Abstract
Chemokines are a small group of related chemoattractant peptides that play an essential role in the homeostatic maintenance of the immune system. They control the recruitment of cells needed for the induction and activation of innate and adaptive immune responses. However, tumors also utilize chemokines to actively progress and evade immunosurveillance. In fact, chemokines are involved directly or indirectly in almost every aspect of tumorigenesis. They mediate survival and metastatic spread of tumors, promote new blood vessel formation (neovascularization) and induce an immunosuppressive microenvironment via recruitment of immunosuppressive cells. As a result, a number of therapeutic strategies have been proposed to target almost every step of the chemokine/chemokine receptor involvement in tumors. Yet, despite occasional success stories, most of them appear to be ineffective or impractical, presumably due to ‘nonspecific’ harm of cells needed for the elimination of tumor escapees and maintenance of immunological memory. The strategy would only be effective if it also promoted antitumor adaptive immune responses capable of combating a residual disease and tumor relapse.
Keywords: angiogenesis, cancer, chemotaxis, metastases, tumor escape
The last few decades have been marked with a number of exciting findings that have deepened our understanding of multistage carcinogenic processes (FIGURE 1). The importance of immunomodulatory cytokines and specifically chemoattractant cytokines, designated chemokines, cannot be overlooked in this multifactorial process. This, in turn, has changed our simplistic view of chemokines as by hitherto recruiter and regulator of the directional migration (chemotaxis) of immune cells. Tumor progression is directly affected by pleiotropic effects of chemokines on survival, proliferation and metastatic spread of malignant cells [1–3]; and indirectly modulated inducing angiogenesis [4,5] or recruiting immunosuppressive cells that lead to escape from immunosurveillance [6–9]. It is striking that these complex functions are controlled by the 8–15 kDa (except fractalkine/CX3CL1)-related peptides with a simple structure. To date, the chemokine family includes approximately 50 secreted peptides that are classified into four super families (two major types CC and CXC and two minor C and CX3C chemokines) on the basis of the first two cysteine residues [10]. Although many other peptides and factors can have chemotactic potency, chemokines are certainly the masterminds that govern cell trafficking and migration. They act through binding and signaling with approximately 18 cell-surface G-protein-coupled 7-transmembrane receptors, designated chemokine receptors.
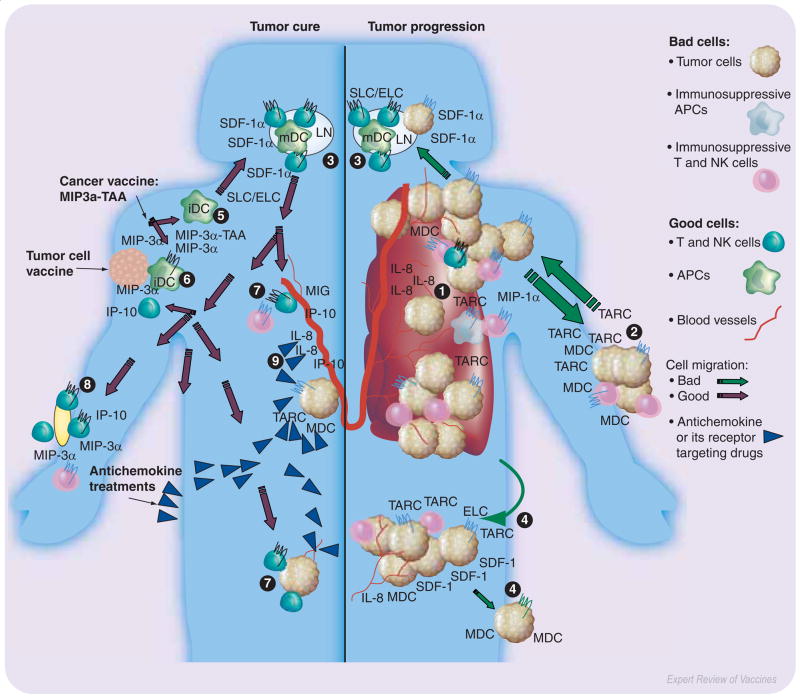
Figure 1. Chemokines are involved in almost every aspect of tumorigenesis and antitumor immunity.
Primary tumors progress producing chemokines (IL-8/CXCL8, MIP-1α/CCL3, TARC/CCL17, MDC/CCL22) that promote neovascularization (1) and escape immunosurveillance via recruitment of immunosuppressive cells, such as T regulatory cells, NK T cells, macrophages and iDC. Tumors metastasize to distant organs, such as skin (2), LN (3), liver (4) and lung, that constitutively produce chemokines (SDF-1/CXCL12, ELC/CCL19 and SLC/CCL21; or TARC/CCL17 and MDC/CCL22). Therefore, to combat the disease and metastatic spread of tumor cells, use of various drugs and specific antibodies that target chemokines or their receptors were proposed, which inhibit neovascularization via expression of angiostatic chemokines (MIG/CXCL9 and IP-10/CXCL10, 9) or depletion of angiogenic chemokines (CXCL8, 9). However, these strategies cannot combat the disease efficiently, particularly relapse, unless they are accompanied with activation of antitumor adaptive immune responses. Immunizations with TAA fused with chemokines (MIP3α-TAA, 5) or with tumor cells modified to express chemokines (MIP-3α/CCL20, IP-10/CXCL10, 6) appear to be potent inducers of antitumor immunity. These vaccines recruit iDCs that take up and present TAAs leading to induction of tumor-specific T-cell responses in secondary lymphoid organs (LN, 3). The activated tumor-specific T cells can efficiently eradicate tumors in the skin (8,2) and other distant sites (7,4). APC: Antigen-presenting cell; DC: Dendritic cell; ELC: EBI1-ligand chemokine; iDC: Immature dendritic cell; IL: Interleukin; IP: Interferon-γ inducible protein; LN: Lymph node; mDC: mature dendritic cellMDC: Monocyte-derived chemokine; MIG: Monokine induged by interferon-γ; MIP: Macrophage inflammatory protein; NK: Natural killer; SDF: Stromal cell-derived factor; SLC: Secondary lymphoid tissue chemokine; TAA: Tumor-associated antigen; TARC: Thymus and activation-regulated chemokine.
The differential and balanced regulation of chemokine and chemokine receptor expressions are an essential part of normal immune function. Impairments in chemokine or chemokine receptor expressions lead to various immunological disorders, including autoimmune diseases and cancer [11,12]. For example, aberrant expression of CCL22 induces prosurvival signaling of the CCR4-expressing tumors and, at the same time, promotes suppression of antitumor immune responses via recruitment of CCR4-expressing suppressive immune cells [13,14]. As a result, overexpression of CCL22 is often associated with severity and a poor disease outcome of cancer patients. This has made chemokines and chemokine receptors attractive targets for cancer therapy [1,15]. However, a genuine and significant interest, particularly from industry, was initiated by the seminal finding that chemokine receptors, such as CCR5 and CXCR4, were also utilized as HIV-1 coreceptors for infection of human CD4+ T cells [16,17]. As a result, ample strategies continue to be reported in the literature, making it almost impossible to overview all of them owing to the limitations of the journal format. Herein, we have tried to summarize and analyze the most ‘interesting’ approaches proposed for cancer immunotherapy, including the use of pharmacological inhibitors, chemokine antagonists and antibodies to block chemokine/chemokine receptor activity. An increasing number of strategies aimed at inhibiting growth and metastastatic spread of tumors are being developed and even tested in humans with intentions either to indirectly affect tumor microenvironment and blood vessel formation, or to directly reduce survival, apoptosis, migration and adhesion of malignant cells. However, these strategies are not exclusively specific for tumors and will be partially effective without elimination of a residual disease. The approach will be partially successful unless it is also supported by induction of adaptive antitumor immune responses, particularly involving CD4+ and CD8+ T cells. In this respect, chemokines have been explored to improve the efficacy of cell-based vaccines by expressing them in tumors to recruit cytotoxic, phagocytic and antigen-presenting cells (APCs) and to elicit potent antitumor adaptive immune responses [18]. Alternatively, chemokines are utilized as carriers for nonimmunogenic tumor antigens to enable their preferential uptake and presentation by the professional APCs [19,20]. However, cancer immunotherapy or vaccines will presumably be most effective on minimal residual disease, since tumor-specific cytotoxic T lymphocytes can be neutralized once they encounter the immunosuppressive microenvironment of bulky tumors or their immunosuppressive macrophages, natural killer cells (NK), natural killer T (NKT) cells, Tregulatory cells (Tregs) and immature dendritic cells (iDCs) [6,13,21,22]. Therefore, the success of chemokine-based therapy depends on a proper engagement of the delicate and ‘double-edged’ balance of activating and suppressive factors and induction of potent adaptive antitumor immune responses.
Chemotactic cytokine–chemokine
Chemokines, originally known as chemotactic cytokines, are secreted and related 8–15-kDa peptides (except fractalkine/CX3CL1) with very simple structures. They are considered master controllers that regulate migration and recruitment of various subsets of immune cells and even malignant cells. To date, the chemokine family consists of approximately 50 chemokines that are classified into four groups, designated CC, CXC, C and CX3C, based on the way the first two conserved cysteines are arranged; and most known chemokines belong to CC and CXC families [10]. There are two nomenclatures used in the literature: one based on given name at the time of chemokine discovery, such as interferon-inducible 10-kDa protein (IP-10) or thymus and activation-regulated chemokine (TARC), and recently adopted a systematic nomenclature that combines structural motifs (CC, CXC, XC and CXC3C) with ‘L’ (for ligand) and the number of the respective gene, such as CXCL10 for IP-10 and CCL17 for TARC (a detailed list is described in [301]). Functionally, chemokines are distinguished as inflammatory (inducible) or homeostatic (constitutive), based on their pathophysiological activities [3]. Inflammatory chemokines are expressed during infection or tissue damage by resident and infiltrated leukocytes. By contrast, homeostatic chemokines are usually produced constitutively in discrete microenvironments and they are involved in maintaining the physiological trafficking of immune cells [3]. The simplistic view of chemokines as recruiters of immune cells and regulators of the directional migration (chemotaxis) is changing owing to the important role they play in the innate and adaptive immune responses [23]. It is also becoming clear that the chemokine network, as an essential part of an intricate system of immunosurveillance, affects and regulates either directly or indirectly, the growth and metastatic spread of malignant cells. Based on the role of chemokines in promotion and suppression of neovascularization, they are also described as angiogenic and angiostatic, respectively.
