Abstract
When caterpillars feed on maize (Zea maize L.) lines with native resistance to several Lepidopteran pests, a defensive cysteine protease, Mir1-CP, rapidly accumulates at the wound site. Mir1-CP has been shown to inhibit caterpillar growth in vivo by attacking and permeabilizing the insect's peritrophic matrix (PM), a structure that surrounds the food bolus, assists in digestion and protects the midgut from microbes and toxins. PM permeabilization weakens the caterpillar defenses by facilitating the movement of other insecticidal proteins in the diet to the midgut microvilli and thereby enhancing their toxicity. To directly determine the toxicity of Mir1-CP, the purified recombinant enzyme was directly tested against four economically significant Lepidopteran pests in bioassays. Mir1-CP LC50 values were 1.8, 3.6, 0.6, and 8.0 ppm for corn earworm, tobacco budworm, fall armyworm and southwestern corn borer, respectively. These values were the same order of magnitude as those determined for the Bacillus thuringiensis toxin Bt-CryIIA. In addition to being directly toxic to the larvae, 60 ppb Mir1-CP synergized sublethal concentrations of Bt-CryIIA in all four species. Permeabilization of the PM by Mir1-CP probably provides ready access to Bt-binding sites on the midgut microvilli and increases its activity. Consequently, Mir1-CP could be used for controlling caterpillar pests in maize using non-transgenic approaches and potentially could be used in other crops either singly or in combination with Bt-toxins.
Introduction
Since the beginning of agriculture, crops have been beleaguered by insect pests. Even now, insect herbivory is responsible for 10 to 20% of major crop losses worldwide [1], [2]. Traditionally, chemical pesticides have been used to control insect damage and at one time it was estimated that approximately 40% of chemical pesticide expenditures have been for controlling Lepidopteran pests alone [3]. Although the use of “hard” chemical insecticides has provided some control, their toxicity to non-target animals and humans poses a serious negative effect on the environment [1], [2], [4], [5]. In the mid-1990s, farmers planted the first transgenic crops expressing the Bt-gene (Bt) that encodes the Δ-endotoxin of Bacillus thuringiensis to control Lepidopteran pests [2], [4]–[6]. By 2006, approximately 30 million hectares of crops expressing Bt-gene were planted globally [7]. Genetically modified, insect-resistant crops, especially cotton and maize, have been a boon to farmers in both the developed and developing world. Because the toxicity of the Δ-endotoxins (Cry-toxins) is generally specific for certain insects, transgenic crops expressing the Cry-toxins have proved to be an “environmentally friendly” method of pest control.
While Bt-crops have been widely accepted by the agricultural community, there are nagging concerns about the durability of the technology [8]–[11]. One concern about the widespread use of transgenic crops expressing a single Bt gene is that the target insect populations will develop resistance to the toxin thus reducing its long-term effectiveness [8]–[11]. It has been demonstrated that field populations of the diamondback moth (Plutella xylostella) have developed resistance to Bt-toxin [12], [13], but resistance against transgenic Bt-toxin expressing plants among other Lepidopteran larvae has yet to be seen in crops [10]. However, recent studies indicate that there are differences in sensitivity to Bt-CryIAc and Bt-CryIIAb2 among field and laboratory colonies of corn earworm and tobacco budworm [14], [15] and European corn borer [16]. It has been suggested that the stacking or pyramiding of genes with different modes of action can significantly increase the number of generations needed for the target insects to become resistant [8], [17], [18], [19].
A series of steps are required for the Bt-toxins to exert their effect on Lepidopteran larvae. First, larvae must ingest the Bt-protoxin, which is converted to its active form by proteases in the alkaline midgut environment [20], [21]. The toxin then must pass through the peritrophic matrix (PM) and bind to specific midgut receptors on the brush border membrane with very high affinity [22], [23]. This is followed by irreversible insertion into the membrane [24], which then forms lytic pores resulting in cell lysis, cessation of feeding and larval death [25]. Experiments using laboratory populations of Lepidoptera suggests that there are three potential resistance mechanisms [26]. The most predominant is the alteration of Bt-toxin binding site on the midgut receptors, which reduces the toxin's ability to bind to the cells [27]. This may be due to decreased binding affinity or a reduction in the number of binding sites. Other possible resistance mechanisms include alterations in the proteolytic processing of the Bt-protoxin and rapid regeneration of the damaged midgut epithelium [27]. To counteract the development of resistance in Lepidopteran field populations, it would be beneficial to have additional resistance technologies with varying modes of action available.
Maize lines with inherent resistance to armyworm (Spodoptera frugiperda) and other Lepidopteran larvae have been developed from exotic germplasm from Antiqua using conventional plant breeding [28], [29]. Within one hour, these lines accumulate maize insect resistance cysteine protease (Mir1-CP) in the whorl in response to Lepidopteran larval feeding [30]. This rapid accumulation occurs because Mir1-CP is located in the maize vascular tissues, which allows it to be rapidly mobilized in response to herbivory [31]. When fall armyworm larvae were fed on transgenic maize callus tissue ectopically producing Mir1-CP, their growth was inhibited approximately 70% [30]. Subsequent scanning electron microscopy (SEM) studies indicated that the peritrophic matrix (PM), of larvae feeding on the transgenic callus was severely damaged and contained numerous holes and fissures [32]. The PM is an extracellular matrix of chitin, glycoproteins and proteoglycans that surrounds the food bolus and protects the midgut epithelium from damage and assists in nutrient uptake and cycling [33]. In vitro studies using purified recombinant Mir1-CP indicated that it completely permeabilizes the PM by digesting PM proteins [34]. These studies also indicated that Mir1-CP was most effective on Lepidopteran larvae belonging to the Noctuidae family, the largest and most economically important familiy of Lepidopterans [34].
