Abstract
Previous studies using genetic and lesion approaches have shown that the neuropeptide Urocortin 1 (Ucn1) is involved in regulating alcohol consumption. Ucn1 is a corticotropin releasing factor (CRF)-like peptide that binds CRF1 and CRF2 receptors. Perioculomotor urocortin-containing neurons (pIIIu), also known as the non-preganglionic Edinger-Westphal nucleus, are the major source of Ucn1 in the brain and are known to innervate the lateral septum. Thus, the present study tested whether Ucn1 could regulate alcohol consumption through the lateral septum. In a series of experiments Ucn1 or CRF were bilaterally injected at various doses into the lateral septum of male C57BL/6J mice. Consumption of 20% v/v ethanol or water was tested immediately after the injections using a modification of a 2-hour limited access sweetener-free “drinking-in-the-dark” procedure. Ucn1 significantly suppressed ethanol consumption when administered prior to the third ethanol drinking session (the expression phase of ethanol drinking) at doses as low as 6 pmol. Ethanol intake was differentially sensitive to Ucn1, as equivalent doses of this peptide did not suppress water consumption. In contrast, CRF suppressed both ethanol and water intake at 40 and 60 pmol, but not at lower doses. Repeated administration of Ucn1 during the acquisition of alcohol consumption showed that 40 pmol (but not 2 or 0.1 pmol) significantly attenuated ethanol intake. Repeated administration of Ucn1 also resulted in a decrease of ethanol intake in sham-injected animals, a finding suggesting that the suppressive effect of Ucn1 on ethanol intake can be conditioned. Taken together, these studies confirm the importance of lateral septum innervation by Ucn1 in the regulation of alcohol consumption.
A variety of evidence has suggested that the neuropeptide corticotropin releasing factor (CRF) plays an important role in the development of alcoholism (Koob and Le Moal, 2001). CRF is known as the prototypical ligand acting on CRF receptors (Vale et al., 1981). However, recent studies indicate that CRF is not the sole ligand of CRF receptors, and that the endogenous neuropeptides urocortin 1 (Ucn1), urocortin 2 (Ucn2, also known as stresscopin-related peptide) and urocortin 3 (Ucn3, also known as stresscopin) also bind these receptors (Vaughan et al., 1995, Hsu and Hsueh, 2001, Lewis et al., 2001, Reyes et al., 2001). Moreover, these studies show that all three urocortins have higher affinity than CRF at CRF2 receptors, and that Ucn1 has higher or equal affinity than CRF at CRF1 receptors. The complexity of the CRF system requires that the potential role of each of the CRF-like peptides be considered in studying the pathogenesis of alcoholism.
While substantial evidence has accumulated that CRF plays a role in withdrawal- and stress-induced increases in alcohol drinking (Sarnyai et al., 2001, Weiss et al., 2001, Olive et al., 2002, Breese et al., 2005, Finn et al., 2007) recent studies show that Ucn1 is also important for controlling alcohol intake (Ryabinin and Weitemier, 2006). Ucn1 in the brain is primarily expressed in the perioculomotor urocortin-containing population of neurons (pIIIu), a brain region also known as the non-preganglionic Edinger-Westphal nucleus (npEW) (Vaughan et al., 1995, Kozicz et al., 1998, Ryabinin et al., 2005, Weitemier et al., 2005, May et al., 2007). The pIIIu sends its projections to the lateral septum, dorsal raphe, spinal cord and several other brain regions, but not brain regions traditionally thought to be involved in the rewarding properties of drugs of abuse, such as the nucleus accumbens or amygdala (Bittencourt et al., 1999, Bachtell et al., 2004, Weitemier et al., 2005). Importantly, it has been repeatedly shown that alcohol consumption in mice and rats leads to preferential induction of the activity marker c-Fos in pIIIu (Topple et al., 1988, Bachtell et al., 1999, Ryabinin et al., 2001, Weitemier et al., 2001). Moreover, genetic studies have shown that the ethanol-preferring C57BL/6J inbred strain has significantly more Ucn1-positive cells than the alcohol-avoiding DBA/2J inbred strain (Bachtell et al., 2002, Weitemier et al., 2005). Similarly, mice selectively-bred for increased alcohol consumption have higher numbers of Ucn1-containing neurons in the pIIIu than mice selectively-bred for low alcohol consumption (Bachtell et al., 2003), with a comparable line difference in mice selectively-bred for high versus low alcohol-induced place preference (Kiianmaa et al., 2003). While the data in rats selectively-bred for differences in alcohol intake are more complex with differences between lines in both directions, a meta-analysis has shown that all of the alcohol-preferring rat lines analyzed to date have higher numbers of Ucn1-containing fibers in the lateral septum than the alcohol-avoiding rats (Turek and Ryabinin, 2005). These findings suggest that Ucn1’s regulation of alcohol consumption occurs through innervation of the lateral septum.
In agreement with genetic studies, lesions of the pIIIu in mice, a procedure leading to a decrease in Ucn1 innervation of the lateral septum, decreased ethanol consumption (Bachtell et al., 2004, Weitemier and Ryabinin, 2005). More recent studies have shown that injections of Ucn1 into the dorsal raphe, another important target of the pIIIu, reduced food and water consumption without affecting alcohol preference (Weitemier and Ryabinin, 2006). Therefore, we hypothesized that the lateral septum was the brain region innervated by Ucn1 that could regulate alcohol consumption.
To test this hypothesis we used a recently developed “drinking-in-the-dark” (DID) procedure. This procedure takes advantage of higher levels of activity, fluid and alcohol consumption that are observed in mice during early hours into the dark portion of the circadian cycle (Freund, 1970, Goldstein and Kakihana, 1977, Kurokawa et al., 2000). Placing the ethanol-containing drinking cylinders onto mouse cages at the peak time of activity allows mice to achieve high levels of ethanol intake during a short period of time with no need of fluid deprivation or sweetened ethanol solutions (Ryabinin et al., 2003b, Sharpe et al., 2005). Alcohol intake in such a procedure leads to high blood ethanol levels accompanied by significant signs of alcohol intoxication, making this a relevant model of human alcohol consumption (Rhodes et al., 2005, Sharpe et al., 2005, Rhodes et al., 2007). At the same time, the relatively short alcohol DID session permits the detection of potentially transient effects of experimental manipulations on ethanol intake.
The first three experiments of this study compared the effects of different doses of CRF and Ucn1 on the expression of alcohol drinking in mice using the DID procedure (during the third drinking session). The fourth experiment tested the effects of repeated Ucn1 administration during the acquisition of alcohol consumption in the first three DID drinking sessions, as well as the possibility of conditioning effects of Ucn1 on ethanol drinking in the fourth drinking session performed without administration of Ucn1.
