Abstract
An anti-poxvirus vaccine based on replicon particles of Venezuelan equine encephalitis virus (VRP) is being developed. The cowpox virus genes encoding structural proteins corresponding to vaccinia virus proteins A33, B5, and A27 were each expressed from VRP. High serum IgG titers against these proteins were generated in BALB/c mice vaccinated with each of these VRP. VRP induced both IgG1 and IgG2a with a strong predominance of IgG2a production. The response is long-lasting, as evidenced by the retention of high anti-B5 serum IgG titers through at least 50 weeks after priming immunization. Mice vaccinated with B5-, A33- or A27-VRP individually or together survived intranasal challenge with cowpox virus, with the multivalent vaccine formulation providing more effective protection from weight loss and clinical signs of illness than the monovalent vaccines. These results demonstrate that VRP may provide an effective alternative to vaccinia virus vaccines against poxvirus infection.
Introduction
The Poxviridae are a family of enveloped viruses with large, linear double stranded DNA genomes ranging in size from 130 to 300 kilobase pairs (kbp) that replicate in the cytoplasm of infected cells. The family is divided into two subgroups: Chordopoxvirinae and Entomopoxvirinae. Chordopoxivirinae have a vertebrate host range and are divided into eight genera, one of which contains the Orthopoxviruses, including camelpox, cowpox, ectromelia (mousepox), monkeypox, raccoonpox, skunkpox, vaccinia, and variola virus, the etiologic agent of smallpox. The orthopoxviruses each have a genome of about 200-kbp typically encoding more than 150 proteins. During the viral life cycle, maturation of the virus results in two major infectious forms of virus: intracellular mature virus particles (MV) and extracellular enveloped virus particles (EV), which have different repertoires of viral envelope proteins (Moss, 2001; Moss, 2006).
Throughout history, smallpox has been one of the most feared human diseases. In 1977, variola virus was eradicated from the natural environment after a World Health Organization vaccination campaign employing live vaccinia virus vaccines (reviewed by Fenner et al., 1988). Although these vaccines were effective against variola virus, they were associated with a high frequency of complications ranging in severity from mild to life-threatening (reviewed by (Fulginiti et al., 2003). Consequently, as smallpox was eradicated around the world, routine vaccination against this disease was discontinued. In the ensuing absence of any natural threat of smallpox, a large proportion of the world’s population has never received vaccination against smallpox or acquired natural resistance to the disease. Individuals vaccinated before the 1980s may also lack protection against orthopoxvirus infection, because vaccinees may lose neutralizing antibodies in the third decade after vaccination (Viner and Isaacs, 2005). Cessation of vaccination and waning immunity has left a large and growing proportion of the population susceptible to infection by the deliberate release of natural wild type or weaponized orthopoxviruses (Henderson, 1999). Furthermore, orthopoxviruses are significant zoonotic pathogens (Hutin et al., 2001; Nalca et al., 2005; Stephenson, 2003). Epidemics of human monkeypox occur sporadically throughout central and west Africa, and an outbreak of monkeypox was reported in the US Midwest in 2003 after infected pet prairie dogs exposed humans to monkeypox virus (Hutin et al., 2001; Nalca et al., 2005; Stephenson, 2003).
The threat of bioterrorist attacks and natural zoonotic orthopoxviral outbreaks has prompted a resumption of vaccination programs to protect certain groups. However, the risks of serious side effects from current vaccinia virus vaccines remain and the resulting low vaccination rates provide the impetus for renewed efforts to develop safe and effective alternatives to live vaccinia virus vaccines.
One strategy to reduce virus-associated complications is to vaccinate not with whole live virus, but with components of the virus known to induce protective immunity. Several MV envelope proteins have been identified as targets of neutralizing antibodies, including A27, L1, H3, and D8 proteins (Davies et al., 2005; Hsiao, Chung, and Chang, 1999; Ichihashi and Oie, 1996; Lin et al., 2000; Rodriguez, Janeczko, and Esteban, 1985; Wolffe, Vijaya, and Moss, 1995). Furthermore, antibodies against B5 protein neutralize EVs (Bell et al., 2004). Several studies have suggested that these targets can be used as subunit vaccines in the form of DNA or purified proteins. His-tagged vaccinia virus A33 and B5 proteins purified from bacteria protected mice against vaccinia virus challenge (Galmiche et al., 1999). B5, L1, A33, and A27 DNA vaccines protected mice from vaccinia virus challenge and non-human primates from lethal monkeypox virus challenge (Hooper et al., 2000; Hooper, Custer, and Thompson, 2003; Hooper et al., 2004; Pulford et al., 2004). Protection was most effective using a combination MV and EV antigens (Hooper, Custer, and Thompson, 2003). Vaccination with purified L1, A33, and B5 proteins also protected mice from vaccinia challenge better than mice immunized with live vaccinia virus (Fogg et al., 2004), and purified A33 protects mice from ectromelia (mousepox) challenge (Fang et al., 2006). Protection with purified proteins is antibody mediated, as passive transfer of monoclonal and polyclonal antibodies also protect mice from lethal vaccinia virus challenge when administered either prophylactically or therapeutically (Lustig et al., 2004).
An alternative vaccine system to deliver subunits of the virus employs single-cycle replicons of Venezuelan equine encephalitis virus (VEE). VEE is an alphavirus within the family Togaviridae with a positive sense 11.5-kbp RNA genome that replicates in the cytoplasm of infected cells (Schlesinger, 2001). The 5′ two-thirds region of the genome encodes non-structural replicase / transcriptase proteins, and the 3′ third region encodes capsid and E1 / E2 glycoproteins downstream of a strong, internal 26S subgenomic mRNA promoter.
VEE replicon particles (VRP) are produced by replacing the structural genes in the VEE genome with heterologous immunogen. The modified VEE genome is co-electroporated into packaging cells with the structural genes provided in trans from two helper RNAs, and VRP containing replicon genomes infect cells and express the transgene, but cannot produce progeny virions (Pushko et al., 1997). In cells infected with VRP in vivo high levels of antigen are produced and both systemic and mucosal immunity are induced in a broad range of species (Davis et al., 2002; Dong et al., 2003; Johnston et al., 2005; Pushko et al., 1997; Schultz-Cherry et al., 2000). VRP directly infect dendritic cells (MacDonald and Johnston, 2000; Moran et al., 2005) and provide adjuvant activity independent of transgene expression (Thompson et al., 2006). VRP expressing gag from HIV clade C have completed Phase I clinical trials in the United States and Africa. Preliminary data analysis indicates that VRP appear to be safe and to elicit antibodies (Callay I, personal communication).
In this study, we have used a cowpox virus/mouse model of infection to determine if the VEE-VRP system will also provide an effective subunit vaccine protective against orthopoxvirus infection.
Results
Expression of cowpox virus genes from VRP
Several vaccinia virus proteins, have been identified as targets of neutralizing antibodies. These include the MV surface proteins A27 (Rodriguez, Janeczko, and Esteban, 1985), L1 (Wolffe, Vijaya, and Moss, 1995), H3 (Lin et al., 2000), and D8 (Hsiao, Chung, and Chang, 1999), as well as the EV surface protein B5 (Aldaz-Carroll et al., 2005; Bell et al., 2004; Galmiche et al., 1999; Law and Smith, 2001). Furthermore, antibodies against A33 are protective in vivo even though they do not elicit neutralizing antibodies (Galmiche et al., 1999). Specifically, vaccinating with a combination of MV and EV antigens protects animals against vaccinia and monkeypox challenge (Fogg et al., 2004; Hooper, Custer, and Thompson, 2003; Hooper et al., 2004).
