Abstract
The chromophore of photoactive yellow protein (PYP) (i.e., 4-hydroxycinnamic acid) has been replaced by an analogue with a triple bond, rather than a double bond (by using 4-hydroxyphenylpropiolic acid in the reconstitution, yielding hybrid I) and by a “locked” chromophore (through reconstitution with 7-hydroxycoumarin-3-carboxylic acid, in which a covalent bridge is present across the vinyl bond, resulting in hybrid II). These hybrids absorb maximally at 464 and 443 nm, respectively, which indicates that in both hybrids the deprotonated chromophore does fit into the chromophore-binding pocket. Because the triple bond cannot undergo cis/trans (or E/Z) photoisomerization and because of the presence of the lock across the vinyl double bond in hybrid II, it was predicted that these two hybrids would not be able to photocycle. Surprisingly, both are able. We have demonstrated this ability by making use of transient absorption, low-temperature absorption, and Fourier-transform infrared (FTIR) spectroscopy. Both hybrids, upon photoexcitation, display authentic photocycle signals in terms of a red-shifted intermediate; hybrid I, in addition, goes through a blue-shifted-like intermediate state, with very slow kinetics. We interpret these results as further evidence that rotation of the carbonyl group of the thioester-linked chromophore of PYP, proposed in a previous FTIR study and visualized in recent time-resolved x-ray diffraction experiments, is of critical importance for photoactivation of PYP.
Keywords: 4-hydroxyphenylpropiolic acid, 7-hydroxycoumarin-3-carboxylic acid, low-temperature spectroscopy, Fourier-transform infrared spectroscopy, locked chromophore
The primary importance of light-induced cis/trans (or E/Z) isomerization of the chromophore of biological photoreceptors, such as rhodopsins, phytochromes, and xanthopsins, for the initiation of signal transduction is generally accepted, although this issue has long been controversial (1–4), almost since the discovery of the molecular basis of vision by Wald in 1968 (5). As an alternative for the now well-accepted concept of light-induced cis/trans isomerization, mechanisms have been proposed, such as light-induced proton transfer (2), that subsequently were largely rejected.
The importance of cis/trans isomerization has been studied not only with several forms of transient spectroscopy but also in experiments in which the retinal chromophore of (bacterio)rhodopsin was replaced through reconstitution by an analogue, equipped with a covalent “bridge” (i.e., forming a five- to eight-membered ring across the double bond), which was anticipated to prevent isomerization (6, 7). Following this approach for bacteriorhodopsin (Brh), Delaney et al. (8) reported that a six-membered ring across C=C(9,10) or C=C(11,12) of retinal does allow formation of the intermediates J and K, albeit with slower kinetics than in Brh containing unmodified retinal. However, even a five-membered ring across C=C(13,14) blocks this primary photochemistry only partially (4). Very recently, it was reported that, by using atomic force microscopy, conformational changes can be detected in the microsecond time domain in Brh hybrids, reconstituted with a modified chromophore that would not be expected to allow primary photochemistry (9). This observation led these authors to conclude that “our data question the current working hypothesis which attributes all primary events in retinal proteins to an initial trans-cis isomerization.”
The interpretation of these experiments, however, is complicated because isomerization across one of the double bonds of retinal, neighboring the one that is locked or modified, may allow for alternative isomerization pathways in the rhodopsins containing a retinal analogue. We therefore have addressed the point of the importance of chromophore isomerization for photoreceptor activation by studying the photoactive yellow protein (PYP) (10). This is a photoreceptor from the purple bacterium Ectothiorhodospira halophila (11), which shows many similarities with the archaeal sensory rhodopsins (12, 13), although PYP contains 4-hydroxycinnamic acid as its chromophore (14, 15) and is water soluble. Activation of PYP function is supposed to proceed through light-induced cis/trans isomerization of the 7,8-vinyl bond of its chromophore (16, 17). The apo form of this photoreceptor can be produced heterologously in Escherichia coli (18), and then can be converted to functional holoprotein through reconstitution with the endogenous chromophore (19) or with analogues, resulting in the formation of hybrids (20).
Here we report reconstitution of PYP with chromophores in which (i) the vinyl double bond of 4-hydroxycinnamic acid is replaced by a triple bond (by using 4-hydroxyphenylpropiolic acid in the reconstitution; the resulting holoprotein is referred to below as hybrid I) or (ii) the vinyl double bond of the chromophore is locked against isomerization by the presence of a covalent “bridge” over the vinyl bond (by using 7-hydroxycoumarin-3-carboxylic acid in the reconstitution; the resulting holoprotein is referred to below as hybrid II). The results obtained show that in both hybrids authentic photocycle signals can be observed. These experiments therefore lead to the conclusion that isomerization across the double bond of the chromophore of the PYP photoreceptor is not a strict prerequisite for photoactivation of PYP.
MATERIALS AND METHODS
Materials.
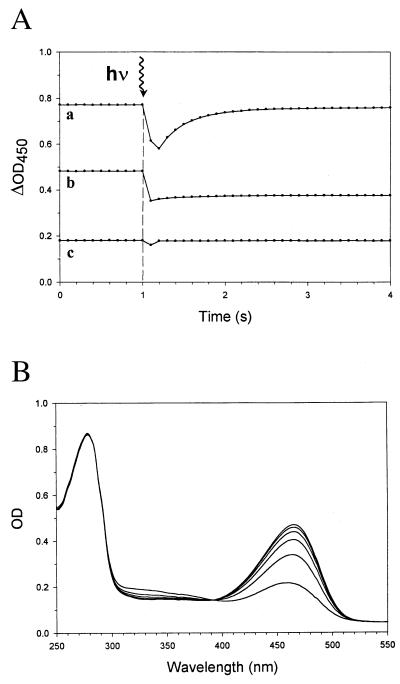
ApoPYP was produced heterologously in E. coli as described previously (18, 20) and was used without prior removal of its polyhistidine tail. This method results in slightly slower kinetics of the recovery step of the photocycle of reconstituted holoPYP (see Fig. 2).