Materials and Methods
Experimental subjects
Drug naive male C57BL/6J mice were either purchased from Jackson Laboratories West (Davis, CA) or obtained from in-house breeding at the Portland VA Medical Center. Mice were housed at 4-5 per cage with food and water available ad libitum on a reverse light:dark cycle (lights off at 8AM). At six to seven weeks of age mice underwent a surgery to implant bilateral cannulae aimed at the lateral septum. Animals were housed individually after surgery until the end of the experiment. Experiments were initiated at 6-8 days following surgery. During the two days prior to experiments, the water bottles were replaced with drinking tubes containing water to habituate mice to drinking from the tubes. Drinking tubes were standard 25 ml graduated cylinders that were fitted with sipper tubes and allowed volumes to be read to the nearest 0.1 ml. Average volume depleted from tubes in control cages were subtracted from individual values for each mouse on each day to control for spillage or evaporation. Mice were handled and weighed on each of these days. A recent 2-hour version of the DID procedure was adapted to investigate the effects of the neuropeptides on alcohol consumption (Rhodes et al., 2005, Rhodes et al., 2007). This version is different from the earlier developed procedure in that animals are not exposed to gradually increasing concentrations of ethanol (Ryabinin et al., 2003, Sharpe et al, 2005), but are immediately offered a 20% ethanol solution. Both versions of the procedure result in behavioral intoxication and blood alcohol levels of 1.5 mg/ml without the need for food or water deprivation (Ryabinin et al., 2003b, Rhodes et al., 2005, Sharpe et al., 2005, Rhodes et al., 2007). The drinking cylinders containing 20% ethanol were replaced with drinking cylinders containing water immediately after the 2-hour drinking session until the next day. All manipulations during the dark phase of the circadian cycle were performed under red light illumination. Procedural details for each experiment are provided in Table 1.
Table 1.
General Experimental Design
| Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|
| Experiment 1 | ||||
| LS injection | No injection | Sham | Vehicle CRF (40 pmol) Ucn1 (40 pmol) |
Not tested |
| Drinking solution (2 hr intake) | Ethanol | Ethanol (baseline) | Ethanol | Not tested |
| Experiment 2 | ||||
| Phase 1 | ||||
| LS injection | No injection | Sham | Vehicle CRF (6, 20 or 60 pmol) |
Sham |
| Drinking solution (2 hr intake) | Ethanol | Ethanol (baseline) | Ethanol | Ethanol |
| Phase 2 | 7 day break between Phase 1 & 2 | |||
| LS injection | No injection | Sham | Vehicle CRF (6, 20 or 60 pmol) |
Sham |
| Drinking solution (2 hr intake) | Water | Water (baseline) | Water | Water |
| Experiment 3 | ||||
| Phase 1 | ||||
| LS injection | No injection | Sham | Vehicle Ucn1 (6, 20 or 60 pmol) |
Sham |
| Drinking solution (2 hr intake) | Ethanol | Ethanol (baseline) | Ethanol | Ethanol |
| Phase 2 | 7 day break between Phase 1 & 2 | |||
| LS injection | No injection | Sham | Vehicle Ucn1 (6, 20 or 60 pmol) |
Sham |
| Drinking solution (2 hr intake) | Water | Water (baseline) | Water | Water |
| Experiment 4 | ||||
| LS injection | Vehicle Ucn1 (0.1, 2 or 40 pmol) |
Vehicle Ucn1 (0.1, 2 or 40 pmol) |
Vehicle Ucn1 (0.1, 2 or 40 pmol) |
Sham |
| Drinking solution (2 hr intake) | Ethanol | Ethanol | Ethanol | Ethanol |
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and disseminated by the U.S. National Institutes of Health and were approved by the local Institutional Animal Care and Use Committee. Every effort was made to minimize the number of animals used and their suffering.
Surgery
Under isoflurane anesthesia, mice were placed into a stereotaxic apparatus (Cartesian Instruments, Sterotaxic Alignment System; David Kopf Inc.) and implanted with 26 gauge stainless steel guide cannulae (Plastics One, Roanoke, VA) directly above the lateral septum. Depending on the experiment, implant coordinates were: AP; range of +0.24 to +0.26 from Bregma; L ±0.35 mm from the midsaggital suture; V; range of -2.0 to -2.5 from the skull surface according to the atlas of Paxinos & Franklin (2001). Once implanted, guide cannulae were secured to an array of four skull screws using dental acrylic and were kept patent with 33 gauge stylets (Plastics One). Animals were allowed a minimum of one week for recovery. For delivery of drugs into the lateral septum, microinjectors made from stainless steel hypodermic tubing (200 μm OD; Small Parts, Miami Lakes, FL) were lowered to a point 0.8 mm beyond the guide cannulae to reach -2.8 to -3.3 mm ventral to the skull.
Experiment 1. Effect of a single dose of Ucn1 or CRF on limited access ethanol consumption
On the first day of the experiment, animals were briefly handled, weighed and placed back into their cage at 2-3 hours into the dark phase. The water-containing drinking cylinders were then replaced with cylinders containing 20% v/v ethanol for two hours. Ethanol consumption was analyzed by measuring the decrease in fluid level. After the ethanol drinking session, water-containing cylinders were placed back onto the cage. At the identical time on Day 2 of the experiment, animals were microinjected with 200 nl/side of vehicle approximately 5-15 min prior to their 2 hours of access to the ethanol-containing cylinders. At the same time on Day 3, separate groups of animals received a microinjection of 40 pmol (20 pmol/side) of Ucn1, CRF or vehicle approximately 5-15 min prior to their 2 hours of ethanol access. Drug infusions were delivered in a volume of 200 nl over 1 minute, followed by a 30 second waiting period to allow diffusion of the peptides away from the tips of the microinjectors. Ucn 1 and CRF peptides were purchased from Phoenix Pharmaceuticals (Burlingame, CA). Since Ucn1 is highly hydrophobic, the vehicle in this experiment was artificial cerebral spinal fluid (ACSF) containing 50% DMSO. ACSF consisted of (in mM) 120 NaCl, 4.8 KCl, 2.5 CaCl2; 1.2 MgCl2; 25 NaHCO3; 1.2 NaH2PO4; pH 7.4.
Experiment 2. Effect of CRF dose response on limited access ethanol and water consumption
This experiment was performed similarly to the one described in Experiment 1, except that only CRF (and not Ucn1) was bilaterally injected in the following doses: 0, 6, 20 or 60 pmol per animal (0, 3, 10 or 30 pmol in 300 nl per side) and that it consisted of two phases. Phase 1 of this experiment was designed to test the effects of CRF on ethanol consumption. Phase 2 was designed to test the effects of CRF on water consumption. Since CRF is not hydrophobic, ACSF (without DMSO) served as the vehicle. As in experiment 1, on Day 1 of Phase 1, consumption of 20% ethanol was measured following handling of animals, on Day 2 following sham-injections, and on Day 3 following injections of CRF. In addition, a 2-hour ethanol drinking session was performed on Day 4 to test if exposure to the peptide during the previous day (i.e. Day 3) had any residual effect on ethanol consumption on the following day. Since water-containing cylinders were replaced after each 2-hour session, we also measured water intake during the 22 hours between ethanol-drinking sessions. This measurement helped to ensure that there were no prolonged effects of the peptides on water consumption following the ethanol drinking sessions. Following the 4 days of ethanol drinking sessions, the animals were given 7 days of rest (water consumption, no handling) prior to Phase 2 of the experiment. Phase 2 was conducted exactly like Phase 1, except that water (instead of a 20% ethanol solution) was used during the 2-hour drinking session. This phase tested whether the effects of CRF were specific to ethanol consumption.
Experiment 3. Effect of Ucn1 dose response on limited access ethanol and water consumption
This experiment was performed as described in Experiment 2 with Phase 1 testing ethanol consumption and Phase 2 testing water consumption. The exception was that Ucn1 (and not CRF) was bilaterally injected at the same doses as in Experiment 2 (0, 6, 20 and 60 pmol per animal). ACSF containing 50% DMSO served as a vehicle. Microinjection parameters were identical to those used in Experiment 2.
Experiment 4. Effect of Ucn1 dose response on the acquisition of limited access ethanol intake with the DID procedure
In this experiment, separate groups of animals were repeatedly injected with Ucn1 in the following doses: 0, 0.1, 2 and 40 pmol per animal (0, 0.05, 1 or 20 pmol in 200 nl per side). Animals were randomly assigned to a dose condition and received a daily microinjection of Ucn1 at 3 hours into the dark cycle on Days 1-3, immediately prior to the 2-hour drinking session with 20% ethanol. ACSF containing 25% DMSO was used as the vehicle in this experiment.