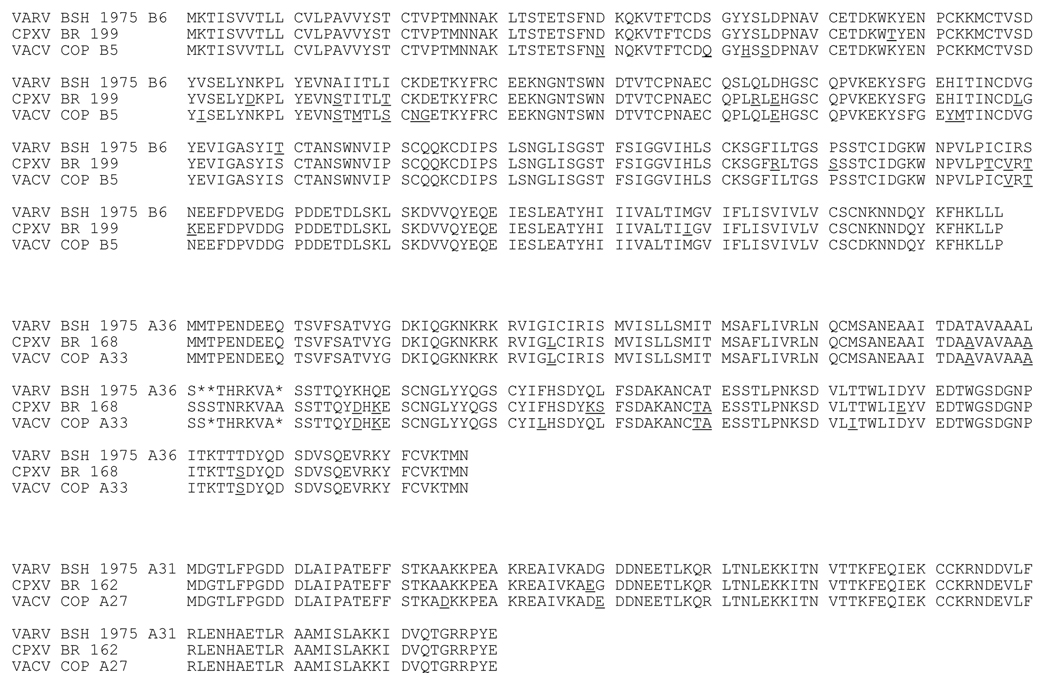
VRP were engineered to express three proteins encoded by the Brighton red strain of cowpox virus (CPXV): two EV antigens, CPXV199 and CPXV168 proteins (similar to vaccinia virus Copenhagen B5 and A33), and one MV antigen, CPXV162 protein (similar to vaccinia virus Copenhagen A27) (Goebel et al., 1990; Massung et al., 1994). In comparing amino acid sequences, CPXV199 protein has 93% identity / 97% similarity to vaccinia virus Copenhagen B5 protein, and 94% identity / 97% similarity to the corresponding protein encoded by variola virus Bangladesh 1975, while vaccinia virus Copenhagen B5 protein also share 93% identity / 97% similary with this variola virus major protein (Figure 1). The CPXV168 protein shares 96% identity / 97% similarity to vaccinia virus Copenhagen A33, and 90% identity / 94% similarity to the corresponding protein of variola virus, while the vaccinia virus A33 protein shares 94% identity / 96% similarity to the corresponding protein encoded by variola virus (Figure 1). The CPXV163 protein shares 97% identity / 98% similarity to vaccinia virus Copenhagen A27 protein, and 98% identity / 100% similarity to the corresponding variola virus MV surface protein. The vaccinia virus Copenhagen A27 protein also shares 97% identity / 98% similarity to the corresponding variola virus protein (Figure 1).
Figure 1. Amino acid comparison of variola, cowpox, and vaccinia virus antigens.
Three genes from cowpox virus Brighton red were cloned into VEE replicon vectors. An amino acid alignment was performed to compare cowpox virus sequences to variola virus Bangladesh 1975 and vaccinia virus Copenhagen sequences. Underlined amino acids indicate a difference in amino acid in comparison to the variola virus sequence. Deletions are indicated with *. The top panel compares variola virus B6 (Bangladesh 1975) with CPXV199 and vaccinia virus Copenhagen B5 proteins. The middle panel compares variola virus A36 (Bangladesh 1975) with CPXV168 and vaccinia virus Copenhagen A33 proteins. The bottom panel compares variola virus A31 (Bangladesh 1975) with CPXV162 and vaccinia virus Copenhagen A27 proteins.
To confirm expression from VRP infection, baby hamster kidney (BHK) cells were infected with VRP at multiplicities of infection (MOI) of 3. Cells were harvested at 12 hours post-infection and the expression of cowpox virus antigens was confirmed by immunoblot with a polyclonal anti-vaccinia virus antibody cross-reactive with cowpox virus proteins. The predicted molecular weights of B5, A33, and A27 are 35 kDa, 20.5 kDa, and 12.6 kDa respectively, and antigens corresponding to the predicted molecular weights were expressed in VRP infected cells (Figure 2).
Figure 2. Expression of cowpox virus genes from VRP infection.
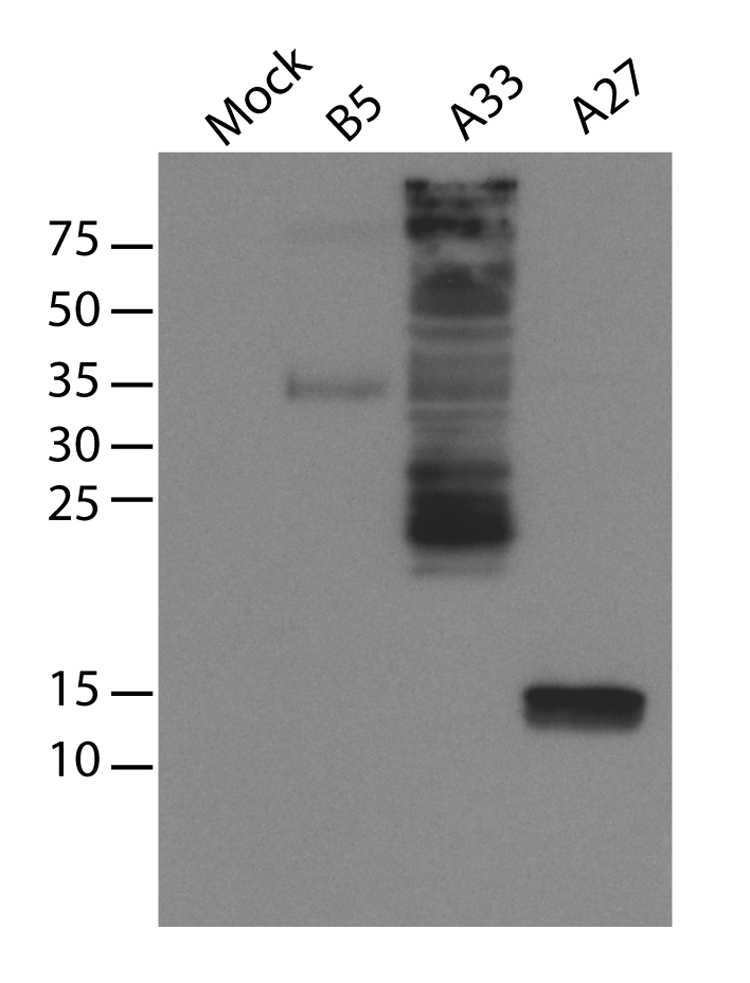
CPXV199 (similar to vaccinia virus B5), CPXV168 (similar to vaccinia virus A33), and CPXV162 (similar to vaccinia virus A27) genes from the cowpox virus (Brighton red) were cloned into VEE replicon vectors and packaged in VRP. BHK cells were infected with VRP and infected cell lysates were used for immunoblot with a polyclonal vaccinia virus antibody.
B5-VRP induces long-lasting, high levels of anti-B5 serum IgG
While T-cell responses contribute to poxvirus immunity, an antibody response is necessary and sufficient to protect macaques against a lethal monkeypox challenge and mice against secondary infection with ectromelia virus (Edghill-Smith et al., 2005; Panchanathan, Chaudhri, and Karupiah, 2006). B5 is an EV envelope protein that contributes to protection against vaccinia virus and monkeypox (Fogg et al., 2004; Hooper, Custer, and Thompson, 2003; Hooper et al., 2004). Furthermore, antibodies against B5 neutralize EV and are the major neutralizing components of human vaccinia immunoglobulin (Bell et al., 2004; Galmiche et al., 1999).
As antibody responses appear to be the major correlate of protection against orthopoxviral disease, the antibody response against a VRP-based vaccine was tested. BALB/c mice were primed with 1 × 106 IU B5-VRP in the rear footpads. Mice were boosted with the same dose of B5-VRP at four and forty-two weeks post-prime. Blood was collected periodically and serum IgG was assayed by B5 endpoint ELISA. ELISAs were performed with purified B5 from vaccinia virus WR. Endpoint titers for B5-VRP vaccinated mice were approximately 104 three weeks post-boost and lost approximately half a log over 38 weeks (Figure 3A). After the second boost, serum IgG titers rebounded. These data indicate that B5 is immunogenic in mice, and when expressed using the VRP system, results in lasting serum antibody titers.
Figure 3. B5-VRP induces long-lasting, high levels of anti-B5 serum IgG.