Mir1-CP is a papain-like cysteine protease that interestingly has amino acid sequence similarity to cysteine proteases from several baculoviruses that infect Lepidopteran larvae such as A. californica and Spodoptera exigua nucleopolyhedrovirus [35], [36]. Another baculovirus metalloprotease, enhancin, attacks the PM of Trichoplusia ni and specifically degrades the integral PM protein, Insect Intestinal Mucin (IIM) [37], [38]. However, Mir1-CP is the only plant defensive cysteine protease that has been shown to directly damage the Lepidopteran PM. Because Mir1-CP acts catalytically, it should have much greater efficacy against larvae than other plant defense proteins such as protease inhibitors and lectins [39].
The idea of stacking other resistance genes with Bt is not new, but to the best of our knowledge there have been no reports of incorporating Bt resistance with naturally occurring plant defense proteins. Mir1-CP and Bt-toxins have different, but potentially complementary toxicity mechanisms. Mir1-CP functions by permeabilizing the PM, whereas Bt-toxins bind the intestinal microvilli. Therefore, this study was undertaken to directly measure the toxicity of Mir1-CP on several different types Lepidopteran larvae and to determine if it acts synergistically with the Bt-toxin CryIIA in bioassays. Mir1-CP and Bt-CryIIA were tested on corn earworm (Helicoverpa zea, Noctuidae) and tobacco budworm (Heliothis virescens, Noctuidae), fall armyworm (Noctuidae) and southwestern corn borer (Diatraea grandiosella, Crambidae). These insects are economically important pests of many crops that are targets of Bt-producing transgenic plants. The results indicated that Mir1-CP had LC50 values on par with those of Bt-CryIIA and that the remarkably low concentration of 60 ppb could dramatically synergize sublethal concentrations of the Bt-toxin.
Results
LC50 values
Table 1 shows the LC50 values for corn earworm, tobacco budworm, fall armyworm, and southwestern corn borer fed either purified Mir1-CP or the processed form of the Bt-toxin, CryIIA. The noctuids, corn earworm, tobacco budworm and fall armyworm were the most sensitive to Mir1-CP with LC50 values ranging from 0.6 to 3.6 ppm. The crambid, southwestern corn borer was less sensitive with a LC50 of 8.0 ppm. The LC50 values for CryIIA were similar among all of the larvae tested and ranged from 0.9 to 1.5 ppm. These values indicate that purified recombinant Mir1-CP is effective at low concentrations and has LC50 values that are the same order of magnitude as those of CryIIA.
Table 1. Probit analysis of LC50 values for several Lepidopteran species fed Mir1-CP and Bt-CryIIA. LC50 values are in ppm and numbers in parenthesis represent the 95% confidence interval.
| Insects | Mir1-CP | Bt-CryIIA | ||||
| *LC50 | Slope (±SE) | R2 | *LC50 | Slope (±SE) | R2 | |
| Corn earworm | 1.8 (1.2–3.2) | 1.202 (0.14) | 0.98 | 0.9 (0.5–1.7) | 1.084 (0.18) | 0.98 |
| Tobacco budworm | 3.6 (3.0–4.5) | 1.461 (0.08) | 0.93 | 1.2 (0.8–1.7) | 1.022 (0.13) | 0.96 |
| Fall armyworm | 0.6 (0.3–1.1) | 0.722 (0.18) | 0.97 | 1.1 (0.8–1.3) | 0.913 (0.06) | 0.94 |
| Southwestern corn borer | 8.0 (6.9–10.1) | 1.250 (0.40) | 0.96 | 1.5 (1.0–3.5) | 0.965 (0.14) | 0.97 |
Combined effects of Mir1-CP and CryIIA on larval growth and mortality
A series of experiments were done for each Lepidopteran species to determine concentrations of Mir1-CP and CryIIA that slightly inhibited larval growth and resulted in limited mortality. When larvae of the four species were reared on artificial diet alone, they all had similar relative growth rates (RGR) of 0.60±0.06 and mortality ranged from 2 to 8% (Fig. 1 to 4).
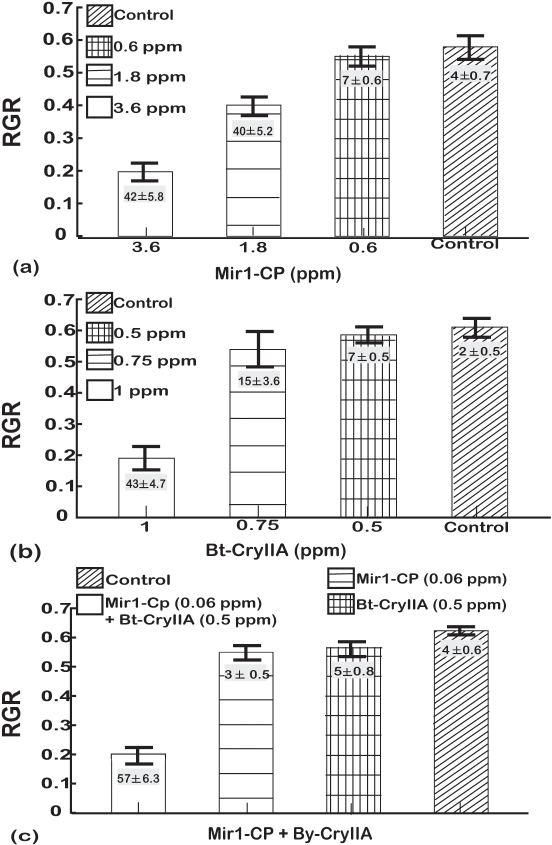
Figure 1. Dose response analysis for corn earworm larvae fed (a) Mir1-CP, (b) Bt-CryIIA, and (c) sub-lethal doses of Bt-CryIIA and Mir1-CP.