Structurally, chemokines contain a high-affinity chemokine receptor-binding site (usually at the N-terminus) and a low-affinity glycosaminoglycan (GAG)-binding region (either distinct or overlapping with a receptor-binding site) [24–27]. GAGs consist of a linear polysaccharide chain linked covalently to protein cores, forming proteoglycans, which are abundantly expressed key components of the endothelial cell surface and extracellular matrix. It has been proposed that, to elicit chemotaxis in vivo, chemokines must be immobilized on endothelial cells or extracellular matrix surfaces by interacting with negatively charged GAGs, which allows increased concentrations of chemokines near the initiating inflammatory or trafficking stimulus [28]. Chemokines on the luminal endothelial surface can activate chemokine receptors of the rolling cells, thus triggering integrin activation. This results in arrest, firm adhesion and transendothelial migration into tissues towards chemokine gradients. Chemokines are often detected in various pathological sites associated with GAGs, for example, interleukin (IL)-8/CXCL8 is found to be complexed with endothelial syndecan-3 in rheumatoid arthritis synovium [29]. The binding with GAGs may not only facilitate a solid-phase chemokine concentration gradient required for directional migration of leukocytes in vivo [30,31] but also modulate activities of CXCL8 and CXCL10 [32,33]. GAGs induce oligomerization of monocyte chemotactic protein (MCP)-1/CCL2, macrophage inflammatory protein (MIP)-1β/CCL4, and regulated on activation, normal T cell expressed and secreted (RANTES)/CCL5 affecting their activity [34,35]. Moreover, association with GAGs, particularly heparin sulphate, is shown to protect stromal cell-derived factor (SDF)-1 from the proteolytic inactivation by CD26/dipeptidyl peptidase IV [36]. Interestingly, binding with GAGs can also result in unexpected activities, such as induction of apoptosis in human T-cell lymphoblastoid cell lines expressing CCR5 [37]. The extra-cellular matrix-bound RANTES and MIP-1β are able to stimulate T-cell adhesion [38], while organ-specific differential expression of GAGs sequester SDF-1/CXL12 affecting metastatic spread of cancer cells [39]. These observations led to proposals to target GAGs, for example using soluble antagonists, to control aberrant inflammation and cancer metastasis [36,39]. Alternatively, the efficacy of dominant-negative antagonists of CCL5 as an anti-inflammatory reagent can be improved by mutating the GAG-binding sites to inhibit formation of oligomers [35].
Chemokines that affect tumor angiogenesis
The salient feature of all solid tumors is their strict dependence on neovascularization and development of new blood vessels through angiogenesis. Access to blood vessels for a sufficient supply of oxygen and nutrients is not only necessary for maintenance of tumor growth, but also for tumor dissemination and metastasis; it is assumed that tumors cannot grow beyond 2 mm in the absence of local angiogenesis. The microenvironment of tumor tissues can be viewed as an important cofactor of the carcinogenic process [40]. The invasive behavior and selection of neoplastic cells are affected by interactions between tumors and the activated microenvironments. The malignant cells recruit vasculature and inflammatory cells through a network of chemokines, cytokines and growth factors. Tumor cells or tumor microenvironments express specific chemokines regulating angiogenesis, growth and metastasis of malignant cells via recruitment effector cells, such as macrophages and lymphocytes (TABLE 1). Angiogenesis is a finely tuned process regulated through a delicate and complex balance between the collective action of proangiogenic (e.g., vascular endothelial growth factor, [VEGF]) and angiostatic (angiogenic inhibitors, such as thrombospondin-1) factors [41]. Since Judah Folkman’s original idea in 1971, a surge of strategies to inhibit tumor angiogenesis has been developed. In particular, anti-VEGF therapy is effective in several preclinical models and Phase I/II or even Phase III human clinical trials [42,43]. This half century-long and never-ending battle continues, to date, by focusing on the improvement of specificity and the reduction of potentially harmful side effects. In this respect, chemokines attract significant interest, as a number of CXC and CC chemokines (CCL1, CCL2 and CCL11) and fractalkine/CX3CL1 modulate angiogenesis both in vitro and in vivo [44–47]. In particular, the so called ELR (Glu–Leu–Arg at the N-terminus) motif containing CXC chemokines, such as CXCL8, and CXCL1–3 (growth-regulated oncogene-[GRO]α, -β and -γ) and epithelial neutrophil activating peptide (ENA)-78/CXCL5, induce angiogenesis directly [44].
Table 1.
Chemokines and tumour angiogenesis.
| Chemokine
|
Chemokine receptor
|
Tumors involved
|
Ref.
|
|
|---|---|---|---|---|
| Promotes (+)angiogensis | Inhibits (−)angiogenesis | |||
| CXCL8 | CXCR1 | + | Ovarian cancer, NSCLC, prostate cancer, glioblastoma, pancreatic cancer, gastric tumors, melanoma | [40,44,57,61,185] |
| ENA-78 (CXCL5) | CXCR2 | + | NSCLC | [44,58] |
| GRO-α (CXCL1) | CXCR2 | + | Melanoma, gastric cancer | [44,59] |
| GRO-β (CXCL2) | CXCR2 | + | Melanoma, esophageal cancer | [44,59] |
| GRO-γ (CXCL3) | CXCR2 | + | Melanoma | [44,59] |
| I-309 (CCL1) | CCR8 | + | - | [44,45] |
| Fractalkine (CX3CL1) | CX3CR1 | + | NSCLC | [47,64] |
| IP-10 (CXCL10) | CXCR3b | - | Various cancers | [44,63] |
| MIG (CXCL9) | CXCR3b | - | - | [44] |
| ITAC (CXCL11) | CXCR3b | - | - | [44] |
| BRAK (CXCL14) | Receptor not known | - | Head and neck, prostate cancer | [121] |
Chemokines affect tumor progression directly or indirectly via promoting neovascularization (angiogenic chemokines). However, some chemokines can inhibit angiogenesis (angiostatic chemokines). Only a representative list of chemokines is shown.
BRAK: Breast- and kidney-expressed chemokine; ENA: Epithelial neutrophil activating peptide; GRO: Growth-regulated oncogene; IP: Interferon-γ inducible protein; ITAC: Interferon-inducible T-cell α chemoattractant; MIG: Monokine induced by interferon-γ; NSCLC: Nonsmall-cell lung cancer.
In pathological angiogenesis, the angiogenic switch is shifted toward the angiogenic factors and, if the imbalance continues, it results in irregular tumor vessel growth. Tumor growth and angiogenesis are often closely linked and associated with a worse disease prognosis for cancer patients [1,48–50]. Tumor growth results in hypoxia that, in turn, induces a profound change in gene expression, particularly angiogenesis-promoting genes, such as VEGF, chemokines (IL-8/CXCL8, MIP-3α/CCL20, MCP-2/CCL8, myeloid progenitor inhibitory factor (MPIF)-1/CCL23 and human chemokine (HCC)-2/CCL15) and chemokine receptors (CCR1, -2 and -5 and CXCR4) [51–53]. Of note, this also means that, even if angiogenic factors and chemokines are not detected in in vitro cultured cells, they could be expressed once cells encounter the hypoxic conditions of solid tumors. Thus, angiogenesis can be further enhanced by chemokines (CCL2, for example) produced at the tumor site that, in turn, leads to the recruitment and retention of macrophages and monocytes that produce a variety of angiogenic factors. This has been documented in a number of different human tumors, such as gastric carcinoma, esophageal squamous cell carcinoma and breast carcinoma [54,55].
Expression of chemokines is positively associated with a poor disease outcome, indicating their useful value as prognostic biomarkers of disease outcome [56] and attractive therapeutic targets. For example, neutralizing anti-ENA78/CXCL5 antibody may be used for reduction of tumor vascularity and angiogenesis associated with CXCL5-expressing human nonsmall-cell lung cancer (NSCLC), as it was able to significantly inhibit tumor growth, vascularity and metastasis of NSCLC cells in severe combined immunodeficiency (SCID) mice [57,58]. Similarly, human cancers, including esophageal tumors and melanoma, highly express GROs/CXCL1–3 [59]; and their vascularization in SCID mice was reduced successfully by the depletion of GROs [60]. Advanced disease stage and poor survival of patients with melanoma, ovarian cancer, prostate and lung cancer is associated with augmented serum levels of CXCL8 [61,62]. Therefore, depletion of CXCL8 appears to be appealing, as it affected growth of various tumors in SCID mice inhibiting tumor-associated vascular density and angiogenic activity [57,58,62].
Chemokines, particularly CXC chemokines (CXCL9, CXCL4 and CXCL10) that do not contain the ELR motifs can exert antiangiogenic/angiostatic properties inhibiting the angiogenic effects of the ELR+ CXC chemokines [44]. Increased levels of IP-10 were detected in fresh specimens of human squamous cell carcinoma and were inversely correlated with tumor growth rate. Intratumorally administered CXCL10 was able to inhibit tumorigenesis and metastasis of NSCLC cells in SCID mice [63,64]. A positive association between expression of IP-10 and improved disease outcome can also be explained by its ability to inhibit production of monocyte-derived CXCL8, CXCL1–3 and CXCL5 [63,65,66]. However, CXCL10 recruits other important effector cells of the adaptive immune system, such as the T helper (Th)-1 type CD4+ T cells and NK cells, that also produce interferon (IFN)-γ and activate keratinocytes, fibroblasts, endothelial cells and mononuclear phagocytes to produce CXCL10. Therefore, CXCL10 is an attractive chemokine to combat tumorigenesis and neovascularization through the shift of a local balance toward angiostatic chemokines. These strategies are potent and safe, although they would only transiently control angiogenesis unless combined with the induction of additional antitumor cellular responses.
Chemokine receptors
Chemokines transmit their signaling via binding with 18 cell-surface G-protein-coupled 7-transmembrane receptors, so-called chemokine receptors, designated CXCR1 through CXCR7, CCR1 through CCR11, CXCR1 and CX3CR1 (based on their specific preference for certain chemokines). Chemokine receptors share 25–80% amino acid homology of 340–370 residues with an acidic N-terminus, conserved ten amino acid sequence in the second intracellular loop, and one cysteine in each of the four extracellular domains. The chemokine binding site is complex and involves several noncontiguous sites, including the N-terminal segment. Although some chemokine receptors bind a single chemokine exclusively and vice versa, such as CXCR5 with BCA-1/CXCL13, CCR6 with MIP-3α/CCL20, CCR9 with TECK/CCL25, and CCR10 with CTACK/CCL27; most chemokine receptors appear to have a high promiscuity and induce redundant functions in vitro after binding with more than one member. For example, CCR7 binds equally well to ELC/MIP-3β/CCL19 and SLC/CCL21, or CCR4 with TARC/CCL17 and monocyte-derived chemokine (MDC)/CCL22, and CXCR3 with IP-10/CXCL10, monokine induced by interferon-γ (MIG)/CXCL9 and IFN-inducible T-cell α chemoattractant (I-TAC)/CXCL11. Despite the fact that the majority of chemokines and chemokine receptors are far more promiscuous (e.g., CCR3 can equally well bind eotaxin-1/CCL11, MCP-2/CCL8, MCP-3/CCL7, MCP-4/CCL13 and RANTES/CCL5), it appears that they are tightly controlled by differential and compartmentalized expression [67]. As a consequence, CD4+ Th1 and Th2 cells can be separated by expression of CCR5 and CXCR3; and CCR4, CCR8 and CCR3, respectively. Moreover, CCR4, CCR8 and CCR10 are expressed on skin-homing CD4+ T cells [68]. The ability of iDCs to migrate to proinflammatory chemokines MIP-1α/CCL3, MIP-1β/CCL4, MCP-1/CCL2, RANTES/CCL5, MCP-3/CCL7 and MIP-3α/CCL20 is associated with the expression of CCR1 [69–71], CCR2, CCR5 and CCR6 [72,73], unlike mature DCs that express CCR7. In fact, expression of CCR7 seems to be utilized by mature DCs to migrate to the secondary lymphoid organs to present processed antigens to the CCR7-expressing naive or central memory T cells, where MIP-3β/CCL19 and SLC/CCL21 are produced constitutively [73,74]. Interestingly, at least two reports suggest that some chemokine receptors may differentiate their ligands regulating their endocytosis. For example, internalization of CCR7 expressed on T cells was only detected after treatment with CCL19 but not CCL21 [75]. Similarly, CCL22, but not CCL17, downregulated expression of CCR4 on cutaneous and Th2-type CD4+ T cells [76]. However, the extent of this type of differentiation remains unknown, as surface expression of chemokine receptors can be regulated by their quick recirculation, or controlled by chemokine oligomerization with GAGs. It is also unclear whether this type of regulation is cell-type specific, as both CCL17 and CCL22 internalized CCR4 expressed on human lymphoblastoid cells equally well [BAATAR ET AL., UNPUBLISHED DATA].
