, lifespan and stress resistance. Here, we extended previous observations by showing that insulin signaling in groups of neurons could promote wildtype lifespan and rescue dauer arrest. These findings suggest equivalency throughout the nervous system for endocrine-like outputs of insulin that regulate these phenotypes. Animals with insulin signaling restored to small groups of cells appeared to have generally weaker rescue of these phenotypes than when insulin signaling were restored to larger groups of cells. A similar result was observed from analysis of daf-2 mosaic animals (Apfeld and Kenyon, 1998). This may reflect a quorum or graded effect of insulin in which insulin signaling within a threshhold number of cells is necessary for reproductive development and wildtype lifespan.
We also examined the effect of insulin on a response to fasting, the FIRE response, within individual intestinal cells. To our knowledge, this is the first study to examine the effects of C. elegans insulin signaling at a cellular level. These studies demonstrated that the lifespan and developmental phenotypes of age-1 mutants could be separated from the altered FIRE response within intestinal cells. These results suggest a model of insulin signaling where endocrine-like outputs of insulin coordinate lifespan and dauer developmental arrest, while cell-intrinsic outputs of insulin affect cellular responses, such as the FIRE response (Fig. 7).
Fig. 7.
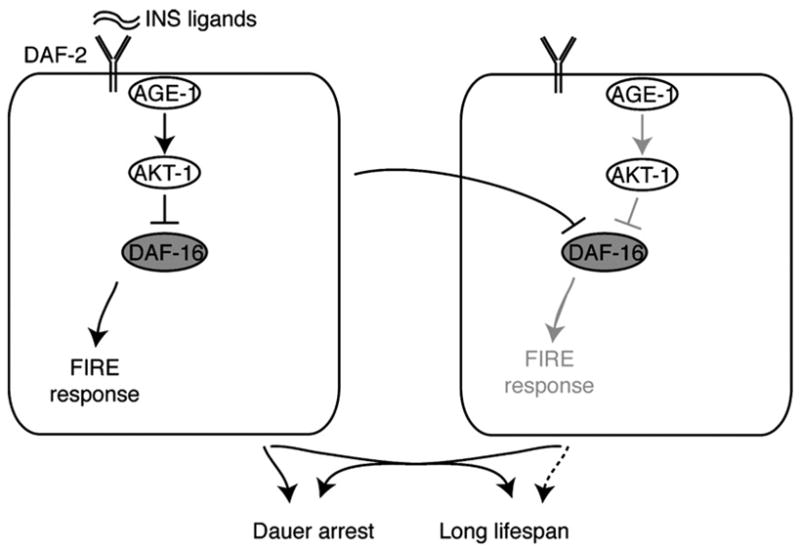
A model for tissue-intrinsic and endocrine effects of insulin. In this model, insulin signaling within one cell (left) activates several daf-16-dependent outputs, including diffusible signals that may impinge on daf-16 activity in distant cells where insulin signaling is inactive (right cell). Insulin regulation of the FIRE response appears to occur preferentially via cell-autonomous pathways. The diffusible outputs of insulin can affect lifespan without necessarily affecting cell-intrinsic outputs in distant cells.
DAF-16/FOXO is the major effector of insulin signaling in C. elegans and is required for dauer formation, stress resistance and long lifespan in insulin-defective mutants (Kenyon et al, 1993; Lin et al, 1997; Ogg et al, 1997). Evidence that AKT directly phosphorylates FOXO proteins suggests that DAF-16 must function in the same cells as AKT-1, and, by extension, AGE-1 and DAF-2 (Brunet et al., 1999; Paradis and Ruvkun, 1998). Previous analysis of daf-16 sites of function revealed that intestine-specific daf-16 expression could increase lifespan of daf-16(mu86); daf-2(e1370) animals (Libina et al., 2003). In contrast, neuron-specific daf-16 expression did not affect lifespan, but did promote dauer arrest. Additive effects of daf-16 activity in these tissues were not investigated. In the present study, we found that intestine-specific or neuron-specific daf-16 expression did not restore long lifespan to daf-16(mg242); age-1(mg109) animals, although daf-16 expression in both tissues did increase lifespan, suggesting that lifespan may also be affected in an incremental fashion by DAF-16 activity in multiple tissues.
