Abstract
The roles of gender and sex hormones in lung function and disease are complex and not completely understood. The present study examined the influence of gender on lung function and respiratory mechanics in naive mice and on acute airway inflammation and hyperresponsiveness induced by intratracheal LPS administration. Basal lung function characteristics did not differ between naive males and females, but males demonstrated significantly greater airway responsiveness than females following aerosolized methacholine challenge as evidenced by increased respiratory system resistance and elastance (p < 0.05). Following LPS administration, males developed more severe hypothermia and greater airway hyperresponsiveness than females (p < 0.05). Inflammatory indices including bronchoalveolar lavage fluid total cells, neutrophils, and TNF-α content were greater in males than in females 6 h following LPS administration (p < 0.05), whereas whole-lung TLR-4 protein levels did not differ among treatment groups, suggesting that differential expression of TLR-4 before or after LPS exposure did not underlie the observed inflammatory outcomes. Gonadectomy decreased airway inflammation in males but did not alter inflammation in females, whereas administration of exogenous testosterone to intact females increased their inflammatory responses to levels observed in intact males. LPS-induced airway hyperresponsiveness was also decreased in castrated males and was increased in females administered exogenous testosterone. Collectively, these data indicate that airway responsiveness in naive mice is influenced by gender, and that male mice have exaggerated airway inflammatory and functional responses to LPS compared with females. These gender differences are mediated, at least in part, by effects of androgens.
Gender differences exist in the risk, incidence, and pathogenesis of various lung diseases in humans (1). Females typically are more susceptible to and/or develop more severe asthma, chronic obstructive pulmonary disease, lung cancer, and other lung conditions. Accumulating epidemiological and experimental data suggest that sex hormones may be important physiological modulators in the lung, and the role of estrogens in asthma has received considerable attention in this regard. However, in general, studies of the effects of sex hormones on lung function and disease pathogenesis are often limited by relatively small sample sizes, subjectivity of outcome measures, and unadjusted comparisons (2), thus making absolute confirmation of hormonal effects elusive.
Studies using a variety of experimental models of lung injury have uncovered underlying gender differences that are believed to result from sex hormone effects on disease pathogenesis. Whereas the role of androgens has not been addressed in detail, estrogens appear to equivocally influence lung injury outcomes depending on the model (3–6). Bacterial LPS, a ubiquitous airborne contaminant and recognized exacerbating factor for the development and severity of asthma (7), causes airway inflammation and hyperresponsiveness in humans and animals. However, surprisingly little has been reported with regard to the potential influence of gender on the pulmonary response to LPS in humans (8) or experimental animals (9). Whereas estrogens were recently shown to at least partially regulate the acute inflammatory response to LPS in mice (10), the potential role of androgens has not been examined. Thus, the roles of gender and sex hormones in the response of the lung to injurious stimuli such as LPS are complex and not completely understood at present.
Lung function in naive mice and in murine lung disease models is another area in which the potential influence of gender has not been extensively investigated. Although various basal lung function parameters were shown to differ between males and females in several strains of mice (11), reports of the comparative pulmonary response of male and female mice to challenge with a bronchoconstrictive agent (e.g., a cholinergic agonist) are lacking. Increased airway responsiveness to cholinergic stimulation is a hallmark of airway diseases such as asthma. Thus, it is of interest and importance to determine whether gender differences exist in airway responsiveness at baseline and/or following lung injury. Knowledge of such potential gender differences is especially pertinent to the design and interpretation of murine studies of lung disease in which functional alterations constitute a key end point.
The present study was undertaken to investigate the influence of gender on lung function and airway responsiveness in naive laboratory mice and on airway inflammation and hyperresponsiveness resulting from LPS administration. Results presented herein indicate that considerable gender differences in airway responsiveness exist in naive mice, and that male sex hormones promote airway inflammation and hyperresponsiveness induced by LPS.
Materials and Methods
Experimental protocol
All studies were conducted in accordance with principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the National Institute of Environmental Health Sciences Animal Care and Use Committee. Male and female C57BL/6 and BALB/c mice (8–10 wk old; Charles River) were used. Naive mice were used for baseline lung function analysis by invasive or noninvasive methods, as described below. For lung inflammation studies, mice were anesthetized with isoflurane/oxygen and were administered 50 µg of LPS (Escherichia coli serotype 0111:B4; Sigma-Aldrich) dissolved in sterile, endotoxin-free saline, or an equivalent volume of sterile saline (75 µl) as a vehicle control, via oropharyngeal aspiration. Briefly, anesthetized mice were suspended by their upper incisors by a rubber band on a 60° incline board. The tongue was gently extended, and the LPS or saline solution was pipetted into the mouth. Brief occlusion of the nose forced the animal to inhale through the mouth, and the solution was aspirated into the respiratory tract in one or two breaths. Animals were subsequently removed from the board and observed closely until fully recovered from anesthesia.
Immediately before aspirations and at regular intervals thereafter, body temperatures were recorded using a Thermalert TH-5 rectal probe (Physi-temp). When performed, gonadectomy of male and female mice occurred 3 wk before experimentation. Similarly, s.c. implantation of pellets containing either 5-α-dihydrotestosterone (DHT3; 15 mg) or placebo into intact female mice, and s.c. implantation of pellets containing either flutamide (25 mg) or placebo into intact male mice, occurred 3 wk before experimentation. All pellets were 60-day release formulations purchased from Innovative Research of America and have been shown to exert androgenic (DHT) and antiandrogenic (flutamide) activity in mice 3–4 wk postimplantation (12).
Analysis of lung function
Respiratory parameters in spontaneously breathing naive mice were determined by barometric whole-body plethysmography (Buxco Electronics). Mice were placed in individual plethysmograph chambers and breathing frequency, tidal volume, minute ventilation, peak inspiratory flow, and peak expiratory flow were determined and averaged over a 30-min period.
Invasive analysis of lung function was performed on naive mice that were anesthetized with urethane (1.5 g/kg, i.p.). Once surgical anesthesia had been established, a tracheostomy was performed and a 19-gauge stainless-steel cannula was inserted into the trachea. Animals were then paralyzed with pancuronium bromide (0.8 mg/kg, i.p.) and placed on a 37°C heating pad, and the cannula was connected to the computer-controlled ventilator. Ventilation was maintained at a rate of 150 breaths/min and a tidal volume of 7.5 ml/kg, with a positive end-expiratory pressure of 3 cm of H2O. Heart rate was monitored with a portable monitor (CardioMonitor; BAS Vetronics) to ensure proper anesthetic depth.
Both the single-compartment model and the constant-phase model of respiratory mechanics were used to assess lung function and airway responses to methacholine. For the single-compartment model, total respiratory system resistance (R) and elastance (E) were determined essentially as described (13). For the constant-phase model, Rn (Newtonian resistance, an indicator of central airways resistance), G (tissue damping, an indicator of peripheral airways and parenchymal resistance), and H (tissue elastance, an indicator of peripheral airways and parenchymal elastance) were determined essentially as described (14). All data points were determined by the FlexiVent software (version 4.0) by using multiple linear regression to fit each data point to the single-compartment or the constant-phase model, as appropriate. Baseline values for each mouse were obtained by applying a 2-s perturbation at a frequency of 2.5 Hz followed by an 8-s pseudorandom perturbation consisting of waveforms of mutually prime frequencies (0.5–19.6 Hz) a total of three times at 30-s intervals; these maneuvers generated data that were fit to the single-compartment and constant-phase models, respectively. The averages of these measurements for each mouse served as its baseline values. Following acquisition of baseline data, airway responsiveness to aerosolized methacholine (0–25 mg/ml saline; delivered by ultrasonic nebulizer) was assessed. Aerosols were delivered for 10 s without altering the ventilatory pattern, after which the 2- and 8-s perturbations were applied consecutively every 30 s for 5 min. Peak responses during each 5-min period were determined, and only values with a coefficient of determination of 0.95 or greater were used. Linear interpolation was used to determine the provocative concentration of methacholine aerosol at which a 200% increase (PC200) over baseline values was observed for R, Rn, and G, and at which a 50% increase (PC50) over baseline values was observed for E and H. Brief occlusion of the expiratory tube was performed before taking baseline measurements and before each aerosol administration to prevent atelectasis and reset the volume history.