Chemokine receptors & cancer metastasis
The formation of metastatic colonies is a nonrandom process that starts early during the growth of the primary tumor, probably increasing with time. The peculiar distribution of metastasis was first recognized by Paget in 1889, who called it ‘seed and soil’. Since then, the idea has not been changed and it essentially indicates that different organs provide growth conditions optimal for specific cancers. Since a first association of breast-cancer metastasis with expression of CXCR4 and CCR7 [1], it is becoming accepted that chemokines and chemokine receptors play a key role determining the metastatic homing site (TABLE 2). It appears that tumors hijack normal trafficking pathways established for the proper function of the immune system by differentially expressing chemokine receptors. For example, expression of CXCR3 is documented on primary melanomas and various lymphomas; such as T-cell and NK-cell lymphomas, chronic lymphocytic leukemia/small lymphocytic lymphoma and splenic marginal zone B-cell lymphoma [77–80]. Similarly, pancreatic, gastric, prostate and NSCLC cancer cells express CCR6, CCR7, CXCR5 and CX3CR1 [81–84]. However, at least 23 different malignancies, including breast cancer, ovarian cancer, melanoma and prostate cancer, express CXCR4 [2]. This allows them to utilize an important network of the CXCL12- and CXCR4-mediated trafficking of hematopoietic progenitors and endothelial cells to preferentially home into lymph nodes [1,85,86]. High levels of CXCL12 or CCL19 and CCL21 not only promote adhesion but also stimulate CXCR4- or CCR7-mediated tyrosine kinases that promote growth of breast cancer cells [1]. It should be noted that, even if receptor expression was not detected in the primary tumors, its expression might be triggered by change in the stromal/tumor microenvironment at the primary or metastatic sites. The hypoxia-inducible factor is shown to upregulate the expression of CXCR4 via inhibition of suppressive effects of the von Hippel–Lindau tumor suppressor protein [87]. Furthermore, CXCR4, in turn, transactivates the metastasis-enhancing receptor tyrosine kinase HER2 that is found in almost a quarter of human breast cancers [88]. In turn, HER2 further upregulates expression of CXCR4 through the inhibition of its degradation [89]. As a result, overexpression of CXCR4 on tumors is often an indicator of poor overall survival of patients [90–92]. Lastly, the hypoxia not only induces expression of CXCR4, as well as CCR1, CCR2 and CCR5, but it also increases retention of the infiltrating leukocytes and tumor cells [51–53].
Table 2.
Chemokines and chemokine receptors in metastasis of solid tumors.
| Primary tumor | Preferred metastasis site | Receptor | Ligand | Ref. |
|---|---|---|---|---|
| Melanoma | Melanocyte extravasation | CXCR1, CXCR2 | CXCL8 | [186,187] |
| Lymph nodes, lung | CXCR3 | IP-10, MIG, I-TAC | [188] | |
| Lymph nodes, liver | CXCR4 | SDF-1 | [2,189] | |
| Skin | CCR10 | CTACK | [1,190] | |
| Lymph nodes | CCR7 | SLC | [190] | |
| Colon | Lymph nodes | CXCR4 | SDF-1 | [91] |
| Lymph nodes, liver | CXCR5 | BCA-1 | [191] | |
| Lymph nodes | CCR7 | SLC | [81,86] | |
| Liver | CCR6 | MIP-3α | [81,192] | |
| CXCR1, CXCR2 | CXCL8 | [193] | ||
| Breast | Bone | CXCR1 | CXCL8 | [194] |
| CXCR2 | CXCL8 | [195] | ||
| Liver | CXCR4 | SDF-1 | [1,195] | |
| Brain | CX3CR1 | CXCL8 | [195] | |
| Pleural space | CCR6 | MIP-3α | [195] | |
| Skin, lymph nodes | CCR7 | SLC | [1,195] | |
| Lung | CXCR3 | IP-10 | [156] | |
| Osteosarcoma | Lung | CXCR4 | SDF-1 | [2,196] |
| Pancreas | Liver, spleen, lymph nodes, stomach | CXCR5 | BCA-1 | [189,197] |
| Lung, liver | CXCR4 | SDF-1 | [2] | |
| Liver | CCR6 | MIP-3α | [82,192] | |
| NSCLC | Pleural space | CXCR4 | SDF-1 | [198] |
| Lymph nodes | CCR7 | SLC | [199] | |
| Nasopharyngeal | Lymph nodes | CXCR4 | SDF-1 | [200] |
| Prostate | CXCR1, CXCR2 | CXCL8 | [97,201–206] | |
| Head and neck | Lymph nodes | CCR7 | SLC and ELC | [83] |
BCA: B cell-attracting chemokine; CTAK: Cutaneous T-cell attracting chemokine; ELC: EBI1-ligand chemokine; IP: Interferon-γ inducible protein; I-TAC: Interferon-inducible T cell α chemoattractant; MIG: Monokine induced by interferon-γ; NSCLC: Nonsmall-cell lung cancer; SDF: Stromal cell-derived factor; SLC: Secondary lymphoid tissue chemokine.
These reports indicate that tumor metastasis may be controlled if their chemokine receptors are blocked [71,93]. The idea has been further fueled by numerous resources created to combat transmission of HIV-1 via inhibition of CXCR4 and CCR5. As a result, therapeutic formulations, such as neomycin B hexa-arginine conjugate (NeoR) that protect human neuroblastoma cells from gp120-induced death, are finding ways to inhibit CXCR4-mediated cancer metastasis [94]. Similarly, antagonist-peptides T22 and T140 that block CXCR4 were shown to inhibit the CXCL12-induced migration of breast cancer cells, leukemia T cells, pancreatic cancer cells, squamous cell lung cancer (SCLC) cells, chronic lymphocytic leukemia B cells, pre-B acute lymphoblastic leukemia cells; promoting significant suppression of pulmonary metastasis of melanoma and breast cancer cells in mice [95,96]. Moreover, bisphosphonates that suppress CXCR-4 expression were also able to suppress tumor invasion both in vitro and in vivo [97,98].
Recent pioneering success in the treatment of people with B-cell lymphomas using a chimeric murine/human monoclonal antibody rituximab [99] ignited a surge of antibody-based strategies that target chemokine receptors. Rituximab binds with B-lymphocyte-restricted differentiation antigen CD20, which is also expressed on the surface of most B-cell non-Hodgkin’s lymphomas (NHL). To date, antibody therapy to a number of chemokines and their receptors is shown to be effective for the inhibition of both primary tumor growth and metastasis in SCID mice, such as anti-CXCL8 antibody against human NSCLC [57,58]; or anti-CXCR4 antibody against NHL [98]. Similarly with rituximab, anti-CCR4 antibody induced death of CCR4-expressing tumors in mice utilizing antibody-dependent cell-mediated cytotoxicity [15,100]. Of note, the clinical potency of antibody-dependent cell-mediated cytotoxicity can be affected by the host Fc receptor (FcR) polymorphism [101], as killing of the CCR4-expressing cells was not uniform in vitro [102]. Taken together, unlike traditional anticancer drugs, strategies that target chemokine receptors are not only less toxic, but also more specific and potent. Even inhibitors with broader specificity such as histone deacetylase inhibitors that downregulate CXCR4 gene expression seem to be nontoxic both in vitro and Phase I clinical trials for acute lymphoblastic leukemia [103]. In addition, targets, such as CXCR4, promote additional benefits inhibiting phosphorylation and transactivation of HER2-neu [88] that, in turn, can downregulate expression of CXCR4 and lead to reduced metastasis and tumor growth [89].
Chemokines & tumor escape from immune surveillance
Aberrant expression of chemokines, particularly CCL2, CCL17, CCL22 and CXCL1–3, and CXCL5, CXCL6, CXCL8 and CXCL12, is often associated with a poor disease prognosis for cancer patients [1,48,49]. A number of chemokines activate chemokine receptors to signal, via two major pathways, the phosphoinositide 3-kinase (PI3K)/Akt and the extracellular signal-regulated (ERK)1/2 kinases [104–106], promoting prosurvival and antiapoptotic signals in various cells, including tumors [107,108]. For example, MIP-1γ/CCL9 has been associated with activation of nuclear factor (NF) κB ligand-induced osteoclast differentiation and survival [109], and CXCL8 with enhancement of proliferation and survival of CXCR1- and CXCR2-expressing endothelial cells [65,47,110]. Recently characterized novel chemokine receptor CXCR7 is shown to provide survival advantages and increased adhesion upon encounter with CXCL12 and CXCL11 [111]. CXCR7-expressing cells expanded more rapidly and were able to grow in suboptimal and serum-deprived tissue culture conditions. In this respect, it is tempting to speculate that an unfavorable disease outcome in patients with adult T-cell leukemia/lymphoma (ATLL), mycosis fungoides and Sézary syndrome associated with CCR4 may be due to the prosurvival and antiapoptotic effects of CCL17 and CCL22 [14,15,112]. Thus, tumors expressing the following chemokine receptors may acquire a higher survival and resistance to apoptosis: CCR1, CCR4 and CCR7, and CXCR1, CXCR2, CXCR4, CXCR5 and CXCR7. At least, their expression is correlated with a poor disease outcome in patients with primary melanoma colorectal cancer, head-and-neck cancer, and Hodgkin lymphoma and NHL [1,77,91,107].
On the other hand, tumors appear to utilize the same machinery as normal immune cells do to evade ‘detection’ or to eliminate abnormal and malignant cells. The evasion of host immune responses was originally documented for viruses, but it is now clear that it plays a significant role in tumor escape [113]. In this process, chemokines regulate recruitment of so-called tumor infiltrating CXCR3- or CCR4-expressing effector CD8+ and CD4+ T cells, leading to overall improvement of survival of patients with stage III melanoma [66]. However, aberrant expression of these chemokines may lead to immunosuppression and tumor progression. For example, overexpression of CXCL9 and CXCL10 in hepatocellular carcinoma (HCC) is shown to down-regulate CXCR3 on CD4+ and CD8+ T cells, leading to reduced T-cell infiltration and impaired host antitumor defenses [114]. Although chemokines are not usually cytotoxic in vitro, they may acquire proapoptotic properties after association with GAGs as shown for the RANTES-induced apoptosis of CCR5-expressing T-cell lines (so far only in vitro) [37]. Moreover, an excessive production of CXCL9, 10 and CXCL11 can be chemorepulsive for DCs, monocytes and neutrophils, instead of chemotaxis [115,116]. Murine B16 melanoma increases its survival by overexpression of CXCL12 that repelled tumor-specific T cells [117]. Tumors also downregulate expression of chemokines that recruit APCs and iDCs, severely damaging their mobility and ability to induce antitumor immune responses [118]. For example, a loss of breast-and kidney-expressed chemokine (BRAK/CXCL14) is associated with reduced infiltration of iDCs and enhanced progression of a number of cancers, including prostate and head-and-neck squamous cell carcinoma [119–121]. However, even if antitumor cytolytic cells manage to infiltrate tumors, their activity can be modulated by the suppressive microenvironment generated by regulatory cells, such as macrophages and suppressive leukocytes, specifically Tregs, recruited to chemokine-expressing tumors. Chemokines participate in Th cell polarization [122]; and importance of activation of Th1 CD4+ cells for induction of long-lasting effector and memory CD8+ T cells that inhibit AIDS progression, eradicate intracellular pathogens or cancer cells has been documented widely [123–126]. However, Th1 responses can be suppressed by overexpression of CCL2 via induction of IL-4-mediated Th2-type cytokine polarization [127], which leads to inhibition of cellular antitumor responses [128,129].