Figure 2.
Transient UV/Vis absorption measurements with holoPYP and hybrids I and II. (A) Absorbance transients, recorded at 450 nm. After 1-sec incubation in the dark, each sample [containing 5 μM holoPYP (trace a), hybrid I (trace b), or hybrid II (trace c)] was exposed to an actinic flash. (B) Absorption spectra of hybrid I, in the seconds time domain, subsequent to an actinic flash. Time series of (six) absorbance spectra of hybrid I, subsequent to an actinic flash, recorded at intervals of 3 min and 45 sec.
7-Hydroxycoumarin-3-carboxylic acid was obtained from Molecular Probes. 4-Hydroxyphenylpropiolic acid (21) was synthesized from 4-t-butyldimethylsilylbenzaldehyde, via 4-t-butyldimethylsilyl-α,α-dibromostyrene. Purity of the final product was checked with 1H NMR (20) and demonstrated to be better than 98%. A small fraction of the contaminants consisted of 4-hydroxycinnamic acid (see Results). All other materials were reagent grade and obtained from commercial sources.
Reconstitution.
Reconstitution of holoPYP and hybrid II was carried out by means of formation of the anhydride derivative of their chromophore, according to Imamoto et al. (19). Hybrid I, because of a more limited availability of its chromophore, was formed with the procedure described by Genick et al. (22), which makes use of chromophore activation via derivatization with carbonyl diimidazole in tetrahydrofuran.
Spectroscopy.
Spectroscopic experiments were routinely carried out in 10 mM Tris⋅HCl, pH 7.0.
UV/Vis (visible) static and transient absorption spectra were recorded with model 8453A and model 8452A Hewlett Packard diode array spectrophotometers, which have a time resolution of 0.1 and 0.5 sec, respectively. To measure light-induced transient absorption spectra, actinic flashes were provided with a conventional photoflash, with an output intensity of 25 W⋅sec.
Low-temperature spectra were recorded as described by Hoff et al. (23). Samples were illuminated with a conventional slide projector, equipped with a 24-V/150-W tungsten lamp and fiber optics, through a narrow-band interference filter with maximal transmission at 460 nm and a bandwidth of 9 nm. The intensity of the output beam was 0.16 mW⋅cm−2.
Fourier-transform infrared (FTIR) spectra were recorded with home-made sandwich cells of 13, 26, or 52 μm thickness, constructed from polyethylene spacers and CaF2 plates. Samples for FTIR measurements were concentrated to between 2.5 and 5 mM, with a 30-kDa cut-off Millipore NMWL filter spin column, at room temperature, in 10 mM Tris⋅HCl, pH 7.0. FTIR difference spectra of white-light photoconverted minus dark-adapted samples were measured on a Bio-Rad FTS-60A IR spectrophotometer and corrected for drift and H2O vapor. Data manipulation was performed with software provided by the manufacturer. Visible light from a 150-W Oriel (Stratford, CT) xenon lamp and fiber optics were used to illuminate the sample in the IR spectrometer.
RESULTS
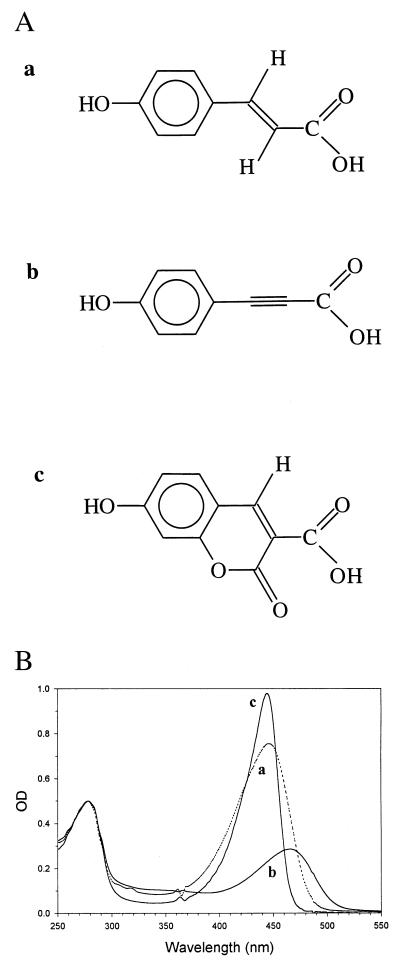
The three proteins formed with the chromophores displayed in Fig. 1A—i.e., holoPYP, hybrid I, and hybrid II—all show a major optical transition in the visible part of the spectrum, with maximal absorbance at 446, 464, and 443 nm, respectively. This indicates that each reconstituted protein contains a highly tuned chromophore, most likely inserted properly into the chromophore-binding pocket, with the phenolic hydroxyl group deprotonated and in hydrogen-bonding contact with Glu-46 (cf. refs. 24 and 25). Nevertheless, some differences are noticeable: Both hybrids have a more symmetrical main absorption band in the visible part of the spectrum [which indicates that they have less pronounced high-energy sidebands, characteristic for holoPYP (23); see further below]. Hybrid II has a much narrower visible absorption band than either holoPYP or hybrid I (full width at half-maximum: 35 nm). Furthermore, its extinction coefficient appears to be slightly higher than the one for holoPYP [45.5 mM−1⋅cm−1 (26)], thus significantly higher than the extinction coefficient of free 7-hydroxycoumarin-3-carboxylic acid, which absorbs maximally at 388 nm, with an extinction coefficient of 32 mM−1⋅cm−1 (27). Hybrid I has a much more red-shifted absorption band than hybrid I, but also a considerably lower extinction coefficient than holoPYP. Because we have not been able to ascertain that reconstitution of this hybrid was complete, we cannot yet precisely calculate its extinction coefficient. Hybrids I and II also fluoresce, with a quantum yield that has increased in hybrid II and decreased in hybrid I, as compared with holoPYP. Furthermore, the Stokes shift of fluorescence is considerably decreased in hybrid II, which is in agreement with a more limited flexibility of the latter chromophore.