However, we note that the daf-16 transgenes generated for our study provided lower levels of daf-16 activity than previous reported (Libina et al., 2003). For example, Pdpy-30:gfp:daf-16 only increased daf-16(mg242); age-1(mg109) lifespan by 25%, although full rescue should have increased lifespan by 46–59% (Table 6). In addition, the previous study reported greater effects of intestinal daf-16 activity on lifespan (Libina et al., 2003). One difference between these studies is the genetic backgound used for the rescue analysis. We examined the effect of daf-16 expression in daf-16(mg242); age-1(mg109) animals, while the previous study examined the daf-16(mu86); daf-2(e1370) background. The daf-16(mg242) allele is a nonsense mutation at amino acid 220 in the DAF-16 DNA binding domain that also affects both the DAF-16a and b forms (Gami and Wolkow, in preparation). The daf-16(mu86) allele is a large, internal deletion in the daf-16 gene which could provide partial daf-16 activity as a result of aberrant splicing. Residual daf-16 activity in the daf-16(mu86) background could complicate interpretation of transgenic activity. Alternatively, there could be AGE-1-independent effectors of DAF-2 signaling that may be active in the age-1(mg109) background, but inactive in the daf-2(e1370) background. In this scenario, the activity of transgenic DAF-16 may be limited by residual DAF-2 signaling, through such AGE-1-independent pathways. Further analysis of daf-16 function and regulation is needed to fully resolve these issues.
FIRE: An assay for monitoring insulin signaling at the cellular level in C. elegans
This work describes a novel assay for measuring cellular effects of insulin signaling by monitoring an intestinal cellular response to fasting. In well-fed animals, gut esterase activity was detectable throughout the intestinal cell cytoplasm, but short-term fasting caused esterase activity to redistribute to the nucleus, which we refer to as the FIRE response. In wildtype animals, the FIRE response was also observed after exposure to high temperature (35°) or to the free-radical generator, paraquat, indicating that the FIRE response may also be regulated by cellular stress (W. Iser and C. Wolkow, unpublished observations). The mechanistic basis for the FIRE response has not yet been determined. One possibility is that this response reflects fasting- and stress-induced alterations in cellular trafficking pathways.
The effects of tissue-restricted insulin signaling on stress resistance have not been investigated to date. Analysis of stress resistance has traditionally been measured by whole-organism survival using assays similar in design to lifespan assays (Larsen, 1993; Lithgow et al., 1995). Many of the genes required for stress resistance in daf-2 mutants are also required for long lifespan (Hsu et al., 2003; Morley and Morimoto, 2004; Walker and Lithgow, 2003). It was therefore expected that stress resistance and lifespan would be linked. Interestingly, our analysis indicates that the FIRE response may be separable from other insulin outputs. For example, neuron-restricted age-1 activity could promote wildtype development and lifespan, but did not affect the altered FIRE response of the age-1(−) background.