The y-axis is the relative growth rate (RGR measured as Δmg/(mgavgd) and vertical bars indicate one standard deviation. The least significant difference (LSD) at the 0.05 confidence level for the RGR values were as follows: Mir1-CP = 0.25, Bt-CryIIA = 0.3, sub-lethal doses of Bt-CryIIA and Mir1-CP = 0.42. Numbers (mean±SD) in the histogram bars are the average percentage mortality for each treatment. The least significant difference (LSD) at the 0.05 confidence level for mortality were as follows: Mir1-CP – 7.4, Bt-CryIIA – 9.5, sub-lethal doses of Bt-CryIIA and Mir1-CP combined – 15.94. Results are based on four independent bioassays. Concentrations are given as parts per million (ppm).
Figure 2. Dose response analysis for tobacco budworm larvae fed (a) Mir1-CP, (b) Bt-CryIIA, and (c) sub-lethal doses of Bt-CryIIA and Mir1-CP.
The y-axis is the relative growth rate (RGR measured as Δmg/(mgavgd) and vertical bars indicate one standard deviation. The least significant difference (LSD) at the 0.05 confidence level for the RGR values were as follows: Mir1-CP – 0.31, Bt-CryIIA – 0.44, sub-lethal doses of Bt-CryIIA and Mir1-CP combined – 0.57. Numbers (mean±SD) in the histogram bars are the average percentage mortality for each treatment. The least significant difference (LSD) at the 0.05 confidence level for mortality were as follows: Mir1-CP – 8.5, Bt-CryIIA – 7.4, sub-lethal doses of Bt-CryIIA and Mir1-CP combined – 15.83. Results are based on four independent bioassays. Concentrations are given as parts per million (ppm).
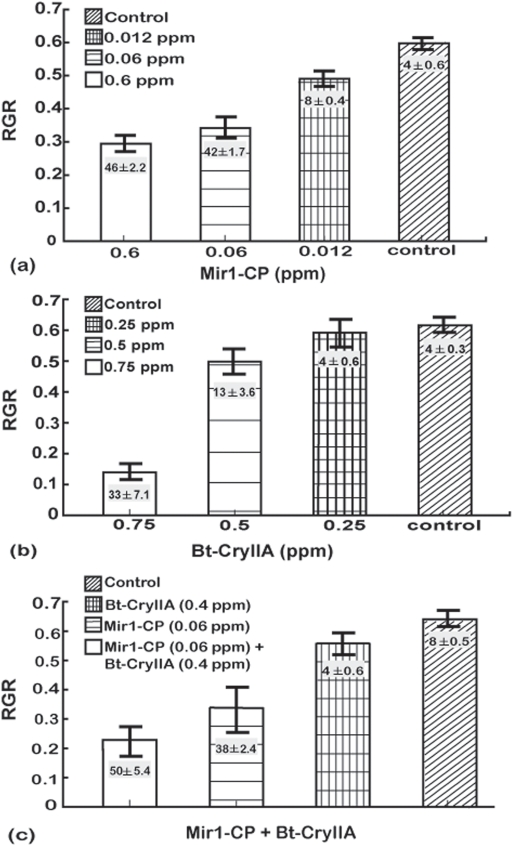
Figure 3. Dose response analysis for fall armyworm larvae fed (a) Mir1-CP, (b) Bt-CryIIA, and (c) sub-lethal doses of Bt-CryIIA and Mir1-CP.
The y-axis is the relative growth rate (RGR measured as Δmg/(mgavgd) and vertical bars indicate one standard deviation. The least significant difference (LSD) at the 0.05 confidence level for the RGR values were as follows: Mir1-CP – 0.14, Bt-CryIIA – 0.37, sub-lethal doses of Bt-CryIIA and Mir1-CP, combined – 0.46. Numbers (mean±SD) in the histogram bars are the average percentage mortality for each treatment. The least significant difference (LSD) at the 0.05 confidence level for mortality were as follows: Mir1-CP – 6.71, Bt-CryIIA – 8.42, sub-lethal doses of Bt-CryIIA and Mir1-CP, combined – 10.01. Results are based on four independent bioassays. Concentrations are given as parts per million (ppm).
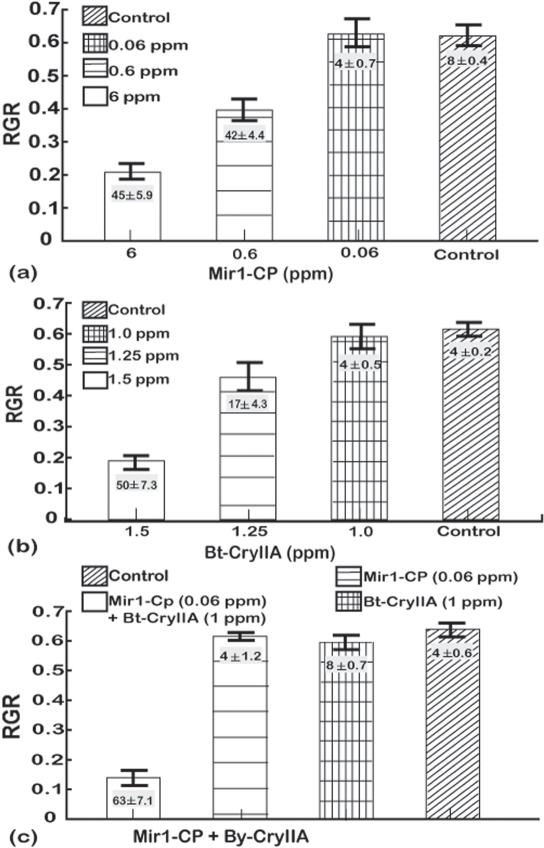
Figure 4. Dose response analysis for southwestern corn borer larvae fed (a) Mir1-CP, (b) Bt-CryIIA, and (c) sub-lethal doses of Bt-CryIIA and Mir1-CP.