Figure 1.
Chemical structure of the chromophores used for the reconstitution of PYP in this investigation and the absorption spectra of holoPYP and the two hybrids. (A) Chemical structure of 4-hydroxycinnamic acid (a), 4-hydroxyphenylpropiolic acid (b), and 7-hydroxycoumarin-3-carboxylic acid (c). (B) Room temperature UV/Vis absorption spectra of apoPYP, reconstituted with the three chromophores shown in A, resulting in (holo)PYP (trace a), hybrid I (trace b), and hybrid II (trace c). The spectrum of hybrid I was recorded after equilibration in the dark for 30 min. The protein concentration in each sample was approximately 10 μM. The three spectra have been normalized to A = 0.5 at 278 nm. The structure of 4-hydroxycinnamic acid and the spectrum of holoPYP are shown for comparison. The irregularities in these spectra at 363 and 488 nm are artifacts, caused by the diode array spectrophotometer.
Next, it was tested whether hybrids I and II would also display photocycle characteristics, by assaying the recovery step of the photocycle with transient absorption spectroscopy (Fig. 2), using holoPYP as a control, and excitation with a conventional photoflash. Trace a of Fig. 2A shows the result of this control experiment. The polyhistidine tail slightly retards the recovery kinetics. Using a monoexponential fit, a rate constant of 3.5 sec−1 is obtained from these data (which is to be compared with 6.5 sec−1 as obtained after removal of the polyhistidine tail; see also ref. 13). Surprisingly, hybrid I did show modified, but authentic, photocycle characteristics, as was concluded from the experiment shown in trace b of Fig. 2A. By measuring during a much more extended period of time, the rate constant for its recovery reaction was determined to be 3.5 (±0.02) × 10−3 sec−1 (i.e., 1000-fold retarded compared with holoPYP). The complete spectra of this actinic flash-induced bleaching process of hybrid I (from 250 to 550 nm, as shown in Fig. 2B), reveal that it is paralleled by an increase in absorbance in the near-UV region, which is indicative for the formation of a blue-shifted (pB)-like intermediate (12, 13). Maximal absorbance of this state is at 326 nm, which is considerably blue-shifted as compared with pB [355 nm; (13, 28)]. It should be noted that in the UV region of this spectrum, no significant changes take place, in contrast to the parallel process in holoPYP (28). In hybrid II this bleaching at the (sub)second time scale has not been detectable (see trace c of Fig. 2A).
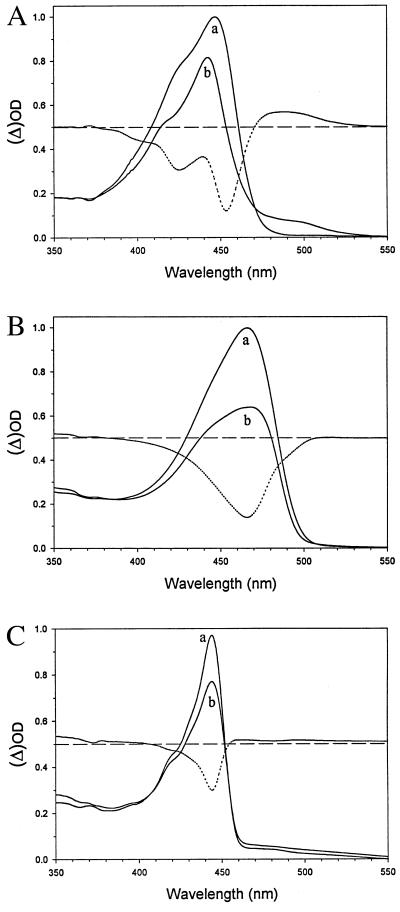
To investigate whether hybrids I and II would show signals characteristic for the formation of pR [the red-shifted photocycle intermediate (12, 13)], we recorded visible absorbance spectra, with and without narrow-band actinic illumination, at 77 K. The resulting difference spectra allow one to demonstrate formation of pR (see Fig. 3) (23, 29). Fig. 3A shows that the conditions selected allow registration of pR formation. The spectra of the dark-equilibrated state of holoPYP in glycerol and at 77 K are slightly red-shifted and significantly sharpened (23, 29). The difference spectrum clearly shows increased absorbance in the range from 470 to 550 nm (indicative of pR formation) and bleaching of the ground state. The sharpening of the absorption band makes it more evident than in the room-temperature spectrum (cf. Fig. 1B) that the visible absorption of the 4-hydroxycinnamic acid chromophore of PYP shows fine structure on the high-energy side of the main maximum. Interestingly, this fine structure becomes somewhat more pronounced upon partial bleaching. In the difference spectrum of holoPYP at least three (sub)maxima can be discerned, with a spacing of ≈1500 (±50) cm−1. Such a spacing is typical for the absorption of many conjugated polyene systems and is related to the excited-state vibrational frequency of a C=C double bond in such a system.
Figure 3.