Invasive analysis of respiratory mechanics and airway responsiveness to methacholine aerosol was also performed in a subgroup of mice 4 h after administration of LPS.
Assessment of LPS-induced airway inflammation
Six hours following oropharyngeal aspiration of saline or LPS, mice were killed with an overdose of sodium pentobarbital (80 mg/kg, i.p.). Bronchoalveolar lavage (BAL) was performed with two 1-ml aliquots of HBSS; recovery was >80% for each mouse. The left lungs were then inflated and fixed with 4% paraformaldehyde and used for preparation of slides for histopathological evaluation, and the right lungs were frozen and stored at −80°C. Recovered BAL fluid was processed and analyzed for total and differential cell counts by routine methods, and for levels of TNF-α, IL-6, and IL-1β using specific ELISA kits according to the manufacturer’s instructions (R&D Systems).
Lung histopathology
Sections of lung (5–6 µm) were stained with H&E, and a semiquantitative histopathologic scoring system was developed and used to analyze the sections as follows: 1) perivascular neutrophils (0, absent; 1, <10 per high power field; 2, 10–50 per high power field; 3, >50 per high power field); 2) perivascular hemorrhage (0, absent; 1, patchy, mild; 2, extensive, mild; 3, extensive, marked); 3) neutrophilic margination in medium-sized vessels (0, absent; 1, present). A total inflammatory score (range, 0–7), taken as the sum of the individual scores, was determined by a pathologist who was blinded to gender and treatment group assignments.
Immunoblotting
Whole-lung lysates were prepared from frozen lung tissues as described previously (15). Western blotting for TLR-4 protein was performed on lysates by using a goat anti-mouse polyclonal Ab (sc-16240; Santa Cruz Biotechnology) at a dilution of 1/100, followed by a HRP-conjugated bovine anti-goat polyclonal Ab (sc-2350; Santa Cruz Biotechnology) at a dilution of 1/1000. Western blotting for cyclooxygenase (COX)-2 protein was performed by using a rabbit anti-mouse polyclonal Ab (160126; Cayman Chemical) at a dilution of 1/2000, followed by a HRP-conjugated bovine anti-rabbit polyclonal Ab (sc-2374; Santa Cruz Biotechnology) at a dilution of 1/5000. Bound Abs were detected and visualized with an enhanced chemiluminescent detection system (Pierce). To account for potential variation in housekeeping gene expression and gel loading, blots were subsequently stripped and reprobed for GAPDH using a goat anti-human polyclonal Ab (sc-20357; Santa Cruz Biotechnology) that also recognizes murine GAPDH. Relative band intensities, expressed as arbitrary units of TLR-4 or COX-2 to GAPDH, were determined by densitometry with a ChemiImager 5500 system (Alpha Innotech Corporation).
Statistical analysis
Data are presented as group means ± SEM. Statistical comparisons among treatment groups were performed by randomized-design two-way ANOVA followed by the Newman-Keuls posthoc test for more than two groups, or by an unpaired Student’s t test for two groups, as appropriate. In all cases, statistical significance was defined as p < 0.05.
Results
Lung function and airway responsiveness in naive mice
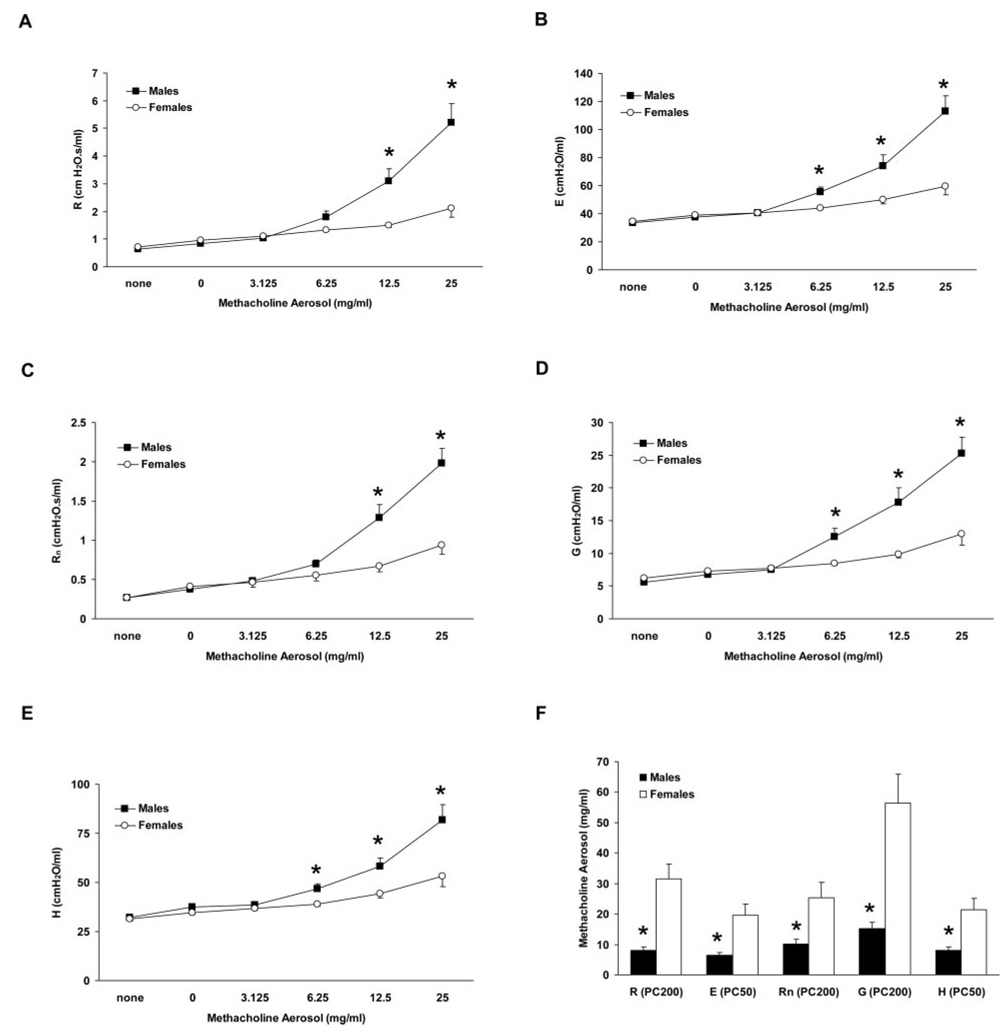
Invasive and noninvasive methods were used to compare baseline lung function and respiratory mechanics in male and female C57BL/6 mice. Naive mice were assessed by noninvasive plethysmography for a variety of respiratory parameters including breathing frequency, tidal volume, and others, and no differences between males and females were observed under basal conditions (Table I). Furthermore, invasive analysis of a separate group of naive mice revealed no baseline differences between genders for parameters fit to the single-compartment (R and E) or the constant-phase (Rn, G, and H) models of respiratory mechanics (Fig. 1, A–E). However, peak values for all of these parameters were significantly greater in males than in females following methacholine aerosol administration (Fig. 1, A–E). Calculated PC200 values for R, Rn, and G, and PC50 values for E and H were all significantly lower in males than in females (Fig. 1 F), reflecting the greater sensitivity of males. Thus, whereas naive male and female mice did not differ with regard to baseline lung function and respiratory mechanics, the airways of male mice were found to be considerably more responsive than those of females to cholinergic stimulation.
