Abstract
We discuss putative mechanisms of membrane protein transport in photoreceptors based on Pde6d and Gucy2e/Gucy2f knockout mice. Knockout of the Pde6d gene encoding PrBP/δ, a prenyl binding protein present in the retina at relatively high levels, was shown to impair transport of G-protein coupled receptor kinase 1 (GRK1) and cone phosphodiesterase α′-subunit (PDE6α′) to the rod and cone outer segments. Other prenylated proteins are minimally affected, suggesting some specificity of interaction. Knockout of the Gucy2e gene encoding guanylate cyclase 1 (GC1) disrupted transport of G-protein coupled receptor kinase 1 (GRK1), cone PDE6α′, cone transducin α- and γ-subunits (cTα and cTγ) to the cone outer segments, while a GC1/GC2 double knockout prevented transport of rod PDE6, but not transducin, GRK1, or rhodopsin, to the rod outer segments. These knockout phenotypes suggest that PrBP/δ functions in extracting prenylated proteins from the endoplasmic reticulum (ER) where they dock after prenylation, and that GC-bearing membranes may co-transport peripheral membrane proteins in vesicles. We conclude that distinct pathways have evolved in rods and cones for transport of integral and peripherally membrane-associated proteins.
Keywords: Phototransduction, Rod and cone photoreceptors, vesicular transport, guanylate cyclase knockout, Pde6d knockout
Introduction
Rod and cone photoreceptors cells receive light and convert it into an electrical signal. To perform this task efficiently, photoreceptors have evolved into highly polarized structures consisting of three distinct areas: the outer segment containing compacted membrane disks housing the phototransduction machinery, the inner segment where biosynthesis occurs, and the synaptic region that transmits excitation by light to a downstream neuron. The inner segment is connected to the outer segment through a fragile cilium, and to the synaptic region by an elongated soma (for review, see http://webvision.med.utah.edu/). In the past, bovine retinas served as a tissue source to isolate biochemically the components comprising the rod phototransduction cascade and to elucidate many of the basic mechanisms (Ridge et al., 2003; Burns & Arshavsky, 2005; Lamb & Pugh, Jr., 2006). In the last 15 years however, an abundance of naturally occurring, transgenic and knockout/knockin mouse models (Hafezi et al., 2000; Chang et al., 2002; Dalke & Graw, 2005) permitted analyses of mechanistic details of rod and cone phototransduction in vivo. The mouse retina is rod-rich, and only about 3% of photoreceptors are cones distributed more or less evenly throughout the retina. Despite this low abundance, cone photoreceptor physiology can be explored readily by manipulation of the mouse genome, by electrophysiology, and sophisticated imaging techniques (Fu & Yau, 2007).
Mouse photoreceptors are postmitotic (withdrawn from the cell cycle) after postnatal day 7. However, the light-sensitive outer segments of rods and cones are renewed roughly every ten days (LaVail, 1976; Young, 1976; Besharse & Hollyfield, 1979). Renewal is achieved by disk assembly/morphogenesis at the proximal end of the outer segment with concomitant disk shedding at the distal end, and phagocytosis of shed disk membrane by the adjacent RPE (Ritter et al., 2004). Daily renewal of ~10% (about 100 disks) of the outer segment membrane requires very active metabolism, i.e., a continuous high rate of biosynthesis to replace OS proteins, and reliable transport and targeting pathways.
Due to their characteristic shape and enormous biosynthetic requirement, photoreceptors are regarded model cells to study protein trafficking. A central question in photoreceptor cell biology concerns post-biosynthesis transport of membrane-associated proteins and the mechanisms of targeting to the outer segments for disk assembly. A rapid and efficient transport system from the ER to the proximal outer segment is essential for cell maintenance and survival. Here, we address the targeting and transport of polypeptides associated with rod and cone phototransduction. Principal components in the rod cascade are: the photoreceptor molecule (rhodopsin), its G-protein (transducin), a cGMP phosphodiesterase (PDE6), and the cation channel/exchanger complex (CNG/NCKX) (Table 1a). Corresponding cone polypeptides are related closely in sequence, structure and function (Table 1b). The regulatory components (GRK1, GC1, GCAP1, and GAP) are shared in rods and cones (Table 1c), but arrestins have rod- and cone-specific versions. Visual pigments, GRKs, and arrestins are single subunit proteins (although they may form homodimers or multimers), whereas transducin, PDE6 and cation channels are hetero-multimeric complexes. These polypeptides are either integral membrane proteins (visual pigments, GCs, channel/exchanger complex), peripherally membrane-associated (T, PDE6 subunits) or associated with integral membrane proteins (arrestin/rhodopsin., GCAPs/GCs). Membrane-associated proteins are considered nondiffusable (exception: arrestins and possibly transducins, under certain conditions of light-induced translocation (Nair et al., 2005; Strissel et al., 2006; Lobanova et al., 2007; Rosenzweig et al., 2007).
Table 1. Membrane-associated phototransduction components. A, rod; B, cone; and C, shared polypeptides.
Column one, photoreceptor polypeptides; column 2, protein symbols; column 3, activity and/or function of polypeptides; column 4, gene symbols. TM, transmembrane protein; PM, peripheral membrane protein, CP, cytosolic protein. GEF, guanine nucleotide exchange factor.