The y-axis is the relative growth rate (RGR measured as Δmg/(mgavgd) and vertical bars indicate one standard deviation. The least significant difference (LSD) at the 0.05 confidence level for the RGR values were as follows: Mir1-CP – 0.15, Bt-CryIIA – 0.23, sub-lethal doses of Bt-CryIIA and Mir1-CP combinded – 0.38. Numbers (mean±SD) in the histogram bars are the average percentage mortality for each treatment. The least significant difference (LSD) at the 0.05 confidence level for mortality were as follows: Mir1-CP – 8.97, Bt-CryIIA – 9.24, sub-lethal doses of Bt-CryIIA and Mir1-CP combined – 16.88. Results are based on four independent bioassays. Concentrations are given as parts per million (ppm).
For corn earworm, 1.80 ppm of Mir1-CP reduced the RGR to 0.18±0.03 and resulted in 39±5.4% mortality (Fig. 1a). Bt-CryIIA (0.75 ppm) reduced the RGR to 0.19±0.02 and the mortality was 47±4.9% (Fig. 1b). Doses of either 0.60 ppm Mir1-CP or 0.25 ppm Bt-CryIIA did not significantly decrease the RGR or percentage mortality relative to the control (Fig. 1a, b). When corn earworm larvae were fed a combination of Mir1-CP and Bt-CryIIA at concentrations (0.06 ppm and 0.5 ppm, respectively) that had little or no effect on the larvae when used singly, the RGR dramatically dropped to 0.20±0.01, and the percentage mortality increased to 61±5.9% (Fig. 1c, Table 2).
Table 2. Relative growth rate (RGR) and percentage mortality of Lepidopteran larvae fed on Mir1-CP, Bt-CryIIA or Mir1-CP and Bt-CryIIA in combination.
| Mir1-CP (ppm) | (±SE) | Bt-CryIIA (ppm) | (±SE) | Combination | (±SE) | P value | |
| Corn earworm | |||||||
| Dose | 0.06 | 0.5 | 0.06+0.5 | ||||
| Mortality (%) | 8 | 0.60 | 4 | 0.60 | 61 | 5.9 | 0.0315 |
| RGR | 0.574 | 0.04 | 0.514 | 0.02 | 0.197 | 0.03 | 0.0370 |
| Tobacco budworm | |||||||
| Dose | 0.06 | 0.5 | 0.06+0.5 | ||||
| Mortality (%) | 3 | 0.50 | 5 | 0.80 | 57 | 6.3 | 0.0034 |
| RGR | 0.562 | 0.03 | 0.574 | 0.02 | 0.201 | 0.02 | 0.0390 |
| Fall armyworm | |||||||
| Dose | 0.06 | 0.4 | 0.06+0.4 | ||||
| Mortality (%) | 38 | 2.4 | 4 | 0.60 | 50 | 5.4 | 0.0123 |
| RGR | 0.333 | 0.08 | 0.568 | 0.05 | 0.22 | 0.03 | 0.0330 |
| Southwestern corn borer | |||||||
| Dose | 0.06 | 1 | 0.06+1 | ||||
| Mortality (%) | 4 | 1.2 | 8 | 0.70 | 63 | 7.1 | 0.0392 |
| RGR | 0.609 | 0.01 | 0.596 | 0.02 | 0.132 | 0.02 | 0.0420 |
RGR = Δmg/(mgavgd); SE = standard error. P values indicate that % mortality and RGR values significantly differ from additivity and show synergy at the 95% confidence level.
Tobacco budworm larvae required 3.6 ppm of Mir1-CP to decrease the RGR to 0.21±0.02 (Fig. 2a) and increase the mortality to 42±5.8%. One ppm of Bt-CryIIA reduced the RGR to 0.20±0.02 (Fig. 2b) and increased the mortality to 43±4.7%. The lower doses of 0.6 ppm Mir1-CP and 0.5 ppm of Bt-CryIIA did not significantly decrease the RGR or percentage mortality (Fig. 2a, b) relative to the control. However, when 0.06 ppm Mir1-CP and 0.5 ppm of Bt-CryIIA were combined, the RGR was far lower (0.19±0.03) and the mortality increased to 57±6.3% (Fig. 2c, Table 2).
The fall armyworm RGR decreased to 0.31±0.04 at 0.60 ppm Mir1-CP and was 0.18±0.03 with 0.75 ppm Bt-CryIIA (Fig. 3a, b). Mortality was 46±2.2% and 33±7.1% at the highest concentrations of Mir1-CP and Bt-CryIIA tested, respectively. When fall armyworm larvae were treated with 0.06 ppm of Mir1-CP, the RGR was 0.33±0.04 and mortality was 42±1.7% (Fig. 1a). Larvae reared on 0.25 ppm Bt-CryIIA had a RGR of 0.64±0.04, which was not significantly different from the control and mortality was the same as the control (4%) (Fig. 3b). At a concentration of 0.5 ppm Bt-CryIIA, the RGR decreased to 0.50±0.06 and mortality was 13±3.6% (Fig. 1b). Therefore, the concentrations of 0.06 ppm Mir1-CP and 0.4 ppm Bt-CryIIA were selected to test the effect of the components in combination (Fig. 3c). When these two doses of Mir1-CP and Bt-CryIIA were combined, the RGR value was reduced to 0.22±0.05 (Fig. 1c) and the percentage mortality increased to 50±5.4%, which was somewhat higher than those of the individual treatments (Table 2).