Consumption of water was measured for 22 hours between ethanol drinking sessions. Finally, at the same time of day on Day 4, animals received a sham injection prior to their 2 hours of ethanol access to test whether repeated experience with ethanol and Ucn1 altered alcohol consumption.
Histology and Data analysis
At the end of the studies, brains were removed and immediately frozen in isopentane on dry ice. Brains were sliced on a cryostat (40 μm sections) and mounted to identify injection tracks. Only animals with confirmed bilateral injection placements in the intermediate and dorsal portions of the lateral septum were included in statistical analyses. There were no animals in which injections were located in the ventral portion of lateral septum. Because the missed locations of microinjections were either complete misses (typically dorsal location) or hit only one side of the lateral septum (resulting in injection of half the peptide dose into the lateral septum), these groups could not be combined as position controls in the statistical analysis.
Ethanol dose (g/kg) and water intake (ml) were the dependent measures that were analyzed. All statistical analyses were performed with the Statview 5 software package (SAS Institute Inc.). Since peptides were injected only during one experimental day for Experiments 1-3, a one-way ANOVA was used to analyze the differences between groups on each experimental day. In Experiments 2 and 3, this analysis was followed by a mixed-design ANOVA comparing groups of animals differing in ethanol or water consumption (between-subject factor) across two days (within-subject factor). In Experiment 4, a mixed design ANOVA (with treatment as between-subject factor and day as within-subject factor) was used for days with repeated peptide treatment. Data from Day 4 of Experiment 4 (with sham injections) were analyzed by a one-way ANOVA. Body weights in each experiment were analyzed by repeated measures ANOVA (treatment as between-subject factor and day as within-subject factor). No significant effects of treatment or treatment by day interactions were observed for body weight, indicating that the effects of treatments on ethanol or water intake were not influenced by body weight (data not shown).
Results
Experiment 1. Effect of a single dose of Ucn1 or CRF on limited access ethanol consumption
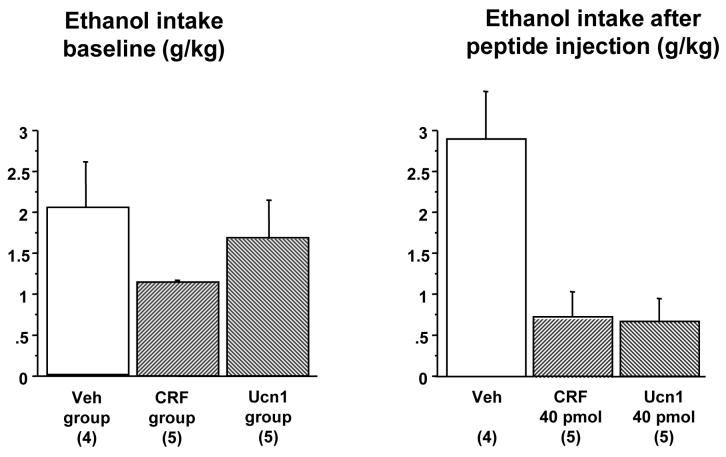
This experiment compared the effect of 40 pmol CRF or Ucn1 on ethanol consumption using the 2-hour “drinking-in-the-dark” procedure during the third ethanol DID session. Of twenty-four mice that began the experiment, one mouse was eliminated because of lost cannula patency. Fourteen animals were confirmed to have correct bilateral placement of injections and were used in the statistical analysis (Figure 1). On the two days prior to peptide microinjection, ethanol consumption did not differ between the groups of animals subsequently assigned to the different treatments [F(2,11)=1.27, p=n.s. on Day 2, Figure 2]. On Day 3, a significant attenuation of ethanol intake was observed after administration of either 40 pmol CRF or 40 pmol Ucn1 [F(2,11)=10.33, p=0.003]. Post-hoc Fisher’s PLSD confirmed a significant difference in intake between the vehicle versus the CRF (p=0.002) and the Ucn1 (p=0.002) groups. Ethanol intake did not differ between the animals injected with CRF or Ucn1. There were no group differences in water intake during the 22 hours after the 2-hour ethanol drinking session [F(2,11)=1.7, p=n.s.], indicating that the peptide injections did not have a prolonged effect on fluid consumption.
Figure 1. Neuroanatomical confirmation of successful injection into the lateral septum.
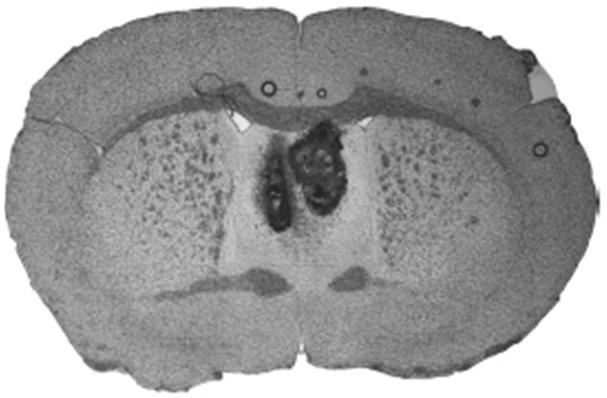
Only data from animals with confirmed bilateral injections were used in the statistical analysis of the behavioral experiments.
Figure 2. Effects of a single dose of CRF or Ucn1 in the lateral septum on limited access ethanol consumption.
Left panel: Baseline intake of 20% v/v ethanol during a 2-hour session on Day 2 of Experiment 1 in three groups of animals after sham injections that were subsequently divided into treatment groups. No significant differences between groups are observed. Right panel: Intake of 20% ethanol during the 2-hour session on Day 3 of Experiment 1 following microinjections of vehicle (Veh), 40 pmol CRF or 40 pmol Ucn1. Both CRF and Ucn1 significantly suppressed ethanol consumption. The number of animals per group is shown in parenthesis.