| A. Rod Component | Symbol | Activity/function | Gene symbol |
|---|---|---|---|
| Rhodopsin (TM) | Rho | Light reception; GEF | RHO |
| Transducin (PM) | Tα
Tβ Tγ |
Activator of rod PDE
Binds phosducin |
GNAT1
GNB1 GNGT1 |
| Phosphodiesterase (PDE6) (PM) | PDE6α
PDE6β PDE6γ |
cGMP hydrolysis
cGMP hydrolysis PDE inhibitor |
PDE6A
PDE6B PDEG |
| Cation channel (TM) | CNGA1
CNGB1 |
cGMP-gated cation channel | CNGA1
CNGB1 |
| Exchanger (TM) | NCKX1 | Cation Exchanger (4 Na+ in; 1 Ca2+/1K+ out); light-insensitive | SLC24A1 |
| Guanylate cyclase (TM) 2 | GC2 | Produces cGMP from GTP | GUCY2F |
| Arrestin (CP) | Arr | Binds to phosphorylated Rho | SAG |
| GC-activating protein 2 (CP) | GCAP2 | GC activator at low Ca2+ | GUCA1B |
| B. Cone Component | Symbol | Activity/function | Gene symbol/ |
| Blue Pigment (TM) | S-opsin | Light reception; GEF | OPN1SW |
| Green Pigment (TM) | M-opsin | Light reception; GEF | OPN1MW |
| Red Pigment (TM) | L-opsin | Light reception; GEF; (not in mouse) | OPN1LW |
| Cone Transducin (PM) | cTα
Gβ3 cTγ |
Activator of cone PDE | GNAT2
GNB3 GNGT2 |
| Cone Phosphodiesterase (PM) | PDE6α′
PDE6γ′ |
cGMP hydrolysis
Inhibitor |
PDE6C
PDE6H |
| Cation channel (TM) | CNGA3
CNGB3 |
cGMP-gated cation channel | CNGA3
CNGB3 |
| Cone Exchanger (TM) | NCKX2 | Cation Exchanger (4 Na+ in; 1 Ca2+/1K+ out); light-insensitive | SLC24A2 |
| Cone arrestin (cytosolic) | cArr | Binds to phosphorylated cone pigments | ARR3 |
| Cone pigment kinase (PM) | GRK7 | Phosphorylates cone pigments (not in mouse) | GPRK7 |
| GC-activating protein 3 | GCAP3 | GC-activator at low Ca2+ (not in mouse) | GUCA1C |
| C. Shared Component | Symbol | Activity/function | Gene symbol |
| Guanylate cyclase 1 (TM) | GC1 | Produces cGMP | GUCY2D (Gucy2e) |
| GC-activating protein 1 (PM) | GCAP1 | Mediates Ca2+ sensitivity of GC1, GC2 | GUCA1A |
| PDEδ (cytosolic) | PrBP/δ | Prenyl binding protein | PDE6D |
| Phosducin (cytosolic) | Pdc | Binds to Tβγ | PDC |
| Rhodopsin Kinase (PM) | GRK1 | Phosphorylates Rho | GRK1 |
| GTPase activating protein (PM)
Regulator of G protein signaling G protein b-subunit 5 RGS9 Anchoring Protein |
RGS9-1 Gβ5L R9AP |
Accelerates GTP hydrolysis of GtαGTP | RGS9
GNB5 R9AP |
ER processing of nascent, prenylated proteins
Membrane association of transducin, PDE6, and GRK1 occurs posttranslationally and is mediated by N-terminal acylation (Tα) or C-terminal prenylation (Tγ, PDE6 catalytic subunits, GRK1). The Tα subunits are acylated heterogeneously (Neubert et al., 1992; Johnson et al., 1994), the Tγ subunit, PDE6α subunit, and GRK1 are farnesylated (C15 side chain) (Lai et al., 1990; Anant et al., 1992; Qin et al., 1992; Inglese et al., 1992), and PDE6β and cone PDE6α′ subunits (Li et al., 1990) are geranylgeranylated (C20 side chain). Prenyl side chains are synthesized in all mammals via the mevalonate pathway and attached to newly synthesized cytosolic proteins carrying a C-terminal CaaX box motif (C = cysteine, a = aliphatic amino acid, X = any amino acid) (Hancock et al., 1991; Magee & Seabra, 2005; McTaggart, 2006). The prenyl chain is attached to the cysteine of the CaaX motif via a thioether bond by cytosolic prenyl transferases (Zhang & Casey, 1996). Prenylated proteins are synthesized in the cytosol, dock to the endoplasmic reticulum (ER) and are further processed by ER-associated proteins (Gelb et al., 2006). The ER-resident processing machinery removes the C-terminal tripeptide aaX and carboxymethylates the C-terminal cysteine, thereby producing a hydrophobic anchor suitable for protein-membrane or protein-protein interaction. A key question concerns mechanisms of targeting and transport of ER-anchored prenylated proteins to the cilium followed by intraflagellar transport through the cilium to nascent outer segments. A current hypothesis is that prenylated proteins docked to the ER require vesicular transport. Transfer to vesicles is thought to be mediated by cytosolic prenyl binding proteins featuring a hydrophobic groove accommodating prenyl side chains (Gelb et al., 2006; McTaggart, 2006), perhaps mediated by small GTP binding proteins. One such protein is PrBP/δ, formerly named PDE6δ (see below).
Biosynthesis of integral membrane proteins and post-Golgi transport
Transmembrane proteins are synthesized by ER-associated ribosomes and exported to the Golgi apparatus, finally emerging in vesicles from the trans-Golgi network (TGN) (reviewed by (Lippincott-Schwartz et al., 2000). A well investigated example is the rod photoreceptor transport of the G-protein coupled receptor (GPCR), rhodopsin, a hepta-spanning polypeptide (Deretic, 2006). Rhodopsin transport occurs sequentially, including budding of vesicular transport carriers carrying rhodopsin from the TGN, vectorial translocation of the carrier through the inner segment towards the basal body involving molecular motors, fusion of the carriers with the plasma membrane near the cilium, and intraflagellar transport through the cilium to the ROS disks. Mechanistic details are complex and incompletely understood. A C-terminal sorting motif (VXPX) regulates budding of rhodopsin containing transport carriers from the TGN (Deretic, 1998) whereupon the VXPX motif is recognized by ARF4, a small GTPase which regulates incorporation of rhodopsin into transport carriers (Deretic et al., 2005). The C-terminal cytoplasmic tail of rhodopsin was shown to interact with cytoplasmic dynein via the dynein light chain Tctex-1 (Tai et al., 2001). Dynein appears to be the molecular motor moving rhodopsin-bearing vesicles along microtubules towards the minus end at the basal body. After fusion with the plasma membrane, rhodopsin transport is thought to be mediated by heterotrimeric kinesin II, a motor powering intraflagellar transport (Marszalek et al., 2000; Vale, 2003). An integral membrane protein, GC, and other membrane proteins (peripherin/rds, ROM1, channel subunits) likely follow similar paths. Targeting signals have been identified in the C-terminal region of S-opsin (Deretic et al., 2005), peripherin/rds (Tam et al., 2004), but not GC1 or GC2. Unresolved questions are whether integral membrane proteins transport in tandem or independently, what the composition of cargoes may be (peripheral membrane proteins?), and exactly which molecular motors facilitate transport.
Prenyl Binding Protein, PrBP/δ
The Pde6d gene encodes PrBP/δ (formerly PDE6δ), a 17 kDa protein that functions as a prenyl binding protein (Cook et al., 2000; Zhang et al., 2004; Zhang et al., 2005). By gene blasting, PrBP/δ orthologs were identified in essentially all animals, e.g., fruitfly, the eyeless C. elegans (Li & Baehr, 1998), and the unicellular protozoan Paramecium (Zhang et al., 2007). PrBP/δ can interact in vitro with a number of prenylated as well as non-prenylated proteins of the Ras and Rho GTPase family (Linari et al., 1999a; Linari et al., 1999b; Hanzal-Bayer et al., 2002), but the physiological significance of these interactions was undetermined. In photoreceptors, PrBP/δ was shown to interact in vitro with RPGR (Becker et al., 1998), the prenyl side chains of rhodopsin kinase (GRK1) (Zhang et al., 2004), and PDE6α and PDE6β subunits (Li & Baehr, 1998; Zhang et al., 2004). Due to its ability to solubilize prenylated proteins, a role in transport was suspected (Norton et al., 2005).