Activity of naïve and effector CD4+ and CD8+ cells, or even DCs, can also be suppressed directly by Tregs via a cell-contact-mediated process [130–132]. Although Tregs comprise 5–10% of all CD4+ T cells, they play a central role in regulation of self-tolerance, such as maternal tolerance to the fetus, autoimmunity and tumor survival [133]. Dysfunction or depletion of Tregs leads to spontaneous onset of various immune or autoimmune disorders, such as organ-specific autoimmune diseases, both in mice and humans [134–136]. Tregs have also been associated with a poor disease outcome in patients with a variety of malignancies [22,68,137,138]. Migration and homing of Tregs are controlled by differential expression of chemokine receptors. For example, natural naive Tregs express CCR7 [139] to presumably home to secondary lymphoid organs [140]; while CXCR4 promotes retention of Tregs in bone marrow [141] and CCR6 in the recruitment of ‘T regulatory effector/memory-like cells’ into the skin [142]. Similarly, CCR4 and 8 are associated with the skin-homing of Tregs [22,68,69,143]. A recent comparison between CCR4-positive and -negative cases in 103 patients with ATLL indicated that, even in the absence of significant clinical characteristics at the time of diagnosis, expression of CCR4 was associated with skin homing. Moreover, an unfavorable disease outcome in ovarian carcinoma and ATLL [144] can also be explained by infiltration of Tregs towards high levels of CCL17 and CCL22 produced at tumor sites [13,14,22,136,145]. In addition, CD4+ T cells recruited at tumor sites are shown to stimulate secretion of chemokines, leading to infiltration and accumulation of neoplastic cells [146]. Taken together, it is tempting to speculate that strategies that target CCL17 and CCL22 or their receptor CCR4 would enable control of the outcome of the disease acting at multiple steps: directly affecting survival and proliferation of tumors and indirectly controlling Tregs and improving antitumor cellular immune responses.
Tumor therapy using chemokines is an attractive but colossal task
The importance of chemokines in antitumor immune responses was demonstrated almost 25 years ago in the pioneering study of Luster and Leder who directly associated tumor eradication with IP-10-mediated recruitment of lymphocytes, neutrophils and monocytes [147]. Since then, a variety of chemokine-based immunotherapeutic strategies have been proposed to combat tumors (FIGURE 1). However, the majority were not translated successfully to the clinic, despite their obvious antitumor potency in inhibition of tumor-mediated neovascularization and metastasis. This is presumably because chemokines and their receptors are also expressed on normal immune cells. Thus, although tumor growth and metastasis can be blocked by targeting their chemokine receptors, normal immune cells, which are required for the clearance of residual tumor cells, would also be affected if they express the same receptors. The importance of antitumor adaptive immune responses in the successful combat of the residual disease preventing relapse is well established. However, most of the reports do not evaluate this issue, since they used SCID mice that lack functional T cells. In addition, the efficacy of the treatments may be affected by the stability of the formulations in the patient’s sera or in the tumor microenvironment. Tumors are shown to overexpress cathepsin-D that specifically cleaves a number of chemokines [148]. Moreover, chemokine activity is regulated by various peptidases, including CD26/dipeptidyl peptidase-IV, which degrade or modify their N- or C-terminal residues [149–151]. In this respect, some tumors, such as Sézary syndrome, cutaneous T-cell lymphoma cells downregulate their CD26/dipeptidyl peptidase-IV expression to enable migration to CXCL12 [149]. Nevertheless, the strategies that target chemokines or their receptors to reduce tumor burden and metastasis attract significant interest as a single treatment modality or in combination with other approaches. The anti-breast cancer effects of neutralizing CXCL8 antibody can be further enhanced in SCID mice when it was combined with anti-epidermal growth factor receptor (EGFR) antibody [152].
Taken together, tumor growth, angiogenesis and metastasis can be inhibited successfully by targeting chemokines and chemokine receptors, leading to reduction of disease severity. For example, since multiple myelomas secrete CCL3 that activates osteoclast stimulating factor [153] and RANKL-mediated bone destruction [154], inhibition of CCL3 was able to prevent bone loss in SCID mice, in addition to the reduction of tumor burden [155]. However, antichemokine or chemokine receptor treatments may suppress normal immune cells, specifically a small precursor pool of antitumor T cells present in cancer patients, thus hampering their beneficial and important therapeutic roles. Furthermore, the treatment will not be effective against tumors that do not express chemokine receptors. Although lung metastasis of CXCR3-expressing mammary tumors was inhibited by treatment with a small-molecular-weight CXCR3 antagonist AMG487, it did not affect local tumor growth and overall survival of mice [156]. It is tempting to speculate that the efficacy of the strategies discussed above would be impartial, unless they also induce robust adaptive anti-tumor immune responses. The task is usually assigned to the ‘professional’ cytolytic T cells working in concert with NK cells.
Chemokine–based cancer vaccines
A primary focus of cancer vaccines is to elicit tumor-specific adaptive immune responses, in particular to activate CD8+ cytotoxic T lymphocytes and Th1 CD4+ cells [125,126]. Cancer vaccines are therapeutic formulations designed to be most effective for the combat of a minimal residual disease, once the bulk of the tumor was reduced by other treatment modalities. However, they may also work as a prophylactic vaccine similarly to the human papillomavirus (HPV) vaccine that protects young women against oncogenic HPV-induced cervical infection and dysplasia [157]. Since human tumors are weakly immunogenic, the major issue is to render them immunogenic. In this respect, chemokines are also a useful tool to induce and modify tumor microenvironment and to promote antitumor adaptive immune responses. They are utilized widely to induce tumor-specific cellular immunity in mice, for example, by transfecting tumors with CXCL12 to recruit DCs [158]. However, chemokines should be used cautiously, since they may also lead to opposite results despite recruitment of DCs. For example, although murine lung carcinomas transduced with CCL22 were able to recruit DCs, NK cells and T cells, resulting in tumor regression [159], others reported only marginal protective effects of CCL17- or CCL22-transduced tumors [160]. In our hands, CCL22 was a very potent Th2-type chemokine that could not facilitate induction of antigen-specific CD8+ T cells [20,161]. This is presumably owing to the fact that CCL22 can attract CCR4-expressing Tregs that suppress antitumor T-cell activity in human ovarian cancers and T-cell leukemia [13,14,68]. CCL3 is also a potent recruiter and activator of DCs [162], but it failed to reduce tumorigenicity CCL3-expressing cells in mice although it significantly increased recruitment of macrophages and neutrophils [163]. These results could also be explained by the recruitment of CCR5-expressing Tregs [164]. Thus, to improve the potency of DC-based vaccines, others proposed to block the activity of the host CCR5, as the vaccine only worked in CCR5-knockout mice [165]. Taken together, chemokines can be used specifically to recruit iDCs and other APCs, such as macrophages, to improve their natural ‘sampling’ process, but their ‘improper’ use in a ‘wrong’ context would lead to immunosuppression through recruitment of various suppressive cells, such as macrophages, NK cells, NKT cells, Tregs and even iDCs [130–132,166].
Activated macrophages and professional APCs, including DCs, elicit tailored immune responses to pathogens and tissue abnormalities, controlling recruitment of Th1/2 polarized T cells via differential expression of chemokines. For example, myeloid DCs secrete CCL17 and CCL22 constitutively, while plasmacytoid DCs do not express them and, instead, produce CCL3 [167]. Thus, the two DCs can differentially recruit CCR4- or CCL5-expressing Tregs and Th2-type cells. This, in turn, may affect the activity of DCs by keeping them suppressed via contact-mediated processes with Tregs, or immunosuppressive cytokines, such as TGF-β and IL-10 and IL-13. The suppression affects an entire local area, including M2-skewed polarization of macrophages capable of downregulating Th1-type CD4+ T-cell and CD8+ T-cell responses [166]. It appears that the suppressive state of cells can be reversed, for example, by ‘danger signals’, which was postulated as a requirement for optimal recognition of self-tumor antigens and induction of proinflammatory rather than tolerogenic responses [168,169]. This induces DC maturation upregulating expression of CD80 and CD86 costimulatory molecules and inducing production of various proinflammatory cytokines and chemokines [170].
Skin DCs and Langerhans’ cells (LCs) constantly sample antigens and self-antigens as part of the normal immunosurveillance process, but do not induce neutralizing immune responses without activating stimuli to mature and express surface costimulatory molecules [171,172]. Tumor-associated antigens (TAAs) are taken up by iDCs and LCs to be processed into small peptides and presented utilizing MHC class I or II machinery. However, most extrinsic antigens are not efficiently taken up and presented to the MHC class I molecules (cross-presentation); instead, they are mostly delivered to the MHC class II-processing machinery. Although cross-presentation can be achieved by loading DCs with large quantities of extrinsic antigens (mg/ml) [173,174], skin DCs and LCs primarily deliver exogenous antigens to the MHC class II processing pathway, while cross-presentation was attributed to only CD8+ DCs [175]. As a result, even if tumors express chemokines that recruit iDCs [93], their antigens may not be efficiently cross-presented to elicit cytolytic CD8+ T cells. On the other hand, antigen presentation can be 100–10,000-fold increased if antigens are taken up using various endocytic cell-surface receptors [176,177]. Thus, we have hypothesized that presentation of weakly immunogenic tumor antigens can be enhanced when they are targeted to chemokine receptors, which may also promote necessary inflammatory conditions at the vaccination site by recruitment of various inflammatory cells [19]. As a result, otherwise nonimmunogenic tumor antigens were efficiently rendered immunogenic when used as a genetic fusion with chemokines, such as CCL7, CCL20 and CXCL10 [19,20]. In fact, we demonstrated that any chemokine, or even β-defensin (an antimicrobial peptide shown to bind CCR6 [178]), was suitable for this purpose as long as they acted via chemokine receptors expressed on iDCs (CCR1, CCR2, CCR5 and CCR6). The breadth of the strategy was apparent by its ability to elicit effector CD4+ and CD8+ T-cell-dependent antitumor immunity and eradication of established A20 lymphoma [19,20]. The response depended on the ability of chemokines to act as a carrier delivering fused TAA to chemokine receptors, indicating that the recruitment of APCs alone without antigen uptake is not sufficient. Although the mechanism of the carrier activity of inflammatory chemokines and defensins is not fully elucidated, we demonstrated recently that chemokines facilitated an efficient delivery of tumor antigens to MHC class I processing and presentation pathways (cross-presentation) [179]. The fate of the internalized chemokine-fused antigens is as follows: a certain proportion of them is degraded in endosomal/lysosomal compartments (for subsequent presentation to MHC class II molecules) [180], while the rest escape to the cytosol where they are degraded by proteasomes to be cross-presented to MHC class I molecules. As a result, naive iDCs were able to stimulate both tumor-specific CD8+ T cells and CD4+ Th cells, despite being only ‘loaded’ with nmol (ng/ml) quantities of chemokine fused TAA. Although cross-presentation by DCs alone may induce tolerance in the absence of activation [172,181], it appears that necessary activating signals were properly engaged on DCs, as mice immunized with chemokine-fused with nonimmunogenic TAA elicited potent T-cell responses in the absence of adjuvants. Although chemokines are not known to induce DCs maturation directly, with the exception of murine β-defensin 2 [182], the chemokine constructs presumably stimulated APCs indirectly by recruitment of proinflammatory cells at the vaccination site.