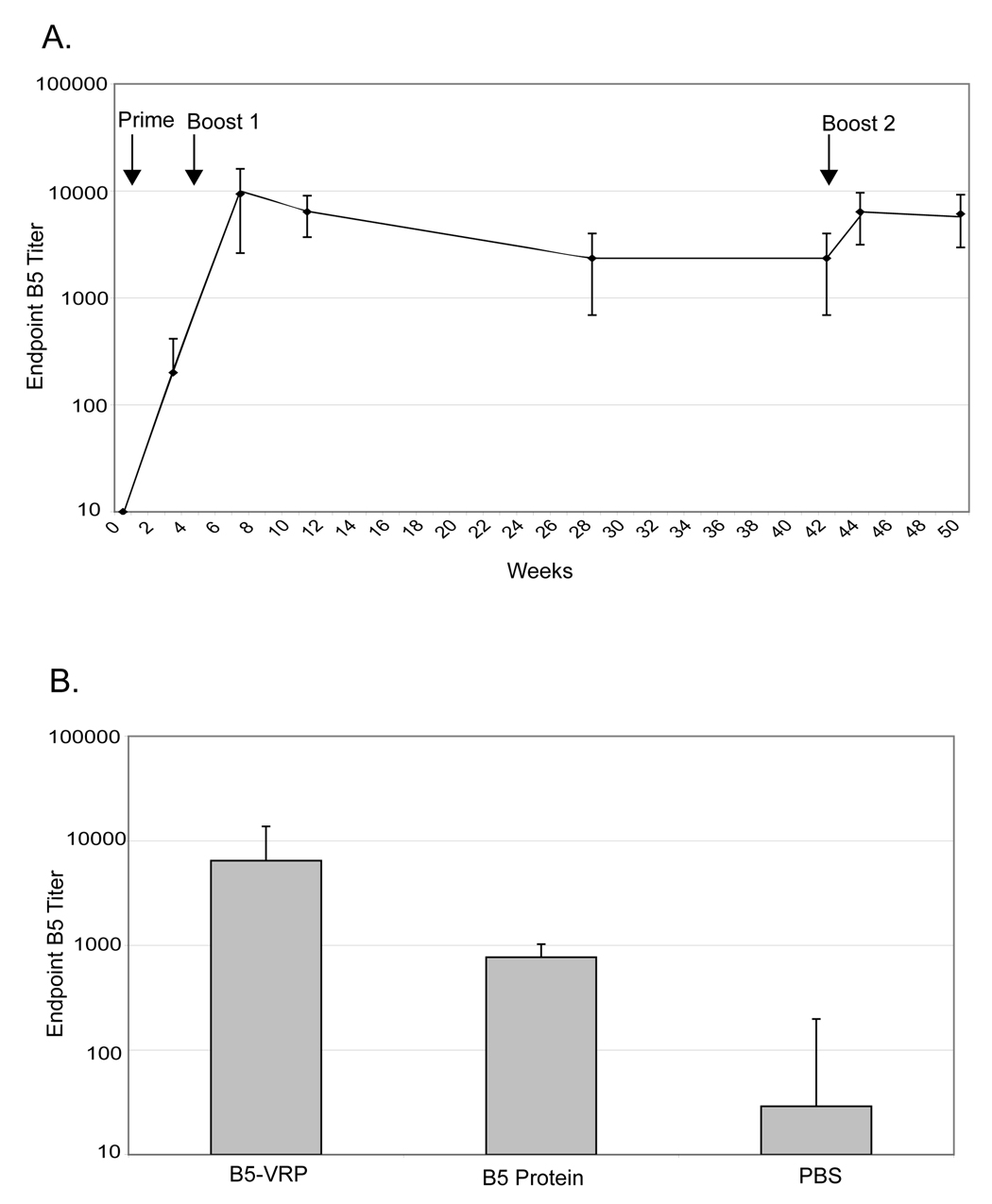
A. Six week-old BALB/c mice were primed with 1 × 106 IU B5-VRP subcutaneously in the rear foot-pad at time 0. Mice were boosted with the same dose at 4 and 42 weeks post-prime. Sera were collected at various time points up to 50 weeks post-prime and were used for B5 ELISA. Serum IgG ELISA titers are reported as the geometric mean of the reciprocal dilution at which the sample absorbance was greater than 0.2 at 450 nm. B. Six week-old BALB/c mice were primed with 1 × 106 IU B5-VRP, 10 µg purified B5, or PBS subcutaneously in the rear foot-pads. Four weeks post-prime, mice were boosted with the same dose of VRP, protein, or PBS. Sera were collected two weeks post-boost and was assayed for B5 serum IgG titers by ELISA.
In order to compare antibody responses from B5-VRP to purified protein, BALB/c mice were primed and boosted one time, four weeks post-prime with 1 × 106 IU B5-VRP in the rear footpads, 10 µg purified B5 protein, or PBS control. Blood was collected three weeks post-boost, and anti-B5 serum IgG was assayed by ELISA. His-tagged B5 protein was used both to immunize mice and coat ELISA plates. To control for the possibility that some of the observed reactivity towards the His-tagged B5 protein was directed to the His-tag itself, ELISA plates were coated with His-peptide, and no background was observed (data not shown). Mice vaccinated with B5-VRP had B5 titers of approximately 1 × 104, 10-fold higher than mice vaccinated with purified B5, confirming that B5 expressed from VRP infection is highly immunogenic in BALB/c mice (Figure 3B).
B5-VRP, A33-VRP, and A27-VRP and a cocktail of these VRP elicit antibody responses in BALB/c mice
In order to examine the effectiveness of VRP vaccines, mice were primed with 1 × 106 B5-VRP, A27-VRP, or A33-VRP individually or as a cocktail of all three plus a fourth non-expressing VRP for a total dose of 4 × 106 IU. VRP expressing the influenza virus hemagglutinin, HA-VRP, were used as an irrelevant vaccine control. Mice were boosted once, 4 weeks post-prime, with the same dose of VRP.
Sera was collected two weeks post-boost and antibodies against B5, A27, and A33 were detected qualitatively when sera from individual mice were used for immunoblot against cell lysates that were infected with cowpox virus. Whole cell lysates from mock infected and cowpox virus Brighton red infected cells were resolved by SDS-PAGE. Sera from individual mice were used as primary antibody. Sera from mice that were bled prior to vaccination (Pre-Bleed) did not reveal any cross reactivity in un-infected or infected cell lysates (Figure 4A, lanes 1 and 2). Sera collected two weeks post-boost from mice vaccinated with HA-VRP also revealed no cross reactivity in either uninfected or infected cell lysates (Figure 4D, lanes 3 and 4). Sera from mice vaccinated with B5-VRP and A33-VRP alone recognize appropriately sized proteins in infected cell lysates, (Figure 4A, lanes 6 and 10). Sera from mice vaccinated with A27-VRP alone also recognized an appropriately sized protein in infected cell lysates (Figure 4A, lane 12). Furthermore, anti-B5, A33, and A27 antibodies were detected in mice vaccinated with the 3X cocktail (Figure 4A, lane 12)
Figure 4. B5-VRP, A33-VRP, and A27-VRP elicit an antibody responses in BALB/c mice.
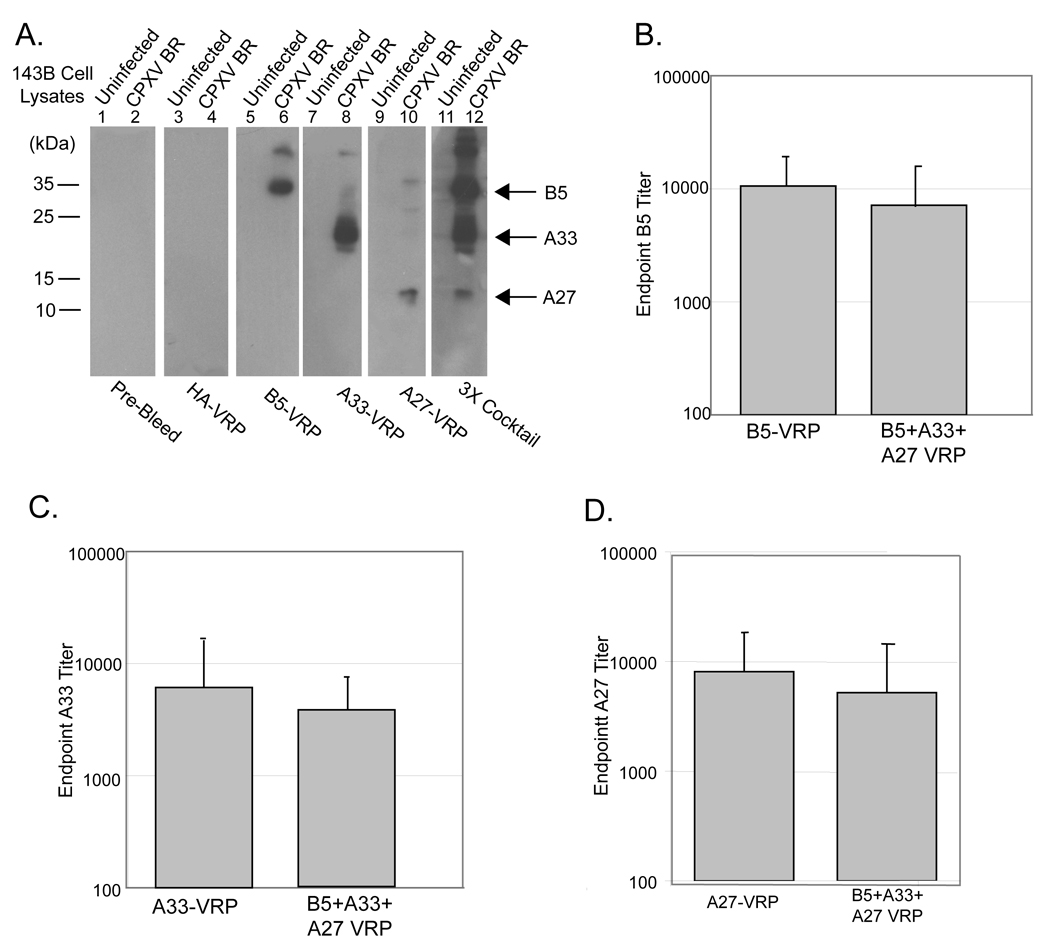
Six-week old BALB/c mice were primed with 1 × 106 IU B5-VRP, A33-VRP, and A27-VRP, individually, or a cocktail of all three VRP plus a fourth non-expressing VRP for a total dose of 4 × 106 IU (3X Cocktail). Mice were boosted at the same dose four weeks post-prime. Sera were collected two weeks post-boost and used for immunoblot and ELISA. A. The presence of anti-cowpox virus antibody in mice vaccinated with B5-VRP, A33-VRP, and A27-VRP, individually, the 3X cocktail, and HA-VRP were detected by immunoblot. Sera from individual mice were used as primary antibodies against uninfected 143B cell lysates and cowpox virus Brighton red infected 143B cell lysates. Sera from mice bled prior to vaccination (Pre-Bleed) were used as a control to detect any non-specific reactivity. B. Sera from mice vaccinated with B5-VRP and the 3X cocktail were used for B5 serum IgG ELISA. C. Sera from mice vaccinated with A33-VRP and the 3X cocktail were used for A33 serum IgG ELISA. D. Sera from mice vaccinated with A27-VRP were used for A27 serum IgG ELISA.