Consequence of deletion of the prenyl binding protein PrBP/δ
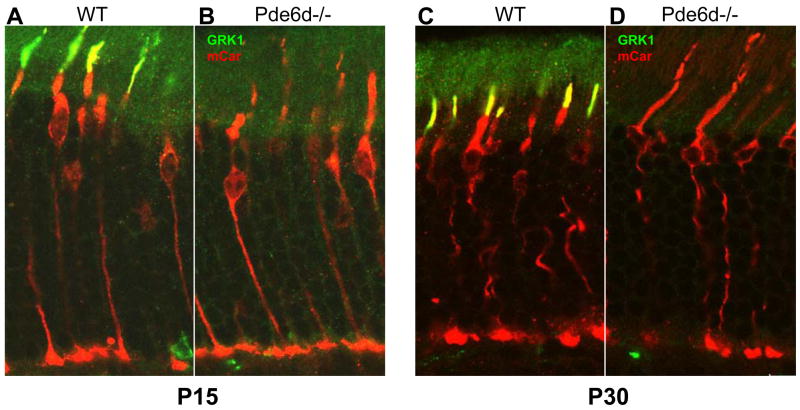
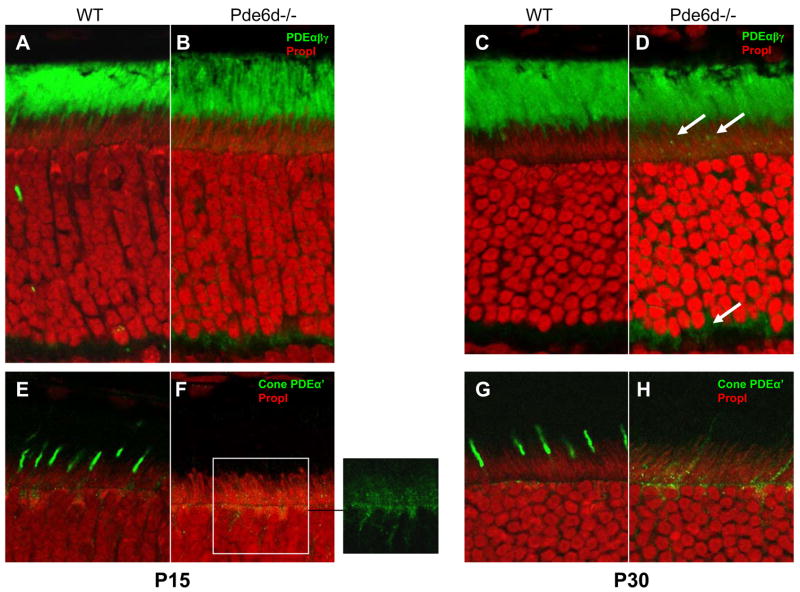
Knockdown of the Pde6d-like gene (C27H5.1) by RNA interference in C. elegans failed to produce an altered phenotype in worm development or behavior (http://www.wormbase.org). In mouse retina, where PrBP/δ is relatively abundant, deletion of the Pde6d gene resulted in an adult mouse of significantly reduced body size but of normal viability, development, and fertility (Zhang et al., 2007). However, Pde6d−/− rod and cone physiology was severely compromised. Mutant rods showed a delay in return to the dark-state and responded more sensitively to dim light flashes in single cell recordings, largely due to depletion of GRK1 in the rod outer segments. In ERG recordings, cone photoreceptor sensitivity was sharply reduced in the mutant. Correspondingly, investigation of subcellular distributions of prenylated phototransduction antigens in Pde6d−/− cones revealed that, farnesylated GRK1 and geranylgeranylated Pde6α′ were absent in outer segments (Zhang et al., 2007). As early as P15, mutant COS revealed undetectable levels of GRK1 (Fig. 1) and Pde6α′ (Fig. 2) although these proteins were present in COS of WT littermate retinas. By contrast, , GRK1 was mistargeted in Pde6d−/− rods, PDE6 subunits were misrouted in part (arrows in Fig. 2D), but farnesylated rod Tγ (and acylated rod Tα) transported to the Pde6d−/− ROS nearly unimpeded. Rhodopsin and transducin subunits were apparently unaffected by deletion of the Pde6d gene (Zhang et al., 2007). Curiously, the GRK1 and cone PDE6α′ transport defects manifest early (p15 and P30, see Figs. 1,2) while rod PDE6 transport mistargets only at later ages (Fig. 2, and Fig. 6B of Zhang et al., 2007). We conclude that PrBP/δ deletion results in defective transport of prenylated proteins to the outer segment; however, transport impedance of variable extent suggests that additional (unidentified) prenyl binding proteins may substitute for PrBP/δ loss in photoreceptors, particularly in transport of rod PDE6 and cone Tγ.
Figure 1. Localization of GRK1 in WT and Pde6d−/− retina.
Frozen sections of WT (A,C), and Pde6d−/− retina (B,D) were probed with polyclonal anti- mCar (red) and monoclonal anti-GRK1 (green) antibodies. mCAR is freely diffusible and distributed throughout cone cells (no light- or dark-adaptation). GRK1 is present in ROS and COS. A,B are P15 retinas and C,D are P30 retinas. Co-localization of mCar and GRK1 is notable in WT P15 and P30 COS (yellow). GRK1 is undetectable in Pde6d−/− COS at P15 and P30. Methods: Immunocytochemistry was performed as described (Zhang et al., 2007).
Figure 2. Localization of rod and cone PDE6 in WT and Pde6d−/− retina.
Frozen sections of WT (A,C,E,G) and Pde6d−/− retinas (B,D,F,H) were probed with anti-rod PDE6 antibody (A–D), of anti-cone PDEα′ antibody (E–H). Nuclei were stained with Propidium iodide (red color in IS results from RNA and mitochondrial DNA). Rod PDE6 is located in ROS (A,C). Note beginning of mislocalization of rod PDE6 to the synaptic region in B,D, as well as to inner segments in D (arrows). Cone PDE6 localizes to COS (E,G), but is mostly mislocalized in Pde6d−/− retina to the ER surrounding nuclei and extending into the IS (inset in F, H). Methods: Immunocytochemistry was performed as described (Zhang et al., 2007).