Taken together, although utilization of chemokines for tumor therapy is a colossal task, our recent understanding of chemokine biology and their role in malignant diseases give rise to exciting hopes and novel ideas. Cancer is a multifactorial disease that requires combination of different strategies and, as such, it would require combination of various strategies. Success of chemokine-based strategies will also depend on their custom tailored use targeting specific stages and particular microenvironment of the disease. However, complete cancer cure may only be possible if the treatment also induces neutralizing adaptive immune responses. This can be achieved by utilizing chemokines as a carrier for TAAs. Tumor protection experiments in murine tumor models indicate that the strategy may have significant clinical value, specifically for eradication of human tumors on minimal residual disease.
Expert commentary & five-year view
The last few decades have been marked with a number of exciting findings that have deepened our understanding of the multistage carcinogenic processes. Identification of specific molecules involved in cancer contributed to the change of ‘the dogma’ for treatment of cancer, with the cytotoxic drugs used in chemotherapy to lose their dominance and to be used alongside targeted therapies, developed with the aim of targeting cancer cells more specifically. Thus, the identification of key molecules for cancer growth and progression represents an exciting and fast expanding area of cancer research. Numerous interesting strategies have been proposed, encouraged by preclinical and clinical results in recent successful treatments of hematological malignancies, utilizing approaches that exploit the therapeutic potential of tumor-specific antibodies and cellular immune effector mechanisms (vaccines). Overall, the concept has hardly left its preclinical testing stage and only a few are being tested successfully in clinics. For example, several promising immunotherapeutics have already reached the market, such as trastuzumab (Herceptin®), bevacizumab (Avastin®), ibritumomab (Zevalin®), tositumomab and iodine I-131 tositumomab (Bexxar®), Campath® and OncoVax®. Promising data are being reported on strategies that inhibit tumor angiogenesis. However, the next generation of treatments would probably be concentrated on their combined use with conventional therapeutic modalities. For example, bevacizumab, an anti VEGF-antibody used in association with chemotherapy, prolongs overall survival and progression-free survival in patients with metastatic colorectal cancer [183]. Efficacy of bevacizumab is also well known in human renal cell carcinoma [184]. Moreover, as underlined in this review, cancer growth and metastasis can also be controlled efficiently using chemotactic and immunomodulatory chemokines. Chemokines/chemokine receptors attract significant attention as anti-cancer strategies, since they are involved in almost every aspect of tumorigenesis and in the complex and multifactorial process of carcinogenesis. Chemokines are ideal targets for the inhibition of tumor angiogenesis, as ELR CXC chemokines exhibit angiogenic/angiostatic properties. The increased levels of IP-10/CCL10 detected in fresh specimens of human squamous cell carcinoma are inversely correlated with tumor growth rate [63,64]. To date, despite their obvious efficacy in the inhibition of tumor-mediated neovascularization and metastasis, the chemokine-based approaches are yet to be translated to the clinic. In fact, this is a colossal task, as chemokines and chemokine receptors are also part of the normal functional immune system. It is tempting to speculate that this is presumably owing to the fact that ‘passive’ immunotherapy may not be fully effective unless it is accompanied with the induction of antitumor adaptive responses capable of clearing residual tumors. In this respect, the use of chemokines to render nonimmunogenic tumor antigens immunogenic by specifically delivering them to iDCs is potentially feasible, thus, attracting significant clinical interest for the development of vaccines against human cancers. Overall, recent advances in the therapeutic utilization of chemokines, fueled by seminal progress in the understanding of their biology and role in carcinogenesis, lead us to believe that chemokine-based strategies hold significant promise for combating cancer successfully.
Key issues
Chemokines are a small group of related chemoattractant peptides that play a crucial role in the innate and adaptive immune responses.
The chemokine network represents an essential part of the immunosurveillance system.
Imbalanced and aberrant chemokine expression affects cancer progression and spread.
Chemokines and chemokine receptors are an attractive target for cancer immunotherapy as they are involved in almost every aspect of tumorigenesis, including growth and metastatic spread of malignant cells, neovascularization and recruitment of immunosuppressive cells.
cA variety of strategies targeting chemokines and chemokine receptors have been proposed, such as the use of pharmacological inhibitors, chemokine antagonists and specific antibodies.
The task is colossal, as antichemokine or chemokine receptor treatments may also affect functions of normal immune cells, thus, hampering their potential therapeutic roles.
The treatment strategy would be partial if it is not accompanied by induction of antitumor adaptive cellular immune responses capable of eradicating residual tumor cells and protecting from disease relapse.
Acknowledgments
We are grateful to Ana Lustig (NIA) for critical reading of the manuscript and helpful comments and Dolgor Baatar (NIA) for technical help and suggestions. This research was supported by the Intramural Research Program of the National Institute on Aging, NIH, USA.
Footnotes
Website
Cytokine Family Database http://cytokine.medic.kumamoto-u.ac.jp
Contributor Information
Chiara Dell’Agnola, Chiara Dell’Agnola, MD, Research Assistant, Department of Clinical and Experimental Medicine, Division of Oncology, University of Verona, Ospedale Policlinico GB Rossi, Piazzale Ludovico Scuro 10, 37134 Verona, Italy, Tel.: +39 045 812 8121 (office), +39 045 812 8502 (secretary), Fax: +39 045 802 7410, chiara.dellagnola@univr.it.
Arya Biragyn, Arya Biragyn, PhD, Head, Principal Investigator, Immunotherapeutics Unit, Laboratory of Immunology, Gerontology Research Center, National Institute on Aging, NIH, 5600 Nathan Shock Drive, Baltimore, MD 21224, USA, Tel.: +1 410 558 8680, Fax: +1 410 558 8284, biragyna@mail.nih.gov.
References
Papers of special note have been highlighted as:
• of interest
• of considerable interest
- 1.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 2•.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. Review on the crucial role of chemokine (C-X-C motif) receptor 4 (CXCR4) in cancer metastases. [DOI] [PubMed] [Google Scholar]
- 3.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 4.Varney ML, Johansson SL, Singh RK. Tumour-associated macrophage infiltration, neovascularization and aggressiveness in malignant melanoma, role of monocyte chemotactic protein-1 and vascular endothelial growth factor-A. Melanoma Res. 2005;15:417–425. doi: 10.1097/00008390-200510000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 6.Nukiwa M, Andarini S, Zaini J, et al. Dendritic cells modified to express fractalkine/CX3CL1 in the treatment of preexisting tumors. Eur J Immunol. 2006;36:1019–1027. doi: 10.1002/eji.200535549. [DOI] [PubMed] [Google Scholar]
- 7.Mendez R, Ruiz-Cabello F, Rodriguez T, et al. Identification of different tumor escape mechanisms in several metastases from a melanoma patient undergoing immunotherapy. Cancer Immunol Immunother. 2007;56:88–94. doi: 10.1007/s00262-006-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumamoto T, Huang EK, Paek HJ, et al. Induction of tumor-specific protective immunity by in situ Langerhans cell vaccine. Nat Biotechnol. 2002;20:64–69. doi: 10.1038/nbt0102-64. [DOI] [PubMed] [Google Scholar]
- 9.Fushimi T, Kojima A, Moore MA, Crystal RG. Macrophage inflammatory protein 3α transgene attracts dendritic cells to established murine tumors and suppresses tumor growth. J Clin Invest. 2000;105:1383–1393. doi: 10.1172/JCI7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacon K, Baggiolini M, Broxmeyer H, et al. Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002;22:1067–1068. doi: 10.1089/107999002760624305. [DOI] [PubMed] [Google Scholar]
- 11.Oppenheim JJ, Yang D, Biragyn A, Howard OM, Plotz P. Chemokine receptors on dendritic cells promote autoimmune reactions. Arthritis Res. 2002;4(Suppl 3):S183–S188. doi: 10.1186/ar574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ploix C, Lo D, Carson MJ. A ligand for the chemokine receptor CCR7 can influence the homeostatic proliferation of CD4 T cells and progression of autoimmunity. J Immunol. 2001;167:6724–6730. doi: 10.4049/jimmunol.167.12.6724. [DOI] [PubMed] [Google Scholar]
- 13.Scrivener S, Goddard RV, Kaminski ER, Prentice AG. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk Lymphoma. 2003;44:383–389. doi: 10.1080/1042819021000029993. [DOI] [PubMed] [Google Scholar]
- 14.Ishida T, Utsunomiya A, Iida S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma, its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9:3625–3634. [PubMed] [Google Scholar]
- 15.Ishida T, Iida S, Akatsuka Y, et al. The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-cell leukemia/lymphoma. Clin Cancer Res. 2004;10:7529–7539. doi: 10.1158/1078-0432.CCR-04-0983. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor, functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 17••.He J, Chen Y, Farzan M, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. Chemokine receptors, such as chemokine (C-C motif) receptor 5 (CCR5) and CXCR4, are utilized as HIV-1 coreceptor for infection of human CD4 T cells, thus supporting the concept that chemokine and chemokine receptors could represent an attractive target for cancer therapy. [DOI] [PubMed] [Google Scholar]
- 18.Coscia M, Biragyn A. Cancer immunotherapy with chemoattractant peptides. Semin Cancer Biol. 2004;14:209–218. doi: 10.1016/j.semcancer.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 19••.Biragyn A, Tani K, Grimm MC, Weeks SD, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotech. 1999;17:253–258. doi: 10.1038/6995. Chemokines can be utilized as carriers for nonimmunogenic tumor antigens, thus representing a new perspective for the development of vaccines against human cancers. [DOI] [PubMed] [Google Scholar]
- 20.Biragyn A, Surenhu M, Yang D, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 21.Burger JA, Kipps TJ. CXCR4, a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 22.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 23.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 24.Lau EK, Paavola CD, Johnson Z, Gaudry, et al. Identification of the glycosaminoglycan binding site of the chemokine CC, MCP-1, implications for structure and function in vivo. J Biol Chem. 2004;279:22294–22305. doi: 10.1074/jbc.M311224200. [DOI] [PubMed] [Google Scholar]
- 25.Stringer SE, Gallagher JT. Specific binding of the chemokine platelet factor 4 to heparan sulfate. J Biol Chem. 1997;272:20508–20514. doi: 10.1074/jbc.272.33.20508. [DOI] [PubMed] [Google Scholar]