Antibody titers were examined by B5, A33, and A27 IgG ELISA. A33 ELISAs were performed with purified His-tagged A33 from vaccinia virus WR, and A27 ELISAs were performed with His-tagged A27 from cowpox virus Brighton red. Serum anti-B5 IgG titers of mice vaccinated with B5-VRP were approximately 104 post-boost and were not statistically different in mice vaccinated with the cocktail of B5, A33, and A27-VRP (p = 0.49) suggesting that immunodominance is not evident when using multiple antigens in the VRP vaccine system (Figure 4B). Similarly, mice vaccinated with A33-VRP alone or as a constituent of the 3X cocktail had A33 ELISA titers of 7 × 10³ and 4 × 10³, respectively (Figure 4C). Mice vaccinated with A27-VRP alone or as a constituent of the 3X cocktail had A27 ELISA titers of 8 × 10³ and 5 × 10³, respectively (Figure 4D).
MV neutralizing antibodies were assayed by plaque reduction neutralization assay. Sera from mice vaccinated with the 3X cocktail VRP reduced plaque formation by 50% at a dilution of approximately 1:500.
B5, A33, and A27-VRP elicit an IgG2a polarized antibody response
Immunoglobulin G subtypes differ in their effector functions and can be indicative of the T helper (Th) response. Th type 2 (Th2) cytokines such as IL-4 induce an isotype switch to IgG1, and Th type 1 (Th1) cytokines, such as IFNγ induce an isotype switch to IgG2a (Finkelman et al., 1990). In order to examine the immunoglobulin G subtypes induced by VRP-vaccination, sera from mice vaccinated with B5-VRP, A33-VRP, or A27-VRP were characterized by means of IgG1 and IgG2a specific ELISAs. Serum anti-B5 IgG1 titers were approximately 9.0 × 10³ post-boost, and serum anti-B5 IgG2a titers were 3.6 × 104 (Figure 5A). Serum anti-A33 IgG1 titers were 2.6 × 10³ post-boost, and serum anti-A33 IgG2a titers were 3.8 × 104 (Figure 5B). Serum anti-A27 IgG1 titers were 500 post-boost, and serum anti-A27 IgG2a titers were 2.3 × 104 (Figure 5C). These data indicate that anti-B5 IgG2a:IgG1 ratio were 4:1, the anti-A33 IgG2a:IgG1 ratio were 14:1, and the anti-A27 IgG2a:IgG1 ration were 40:1 in mice vaccinated with VRP.
Figure 5. B5, A33, and A27-VRP elicit an IgG2a polarized antibody response.
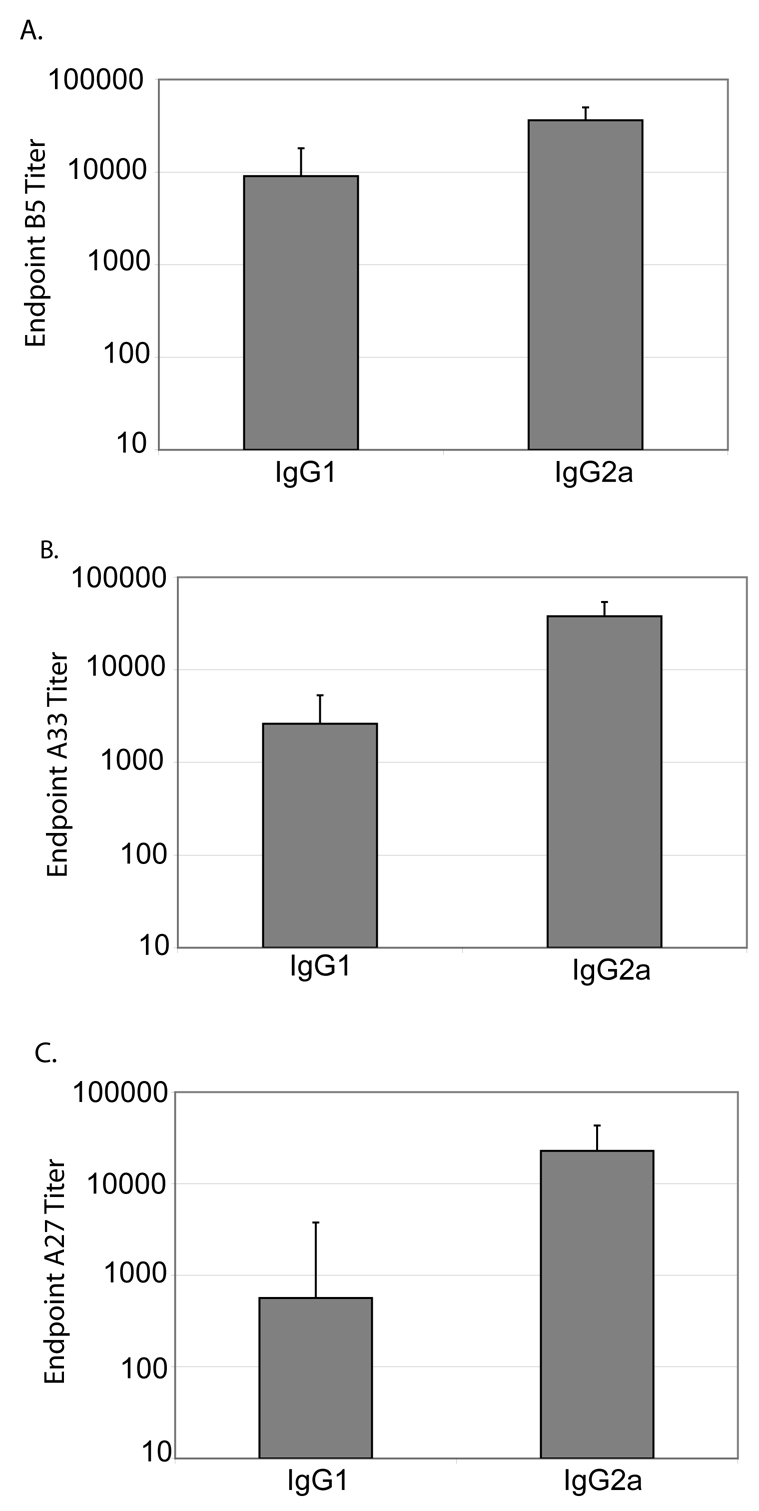
Six-week old BALB/c mice were primed with 1 × 106 IU B5-VRP or A33-VRP. Mice were boosted at the same dose four weeks post-prime. Sera were collected two weeks post-boost and used for IgG subtype ELISAs. A. Sera from mice vaccinated with B5-VRP B5 serum IgG1 and IgG2a ELISA. B. Sera from mice vaccinated with A33-VRP were used for A33 serum IgG1 and IgG2a ELISA. C. Sera from mice vaccinated with A27-VRP were used for A27 serum IgG1 and IgG2a ELISA.
Intranasal challenge of adult BALB/c mice with cowpox virus Brighton red
Clinical signs of illness in 6–8 week old BALB/c mice begin 3–6 days after intranasal challenge with cowpox virus, and the mice typically succumb to lethal doses of virus 4–10 days after infection (Thompson et al., 1993). Infection induces peribronchial and peribronchiolar hemorrhage, bronchial and bronchiolar epithelial cell hyperplasia, and alveolar hemorrhage and edema (Thompson et al., 1993). However, mice older than 6–8 weeks are more resistant to infection than younger mice, necessitating the establishment of a model involving the intranasal challenge of 14–16 weeks old BALB/c mice with cowpox virus to allow time for a standard prime-boost regimen.
In this model system, virus infected mice began to lose weight at approximately 5 days post-challenge with peak weight loss at 10 days post-challenge (Figure 6A). All mice that lost more than 20% of their body weight were euthanized according to the established animal protocol, though surviving mice started to regain weight at 12 days post-challenge. The degree of weight loss was dose dependent and animals challenged with doses greater than 1 × 10³ pfu lost a statistically significant amount of weight in comparison to mock challenged animals. Infected mice exhibited ruffling of the fur, conjunctivitis in the eye proximal to the challenged nostril, decreased activity, and labored breathing (Figure 6B). Similarly, linical signs of illness were apparent by approximately 5 days post challenge and were dose dependent. Five of 8 mice challenged with 1 × 106 pfu cowpox virus and 5 of 8 mice challenged with 1 × 105 pfu cowpox virus survived challenge. All of the mice challenged with 1 × 104 pfu or 1 × 10³ pfu cowpox virus survived. Multiple repetitions of intranasal challenge with cowpox virus Brighton red have indicated that this model is both predictable and reproducible, providing an excellent screening mechanism for candidate vaccines.
Figure 6. Intranasal challenge of adult BALB/c mice with cowpox virus (Brighton red).