Consequence of GC1 and GC2 deletions
Guca2e and Guca2f genes encode the photoreceptor-specific guanylate cyclases GC1 and GC2, respectively. Both GCs are integral membrane proteins, closely related, with a single transmembrane domain. GCs produce cGMP, the internal transmitter of phototransduction in rods and cones. The Ca2+-sensitivity of GC enzymatic activity is mediated by GC-activating proteins (GCAPs) (reviewed by (Palczewski et al., 2004). GC1 null alleles cause a rod/cone dystrophy in human (Leber congenital amaurosis (Perrault et al., 1996) and chicken (Semple-Rowland et al., 1998) indicating that GC1 mediates both rod and cone vision in these species. Guca2e was deleted by gene targeting and the results showed that cones were nonfunctional in the GC1 knockout mouse (Yang et al., 1999). Rod photoreceptors functioned, presumably due to presence of an alternate GC (GC2), but with reduced ERG amplitudes. The GC2 knockout mouse had essentially no phenotype (Baehr et al., 2007) consistent with the presence of GC1 in rods and cones. Simultaneous ablation of GC1 and GC2 abolishes phototransduction altogether, excluding a role for additional GCs in phototransduction. Electron microscopy of GC1/GC2 double knockout (GCdko) retinas showed that rod and cone outer segments were present, but severely disorganized at age one month. These observations were interpreted to indicate that mouse rods rely on two GCs (and two GCAPs) while in human rods, GC2 has no obvious function.
Downregulation of GCAPs and PDE6 in GC double knockout rods
Several peripherally membrane-associated phototransduction components were either absent, mistargeted or severely reduced in GC1−/− and GCdko photoreceptor outer segments (Baehr et al., 2007). Knockout of both GCs does not apparently affect the rhodopsin transport pathway since rhodopsin was found at near normal levels in GCdko ROS. Transport of rhodopsin and GC may therefore occur independently, as shown in Fig. 5A, although co-transport in a vesicle carrying Rho:GC in a ratio of 100:1 cannot be excluded. Importantly, another structural protein, peripherin/rds, is present in GCdko ROS (Fig. 4) thereby confirming that it, too, transports independently of GC1/GC2. Previous work suggested that rhodopsin and peripherin/rds transport independently based on morphological analysis of normal and mutant photoreceptors (Fariss et al., 1997; Lee et al., 2006). In contrast, knockout of rhodopsin prevents ROS formation and precludes rhodopsin-associated transport (Humphries et al., 1997; Lem et al., 1999). Deletion of one of two GCs has no recognizable effect on rod morphology at early age, but in GC1−/− rods, GCAP1 levels are reduced (Coleman et al., 2004) and in GC double knockouts, both GCAPs are severely downregulated. GCAP1 and PDE6α-subunit mRNA were measured at normal levels in mutant animals. Surprisingly, PDE6 was undetectable in six-weeks-old GCdko ROS which was likely due to PDE6 instability and/or lack of transport to the outer segment (Baehr et al., 2007).
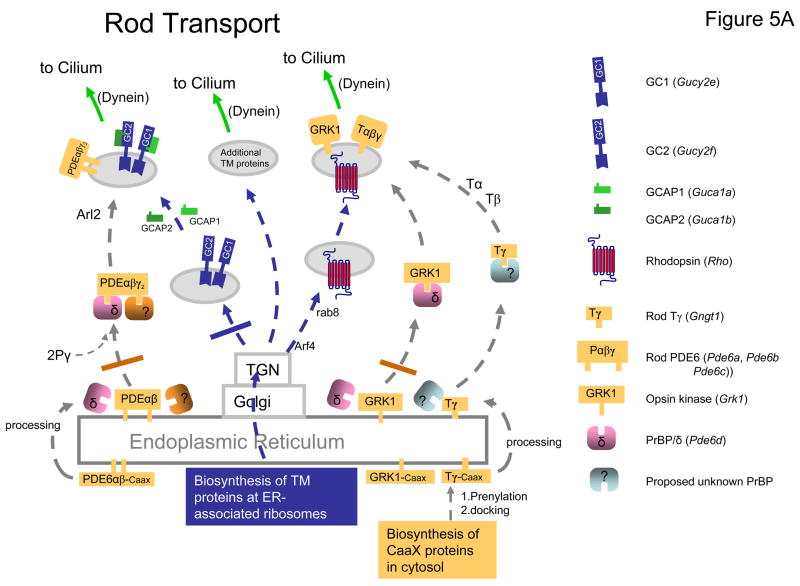
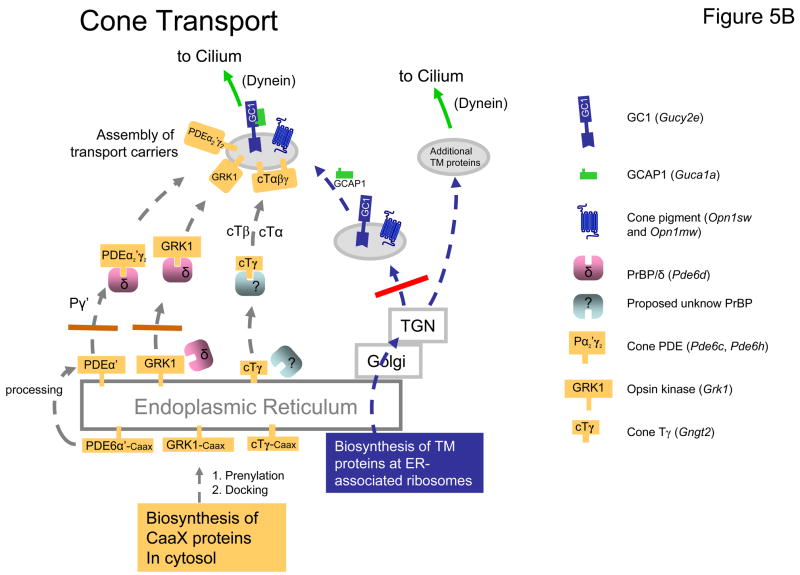
Figure 5. Putative model of post-biosynthesis transport of membrane proteins in rods and cones.