- 26.Stringer SE, Forster MJ, Mulloy B, Bishop CR, Graham GJ, Gallagher JT. Characterization of the binding site on heparan sulfate for macrophage inflammatory protein 1α. Blood. 2002;100:1543–1550. [PubMed] [Google Scholar]
- 27.Spillmann D, Witt D, Lindahl U. Defining the interleukin-8-binding domain of heparan sulfate. J Biol Chem. 1998;273:15487–15493. doi: 10.1074/jbc.273.25.15487. [DOI] [PubMed] [Google Scholar]
- 28.Lortat-Jacob H, Grosdidier A, Imberty A. Structural diversity of heparan sulfate binding domains in chemokines. Proc Natl Acad Sci USA. 2002;99:1229–1234. doi: 10.1073/pnas.032497699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson AM, Gardner L, Shaw JG, et al. Induction of a CXCL8 binding site on endothelial syndecan-3 in rheumatoid synovium. Arthritis Rheum. 2005;52:2331–2342. doi: 10.1002/art.21222. [DOI] [PubMed] [Google Scholar]
- 30.Ali S, Palmer AC, Banerjee B, Fritchley SJ, Kirby JA. Examination of the function of RANTES, MIP-1α, MIP-1β following interaction with heparin-like glycosaminoglycans. J Biol Chem. 2000;275:11721–11727. doi: 10.1074/jbc.275.16.11721. [DOI] [PubMed] [Google Scholar]
- 31.Kuschert GS, Coulin F, Power CA, et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 32.Webb LM, Ehrengruber MU, Clark-Lewis I, Baggiolini M, Rot A. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc Natl Acad Sci USA. 1993;90:7158–7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proudfoot AE, Handel TM, Johnson Z, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci USA. 2003;100:1885–1890. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson Z, Kosco-Vilbois MH, Herren S, et al. Interference with heparin binding and oligomerization creates a novel anti-inflammatory strategy targeting the chemokine system. J Immunol. 2004;173:5776–5785. doi: 10.4049/jimmunol.173.9.5776. [DOI] [PubMed] [Google Scholar]
- 36.Sadir R, Imberty A, Baleux F, Lortat-Jacob H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem. 2004;279:43854–43860. doi: 10.1074/jbc.M405392200. [DOI] [PubMed] [Google Scholar]
- 37.Murooka TT, Wong MM, Rahbar R, Majchrzak-Kita B, Proudfoot AE, Fish EN. CCL5-CCR5-mediated apoptosis in T cells: Requirement for glycosaminoglycan binding and CCL5 aggregation. J Biol Chem. 2006;281:25184–25194. doi: 10.1074/jbc.M603912200. [DOI] [PubMed] [Google Scholar]
- 38.Gilat D, Hershkoviz R, Mekori YA, Vlodavsky I, Lider O. Regulation of adhesion of CD4+ T lymphocytes to intact or heparinase-treated subendothelial extracellular matrix by diffusible or anchored RANTES and MIP-1β. J Immunol. 1994;153:4899–4906. [PubMed] [Google Scholar]
- 39.Dowsland MH, Harvey JR, Lennard TW, Kirby JA, Ali S. Chemokines and breast cancer, a gateway to revolutionary targeted cancer treatments? Curr Med Chem. 2003;10:579–592. doi: 10.2174/0929867033457944. [DOI] [PubMed] [Google Scholar]
- 40.Shurin MR, Shurin GV, Lokshin A, et al. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells, friends or enemies? Cancer Metastasis Rev. 2006;25:333–356. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 41.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 42.Zilberberg L, Shinkaruk S, Lequin O, et al. Structure and inhibitory effects on angiogenesis and tumor development of a new vascular endothelial growth inhibitor. J Biol Chem. 2003;278(37):35564–35573. doi: 10.1074/jbc.M304435200. [DOI] [PubMed] [Google Scholar]
- 43••.Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3:263–276. doi: 10.1517/14712598.3.2.263. Underlies the potential clinical impact of targeting angiogenesis in tumor growth and progression, particularly focusing on anti-vascular endothelial growth factor therapy in several preclinical models and also in human clinical trials. [DOI] [PubMed] [Google Scholar]
- 44.Homey B, Muller A, Zlotnik A. Chemokines, agents for the immunotherapy of cancer? Nature Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 45.Bernardini G, Spinetti G, Ribatti D, et al. I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood. 2000;96:4039–4045. [PubMed] [Google Scholar]
- 46.Salcedo R, Young HA, Ponce ML, et al. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166:7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 47.Volin MV, Woods JM, Amin MA, Connors MA, Harlow LA, Koch AE. Fractalkine, a novel angiogenic chemokine in rheumatoid arthritis. Am J Pathol. 2001;159:1521–1530. doi: 10.1016/S0002-9440(10)62537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wierda WG, Johnson MM, Do KA, et al. Plasma interleukin 8 level predicts for survival in chronic lymphocytic leukaemia. Br J Haematol. 2003;120:452–456. doi: 10.1046/j.1365-2141.2003.04118.x. [DOI] [PubMed] [Google Scholar]
- 49.White ES, Flaherty KR, Carskadon S, et al. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer, role in angiogenesis and prognosis. Clin Cancer Res. 2003;9:853–860. [PubMed] [Google Scholar]
- 50.Belperio JA, Keane MP, Arenberg DA, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 51.Bosco MC, Puppo M, Santangelo C, et al. Hypoxia modifies the transcriptome of primary human monocytes, modulation of novel immune-related genes and identification of CC-chemokine ligand 20 as a new hypoxia-inducible gene. J Immunol. 2006;177:1941–1955. doi: 10.4049/jimmunol.177.3.1941. [DOI] [PubMed] [Google Scholar]
- 52.Bosco MC, Reffo G, Puppo M, Varesio L. Hypoxia inhibits the expression of the CCR5 chemokine receptor in macrophages. Cell Immunol. 2004;228:1–7. doi: 10.1016/j.cellimm.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohta M, Kitadai Y, Tanaka S, et al. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol. 2003;22:773–778. [PubMed] [Google Scholar]
- 55.Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765–770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 56••.Ueno T, Toi M, Saji H, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–3289. Based on the observation that chemokine expression is positively associated with a poor disease outcome, this work suggests that chemokines could represent prognostic biomarkers of disease outcome. This is particularly true for diseases, such as melanoma, ovarian cancer, prostate and lung cancer, associated with augmented serum levels of CXCL8. [PubMed] [Google Scholar]
- 57.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest. 1996;97:2792–2802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arenberg DA, Keane MP, DiGiovine B, et al. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest. 1998;102:465–472. doi: 10.1172/JCI3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66:3071–3077. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- 60.Luan J, Shattuck-Brandt R, Haghnegahdar H, et al. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- 61••.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. Based on the observation that chemokine expression is positively associated with a poor disease outcome, this work suggests that chemokines could represent prognostic biomarkers of disease outcome. This is particularly true for diseases, such as melanoma, ovarian cancer, prostate and lung cancer, associated with augmented serum levels of CXCL8. [DOI] [PubMed] [Google Scholar]
- 62.Huang S, Mills L, Mian B, et al. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161:125–134. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Arenberg DA, Kunkel SL, Polverini PJ, et al. Interferon-γ-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184:981–992. doi: 10.1084/jem.184.3.981. The key role of CXCL10 is pointed out by showing the possibility to combat tumorigenesis through the shift of a local balance toward angiostatic chemokines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Arenberg DA, Zlotnick A, Strom SR, Burdick MD, Strieter RM. The murine chemokine CC, 6C-kine, inhibits tumor growth and angiogenesis in a human lung cancer SCID mouse model. Cancer Immunol Immunother. 2001;49:587–592. doi: 10.1007/s002620000147. The key role of CXCL10 is pointed out by showing the possibility to combat tumorigenesis through the shift of a local balance toward angiostatic chemokines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Addison CL, Arenberg DA, Morris SB, et al. The chemokine XC, monokine induced by interferon-γ, inhibits non-small cell lung carcinoma tumor growth and metastasis. Hum Gene Ther. 2000;11:247–261. doi: 10.1089/10430340050015996. [DOI] [PubMed] [Google Scholar]
- 66.Mullins IM, Slingluff CL, Lee JK, et al. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- 67.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses, lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291–298. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 68.Sebastiani S, Allavena P, Albanesi C, et al. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol. 2001;166:996–1002. doi: 10.4049/jimmunol.166.2.996. [DOI] [PubMed] [Google Scholar]
- 69•.Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. Despite the fact that the majority of chemokines and chemokine receptors are promiscuous, they are tightly controlled by differential and compartmentalized expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stine JT, Wood C, Hill M, et al. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood. 2000;95:1151–1157. [PubMed] [Google Scholar]
- 71.Bendall L. Chemokines and their receptors in disease. Histol Histopathol. 2005;20:907–926. doi: 10.14670/HH-20.907. [DOI] [PubMed] [Google Scholar]
- 72.Sallusto F, Palermo B, Lenig D, et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29:1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 73.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campbell MJ, Esserman L, Byars NE, Allison AC, Levy R. Idiotype vaccination against murine B cell lymphoma. Humoral and cellular requirements for the full expression of antitumor immunity. J Immunol. 1990;145:1029–1036. [PubMed] [Google Scholar]
- 75.Bardi G, Lipp M, Baggiolini M, Loetscher P. The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur J Immunol. 2001;31:3291–3297. doi: 10.1002/1521-4141(200111)31:11<3291::aid-immu3291>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 76.Mariani M, Lang R, Binda E, Panina-Bordignon P, D’Ambrosio D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur J Immunol. 2004;34:231–240. doi: 10.1002/eji.200324429. [DOI] [PubMed] [Google Scholar]
- 77.Longo-Imedio MI, Longo N, Trevino I, Lazaro P, Sanchez-Mateos P. Clinical significance of CXCR3 and CXCR4 expression in primary melanoma. Int J Cancer. 2005;117:861–865. doi: 10.1002/ijc.21269. [DOI] [PubMed] [Google Scholar]
- 78.Ishida T, Inagaki H, Utsunomiya A, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res. 2004;10:5494–5500. doi: 10.1158/1078-0432.CCR-04-0371. [DOI] [PubMed] [Google Scholar]
- 79.Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood. 2000;95:627–632. [PubMed] [Google Scholar]
- 80.Trentin L, Agostini C, Facco M, et al. The chemokine receptor CXCR3 is expressed on malignant B cells and mediates chemotaxis. J Clin Invest. 1999;104:115–121. doi: 10.1172/JCI7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubie C, Frick VO, Wagner M, et al. Enhanced expression and clinical significance of CC-chemokine MIP-3α in hepatocellular carcinoma. Scand J Immunol. 2006;63:468–477. doi: 10.1111/j.1365-3083.2006.001766.x. [DOI] [PubMed] [Google Scholar]