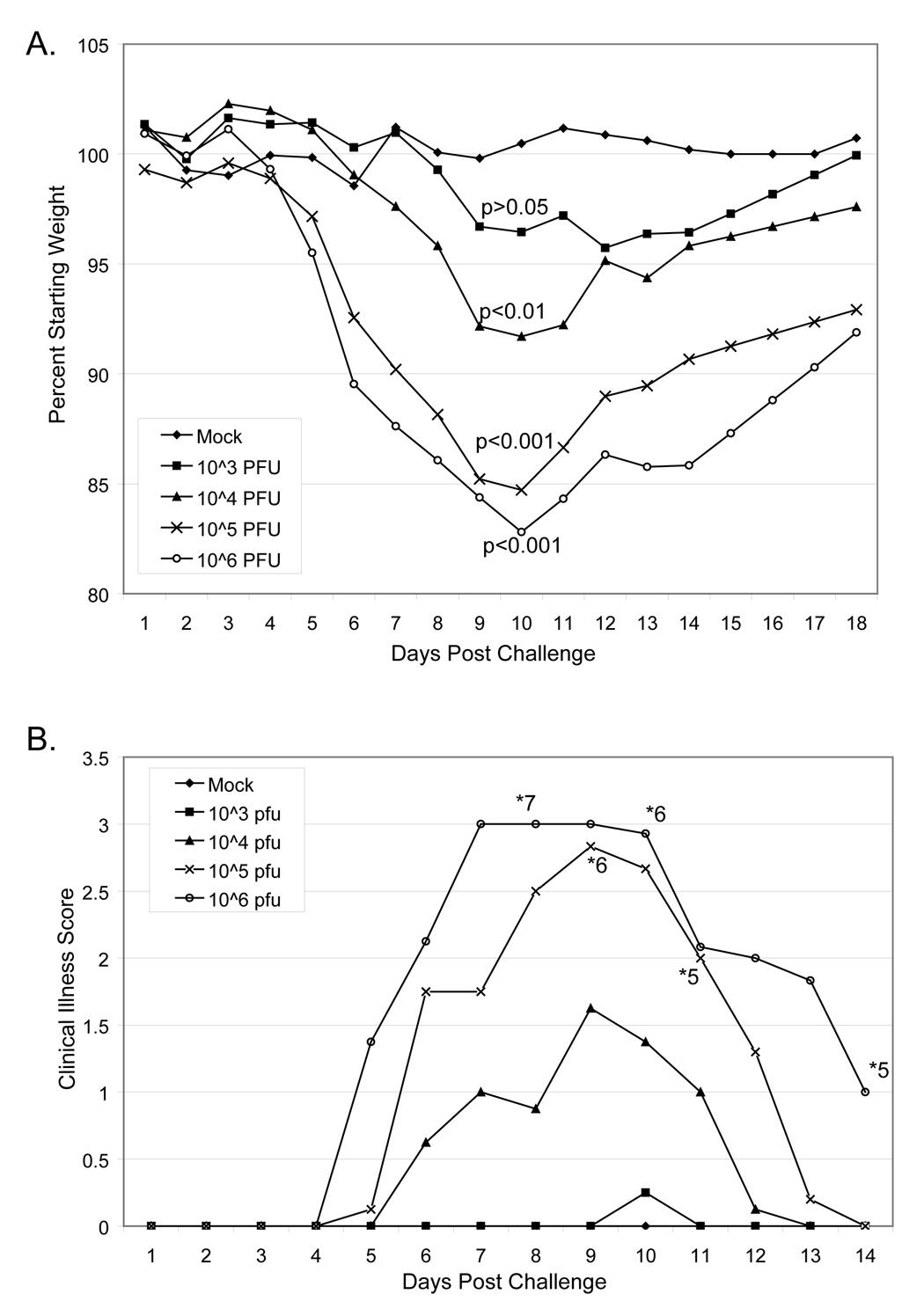
A. Female BALB/c mice 14–16 weeks old were anesthetized with Ketamine / xylazine intraperitoneal. Eight mice per group were challenged with indicated doses of cowpox virus introduced into one nostril in a total volume of 5 µL. Mock challenged mice were anesthetized and given diluent intranasally. Mice were monitored daily for weight loss. Mice that lost more than 20% body weight were euthanized. Statistics shown are in comparison to mock challenged mice. B. Challenged mice were also monitored daily for clinical signs of illness. 0 indicates no clinical signs of illness; 1 indicates slight ruffling of fur; 2 indicates moderate signs of illness including ruffled fur, decreased activity, and conjunctivitis, 3 indicates severe signs of illness including ruffled fur, lethargy, labored breathing, hunched posture, and noticeable weight loss; and 4 indicates non-responsive or moribund. The number of surviving mice per group of 8 are noted with *.
Mice vaccinated with VRP are protected from intranasal challenge with cowpox virus Brighton red
In order to assay the protective capacity of the individual VRP vaccines or the 3X cocktail, BALB/c mice were primed and boosted once four weeks post prime with 1 × 106 IU B5-VRP, A27-VRP, or A33-VRP individually or as a cocktail of all three. HA-VRP were used as an irrelevant vaccine control. Three weeks post-boost, mice were anesthetized and challenged intranasally with 1 × 106 pfu cowpox virus Brighton red. Age-matched, unvaccinated BALB/c mice were anesthetized and challenged, in parallel, with an equal volume of diluent to serve as a mock-challenged control group. Mice were monitored daily for weight loss and clinical signs of illness. Mock challenged mice did not lose any weight over the course of the 18 day experiment or show any signs of clinical illness (Figure 7A and 7B). Mice that were vaccinated with the irrelevant HA-VRP exhibited significant weight loss as well as clinical signs of illness (Figure 7A and 7B). Five of eight mice vaccinated with HA-VRP were euthanized due to weight loss in excess of 20% and severe signs of illness (Figure 7B). In contrast, mice vaccinated with A33-VRP, A27-VRP, and B5-VRP alone lost significantly less weight than HA-VRP vaccinated mice (Figure 7A). Only one mouse of eight vaccinated with B5-VRP required euthanasia and none vaccinated with A33-VRP or A27-VRP required euthanasia (Figure 7B). Mice vaccinated with the 3X cocktail lost very little weight and showed only mild signs of illness (Figure 7A and 7B). None of the mice vaccinated with the 3X cocktail required euthanasia. These data indicate that B5-VRP, A33-VRP, and A27-VRP individually protect mice against mortality and severe morbidity after cowpox virus challenge and that these VRP vaccines work most effectively in combination.
Figure 7. Mice vaccinated with VRP are protected from intranasal challenge with cowpox virus (Brighton red).
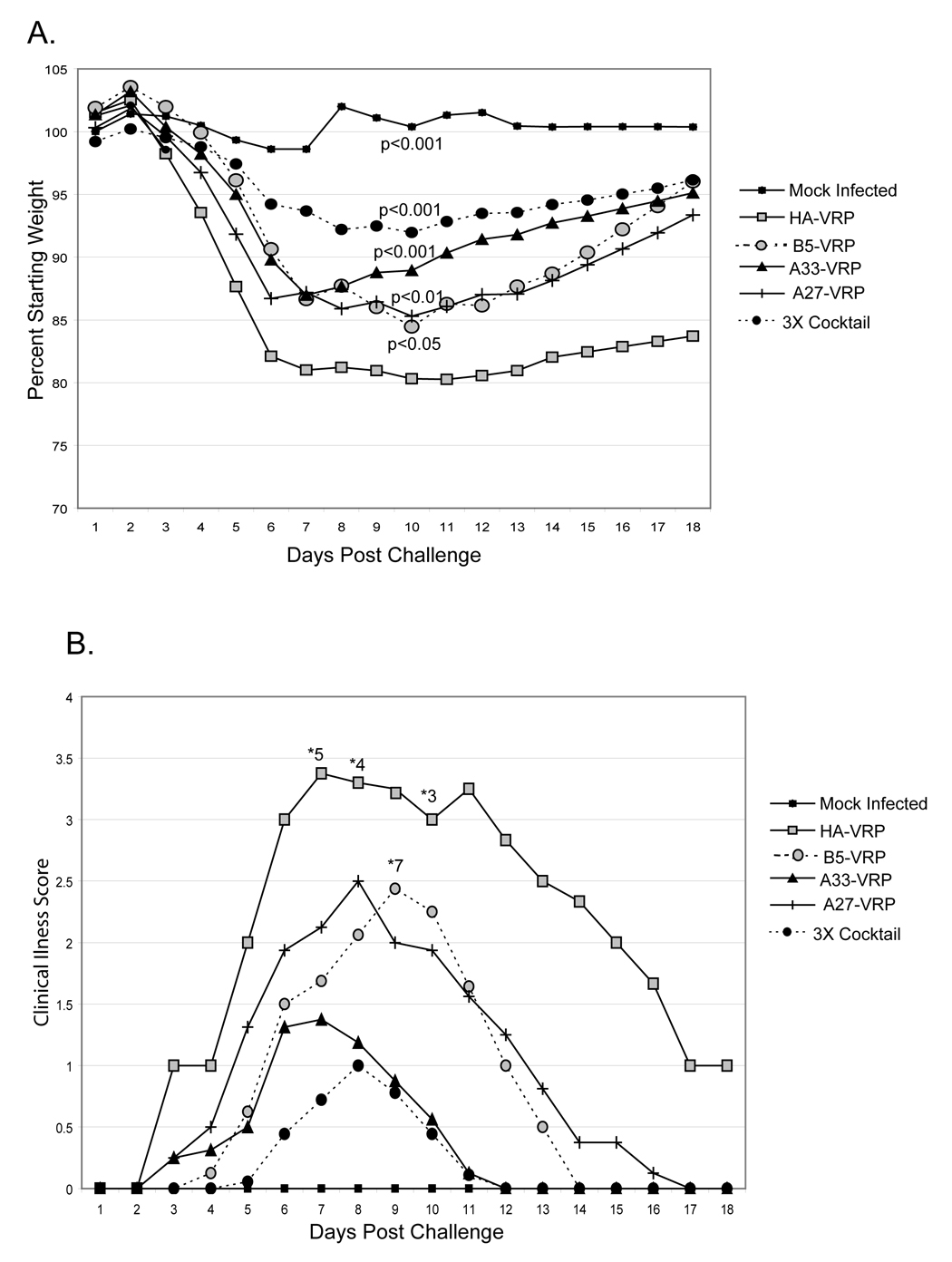
Six-week old BALB/c mice were primed with 1 × 106 IU B5-VRP, A33-VRP, and A27-VRP, or a cocktail of all three VRP (3X Cocktail). Mice were also primed and boosted with 1 × 106 IU HA-VRP as an irrelevant vaccine control. Three weeks post-boost, mice were anesthetized with Ketamine / xylazine and challenged with 1 × 106 pfu cowpox virus Brighton red. A group of unvaccinated mice was also challenged with 5 µL diluent to serve as mock challenge controls. A. Mice were monitored daily for weight loss. Statistics shown are in comparison to HA-VRP vaccinated mice challenged with 1 × 106 pfu cowpox virus Brighton red. B. Challenged mice were monitored daily for clinical signs of illness. Values are defined in Figure 5. The number of surviving mice per group of 8 are noted with *.