The southwestern corn borer was more recalcitrant to both Mir1-CP and Bt-CryIIA. Six ppm of Mir1-CP were required to reduce the RGR to 0.21±0.02 and increase the mortality to 45±5.9% (Fig. 4a). A concentration of 1.5 ppm Bt-CryIIA was needed to reduce the RGR to 0.19±0.02 and increase the mortality to 50±7.3% (Fig. 4b). When used singly, the concentrations 0.06 ppm of Mir1-CP and 1 ppm Bt-CryIIA did not significantly change the RGR or mortality (4% for each Fig. 4c) relative to the control. In combination, however, the effect was much greater as the RGR was reduced to 0.13±0.03 and the mortality increased to 63±7.1% (Fig. 4c, Table 2).
The data for all four lepidopteran species indicates that 0.06 ppm of Mir1-CP can synergize sublethal concentrations of Bt-CryIIA resulting in significantly lower growth rates and higher mortality.
We suggest there are two possible ways that Mir1-CP could synergize Bt-endotoxin. First, Mir1-CP, which permeabilizes the PM, likely facilitates Bt-CryIIA movement though the PM allowing greater access to the epithelial cells. Alternatively, Mir1-CP might process the Bt-CryIIA protoxin to the toxic form in the bioassay or the midgut. Because the processed form of the toxin was used in the bioassays, the second possibility is unlikely. Nevertheless, the ability of Mir1-CP to process the Bt-CryIIA protoxin to its mature form was tested by incubating the protoxin with Mir1-CP. SDS-PAGE analysis indicated that Mir1-CP partially cleaved the protoxin after 8 hours of incubation (Fig. 5, lane 3) and completely cleaved it after 24 hours (Fig. 5, lane 4). However, it did not affect the mobility of the processed form that was used in the bioassays (Fig. 5, lane 5). The low molecular weight band (Fig. 5, lanes 3, 4, and 5) is Mir1-CP. If Mir1-CP is capable of processing the protoxin in vivo, it might enhance its synergistic effects.
Figure 5. SDS-PAGE analysis of Bt-CryIIA protoxin and its processed form incubated with 0.06 ppm of Mir1-CP.

Lane M – markers; lane 1 -processed Bt-CryIIA; lane 2 – unprocessed Bt-CryIIA protoxin; lane 3 - unprocessed Bt-CryIIA protoxin incubated with Mir1-CP for 8 hr; lane 4 – unprocessed Bt-CryIIA protoxin incubated with Mir1-CP for 24 hr; lane 5 – processed Bt-CryIIA toxin incubated with Mir1-CP for 8 hr. Numbers on the left margin refer to the molecular mass of the marker bands in kD. Arrow A indicates the position of the protoxin, arrow B indicates the position of the processed toxin and arrow C marks the position of Mir1-CP (molecular mass of 33 kD) added to the incubation.
Discussion
Our study demonstrates that Mir1-CP, a natural defense cysteine protease found in maize [29], [30], reduces the growth of Lepidopteran larvae when directly added to artificial diet and synergizes the effects of the Bt-toxin CryIIA. LC50 values for Mir1-CP ranged from 0.9 to 8.0 ppm and were in the same range as those of Bt-CryIIA. In fact, Mir-CP appears to be considerably more toxic than other plant-derived defense proteins. For example, protease inhibitors do not act catalytically and their effective concentrations range from 1 to 5% of the total protein in the diet [39]. There was a 10-fold range in sensitivity to Mir1-CP among the Lepidopteran species tested and Southwestern corn borer was the least sensitive to the protease. The LC50 values correlate well with in vitro permeability studies, which also indicated that PM in the Noctuidae were more sensitive to Mir1-CP degradation than those of the Crambidae [34]. Preliminary evidence suggests that that the Southwestern corn borer PM may contain one or more proteins that are resistant to Mir1-CP attack (Luthe and Mohan, unpublished data).
Both scanning electron microscopy (SEM) and in vitro permeability experiments indicated that the PM is the target of Mir1-CP [32], [34]. SEM indicated that there were many cracks and fissures in the PM of fall armyworm larvae that fed on plant material containing Mir1-CP [32]. The in vitro studies indicated that the PM could be completely permeabilized by Mir1-CP in a concentration dependent manner [34]. Therefore, it appears that Mir1-CP exerts its effect on these Lepidopterans by making holes in the PM. Damage to the PM results in the loss of the midgut protective barrier, disrupts nutrient cycling in the midgut and slows larval growth [33]. The Bt-endotoxins have a different toxicity mechanism than Mir1-CP and act by binding to receptors in the midgut cells and forming lytic pores [24], [40]. Because the processed form of BT-CryIIA was used in this study, Mir1-CP probably synergized the activity of Bt-CryIIA, by increasing PM permeability and facilitating toxin movement to the microvilli. Consequently, it is likely that Mir1-CP will also synergize other Bt-toxins including CryIAb and CryIAc, which are typically used in transgenic crops. Our data also indicate that Mir1-CP processes the protoxin to its mature form in vitro. If Mir1-CP were deployed in transgenic plants expressing the protoxin, it might have dual functions: processing the protoxin and perforating the PM.