Light-induced formation of pR and a pR-like intermediate in PYP (A), hybrid I (B), and hybrid II (C), at low temperature (77 K). Each protein was dissolved, at a concentration of 20 μM, in a buffer of 10 mM Tris⋅HCl, pH 7.0, containing 50% (vol/vol) glycerol. Samples were frozen in 1-cm acrylic cuvettes in the dark. After recording of the dark spectra (a traces), each sample was illuminated in the cryostat, as described in Materials and Methods, for 20 min, to induce pR formation (b traces). The absorbance in both spectra was set to zero at 600 nm for background subtraction. The dotted line in each panel represents the difference spectrum between traces a and b.
The fact that the fine structure appears more pronounced after bleaching and shows up most clearly in the difference spectrum implies that the absorption of PYP in glycerol at 77 K is inhomogeneously broadened and that the monochromatic bleaching light selectively excites a subset of molecules. It appears quite likely that this phenomenon relates to different conformers of the protein and/or various aggregates that are frozen into the water/glycerol matrix (see also Discussion).
In hybrid I (Fig. 3B) the visible absorption band also sharpens and undergoes a slight red shift at 77 K, but no clear fine structure of the absorption band can be discerned. Bleaching of this hybrid occurs very efficiently. Its bandshape clearly changes upon bleaching, demonstrating that also for hybrid I this band is inhomogeneously broadened and that spectral selectivity of bleaching can be achieved. For hybrid II (Fig. 3C) again the sharpening and slight red-shift occur at 77 K, but in this case the change in bandshape upon bleaching appears to be minor, if any. It should be noted that in this case the extent of bleaching is smaller. Although much less pronounced than for holoPYP, also for hybrid II some indication for vibrational fine structure is evident in the low-temperature spectra (with a spacing similar to that in holoPYP and hybrid I).
Whereas hybrid I (Fig. 3B) and hybrid II (Fig. 3C) both display bleaching of the main absorption band by actinic illumination, with neither hybrid was evidence for the formation of a red-shifted intermediate obtained, in contrast to holoPYP, which clearly shows this intermediate with an absorbance maximum at ≈490 nm. The small increase observed in the absorbance of hybrid II in the 500-nm region does not show the typical characteristics of a specific absorption band. However, we interpret these low-temperature (difference) spectra as evidence that a pR-like state is formed in both hybrids, but that this latter state absorbs at a wavelength similar to that of its corresponding pG state, albeit with a considerably decreased extinction coefficient. This interpretation is fully in line with the results of room-temperature nanosecond transient absorbance measurements. In these latter experiments also, hybrid I does not show the increase in absorbance in the 500-nm region in the nanosecond to microsecond time scale (typical for pR formation in holoPYP), prior to formation of the blue-shifted intermediate, as shown in Fig. 2. Hybrid II shows only a transient bleaching of the ground state in the micro- to millisecond time scale; signals from a pB-like state could not be detected in the latter hybrid.
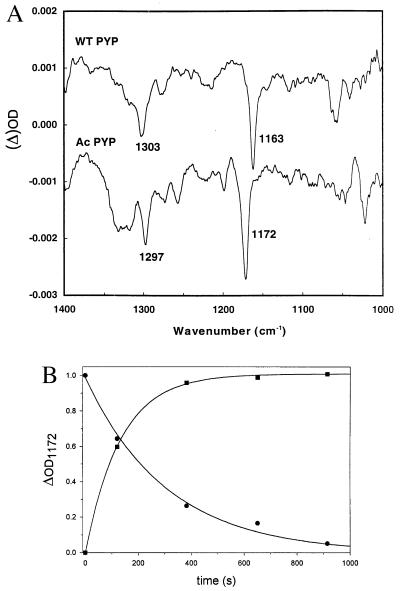
To obtain further evidence that authentic photocycle signals had been obtained, particularly from hybrid I, light − dark (i.e., pB − pG) FTIR difference spectra were recorded in solution, again using holoPYP as a control (Fig. 4). Steady-state accumulation of pB was accomplished by illumination of the sample in the IR spectrophotometer. For holoPYP, because of the relatively low light intensity available, a maximal conversion of 15% of the pigment was achieved. For hybrid I, at least in part because of its much slower recovery rate, a maximal conversion of 40% of the pigment into the pB-like state was achieved during steady-state illumination. For hybrid I, this level of photoconversion was calculated from UV/Vis detection of photoconversion in the FTIR cell, directly after removal of the sample from the FTIR spectrometer, and from the complete conversion, obtained with much higher light intensities than obtainable within the FTIR set-up, outside the spectrometer. Similar difference spectra were obtained from samples of holoPYP and hybrid I incubated at pH 5.0.
Figure 4.
FTIR pG − pB difference spectra of PYP and hybrid I (A) and the kinetics of the transition between pG and the pB-like intermediate of hybrid I (B). (A) FTIR difference spectra of PYP (upper trace) and hybrid I (lower trace), before minus after illumination (i.e., pG − pB spectra) were recorded as described in Materials and Methods, at pH 7.0. By using a combination of UV/Vis and IR spectroscopy, it could be derived that the levels of photoconversion of pG into the pB intermediate in the IR spectrometer, for PYP and hybrid I, were 10% and 40%, respectively. (B) A time series of spectra was measured by averaging 381 scans for each time point, during light-induced formation, and dark decay, of the pB-like intermediate of hybrid I, over a period of 20 min. Acquisition of each spectrum takes ≈4 min. The data obtained were fitted to an exponential rise (▪) or decay (•) function.