Experiment 2. Effect of CRF dose response on limited access ethanol and water consumption
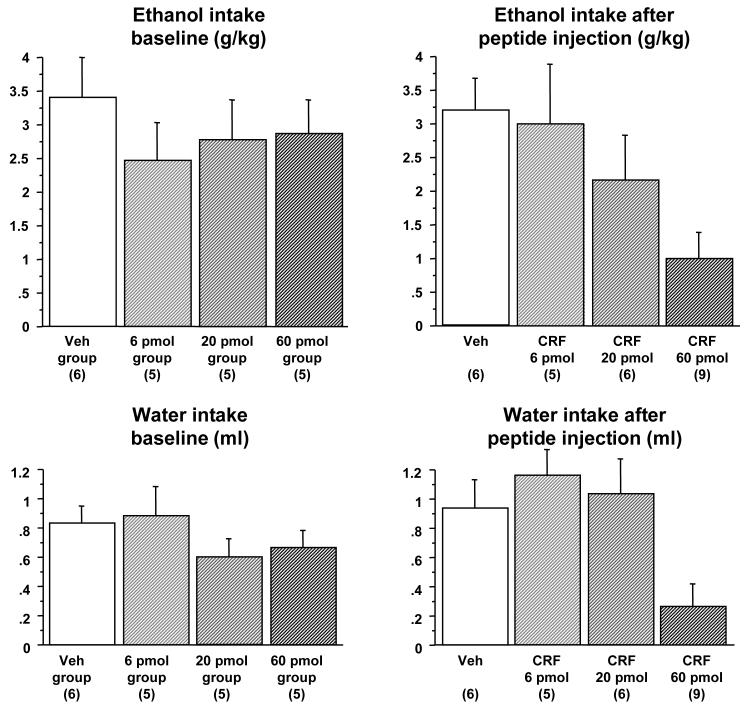
This experiment tested the effect of three different doses of CRF on ethanol or water consumption during the third DID session in Phase 1 or Phase 2 of the experiment, respectively. Of the forty-three mice that began the experiment, twenty-six animals were confirmed to have correct bilateral placement of injections and were used in statistical analysis. On the two days prior to CRF microinjection, there were no differences in ethanol intake between the groups of animals subsequently assigned to the different CRF dose groups [F(3,22)=0.43, p=n.s. for Day 2, Figure 3]. On Day 3, a significant attenuation of ethanol intake was observed after administration of the 60 pmol dose of CRF, but not after other doses of this peptide [F(3,22)=3.5, p=0.033]. Post-hoc Fisher’s PLSD confirmed that there was a significant difference in intake between the animals injected with 60 pmol CRF versus the vehicle-injected animals (p=0.009) and versus the animals injected with 6 pmol CRF. Ethanol consumption was also analyzed by a repeated measures ANOVA, comparing animals treated with 60 pmol of CRF versus all other animals (between-subjects comparison) across the baseline day and day of treatment (within-subject comparison). This analysis showed a significant effect of treatment [F(1,24)=4.3, p=0.049], significant effect of day [F(1,24)=6.7, p=0.016] and a significant interaction between these factors [F(1,24)]=5.09, p=0.034], confirming that differences in ethanol consumption between animals treated with the high dose of CRF versus other groups were not due to innate differences in ethanol consumption between individual mice. Additional analysis showed that there were no group differences in water intake during the 22 hours after the 2-hour ethanol drinking session [F(3,21)=1.58, p=n.s.], indicating a lack of prolonged effects of peptide injections on water consumption. No residual effects of peptide injections were observed on ethanol consumption during the DID session after sham injections on Day 4 of the experiment [F(3,22)=1.37, p=n.s., data not shown].
Figure 3. Dose dependent suppression of limited access ethanol and water consumption following microinjection of CRF into the lateral septum.
Top left panel: Baseline intake of 20% v/v ethanol during a 2-hour session on Day 2 of Phase 1 of Experiment 2 following sham injections in four groups of animals that were subsequently divided into dose groups. No significant differences between groups are observed in baseline 2 hr ethanol intake. Top right panel: Intake of 20% ethanol during a 2-hour session on Day 3 of Phase 1 of Experiment 2 following microinjections of vehicle (Veh), 6, 20 or 60 pmol of CRF. The highest dose of CRF significantly attenuated ethanol intake. Bottom left panel: Intake of water during a 2-hour session on Day 2 of Phase 2 of Experiment 2 following sham injections in four groups of animals that were subsequently divided into dose groups. No significant differences are observed in baseline 2 hr water intake. Bottom right panel: Intake of water during a 2-hour session on Day 3 of Phase 2 of Experiment 2 following injections of Veh, 6, 20 or 60 pmol of CRF. The highest dose of CRF significantly attenuated water intake. The number of animals per group is shown in parenthesis.
Phase 2 of the experiment replicated Phase 1, except that water (not ethanol) was used during all DID sessions. During the two days preceding CRF microinjection, there were no differences in water intake between the groups of animals that were subsequently assigned to the different dose groups [F (3,22)=0.85, p=n.s. for Day 2], indicating lack of residual effects from the previous phase of the experiment. On Day 3, a significant attenuation of water intake was observed after administration of the 60 pmol dose of CRF, but not after other doses of this peptide [F(3,22)=5.31, p=0.007]. Post-hoc Fisher’s PLSD confirmed that water intake was significantly decreased in the animals injected with the 60 pmol dose of CRF, when compared with values in the animals injected with vehicle (p=0.015), 6 pmol of CRF (p=0.003), and 20 pmol of CRF (p=0.006). These data indicated that the intra-lateral septum injection of CRF attenuated intake during the DID sessions in a non-specific manner, inhibiting intake of both ethanol and water. Water consumption was also analyzed by repeated measures ANOVA, comparing animals treated with 60 pmol of CRF versus all other mice (between-subjects) across the baseline day and day of treatment (within-subjects). This analysis showed a significant effect of treatment [F(1,24)=13.3, p=0.0012], no effect of day [F(1,24)=0.28, p=n.s.] and a significant interaction between these factors [F(1,24)]=7.64, p=0.011], confirming that differences in water consumption between animals treated with the high dose of CRF versus other groups were not due to innate differences in water consumption between individual animals. Similar to Phase 1 of this experiment, there were no prolonged effects of CRF during the 22 hours after the 2-hour water DID session [F(3,22)=1.89, p=n.s.] and no residual effects of CRF on water intake after sham injections on Day 4 [F(3,22)=2.55, p=n.s., data not shown].
Experiment 3. Effect of Ucn1 dose response on limited access ethanol and water consumption
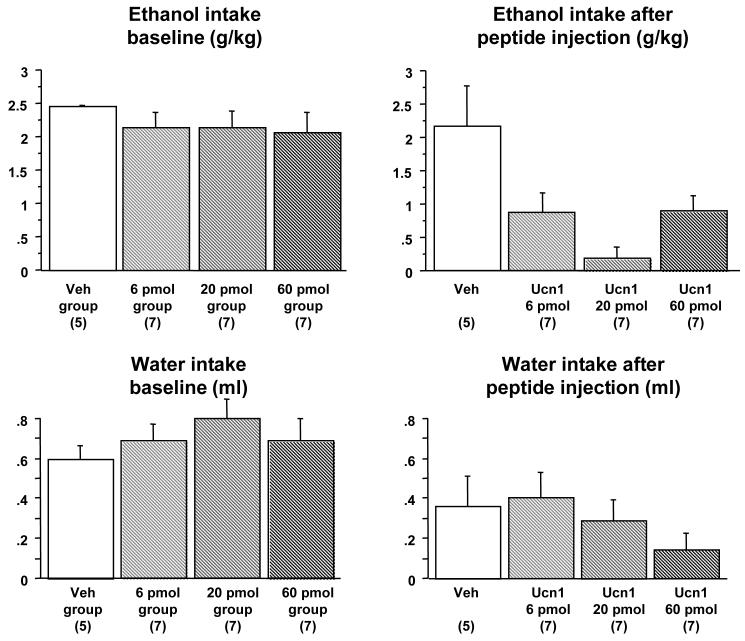
This experiment tested the effect of three different doses of Ucn1 on ethanol or water consumption during the third DID session in Phase 1 or Phase 2 of the experiment, respectively. Of the forty-nine mice that began the experiment, five animals were eliminated from the study because of lack of cannulae patency. Twenty-six animals were confirmed to have correct bilateral placement of injections and were used in the statistical analysis. During the two days preceding Ucn1 microinjection, there were no differences in ethanol intake between the groups of animals subsequently assigned to different dose groups [F(3,22)=0.41, p=n.s. for Day 2, Figure 4]. On Day 3, a significant attenuation of ethanol intake was observed after administration of all doses of Ucn1 [F(3,22)=5.39, p=0.006]. Post-hoc Fisher’s PLSD confirmed that ethanol intake in the vehicle-injected animals was significantly higher than values in the mice microinjected with 6 pmol (p=0.016), 20 pmol (p=0.0006), and 60 pmol (p=0.017) of Ucn1. Ethanol intake did not differ between the animals injected with the different doses of Ucn1. Thus, Ucn1 appeared to have higher potency to inhibit ethanol consumption than CRF. Ethanol consumption was also analyzed by repeated measures ANOVA, comparing animals treated with vehicle versus all animals treated with Ucn1 (between-subjects) across the baseline day and day of treatment (within-subjects). This analysis showed a significant effect of treatment [F(1,24)=12.9, p=0.0015], a significant effect of day [F(1,24)=9.67, p=0.0048] and a significant interaction between these factors [F(1,24)]=5.19, p=0.032], confirming that differences in ethanol consumption between animals treated with Ucn1 versus vehicle were not due to innate differences in water consumption between individual animals. There were no significant group differences in water intake during the 22 hours after the 2-hour ethanol drinking session [F(3,22=2.63, p=n.s.], suggesting a lack of prolonged effect of peptide microinjections on water consumption. Likewise, there were no residual effects of peptide injections on ethanol consumption during the DID session after sham injections on Day 4 of the experiment [F(3,22)=0.16, p=n.s., data not shown].