Table I.
Baseline respiratory parameters in naive male and female C57BL/6 mice assessed using noninvasive whole-body plethysmographya
| Gender | n | f | Vt | MV | PIF | PEF |
|---|---|---|---|---|---|---|
| Male | 21 | 492 ± 15 | 0.23 ± 0.01 | 110.2 ± 7.7 | 8.2 ± 0.6 | 5.3 ± 0.4 |
| Female | 20 | 509 ± 13 | 0.22 ± 0.01 | 107.6 ± 6.0 | 8.2 ± 0.5 | 5.2 ± 0.3 |
Abbreviations: n, Number of mice; f, breathing frequency (breaths/minute); Vt, tidal volume (milliliters/breath); MV, minute ventilation (milliliters/minute); PIF, peak inspiratory flow (milliliters/second); PEF, peak expiratory flow (milliliters/second).
FIGURE 1.
Analysis of respiratory mechanics and airway responsiveness in mechanically ventilated naive C57BL/6 mice. A–E, Airway responsiveness to aerosolized methacholine was greater in naive male mice than in naive female mice as determined by parameters fit to the single-compartment (A and B) and constant-phase (C–E) models of respiratory mechanics. F, Calculated provocative concentrations of methacholine aerosol for resistance (R), elastance (E), Newtonian resistance (Rn), tissue damping (G), and tissue elastance (H) were lower in males than in females, indicative of increased sensitivity to methacholine. *, p < 0.05 vs females, n = 23 males and 17 females.
LPS-induced hypothermia and airway inflammation
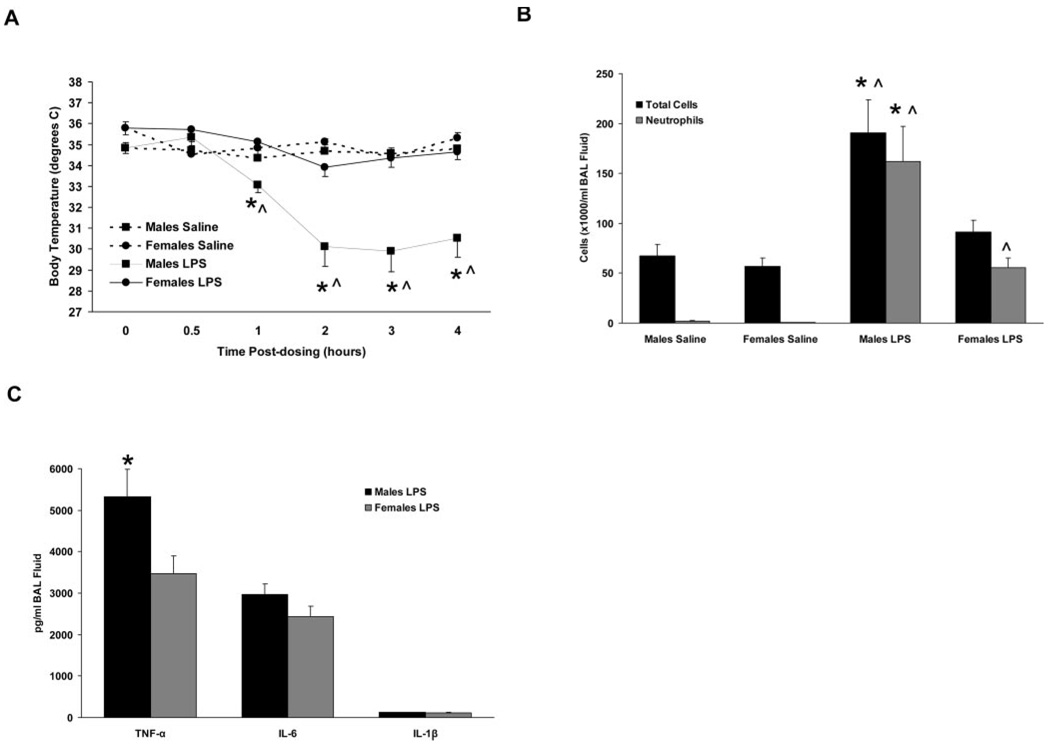
Intratracheal instillation of saline did not significantly alter the body temperature of male or female C57BL/6 mice during the 4-h course of measurements (Fig. 2A). Instillation of LPS resulted in a severe hypothermic response in male mice that began 1 h postdosing and continued for the duration of the measurement period. In contrast, female mice demonstrated a blunted hypothermic response to LPS, and temperatures were not statistically different from those of saline-treated female mice at any of the time points examined (Fig. 2A). These results are consistent with those of a previous study demonstrating gender differences in body temperature changes following intranasal LPS administration in mice (9).
FIGURE 2.
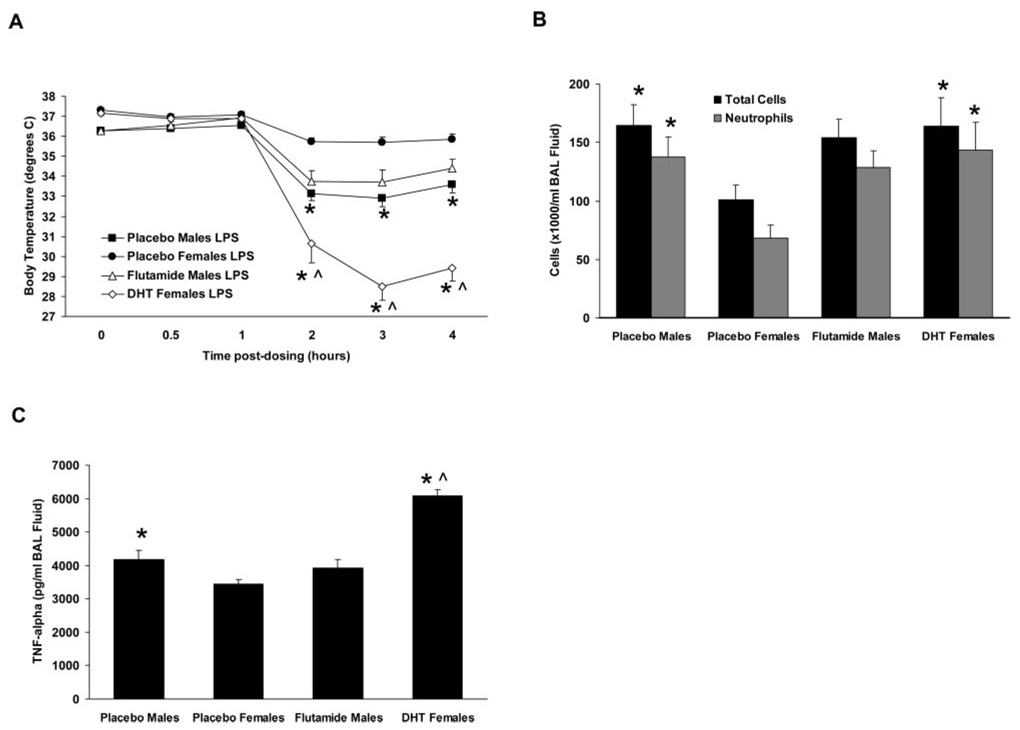
Inflammatory responses of intact C57BL/6 mice to airway administration of saline or LPS. A, Body temperatures were reduced in male mice treated with LPS compared with females treated with LPS and to saline-treated males and females. B and C, BAL fluid cells (B) and TNF-α content (C) were increased in male mice treated with LPS compared with all other treatment groups. *, p < 0.05 vs LPS-treated females. ^, p < 0.05 vs saline-treated animals of the same gender, n = 7–13 per group.
