Abstract
Accumulation of CD28nullCD8+ T cells and the defects of these cells in response to antigenic stimulation are the hallmarks of age-associated decline of T cell function. However, the mechanism of these age-associated changes is not fully understood. In this study, we report an analysis of the growth of human CD28null and CD28+CD8+ memory T cells in response to homeostatic cytokine IL-15 in vitro. We showed that 1) there was no proliferative defect of CD28nullCD8+ memory T cells in response to IL-15 compared with their CD28+ counterparts; 2) stable loss of CD28 expression occurred in those actively dividing CD28+CD8+ memory T cells responding to IL-15; 3) the loss of CD28 was in part mediated by TNF-α that was induced by IL-15; and 4) CCL4 (MIP-1β), also induced by IL-15, had a significant inhibitory effect on the growth of CD28null cells, which in turn down-regulated their expression of CCL4 receptor CCR5. Together, these findings demonstrate that CD28nullCD8+ memory T cells proliferate normally in response to IL-15 and that IL-15 and its induced cytokines regulate the generation and growth of CD28nullCD8+ T cells, suggesting a possible role of IL-15 in the increase in CD28nullCD8+ T cells that occurs with aging.
An essential part of the adaptive immune response, CD8+ T cells mediate protection against cancer and infection by intracellular pathogens. The function of CD8+ T cells depends on their ability to respond to the engagement of TCRs and costimulatory receptors with their respective ligands on APCs. CD28, a key costimulatory receptor for T cell activation, participates in a range of events from the amplification of the TCR signals to the induction of essential cytokine secretion such as IL-2 (1, 2). Without signals from CD28 generated by binding to B7 family members on APC, the interaction between TCR and the Ag/MHC complex alone is not sufficient to completely activate T cells and therefore an efficient T cell response is not generated.
Loss of CD28 expression on CD8+ T cells is a hallmark of the age-associated decline of T cell function (3-5). Because CD28nullCD8+ T cells are absent in the newborn (3, 6), increase with chronic infection (7, 8), and have shorter telomere length than their CD28+ counterparts (9, 10), it is generally believed that CD28nullCD8+ T cells are derived from CD28+CD8+ T cells. As a consequence of repeated stimulation, CD28nullCD8+ T cells exhibit a reduced proliferative response to TCR cross-linking and mitogen (PHA) (3, 11-15); they are also associated with replicative senescence in long-term culture (16-18). Despite their proliferative defects, CD28nullCD8+ T cells retain or even enhance their cytotoxic capacities (3, 4, 19). However, the mechanisms underlying age-associated accumulation of CD28nullCD8+ memory T cells are not fully understood. As memory T cells are maintained by homeostatic cytokines such as IL-15 in an Ag-independent manner (20-22), it is of great interest to determine the responsiveness of CD28nullCD8+ memory T cells to homeostatic cytokines.
A transient down-regulation of CD28 expression on T cells occurs after antigenic stimulation (23). Recently, down-regulation of CD28 in T cells by cytokines sharing the common γ-chain (γc)3 receptors including IL-15 has been reported (18); however, it is unclear whether such down-regulation is transient or stable and what mechanisms are responsible for these cytokine-mediated down-regulations of CD28. Furthermore, TNF-α, a proinflammatory cytokine secreted by various cells including T cells, has also been shown to down-regulate CD28 expression in CD4+ T cells (24). Whether TNF-α can down-regulate CD28 expression in CD8+ T cells and whether other cytokines are also involved in the regulation of CD28 expression remain to be determined.
In this report, we showed that CD28null and CD28+CD8+ memory T cells exhibited comparable growth under IL-15 stimulation in vitro. Furthermore, we showed that IL-15-mediated proliferation resulted in a stable loss of CD28 expression in CD28+CD8+ memory T cells in part through the induction of TNF-α secretion. Surprisingly, we found that IL-15-induced production of CCL4 had a significant inhibitory effect on the growth of CD28nullCD8+ memory T cells and a less inhibitory effect on that of CD28+ cells. Finally, we showed that peripheral blood of older donors was characterized by high basal levels of TNF-α and an increase in the number of CD28nullCD8+ memory T cells, and that high basal levels of CCL4 in peripheral blood of older donors was associated with the down-regulation of CCR5 (a receptor for CCL4) on these CD28nullCD8+ memory cells. Together, our findings suggest that CD28nullCD8+ memory T cells can be generated by cytokine-mediated down-regulation of CD28 expression in CD28+CD8+ memory T cells and that their accumulation may result from the dynamic interactions among cytokines induced by IL-15.
Materials and Methods
Isolation of CD28null and CD28+ memory phenotype CD8+ T cells from human peripheral blood
CD28null and CD28+ memory phenotype CD8+ T cells were isolated from peripheral blood of normal volunteers by immunomagnetic separation and by cell sorting as previously described (25). In brief, blood was obtained from healthy adults and aged volunteers of the National Institute on Aging Clinical Research Branch under Institutional Review Board-approved protocols (MRI2003-054 and GRC98-12-28-01) and mononuclear cells were isolated by Ficoll gradient centrifugation (ICN Biomedicals). Memory phenotype CD8+ T cells were then enriched by removing other types of cells through incubation with a panel of mouse mAbs against CD4, CD19, CD11b, CD14, CD16, MHC class II, erythrocytes, platelets, and CD45RA. Ab-bound cells were subsequently removed by incubation with anti-mouse IgG-conjugated magnetic beads (Qiagen). These enriched memory phenotype CD8+ cells were either separated into CD8+CD45RA−CD28null and CD8+CD45RA−CD28+ memory T cells by a cell sorter (MoFlo; Dako-Cytomation) or further purified into CD8+ memory T cells through positive immunomagnetic selection by a human CD8 multisort kit (Miltenyi Biotec). In experiments comparing young (age 19–29) and old (age 76–86) donors, total CD8+ T cells were isolated directly by anti-CD8 beads as described above from PBMC. The purities of both CD8 subsets were over 96%.
Analysis of cell surface receptors and intracellular proteins
Fluorescent dye-labeled Abs against CD8-Tricolor, CD28-PE, CD95-PE, CD95L-PE, Bcl2-PE, mouse IgG1-control-PE, CD45RA-FITC, and mouse-IgG1-control-FITC were obtained from Caltag Laboratories. Anti-IL-15Rα was purchased from R&D Systems and was FITC labeled by a Fluorescein Protein Labeling kit from Pierce. Abs against CCR5-FITC, CD28-PE, CD122-PE, CD132-PE, and CD45RA-allophycocyanin were purchased from BD Biosciences. Expression of CD28 on CD8 T cells were determined by two different mAbs from Caltag Laboratories and BD Biosciences and similar results were obtained. Also isotype- and fluorescent dye-matched IgG controls were used in FACS staining. For surface marker analysis, freshly purified and IL-15-treated CD8+ memory phenotype cell subsets were incubated with three or four different fluorescent dye-conjugated Abs and prepared for FACS analysis according to manufacturers’ instructions. For intracellular protein analysis, cells were fixed, washed, and permeabilized by Cytofix and Cytoperm (BD Biosciences), and data were acquired by FACScan or FACSCalibur and analyzed by CellQuest Pro software (BD Biosciences).
Proliferation assay
We used a cell division tracking dye, CFSE (Invitrogen Life Technologies), to measure the proliferation of cells as previously described (26). In brief, sorted CD28null and CD28+CD8+ memory phenotype T cells were incubated with 5 μM/ml CFSE for 10 min at 37°C, washed with RPMI 1640 once, and cultured at 1 × 106 cells/ml in RPMI 1640 supplemented with 10% FBS and penicillin (10 U/ml)/streptomycin (10 μg/ml; Invitrogen Life Technologies) in the presence of recombinant human IL-15 (50 ng/ml or specified) (PeproTech), anti-CD3 alone, or anti-CD3 plus anti-CD28 (Invitrogen Life Technologies) either on 24-well or 48-well flat-bottom plates (BD Biosciences), dependent on the number of cells available. The proliferative responses of CD28null and CD28+CD8+ memory T cells to anti-CD3 or anti-CD3/CD28 were analyzed on day 4 and to IL-15 were analyzed on days 5, 10, and 15 by FACScan. The division rate was analyzed by software ModFit LT 3.0 Mac (Verity House Software).