Discussion
In this report, cowpox virus structural antigens have been expressed from VEE replicon particles. VRP efficiently expressed poxvirus antigens in cell culture, elicited long lasting serum IgG titers in mice, and protected mice from intranasal cowpox virus challenge, validating the concept of VRP-based poxvirus vaccines.
As a result of cessation of routine vaccination against smallpox, a majority of the world’s population may now be susceptible to fatal orthopoxvirus outbreaks, either through naturally occurring exposures or through bioterrorist attacks. Common side effects from vaccination with the current vaccine include fever, malaise, and enlarged lymph nodes (Bray and Wright, 2003). Other more serious complications include generalized vaccinia, eczema vaccinatum, postvaccinal encephalopathy, and progressive vaccinia, which can be severe or fatal (Bray and Wright, 2003). Furthermore, vaccination of healthcare workers in 2003 has suggested a possible link between vaccinia vaccination and cardiac events (Casey et al., 2005).
Current vaccines may not be effective against genetically engineered poxviruses. Mice that are genetically resistant to ectromelia virus are susceptible to ectromelia virus expressing murine IL-4 (Jackson et al., 2001). Mice previously immunized with vaccinia virus were also susceptible to the IL-4 expressing ectromelia virus, indicating that the engineered poxvirus overcame otherwise protective pre-existing immunity.
The danger of vaccinia vaccination combined with the potential that new poxviruses may be engineered to bypass existing poxvirus immunity emphasizes the need to develop multiple new approaches to vaccinate against poxvirus disease. Ideally, new vaccine technologies would be safer than vaccinia and could be developed quickly should an engineered poxvirus emerge. Several second generation poxvirus vaccines are currently being developed including tissue culture produced vaccinia, modified vaccinia Ankara, purified proteins, and DNA-based vaccines (Earl et al., 2004; Fogg et al., 2004; Hooper et al., 2000; Hooper, Custer, and Thompson, 2003; Phelps et al., 2005).
Studies using purified B5, A33, and L1 in combination with Ribi or saponin adjuvants protected mice from vaccinia virus challenge better than live vaccinia virus vaccination (Fogg et al., 2004). B5-VRP elicited even higher serum IgG titers than purified B5 without adjuvant, suggesting that poxvirus antigen-expressing VRP may be more immunogenic than purified protein alone (Fig. 4B). Furthermore, the 3X-VRP cocktail protected mice after just one prime and one boost (Figure 7A and 7B), as opposed to the 3–4 shots needed for protection with purified proteins (Fogg et al., 2004). Currently, there is only one FDA approved adjuvant, alum, which works well as a systemic adjuvant, but not well as a mucosal adjuvant. VRP vaccination is efficient without the addition of adjuvants. VRP display adjuvant activity independent of antigen expression, bypassing the need to use additional adjuvants (Thompson et al., 2006). Systemic adjuvant activity provided by VRP is at least equivalent to cholera toxin and CpG DNA, and mucosal adjuvant activity surpasses that of cholera toxin and CpG DNA (Thompson et al., 2006).
Vaccination with purified poxvirus proteins induce a strong IgG1, Th2 response (Fogg et al., 2004). In contrast, vaccination of mice with live vaccinia virus yields a IgG2a dominant response (Fogg et al., 2004). Vaccination with B5-VRP, and A33-VRP yielded IgG2a:IgG1 ratios that were remarkably similar to those in mice vaccinated with live vaccinia virus. Vaccination with live vaccinia virus elicits anti-B5 IgG2a:IgG1 ratios of approximately 5:1 and anti-A33 IgG2a:IgG1 ratios of approximately 13:1 (Figure 5), which were very similar to the ratios in VRP vaccinated mice (Fogg et al., 2004). Th1 cytokines, such as IFNγ induce an isotype switch to IgG2a, indicating that both VRP and vaccinia vaccination induce a strong Th1 response (Finkelman et al., 1990). Notably, vaccine naïve patients who received the Aventis Pasteur smallpox vaccine also exhibited a strong Th1 cytokine response with extremely elevated levels of IFNγ one week post-immunization (Rock et al., 2004). IgG1 and IgG2a have different activities potentially through their affinities for activating versus inhibitory Fc receptors (Nimmerjahn and Ravetch, 2005). IgG2a has also been shown to be the IgG subclass that strongly correlates with protection against viral infections including Herpes simplex virus, yellow fever virus, and lactate dehydrogenase-elevating virus (Ishizaka et al., 1995; Markine-Goriaynoff and Coutelier, 2002; Schlesinger, Foltzer, and Chapman, 1993).
DNA vaccines encoding B5, A33, A27, and L1 have also protected animals from poxviral diseases (Hooper et al., 2000; Hooper, Custer, and Thompson, 2003; Hooper et al., 2004; Pulford et al., 2004). Although, DNA vaccines at doses up to 5 mg do not elicit robust antibody responses, antibody responses can be improved through particle-mediated epidermal delivery or inclusion of adjuvants (Dean, Haynes, and Schmaljohn, 2005). VRP potentially elicit strong antibody responses without the use of specialized delivery devices or adjuvants. Furthermore, VRP are especially attractive as potential vaccine vectors because they can directly infect human dendritic cells, process antigens, and present antigens within the draining lymph nodes. Specifically, human dendritic cells can be infected ex vivo, which leads to DC maturation and cytokine secretion (Moran et al., 2005).
Despite the ability of the 3X-VRP cocktail to protect mice from significant weight loss and illness (Figure 7A and 7B), mice vaccinated with the 3X-VRP cocktail still lost statistically significant weight in comparison to the mock challenged mice. These data suggest that the VRP poxvirus vaccine still needs improvement, possibly through the inclusion of additional antigens. Several other structural antigens have been identified as either targets of neutralizing antibodies and as protective immunogens (Davies et al., 2005; Fogg et al., 2004; Hooper et al., 2000; Hooper, Custer, and Thompson, 2003; Hooper et al., 2004; Hsiao, Chung, and Chang, 1999).
Mice have been successfully vaccinated with cocktails of up to 9 different VRP without any apparent adverse effects or lost reactivity to individually expressed poxvirus antigens, demonstrating the ability to include multiple antigens within a potential poxvirus vaccine product. (Thornburg and Johnston, unpublished data). Furthermore, individual VRP can be engineered to express several antigens, thereby facilitating manufacturing. These data suggest that VRP-poxvirus vaccines may provide a safe, viable alternative to live vaccinia virus.
Materials and Methods
Construction of VRP encoding cowpox virus structural proteins
Coding regions of the CPXV162, CPXV168, and CPXV199 open reading frames corresponding to vaccinia virus genes A27, A33, and B5 were amplified by PCR and inserted into a VEE replicon plasmid pVR21 (Balasuriya et al., 2000; Pushko et al., 1997). Cowpox virus DNA was amplified by overlap PCR with BD Advantage™ HF 2 PCR reagents (Clontech, Becton Dickinson). In each case, the upstream PCR fragment containing the 26S VEE structural gene promoter was generated from primers VR21F and VR21R#2 (Table 1). The coding region of CPXV162 (A27) was generated with primers VR21F162 and R162 (Table 1); the coding region of CPXV168 (A33) was generated with primers F168 and 168R (Table 1); and the coding region of CPXV199 (B5) was generated with primers VR21F199 and R199VR21. Overlap PCR products were cloned into pVR21 from ApaI to either AscI (CPXV199) or ClaI (CPXV 162 and CPXV 168). The sequence of each construct was confirmed by DNA sequence analyses.
Table 1.