To determine the extent of airway inflammation, BAL was performed 6 h following LPS administration, and the resulting BAL fluid was examined for cell populations and for levels of proinflammatory cytokines. LPS-treated male mice had a significantly increased number of total BAL fluid cells compared with saline-treated mice and to LPS-treated female mice (Fig. 2B). Conversely, LPS-treated female mice had total BAL fluid cell numbers that were not significantly different from saline-treated mice, but demonstrated increased neutrophil content. Neutrophils accounted for 80% of the total BAL fluid cells in male LPS-treated mice and for 55% in females, corresponding to absolute neutrophil counts of 1.61 ± 0.35 × 105/ml BAL fluid in males and 0.55 ± 0.10 × 105/ml in females (p < 0.05; Fig. 2B). No differences in BAL fluid cell population were observed between saline-treated male and female mice.
Airway levels of several proinflammatory cytokines increase following exposure to LPS, likely contributing to the development and/or progression of LPS-induced airway inflammation. We measured BAL fluid levels of TNF-α, IL-6, and IL-1β at 6 h postdosing using specific ELISA kits, and found that all were below detectable levels in samples from saline-treated mice. Following LPS treatment, the BAL fluid level of TNF-α was elevated in both genders, but was significantly greater in males than in females (p < 0.05; Fig. 2C). BAL fluid levels of IL-6 and IL-1β were not significantly different between males and females following LPS treatment (Fig. 2C).
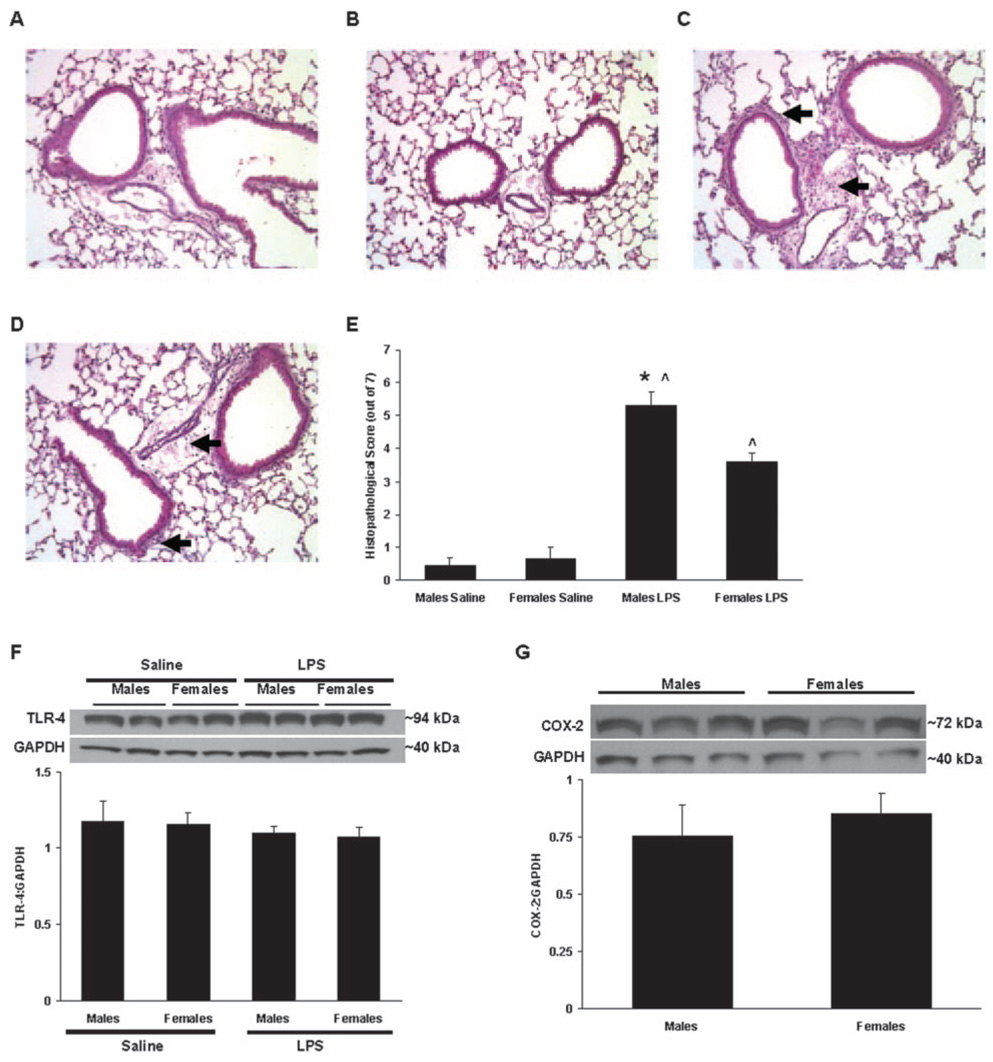
Histopathological evaluation of lung sections was performed using a semiquantitative scoring system to objectively compare the degree of inflammation among treatment groups. Representative lung sections from each treatment group are shown (Fig. 3, A–D). Saline-treated male and female mice had low histopathological scores, reflective of the normal lung architecture and relative lack of inflammation in these mice (Fig. 3E). In contrast, male and female mice treated with LPS demonstrated considerable perivascular and peribronchiolar hemorrhage and neutrophilic inflammation, which resulted in significantly increased scores compared with the saline-treated groups (Fig. 3E). However, these inflammatory parameters were found to be less severe in females, as reflected by the lower histopathological score of LPS-treated females compared with LPS-treated males (p < 0.05; Fig. 3E).
FIGURE 3.
Representative lung sections stained with H&E from C57BL/6 mice administered saline or LPS. Male (A and C) and female (B and D) mice were administered saline (A and B) or LPS (C and D), and tissues were collected 6 h later. Areas of peribronchiolar and perivascular inflammatory cell influx are indicated by arrows. E, Total histopathological scores were determined for mice administered saline or LPS (n = 6–13 per group). F, Whole-lung TLR-4 protein content in saline- and LPS-treated mice. Representative immunoblots from two mice per group are shown for TLR-4 (~94 kDa) and GAPDH (~40 kDa) (n = 4 per group). G, Whole-lung COX-2 protein content in LPS-treated mice. Representative immunoblots from three mice per group are shown for COX-2 (~72 kDa) and GAPDH (~40 kDa). *, p < 0.05 vs LPS-treated females. ^, p < 0.05 vs saline-treated animals of the same gender.