RT-PCR
Total RNA were extracted from freshly isolated and IL-15-cultured sorted CD28null and CD28+CD8+ memory T cells as well as from anti-CD3/CD28 Ab-stimulated CD28null and CD28+CD8+ memory T cells by a standard procedure as previously described (27). In brief, RNA were extracted from cells by Stat 60 (Tel-Test) based on manufacturers’ protocol and quantified by Nanodrop (Nanodrop Technologies). cDNA synthesis was conducted using 1 μg of total RNA with reverse transcriptase (Super-Script II; Invitrogen Life Technologies) based on manufacturer’s instructions. mRNA level of CD28 (forward: 5′-AGGCTGCTCTTGGCTCTCAACT-3′ and backward: 5′-ACCGCATTGTCGTACGCTACA-3′), IL-2 (forward: 5′-AAGAATCCCAAACTCACCAGGAT-3′ and backward: 5′-TAGACACTGAAGCTGTTTCAGTTCTG-3′) and CD25 (forward: 5′-GACCCAGCCCCAGCTCAT-3′ and backward: 5′-CTTGCCTGAGGCTTCTCTTCAC-3′) were determined by real-time quantitative RT-PCR and normalized based on the level of β-actin (forward: 5′-CCTGGCACCCAGCACAA-3′ and backward: 5′-GCCGATCCACACGGAGTACT-3′). Specifically, PCR was conducted in 25-μl total volume with 0.1 μM primers using a SyBr Green kit on ABI Prism 7700 (Applied Biosystems) for cycles specified in the figure legends. The specific amplification of RT-PCR products was confirmed by agarose (2.5%) gel electrophoresis. Serial dilutions of cDNA at 1/1, 1/4, and 1/16 were used for quantitation and the images were taken by the Fluor gel imaging system (Alpha Innotech).
TNF-α neutralization and rTNF-α
In the TNF-α neutralization experiments, 10, 20, and 50 μg/ml anti-TNF-α Ab (Remicade; Centocor) or 20 μg/ml Enbrel (Immunex), a soluble TNF-α receptor, was added to the culture of CD28+CD8+ memory T cells for the initial screening. Similar results were obtained with Remicade and Enbrel, so we used 20 μg/ml Remicade in the subsequent experiments. Neutralizing Abs to GM-CSF (1 μg/ml), IFN-γ (200 ng/ml), IL-5 (100 ng/ml), IL-6 (500 ng/ml), IL-8 (10 μg/ml), and IL-13 (200 ng/ml) (PeproTech), were used according to manufacturers’ instructions and/or our own screening. Species-matched IgG of equal concentrations were used as control. rTNF-α was used at 200 ng/ml based on initial titration (PeproTech). Neutralizing Abs and recombinant cytokines were added on days 0, 7, and 14 to cell culture. In some experiments, Abs and cytokines were replaced twice a week. CD28 expression in cultured memory CD8 T cells was analyzed by FACScan at days 0, 7, 14, and 21.
Neutralization of MIP-1β and rMIP-1β
In MIP-1β-neutralizing experiments, 6 μg/ml polyclonal anti-MIP-1β Ab (PeproTech) was added to the culture of CD28+CD8+ memory phenotype T cells according to manufacturers’ instructions and our own testing. In addition, we also used a monoclonal anti-MIP-1β Ab (6 μg/ml; R&D Systems) to verify the specificity of anti-MIP-1β effects. The results from both Abs were similar and we used the polyclonal anti-MIP-1β Abs in subsequent experiments. A total of 500 ng/ml rMIP-1β (PeproTech) was used based on initial titration. CD28 expression was analyzed as described above. In experiments that examined the MIP-1β effect on CD28null and CD28+CD8+ memory T cells, CD8+ memory T cells were purified, cultured for 14 days in the presence of IL-15, and sorted into CD28null and CD28+CD8+ subsets. Both subsets were labeled with CFSE and cultured with 50% fresh medium and 50% pooled conditioned medium was collected from IL-15-cultured CD8 memory T cells of 10 donors. Each subset was further divided into two pools: IL-15 culture alone and IL-15 culture with anti-MIP-1β Ab. Cells were cultured for an additional 14 days and their proliferation rates were analyzed by FACScan and software ModFit LT 3.0 Mac.
Measurement of cytokines in culture supernatant and plasma
Culture supernatants of IL-15-treated CD28+CD8+ memory phenotype T cells with various neutralizing Abs and cytokines were collected at days 7, 14, and 21 and the concentration of 17 cytokines (G-CSF, GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, TNF-α, MCP-1, and MIP-1β) were measured by BioPlex protein array system (Bio-Rad) according to manufacturers’ instructions. Plasma samples were isolated from whole blood by centrifuging at 1,400 rpm for 20 min and were frozen at −20°C. The samples were thawed the day before measurement and were spun at 14,000 rpm for 5 min to remove precipitated proteins. Cytokines were measured by both BioPlex protein array system (TNF-α and MIP-1β) and LincoPlex Human Cytokine 22-plex kit (shares 16 cytokines with BioPlex and 6 additional cytokines, i.e., eotaxin, IL-1α, IL-15, MIP-1α, IFN-γ-inducible protein 10, and RANTES) (Linco Research) according to manufacturers’ instructions.
Statistical analysis
The differences of biological parameters between CD28null and CD28+CD8+ memory T cells were analyzed by a two-tailed Student’s t test. The differences of biological parameters between donors with a high percentage of CD28nullCD8+ T cells and donors with a low percentage of CD28nullCD8+ T cells were analyzed by a one-tailed Student’s t test.
Results
The response of CD28nullCD8+ memory T cells to homeostatic cytokine IL-15 is not impaired
A prominent defect of CD28nullCD8+ T cells is its poor proliferative response to antigenic stimulations (4, 28), yet the number of CD28nullCD8+ T cells increases in peripheral blood with age. Because the maintenance of memory CD8+ T cells is dependent on homeostatic cytokines, we wanted to determine whether there was any defect in the proliferation of CD28nullCD8+ T cells to homeostatic cytokines such as IL-15. We isolated CD28null and CD28+ memory CD8+ T cells from healthy adults (Fig. 1A) and cultured them in the presence of IL-15. To rule out a transient down-regulation of surface expression of CD28 in these CD28nullCD8+ memory T cells, we analyzed the transcripts of CD28 by real-time RT-PCR and confirmed the absence of CD28 mRNA in CD28nullCD8+ memory T cells (Fig. 1B). To further analyze CD28nullCD8+ T cells, we compared the expression levels of IL-15 receptors (IL-15Rα, IL-2/IL-15β, and γc) and apoptosis/survival markers (Fas, Fas ligand, and Bcl2) before and after IL-15 treatment and found that both IL-15 receptors and apoptosis/survival markers were similarly expressed between CD28null and CD28+CD8+ T cells, independent of the age of the donors (Fig. 1, C and D). To rule out the potential function of residual CD28 in CD28nullCD8+ memory T cells, we analyzed the IL-2 expression by real-time RT-PCR and confirmed that our CD28nullCD8+ memory T cells lacked the ability to transcribe IL-2 in response to the stimulation by anti-CD3 plus anti-CD28 Abs (data not shown).
FIGURE 1.