Figure 4. Dose response of Ucn1’s suppressive effects on limited access ethanol but not water consumption following intra-lateral septum microinjection.
Top left panel: Baseline intake of 20% v/v ethanol during a 2-hour session on Day 2 of Phase 1 of Experiment 3 following sham injections in four groups of animals that were subsequently divided into dose groups. No significant differences between groups are observed in baseline 2 hr ethanol intake. Top right panel: Intake of 20% ethanol during a 2-hour session on Day 3 of Phase 1 of Experiment 2 following injections of vehicle (Veh), 6, 20 or 60 pmol of Ucn1. All three doses of Ucn1 significantly attenuated ethanol intake. Bottom left panel: Intake of water during a 2-hour session on Day 2 of Phase 2 of Experiment 3 following sham injections in four groups of animals that were subsequently divided into dose groups. No significant differences are observed in baseline 2 hr water consumption. Bottom right panel: Intake of water during a 2-hour session on Day 3 of Phase 2 of Experiment 2 following injections of Veh, 6, 20 or 60 pmol of Ucn1. No significant differences between groups were found. The number of animals per group is shown in parenthesis.
Phase 2 of the experiment replicated Phase 1, except that water (not ethanol) was used during all DID sessions. During the two days preceding Ucn1 microinjection, there were no differences in water intake between the groups of animals that were subsequently assigned to the different dose groups [F(3,22)=0.67, p=n.s. for Day 2], indicating a lack of any carry-over effects from the previous phase of the experiment. There were no significant effects of Ucn1 on water intake during the DID session on Day 3 [F(3,22)=1.04, p=n.s.], nor were there any effects of the peptide on water consumption during the 22 hours after the DID water drinking session [F(3,22)=2.59, p=n.s., data not shown]. In agreement with these findings, a repeated measures ANOVA, comparing animals treated with vehicle versus all animals treated with Ucn1 (between-subjects) across the baseline day and day of treatment (within-subjects) showed no significant effect of treatment [F(1,24)=0.05, p=n.s.], a significant effect of day [F(1,24)=12.4, p=0.002] and no significant interaction between these factors [F(1,24) =1.12, p=n.s.]. These results indicated that Ucn1 inhibited intake in a specific manner, such that ethanol, but not water intake was affected by Ucn1 microinjection into the lateral septum.
Experiment 4. Effect of Ucn1 dose response on the acquisition of limited access ethanol intake with the DID procedure
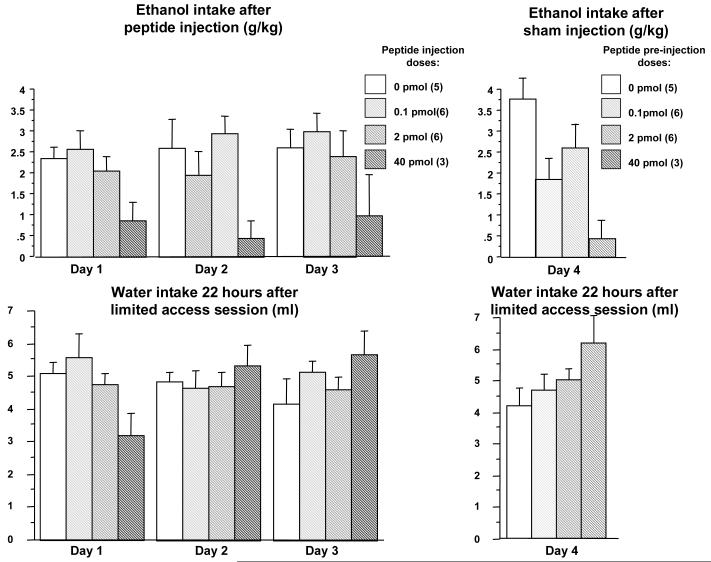
This experiment tested the effects of intra-lateral septum administration of Ucn1 on ethanol intake during the first three sessions of DID, as well as potential conditioning effects of repeated Ucn1 administration on subsequent ethanol intake. It also explored a wider dose range of the peptide. Of thirty-two mice that began the experiment, twenty animals were confirmed to have correct bilateral placement of injections and were used in the statistical analysis. A mixed-design repeated measures ANOVA performed on ethanol intake during the first 3 days of ethanol intake, showed a significant effect of Ucn1 dose [F(3,16)=4.84, p=0.014], but not of day [F(2,32)=0.41, p=n.s.], and no dose by day interaction [F(6,32)=0.15, p=n.s., Figure 5]. Post-hoc Fisher PLSD analysis of average intake over the three DID sessions showed that ethanol intake in the mice microinjected with the 40 pmol dose of Ucn1 was significantly lower than values in mice injected with vehicle (p=0.008), 2 pmol (p=0.026) and 0.1 pmol (p=0.002) of Ucn1. These data indicated that Ucn1 suppressed ethanol intake during the acquisition phase of the DID procedure. Ethanol intake on the fourth DID session (after animals received sham injections) was also significantly attenuated [F(3,16)=5.08, p=0.012]. Fisher PLSD performed on the Day 4 data confirmed that ethanol intake in the mice that had previously received 40 pmol of Ucn1 was significantly lower, versus animals that had previously received vehicle (p=0.002) and 2 pmol of Ucn1 (p=0.023). On Day 4, there also was a significant difference in ethanol intake between animals that had previously been injected with vehicle and animals previously injected with 0.1 pmol of Ucn1 (p=0.021). These data suggested that there might be a conditioning effect of repeated Ucn1 injections on subsequent ethanol intake.
Figure 5. Effects of repeated intra-lateral septum microinjection of Ucn1 on limited access ethanol consumption.
Top left panel: Intake of 20% v/v ethanol during 2-hour sessions on Days 1-3 of Experiment 4 following injections of 0 (vehicle), 0.1, 2 or 40 pmol of Ucn1. The highest dose of Ucn1 had suppressive effects on ethanol consumption. Top right panel: Intake of 20% ethanol during a 2-hour session following a sham injection on Day 4 of Experiment 4 (these animals had received 0, 0.1, 2 or 40 pmol of Ucn1 during the three days preceding Day 4, but not on Day 4). Animals with a history of repeated administration of 40 pmol of Ucn1 showed significantly lower intakes of ethanol. Bottom left panel: Intake of water during the 22 hours after the 2-hour ethanol drinking session during Days 1-3 of Experiment 4 (as a control for long-term effects of Ucn1). No significant differences between groups are observed. Bottom right panel: Intake of water during the 22 hours after the 2-hour ethanol drinking session following sham injections on Day 4 of Experiment 4. No significant effects are observed. The number of animals per group is shown in parenthesis (top panels).