Whole-lung TLR-4 and COX-2 expression
LPS recognition and signaling involves a complex of proteins including TLR-4, MD-2, CD14, MyD88, and others. TLR-4 is intimately involved in the recognition of LPS, and mice genetically deficient in TLR-4 are resistant to the airway inflammatory effects of LPS (16). Thus, TLR-4 protein levels in whole-lung homogenates were examined to determine whether differential expression was underlying the observed gender differences in LPS responses. Immunoblot analysis revealed that naive (i.e., saline-treated) male and female C57BL/6 mice expressed equivalent amounts of whole-lung TLR-4 protein and that these levels did not appreciably change following LPS administration (Fig. 3F), indicating that differential TLR-4 expression before or after LPS exposure was not likely responsible for the observed gender differences in the inflammatory response to LPS. Similarly, whole-lung levels of COX-2 protein did not differ between LPS-treated male and female mice (Fig. 3G; constitutive COX-2 expression is low and was therefore not examined in lungs from naive mice), suggesting that differential induction of this acute-phase protein in response to LPS did not account for the observed differences in inflammation.
LPS-induced airway inflammation in gonadectomized and hormone-treated mice
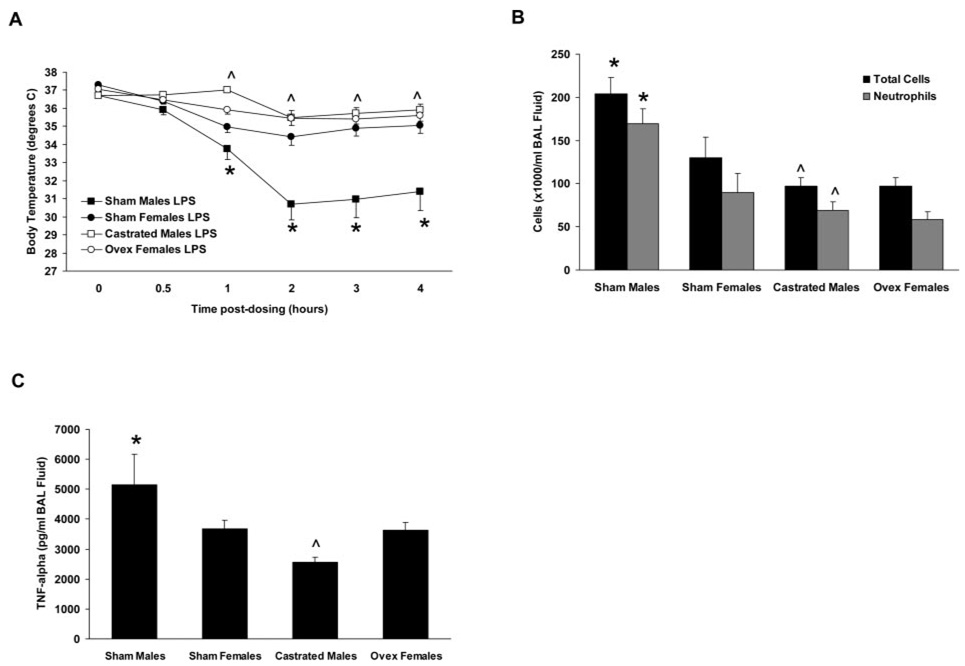
To delineate the contributions of sex hormones to LPS-induced airway inflammation, gonadectomy of male and female C57BL/6 mice was performed 3 wk before administration of LPS or saline. Sham-operated mice served as controls. Gonadectomy did not alter any of the outcomes measured in saline-treated male and female mice (data not shown). LPS-induced hypothermia was attenuated in castrated male mice (Fig. 4A), as was the influx of BAL fluid neutrophils and TNF-α content 6 h following LPS administration (p < 0.05; Fig. 4, B and C). In contrast, the inflammatory responses of ovariectomized female mice to LPS did not differ from those of sham-operated female mice (Fig. 4, A–C). These data suggest that, in this model, male sex hormones promote the inflammatory responses of the murine lung to LPS challenge, whereas female sex hormones do not influence these responses to a significant extent.
FIGURE 4.
Inflammatory responses to airway administration of LPS in gonadectomized and sham-operated C57BL/6 mice. A, Castration of male mice resulted in a reduced hypothermic response following LPS administration relative to sham-operated male mice, whereas ovariectomized female mice did not exhibit an altered response relative to sham-operated females. B and C, Castrated male mice exhibited decreased BAL fluid cells (B) and TNF-α content (C) in response to LPS administration relative to sham-operated male mice, whereas ovariectomized female mice did not exhibit altered responses relative to sham-operated females. *, p < 0.05 vs sham-operated females. ^, p < 0.05 vs sham-operated males. n = 8–9 per group.
To further examine the roles of male sex hormones, LPS was administered to male C57BL/6 mice implanted with pellets containing the androgen receptor antagonist flutamide, and to female C57BL/6 mice implanted with pellets containing DHT to increase their levels of circulating androgens. Mice implanted with placebo pellets served as controls. Implantation of placebo pellets did not alter any of the outcomes measured in saline-treated male and female mice (data not shown). Similarly, flutamide treatment did not alter the inflammatory outcomes in LPS-treated male mice (Fig. 5, A–C), suggesting that the unfavorable effects of androgens in this model are not mediated via the androgen receptor. However, the partial agonist activity of flutamide (and of its primary metabolite hydroxyflutamide) on the androgen receptor suggest caution in the interpretation of these data. Administration of DHT to female mice resulted in a significant augmentation of their inflammatory responses to LPS such that they resembled and often surpassed those of intact males (Fig. 5, A–C), further supporting an important role for androgens in mediating LPS responses in this model.
FIGURE 5.
Inflammatory responses to airway administration of LPS in intact male C57BL/6 mice treated with flutamide and in intact female C57BL/6 mice treated with DHT. A, Treatment of male mice with flutamide did not alter the hypothermic response relative to placebo-treated male mice. In contrast, female mice treated with DHT exhibited a more severe hypothermia following LPS administration relative to placebo-treated female mice. B and C, BAL fluid cells (B) and TNF-α content (C) were not altered in flutamide-treated male mice relative to placebo-treated male mice in response to LPS, whereas DHT-treated female mice exhibited increases in both parameters relative to placebo-treated female mice in response to LPS. *, p < 0.05 vs placebo-treated females. ^, p < 0.05 vs placebo-treated males. n = 8–14 per group.
LPS-induced airway hyperresponsiveness
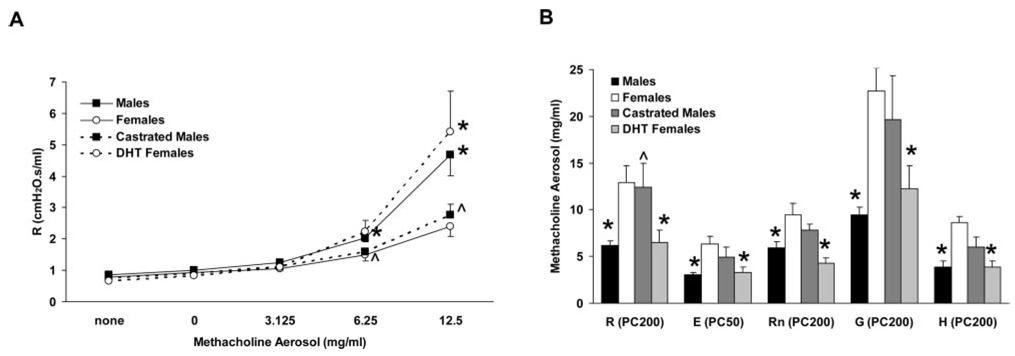
In addition to eliciting an inflammatory response, LPS induces airway hyperresponsiveness in humans and experimental animals (8, 16, 17). Given the observed detrimental role of androgens in the inflammatory response to LPS, we sought to determine the potential contribution of androgens to LPS-induced airway hyperresponsiveness. Thus, respiratory mechanics and airway responsiveness to aerosolized methacholine were examined 4 h following LPS administration in a separate group of C57BL/6 mice comprised of intact males and females, castrated males, and females implanted with DHT pellets. In the absence of aerosol challenge, no baseline differences were observed between the groups for the various parameters measured (Fig. 6A and data not shown). Airway hyperresponsiveness was evident in LPS-treated intact male and female mice, as these groups demonstrated peak R values following 6.25 and 12.5 mg/ml methacholine (Fig. 6A) that were similar to those observed in naive males and females following exposure to 12.5 and 25 mg/ml methacholine, respectively (Fig. 1A). Compared with intact male and female mice, respectively, LPS-induced airway hyperresponsiveness was reduced in castrated males and was increased in DHT-treated females (Fig. 6A). Increased sensitivity to methacholine in LPS-treated male mice compared with LPS-treated female mice was confirmed by the significantly lower PC200 and PC50 values for males (p < 0.05; Fig. 6B). Castration reduced the sensitivity of LPS-treated males to methacholine (i.e., increased PC200 and PC50 values), whereas DHT administration enhanced the sensitivity of LPS-treated females such that PC200 and PC50 values resembled those of intact males (Fig. 6B). Collectively, these data suggest that androgens promote airway hyperresponsiveness following LPS administration.