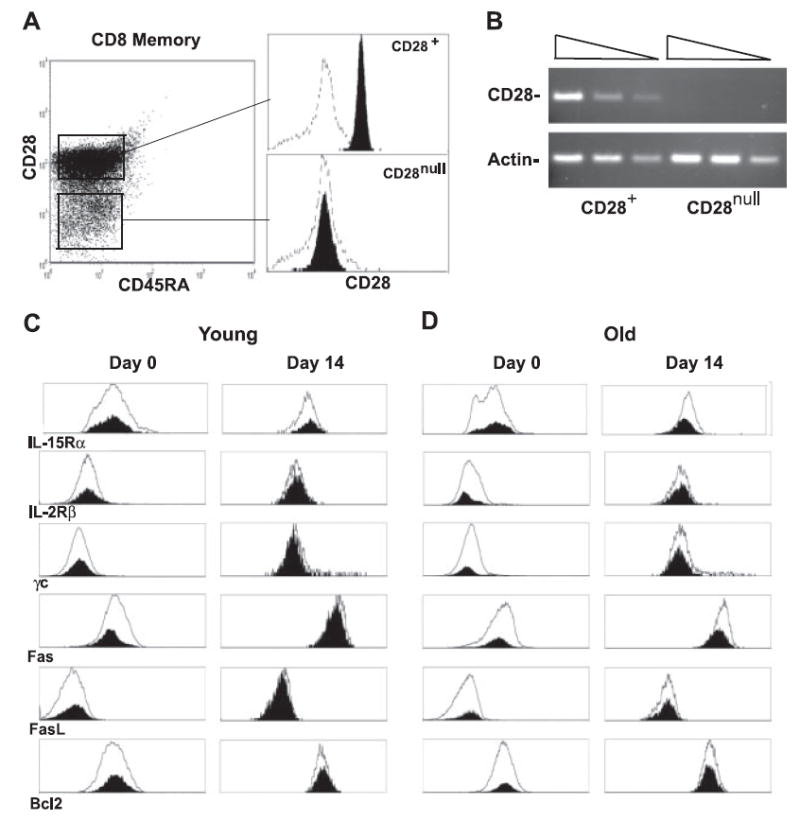
IL-15 induced similar cellular responses between CD28null and CD28+CD8+ memory phenotype T cells in vitro. A, Isolation of CD28null and CD28+CD8+ memory phenotype T cells (filled). Negative control (line) is stained with IgG control Ab. B, CD28 and β-actin gene expressions of freshly isolated CD28null and CD28+CD8+ memory phenotype T cells. A total of 39 cycles of PCR were performed to amplify CD28 and 33 cycles of PCR were performed to amplify β-actin. A serial 4-fold dilution of cDNA was used. C, Expression of IL-15 receptors and apoptosis and survival markers of CD28null (shaded) and CD28+ (line) CD8+ T cells of young donors before and after treatment with IL-15 for 14 days are shown in the histogram. FACS analyses were done on days 0 and 14 after IL-15 treatment by FACS with specific Abs. D, Expression of IL-15 receptors and apoptosis and survival markers of CD28null (filled) and CD28+ (line) CD8+ T cells of old donors before and after treatment with IL-15 for 14 days in are shown in the histogram. In both C and D, representative data are presented from four independent donors. The mean age for young donors is 23 ± 2 and for old donors it is 82 ± 2.
Although CD28null and CD28+CD8+ memory T cells showed similar cellular expressions, the proliferative deficiency in response to the cross-linking of TCR alone (anti-CD3) or the cross-linking of TCR in conjunction with costimulatory receptor CD28 (anti-CD3 plus anti-CD28) was obvious in CD28nullCD8+ memory T cells. The proliferation indexes (PI) were 1.3 and 1.0 for CD28+ and CD28null cells after anti-CD3 stimulation for 4 days, respectively, and were 2.5 and 1.6 for CD28+ and CD28null cells after anti-CD3/28 stimulation for 4 days, respectively (p < 0.01, n = 6) (Fig. 2A). In contrast, CD28nullCD8+ memory T cells proliferated at a comparable rate to their CD28+ counterparts in response to IL-15 at 50 ng/ml (Fig. 2A) and at a lower concentration (5 ng/ml) (data not shown) in vitro. The average PI at day 14 after IL-15 culture was 5.6 for CD28+ cell and 4.9 CD28null cells (p = 0.6, n = 6). These findings suggest that maintenance of CD28nullCD8+ memory T cells in vivo may be mediated by homeostatic cytokines such as IL-15. To further determine whether age influences IL-15-induced cell division of CD28nullCD8+ T cells, we compared CD8+ T cells from young and old donors in response to IL-15 in vitro. We found that CD28+ and CD28nullCD8+ T cells from both age groups had similar proliferation rates: PI was 2.72 and 2.53 for CD28+ and CD28nullCD8+ T cells of young donors, respectively, and PI was 2.77 and 2.74 for CD28+ and CD28nullCD8+ T cells of old donors, respectively. This suggests that age does not alter the proliferation rates of CD28nullCD8+ T cells in response to IL-15 (Fig. 2B).
FIGURE 2.
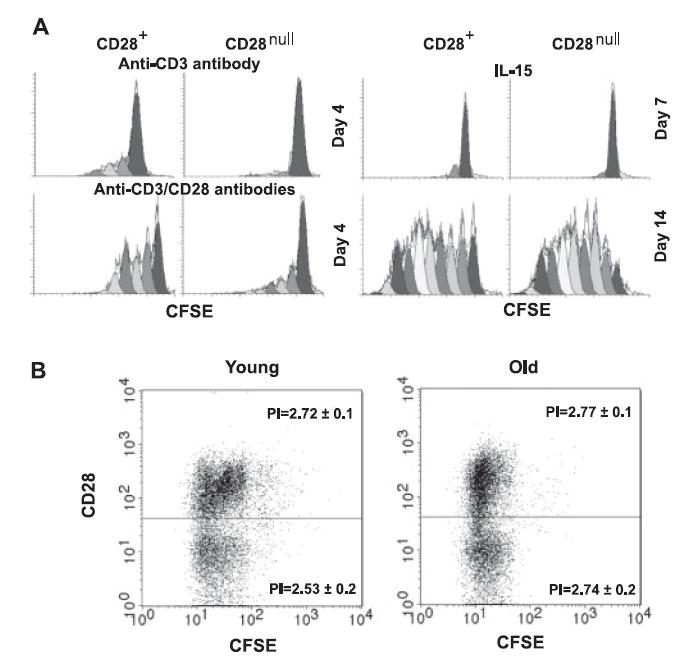
A, Cell division profiles of CD28null and CD28+CD8+ memory T cells stimulated with anti-CD3 and anti-CD3/CD28 Abs at day 4. CFSE was labeled at day 0 and collected at day 4 by FACScan. Cell division profiles of CD28null and CD28+CD8+ memory T cells stimulated with IL-15 at days 7 and 15. Data were collected by FACScan. A representative histogram is shown from six independent donors prepared by Modfit software. B, Cell division profiles of IL-15-treated CD28null and CD28+CD8+ T cells isolated from young and old donors at day 14. CFSE was labeled at day 0 and data were collected at day 14 by FACScan. Representative figures are shown from eight independent donors (four young donors and four old donors) prepared by CellQuest Pro.
IL-15 induces generation of CD28null cells from CD28+CD8+ memory T cells in vitro
Loss of CD28 expression in CD28+CD8+ T cells has been associated with repeated antigenic stimulation during chronic infection and with aging in vivo, and under mitogenic stimulation in vitro (7, 18, 29). Recently, one report showed that γc sharing cytokines including IL-15 are capable of down-regulating CD28 expression in CD28+CD8+ T cells (18). However, it is unclear whether the cytokine-mediated loss of CD28 expression in CD8+ T cells is transient or stable and what mechanisms underlie such a loss. To address these questions, we analyzed the kinetic expression of CD28 on highly pure CD28+CD8+ memory T cells cultured with IL-15. We found that CD28 expression was relatively stable during the initial few rounds of cell divisions (Fig. 3A); the average ratio of CD28null to CD28+CD8+ memory T cells was 0.43 for the cells that had undergone fewer than five cell divisions (n = 11) (Fig. 3B). In contrast, we found that a significant loss of CD28 expression occurred after the fifth cell division; the average ratio of CD28null to CD28+CD8+ memory T cell was 1.4 (Fig. 3B). To determine whether loss of CD28 expression was limited to the surface expression or occurred at the transcription level, we sorted CD28nullCD8 T cells generated after IL-15 treatment and analyzed the transcripts of CD28 by real-time RT-PCR. We found that CD28 mRNA was absent in CD28nullCD8+ memory T cells (Fig. 3C). We then analyzed induction of IL-2 and IL-2Rα (or CD25) expression as the function of CD28 and demonstrated that these CD28nullCD8+ memory T cells transcribed neither IL-2 nor CD25 in response to anti-CD3/CD28 stimulation (Fig. 3D). Furthermore, we found that loss of CD28 expression was stable in IL-15-generated CD28nullCD8+ memory T cells over a month (data not shown). These findings suggest that IL-15-mediated down-regulation of CD28 expression occurred primarily in actively dividing CD28+CD8+ memory T cells and that IL-15-induced loss of CD28 expression in CD8+ memory T cells was stable under continuous IL-15 stimulation.