Oligonucleotide primers used in the construction of the expression plasmids.
| Primer | Sequence (5′ to 3′) |
|---|---|
| VR21F | CAAAGCTGCGCAGCTTTCC |
| VR21R#2 | CTTGGCGGACTAGACTATGTCGTAGTCCATTCAGGTTAGCCG |
| VR21F162 | CATAGTCTAGTCCGCCAAGATGGACGGAACTCTTTTCCCCGGAG |
| R162 | TGGCATCGATTATTCATATGGTCGCCGTCCAGTC |
| F168 | CATAGTCTAGTCCGCCAAGATGATGACACCAGAAAACGACGAAGAG |
| 168R | AGCCATCGATTAGTTCATTGTTTTAACACAAAAATACTTTCTAACTTCTTGTG |
| VR21F199 | AGTCTAGTCCGCCAAGATGAAAACGATTTCCGTTGTTACGTTGTTATGCG |
| R199VR21 | CGTAGGCGCGCCTCACGGCAGCAATTTATGGAAC |
| B5R5 | TTCAGCTAGCGTCGACGACCATGAAAACGATTTCCGTTGTTACG |
| B5R3P | TTGTGGTACCTTAGTGATGATGGTGGTGATGATGATAAGTTGCTTCTAACGATTCTATTTCTTG |
| 162f5pPet40 | CTAACCATGGACGGAACTCTTTTCCCCGGAGATGAC |
| 162TPpet40 | GCATCTCGAGTTCATATGGTCGCCGTCCAGTCTGAACATC |
Cells and Viruses
BHK cells were used for VRP production and infections. BHK cells were maintained in MEM alpha (Gibco) supplemented with 10% FBS or DCS, L-glutamine and tryptose -phosphate. VRP production has been described previously (Davis et al., 2000). Briefly, pVR21 encoding the VEE nonstructural proteins, an encapsidation signal, and the transgene downstream of a 26S promoter, and two helper plasmids encoding VEE capsid and VEE E1/E2 glycoproteins were linearized and transcribed using the mMessage in vitro transcription kit (Ambion). The attenuated V3014 glycoproteins were used to package all VRP (Bernard, Klimstra, and Johnston, 2000). Transcripts were electroporated into BHK cells. VRP were recovered from culture supernatants and were purified by ultra-centrifugation through a 20% sucrose cushion. Concentrated VRP were resuspended in PBS.
VRP were titered by indirect immunofluorescent staining of BHK cells. BHK cells were infected with VRP and were fixed with cold methanol. Cells were permeablized with Tris Buffered Saline-HCl pH 7.6 + 0.1% Triton X-100 + 3% bovine serum albumin (BSA) and blocked with 5% normal horse serum and 5% BSA. Positive cells were detected with mouse polyclonal antiserum directed against the VEE nonstructural proteins, biotinylated horse antimouse (Vector Laboratories), and Texas red-conjugated streptavidin (Molecular Probes). Each fluorescent cell detected by microscopy was considered infected by a single infectious unit (IU).
Thymidine kinase negative human osteosarcoma 143B cells (ATCC) were used to culture the Brighton red strain of cowpox virus. 143B cells were maintained in MEM (Gibco) supplemented with L-glutamine, non-essential amino acids, and 10% FBS.
Western Blots
In order to detect transgene expression from VRP infection, BHK cells were infected at an MOI of 3 at 37°C for 1 hour. Twelve hours post-infection, cells were harvested by scraping and were lysed with RIPA buffer (10 mM Tris-HCl pH 8.0, 140 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% deoxycholic acid) supplemented with protease inhibitor cocktail (Sigma). Equal amounts of proteins were resolved by SDS-PAGE on a 13% polyacrylamide gel, and then transferred to Optitran nitrocellulose membrane. Poxvirus antigens were detected with rabbit anti-vaccinia virus primary antibody (Biodesign). Horseradish peroxidase-conjugated α-rabbit (ECL) secondary antibody was used to detect the primary antibody, and blots were developed using the Supersignal West Pico Chemiluminescence system (Pierce).
In order to detect antibody against cowpox virus proteins in individual mouse sera, 143B cells were infected with cowpox virus at a multiplicity of infection of 1 pfu/cell. Cells were harvested at 16 hours post-infection and lysed in RIPA buffer. Proteins in the lysates were resolved by SDS PAGE in 15% gels, and then transferred to Optitran nitrocellulose membranes. Mouse sera were diluted 1:200 in 5% milk and were incubated with membranes overnight at 4°C prior to development as above.
Mouse vaccinations and challenges
Five to six week-old female BALB/c mice received a primary inoculation with 1 × 106 to 4 × 106 IU VRP in a total volume of 20 µL diluted in PBS. Ten µL were inoculated into each rear footpad. Mice were boosted at the same dose four weeks post-prime. Blood was collected in Microtainer serum separator tubes (Becton Dickinson) by tail vein puncture pre-prime, immediately pre-boost, and two weeks post-boost. Serum was separated as directed by the manufacturer. Three weeks post-boost, mice were anesthetized with 1.3 mg Ketamine / 0.38 mg xylazine intraperitoneal, and challenged with cowpox virus Brighton red at doses ranging from 1 × 10³ pfu – 1 × 106 pfu diluted in PBS in a total volume of 5 µL into one nostril. Mock challenged mice also were anesthetized, and 5 µL of PBS were loaded into one nostril. Mice were weighed at the time of challenge to establish a 100% weight. Weight and clinical signs of illness were monitored daily for three weeks post-challenge.
Expression and purification of B5, A33, and A27 proteins
B5 protein
Primers B55 and B53P (Table 1) were used to amplify the ectodomain (amino acids 1 to 278) of the B5 protein of vaccinia virus WR, with the addition of 5′ Nhe I and 3′ Kpn I restriction endonuclease sites as well as a His6-tag. The amplified fragment was cloned into pcDNA3.1+ (Invitrogen). Human 293T cells were transfected with the His-B5 expression construct, and recombinant B5 protein was purified with a nickel column from culture supernatant.
A33 protein
The fragment of the gene encoding the ectodomain (amino acids 81 to 185) of vaccinia virus WR A33 protein was synthesized de novo (Blue Heron) together with the human CD5 signal peptide (Aruffo et al., 1990) at the N-terminus and His-6 tag at the C-terminus. The synthetic gene was then cloned into pcDNA3.1+ (Invitrogen). Human 293T cells were transfected with the His-A33 expression construct, and recombinant A33 protein was purified with a nickel column from culture supernatant.
A27 protein
The entire coding region of the A27 gene of cowpox virus (CPXV162) was PCR amplified using primers 162f5pPet40 and 162TPpet40, and was cloned into plasmid pET-40b(+) (Novagen) NcoI-XhoI (Table 1). The resulting DsbC-A27 fusion protein contains both N-and C-terminal His tags. Rosetta™ DE3 E.coli (Novagen) were transformed with pET-40b encoding the A27 fusion protein, protein expression was induced by treatment with 1mM IPTG, and the His-tagged protein was extracted and purified with Nickel affinity resin, as described by the manufacturers (Novagen). The A27 protein was dialyzed against PBS and the purity and size of the protein was confirmed by SDS-PAGE analysis.
ELISA
Immulon 96 well plates (Fisher) were coated with purified B5, A33, or A27 (200 ng per well) in carbonate buffer (7.5 mM Na2CO3/17.4 mM NaHCO3) overnight at 4°C. Wells were washed, blocked with 3% BSA/PBS or 10% Blocking Buffer (Sigma), and incubated with diluted sera. Antibody was detected with horseradish peroxidase conjugated goat anti-mouse IgG (Sigma), goat anti-mouse IgG1 (Southern Biotech), or goat anti-mouse IgG2a (Southern Biotech) and antibody reactivity was detected with o-Phenylenediamine dihydrochloride (Sigma) as directed by the manufacturer. Serum IgG endpoint titers are reported as the reciprocal of the highest dilution that resulted in an OD450 ≥0.2.
Statistical Analysis
Statistical analysis utilized the Tukey-Kramer Multiple Comparisons test using GraphPad InStat 3 software.