FIGURE 6.
Analysis of respiratory mechanics and airway responsiveness in mechanically ventilated C57BL/6 mice following administration of LPS. A, Total respiratory system resistance, determined by the single-compartment model of respiratory mechanics, demonstrated increased responsiveness to aerosolized methacholine in males and DHT-treated females in comparison to females and castrated males. B, Provocative concentrations for R, E, Rn, G, and H demonstrated that males were more sensitive than females to aerosolized methacholine. Castration reduced the sensitivity of males (increased PC values), whereas DHT administration increased the sensitivity of females (decreased PC values). *, p < 0.05 vs females. ^, p < 0.05 vs males, n = 7–8 per group.
Gender differences in airway responsiveness and LPS-induced airway inflammation are not mouse strain dependent
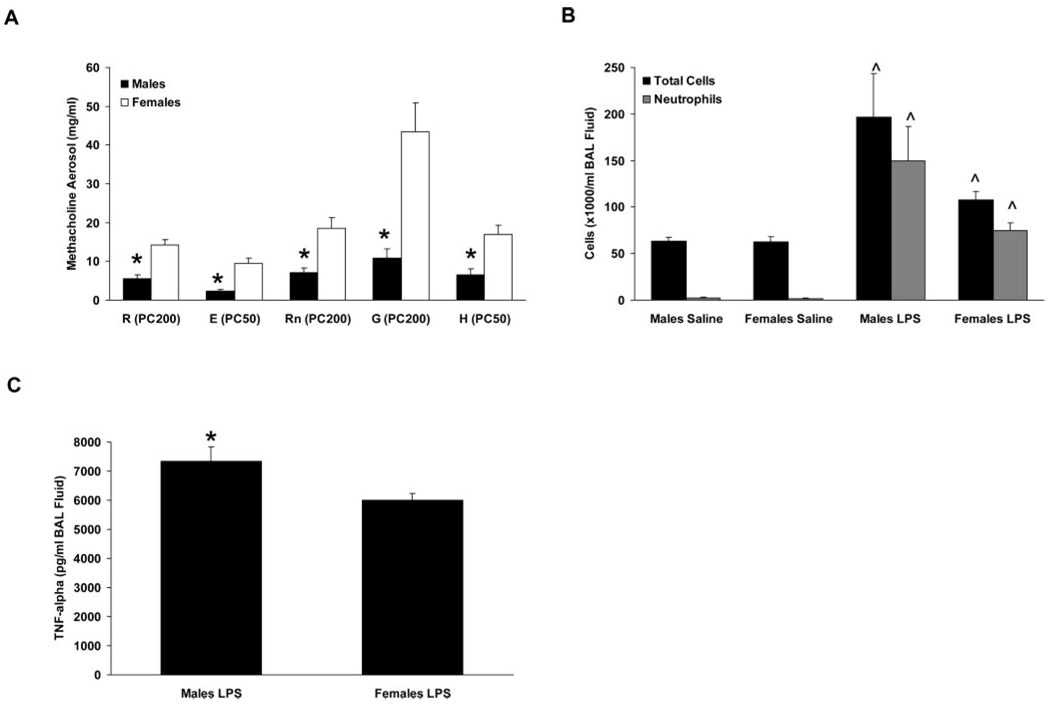
Data reported thus far were generated in studies conducted with C57BL/6 mice. To determine whether the observed gender differences in airway responsiveness and LPS-induced inflammation were strain dependent, additional studies were performed using BALB/c mice, another inbred strain that is commonly used in models of inflammatory lung diseases. Naive male and female BALB/c mice differed in their airway responses to methacholine aerosol administration, with males being more sensitive than females (as indicated by lower PC values) for all parameters measured (Fig. 7A). Similarly, LPS-induced inflammation was more severe in BALB/c males than in females as assessed by quantifying BAL fluid cell counts (p = 0.06 for total cells and p = 0.05 for neutrophils in LPS-treated males vs females; Fig. 7B) and TNF-α levels (Fig. 7C), and LPS-induced airway hyperresponsiveness was also greater in BALB/c males than in females (PC200 values for R were 3.2 ± 0.3 vs 7.0 ± 0.8 mg/ml for LPS-treated males and females, respectively; p < 0.05). These data indicate that the primary gender-related differences observed in C57BL/6 mice were also evident in BALB/c mice, suggesting that mouse strain did not significantly influence the observed phenotypes.
FIGURE 7.
Gender differences in airway responsiveness and LPS-induced airway inflammation in BALB/c mice. A, Calculated provocative concentrations of methacholine aerosol for resistance (R), elastance (E), Newtonian resistance (Rn), tissue damping (G), and tissue elastance (H) were lower in naive males than in females, indicative of increased sensitivity to methacholine. *, p < 0.05 vs females. n = 9 per group. B and C, BAL fluid cells (B) and TNF-α content (C) were increased in LPS-treated males compared with females. *, p < 0.05 vs LPS-treated females. ^, p < 0.05 vs saline-treated animals of the same gender. n = 9–22 per group.
Discussion
The extensive use of mice as a model to study human lung diseases underscores the importance of elucidating factors that contribute to the response of the murine lung to injurious agents. Considerable experimental and clinical data indicate that female sex hormones may influence lung function, airway responsiveness, and inflammation (4, 5, 8, 18); however, their effects appear to vary depending on the experimental system used and the end points analyzed. Indeed, whereas estradiol has been shown to reduce airway responsiveness in rats (18), equivocal effects of estrogens have been reported in animal models of lung injury and include anti-inflammatory (3, 5), proinflammatory (4), and profibrotic (6) outcomes. Conversely, much less is known regarding the influence of male sex hormones on lung function and disease. Thus, the present study was undertaken with two main objectives. The first objective was to determine whether there is a gender difference in lung function and airway responsiveness in naive C57BL/6 mice, an inbred strain commonly used as the background for transgenic and knockout lines and that is extensively used in a variety of lung disease models. Although this information would seem to be important in the design and interpretation of murine studies involving lung function analysis, this issue has not been adequately addressed in the scientific literature. The second objective was to investigate the potential contribution of gender to the development of airway inflammation and hyperresponsiveness in a murine model of nonallergic lung inflammation induced by LPS. Exposure of mice to LPS (via inhalation, intratracheal, or intranasal administration) is extensively used as a model to study mechanisms of acute lung injury and inflammation, and identification of factors contributing to LPS responsiveness could have implications for the treatment of inflammatory lung disease.