FIGURE 3.
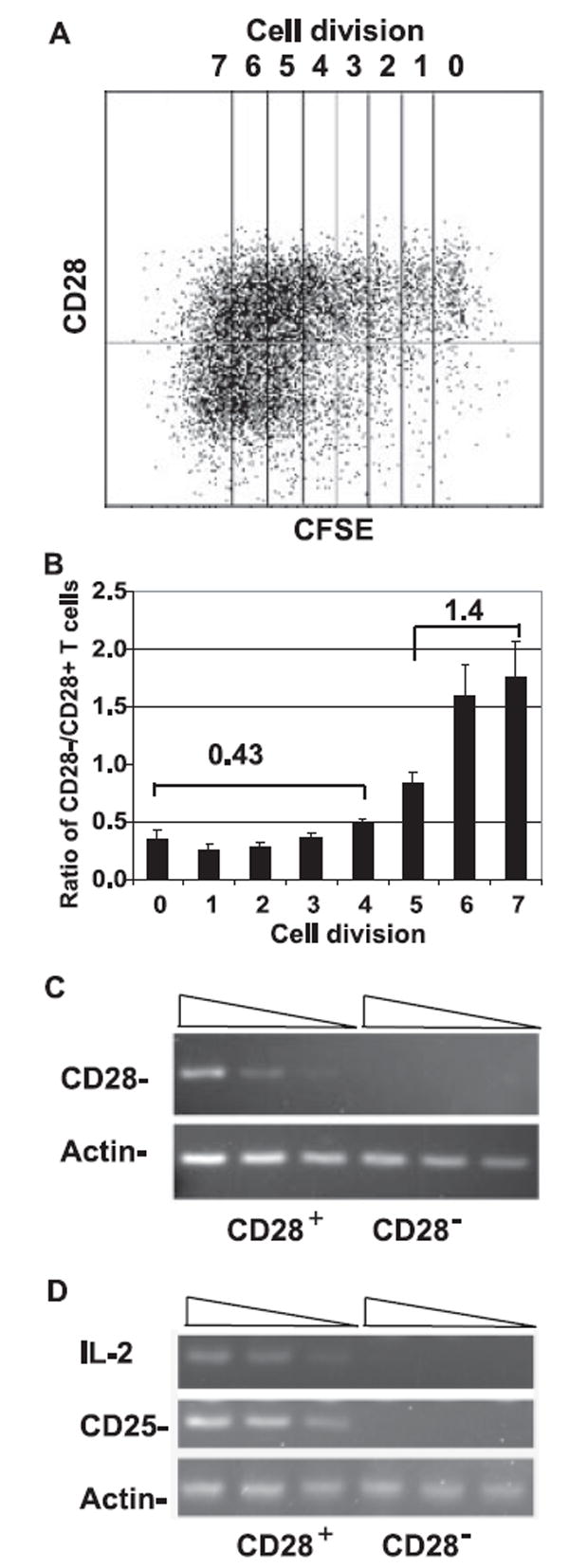
Loss of CD28 expression in CD28+CD8+ memory phenotype T cells after IL-15 treatment. A, Loss of CD28 expression in frequently dividing CD28+CD8+ memory T cells. Representative data of 11 independent experiments are shown. Freshly sorted CD28+CD8+ memory T cells were labeled with CFSE and cultured in the presence of 50 ng/ml IL-15 for 15 days. Cells were collected on days 5, 10, and 15 and CD28 expressions were analyzed by FACScan. B, The quantitative analysis of loss of CD28 expression in CD28+CD8+ memory phenotype T cells as a function of cell division. The ratios of CD28null to CD28+CD8+ memory T cells of each cell division are presented as mean ± SEM (n = 11). The mean ratios of CD28null/CD28+CD8+ T cells that underwent fewer than four and more than five cell divisions were 0.43 and 1.4, respectively. C, Lack of CD28 transcription in IL-15-generated CD28nullCD8+ memory T cells. CD8 memory T cells were cultured under IL-15 for 14 days before being sorted into CD28null and CD28+ subsets. CD28 expression and β-actin expression were analyzed by 39 cycles and 33 cycles of real-time RT-PCR, respectively. A 4-fold serial dilution of cDNA was applied in the analysis. D, Lack of IL-2 and IL-2Rα (CD25) expression in IL-15-cultured CD28nullCD8+ memory T cells after stimulation. Cells were stimulated with anti-CD3/CD28 beads for 16 h. Real-time RT-PCR was performed with 4-fold serial-diluted cDNA. Forty cycles of PCR were performed to amplify IL-2 and 35 cycles of PCR were performed to amplify CD25 and β-actin.
Generation of CD28null cells is unaffected by IL-15-induced GM-CSF, IFN-γ, IL-5, IL-6, IL-8, and IL-13
Our previous studies showed that IL-15 is capable of inducing production of effector cytokines in CD8+ memory T cells (25, 30), including TNF-α, which has been recently reported to induce down-regulation of CD28 expression in CD4+ T cells (29). To understand the role of IL-15-induced cytokines in regulation of CD28 expression, we measured 17 cytokines in the supernatant of IL-15-treated CD28+CD8+ memory T cells and found that 8 cytokines were highly induced in the IL-15-treated culture supernatant of CD8+ memory T cells (Table I). Neutralizing Abs specific to each of the eight cytokines were applied individually in the culture. With optimal concentrations of the Abs, we confirmed the neutralization of each cytokine by measurement of Ab-treated supernatants (data not shown) but we did not observe significant change of the percentage of CD28null cells in IL-15-cultured CD8+ memory T cells in the absence of individual cytokine GM-CSF, IFN-γ, IL-5, IL-6, IL-8, and IL-13 (Table I). We did not observe changes of the percentage of CD28null cells in IL-15-cultured CD8+ memory T cells after blocking all six cytokines simultaneously (data not shown). This suggests that IL-15-induced GM-CSF, IFN-γ, IL-5, IL-6, IL-8, and IL-13 at least did not participate in the generation of CD28nullCD8+ T cells in vitro individually.
Table I.
Summary of highly expressed cytokines in the supernatant of IL-15-cultured CD28+CD8+ memory T cells and their involvement in the regulation of CD28nullCD8+ memory T cells
| Cytokinea | Day 7 | Day 14 | Day 21 | Neutralizing Abs | Ab Effect on CD28null (%) |
|---|---|---|---|---|---|
| GM-CSF | 22.2 ± 7.0b | 30.6 ± 10.7 | 28.5 ± 11.7 | + | − |
| IFN-γ | 161.4 ± 65.3 | 79.3 ± 16.3 | 26.7 ± 5.3 | + | − |
| IL-5 | 41.2 ± 35.3 | 104.3 ± 85.0 | 37.8 ± 30.8 | + | − |
| IL-6 | 1037.5 ± 677.1 | 461.6 ± 302.5 | 224.6 ± 149.7 | + | − |
| IL-8 | 5883.7 ± 4150.7 | 1179.6 ± 753.6 | 267.3 ± 217.2 | + | − |
| IL-13 | 202.6 ± 173.3 | 407.7 ± 332.8 | 233.3 ± 190.7 | + | − |
| MIP-1β | 238.3 ± 25.5 | 120.4 ± 25.4 | 137.9 ± 29.9 | + | + |
| TNF-α | 14.4 ± 2.8 | 14.2 ± 2.5 | 8.4 ± 2.6 | + | + |
The concentration of cytokines was picograms per milliliter.
Data were presented as mean ± SEM (n = 5).