A mixed-design repeated measures ANOVA on water intake during the 22 hours following the 2-hour ethanol DID session showed no significant effect of Ucn1 dose [F(3,16)=0.24, p=n.s.] and no significant effect of day [F(2,32)=0.76, p=n.s.]. A significant interaction between day and dose was observed [F(6,32)=5.45, p=0.0006], reflecting a tendency towards lower intake of water on Day 1 in animals microinjected with 60 pmol of Ucn1. However, this tendency was not statistically significant when a post-hoc one-way ANOVA was performed on water intake on data from Day 1 [F(3,16)=2.58, p=n.s.]. Similarly, no significant effects of preceding repeated administration of Ucn1 on the 22-hour water intake were observed on Day 4 [F(3,16)=1.97, p=n.s.]. Taken together these data indicated that Ucn1 has suppressive effects on ethanol intake during the acquisition of limited access ethanol intake with the DID procedure, that repeated Ucn1 injections may lead to conditioning of the effect on ethanol intake, and that Ucn1 injections had no long-term effects on water consumption.
Discussion
The results of these experiments provide strong evidence for the involvement of the CRF/urocortin system in the regulation of alcohol consumption. Importantly, while the majority of studies in this field focus on the importance of CRF, our studies clearly show that Ucn1 could play an equal, if not a greater role in the regulation of alcohol intake. Overall, intra-lateral septum injection of Ucn1 significantly decreased alcohol consumption at picomolar doses in contrast to water, which was not affected by Ucn1 at these doses. Ethanol consumption appeared differentially sensitive to Ucn1 because CRF decreased ethanol consumption only at the highest dose (60 pmol), and both ethanol and water consumption were affected by CRF microinjections. Ucn1 was capable of suppressing ethanol intake both when it was administered prior to the third ethanol drinking session, presumably influencing the expression of ethanol intake, and when it was administered during the first three ethanol drinking sessions, presumably affecting the acquisition of ethanol self-administration. Moreover, while injections of this peptide did not have significant prolonged effects on fluid consumption beyond the 2-hour ethanol drinking session, a history of repeated Ucn1 injections led to decreased ethanol intake in the absence of Ucn1, which may represent a conditioned response. Taken together, these data suggest that Ucn1 affects the regulatory processes governing limited access ethanol consumption.
Since Ucn1 is a peptide related to CRF, much attention has focused on its potential involvement in the regulation of anxiety and the stress response (Bale and Vale, 2004, Gysling et al., 2004, Kozicz, 2007). While the data on its involvement in the regulation of these responses remain not without contradictions, with different results depending on the species and stress paradigm (Weninger et al., 2000, Vetter et al., 2002, Wang et al., 2002, Gaszner et al., 2004, Kozicz et al., 2004, Turek and Ryabinin, 2005), it is important to consider whether anxiety or stress could contribute to the results observed in the present studies. In fact, several studies have shown that intra-lateral septum injection of CRF or related peptides could induce anxiety-related responses (Radulovic et al., 1999, Henry et al., 2006), while intra-lateral septum injection of CRF2 receptor antagonists could attenuate them (Radulovic et al., 1999, Bakshi et al., 2002). However, the threshold doses of Ucn1 that were capable of decreasing ethanol consumption in our procedure (6 pmol) are substantially lower than doses of CRF (100 but not 20 pmol) or doses of Ucn2 (240 but not 48 pmol) that have been reported to increase anxiety-like behavior in mice (Radulovic et al., 1999, Henry et al., 2006).
It has been suggested that exposure to immobilization stress can increase the sensitivity of anxiety responses in mice to intra-lateral septum Ucn2 injections. Although it is unlikely that mice in our procedure experience levels of anxiety similar to those produced by immobilization stress, it is important to note that our 6 pmol dose was lower than the dose of Ucn2 necessary to produce an increase in anxiety in mice stressed by immobilization (48, but not 4.8 pmol, (Henry et al., 2006)). Theoretically it is possible that Ucn1 used in our study would be more potent than CRF or Ucn2 in producing anxiety, by acting on both CRF1 and CRF2 receptors. However, previous studies have shown that the effects of stress-induced anxiety are mediated by CRF2, and not CRF1 receptors (Henry et al., 2006, Todorovic et al., 2007). Therefore, it is unlikely that activation of CRF1 and CRF2 receptors by Ucn1 would have additive effects exceeding its action at CRF2 receptors.
Taking into account the above mentioned doses of peptides required for anxiogenic effects, it is possible that the non-specific effects of the highest doses of CRF on ethanol and water consumption observed in our experiments were mediated by increased anxiety following these injections. In contrast, Ucn1 did not exert a non-specific effect on fluid consumption, exhibiting a selective effect on ethanol intake. Therefore, it is much more likely that the effects of Ucn1 on ethanol intake were not mediated by concomitant effects on anxiety. This conclusion concurs with early studies showing that intracerebroventricular administration of Ucn1 was more effective than CRF at reducing food intake, but less effective at increasing anxiety (Spina et al., 1996).
Since the selectivity of the effects of Ucn1 on ethanol consumption is suggested by lack of effects on water intake in Experiment 3, the possibility of test-order effects should be acknowledged. Theoretically, one can envision that a single exposure to picomolar doses of Ucn1 (as low as 6 and 20 pmol) could lead to insensitivity of lateral septum to subsequent administrations of this peptide. However, such desensitization did not occur in the Experiment 4, even with a higher (40 pmol) dose of Ucn1. Therefore, we believe it is unlikely that test-order effects contributed to the selectivity of Ucn1’s effect on ethanol consumption (and lack of effect on water consumption with subsequent microinjection) in Experiment 3.
Interestingly, it has been shown that intra-lateral septum injections of CRF-like peptides can modulate learning independently of their effects of anxiety (Radulovic et al., 1999). Importantly, these effects were mediated by lower doses of CRF and Ucn1 (20 pmol). Therefore, we cannot exclude the possibility that Ucn1’s effect on alcohol consumption during acquisition of alcohol drinking (Experiment 4) could be influenced by Ucn1’s effect on learning. While an effect of repeated administration on learning might contribute to the putative conditioned suppression of ethanol intake following the sham injection in Experiment 4, the potential effect on learning cannot account for the Ucn1-mediated suppression of ethanol intake following a single administration of the peptide (e.g., Day 3 of Experiments 1 and 3 or Day 1 of Experiment 4). Therefore, it seems more likely that Ucn1 influenced ethanol intake by interfering with the regulatory processes governing alcohol consumption.
The role of the lateral septum in the reinforcing properties of food or drugs of abuse has been studied less than that of the more traditionally investigated “reward” circuits. Therefore, it is important to consider whether the observed effects of intra-lateral septum injections were mediated by leakage of Ucn1 into other brain regions or into the ventricles. Intracerebroventricular effects of peptides in our studies are unlikely because substantially higher doses of Ucn1 are needed to produce significant effects on behavioral responses (Swiergiel and Dunn, 1999, Bradbury et al., 2000, Sinnayah et al., 2003). The effects are also not likely to be mediated by Ucn1 leakage to other brain regions because the majority of other forebrain regions contain higher levels of CRFR1 than CRFR2 receptors, and one would expect stronger or equal effects of CRF than Ucn1 in these brain regions (Potter et al., 1994, Chalmers et al., 1995, Perrin et al., 1995, Turnbull and Rivier, 1997, Steckler and Holsboer, 1999, Fekete and Zorrilla, 2006). In light of these facts, the role of the lateral septum in consumption of ethanol needs to be given strong consideration.