Airway responsiveness and basal lung function in naive mice were determined using invasive and noninvasive methods. Invasive measurements revealed no differences in baseline R, E, Rn, G, or H values between genders. The lack of any differences in basal respiratory mechanics between male and female C57BL/6 mice is consistent with the findings of a previous study (11). Noninvasive measurements also revealed no differences in various baseline respiratory parameters including breathing frequency, tidal volume, and others. However, following aerosol challenges with the cholinergic agonist methacholine, female mice were observed to be less responsive than male mice as assessed by the single-compartment and constant-phase models of respiratory mechanics. Combined, these findings indicate that naive male and female C57BL/6 mice do not differ with regard to lung function at baseline despite known differences in lung and airway size and alveolar number and volume (11, 19), but that considerable differences exist between males and females in central and peripheral airways responsiveness to cholinergic stimulation. Similar observations were made with BALB/c mice, suggesting that the gender disparity in airway responsiveness is not strain dependent.
Many laboratory studies investigating LPS-induced lung inflammation in mice use only one gender or do not consider potential differences between the genders if both are used, thus making comparisons among various studies difficult. A recent publication described a suppressive effect of ovarian hormones on LPS-induced acute lung inflammation in C57BL/6 mice that was associated with a reduction of airway IL-1β levels (10). In the present study, considerable gender differences in the acute airway inflammatory response to LPS were observed in C57BL/6 mice, in that males exhibited a greater degree of neutrophilic influx and TNF-α content in BAL fluid than females, and demonstrated a greater degree of inflammation as assessed histologically. Analogous gender differences in LPS-induced airway inflammation were observed in BALB/c mice, indicative of a gender-specific response in this model that does not appear to be mouse strain dependent. We considered the possibility that these gender differences may have resulted from differential pulmonary expression of proteins involved in LPS recognition and signaling or in the generation of proinflammatory mediators such as PGs. However, whole-lung protein levels of the LPS receptor TLR-4 did not differ between naive males and females or between males and females that had been administered LPS. Similarly, whole-lung COX-2 protein levels did not differ between LPS-treated male and female mice. Nevertheless, we cannot rule out lung cell-specific differences in expression of TLR-4 and/or COX-2, or differential expression of other molecules involved in LPS signaling (e.g., MD-2, CD14, others) or PG biosynthesis (e.g., PGE synthases, PGI synthase) as contributing to the observed gender differences.
Protective effects of estrogens in female mice, detrimental effects of androgens in male mice, or some combination thereof may have contributed to the observed gender differences in LPS-induced inflammation. Protective effects of estrogens seemed the most likely explanation, given that estrogens have demonstrated potent anti-inflammatory activity in other experimental lung injury models (3, 5, 10) and were recently shown to decrease LPS-induced airway inflammation in mice (10). However, androgen-mediated promotion of inflammation has been described in murine models of cutaneous wound healing (20) and ischemic renal injury (21), underscoring the potential for a significant contribution by male sex hormones. To investigate the relative contribution of sex hormones to the observed gender differences in LPS-induced lung inflammation, male and female C57BL/6 mice were castrated and ovariectomized, respectively, 3 wk before administration of LPS. A separate group of intact female mice was implanted 3 wk before study with s.c. pellets containing DHT to increase circulating androgen levels. Removal of the ovaries did not appreciably alter the airway inflammatory responses of female mice to LPS, suggesting that effects of estrogens alone did not account for the observed gender differences. Conversely, castration of male mice diminished the inflammatory responses to LPS whereas administration of DHT to intact female mice enhanced their responses to levels that were similar to those of intact males. Whereas Speyer et al. (10) observed a detrimental effect of ovariectomy on LPS-induced airway inflammation, we did not observe such a phenomenon. Differences between the results of these studies may be explained in part by differences in methodology, including the LPS doses administered. Collectively, results from the present study suggest that androgens possess proinflammatory properties in LPS-induced lung inflammation and are responsible, at least in part, for the observed gender differences in this murine model.
We surmised that the influence of androgens on LPS-induced airway inflammation may have been due to genomic effects mediated through interaction with the androgen receptor, leading to alteration of the transcription of genes important to airway inflammation. However, male C57BL/6 mice treated with the androgen receptor antagonist flutamide for 3 wk before study did not exhibit altered inflammatory responses to LPS. This suggests that, in contrast to the effects of castration, 3 wk of reduced androgen signaling due to receptor blockade was insufficient to alter the expression of genes critical to the inflammatory effects of LPS in the lung. This result may be due in part to the partial agonist activity of the flutamide metabolite hydroxyflutamide (22). Alternatively, the effects of androgens that are important to the inflammatory response to LPS may be nongenomic, similar to the detrimental effects ascribed to androgens in ischemic renal inflammation and injury (21). These effects may potentially be mediated through the recently described plasma membrane androgen receptor, which has been identified on various cell types including T cells (23) and macrophages (24) and the activation of which increases intracellular Ca2+ levels. However, its expression in the lung and potential involvement in LPS-induced lung inflammatory responses are unknown at present.
Having demonstrated that androgens promote the airway inflammatory response, we examined whether they also influence airway hyperresponsiveness induced by LPS. Both LPS-treated male and female C57BL/6 and BALB/c mice exhibited airway hyperresponsiveness to cholinergic stimulation, but males were more sensitive than females as evidenced by their lower PC200 values for R, Rn, and G, and lower PC50 values for E and H. Similar to the effects on inflammatory end points, alteration of androgen levels via castration of male mice and exogenous administration of DHT to female mice resulted in reduced and increased sensitivity to methacholine, respectively. These data extend the effects of androgens in the pulmonary response to LPS to include functional alterations characterized by increased airway responsiveness to cholinergic stimulation.
An interesting observation in the present study was the effect of LPS administration on body temperature. Rapid and severe hypothermia ensued in males in response to LPS, whereas the body temperature of females did not decline to the same extent. Whereas ovariectomy of female mice did not significantly alter body temperature following LPS, castration of male mice reduced the hypothermic response to LPS. In addition, administration of DHT to female mice resulted in a significant hypothermic response to LPS. The relationship between body temperature and inflammatory and functional responses to LPS in this and other studies is unclear. Studies in male rats have reported seemingly contradictory findings regarding the effects of body temperature on LPS-induced lung inflammation, with hypothermia shown to be both beneficial (25) and detrimental (26) to the inflammatory outcome. Furthermore, a previous study in mice (9) reported hypothermia following intranasal LPS administration that was more severe in males than in females, although with the exception of increased BAL fluid IL-6 content in males, no gender differences were observed for lung inflammatory parameters. At this time, we do not have a mechanistic explanation for the exaggerated hypothermic response to LPS that was observed in intact male mice and in female mice treated with DHT, but the data clearly suggest a role for androgens.
In summary, we have demonstrated that the airways of naive male mice of the C57BL/6 and BALB/c strains are considerably more responsive than those of females to cholinergic stimulation. These data provide important information to researchers studying murine lung function and murine models of lung disease in which airway responsiveness is a critical end point. Furthermore, we offer evidence that androgens are detrimental in the pathogenesis of LPS-induced airway inflammation and hyperresponsiveness, and suggest that gender be carefully considered when designing and interpreting murine studies of the pulmonary effects of LPS. Finally, we propose that sex hormones may provide novel targets for therapeutic intervention in inflammatory lung disease.