This study shows that Mir1-CP is effective against Lepidopteran pests and may be one of the most toxic endogenous plant defense proteins known and that only 60 ppb of Mir1-CP are required to synergize the effects of Bt-CryIIA concentrations ranging from 0.40 to 1.0 ppm. The synergism between Mir1-CP and Bt-CryIIA suggests that stacking these two genes in transgenic plants could enhance the effectiveness of Bt-toxin, potentially reduce the development of insect resistance to this class of toxins, and ultimately result in more sustainable control of Lepidopteran pests.
Experimental protocol
Chemicals and reagents
All chemicals and reagents were purchased from Fisher Biotech (Fairlawn, NJ). Purified 33-KDa Mir1-CP was prepared as previously described by Mohan et al. [31]. Processed 68-KDa Bt-CryIIA toxin and its unprocessed 143-KDa form were obtained from Monsanto Plant Science/Regulatory Sciences (St. Louis, MO).
The two Bt-CryIIA proteins were dissolved in 50 mM potassium phosphate, 50 mM sodium chloride, 1 mM EDTA, with at final pH of 10.8,
Insect rearing
Larvae of fall armyworm (Spodoptera frugiperda), southwestern corn borer (Diatraea grandiosella), corn earworm (Helicoverpa zea) and tobacco budworm (Heliothis virescens) were obtained from laboratory colonies maintained by the USDA-ARS Corn Host Plant Resistance Research Unit at Mississippi State University. They were reared on artificial diet [31] under a photoperiod of 16∶8 at 26.6°C.
Insect feeding bioassays
Dose response bioassays were conducted using second instar larvae of each of the Lepidopteran species. Artificial diet (approximately 250 µl) was placed in 96 well plates and the surface was covered with 50 µl solutions containing dilutions of either recombinant Mir1-CP, processed Bt-CryIIA or a combination of both. Untreated wells served as controls. The plates were air dried for 3–4 h and covered with Saran Wrap®. Prior to the bioassay, larvae were weighed and placed in individual wells and the plates were covered with Breath Easy™ (USA Scientific Inc, FL, USA). Each bioassay treatment involved 24 larvae and was performed with three replicates. Larvae were incubated at 30°C/48 h in 16∶8 photoperiod before final larval weight was measured. Previous experiments indicated that purified Mir1-CP lost only 20% of its activity after incubation at 30°C for 7 days (Pechan and Luthe, unpublished data). Consequently, Mir1-CP should retain a major portion of activity during the 48 hr bioassay. Each bioassay treatment condition was repeated independently three to five times. The larval relative growth rate (RGR) [41] was calculated using the formula RGR = ((final larval weight)-(initial larval weight))/((average larval weight) x time) and is reported as Δmg/(mgavg d).
Experiments were conducted using a randomized complete block design with a factorial structure of Mir1-CP and Bt-CryIIA and statistical analyses were done using SAS® software (Cary, NC). Statistically significant departures from additivity were used to determine if there was a synergistic interaction between Mir1-CP and Bt-CryIIA for both RGR and the percentage mortality.
Toxicity analysis (LC50)
Dose response analysis was conducted to determine the LC50 for each of the Lepidopteran species. At least 24 larvae of each Lepidopteran species were tested against each concentration of either Mir1-CP or Bt-CryIIA toxin. Larvae were incubated at 30°C/48 h in 16∶8 photoperiod. Mortality was recorded after 24 h and each experimental condition was replicated at least four times. The LC50 was calculated using PROC PROBIT (SAS Institute 1998) [42]. Variability was examined using a one-way analysis of variance (ANOVA) (PROC ANOVA, SAS Institute 1998) [42]. Confidence intervals (CIs) were calculated using the Robertson and Preisler (1992) [43] methods as explained by Ali et al., (2006) [15]. The Mir1-CP concentrations tested, regression equation and coefficient (R2) for each species are given in parentheses following the insect name: corn earworm (1, 2, 3, 4, ppm; y = 2.4269x - 7.9283; R2 = 0.9869), tobacco budworm (1.2, 2.4, 3.6, 4.8 ppm; y = 1.3305x - 4.7327; R2 = 0.9349), fall armyworm (0.3, 0.6, 0.9, 1.2 ppm; y = 2.2104x - 6.1873; R2 = 0.9796), southwestern corn borer (3, 6, 9, 12 ppm; y = 0.4890x - 1.7891; R2 = 0.9605). The concentrations Bt-CryIIA concentrations tested, regression equation and coefficient (R2) for each species are given in parentheses following the insect name: corn earworm (0.75, 1.5, 2.1 ppm; y = 2.666 x - 7.8445; R2 = 0.9849), tobacco budworm (0.75, 1.5, 2.1 ppm; y = 3.1959x – 9.7953; R2 = 0.9658), fall armyworm (0.5, 1.0, 1.5 ppm; y = 2.2021x – 6.6875; R2 = 0.9435), southwestern corn borer (1.0, 2.0, 3.0 ppm; 7.8146x -24.847; R2 = 0.9759).
Processing of Bt-CryIIA protoxin
To determine if MIr1-CP could cleave the Bt-CryIIA protoxin, 12 µg of either the protoxin or processed Bt-CryII were incubated for 8 and 24 hr with 3 µg of Mir1-CP in a total volume of 15 µl water at room temperature. The sample was diluted (1∶1) with sample buffer [42] and boiled for 5 min. Samples containing 7.5 µg of protein were then analyzed by SDS-PAGE [44]. The gel was stained with colloidal Coomassie Blue as described by Neuhoff et al. [45]. Multimark™ Multi-colored protein ladder (Novex, CA) was used to determine the molecular weight of proteins in SDS-PAGE.