- 82.Kimsey TF, Campbell AS, Albo D, Wilson M, Wang TN. Co-localization of macrophage inflammatory protein-3α (Mip-3α) and its receptor, CCR6, promotes pancreatic cancer cell invasion. Cancer J. 2004;10:374–380. doi: 10.1097/00130404-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 83.Wang J, Xi L, Hunt JL, Gooding W, et al. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res. 2004;64:1861–1866. doi: 10.1158/0008-5472.can-03-2968. [DOI] [PubMed] [Google Scholar]
- 84.Shulby SA, Dolloff NG, Stearns ME, Meucci O, Fatatis A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res. 2004;64:4693–4698. doi: 10.1158/0008-5472.CAN-03-3437. [DOI] [PubMed] [Google Scholar]
- 85.Cabioglu N, Yazici MS, Arun B, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 86.Gunther K, Leier J, Henning G, et al. Prediction of lymph node metastasis in colorectal carcinoma by expression of chemokine receptor CCR7. Int J Cancer. 2005;116:726–733. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- 87.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 88.Cabioglu N, Summy J, Miller C, et al. CXCL-12/stromal cell-derived factor-1α transactivates HER2-neu in breast cancer cells by a novel pathway involving Src kinase activation. Cancer Res. 2005;65:6493–6497. doi: 10.1158/0008-5472.CAN-04-1303. [DOI] [PubMed] [Google Scholar]
- 89.Li YM, Pan Y, Wei Y, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 90.Kodama J, Hasengaowa, Kusumoto T, et al. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann Oncol. 2006;18:70–76. doi: 10.1093/annonc/mdl342. [DOI] [PubMed] [Google Scholar]
- 91.Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 92.Ottaiano A, Franco R, Aiello TA, et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II–III colorectal cancer patients. Clin Cancer Res. 2006;12:2795–2803. doi: 10.1158/1078-0432.CCR-05-2142. [DOI] [PubMed] [Google Scholar]
- 93•.Proudfoot EA. Chemokine receptors, multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106–115. doi: 10.1038/nri722. Focusing on mechanisms and pathways of cross-presentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Catani MV, Corasaniti MT, Ranalli M, et al. The Tat antagonist neomycin B hexa-arginine conjugate inhibits gp-120-induced death of human neuroblastoma cells. J Neurochem. 2003;84:1237–1245. doi: 10.1046/j.1471-4159.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- 95.Takenaga M, Tamamura H, Hiramatsu K, et al. A single treatment with microcapsules containing a CXCR4 antagonist suppresses pulmonary metastasis of murine melanoma. Biochem Biophys Res Commun. 2004;320:226–232. doi: 10.1016/j.bbrc.2004.05.155. [DOI] [PubMed] [Google Scholar]
- 96.Tamamura H, Fujii N. The therapeutic potential of CXCR4 antagonists in the treatment of HIV infection, cancer metastasis and rheumatoid arthritis. Expert Opin Ther Targets. 2005;9:1267–1282. doi: 10.1517/14728222.9.6.1267. [DOI] [PubMed] [Google Scholar]
- 97.Miwa S, Mizokami A, Keller ET, Taichman R, Zhang J, Namiki M. The bisphosphonate YM529 inhibits osteolytic and osteoblastic changes and CXCR-4-induced invasion in prostate cancer. Cancer Res. 2005;65:8818–8825. doi: 10.1158/0008-5472.CAN-05-0540. [DOI] [PubMed] [Google Scholar]
- 98.Bertolini F, Dell’Agnola C, Mancuso P, et al. CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin lymphoma. Cancer Res. 2002;62:3106–3112. [PubMed] [Google Scholar]
- 99••.Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–2195. New perspective for the use of anti-CD20 antibodies in the hematological clinical setting. [PubMed] [Google Scholar]
- 100.Niwa R, Shoji-Hosaka E, Sakurada M, et al. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004;64:2127–2133. doi: 10.1158/0008-5472.can-03-2068. [DOI] [PubMed] [Google Scholar]
- 101.Boot JH, Geerts ME, Aarden LA. Functional polymorphisms of Fc receptors in human monocyte-mediated cytotoxicity towards erythrocytes induced by murine isotype switch variants. J Immunol. 1989;142:1217–1223. [PubMed] [Google Scholar]
- 102.Kim DH, Jung HD, Kim JG, et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood. 2006;108:2720–2725. doi: 10.1182/blood-2006-01-009480. [DOI] [PubMed] [Google Scholar]
- 103.Crazzolara R, Bernhard D. CXCR4 chemokine receptors, histone deacetylase inhibitors and acute lymphoblastic leukemia. Leuk Lymphoma. 2005;46:1545–1551. doi: 10.1080/10428190500215027. [DOI] [PubMed] [Google Scholar]
- 104.Sanchez-Sanchez N, Riol-Blanco L, de la Rosa G, et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood. 2004;104:619–625. doi: 10.1182/blood-2003-11-3943. [DOI] [PubMed] [Google Scholar]
- 105.Wang J, Zhang X, Thomas SM, et al. Chemokine receptor 7 activates phosphoinositide-3 kinase-mediated invasive and prosurvival pathways in head and neck cancer cells independent of EGFR. Oncogene. 2005;24:5897–5904. doi: 10.1038/sj.onc.1208740. [DOI] [PubMed] [Google Scholar]
- 106.Cronshaw DG, Owen C, Brown Z, Ward SG. Activation of phosphoinositide 3-kinases by the CCR4 ligand macrophage-derived chemokine is a dispensable signal for T lymphocyte chemotaxis. J Immunol. 2004;172:7761–7770. doi: 10.4049/jimmunol.172.12.7761. [DOI] [PubMed] [Google Scholar]
- 107.Muller A, Sonkoly E, Eulert C, et al. Chemokine receptors in head and neck cancer, association with metastatic spread and regulation during chemotherapy. Int J Cancer. 2006;118:2147–2157. doi: 10.1002/ijc.21514. [DOI] [PubMed] [Google Scholar]
- 108.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 109.Okamatsu Y, Kim D, Battaglino R, Sasaki H, Spate U, Stashenko P. MIP-1γ promotes receptor-activator-of-NF-κ B-ligand-induced osteoclast formation and survival. J Immunol. 2004;173:2084–2090. doi: 10.4049/jimmunol.173.3.2084. [DOI] [PubMed] [Google Scholar]
- 110.Salcedo R, Resau JH, Halverson D, et al. Differential expression and responsiveness of chemokine receptors (CXCR1–3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 2000;14:2055–2064. doi: 10.1096/fj.99-0963com. [DOI] [PubMed] [Google Scholar]
- 111.Burns JM, Summers BC, Wang Y, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yoshie O, Fujisawa R, Nakayama T, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99:1505–1511. doi: 10.1182/blood.v99.5.1505. [DOI] [PubMed] [Google Scholar]
- 113.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 114.Liu YQ, Poon RT, Hughes J, Li QY, Yu WC, Fan ST. Desensitization of T lymphocyte function by CXCR3 ligands in human hepatocellular carcinoma. World J Gastroenterol. 2005;11:164–170. doi: 10.3748/wjg.v11.i2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kohrgruber N, Groger M, Meraner P, et al. Plasmacytoid dendritic cell recruitment by immobilized CXCR3 ligands. J Immunol. 2004;173:6592–6602. doi: 10.4049/jimmunol.173.11.6592. [DOI] [PubMed] [Google Scholar]
- 116.Ogilvie P, Paoletti S, Clark-Lewis I, Uguccioni M. Eotaxin-3 is a natural antagonist for CCR2 and exerts a repulsive effect on human monocytes. Blood. 2003;102:789–794. doi: 10.1182/blood-2002-09-2773. [DOI] [PubMed] [Google Scholar]
- 117.Vianello F, Papeta N, Chen T, et al. Murine B16 melanomas expressing high levels of the chemokine stromal-derived factor-1/CXCL12 induce tumor-specific T cell chemorepulsion and escape from immune control. J Immunol. 2006;176:2902–2914. doi: 10.4049/jimmunol.176.5.2902. [DOI] [PubMed] [Google Scholar]
- 118.Remmel E, Terracciano L, Noppen C, et al. Modulation of dendritic cell phenotype and mobility by tumor cells in vitro. Hum Immunol. 2001;62:39–49. doi: 10.1016/s0198-8859(00)00221-4. [DOI] [PubMed] [Google Scholar]
- 119.Frederick MJ, Henderson Y, Xu X, et al. In vivo expression of the novel CXC chemokine BRAK in normal and cancerous human tissue. Am J Pathol. 2000;156:1937–1950. doi: 10.1016/S0002-9440(10)65067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shurin GV, Ferris RL, Tourkova IL, et al. Loss of new chemokine CXCL14 in tumor tissue is associated with low infiltration by dendritic cells (DC), while restoration of human CXCL14 expression in tumor cells causes attraction of DC both in vitro and in vivo. J Immunol. 2005;174:5490–5498. doi: 10.4049/jimmunol.174.9.5490. [DOI] [PubMed] [Google Scholar]
- 121.Shellenberger TD, Wang M, Gujrati M, et al. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004;64:8262–8270. doi: 10.1158/0008-5472.CAN-04-2056. [DOI] [PubMed] [Google Scholar]
- 122.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 123.Shearer GM, Clerici M. Protective immunity against HIV infection, has nature done the experiment for us? Immunol Today. 1996;17:21–24. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 124.Rowland-Jones S, Sutton J, Ariyoshi K, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 125.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 126.Melief CJ, Kast WM. T-cell immunotherapy of tumors by adoptive transfer of cytotoxic T lymphocytes and by vaccination with minimal essential epitopes. Immunol Rev. 1995;145:167–177. doi: 10.1111/j.1600-065x.1995.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 127.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 128.Mendel IE, Shevach M. The IL-10-producing competence of Th2 cells generated in vitro is IL-4 dependent. Eur J Immunol. 2002;32:3216–3224. doi: 10.1002/1521-4141(200211)32:11<3216::AID-IMMU3216>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 129.Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 130.Sakaguchi S. Regulatory T cells, key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 131.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004;172:2778–2784. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 132.Cavani A, Nasorri F, Prezzi C, Sebastiani S, Albanesi C, Girolomoni G. Human CD4+ T lymphocytes with remarkable regulatory functions on dendritic cells and nickel-specific Th1 immune responses. J Invest Dermatol. 2000;114:295–302. doi: 10.1046/j.1523-1747.2000.00881.x. [DOI] [PubMed] [Google Scholar]
- 133.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 134.Alyanakian MA, You S, Damotte D, et al. Diversity of regulatory CD4+T cells controlling distinct organ-specific autoimmune diseases. Proc Natl Acad Sci USA. 2003;100:15806–15811. doi: 10.1073/pnas.2636971100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands, implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 137•.Woo EY, Yeh H, Chu CS, et al. Cutting edge regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. Role played by T regulatory cells (Tregs) in disease outcome is explored and shown. [DOI] [PubMed] [Google Scholar]
- 138.Beyer M, Kochanek M, Darabi K, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 139.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naïve CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 141.Zou L, Barnett B, Safah H, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 142.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 143•.Colantonio L, Iellem A, Sinigaglia F, D’Ambrosio D. Skin-homing CLA+ T cells and regulatory CD25+ T cells represent major subsets of human peripheral blood memory T cells migrating in response to CCL1/I-309. Eur J Immunol. 2002;32:3506–3514. doi: 10.1002/1521-4141(200212)32:12<3506::AID-IMMU3506>3.0.CO;2-#. Chemokine receptors and Tregs are strictly interacting in an intricate and functional network, as presented in this work, in which CCR4 and CCR8 are associated with skin homing of Tregs. [DOI] [PubMed] [Google Scholar]