IL-15-induced TNF-α down-regulates CD28 in CD28+CD8+ memory T cells
To determine whether TNF-α mediates down-regulation of CD28 expression in CD28+CD8+ memory T cells, we used neutralizing anti-TNF-α Ab to block the TNF-α in the culture medium and found a significant decline in loss of CD28 expression in anti-TNF-α-Ab-treated cells (24.8%) as compared with the control (30.4%) at day 14 (p = 0.002, n = 12) (Fig. 4, A and B). In the presence of anti-TNF-α Ab, the number of CD28nullCD8+ memory T cells among cells was ~15% less than that of the control after 14 days (Table II). To confirm the neutralizing effect of anti-TNF-α Ab, we tested different concentrations of Ab (10, 20, and 50 μg/ml) and measured TNF-α in the supernatant of Ab-treated cells. We found no detectable levels of TNF-α in the anti-TNF-α-Ab-treated culture supernatant (data not shown).
FIGURE 4.
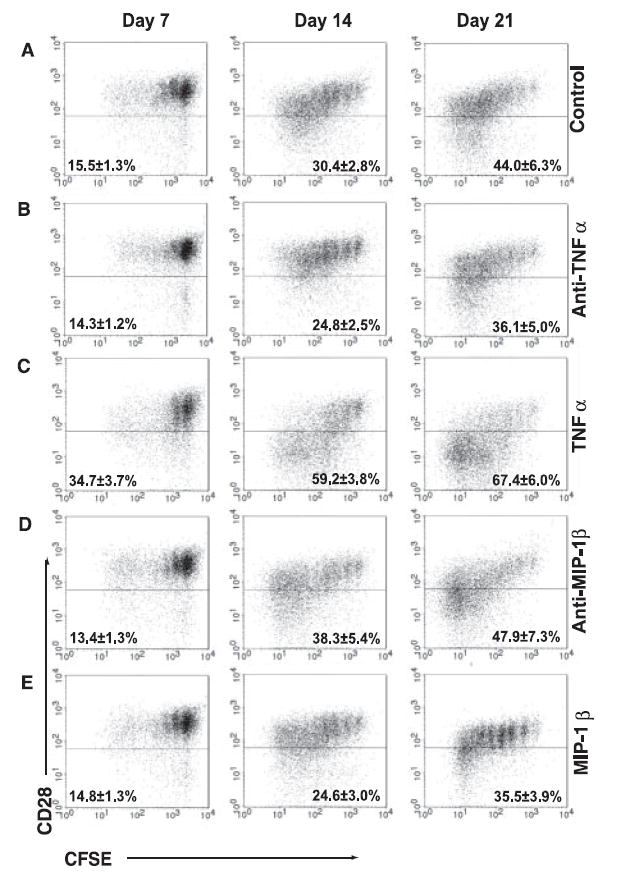
IL-15-induced TNF-α and MIP-1β influence the number of CD28null cells in CD28+CD8+ memory T cells. A, Generation of CD28null cells from CD28+CD8+ memory T cells after IL-15 treatment. CD28+CD8+ memory T cells isolated from cell sort were labeled with CFSE and cultured in the presence of IL-15. B, Reduction of CD28null cells by blocking TNF-α with Ab in IL-15-treated CD28+CD8+ memory T cells. Anti-TNF-α treatment (20 μg/ml) was added along with IL-15 to freshly sorted CD28+CD8+ memory T cells. C, Increase of CD28null cells by supplement of TNF-α in IL-15-treated CD28+CD8+ memory T cells. rTNF-α (200 ng/ml) was added along with IL-15 to freshly sorted CD28+CD8+ memory T cells. D, Increase of CD28null cells in the presence of anti-MIP-1β-neutralizing Ab in IL-15-treated CD28+CD8+ memory T cells. Anti-MIP-1β (6 μg/ml) was added along with IL-15 to sorted CD28+CD8+ memory T cells. E, Reduction of CD28null cells in the presence of rMIP-1β. rMIP-1β (500 ng/ml) was added along with IL-15. In all culture conditions, cells were collected at days 7, 14, and 21 and the rate of cell division and CD28 expression were analyzed by FACScan. The representative plots and the mean ± SEM from 5 to 13 independent donors are shown.
Table II.
Effects of TNF-α and MIP-1β on the changes of the percentage of CD28nullCD8+ memory T cells under IL-15a
| Condition | Day 7 (%) | Day 14 (%) | Day 21 (%) |
|---|---|---|---|
| IL-15 + Anti-TNF-α/IL-15 | 94 ± 6 | 85 ± 4b | 90 ± 3b |
| IL-15 + TNF-α/IL-15 | 216 ± 26b | 216 ± 18b | 185 ± 14b |
| IL-15 + Anti-MIP-1β/IL-15 | 83 ± 9 | 133 ± 10b | 125 ± 8b |
| IL-15 + MIP-1β/IL-15 | 98 ± 5 | 100 ± 3 | 90 ± 8 |
| IL-15 + Anti-TNF-α + MIP-1β/IL-15 | 98 ± 4 | 87 ± 2b | 82 ± 8 |
| IL-15 + TNF-α + Anti-MIP-1β/IL-15 | 190 ± 16b | 214 ± 18b | 201 ± 22b |
CD28+CD8+ memory T cells were cultured with IL-15 (50 ng/ml) in the presence or absence of neutralizing Abs or recombinant TNF-α or MIP-1β or a combination of Ab and recombinant TNF-α or MIP-1β for a given time. The percentage of CD28nullCD8+ memory T cells in the culture was determined by FACS analysis. The relative percentages of CD28nullCD8+ memory T cells under the various conditions to the IL-15 control are presented as mean ± SEM (n = 5–13).
Results are statistically significant compared to IL-15 alone by Student’s t test.
To confirm the effect of TNF-α in down-regulation of CD28 expression in CD8+ memory T cells, we tested the effect of added rTNF-α. The percentage of CD28nullCD8+ memory T cells in cultures containing IL-15 plus 200 ng/ml rTNF-α was significantly higher than that in control cultures (IL-15 alone) at day 14 (59.2 vs 30.4% in control cultures with IL-15 alone, p = 7.1 × 10−6) and day 21 (67.4 vs 44% in control, p = 0.001) (Fig. 4C and Table II). Furthermore, the loss of CD28 expression induced by exogenous TNF-α is dosage dependent, and a significant level of down-regulation of CD28 expression was observed at 50 ng/ml rTNF-α (data not shown). A high concentration of exogenous TNF-α provided at the beginning of culture accelerated CD28 down-regulation as early as 7 days of culture (Fig. 4C and Table II). Together, these findings indicate that TNF-α was capable of down-regulating CD28 expression in CD28+CD8+ memory T cells.
IL-15-induced MIP-1β has a significant negative effect on the proliferation of CD28null but not CD28+CD8+ memory T cells
As neutralization of TNF-α resulted in an ~15% reduction in the number of CD28null cells induced by IL-15, we speculated that other IL-15-induced cytokines might play a role in regulation of CD28 expression and the growth of CD28null and CD28+CD8+ memory T cells. MIP-1β, a chemokine involved in T cell migration and proliferation (31), was highly induced by IL-15 (Table I). When MIP-1β was blocked by neutralizing polyclonal anti-MIP-1β Ab in IL-15 cultures (Fig. 4D), we found a significant increase in the number of CD28null cells at day 14 (38.3 vs 30.4% in control cultures with IL-15 alone, p = 0.03) and at day 21 (47.9 vs 44% in control, p = 0.01) (Fig. 4D and Table II). A monoclonal anti-MIP-1β blocking Ab had a similar effect (data not shown). When we tested the effect of added rMIP-1β in the culture, we noticed a decreased percentage of frequently dividing cells as compared with the control but they did not reach statistical significance (Fig. 4E and Table II). However, we did not observe an additive effect of reducing CD28null cells when both anti-TNF-α Ab and rMIP-1β were applied in the IL-15 culture (Table II).