Acknowledgments
We thank Monsanto Plant Science (St. Louis, MO) for the Bt-CryIIA and Drs. Howard Chambers and Patrick Gerrard for assistance with probit analysis for LC50 and statistic analysis, respectively.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was funded by National Science Foundation Awards IBN-0131328 and IOS-0641219.
References
- 1.Ferry N, Edwards MG, Gatehouse JA, Gatehouse AM. Plant-insect interactions: molecular approaches to insect resistance. Curr Opin Biotechnol. 2004;15:155–161. doi: 10.1016/j.copbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Ferry N, Edwards MG, Gatehouse J, Capell T, Christou P, Gatehouse AM. Transgenic plants for insect pest control: a forward looking scientific perspective. Transgenic Res. 2006;15:13–19. doi: 10.1007/s11248-005-4803-x. [DOI] [PubMed] [Google Scholar]
- 3.Boulter D. Insect pest control by copying nature using genetically engineered crops. Phytochemistry Phytochemistry. 1993;34:1453–1466. doi: 10.1016/s0031-9422(00)90828-8. [DOI] [PubMed] [Google Scholar]
- 4.Huesing J, English L. The impact of Bt crops on the developing world. AgBioForum. 2004;7:84–95. [Google Scholar]
- 5.Christou P, Capell T, Kohli A, Gatehouse JA, Gatehouse AM. Recent developments and future prospects in insect pest control in transgenic crops. Trends Plant Sci. 2006;11:302–308. doi: 10.1016/j.tplants.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Brookes GA. GM crops: the global economic and environmental impact – the first 9 years 1996–2004. AgBioForum. 2005;8:187–196. [Google Scholar]
- 7.James C. Global status of commercialized Biotech/GM Crops (International Service for the Acquisition of Agri-Biotech Applications, Ithaca NY). 2006 [Google Scholar]
- 8.Gould F. Bt-resistance management–theory meets data. Nat. Biotechnol. 2003;21:1450–1451. doi: 10.1038/nbt1203-1450. [DOI] [PubMed] [Google Scholar]
- 9.Tabashnik BE, Gould F, Carriere Y. Delaying evolution of insect resistance to transgenic crops by decreasing dominance and heritability. J Evol Biol. 2004;17:904–912. doi: 10.1111/j.1420-9101.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- 10.Tabashnik BE, Carriere Y, Dennehy TJ, Morin S, Sisterson MS, et al. Insect resistance to transgenic Bt crops: lessons from the laboratory and field. J Econ Entomol. 2003;96:1031–1038. doi: 10.1603/0022-0493-96.4.1031. [DOI] [PubMed] [Google Scholar]
- 11.Heckel DG, Gahan LJ, Baxter SW, Zhao JZ, Shelton AM, et al. The diversity of Bt resistance genes in species of Lepidoptera. J Invertebr Pathol. 2007;95:192–197. doi: 10.1016/j.jip.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Hama H, Suzuki K, Tanaka H. Inheritance and stability of resistance to Bacillus thuringiensis formulations in the diamondback moth Plutella xylostella (Linnaeus) (Lepidoptera: Yponomeuidae). J Appl Entomol Zoo. 1992;27:355–362. [Google Scholar]
- 13.Tabashnik BE, Cushing NL, Finson N, Johnson MW. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol. 1990;83:1671–1676. [Google Scholar]
- 14.Ali MI, Luttrell RG, Young SY. Susceptibilities of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) populations to Cry1Ac insecticidal protein. J Econ Entomol. 2006;99:164–175. doi: 10.1093/jee/99.1.164. [DOI] [PubMed] [Google Scholar]
- 15.Ali MI, Luttrell RG. Susceptibility of bollworm and tobacco budworm (Lepidoptera: Noctuidae) to Cry2Ab2 insecticidal protein. J Econ Entomol. 2007;100:921–931. doi: 10.1603/0022-0493(2007)100[921:sobatb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Chaufaux J, Seguin M, Sawson JJ, Bourguet D, Siegfried BD. Chronic exposure of the European corn borer (Lepidoptera: Crambidae) to Cry1Ab Bacillus thuringiensis toxin. J Econ Entomol. 2001;94:1564–1569. doi: 10.1603/0022-0493-94.6.1564. [DOI] [PubMed] [Google Scholar]
- 17.Roush RT. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Phil Trans Roy Soc London B. 1998;353:1777–1786. [Google Scholar]
- 18.Zhao JZ, Cao J, Li Y, Collins HL, Roush RT, Earle ED, et al. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat Biotechnol. 2003;21:1493–1497. doi: 10.1038/nbt907. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Hau G, Jurat-Fuentes JL, Abdullah MA, Adang MJ. Synergism of Bacillus thuringiensis toxins by a fragment of a toxin-binding cadherin. Proc Natl. Acad Sci (USA) 2007;104:13901–13906. doi: 10.1073/pnas.0706011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecadet MM, Martouret D. Enzymatic hydrolysis of the crystals of Bacillus thuringiensis by the proteases of Pieris brassicae, I. Toxicity of the different fractions of the hydrolysate for larvae of Pieris brassicae. J Inv Pathol. 1967;9:310–321. [Google Scholar]
- 21.Tojo A, Aizawa K. Dissolution and degradation of Bacillus thuringiensis delta-endotoxin by gut juice protease of the silkworm Bombyx mori. Appl Env Microbiol. 1983;45:576–580. doi: 10.1128/aem.45.2.576-580.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann C, Luthy P, Hutter R, Pliska V. Binding of the delta-endotoxin from Bacillus thuringiensis to brush-border membrane besicles of the cabbage butterfly (Pieris brassicae). Euro J Biochem. 1988;173:85–91. doi: 10.1111/j.1432-1033.1988.tb13970.x. [DOI] [PubMed] [Google Scholar]