The FTIR difference spectrum, here displayed from 1400 to 1000 cm−1, has a suboptimal signal-to-noise ratio, in part because of the relatively inefficient photoconversion conditions used in this experiment (Fig. 4). Nevertheless, this figure clearly shows two of the most typical features of the pB − pG difference spectrum, characteristic for holoPYP (ref. 30; A. Xie, W. D. Hoff, and K.J.H., unpublished experiments) at 1163 and 1303 cm−1. Both features are characteristic for phenolates. This interpretation has been documented for (para-substituted) phenols, tyrosine, etc. (in both their neutral and anionic forms) by FTIR and resonance Raman spectroscopy (31–33). In the nomenclature of vibrational modes derived for benzene (34), these features have been assigned to the Y9a (C—H in-plane bending) and Y7a′ (predominantly C—O stretch) modes of the phenolate group, respectively. For hybrid I, a very similar difference spectrum was obtained, with the corresponding main peaks at 1172 and 1297 cm−1, indicating that in this hybrid also a protonated chromophore is transiently formed. The shift from 1163 to 1172 cm−1 was predicted on the basis of pH-titration studies of ester model compounds of the chromophore of hybrid I.
The rate of formation and decay of the signal at 1172 cm−1 was recorded in a series of measurements in which a smaller number of transients was averaged, to allow for time resolution of the FTIR measurements. The pB state of hybrid I was populated with a rate of ≈0.01 sec−1, which is dependent on the intensity of actinic illumination. For the recovery of the ground state of hybrid I, a rate constant of 3.2 (±0.5) × 10−3 sec−1 was obtained (Fig. 4B), in close agreement with the rate of recovery of the ground state of this pigment, measured by using visible absorbance measurements (Fig. 2A).
Additional typical characteristics of the pB − pG FTIR difference spectra, such as the signals from Glu-46 at 1739 cm−1, were recorded, but are not shown in Fig. 4. FTIR, as a technique, was selected for these measurements because we anticipated to be able also to detect the C☰C triple bond of hybrid I, of which the stretch vibration is expected to absorb at ≈2200 cm−1, as was concluded from measurements on derivatives of 4-hydroxyphenylpropiolic acid at neutral and alkaline pH. This is a region with little absorbance originating from the apoprotein. However, this band was not detected in the spectra. The explanation for this lack of detection may be related to the relatively large width of this latter band, as compared with the Y9a marker band at 1172 cm−1.
Because bleaching of hybrid II with continuous actinic illumination at room temperature could not be accomplished (presumably because of its very rapid recovery rate; see also Fig. 2A), no FTIR measurements with this hybrid have been performed. With hybrid I, care should be taken to allow full relaxation of the pigment to the ground state before measurements are started, because ambient light already causes significant accumulation of the pB-like intermediate.
1H NMR analysis (see Materials and Methods) indicated that a small fraction of the impurities in 4-hydroxyphenylpropiolic acid consisted of 4-hydroxycinnamic acid. Because apoPYP might selectively recognize this contaminant in the reconstitution experiments, we have carried out chromophore reextraction experiments. These, however, have not allowed us to make an estimate of the maximal level of contamination of the reconstituted chromophore in hybrid I, because only very small (far less than stoichiometric) amounts of 4-hydroxyphenylpropiolic acid were released by alkaline hydrolysis. This limited release may be because of a nucleophilic attack of the liberated thiolate anion of Cys-69 on the triple bond of the 4-hydroxyphenylpropiolic acid. Nevertheless, the results presented in Figs. 2A and 4 lead to the conclusion that the level of contamination by 4-hydroxycinnamic acid was below the level of detection of the techniques used in these experiments (estimated to be 5%).
Besides the two chromophore analogues described in this study, more have been made and tested. In addition to the ones reported in ref. 20, a particularly relevant one for this report is a ring-locked chromophore in which the α-pyrone ring of 7-hydroxycoumarin-3-carboxylic acid was replaced by a benzene ring (i.e., 2-carboxy-6-hydroxynaphthalene). However, we have not succeeded in reconstituting any functional or tuned pigment with this analogue. The same applies to reconstitution experiments with 2- and 3-hydroxycinnamic acid anhydride. Chromophore analogues that were successfully used to form hybrid PYPs include chromophores with a deuterated and a brominated vinyl bond. The latter, because of its increased capacity to scatter electrons, should prove useful in (time-resolved) x-ray diffraction studies.
DISCUSSION
The role of cis/trans isomerization as the chemical basis of the primary reaction of photoperception in sensory transduction in the rhodopsin-based visual process is under intense discussion. Understanding this system, however, is complicated by the polyenic character of retinal, which makes it necessary to consider isomerization across alternative bonds when this process across one particular bond is prohibited by chemical derivatization of the chromophore (9). Furthermore, both visual rhodopsin and Brh hold their chromophore—i.e., retinal—bound in a distorted conformation, which may significantly affect its basic photochemistry (35, 36) and thus complicate comparisons with model compounds.
We have therefore decided to study the role of chromophore isomerization in a member of the xanthopsin protein family (18), PYP, from Ectothiorhodospira halophila (10). The 4-hydroxycinnamic acid chromophore of this photoreceptor contains a unique double bond, which is subject to isomerization upon photoactivation (16, 17). On the basis of FTIR measurements, it has been proposed that this isomerization process involves a rotation across two bonds: the 7,8-vinyl bond of the chromophore and the S—C single bond through which the chromophore is linked to the apoprotein (37). Very recently, the atomic displacements during nanosecond laser activation of PYP have been revealed through subjecting crystals of this photoreceptor to time-resolved x-ray diffraction experiments (38). These experiments clearly demonstrated that the change in the position of the aromatic ring of the chromophore of PYP is very small: it can best be described as a rotation of 60°, within the plane in which it is clamped in its binding pocket, between the aromatic amino acid side chains (i.e., Phe-62 and Phe-96) on one side, and the hydrogen-bonding Arg-52 and Tyr-98 on the other. The largest atomic displacement that is seen in these diffraction experiments, however, is the trans-to-cis displacement of the vinyl bond of the chromophore and—in particular—the rotation of the carbonyl group of the chromophore across the long axis through the chromophore and Cys-69.