A, model of rod transport based on GCdko and PrBP/δ deletion phenotypes. TM proteins (GC1, Rhodopsin, and others) follow the secretory pathway (blue arrows). They emerge from the TGN in vesicles to be transported to the base of the cilium by molecular motors (e.g., dynein). Deletion of both GC1 and GC2 prevents formation of GC vesicles (blue bar). As a consequence, PDE6 and GCAPs, shown associated with GC-bearing vesicles in WT rods, are not transported to the ROS. RK (GRK1) and T are unaffected by GC1/GC2 deletion and are shown tentatively associated with rhodopsin bearing vesicles. The Caax-box containing proteins RK (GRK1), Pαβ (rod PDE6), and Tγ (yellow) are synthesized in the cytosol and dock to the ER after prenylation. Prenylated proteins may be extracted from the ER by prenyl binding proteins and loaded onto the transport carriers. An identified prenyl binding protein is PrBP/δ (purple), additional prenyl binding proteins are proposed to exist (? in brown and purple). Deletion of PrBP/δ prevents RK from attaching to the GC-vesicles (brown bar). Deletion of PrBP/δ has only partial effect on extraction of PDE which has two distinct prenyl side chains, perhaps accommodating two different prenyl binding proteins. B, model of cone transport based on consequences of GC1 and PrBP/δ deletion. Cone pigments and GC1 are shown to transport together, but other TM proteins may transport independently (blue arrows). Deletion of GC1 (red bar), the only GC in cones, prevents GCAP1, GRK1, cone T, and cone PDEα′ to be transported to the COS. Deletion of PrBP/δ prevents transport of GRK1 and cone PDEα′ (gray bar), but not cone transducin suggesting the presence of an alternate prenyl binding protein (? green).
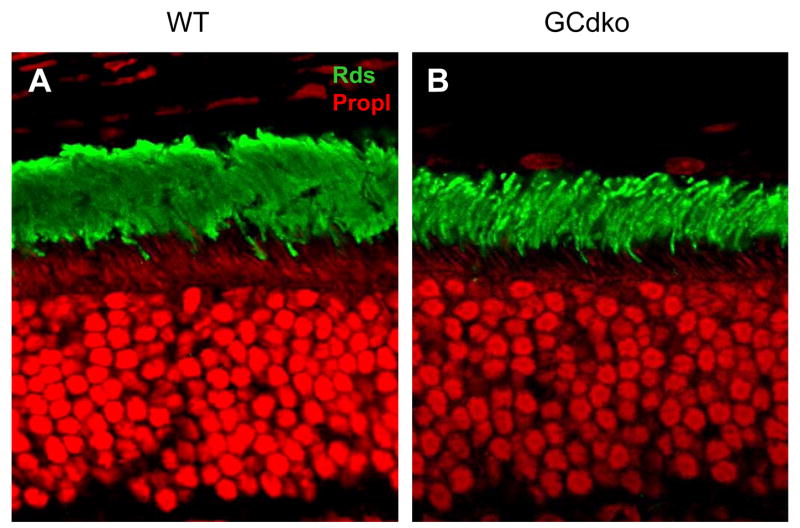
Figure 4. Localization of Rds/peripherin.
Frozen sections of WT (A) and GCdko retinas (B) were probed with anti- peripherin/rds/antibody. Knockout of both GCs does not affect transport of peripherin/rds to rod and cone outer segments. Methods: Immunocytochemistry was performed as described (Baehr et al., 2007). The anti-RDS antibody was kindly provided by Dr. Andrew F. X. Goldberg, Eye Research Institute, Oakland University, Rochester, MI).
Effect of GC1 knockout on cones
Absence of GC1 affects peripheral membrane proteins of cones most profoundly. Cone Tα and cone PDEα′ were downregulated in immunoblots and undetectable by immunocytochemistry in GC1−/− COS (Baehr et al., 2007). Thus, cone transducin and cone PDE stabilities appear dependent on the presence of GC1. S- and M/L-cone pigments transport in part to the GC1−/− COS, but are also localized to vesicles that appear detached from the cone inner segments (Baehr et al., 2007). Mislocalization of pigments to the inner segments and cone synaptic pedicles of GCdko retinas was also evident (Figure 3). Cone pigment mislocalizations have been observed previously in Cnga3−/− (Michalakis et al., 2005) and RPE65−/− cones (Rohrer et al., 2005). In Cnga3−/− cones, GC1, GCAP1 and cone Tα polypeptides were downregulated similarly as observed in GC1−/− and GCdko cones. GCAP1, GCAP2, rod PDEα, and cone Tα GC1−/− retina mRNA levels, relative to WT retina mRNA levels, were essentially identical (Baehr et al., 2007). Microarray analysis verified that the transcript levels of the downregulated proteins (and all other phototransduction proteins) in GCdko retina were essentially unchanged (SK, HZ, and WB, unpublished). Thus, downregulation occurs posttranslationally.
Figure 3. Localization of ML-opsin in WT and GC knockout retina.
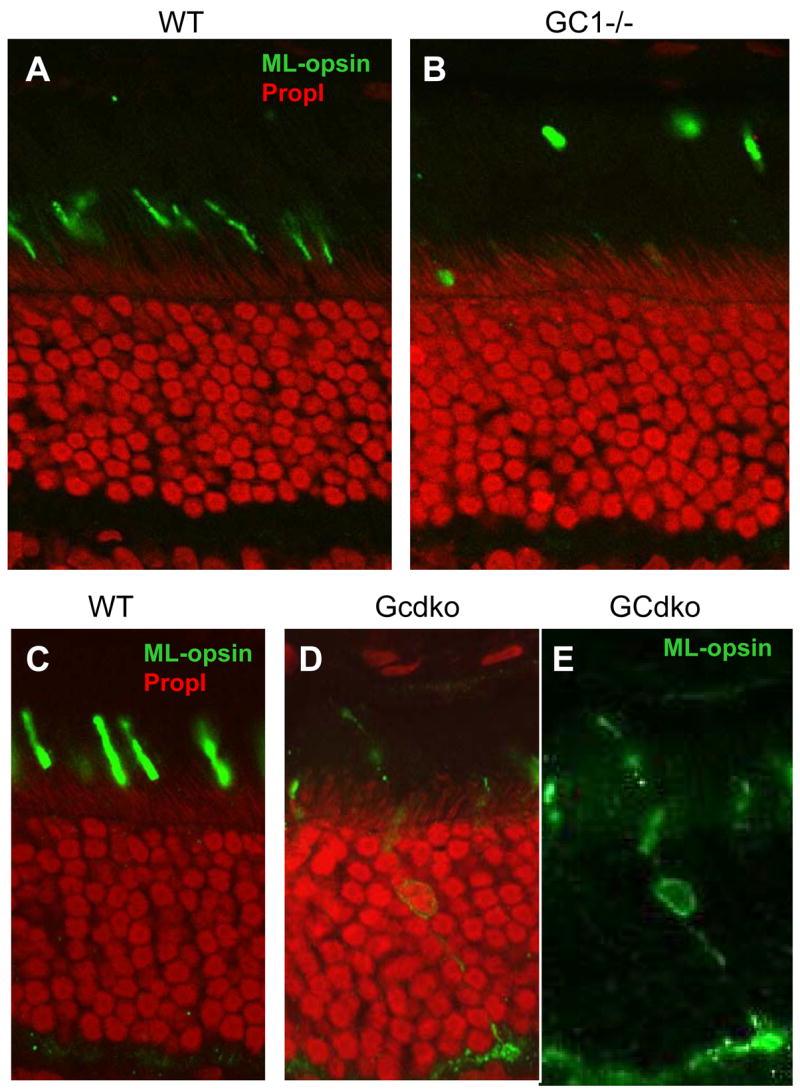
Frozen sections of WT retina (A,C), GC1−/− (B), and GCdko retina (D,E) were probed with polyclonal anti- ML-opsin (green) antibody. GC1−/− COS werefound unstable, and ML-opsin containing particles were detached from the rest of the COS (B). In cones of the GCdko retina, some cones show mislocalization of ML-opsin (C,D). In panel E, staining with propidium iodide was subtracted to visualize mislocalization of ML-opsin in the IS, soma, axon and pedicles in the GCdko retina. Methods: Immunocytochemistry was performed as described (Baehr et al., 2007).