Acknowledgments
The authors would like to thank Nancy Davis for critical review of the manuscript and Ande West for expert technical assistance. This work was supported by grant 1U54AI057157 from the National Institutes of Health to the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense (SERCEB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Hirao L, Isaacs SN, Moss B, Eisenberg RJ, Cohen GH. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J Virol. 2005;79(10):6260–6271. doi: 10.1128/JVI.79.10.6260-6271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Balasuriya UB, Heidner HW, Hedges JF, Williams JC, Davis NL, Johnston RE, MacLachlan NJ. Expression of the two major envelope proteins of equine arteritis virus as a heterodimer is necessary for induction of neutralizing antibodies in mice immunized with recombinant Venezuelan equine encephalitis virus replicon particles. J Virol. 2000;74(22):10623–10630. doi: 10.1128/jvi.74.22.10623-10630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E, Shamim M, Whitbeck JC, Sfyroera G, Lambris JD, Isaacs SN. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology. 2004;325(2):425–431. doi: 10.1016/j.virol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Bernard KA, Klimstra WB, Johnston RE. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology. 2000;276(1):93–103. doi: 10.1006/viro.2000.0546. [DOI] [PubMed] [Google Scholar]
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36(6):766–774. doi: 10.1086/374244. [DOI] [PubMed] [Google Scholar]
- Casey CG, Iskander JK, Roper MH, Mast EE, Wen XJ, Torok TJ, Chapman LE, Swerdlow DL, Morgan J, Heffelfinger JD, Vitek C, Reef SE, Hasbrouck LM, Damon I, Neff L, Vellozzi C, McCauley M, Strikas RA, Mootrey G. Adverse events associated with smallpox vaccination in the United States, January–October 2003. Jama. 2005;294(21):2734–2743. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, Hirst S, Villarreal L, Felgner PL, Crotty S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol. 2005;79(18):11724–11733. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NL, Caley IJ, Brown KW, Betts MR, Irlbeck DM, McGrath KM, Connell MJ, Montefiori DC, Frelinger JA, Swanstrom R, Johnson PR, Johnston RE. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J Virol. 2000;74(1):371–378. doi: 10.1128/jvi.74.1.371-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NL, West A, Reap E, MacDonald G, Collier M, Dryga S, Maughan M, Connell M, Walker C, McGrath K, Cecil C, Ping LH, Frelinger J, Olmsted R, Keith P, Swanstrom R, Williamson C, Johnson P, Montefiori D, Johnston RE. Alphavirus replicon particles as candidate HIV vaccines. IUBMB Life. 2002;53(4–5):209–211. doi: 10.1080/15216540212657. [DOI] [PubMed] [Google Scholar]
- Dean HJ, Haynes J, Schmaljohn C. The role of particle-mediated DNA vaccines in biodefense preparedness. Adv Drug Deliv Rev. 2005;57(9):1315–1342. doi: 10.1016/j.addr.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Dong M, Zhang PF, Grieder F, Lee J, Krishnamurthy G, VanCott T, Broder C, Polonis VR, Yu XF, Shao Y, Faix D, Valente P, Quinnan GV., Jr Induction of primary virus-cross-reactive human immunodeficiency virus type 1-neutralizing antibodies in small animals by using an alphavirus-derived in vivo expression system. J Virol. 2003;77(5):3119–3130. doi: 10.1128/JVI.77.5.3119-3130.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, Eisenberg RJ, Hartmann CJ, Jackson DL, Kulesh DA, Martinez MJ, Miller DM, Mucker EM, Shamblin JD, Zwiers SH, Huggins JW, Jahrling PB, Moss B. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(6979):182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, Nalca A, Hooper JW, Whitehouse CA, Schmitz JE, Reimann KA, Franchini G. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- Fang M, Cheng H, Dai Z, Bu Z, Sigal LJ. Immunization with a single extracellular enveloped virus protein produced in bacteria provides partial protection from a lethal orthopoxvirus infection in a natural host. Virology. 2006;345(1):231–243. doi: 10.1016/j.virol.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Fogg C, Lustig S, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78(19):10230–10237. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clin Infect Dis. 2003;37(2):251–271. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- Galmiche MC, Goenaga J, Wittek R, Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999;254(1):71–80. doi: 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- Goebel SJ, Johnson GP, Perkus ME, Davis SW, Winslow JP, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179(1):247–266. 517–563. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Henderson DA. The looming threat of bioterrorism. Science. 1999;283(5406):1279–1282. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266(2):329–339. doi: 10.1006/viro.1999.0096. [DOI] [PubMed] [Google Scholar]
- Hooper JW, Custer DM, Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306(1):181–195. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper JW, Thompson E, Wilhelmsen C, Zimmerman M, Ichou MA, Steffen SE, Schmaljohn CS, Schmaljohn AL, Jahrling PB. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78(9):4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao JC, Chung CS, Chang W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J Virol. 1999;73(10):8750–8761. doi: 10.1128/jvi.73.10.8750-8761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutin YJ, Williams RJ, Malfait P, Pebody R, Loparev VN, Ropp SL, Rodriguez M, Knight JC, Tshioko FK, Khan AS, Szczeniowski MV, Esposito JJ. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7(3):434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y, Oie M. Neutralizing epitope on penetration protein of vaccinia virus. Virology. 1996;220(2):491–494. doi: 10.1006/viro.1996.0337. [DOI] [PubMed] [Google Scholar]
- Ishizaka ST, Piacente P, Silva J, Mishkin EM. IgG subtype is correlated with efficiency of passive protection and effector function of anti-herpes simplex virus glycoprotein D monoclonal antibodies. J Infect Dis. 1995;172(4):1108–1111. doi: 10.1093/infdis/172.4.1108. [DOI] [PubMed] [Google Scholar]
- Jackson RJ, Ramsay AJ, Christensen CD, Beaton S, Hall DF, Ramshaw IA. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J Virol. 2001;75(3):1205–1210. doi: 10.1128/JVI.75.3.1205-1210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE, Johnson PR, Connell MJ, Montefiori DC, West A, Collier ML, Cecil C, Swanstrom R, Frelinger JA, Davis NL. Vaccination of macaques with SIV immunogens delivered by Venezuelan equine encephalitis virus replicon particle vectors followed by a mucosal challenge with SIVsmE660. Vaccine. 2005;23(42):4969–4979. doi: 10.1016/j.vaccine.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Law M, Smith GL. Antibody neutralization of the extracellular enveloped form of vaccinia virus. Virology. 2001;280(1):132–142. doi: 10.1006/viro.2000.0750. [DOI] [PubMed] [Google Scholar]
- Lin CL, Chung CS, Heine HG, Chang W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J Virol. 2000;74(7):3353–3365. doi: 10.1128/jvi.74.7.3353-3365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig S, Fogg C, Whitbeck JC, Moss B. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology. 2004;328(1):30–35. doi: 10.1016/j.virol.2004.07.024. [DOI] [PubMed] [Google Scholar]
- MacDonald GH, Johnston RE. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J Virol. 2000;74(2):914–922. doi: 10.1128/jvi.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markine-Goriaynoff D, Coutelier JP. Increased efficacy of the immunoglobulin G2a subclass in antibody-mediated protection against lactate dehydrogenase-elevating virus-induced polioencephalomyelitis revealed with switch mutants. J Virol. 2002;76(1):432–435. doi: 10.1128/JVI.76.1.432-435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massung RF, Liu LI, Qi J, Knight JC, Yuran TE, Kerlavage AR, Parsons JM, Venter JC, Esposito JJ. Analysis of the complete genome of smallpox variola major virus strain Bangladesh-1975. Virology. 1994;201(2):215–240. doi: 10.1006/viro.1994.1288. [DOI] [PubMed] [Google Scholar]
- Moran TP, Collier M, McKinnon KP, Davis NL, Johnston RE, Serody JS. A novel viral system for generating antigen-specific T cells. J Immunol. 2005;175(5):3431–3438. doi: 10.4049/jimmunol.175.5.3431. [DOI] [PubMed] [Google Scholar]
- Moss B. Poxviridae: the viruses and their replication. In: DM K, Howley PM, editors. Fields virology. 4th ed. Philadelphia, Pa: 2 Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Moss B. Poxvirus entry and membrane fusion. Virology. 2006;344(1):48–54. doi: 10.1016/j.virol.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41(12):1765–1771. doi: 10.1086/498155. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310(5753):1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- Panchanathan V, Chaudhri G, Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J Virol. 2006;80(13):6333–6338. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps A, Gates AJ, Hillier M, Eastaugh L, Ulaeto DO. Comparative efficacy of replicating smallpox vaccine strains in a murine challenge model. Vaccine. 2005;23(27):3500–3507. doi: 10.1016/j.vaccine.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Pulford DJ, Gates A, Bridge SH, Robinson JH, Ulaeto D. Differential efficacy of vaccinia virus envelope proteins administered by DNA immunisation in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine. 2004;22(25–26):3358–3366. doi: 10.1016/j.vaccine.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239(2):389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- Rock MT, Yoder SM, Talbot TR, Edwards KM, Crowe JE., Jr Adverse events after smallpox immunizations are associated with alterations in systemic cytokine levels. J Infect Dis. 2004;189(8):1401–1410. doi: 10.1086/382510. [DOI] [PubMed] [Google Scholar]
- Rodriguez JF, Janeczko R, Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985;56(2):482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger JJ, Foltzer M, Chapman S. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology. 1993;192(1):132–141. doi: 10.1006/viro.1993.1015. [DOI] [PubMed] [Google Scholar]
- Schlesinger S, S M. Togaviridae: The Viruses and Their Replication. In: DM K, Howley PM, editors. Fields virology. 4th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Schultz-Cherry S, Dybing JK, Davis NL, Williamson C, Suarez DL, Johnston R, Perdue ML. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses. Virology. 2000;278(1):55–59. doi: 10.1006/viro.2000.0635. [DOI] [PubMed] [Google Scholar]
- Stephenson J. Monkeypox outbreak a reminder of emerging infections vulnerabilities. Jama. 2003;290(1):23–24. doi: 10.1001/jama.290.1.23. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Whitmore AC, Konopka JL, Collier ML, Richmond EM, Davis NL, Staats HF, Johnston RE. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc Natl Acad Sci U S A. 2006;103(10):3722–3727. doi: 10.1073/pnas.0600287103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JP, Turner PC, Ali AN, Crenshaw BC, Moyer RW. The effects of serpin gene mutations on the distinctive pathobiology of cowpox and rabbitpox virus following intranasal inoculation of Balb/c mice. Virology. 1993;197(1):328–338. doi: 10.1006/viro.1993.1594. [DOI] [PubMed] [Google Scholar]
- Viner KM, Isaacs SN. Activity of vaccinia virus-neutralizing antibody in the sera of smallpox vaccinees. Microbes Infect. 2005;7(4):579–583. doi: 10.1016/j.micinf.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wolffe EJ, Vijaya S, Moss B. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology. 1995;211(1):53–63. doi: 10.1006/viro.1995.1378. [DOI] [PubMed] [Google Scholar]