This carbonyl group is therefore subject to a crankshaft-like motion, in which the initial hydrogen bond to the backbone-N-H of the cysteine is disrupted and a new hydrogen bond to Tyr-98 is transiently formed (38). Subsequent recovery of the ground state thus includes nonphotochemical cis/trans reisomerization of the double bond of the chromophore. In the absence of the apoPYP protein this reisomerization process displays a very high activation barrier (the isolated 4-hydroxycinnamic acid chromophore is thermally stable in both the cis and the trans forms). His-108 of the apoprotein may be of critical importance in the “catalysis” of the latter process by the apoprotein.
We have investigated the (in)dispensability of trans/cis isomerization of the chromophore for activation of the PYP photoreceptor (i) by replacing the double bond of the endogenous chromophore by a triple bond and (ii) by using a chromophore with a locked double bond. Both modified chromophores have no possibility to undergo trans/cis isomerization across the double bond that corresponds with the 7,8-vinyl bond of the authentic chromophore. Nevertheless, the resulting hybrid pigments do show absorption characteristics and photoactivity that are typical for holoPYP. Of the two, hybrid II is most restricted in its photochemistry. Formation of a pR-like intermediate could be demonstrated at 77 K, albeit the pR-like intermediate formed does not show a shift in its absorption maximum with respect to its corresponding pG. Hybrid I behaved similarly in this respect; however, from the latter also authentic signals originating from a pB-like intermediate could be recorded (see Figs. 2 and 4). These latter results strongly argue that the pB-like intermediate of hybrid I also contains a protonated chromophore (20, 24, 25, 30, 37).
These observations can be interpreted within the framework of a model that assumes that photoactivation induces rotation of the carbonyl group of the chromophores (see above). In both hybrids the carbonyl function of the chromophore is part of an extended conjugated system, ranging from the phenolate oxygen to the sulfur atom of Cys-69, just as in holoPYP. After photoactivation of both hybrids, dipole formation in both chromophores leads to C=O rotation, which will be very much sterically limited in hybrid II, because of the presence of the second ring of the chromophore; most likely to such an extent that the C=O group cannot reach the stabilizing position in which it can form a hydrogen bond with Tyr-98 (38). Hence, a very rapid decay from the pR-like state, back to the ground state, will occur in this hybrid, which is in agreement with preliminary results of nanosecond time-resolved transient absorption spectroscopy experiments (see Results). In contrast, the C=O group of the chromophore of hybrid I does reach a similar position as in holoPYP, which therefore subsequently leads to disruption of the hydrogen-bonding network of the phenolate oxygen and to partial unfolding of hybrid I into a pB-like signaling state (39). Return from this state may be dependent on interaction with, and catalysis by, side chains from the apoprotein (40). Subångström changes in the mutual distance of the atoms involved can already dramatically slow down this catalytic function (41), which may explain why the overall recovery reaction of hybrid I is 1,000-fold retarded, even though this process in hybrid I does not require the reisomerization of a double bond. Nevertheless, this retardation can be exploited as a convenient alternative for lowering the pH in holoPYP (28).
These experiments unequivocally demonstrate that trans/cis isomerization of the vinyl bond of the chromophore of PYP is not required for photoactivation of this photoreceptor.
As discussed above, hybrid II most likely, upon photoactivation, goes through a short-circuited photocycle in which the (locked) chromophore remains deprotonated. Consequently, no major rearrangements in the backbone structure of PYP are involved in this process and thus the photocycle can be completed very rapidly. Evidence for other forms of short-circuiting of the photocycle of holoPYP is available: Both from pR and from pB, the ground state can be recovered in a photochemical reaction (23, 42). In addition, short-circuiting of the major conformational transitions associated with the formation of the signaling state of pB has also been obtained. First, limited hydration of PYP leads to a photocycle with only limited conformational rearrangements, as reflected in the changes in various parts of the IR spectrum of the protein (43). Furthermore, comparison of the structural rearrangements associated with pB formation, as determined with time-resolved x-ray diffraction and 1H NMR spectroscopy (refs. 17 and 38; G. Rubinstein, G. W. Vuister, F. A. A. Mulder, P. E. Düx, R. Boelens, K.J.H., and R. Kaptein, unpublished results), lead to the same conclusion.
The transient absorption spectra and the pB − pG FTIR difference spectra (Figs. 2 and 4) suggest that intramolecular proton transfer remains a key step in the photocycle of hybrid I. The strongly prolonged recovery times of this hybrid may help to further characterize the path of the proton that is transferred while PYP progresses through its photocycle.
The low-temperature spectra (Fig. 3) show a striking fine structure in the spectrum of the PYP molecules that were photoconverted to an intermediate state at 77 K (see also refs. 23 and 29). These results suggest that the sample of PYP molecules, frozen in glycerol, contains a very heterogeneous population of molecules, of which only a subpopulation is excited. From the absence of well-resolved sidebands in the absorbance spectrum of PYP in buffer at room temperature, one is led to conclude that a similar heterogeneity exists under those conditions. The results of 1H NMR experiments, which have led to a resolution of the structure of PYP in solution, have already revealed some forms of heterogeneity (44). It will be of great interest to resolve this relation between (variations in) PYP structure and its absorbance characteristics.