Putative model for membrane protein transport in rods
All downregulated polypeptides cited above can be expressed stably in vitro (although PDE is biologically inactive). Posttranslational downregulation in-vivo may be the result of polypeptide instability, e.g., it is known that GCAPs are susceptible to proteolysis in low free Ca2+ (Coleman et al., 2004), and GRK1 is labile without PrBP/δ (Zhang et al., 2007). Stability and lifetime of transducin or PDE subunits in vivo may depend on cGMP levels, unidentified cGMP-dependent pathways, or precise levels of free Ca2+. Alternatively, downregulation may result from failed vesicle formation and transport to the OS (Fig. 5). We presume that in WT photoreceptors, prenylated cone PDE6α′, farnesylated GRK1 and farnesylated rod and cone Tγ depend on a prenyl binding protein for extraction from the ER and delivery to a transport carrier. Subsequently, prenylated proteins may co-transport to the outer segments using vesicles with a transmembrane protein as guide, either rhodopsin or GC1/GC2, or another integral membrane protein (Fig. 5A). In the absence of PrBP/δ, selected prenylated proteins fail to be delivered to a carrier and do not transport (Zhang et al., 2007). In rods, GRK1 is largely absent in Pde6d−/− ROS. In cones (Fig. 5B), PrBP/δ deletion affects both GRK1 and cone PDE6 equally although the former is farnesylated and the latter geranylgeranylated. We propose that in the absence of PrBP/δ, solubilization of these proteins is inhibited and transport to the COS impeded. Mechanisms of delivery of prenylated proteins as cargo to a vesicular transport carrier are unknown. Since PrBP/δ cocrystallized with Arl2 (Hanzal-Bayer et al., 2002), an Arf-like small GTPase (Table 2D), it is conceivable that Arl2 or its close cousin Arl3 may play a role in docking to vesicles. Since rod PDE, rod and cone Tγ are not or only weakly affected, additional prenyl binding proteins may be present and able to substitute.
Putative transport in cones
Deletion of GC1 prevents transport of cone T, GRK1 and cone PDE to the COS. We speculate that in the GC1 knockout, formation of GC1-containing post-TGN transport carriers is prevented, and cone transducin, GRK1, and cone PDE lose their transport carrier. GC1 deletion also affects targeting of cone pigments, therefore GC1 and cone pigments may be present on the same carrier (Fig. 5B). Fate of polypeptides unable to be delivered to their destination appears to be degradation by undefined mechanisms. For simplicity, as transport carriers we chose post-TGN vesicles. Intermediate transport vesicles (e.g., ER to Golgi) cannot be excluded but seem less likely since the Golgi is the organelle where proteins are modified and sorted. We envision that GCs, rhodopsin, and other transmembrane proteins (peripherin/rds, CNG subunits) transport independently to the base of the cilium after emerging from the trans-Golgi network. However, downregulation of cone opsins in GC1−/− and Cnga3−/− cones suggests a link in transport and/or targeting of these proteins. Most likely GC1 (and GC2) carry a cytoplasmic sorting signal similarly as observed for rhodopsin, peripherin/rds, and S-cone pigment (Ritter et al., 2004; Deretic et al., 2005). Once assembled, the transport carrier moves along microtubules using motors (e.g., dynein-1) and delivers its cargo to the distal end of the inner segment for intraflagellar transport through the connecting cilium.
What triggers retina degeneration in GC and PrBP/δ knockouts?
GC1−/− cones lack GC1, GCAP1, GRK1 and the entire phototransduction cascade, while GCdko rods lack PDE, but not rhodopsin, transducin and GRK1. Electronmicrographs show that the COS and ROS are disorganized and unstable. We hypothesize that GCs may have a structural role in stabilizing the rod and cone disk membranes. The extracellular domains of GCs are large (about 400 amino acids, excluding the leader sequence) and have no known function, but still have sequence similarity to extracellular domains of ANF receptors (atrial natriuretic factor receptor guanylate cyclases) (Margulis et al., 1993). In rod disks, the extracellular domains of GC1 and GC2 are packed tightly into the lumen and may contribute to the flattened disk structure since in their absence, disks appear to form incorrectly and are not maintained (Baehr et al., 2007). Photoreceptors without enzymes that produce cGMP are unable to signal, i.e., unable to support phototransduction because essentially all channels are closed for lack of cGMP. Therefore, the cell is hyperpolarized and in a state equivalent to “constant light,” a situation that may produce a trigger for apoptosis (Fain & Lisman, 1999). Absence of nearly all phototransduction components likely further compounds the structural destabilization.
In the Pde6d−/− ROS, the only known deficiency is lack of GRK1, an enzyme essential for phosphorylation of rhodopsin, and a minor mistargeting of PDE6. Physiologically, the Pde6d−/− rod behaves like a Grk1 knockdown with delay of dark-adaptation. In the Pde6d−/− COS, cone PDE is at very low levels, but a much attenuated cone response can still be recorded. Since GC1 and GCAPs are present at normal levels, cGMP may rise to toxic levels. Elevated levels of cGMP have been linked to a variety of retina degenerations, e.g., the rd mouse (Farber & Lolley, 1974), Irish setter (Suber et al., 1993), as well as dominant cone dystrophies based on GCAP1 mutations (Olshevskaya et al., 2004).