We found that the FIRE response was partially affected by age-1 expression from some neuron-restricted promoters, such as flp-1 and unc-4 (Table 7). This could reflect promiscuous intestinal expression of these promoters or weak regulation of the FIRE response by the endocrine-like outputs of neuronal insulin signaling. To address this issue, we investigated whether there was an obvious correlation between lifespan and FIRE among individual age-1 and daf-16 transgenic lines (Fig. 6, Table 7). First, we found that the Pric-19:age-1 and Pric-19:gfpdaf-16 transgenes did not strongly affect FIRE, although Pric-19:age-1 rescued long lifespan of age-1 (−) animals (Figs. 6A, D). This suggests that the Pric-19 promoter is specifically expressed in the nervous system, and is consistent with the hypothesis that FIRE is an intestine-specific phenotype. Second, the strongest effects on FIRE were observed in lines with intestinal age-1 or daf-16 expression, as well as lines with Punc-4:age-1 and Pflp-1: age-1 (Figs. 6 B, C, D). This suggests that the unc-4 and flp-1 promoters may also be intestinally expressed. We note that the Punc-4:age-1 and Pflp-1:age-1 lines with the strongest effect on FIRE also significantly shortened lifespan, which was not observed in Pspl-1:age-1 lines. Although the basis for shortened lifespan in Punc-4:age-1 and Pflp-1:age-1 lines is not yet known, it appears to correlate with strong effects on FIRE.
Lifespan may result from incorporation of cell-autonomous and endocrine-like outputs of insulin signaling
Our transgenic experiments support the existence of a non-cell autonomous, endocrine-like pathway through which insulin promotes reproductive development and wildtype lifespan. Furthermore, our studies show that this endocrine-like effect can arise from insulin signaling in a number of different tissues and has a graded effect on lifespan and development. However, we found that tissue-restricted daf-16 expression did not affect lifespan as strongly as daf-16 expression in multiple tissues. This may reflect a requirement for daf-16 activity in most or all tissues in order for insulin signaling decrements to extend lifespan. One model to accommodate this data may be that insulin signaling regulates the production of diffusible factors that impinge on DAF-16 activity throughout the body. Consistent with this idea, DAF-16 nuclear localization was affected non-cell autonomously by DAF-18/PTEN activity, suggesting that insulin signaling can affect DAF-16 activity in distant cells (Masse et al., 2005).
One pathway for the non-cell autonomous regulation of DAF-16 could be feed-back regulation of insulin (ins) production or secretion. Insulin signaling has been shown to regulate expression of at least one insulin-like ligand, ins-7 (Murphy et al., 2003). However, such feedback regulation of insulin ligands cannot explain how neuron-restricted insulin signaling could rescue lifespan when non-neuronal tissues lacked a functional insulin signaling pathway. Therefore, we favor an alternative model in which DAF-2 signaling can regulate DAF-16 in the same cells, via AKT phosphorylation, and in distant cells, through a diffusible signal. Indeed, several pathways can regulate DAF-16 independently of DAF-2, including Jun N-terminal kinase JNK-1, beta-catenin BAR-1 and the heat-shock transcription factor HSF-1 (Essers et al., 2005; Hsu et al., 2003; Oh et al., 2005). Interactions between these proteins and insulin signaling could provide pathways for coordinating DAF-16 activity throughout the body in response to insulin signaling in a subset of cells.
Supplementary Material
Acknowledgments
We thank the C. elegans Genetics Center for providing strains, Dr. Y. Kohara (National Institute of Genetics, Japan) for cDNA clones and Dr. A. Fire (Stanford University, Palo Alto, CA) for pPD95.75 and pPD95.02. We are grateful to the Wolkow lab, Gary Ruvkun and NIA colleagues for helpful discussions. This work was supported by the National Institute on Aging Intramural Research Program and by a New Scholar in Aging Award from the Ellison Medical Foundation (C.A.W.).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2006.04.467.