The results obtained with hybrids I and II support the idea that the fine structure in the visible absorption band of holoPYP is related to a vibrational progression, connected to the double bond (cf. ref. 45). Thus it is also weakly detectable in hybrid II, but not in hybrid I. The wavelength maxima of the intermediates of hybrid I reveal an extremely large magnitude of chromophore tuning, as induced by protonation: from 464 nm (in pG) to 326 nm (in the pB-like intermediate). This magnitude is unprecedented in the previously characterized hybrids (20).
CONCLUSION
The results presented in this study unequivocally demonstrate that trans/cis isomerization of the vinyl bond of the chromophore of PYP is dispensable for activation of the PYP photoreceptor and suggest that rotation of its carbonyl group may be of higher importance. Furthermore, the results obtained suggest that in hybrid I intramolecular proton transfer still takes place and that the dark reisomerization of the chromophore is strongly retarded.
Acknowledgments
Note. The x-ray structure of a pR-like intermediate of holoPYP was recently published by Genick et al. (46). These data are in agreement with the model describing the initial photoisomerization of the chromophore of PYP as a rotation of the carbonyl group of the chromophore around the long axis of the 4-hydroxycinnamic acid (37). This study even provides an explanation for the apparent contradiction between the results obtained with FTIR (37) and time-resolved Laue diffraction experiments (38), through the difference between pR77 K and pRRT.
ABBREVIATIONS
- PYP
photoactive yellow protein
- Brh
bacteriorhodopsin
- hybrid I
apoPYP combined with 4-hydroxyphenylpropiolic acid
- hybrid II
apoPYP combined with 7-hydroxycoumarin-3-carboxylic acid
- FTIR
Fourier-transform infrared
- pG
pR, and pB, dark-adapted ground state, red-shifted, and blue-shifted photocycle intermediate of PYP, respectively
References
- 1.Warshel A. Nature (London) 1976;260:679–683. doi: 10.1038/260679a0. [DOI] [PubMed] [Google Scholar]
- 2.Peters K, Applebury M L, Rentzepis P M. Proc Natl Acad Sci USA. 1977;74:3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peteanu L A, Schoenlein R W, Wang Q, Mathies R A, Shank C V. Proc Natl Acad Sci USA. 1993;90:11762–11766. doi: 10.1073/pnas.90.24.11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delaney J K, Brack T L, Atkinson G H, Ottolenghi M, Steinberg G, Sheves M. Proc Natl Acad Sci USA. 1995;92:2101–2105. doi: 10.1073/pnas.92.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wald G. Nature (London) 1968;219:800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- 6.Kandori H, Matuoka S, Shichida Y, Yoshizawa T, Ito M, Tsukida K, Balogh-Nair V, Nakanishi K. Biochemistry. 1989;28:6460–6467. doi: 10.1021/bi00441a045. [DOI] [PubMed] [Google Scholar]
- 7.Hu S, Franklin P J, Wang J, Ruiz Silva B E, Derguini F, Nakanishi K, Chen A H. Biochemistry. 1994;33:408–416. doi: 10.1021/bi00168a004. [DOI] [PubMed] [Google Scholar]
- 8.Delaney J K, Atkinson G H, Sheves M, Ottolenghi M. J Phys Chem. 1995;99:7801–7805. [Google Scholar]
- 9.Rousso I, Khachatryan E, Gat Y, Brodsky I, Ottolenghi M, Sheves M, Lewis A. Proc Natl Acad Sci USA. 1997;94:7937–7941. doi: 10.1073/pnas.94.15.7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer T E. Biochim Biophys Acta. 1985;806:175–183. doi: 10.1016/0005-2728(85)90094-5. [DOI] [PubMed] [Google Scholar]
- 11.Sprenger W W, Hoff W D, Armitage J P, Hellingwerf K J. J Bacteriol. 1993;175:3096–3104. doi: 10.1128/jb.175.10.3096-3104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer T E, Yakali E, Cusanovich M A, Tollin G. Biochemistry. 1987;26:418–423. doi: 10.1021/bi00376a012. [DOI] [PubMed] [Google Scholar]
- 13.Hoff W D, Van Stokkum I H M, Van Ramesdonk J, Van Brederode M E, Brouwer A M, Fitch J C, Meyer T E, Van Grondelle R, Hellingwerf K J. Biophys J. 1994;67:1691–1705. doi: 10.1016/S0006-3495(94)80643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoff W D, Düx P, Hård K, Devreese B, Nugteren-Roodzant I M, Crielaard W, Boelens R, Kaptein R, Van Beeumen J, Hellingwerf K J. Biochemistry. 1994;33:13959–13962. doi: 10.1021/bi00251a001. [DOI] [PubMed] [Google Scholar]
- 15.Baca M, Borgstahl G E O, Boissinot M, Burke P M, Williams W R, Slater K A, Getzoff E D. Biochemistry. 1994;33:14369–14377. doi: 10.1021/bi00252a001. [DOI] [PubMed] [Google Scholar]