Acknowledgments
This work was supported by the National Institutes of Health (NIH grant EY08123, P30 EY014800-01A2), by a Center grant from the Foundation Fighting Blindness, Inc., and the Knights Templar Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Anant JS, Ong OC, Xie H, Clarke S, O’Brien PJ, Fung BKK. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992;267:687–690. [PubMed] [Google Scholar]
- Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of Guanylate Cyclase 1 (GC1) and Guanylate Cyclase 2 (GC2) in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Linari M, Manson F, Wright A, Meindl A, Meitinger T. The delta subunit of rod phosphodiesterase interacts with the RCC1 homologpus domain of RPGR. Invest Ophthalmol & Vis Sci. 1998;39:S953. [Google Scholar]
- Besharse JC, Hollyfield JG. Turnover of mouse photoreceptor outer segments in constant light and darkness. Invest Ophthalmol Vis Sci. 1979;18:1019–1024. [PubMed] [Google Scholar]
- Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. doi: 10.1016/j.neuron.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Research. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- Coleman JE, Zhang Y, Brown GA, Semple-Rowland SL. Cone cell survival and downregulation of GCAP1 protein in the retinas of GC1 knockout mice. Invest Ophthalmol Vis Sci. 2004;45:3397–3403. doi: 10.1167/iovs.04-0392. [DOI] [PubMed] [Google Scholar]
- Cook TA, Ghomashchi F, Gelb MH, Florio SK, Beavo JA. Binding of the delta subunit to rod phosphodiesterase catalytic subunits requires methylated, prenylated C-termini of the catalytic subunits. Biochemistry. 2000;39:13516–13523. doi: 10.1021/bi001070l. [DOI] [PubMed] [Google Scholar]
- Dalke C, Graw J. Mouse mutants as models for congenital retinal disorders. Exp Eye Res. 2005;81:503–512. doi: 10.1016/j.exer.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Deretic D. Post-Golgi trafficking of rhodopsin in retinal photoreceptors. Eye. 1998;12(Pt 3b):526–530. doi: 10.1038/eye.1998.141. [DOI] [PubMed] [Google Scholar]
- Deretic D. A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vision Research. 2006;46:4427–4433. doi: 10.1016/j.visres.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Deretic D, Williams AH, Ransom N, Morel V, HArgrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4) Proc Natl Acad Sci USA. 2005;102:3301–3306. doi: 10.1073/pnas.0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Lisman JE. Light, Ca2+, and photoreceptor death: new evidence for the equivalent- light hypothesis from arrestin knockout mice [In Process Citation] Invest Ophthalmol Vis Sci. 1999;40:2770–2772. [PubMed] [Google Scholar]
- Farber DB, Lolley RN. Cyclic guanosine monophosphate: elevation in degenerating photoreceptor cells of the C3H mouse retina. Science. 1974;186:449–451. doi: 10.1126/science.186.4162.449. [DOI] [PubMed] [Google Scholar]
- Fariss RN, Molday RS, Fisher SK, Matsumoto B. Evidence from normal and degenerating photoreceptors that two outer segment integral membrane proteins have separate transport pathways. J Comp Neurol. 1997;387:148–156. doi: 10.1002/(sici)1096-9861(19971013)387:1<148::aid-cne12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Arch. 2007 doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb MH, Brunsveld L, Hrycyna CA, Michaelis S, Tamanoi F, Van Voorhis WC, Waldmann H. Therapeutic intervention based on protein prenylation and associated modifications. Nat Chem Biol. 2006;2:518–528. doi: 10.1038/nchembio818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi F, Grimm C, Simmen BC, Wenzel A, Reme CE. Molecular ophthalmology: an update on animal models for retinal degenerations and dystrophies. Br J Ophthalmol. 2000;84:922–927. doi: 10.1136/bjo.84.8.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Cadwallader K, Paterson H, Marshall CJ. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991;10:4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzal-Bayer M, Renault L, Roversi P, Wittinghofer A, Hillig RC. The complex of Arl2-GTP and PDE delta: from structure to function. EMBO J. 2002;21:2095–2106. doi: 10.1093/emboj/21.9.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nature Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Inglese J, Koch WJ, Caron MG, Lefkowitz RJ. Isoprenylation in regulation of signal transduction by G-protein- coupled receptor kinases. Nature. 1992;359:147–150. doi: 10.1038/359147a0. [DOI] [PubMed] [Google Scholar]
- Johnson RS, Ohguro H, Palczewski K, Hurley JB, Walsh KA, Neubert TA. Heterogeneous N-acylation is a tissue- and species-specific posttranslational modification. J Biol Chem. 1994;269:21067–21071. [PubMed] [Google Scholar]
- Lai RK, Perez-Sala D, Cañada FJ, Rando RR. The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci USA. 1990;87:7673–7677. doi: 10.1073/pnas.87.19.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci. 2006;47:5138–5152. doi: 10.1167/iovs.06-0849. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Lee ES, Burnside B, Flannery JG. Characterization of peripherin/rds and rom-1 transport in rod photoreceptors of transgenic and knockout animals. Invest Ophthalmol Vis Sci. 2006;47:2150–2160. doi: 10.1167/iovs.05-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem J, Krasnoperova NV, Calvert PD, Kosaras B, Cameron DA, Nicol Makino CL, Sidman RL. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci USA. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Baehr W. Expression and characterization of human PDEδ and its Caenorhabditis elegans ortholog CEδ. FEBS Lett. 1998;440:454–457. doi: 10.1016/s0014-5793(98)01501-4. [DOI] [PubMed] [Google Scholar]
- Li T, Volpp K, Applebury ML. Bovine cone photoreceptor cGMP phosphodiesterase structure deduced from a cDNA clone. Proc Natl Acad Sci USA. 1990;87:293–297. doi: 10.1073/pnas.87.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M, Hanzal-Bayer M, Becker J. The delta subunit of rod specific cyclic GMP phosphodiesterase, PDE delta, interacts with the Arf-like protein Arl3 in a GTP specific manner. FEBS Lett. 1999a;458:55–59. doi: 10.1016/s0014-5793(99)01117-5. [DOI] [PubMed] [Google Scholar]