- 23.Van Rie J, McGaughey WH, Johnson DE, Barnett BD, Van Mellaert H. Mechanism of insect resistance to the microbial insecticide, Bacillus thuringiensis. Science. 1990;247:72–74. doi: 10.1126/science.2294593. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins JL, Lee MK, Valaitis AP, Curtiss A, Dean DH. Bivalent sequential binding model of a Bacillus thuringiensis toxin to gypsy moth aminopeptidase N receptor. J Biol Chem. 2000;275:14423–14431. doi: 10.1074/jbc.275.19.14423. [DOI] [PubMed] [Google Scholar]
- 25.Luthy P, Ebersold HR. Bacillus thuringiensis delta-endotoxin: Histopathology and molecular mode of action. in Bacillus thuringiensis delta-endotoxin: Histopathology and molecular mode of action (Allanhe LC, Osmum and Co, Montclair NJ). 1981 [Google Scholar]
- 26.Hofte H, Whiteley HR. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989;53:242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferre J, Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Ann Rev Entomol. 2002;47:501–533. doi: 10.1146/annurev.ento.47.091201.145234. [DOI] [PubMed] [Google Scholar]
- 28.Davis FM, Williams WP, Mihm JA, Barry BD, Overman JL, Wiseman BR, Riley TJ. Resistance to multiple lepidopterous species in tropical derived corn germplasm. Miss Agri Exp St Tech Bull. 1988;157:1–6. [Google Scholar]
- 29.Williams WP, Buckley PM, Hedin PA, Davis FM. Laboratory bioassay for resistance in corn to fall armyworm and southwestern corn borer. J Econ Entomol. 1990;83:1578–1581. [Google Scholar]
- 30.Pechan T, Ye L, Chang Y-M, Mitra A, Lin L, Davis FM, et al. A unique 33-KD cysteine protease accumulates in response to larval feeding in maize genotype resistant to fall armyworm and other Lepidoptera. Plant Cell. 2000;12 doi: 10.1105/tpc.12.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez L, Camas A, Ankala A, Shivaji R, Williams WP, et al. Mir1-CP a novel defense cysteine protease accumulates in maize phloem in response to herbivory. Planta. 2007;226:517–527. doi: 10.1007/s00425-007-0501-7. [DOI] [PubMed] [Google Scholar]
- 32.Pechan T, Cohen A, Williams WP, Luthe DS. Insect Feeding mobilizes a unique plant defense protease that disrupts the peritrophic matrix of caterpillars. Proc Natl Aca Sci USA. 2002;99:13319–13323. doi: 10.1073/pnas.202224899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang P, Granados RR. Molecular structure of the peritrophic membrane (PM): identification of potential PM target sites for insect control. Arch Insect Biochem Physiol. 2001;47:110–118. doi: 10.1002/arch.1041. [DOI] [PubMed] [Google Scholar]
- 34.Mohan S, Ma PWK, Pechan T, Bassford ER, Williams WP, Luthe DS. Degradation of S. frugiperda peritrophic matrix by an inducible maize cysteine protease. J Ins Physiol. 2006;52:21–28. doi: 10.1016/j.jinsphys.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Rawlings ND, Pearl LH, Buttle DJ. The baculovirus Autographa californica nuclear polyhedrosis virus genome induces a papain like sequence. J Biol Chem. 1992;373:1211–1215. doi: 10.1515/bchm3.1992.373.2.1211. [DOI] [PubMed] [Google Scholar]
- 36.Wi FIJ, Van Strien EA, Helcens JG, Broer R, Zuidema D, et al. Sequence and organization of Spodoptera exigua multicapsid nucleopolyhedrosis genome. J Gen Virol. 1999;80:3289–3304. doi: 10.1099/0022-1317-80-12-3289. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Granados RR. An intestinal mucin is the target substrate for a baculovirus enhancin. Proc Natl Aca Sci USA. 1997a;94:6977–6982. doi: 10.1073/pnas.94.13.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P, Granados RR. Molecular cloning and sequencing off a novel invertebrate intestinal mucin. J Biol Chem. 1997b;272:16663–16669. doi: 10.1074/jbc.272.26.16663. [DOI] [PubMed] [Google Scholar]
- 39.Gatehouse AMR, Shi Y, Powell KS, Brough C, Hilder VA, Hamilton WDO, Newell CA, Merryweather A, Boulter D, Gatehouse JA. Approaches to insect resistance using transgenic plants. Phil Trans Roy Soc London B. 1993;342:279–286. [Google Scholar]
- 40.Knowles BH, Ellar DJ. Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensis delta-endotoxins with different insect specificity. Biochim Biophys Acta. 1987;924:509–518. [Google Scholar]
- 41.Hoffmann WA, Poorter H. Avoiding bias in calculations of relative growth rate. Ann Bot. 2002;80:37–42. doi: 10.1093/aob/mcf140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.SAS Institute Inc. 1998 in SAS/STAT User's Guide, Version 8, 4th Edition, (SAS Institute). [Google Scholar]
- 43.Robertson JL, Preisler HK. 1992 in Pesticide Bioassays with Arthropods. (CRC Press Boca Raton,Fl) [Google Scholar]
- 44.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 45.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]