To examine the action of MIP-1β in IL-15-cultured memory CD8+ T cells, we isolated CD28null and CD28+ cells from IL-15-treated CD28+CD8+ memory T cells that had been cultured for 2 wk and cultured them separately using the MIP-1β-containing conditional medium in the presence or absence of anti-MIP-1β Abs for another 14 days. We found a significant increase in CD28null cell proliferation in the presence of anti-MIP-1β Ab as compared with that of nonspecific Ab control (PI was 2.6 for anti-MIP-1β treated vs 2.0 for the control, p = 0.05, n = 10) (Fig. 5A). Although a slight increase in the proliferation of CD28+ cells in the presence of anti-MIP-1β Ab as compared with that of nonspecific Ab control was also observed, it did not reach statistical significance (PI was 2.2 for anti-MIP-1β treated vs 1.9 for the control, p = 0.19, n = 10) (Fig. 5B). To understand the differential effect of MIP-1β on the growth of memory T cell subsets, we analyzed the expression of CCR5, a MIP-1β receptor, and found that exposure of MIP-1β in vitro resulted in down-regulation of CCR5 in both CD28null and CD28+CD8+ memory T cells (Fig. 5C). Furthermore, we found a significantly lower level of CCR5 expression in CD28null than in CD28+ memory T cells ex vivo (Fig. 5D). As lower levels of CCR5 were expressed in CD28nullCD8+ T cells than in their CD28+ counterparts, the inhibitory effect of increased MIP-1β on the growth of CD28nullCD8+ T cells was minimized. These findings suggest that the growth of CD28nullCD8+ T cells was affected by both positive and negative effects of cytokines, and that the effects of these cytokines were also regulated by the expression of their corresponding receptors.
FIGURE 5.
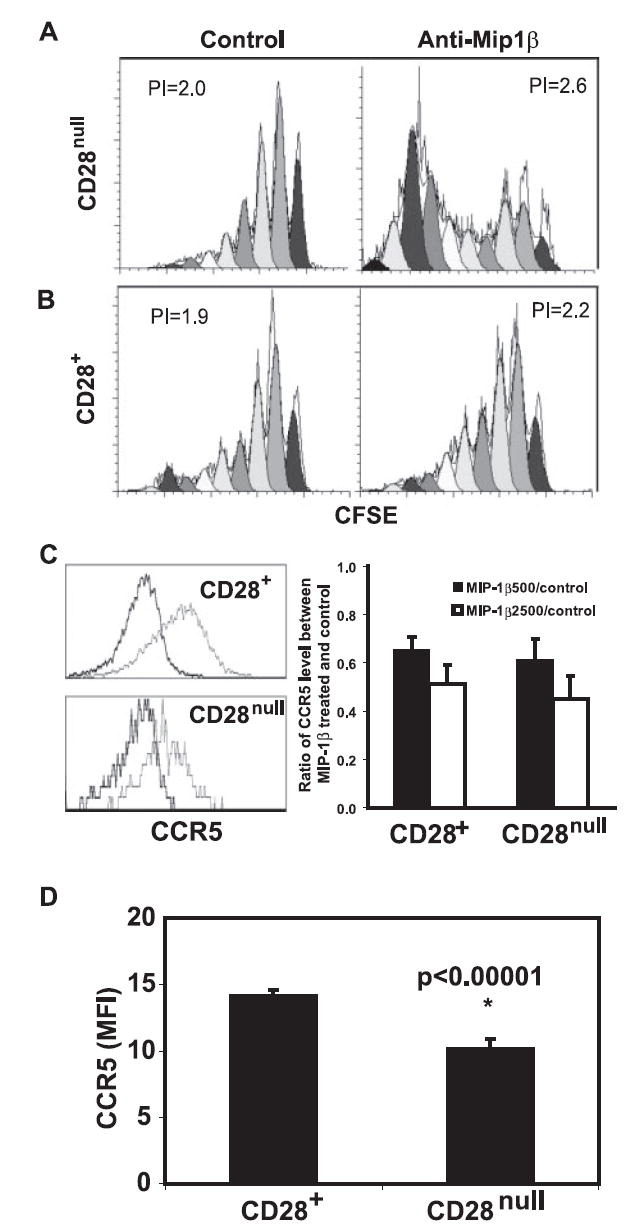
Inhibitory effect of MIP-1β on proliferation of CD28nullCD8+ memory T cells. CD28+CD8+ memory T cells were cultured for 14 days in the presence of IL-15 and sorted into CD28null and CD28+ subsets. Both subsets were labeled with CFSE and cultured with 50% fresh medium and 50% pooled conditional medium collected from IL-15-cultured CD8+ memory T cells of 10 donors. Cells were cultured for 14 days and analyzed by FACScan and software ModFit. A, Anti-MIP-1β treatment had a significant inhibitory effect on proliferation of CD28nullCD8+ memory T cells. A representative histogram is shown. B, Anti-MIP-1β treatment had a less inhibitory effect on proliferation of CD28+CD8+ memory T cells. A representative histogram is shown. The data in A and B were derived from 10 independent donors. C, Down-regulation of CCR5 expression in CD28null and CD28+CD8+ memory T cells in vitro after exposure to rMIP-1β at 500 ng/ml and 2500 ng/ml for 7 days. On the left, histograms of CCR5 expression levels between rMIP-1β treated (2500 ng/ml, bold line) and control (light line) for both CD28null and CD28+CD8+ T cells. On the right, the ratios of CCR5 expression between rMIP-1β treated and untreated CD8 subsets were presented as mean ± SE (n = 4). D, Decrease of CCR5 expression in CD28nullCD8+ T cells ex vivo. The levels of CCR5 on both CD28null and CD28+CD8+ T cells were analyzed by FACSCalibur, and data were presented as mean ± SEM (n = 65).
TNF-α and MIP-1β regulate each other in balancing their effects on CD28nullCD8+ memory T cells
As shown above, it appears that IL-15 induced two antagonist cytokines, TNF-α and MIP-1β, for regulating CD28nullCD8+ memory T cell generation and maintenance. To determine whether TNF-α regulates MIP-1β, we measured MIP-1β in the supernatant from either rTNF-α or anti-TNF-α-treated cultures and found that the levels of MIP-1β in the culture supernatant correlated with the level of TNF-α (Fig. 6A). This suggests that the production of MIP-1β was regulated by the level of TNF-α in the supernatant. To examine whether the level of MIP-1β reciprocally regulates TNF-α, we measured TNF-α in the supernatant from either anti-MIP-1β- or rMIP-1β-treated cultures and found that anti-MIP-1β resulted in an increase of TNF-α level in the supernatant (Fig. 6B). This suggests that the level of MIP-1β negatively regulated the level of TNF-α in IL-15-cultured CD8+ memory T cells. To further determine whether the levels of TNF-α correlate with the level of MIP-1β in vivo, we analyzed the concentration of TNF-α, MIP-1β, and the percentage of CD28nullCD8+ T cells in the blood of healthy young (age 18–34, n = 15) and old (age over 70, n = 59) donors. We detected significantly higher levels of TNF-α (p = 0.001) (Fig. 7A) correlating with a significantly higher level of MIP-1β (p = 0.01) (Fig. 7B), along with an increase in the percentages of CD28nullCD8+ T cells (p = 0.001) (Fig. 7C) in the old-age group. These findings suggest that cytokine production is tightly regulated by feedback mechanisms and that alteration of the balance of these cytokines may explain the accumulation of CD28nullCD8+ T cells with age.
FIGURE 6.
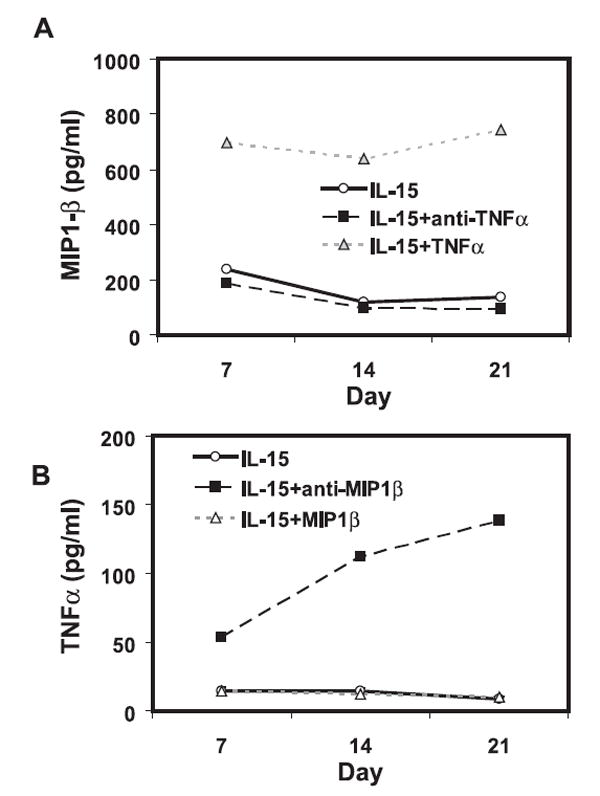
Production of TNF-α and MIP-1β by IL-15-treated CD8+ memory T cells in vitro. A, The level of TNF-α is correlated with the level of MIP-1β in the supernatant of CD28+CD8+ memory T cells. The concentrations of MIP-1β in the supernatant of sorted CD28+CD8+ memory T cells that were cultured with IL-15 in the presence of either anti-TNF-α Ab or rTNF-α were determined by BioPlex assay. The mean of five donors is shown. B, MIP-1β negatively regulates level of TNF-α in CD28+CD8+ memory T cells. The concentrations of TNF-α in the supernatant of sorted CD28+CD8+ memory T cells that were cultured with IL-15 in the presence of either anti-MIP-1β Ab or rMIP-1β were determined by BioPlex assay. The mean of seven donors is shown.