In agreement with this role, lesions of the lateral septum have been shown to increase water consumption (Iovino and Steardo, 1985, Taghzouti et al., 1985, Vasudev et al., 1985) and food consumption in rats (King and Nance, 1986, Oliveira et al., 1990). This suggests that the lateral septum has suppressive effects on consummatory behaviors. Since lateral septal neurons are preferentially GABAergic, lesions of this region would presumably disinhibit the hypothalamus, a long-proposed integrator of consummatory responses (Leibowitz, 1992, Elmquist et al., 1998, Inui, 1999). However, interpretation of these data are complicated by the fact that the lateral septum is involved with other functions (including anxiety, as described above), which would also contribute to the regulation of consummatory behaviors. In this regard, it is important to note that one study to date investigated the effects of Ucn1 and CRF microinjection into the lateral septum on food consumption. The results demonstrated that relatively low doses of Ucn1 injected into the lateral septum (3-30 pmol) suppressed food consumption in rats, and that this suppressive effect was longer lasting than that of equimolar CRF (Wang and Kotz, 2002). No study prior to the present one has investigated the effects of intra-septal injections of any substances on the reinforcing properties of addictive drugs. A study using brain slices has shown that Ucn1 suppresses glutamatergic activation of neurons in the lateral septum, and that repeated cocaine administration in rats reverses this effect (Liu et al., 2004, Liu et al., 2005). The involvement of the lateral septum in the reinforcing effects of drugs of abuse clearly deserves further investigation.
Given that Ucn1 exerted stronger and more specific effects on ethanol consumption than CRF, it seems more likely that these effects are mediated through CRF2 and not CRF1 receptors. Since the CRF2 receptor has high affinity to Ucn1, Ucn2 and Ucn3, it is important to note that not only Ucn1, but also Ucn3, innervate this brain structure (Li et al., 2002). Therefore, we cannot exclude the possibility that the consumption of alcohol could be regulated by either Ucn1 or Ucn3. However, several studies that mapped expression of the immediate early gene product c-Fos have shown that self-administration of alcohol leads to consistent activation of the pIIIu, the major source of Ucn1 in the brain, but not brain areas containing Ucn3 (Topple et al., 1988, Bachtell et al., 1999, Ryabinin et al., 2003a, Sharpe et al., 2005). Therefore, it seems likely that Ucn1 from the pIIIu contributes to the regulation of alcohol consumption.
An important consideration is that previous studies using genetic and lesion approaches correlated higher number of Ucn1-containing neurons in the pIIIu with higher ethanol intake (Ryabinin and Weitemier, 2006). In contrast, our present study shows that application of Ucn1 suppresses ethanol intake. One potential explanation for this apparent discrepancy is that if Ucn1 contributes to a reward signal associated with ethanol, exogenous application of Ucn1 could signal that the reward already has been achieved, resulting in no further effort to obtain it (by alcohol consumption). However, in this case one would expect low doses of Ucn1 to increase ethanol intake during acquisition of this behavior. Since our study included very low doses of Ucn1, this reasoning seems unlikely. As an alternative explanation, since pIIIu neurons contain other peptides besides Ucn1 as potential regulators of the rewarding properties of drugs of abuse [including CART, CCK and neuropeptide B (Maciewicz et al., 1984, Innis and Aghajanian, 1986, Couceyro et al., 1997, Kozicz, 2003, Tanaka et al., 2003)], it is possible that synergistic actions of these peptides are needed to increase the rewarding properties of alcohol. Lesions of the pIIIu or a study correlating the number of cells in the pIIIu with alcohol drinking cannot directly test this possibility, but can only point to an importance of the brain region in this behavior.
Taken together, our experiments show that the neuropeptide Ucn1 acts to suppress alcohol consumption through the lateral septum. Further studies will be required to decipher the exact role of the complex neurocircuits expressing Ucn1 in the regulation of the actions of alcohol and other drugs of abuse.
Acknowledgments
This work was supported by NIH grants AA013738 (AER), INIA Consortium grants AA013478 (DAF) and AA016647 (AER), and the Department of Veterans Affairs (DAF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Strain differences in urocortin expression in the Edinger-Westphal nucleus and its relation to alcohol-induced hypothermia. Neuroscience. 2002;113:421–434. doi: 10.1016/s0306-4522(02)00174-4. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci. 2003;23:2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus in C57BL/6J mice disrupt ethanol-induced hypothermia and ethanol consumption. Eur J Neurosci. 2004;20:1613–1623. doi: 10.1111/j.1460-9568.2004.03594.x. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- Bradbury MJ, McBurnie MI, Denton DA, Lee KF, Vale WW. Modulation of urocortin-induced hypophagia and weight loss by corticotropin-releasing factor receptor 1 deficiency in mice. Endocrinol. 2000;141:2715–2724. doi: 10.1210/endo.141.8.7606. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, D.A. L, O’Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couceyro PR, Koylu EO, Kuhar MJ. Further studies on the neuroanatomical distribution of CART by in situ hybridization. J Chem Neuroanat. 1997;12:229–241. doi: 10.1016/s0891-0618(97)00212-3. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2006;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Freund G. Alcohol consumption and its circadian distribution in mice. J Nutr. 1970;100:3–36. doi: 10.1093/jn/100.1.30. [DOI] [PubMed] [Google Scholar]
- Gaszner B, Csernus V, Kozicz T. Urocortinergic neurons respond in a differentiated manner to various acute stressors in the Edinger-Westphal nucleus in the rat. J Comp Neurol. 2004;480:170–179. doi: 10.1002/cne.20343. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Kakihana R. Circadian rhythms of ethanol consumption by mice: a simple computer analysis of chronopharmacology. Psychopharmacol. 1977;52:41–45. doi: 10.1007/BF00426598. [DOI] [PubMed] [Google Scholar]
- Gysling K, Forray MI, Haeger P, Daza C, Rojas R. Corticotropin-releasing hormone and urocortin: redundant or distinctive functions? Brain Res Rev. 2004;47:116–125. doi: 10.1016/j.brainresrev.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- Innis RB, Aghajanian GK. Cholecystokinin-containing and nociceptive neurons in rat Edinger-Westphal nucleus. Brain Res. 1986;363:230–238. doi: 10.1016/0006-8993(86)91008-5. [DOI] [PubMed] [Google Scholar]
- Inui A. Feeding and body-weight regulation by hypothalamic neuropeptides-mediation of the actions of leptin. Trends Neurosci. 1999;22:62–67. doi: 10.1016/s0166-2236(98)01292-2. [DOI] [PubMed] [Google Scholar]
- Iovino M, Steardo L. Thirst and vasopressin secretion following central administration of angiotensin II in rats with lesions of the septal area and subfornical organ. Neurosci. 1985;15:61–67. doi: 10.1016/0306-4522(85)90123-x. [DOI] [PubMed] [Google Scholar]
- Kiianmaa K, Hyytia P, Samson HH, Egel JA, Svensson L, Soderpalm B, Larsson A, Colombo G, Vacca G, Finn DA, Bachtell RK, Ryabinin AE. New neuronal networks involved in ethanol reinforcement. Alcohol Clin Exp Res. 2003;27:209–219. doi: 10.1097/01.ALC.0000051020.55829.41. [DOI] [PubMed] [Google Scholar]
- King TR, Nance DM. Neuroestrogenic control of feeding behavior and body weight in rats with kainic acid lesions of lateral septal area. Physiol Behav. 1986;37:475–481. doi: 10.1016/0031-9384(86)90209-x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kozicz T. Neurons colocalizing urocortin and cocaine and amphetamine-regulated transcript immunoreactivities are induced by acute lipopolysaccharide stress in the Edinger-Westphal nucleus in the rat. Neurosci. 2003;116:315–320. doi: 10.1016/s0306-4522(02)00772-8. [DOI] [PubMed] [Google Scholar]