- 144.Hisaeda H, Maekawa Y, Iwakawa D, et al. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 145.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ghia P, Strola G, Granziero L, et al. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. Eur J Immunol. 2002;32:1403–1413. doi: 10.1002/1521-4141(200205)32:5<1403::AID-IMMU1403>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 147.Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med. 1993;178:1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wolf M, Clark-Lewis I, Buri C, Langen H, Lis M, Mazzucchelli L. Cathepsin D specifically cleaves the chemokines macrophage inflammatory protein-1 α, macrophage inflammatory protein-1 β, SLC that are expressed in human breast cancer. Am J Pathol. 2003;162:1183–1190. doi: 10.1016/s0002-9440(10)63914-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Narducci MG, Scala E, Bresin A, et al. Skin homing of Sezary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood. 2006;107:1108–1115. doi: 10.1182/blood-2005-04-1492. [DOI] [PubMed] [Google Scholar]
- 150.Proost P, Menten P, Struyf S, Schutyser E, De Meester I, Van Damme J. Cleavage by CD26/dipeptidyl peptidase IV converts the chemokine LD78β into a most efficient monocyte attractant and CCR1 agonist. Blood. 2000;96:1674–1680. [PubMed] [Google Scholar]
- 151.Shioda T, Kato H, Ohnishi Y, et al. Anti-HIV-1 and chemotactic activities of human stromal cell-derived factor 1α (SDF-1α) and SDF-1β are abolished by CD26/dipeptidyl peptidase-IV-mediated cleavage. Proc Natl Acad Sci USA. 1998;95:6331–6336. doi: 10.1073/pnas.95.11.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Salcedo R, Martins-Green M, Gertz B, Oppenheim JJ, Murphy WJ. Combined administration of antibodies to human interleukin 8 and epidermal growth factor receptor results in increased antimetastatic effects on human breast carcinoma xenografts. Clin Cancer Res. 2002;8:2655–2665. [PubMed] [Google Scholar]
- 153.Choi SJ, Cruz JC, Craig F, et al. Macrophage inflammatory protein 1α is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96:671–675. [PubMed] [Google Scholar]
- 154.Abe M, Hiura K, Wilde J, et al. Role for macrophage inflammatory protein (MIP)-1α and MIP-1β in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100:2195–2202. [PubMed] [Google Scholar]
- 155.Choi SJ, Oba Y, Gazitt Y, et al. Antisense inhibition of macrophage inflammatory protein 1-α blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108:1833–1841. doi: 10.1172/JCI13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Walser TC, Rifat S, Ma X, et al. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66:7701–7707. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- 157.Schiller JT, Lowy DR. Prospects for cervical cancer prevention by human papillomavirus vaccination. Cancer Res. 2006;66:10229–10232. doi: 10.1158/0008-5472.CAN-06-0630. [DOI] [PubMed] [Google Scholar]
- 158.Fushimi T, O’Connor TP, Crystal RG. Adenoviral gene transfer of stromal cell-derived factor-1 to murine tumors induces the accumulation of dendritic cells and suppresses tumor growth. Cancer Res. 2006;66:3513–3522. doi: 10.1158/0008-5472.CAN-05-1493. [DOI] [PubMed] [Google Scholar]
- 159.Guo J, Wang B, Zhang M, et al. Macrophage-derived chemokine gene transfer results in tumor regression in murine lung carcinoma model through efficient induction of antitumor immunity. Gene Ther. 2002;9:793–803. doi: 10.1038/sj.gt.3301688. [DOI] [PubMed] [Google Scholar]
- 160.Okada N, Gao JQ, Sasaki A, et al. Anti-tumor activity of chemokine is affected by both kinds of tumors and the activation state of the host’s immune system, implications for chemokine-based cancer immunotherapy. Biochem Biophys Res Commun. 2004;317:68–76. doi: 10.1016/j.bbrc.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 161.Biragyn A, Belyakov IM, Chow YH, Dimitrov DS, Berzofsky JA, Kwak LW. DNA vaccines encoding HIV-1 gp120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. 2002;100:1153–1159. doi: 10.1182/blood-2002-01-0086. [DOI] [PubMed] [Google Scholar]
- 162.Trifilo MJ, Lane TE. The CC chemokine ligand 3 regulates CD11c+ CD11b+ CD8α dendritic cell maturation and activation following viral infection of the central nervous system, implications for a role in T cell activation. Virology. 2004;327:8–15. doi: 10.1016/j.virol.2004.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nakashima E, Oya A, Kubota Y, et al. A candidate for cancer gene therapy, MIP-1α gene transfer to an adenocarcinoma cell line reduced tumorigenicity and induced protective immunity in immunocompetent mice. Pharm Res. 1996;13:1896–1901. doi: 10.1023/a:1016057830271. [DOI] [PubMed] [Google Scholar]
- 164.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 165.Ng-Cashin J, Kuhns JJ, Burkett SE, et al. Host absence of CCR5 potentiates dendritic cell vaccination. J Immunol. 2003;170:4201–4208. doi: 10.4049/jimmunol.170.8.4201. [DOI] [PubMed] [Google Scholar]
- 166.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 167.Penna G, Vulcano M, Roncari A, Facchetti F, Sozzani S, Adorini L. Cutting edge, differential chemokine production by myeloid and plasmacytoid dendritic cells. J Immunol. 2002;169:6673–6676. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- 168.George TC, Bilsborough J, Viney JL, Norment AM. High antigen dose and activated dendritic cells enable Th cells to escape regulatory T cell-mediated suppression in vitro. Eur J Immunol. 2003;33:502–511. doi: 10.1002/immu.200310026. [DOI] [PubMed] [Google Scholar]
- 169.Matzinger P. An innate sense of danger. Semin Immunol. 2002;10(5):399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 170.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343(5):338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 171.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 172.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 173.Delamarre L, Holcombe H, Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J Exp Med. 2003;198:111–122. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lizee G, Basha G, Tiong J, et al. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat Immunol. 2003;4:1065–1073. doi: 10.1038/ni989. [DOI] [PubMed] [Google Scholar]
- 175•.Heath WR, Belz GT, Behrens GM, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. Focuses on mechanisms and pathways of cross-presentation. [DOI] [PubMed] [Google Scholar]
- 176.Zaliauskiene L, Kang S, Sparks K, et al. Enhancement of MHC class II-restricted responses by receptor-mediated uptake of peptide antigens. J Immunol. 2002;169:2337–2345. doi: 10.4049/jimmunol.169.5.2337. [DOI] [PubMed] [Google Scholar]
- 177.Mahnke K, Guo M, Lee S, et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–684. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang D, Chertov O, Bykovskaia SN, et al. β-defensins, linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 179.Schiavo R, Baatar D, Olkhanud P, et al. Chemokine receptor targeting efficiently directs antigens to MHC class I pathways and elicits antigen-specific CD8+ T-cell responses. Blood. 2006;107:4597–4605. doi: 10.1182/blood-2005-08-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Biragyn A, Ruffini PA, Coscia M, et al. Chemokine receptor-mediated delivery directs self-tumor antigen efficiently into the class II processing pathway in vitro and induces protective immunity in vivo. Blood. 2004;104:1961–1969. doi: 10.1182/blood-2004-02-0637. [DOI] [PubMed] [Google Scholar]
- 181.Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 crosslinking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Biragyn A, Ruffini PA, Leifer CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 183.Kozloff M, Hainsworth J, Badarinath S, et al. Survival of patients (pts) with mCRC treated with bevacizumab in combination with chemotherapy: results from the BRiTE registry. Gastrointestinal Cancers Symposium; FL, USA. 2007. Abstract No 375. [Google Scholar]
- 184.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 186.Varney ML, Li A, Dave BJ, Bucana CD, Johansson SL, Singh RK. Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different metastatic potential and their role in interleukin-8 (CXCL-8)-mediated modulation of metastatic phenotype. Clin Exp Metastasis. 2003;20:723–731. doi: 10.1023/b:clin.0000006814.48627.bd. [DOI] [PubMed] [Google Scholar]
- 187.Varney ML, Johansson SL, Singh RK. Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol. 2006;125:209–216. doi: 10.1309/VPL5-R3JR-7F1D-6V03. [DOI] [PubMed] [Google Scholar]
- 188.Kawada K, Sonoshita M, Sakashita H, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 189.Scala S, Giuliano P, Ascierto PA, et al. Human melanoma metastases express functional CXCR4. Clin Cancer Res. 2006;12:2427–2433. doi: 10.1158/1078-0432.CCR-05-1940. [DOI] [PubMed] [Google Scholar]
- 190.Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. J Dermatol Sci. 2004;36:71–78. doi: 10.1016/j.jdermsci.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 191.Meijer J, Zeelenberg IS, Sipos B, Roos E. The CXCR5 chemokine receptor is expressed by carcinoma cells and promotes growth of colon carcinoma in the liver. Cancer Res. 2006;66:9576–9582. doi: 10.1158/0008-5472.CAN-06-1507. [DOI] [PubMed] [Google Scholar]
- 192.Dellacasagrande J, Schreurs OJ, Hofgaard PO, et al. Liver metastasis of cancer facilitated by chemokine receptor CCR6. Scand J Immunol. 2003;57:534–544. doi: 10.1046/j.1365-3083.2003.01263.x. [DOI] [PubMed] [Google Scholar]
- 193.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–3304. [PubMed] [Google Scholar]
- 194.Bendre MS, Gaddy-Kurten D, Mon-Foote T, et al. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res. 2002;62:5571–5579. [PubMed] [Google Scholar]
- 195.Andre F, Cabioglu N, Assi H, et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol. 2006;17:945–951. doi: 10.1093/annonc/mdl053. [DOI] [PubMed] [Google Scholar]
- 196.Perissinotto E, Cavalloni G, Leone F, et al. Involvement of chemokine receptor 4/stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin Cancer Res. 2005;11:490–497. [PubMed] [Google Scholar]
- 197.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 198.Liu YL, Yu JM, Song XR, Wang XW, Xing LG, Gao BB. Regulation of the chemokine receptor CXCR4 and metastasis by hypoxia-inducible factor in non small cell lung cancer cell lines. Cancer Biol Ther. 2006;5:1320–1326. doi: 10.4161/cbt.5.10.3162. [DOI] [PubMed] [Google Scholar]
- 199.Takanami I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer, correlation with lymph node metastasis. Int J Cancer. 2003;105:186–189. doi: 10.1002/ijc.11063. [DOI] [PubMed] [Google Scholar]
- 200.Hu J, Deng X, Bian X, et al. The expression of functional chemokine receptor CXCR4 is associated with the metastatic potential of human nasopharyngeal carcinoma. Clin Cancer Res. 2005;11:4658–4665. doi: 10.1158/1078-0432.CCR-04-1798. [DOI] [PubMed] [Google Scholar]
- 201.Murphy C, McGurk M, Pettigrew J, et al. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res. 2005;11:4117–4127. doi: 10.1158/1078-0432.CCR-04-1518. [DOI] [PubMed] [Google Scholar]
- 202.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 203.Sun YX, Schneider A, Jung Y, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 204.Lu Y, Cai Z, Galson DL, et al. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 205.Loberg RD, Day LL, Harwood J, et al. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kim SJ, Uehara H, Karashima T, McCarty M, Shih N, Fidler IJ. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001;3:33–42. doi: 10.1038/sj.neo.7900124. [DOI] [PMC free article] [PubMed] [Google Scholar]