- 16.Kort R, Vonk H, Xu X, Hoff W D, Crielaard W, Hellingwerf K J. FEBS Lett. 1996;382:73–78. doi: 10.1016/0014-5793(96)00149-4. [DOI] [PubMed] [Google Scholar]
- 17.Genick U K, Borgstahl G E, Ng K, Ren Z, Pradervand C, Burke P M, Srajer V, Teng T Y, Schildkamp W, McRee D E, Moffat K, Getzoff E D. Science. 1997;275:1471–1475. doi: 10.1126/science.275.5305.1471. [DOI] [PubMed] [Google Scholar]
- 18.Kort R, Hoff W D, Van West M, Kroon A R, Hoffer S M, Vlieg K H, Crielaard W, Van Beeumen J J, Hellingwerf K J. EMBO J. 1996;15:3209–3218. [PMC free article] [PubMed] [Google Scholar]
- 19.Imamoto Y, Ito T, Kataoka M, Tokunaga F. FEBS Lett. 1995;374:157–160. doi: 10.1016/0014-5793(95)01096-w. [DOI] [PubMed] [Google Scholar]
- 20.Kroon A R, Hoff W D, Fennema H, Gijzen J, Koomen G-J, Verhoeven J W, Crielaard W, Hellingwerf K J. J Biol Chem. 1996;271:31949–31956. doi: 10.1074/jbc.271.50.31949. [DOI] [PubMed] [Google Scholar]
- 21.Babin P, Dunogues J, Petraud M. Tetrahedron. 1981;37:1131–1139. [Google Scholar]
- 22.Genick U K, Devanathan S, Meyer T E, Canestrelli I L, Williams E, Cusanovich M A, Tollin G, Getzoff E D. Biochemistry. 1997;36:8–14. doi: 10.1021/bi9622884. [DOI] [PubMed] [Google Scholar]
- 23.Hoff W D, Kwa S S, van Grondelle R, Hellingwerf K J. Photochem Photobiol. 1992;56:529–539. [Google Scholar]
- 24.Borgstahl G E O, Williams D R, Getzoff E D. Biochemistry. 1995;34:6278–6287. doi: 10.1021/bi00019a004. [DOI] [PubMed] [Google Scholar]
- 25.Kim M, Mathies R A, Hoff W D, Hellingwerf K J. Biochemistry. 1995;34:12669–12672. doi: 10.1021/bi00039a024. [DOI] [PubMed] [Google Scholar]
- 26.Meyer T E, Tollin G, Hazzard J H, Cusanovich M A. Biophys J. 1989;56:559–564. doi: 10.1016/S0006-3495(89)82703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haugland R P. Handbook of Fluorescent Probes and Research Chemicals. Eugene, OR: Molecular Probes; 1992. pp. 81–88. [Google Scholar]
- 28.Hoff W D, van Stokkum I H M, Gural J, Hellingwerf K J. Biochim Biophys Acta. 1997;1322:151–162. [Google Scholar]
- 29.Imamoto Y, Kataoka M, Tokunaga F. Biochemistry. 1996;35:14047–14053. doi: 10.1021/bi961342d. [DOI] [PubMed] [Google Scholar]
- 30.Imamoto Y, Mihara K, Hisatomi O, Kataoka M, Tokunaga F, Bojkova N, Yoshihara K. J Biol Chem. 1997;272:12905–12908. doi: 10.1074/jbc.272.20.12905. [DOI] [PubMed] [Google Scholar]
- 31.Dollinger G, Eisenstein L, Lin S-L, Nakanishi K, Termini J. Biochemistry. 1986;25:6524–6533. doi: 10.1021/bi00369a028. [DOI] [PubMed] [Google Scholar]
- 32.Harada I, Takeuchi H. In: Spectroscopy of Biological Systems. Clark R J H, Hester R E, editors. New York: Wiley; 1986. pp. 113–175. [Google Scholar]
- 33.Jakobsen R J, Brewer E J. J Appl Spectrosc. 1962;16:32–35. [Google Scholar]
- 34.Wilson E B. Phys Rev. 1934;45:706–714. [Google Scholar]
- 35.Jaeger S, Lewis J W, Zvyaga T A, Szundi I, Sakmar T P, Kliger D S. Proc Natl Acad Sci USA. 1997;94:8557–8562. doi: 10.1073/pnas.94.16.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pebay-Peyroula E, Rummel G, Rosenbusch J P, Landau E M. Science. 1997;277:1676–1681. doi: 10.1126/science.277.5332.1676. [DOI] [PubMed] [Google Scholar]
- 37.Xie A, Hoff W D, Kroon A R, Hellingwerf K J. Biochemistry. 1996;35:14671–14678. doi: 10.1021/bi9623035. [DOI] [PubMed] [Google Scholar]
- 38.Perman B, Srajer V, Ren Z, Teng T, Pradervand C, Ursby T, Bourgeois D, Schotte F, Wulff M, Kort R, Hellingwerf K, Moffat K. Science. 1998;279:1946–1950. doi: 10.1126/science.279.5358.1946. [DOI] [PubMed] [Google Scholar]
- 39.Van Brederode M E, Hoff W D, Van Stokkum I H M, Groot M-L, Hellingwerf K J. Biophys J. 1996;71:365–380. doi: 10.1016/S0006-3495(96)79234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hellingwerf K J, Hoff W D, Crielaard W. Mol Microbiol. 1996;21:683–693. doi: 10.1046/j.1365-2958.1996.411402.x. [DOI] [PubMed] [Google Scholar]
- 41.Carey P R, Tonge P J. Acc Chem Res. 1995;28:8–13. [Google Scholar]
- 42.Miller A, Leigeber H, Hoff W D, Hellingwerf K J. Biochim Biophys Acta. 1992;1141:190–196. [Google Scholar]
- 43.Hoff W D, Xie A, Hellingwerf K J. Biophys J. 1998;74:A291. (abstr.). [Google Scholar]
- 44.Düx P E. Ph.D. thesis. Utrecht, The Netherlands: Utrecht Univ.; 1997. , ISBN 90–393-1583–3. [Google Scholar]
- 45.Takahashi T, Yan B, Mazur F, Derguini F, Nakanishi K, Spudich J L. Biochemistry. 1990;29:8467–8474. doi: 10.1021/bi00488a038. [DOI] [PubMed] [Google Scholar]
- 46.Genick U K, Soltis S M, Kuhn P, Canestrelli I L, Getzoff E D. Nature (London) 1998;392:206–209. doi: 10.1038/32462. [DOI] [PubMed] [Google Scholar]