- Kozicz T. On the role of urocortin 1 in the non-preganglionic Edinger-Westphal nucleus in stress adaptation. Gen Comp Endocrinol. 2007;153:235–240. doi: 10.1016/j.ygcen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Korosi A, Korsman C, Tilburg-Ouwens D, Groenink L, Veening J, van Der Gugten J, Roubos E, Olivier B. Urocortin expression in the Edinger-Westphal nucleus is down-regulated in transgenic mice over-expressing neuronal corticotropin-releasing factor. Neuroscience. 2004;123:589–594. doi: 10.1016/j.neuroscience.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Ahino K, Kanda K. A new apparatus for studying feeding and drinking in the mouse. Physiol Behav. 2000;70:105–112. doi: 10.1016/s0031-9384(00)00226-2. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. Neurochemical-neuroendocrine systems in the brain controlling macronutrient intake and metabolism. Trends Neurosci. 1992;15:491–497. doi: 10.1016/0166-2236(92)90101-d. [DOI] [PubMed] [Google Scholar]
- Lewis K, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezkjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, and additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in the rat brain: partial overlap with sites of type 2 corticotropin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Corticotropin-releasing factor and urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24:4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Chronic cocaine administration switches corticotropin-releasing factor 2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci. 2005;25:577–583. doi: 10.1523/JNEUROSCI.4196-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciewicz R, Phipps BS, Grenier J, Poletti CE. Edinger-Westphal nucleus: cholecystokinin immunocytochemistry and projections to spinal cord and trigeminal nucleus in the cat. Brain Res. 1984;299:139–145. doi: 10.1016/0006-8993(84)90796-0. [DOI] [PubMed] [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distribution of urocortin containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2007 doi: 10.1002/cne.21514. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Oliveira LA, Gentil CG, Covian MR. Role of the lateral septum in feeding behavior elicited by electrical stimulation of the lateral hypothalamus in the rat. Braz J Med Biol Res. 1990;23:49–58. [PubMed] [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezkjian L, Sawchenko P, Vale W. Identification a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci U S A. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake K, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;31:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr., Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Bachtell RK, Freeman P, Risinger FO. ITF expression in mouse brain during acquisition of alcohol self-administration. Brain Res. 2001;890:192–195. doi: 10.1016/s0006-8993(00)03251-0. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involvement of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology (Berl) 2003a;165:296–305. doi: 10.1007/s00213-002-1284-y. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Sharpe AL, Tsivkovskaia NO, Weitemier AZ. Ethanol self-administration during the circadian dark phase. Alcohol Clin Exp Res; Supplement, Abstr. Annual RSA meeting; 2003b.p. 183A. [Google Scholar]
- Ryabinin AE, Tsivkovskaia NO, Ryabinin SA. Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neurosci. 2005;134:1317–1317. doi: 10.1016/j.neuroscience.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Rev. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29:1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Sinnayah P, Blair-West JR, McBurnie MI, McKinley MJ, Oldfield BJ, Rivier J, Vale WW, Walker LL, Weisinger RS, Denton DA. The effect of urocortin on ingestive behaviours and brain Fos immunoreactivity in mice. Eur J Neurosci. 2003;18:373–382. doi: 10.1046/j.1460-9568.2003.02760.x. [DOI] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RKW, Basso AM, Rivier J, Vale W, Koob GF. Appetite-supressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Steckler T, Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. CRF-deficient mice respond like wild-type to hypophagic stimuli. Pharmacol Biochem Behav. 1999;64:59–64. doi: 10.1016/s0091-3057(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Taghzouti K, Simon H, Tazi A, Dantzer R, Le Moal M. The effect of 6-OHDA lesion of lateral septum on schedule-induced polydipsia. Behav Brain Res. 1985;15:1–8. doi: 10.1016/0166-4328(85)90012-9. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yoshida T, Miyamato N, Motoike T, Kurosu H, Shibata K, Yamanaka A, Williams SC, Richardson JA, Tsujino N, Garry MG, Lerner MR, King DS, O’Dowd BF, Sakurai T, Yanagisawa M. Characterization of a family of endogenous neuroopeptide ligands for the G protein-couple receptors GPR7 and GPR8. Proc Natl Acad Sci U S A. 2003;100:6251–6256. doi: 10.1073/pnas.0837789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic C, Radulovic J, Jahn O, Radulovic M, Sherrin T, Hippel C, Spiess J. Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety. Eur J Neurosci. 2007;25:3385–3397. doi: 10.1111/j.1460-9568.2007.05592.x. [DOI] [PubMed] [Google Scholar]
- Topple AN, Hunt GE, McGregor IS. Possible neural substrates of beer-craving in rats. Neurosci Lett. 1988;252:99–102. doi: 10.1016/s0304-3940(98)00574-6. [DOI] [PubMed] [Google Scholar]
- Turek VF, Ryabinin AE. Expression of c-Fos in the mouse Edinger-Westphal nucleus following ethanol administration is not secondary to hypothermia or stress. Brain Res. 2005;1063:132–139. doi: 10.1016/j.brainres.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Med. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- Vale WW, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates sectretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vasudev R, Gentil CG, Covian MR. Taste preferences in a free-choice situation following electrical stimulation and lesion of septal area in rats. Physiol Behav. 1985;34:619–624. doi: 10.1016/0031-9384(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt JC, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy DA, Rivier C, Vale WW. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, Marchuk Y, Koob GF, Heineman SF, Vale W, Lee KF. Urocortin-deficient mice show hearing impariment and increased anxiety-like behavior. Nat Genetics. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- Wang C, Kotz CM. Urocortin in the lateral septal area modulates feeding induced by orexin A in the lateral hypothalamus. Am J Physiol Reg Integr Comp Physiol. 2002;283:R358–R367. doi: 10.1152/ajpregu.00558.2001. [DOI] [PubMed] [Google Scholar]
- Wang X, Su H, Copenhagen LD, Vaishnav S, Pieri F, Shope CD, Brownell WE, De Biasi M, Paylor R, Bradley A. Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control but an impaired acoustic startle response. Mol Cell Biol. 2002;22:6605–6610. doi: 10.1128/MCB.22.18.6605-6610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu Z, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus alter food and water consumption. Behav Neurosci. 2005:119. doi: 10.1037/0735-7044.119.5.1235. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Urocortin 1 in the dorsal raphe regulates food and fluid consumption, but not ethanol preference in C57BL/6J mice. Neurosci. 2006;137:1439–1445. doi: 10.1016/j.neuroscience.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Tsivkovskaia NO, Ryabinin AE. Urocortin 1 distribution in mouse brain is strain-dependent. Neuroscience. 2005;132:729–740. doi: 10.1016/j.neuroscience.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Woerner A, Backstrom P, Hyytia P, Ryabinin AE. Expression of c-Fos in Alko alcohol rats responding for ethanol in an operant paradigm. Alcohol Clin Exp Res. 2001;25:704–710. [PubMed] [Google Scholar]
- Weninger SC, Peters LL, Majzoub JA. Urocortin expression in the Edinger-Westphal nucleus is up-regulated by stress and corticotropin-releasing hormone deficiency. Endocrinology. 2000;141:256–263. doi: 10.1210/endo.141.1.7277. [DOI] [PubMed] [Google Scholar]