FIGURE 7.
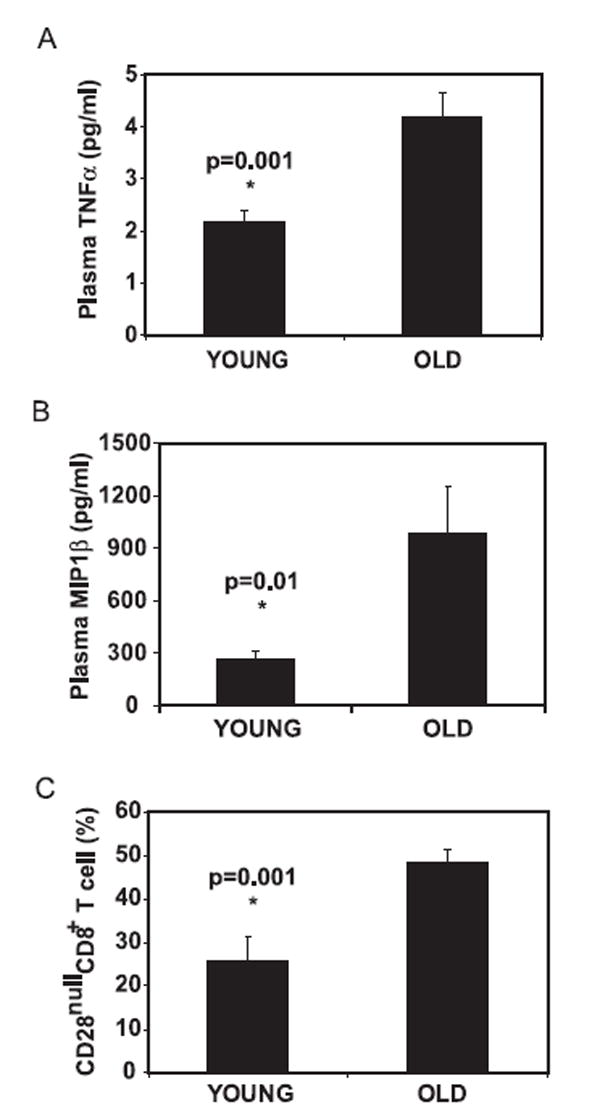
Levels of TNF-α and MIP-1β in peripheral blood. A, Increased concentration of TNF-α. B, Increase of MIP-1β in blood plasma in old donors. C, Increase of the percentage of CD28nullCD8+ memory T cells with age. Whole blood was collected from young (age <34, n = 15) and old (age >70, n = 59) donors. Plasma was isolated and cytokine level was measured by BioPlex and LincoPlex protein array systems, and the percentage of CD28nullCD8+ memory T cells was determined by FACS analysis.
Discussion
The deficiency of activation-induced proliferation of CD28nullCD8+ T cells has been considered a key defect in age-associated T cell dysfunction. In this regard, it was somewhat surprising that CD28null and CD28+CD8+ T cells had a similar growth rate in response to IL-15 (Fig. 2). This suggests that the IL-15-mediated growth pathway is intact in CD28nullCD8+ memory T cells, providing a plausible explanation for the age-associated expansion of CD28nullCD8+ T cells mediated by homeostatic cytokines. Furthermore, the fact that IL-15 and its induced cytokines are capable of down-regulating CD28 expression points out the prominent role of these cytokines in generation and maintenance of CD28nullCD8+ T cells with age.
Despite the identification of homeostatic cytokine in regulation of CD28 expression in CD28+CD8+ memory T cells, the mechanisms of their action are not fully understood. TNF-α, which has been shown to down-regulate CD28 expression in CD4+ T cells through alteration of CD28 promoter activity, accounts for ~15% of the CD28nullCD8+ memory T cells in culture with IL-15, as indicated by the effect of neutralizing anti-TNF-α Ab (Fig. 4). We did not detect effects on CD28 expression by individual neutralization of other IL-15-induced cytokines (GM-CSF, IFN-γ, IL-5, IL-6, IL-8, and IL-13) alone or in combination (Table I). Although it was somewhat surprising that anti-MIP-1β and TNF-α did not have an additive effect when added to the culture together (Table II), it should be noted that the lack of MIP-1β led to an increase in TNF-α and TNF-α was not the sole factor that determined CD28 down-regulation. Moreover, TNF-α and MIP-1β seem to regulate CD28 expression through different mechanisms; TNF-α directly regulates CD28 down-regulation while MIP-1β regulates cell proliferation. Because IL-15 serves primarily as a growth factor for CD8+ memory T cells in this culture system, it is difficult to distinguish its growth function from its potential roles in CD28 expression. Whether the down-regulation of CD28 expression in the remaining CD8+ memory T cells is mediated by IL-15 directly or by IL-15-induced cytokines in addition to TNF-α remains to be determined.
The finding that MIP-1β delivers an inhibitory effect on the growth of CD28nullCD8+ T cells is unexpected (Fig. 5). It appears that IL-15 exerts a paradoxical effect on CD8+ memory T cells. IL-15 induces TNF-α, which promotes generation of CD28null T cells, while it also induces MIP-1β, a chemokine that inhibits the growth of CD28nullCD8+ memory T cells. Strikingly, the level of TNF-α appears to positively regulate the level of MIP-1β while the level of MIP-1β appears to negatively regulate the level of TNF-α (Fig. 6). Furthermore, the observed reduction in expression of CCR5 in CD28nullCD8+ memory T cells in vitro and in vivo potentially acts to minimize the negative growth impact of MIP-1β on these cells. Together, these data suggest that a tightly regulated feedback system may operate here to balance the effects of cytokines on the growth of CD8+ memory T cells. The immune system is constantly adjusting and balancing effects that may be beneficial and detrimental in specific circumstances. In this case, the undesirable effect of IL-15-mediated homeostatic proliferation is down-regulation of CD28. By producing MIP-1β, IL-15 indirectly modulates the accumulation of CD28null T cells. Further modulating the overall outcome of these events, expression of CCR5 is reduced on CD28nullCD8+ memory T cells, making them less susceptible to inhibition by MIP-1β.
The factors that contribute to the accumulation of CD28nullCD8+ memory T cells with age are complex. Although we have shown that IL-15-induced TNF-α and MIP-1β are capable of regulating CD28nullCD8+ T cells, other cytokines have also been linked to CD28 regulation. IL-12 and IL-4 are able to slow down the loss of CD28 expression in CD8+ T cells and/or stimulate CD28null CD4+ T cells to re-express CD28 (32, 33). Additional experiments are needed to determine the interactions and mechanisms among these cytokines in regulation of CD28 expression and CD28nullCD8+ T cells. Our findings presented in this report suggest that homeostatic cytokine IL-15 is a cause of the generation and accumulation of CD28nullCD8+ memory T cells. Based on previous reports and present findings, we propose a two-step and feedback model for age-associated accumulation of CD28nullCD8+ memory T cells (Fig. 8). At the first step, loss of CD28 expression occurs in CD28+CD8+ T cells as a correlate of proliferation induced by either antigenic stimulation and/or homeostatic cytokines such as IL-15. The majority of T cells may re-express CD28 after the removal of such stimulations. Only those T cells with stable loss of CD28 expression, probably influenced by the continuous presence of the stimulators, TNF-α and possible additional cytokines, enter the second phase. During the second phase, the proliferative ability of CD28nullCD8+ memory T cells to stimulate Ag is impaired but remains intact to homeostatic cytokines. The cytokine-mediated growth of CD28nullCD8+ memory T cells appears to be complex as well. Both positive (IL-15) and negative (MIP-1β) growth factors for CD28nullCD8+ memory T cells exist and the positive growth effects of CD28nullCD8+ memory T cells eventually prevail with the advance of age. A better understanding of the mechanisms behind the generation and growth of CD28nullCD8+ memory T cells may facilitate the development of therapeutic means to improve the immune function in the elderly.