Acknowledgments
We are grateful to Herman Price, Clark Colegrove, and Ron Boone for expert technical assistance, to Sandy Ward for help with cell differential counting, and to Drs. Dori Germolec and Farhad Imani for helpful suggestions during preparation of this manuscript. These studies were conducted in part at the National Institute of Environmental Health Sciences Inhalation Facility under contract to Alion Science and Technology.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. J.W.C. is the recipient of a Research Fellowship Award from the Davies Charitable Foundation, and of a Senior Research Training Fellowship from the American Lung Association of North Carolina.
Abbreviations used in this paper: DHT, 5-α-dihydrotestosterone; BAL, bronchoalveolar lavage; COX, cyclooxygenase.
Disclosures The authors have no financial conflict of interest.
References
- 1.Caracta CF. Gender differences in pulmonary disease. Mt. Sinai J. Med. 2003;70:215–224. [PubMed] [Google Scholar]
- 2.Haggerty CL, Ness RB, Kelsey S, Waterer GW. The impact of estrogen and progesterone on asthma. Ann. Allergy Asthma Immunol. 2003;90:284–291. doi: 10.1016/S1081-1206(10)61794-2. [DOI] [PubMed] [Google Scholar]
- 3.Cuzzocrea S, Santagati S, Sautebin L, Mazzon E, Calabro G, Serraino I, Caputi AP, Maggi A. 17β-Estradiol antiinflammatory activity in carrageenan-induced pleurisy. Endocrinology. 2000;141:1455–1463. doi: 10.1210/endo.141.4.7404. [DOI] [PubMed] [Google Scholar]
- 4.Ligeiro de Oliveira AP, Oliveira-Filho RM, da Silva ZL, Borelli P, Tavares de Lima W. Regulation of allergic lung inflammation in rats: interaction between estradiol and corticosterone. Neuroimmunomodulation. 2004;11:20–27. doi: 10.1159/000072965. [DOI] [PubMed] [Google Scholar]
- 5.Shirai M, Sato A, Chida K. The influence of ovarian hormones on the granulomatous inflammatory process in the rat lung. Eur. Respir. J. 1995;8:272–277. doi: 10.1183/09031936.95.08020272. [DOI] [PubMed] [Google Scholar]
- 6.Gharaee-Kermani M, Hatano K, Nozaki Y, Phan SH. Gender-based differences in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 2005;166:1593–1606. doi: 10.1016/S0002-9440(10)62470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, Pauwels R, Sergysels R. Severity of asthma is related to endotoxin in house dust. Am. J. Respir. Crit. Care Med. 1996;154:1641–1646. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 8.Kline JN, Cowden JD, Hunninghake GW, Schutte BC, Watt JL, Wohlford-Lenane CL, Powers LS, Jones MP, Schwartz DA. Variable airway responsiveness to inhaled lipopolysaccharide. Am. J. Respir. Crit. Care Med. 1999;160:297–303. doi: 10.1164/ajrccm.160.1.9808144. [DOI] [PubMed] [Google Scholar]
- 9.Tesfaigzi Y, Rudolph K, Fischer MJ, Conn CA. Bcl-2 mediates sex-specific differences in recovery of mice from LPS-induced signs of sickness independent of IL-6. J. Appl. Physiol. 2001;91:2182–2189. doi: 10.1152/jappl.2001.91.5.2182. [DOI] [PubMed] [Google Scholar]
- 10.Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, Ward PA. Regulatory effects of estrogen on acute lung inflammation in mice. Am. J. Physiol. 2005;288:C881–C890. doi: 10.1152/ajpcell.00467.2004. [DOI] [PubMed] [Google Scholar]
- 11.Reinhard C, Eder G, Fuchs H, Ziesenis A, Heyder J, Schulz H. Inbred strain variation in lung function. Mamm. Genome. 2002;13:429–437. doi: 10.1007/s00335-002-3005-6. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Graves J, Bradbury JA, Zhao Y, Swope DL, King L, Qu W, Clark J, Myers P, Walker V, et al. Regulation of mouse renal CYP2J5 expression by sex hormones. Mol. Pharmacol. 2004;65:730–743. doi: 10.1124/mol.65.3.730. [DOI] [PubMed] [Google Scholar]
- 13.Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, Tiano HF, Lee CA, Langenbach R, Roggli VL, Zeldin DC. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J. Clin. Invest. 1999;104:721–732. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 15.Zeldin DC, Wohlford-Lenane C, Chulada P, Bradbury JA, Scarborough PE, Roggli V, Langenbach R, Schwartz DA. Airway inflammation and responsiveness in prostaglandin H synthase-deficient mice exposed to bacterial lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 2001;25:457–465. doi: 10.1165/ajrcmb.25.4.4505. [DOI] [PubMed] [Google Scholar]
- 16.Hollingsworth JW, 2nd, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am. J. Respir. Crit. Care Med. 2004;170:126–132. doi: 10.1164/rccm.200311-1499OC. [DOI] [PubMed] [Google Scholar]
- 17.Michel O, Dentener M, Corazza F, Buurman W, Rylander R. Healthy subjects express differences in clinical responses to inhaled lipopolysaccharide that are related with inflammation and with atopy. J. Allergy Clin. Immunol. 2001;107:797–804. doi: 10.1067/mai.2001.114249. [DOI] [PubMed] [Google Scholar]
- 18.Degano B, Prevost MC, Berger P, Molimard M, Pontier S, Rami J, Escamilla R. Estradiol decreases the acetylcholine-elicited airway reactivity in ovariectomized rats through an increase in epithelial acetylcholinesterase activity. Am. J. Respir. Crit. Care Med. 2001;164:1849–1854. doi: 10.1164/ajrccm.164.10.2102009. [DOI] [PubMed] [Google Scholar]
- 19.Massaro GD, Mortola JP, Massaro D. Sexual dimorphism in the architecture of the lung’s gas-exchange region. Proc. Natl. Acad. Sci. USA. 1995;92:1105–1107. doi: 10.1073/pnas.92.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J. Clin. Invest. 2002;110:615–624. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J. Biol. Chem. 2004;279:52282–52292. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- 22.Wong C, Kelce WR, Sar M, Wilson EM. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J. Biol. Chem. 1995;270:19998–20003. doi: 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]
- 23.Benten WP, Lieberherr M, Sekeris CE, Wunderlich F. Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. FEBS Lett. 1997;407:211–214. doi: 10.1016/s0014-5793(97)00346-3. [DOI] [PubMed] [Google Scholar]
- 24.Guo Z, Benten WP, Krucken J, Wunderlich F. Nongenomic testosterone calcium signaling: genotropic actions in androgen receptor-free macrophages. J. Biol. Chem. 2002;277:29600–29607. doi: 10.1074/jbc.M202997200. [DOI] [PubMed] [Google Scholar]
- 25.Lim CM, Kim MS, Ahn JJ, Kim MJ, Kwon Y, Lee I, Koh Y, Kim DS, Kim WD. Hypothermia protects against endotoxin-induced acute lung injury in rats. Intensive Care Med. 2003;29:453–459. doi: 10.1007/s00134-002-1529-6. [DOI] [PubMed] [Google Scholar]
- 26.Wang GC, Chi WM, Perng WC, Huang KL. Body temperature control in sepsis-induced acute lung injury. Chin. J. Physiol. 2003;46:151–157. [PubMed] [Google Scholar]