References
- Apfeld J, Kenyon C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell. 1998;95:199–210. doi: 10.1016/s0092-8674(00)81751-1. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch B, Antebi A. Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development. 2004;131:1765–1776. doi: 10.1242/dev.01068. [DOI] [PubMed] [Google Scholar]
- Halaschek-Wiener J, Khattra JS, McKay S, Pouzyrev A, Stott JM, Yang GS, Holt RA, Jones SJ, Marra MA, Brooks-Wilson AR, Riddle DL. Analysis of long-lived C. elegans daf-2 mutants using serial analysis of gene expression. Genome Res. 2005;15:603–615. doi: 10.1101/gr.3274805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Hertweck M, Gobel C, Baumeister R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell. 2004;6:577–588. doi: 10.1016/s1534-5807(04)00095-4. [DOI] [PubMed] [Google Scholar]
- Holt SJ, Riddle DL. SAGE surveys C. elegans carbohydrate metabolism: evidence for an anaerobic shift in the long-lived dauer larva. Mech Ageing Dev. 2003;124:779–800. doi: 10.1016/s0047-6374(03)00132-5. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy C, Kenyon C. Regulation of aging and age-related disease by DAF-16 and Heat-Shock Factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Johnson T. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990;249:908–912. doi: 10.1126/science.2392681. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Mitchell DH, Kline S, Kemal R, Foy J. Arresting development arrests aging in the nematode Caenorhabditis elegans. Mech Ageing Dev. 1984;28:23–40. doi: 10.1016/0047-6374(84)90150-7. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ, Roots L. A “Direct-Coloring” thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-I and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Lithgow G, White T, Melov S, Johnson T. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse I, Molin L, Billaud M, Solari F. Lifespan and dauer regulation by tissue-specific activities of Caenorhabditis elegans DAF-18. Dev Biol. 2005;286:91–101. doi: 10.1016/j.ydbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Horvitz HR. Genes required for GABA function in Caenorhabditis elegans. Nature. 1993;364:334–337. doi: 10.1038/364334a0. [DOI] [PubMed] [Google Scholar]
- Mendel J, Heinecke K, Fyrst H, Saba JD. Sphingosine phosphate lyase expression is essential for normal development in Caenorhabditis elegans. J Biol Chem. 2003;278:22341–22349. doi: 10.1074/jbc.M302857200. [DOI] [PubMed] [Google Scholar]
- Miller DM, Shen MM, Shamu CE, Burglin TR, Ruvkun G, Dubois ML, Ghee M, Wilson L. C. elegans unc-4 gene encodes a homeodomain protein that determines the pattern of synaptic input to specific motor neurons. Nature. 1992;355:841–845. doi: 10.1038/355841a0. [DOI] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Murphy C, McCarroll S, Bargmann C, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–284. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nanji M, Hopper NA, Gems D. LET-60 RAS modulates effects of insulin/IGF-1 signaling on development and aging in Caenorhabditis elegans. Aging Cell. 2005;4:235–245. doi: 10.1111/j.1474-9726.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- Nelson LS, Rosoff ML, Li C. Disruption of a neuropeptide gene, flp-1, causes multiple behavioral defects in Caenorhabditis elegans. Science. 1998;281:1686–1690. doi: 10.1126/science.281.5383.1686. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 1999;13:1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon M, Peng XR, Spence AM, Plasterk RH, Dosch HM. The diabetes autoantigen ICA69 and its Caenorhabditis elegans homologue, ric-19, are conserved regulators of neuroendocrine secretion. Mol Biol Cell. 2000;11:3277–3288. doi: 10.1091/mbc.11.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Ruvkun G. Regulation of Caenorhabditis elegans RNA interference by the daf-2 insulin stress and longevity signaling pathway. Cold Spring Harb Symp Quant Biol. 2004;69:429–431. doi: 10.1101/sqb.2004.69.429. [DOI] [PubMed] [Google Scholar]
- Warren CE, Krizus A, Dennis JW. Complementary expression patterns of six nonessential Caenorhabditis elegans core 2/I N-acetylglucosaminyltransferase homologues. Glycobiology. 2001;11:979–988. doi: 10.1093/glycob/11.11.979. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN. Mutations in the Caenorhabditis elegans unc-4 gene alter the synaptic input to ventral cord motor neurons. Nature. 1992;355:838–841. doi: 10.1038/355838a0. [DOI] [PubMed] [Google Scholar]
- Wolkow CA, Kimura K, Lee M, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.