FIGURE 8.
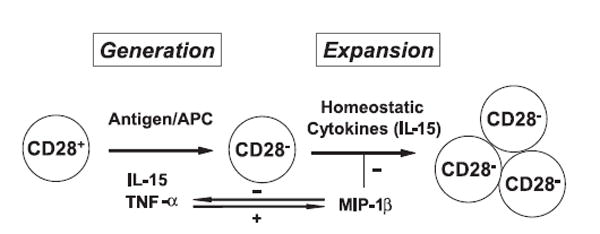
A two-step model of the accumulation of CD28nullCD8+ memory T cells with age. 1) Stable down-regulation of CD28 expression occurs in CD28+CD8+ memory T cells induced by either repeated antigenic stimulation or cytokines such as IL-15 and TNF-α and 2) proliferation of CD28null CD8+ memory T cells is induced by homeostatic cytokine (IL-15) but is also impeded by other negative growth regulators such as MIP-1β. The cytokines acting to enhance or inhibit generation and expansion of CD28nullCD8+ memory T cells are coregulated, such as the feedback regulation of TNF-α and MIP-1β, to reach a homeostatic balance. With age, the net effects for increase of CD28nullCD8+ memory T cells prevail.
Acknowledgments
We are grateful to Drs. Richard Hodes and Dan Longo for their critical reading of the manuscript. We thank Bob Wersto, Joe Chrest, and Christa Morris of the Flow Cytometry Unit for performing cell sorting and analysis, Drs. Shari Ling for obtaining Remicade, Bob Pyle for assistance of measuring cytokines, and Karen Madara and her staff of the Apheresis Unit for providing blood samples.
Footnotes
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Abbreviations used in this paper: γc, common γ-chain; PI, proliferation index.
Disclosures The authors have no financial conflict of interest.
References
- 1.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 2.Riley JL, June CH. The CD28 family: a T cell rheostat for therapeutic control of t cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 3.Azuma M, Phillips JH, Lanier LL. CD28− T lymphocytes: antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- 4.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Posnett DN, Edinger JW, Manavalan JS, Irwin C, Marodon G. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+ Int Immunol. 1999;11:229–241. doi: 10.1093/intimm/11.2.229. [DOI] [PubMed] [Google Scholar]
- 7.Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV. Shortened telomeres in the expanded CD28−CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Hazzan M, Labalette M, Noel C, Lelievre G, Dessaint JP. Recall response to cytomegalovirus in allograft recipients: mobilization of CD57+, CD28+ cells before expansion of CD57+ Transplantation. 1997;63:693–698. doi: 10.1097/00007890-199703150-00014. [DOI] [PubMed] [Google Scholar]
- 9.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28−CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 10.Batliwalla FM, Rufer N, Lansdorp PM, Gregersen PK. Oligoclonal expansions in the CD8+CD28− T cells largely explain the shorter telomeres detected in this subset: analysis by flow FISH. Hum Immunol. 2000;61:951–958. doi: 10.1016/s0198-8859(00)00157-9. [DOI] [PubMed] [Google Scholar]
- 11.Nociari MM, Telford W, Russo C. Postthymic development of CD28−CD8+ T cell subset: age-associated expansion and shift from memory to naive phenotype. J Immunol. 1999;162:3327–3335. [PubMed] [Google Scholar]
- 12.Scheuring UJ, Sabzevari H, Theofilopoulos AN. Proliferative arrest and cell cycle regulation in CD8+CD28− versus CD8+CD28+ T cells. Hum Immunol. 2002;63:1000–1009. doi: 10.1016/s0198-8859(02)00683-3. [DOI] [PubMed] [Google Scholar]
- 13.Brzezinska A, Magalska A, Szybinska A, Sikora E. Proliferation and apoptosis of human CD8+CD28+ and CD8+CD28− lymphocytes during aging. Exp Gerontol. 2004;39:539–544. doi: 10.1016/j.exger.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, Schonitzer D, Grubeck-Loebenstein B. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tortorella C, Pisconti A, Piazzolla G, Antonaci S. APC-dependent impairment of T cell proliferation in aging: role of CD28− and IL-12/IL-15-mediated signaling. Mech Ageing Dev. 2002;123:1389–1402. doi: 10.1016/s0047-6374(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 16.Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, Kronenberg M, Cohen D, Schachter F. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–609. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 17.Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, June CH. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 18.Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8+ T cells is induced by IL-2 receptor γ chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12:1005–1013. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 19.Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+CD28− T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 21.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 22.Li XC, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek TR, Strom TB. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 23.Linsley PS, Bradshaw J, Urnes M, Grosmaire L, Ledbetter JA. CD28 engagement by B7/BB-1 induces transient down-regulation of CD28 synthesis and prolonged unresponsiveness to CD28 signaling. J Immunol. 1993;150:3161–3169. [PubMed] [Google Scholar]
- 24.Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-α. J Immunol. 2001;167:3231–3238. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 25.Fann M, Chiu WK, Wood WH, III, Levine BL, Becker KG, Weng NP. Gene expression characteristics of CD28null memory phenotype CD8+ T cells and its implication in T-cell aging. Immunol Rev. 2005;205:190–206. doi: 10.1111/j.0105-2896.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Zhi W, Wareski P, Weng NP. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro. J Immunol. 2005;174:4019–4024. doi: 10.4049/jimmunol.174.7.4019. [DOI] [PubMed] [Google Scholar]
- 27.Hess K, Yang Y, Golech S, Sharov A, Becker KG, Weng NP. Kinetic assessment of general gene expression changes during human naive CD4+ T cell activation. Int Immunol. 2004;16:1711–1721. doi: 10.1093/intimm/dxh172. [DOI] [PubMed] [Google Scholar]
- 28.Sansoni P, Fagnoni F, Vescovini R, Mazzola M, Brianti V, Bologna G, Nigro E, Lavagetto G, Cossarizza A, Monti D, et al. T lymphocyte proliferative capability to defined stimuli and costimulatory CD28 pathway is not impaired in healthy centenarians. Mech Ageing Dev. 1997;96:127–136. doi: 10.1016/s0047-6374(97)01887-3. [DOI] [PubMed] [Google Scholar]
- 29.Lewis DE, Merched-Sauvage M, Goronzy JJ, Weyand CM, Vallejo AN. Tumor necrosis factor-α and CD80 modulate CD28 expression through a similar mechanism of T-cell receptor-independent inhibition of transcription. J Biol Chem. 2004;279:29130–29138. doi: 10.1074/jbc.M402194200. [DOI] [PubMed] [Google Scholar]
- 30.Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci USA. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taub DD, Ortaldo JR, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. β Chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J Leukocyte Biol. 1996;59:81–89. doi: 10.1002/jlb.59.1.81. [DOI] [PubMed] [Google Scholar]
- 32.Warrington KJ, Vallejo AN, Weyand CM, Goronzy JJ. CD28 loss in senescent CD4+ T cells: reversal by interleukin-12 stimulation. Blood. 2003;101:3543–3549. doi: 10.1182/blood-2002-08-2574. [DOI] [PubMed] [Google Scholar]
- 33.Labalette M, Leteurtre E, Thumerelle C, Grutzmacher C, Tourvieille B, Dessaint JP. Peripheral human CD8+CD28+T lymphocytes give rise to CD28− progeny, but IL-4 prevents loss of CD28 expression. Int Immunol. 1999;11:1327–1336. doi: 10.1093/intimm/11.8.1327. [DOI] [PubMed] [Google Scholar]