- Linari M, Ueffing M, Manson F, Wright A, Meitinger T, Becker J. The retinitis pigmentosa GTPase regulator, RPGR, interacts with the delta subunit of rod cyclic GMP phosphodiesterase. Proc Natl Acad Sci USA. 1999b;96:1315–1320. doi: 10.1073/pnas.96.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Roberts TH, Hirschberg K. Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol. 2000;16:557–589. doi: 10.1146/annurev.cellbio.16.1.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanova ES, Finkelstein S, Song H, Tsang SH, Chen CK, Sokolov M, Skiba NP, Arshavsky VY. Transducin translocation in rods is triggered by saturation of the GTPase-activating complex. J Neurosci. 2007;27:1151–1160. doi: 10.1523/JNEUROSCI.5010-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee T, Seabra MC. Fatty acylation and prenylation of proteins: what’s hot in fat. Curr Opin Cell Biol. 2005;17:190–196. doi: 10.1016/j.ceb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Margulis A, Goraczniak RM, Duda T, Sharma RK, Sitaramayya A. Structural and biochemical identity of retinal rod outer segment membrane guanylate cyclase. Biochem Biophys Res Commun. 1993;194:855–861. doi: 10.1006/bbrc.1993.1900. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- McTaggart SJ. Isoprenylated proteins. Cell Mol Life Sci. 2006;63:255–267. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Geiger H, Haverkamp S, Hofmann F, Gerstner A, Biel M. Impaired opsin targeting and cone photoreceptor migration in the retina of mice lacking the cyclic nucleotide-gated channel CNGA3. Invest Ophthalmol Vis Sci. 2005;46:1516–1524. doi: 10.1167/iovs.04-1503. [DOI] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Mendez A, Gurevich EV, Kennedy MJ, Shestopalov VI, Vishnivetskiy SA, Chen J, Hurley JB, Gurevich VV, Slepak VZ. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005;46:555–567. doi: 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert TA, Johnson RS, Hurley JB, Walsh KA. The rod transducin α subunit amino terminus is heterogeneously fatty acylated. J Biol Chem. 1992;267:18274–18277. [PubMed] [Google Scholar]
- Norton AW, Hosier S, Terew JM, Li N, Dhingra A, Vardi N, Baehr W, Cote RH. Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors. J Biol Chem. 2005;280:1248–1256. doi: 10.1074/jbc.M410475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshevskaya EV, Calvert PD, Woodruff ML, Peshenko IV, Savchenko AB, Makino CL, Ho YS, Fain GL, Dizhoor AM. The Y99C mutation in guanylyl cyclase-activating protein 1 increases intracellular Ca2+ and causes photoreceptor degeneration in transgenic mice. Journal of Neuroscience. 2004;24:6078–6085. doi: 10.1523/JNEUROSCI.0963-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Sokal I, Baehr W. Guanylate cyclase-activating proteins: structure, function, and diversity. Biochem Biophys Res Commun. 2004;322:1123–1130. doi: 10.1016/j.bbrc.2004.07.122. [DOI] [PubMed] [Google Scholar]
- Perrault I, Rozet JM, Calvas P, Gerber S, Camuzat A, Dollfus H, Chatelin S, Souied E, Ghazi I, Leowski C, Bonnermaison M, Le Paslier D, Frezal J, Dufier JL, Pittler SJ, Munnich A, Kaplan J. Retinal-specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nature Genet. 1996;14:461–464. doi: 10.1038/ng1296-461. [DOI] [PubMed] [Google Scholar]
- Qin N, Pittler SJ, Baehr W. In vitro isoprenylation and membrane association of mouse rod photoreceptor cGMP phosphodiesterase α and β subunits expressed in bacteria. J Biol Chem. 1992;267:8458–8463. [PubMed] [Google Scholar]
- Ridge KD, Abdulaev NG, Sousa M, Palczewski K. Phototransduction: crystal clear. Trends Biochem Sci. 2003;28:479–487. doi: 10.1016/S0968-0004(03)00172-5. [DOI] [PubMed] [Google Scholar]
- Ritter LM, Boesze-Battaglia K, Tam BM, Moritz OL, Khattree N, Chen SC, Goldberg AF. Uncoupling of photoreceptor peripherin/rds fusogenic activity from biosynthesis, subunit assembly, and targeting: a potential mechanism for pathogenic effects. J Biol Chem. 2004;279:39958–39967. doi: 10.1074/jbc.M403943200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46:3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- Rosenzweig DH, Nair KS, Wei J, Wang Q, Garwin G, Saari JC, Chen CK, Smrcka AV, Swaroop A, Lem J, Hurley JB, Slepak VZ. Subunit dissociation and diffusion determine the subcellular localization of rod and cone transducins. J Neurosci. 2007;27:5484–5494. doi: 10.1523/JNEUROSCI.1421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple-Rowland SL, Lee NR, Van-Hooser JP, Palczewski K, Baehr W. A null mutation in the photoreceptor guanylate cyclase gene causes the retinal degeneration chicken phenotype. Proc Natl Acad Sci USA. 1998;95:1271–1276. doi: 10.1073/pnas.95.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strissel KJ, Sokolov M, Trieu LH, Arshavsky VY. Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J Neurosci. 2006;26:1146–1153. doi: 10.1523/JNEUROSCI.4289-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suber ML, Pittler SJ, Qin N, Wright GC, Holcombe V, Lee RH, Craft CM, Lolley RN, Baehr W, Hurwitz RL. Irish setter dogs affected with rod/cone dysplasia contain a nonsense mutation in the rod cGMP phosphodiesterase beta subunit gene. Proc Natl Acad Sci USA. 1993;90:3968–3972. doi: 10.1073/pnas.90.9.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Sung CH. Cytoplasmic dynein regulation by subunit heterogeneity and its role in apical transport. J Cell Biol. 2001;153:1499–1509. doi: 10.1083/jcb.153.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Papermaster DS. The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell. 2004;15:2027–2037. doi: 10.1091/mbc.E03-09-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- Yang RB, Robinson SW, Xiong WH, Yau KW, Birch DG, Garbers DL. Disruption of a retinal guanylyl cyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. Journal of Neuroscience. 1999;19:5889–5897. doi: 10.1523/JNEUROSCI.19-14-05889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci. 1976;15:700–725. [PubMed] [Google Scholar]
- Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hosier S, Terew JM, Zhang K, Cote RH, Baehr W. Assay and functional properties of PrBP(PDEdelta), a prenyl-binding protein interacting with multiple partners. Methods Enzymol. 2005;403:42–56. doi: 10.1016/S0076-6879(05)03005-3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li S, Doan T, Rieke F, Detwiler PB, Frederick JM, Baehr W. Deletion of PrBP/{delta} impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0701681104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu XH, Zhang K, Chen CK, Frederick JM, Prestwich GD, Baehr W. Photoreceptor cGMP phosphodiesterase delta subunit (PDEdelta) functions as a prenyl-binding protein. J Biol Chem. 2004;279:407–413. doi: 10.1074/jbc.M306559200. [DOI] [PubMed] [Google Scholar]