Abstract
Cells in glucose-limited Saccharomyces cerevisiae cultures differentiate into quiescent (Q) and nonquiescent (NQ) fractions before entering stationary phase. To understand this differentiation, Q and NQ cells from 101 deletion-mutant strains were tested for viability and reproductive capacity. Eleven mutants that affected one or both phenotypes in Q or NQ fractions were identified. NQ fractions exhibit a high level of petite colonies, and nine mutants affecting this phenotype were identified. Microarray analysis revealed >1300 mRNAs distinguished Q from NQ fractions. Q cell-specific mRNAs encode proteins involved in membrane maintenance, oxidative stress response, and signal transduction. NQ-cell mRNAs, consistent with apoptosis in these cells, encode proteins involved in Ty-element transposition and DNA recombination. More than 2000 protease-released mRNAs were identified only in Q cells, consistent with these cells being physiologically poised to respond to environmental changes. Our results indicate that Q and NQ cells differentiate significantly, with Q cells providing genomic stability and NQ cells providing nutrients to Q cells and a regular source of genetic diversity through mutation and transposition. These studies are relevant to chronological aging, cell cycle, and genome evolution, and they provide insight into complex responses that even simple organisms have to starvation.
INTRODUCTION
Most cells on earth exist in a nondividing, quiescent state, often typified by low metabolic activity and arrest in an unbudded, relatively unstudied state (Gray et al., 2004). In eukaryotes, this state is also referred to as G0, and it is exemplified by stem cells, neurons, eggs, and spores. We know less about the quiescent state than about dividing cells, because both this state and the biogenesis of cells in this state have been difficult to study. However, understanding this state is important, because it plays a critical role in normal metazoan development and disease, including the development of cancer stem cells in solid tissues (Kim et al., 2005; Suda et al., 2005; Abbott, 2006) and the persistence of long-lived spores in infections such as tuberculosis, cryptosporesis, and anthracis (Murray, 1999; Gray et al., 2004). Finally, the quiescent state is important in the environment, including the 99.9% of all microbes that exist in an unculturable, quiescent state (Kaeberlein et al., 2002). Fortunately, several recent developments, including the ability to use genome-scale tools to understand this state, have facilitated studies into the quiescent state, especially in yeast (Allen et al., 2006; Yang et al., 2006).
We recently reported the isolation of quiescent (Q) and nonquiescent (NQ) cells from stationary phase (SP) yeast cultures (Allen et al., 2006). These cells were also observed in long-term, unfractionated, SP cultures by electron microscopy (Yang et al., 2006). In cultures grown in rich, glucose-based medium, Q cells are dense, more thermotolerant, and can be separated from NQ cells 1–2 d after glucose exhaustion. Q cells are also predominantly daughter cells that are synchronous upon reentry into the cell cycle. NQ cells, in contrast, retain viability but rapidly lose the ability to reproduce, with ∼50% of the viable cells unable to produce daughters at 7 d. By days 14 and 21, almost 90% of the viable NQ cells have lost their ability to reproduce, and ∼50% are apoptotic or necrotic.
To increase our understanding of this differentiation, we carried out comprehensive analyses Q and NQ cells from SP cultures, by using wild type, parental, and 101 mutants from the yeast deletion set (Winzeler et al., 1999). We report the identification of 11 mutants that affect viability and/or reproductive capacity of NQ cells and/or Q cells. Microarray analysis led to the identification of >1300 mRNAs that differentiate these two cell types, ∼1100 more than in our previous study (Allen et al., 2006). Finally, we determined that most mRNAs released by protease treatment, identified previously in unfractionated SP cultures (Aragon et al., 2006) were present in Q cells. The largest single group of mRNAs released by proteinase K treatment in Q cells encodes proteins required for Ty-element transposition and DNA recombination. This and previous data (Aragon et al., 2006) suggest that Q cells are poised for a variety of physiological responses to environmental conditions, including apoptosis, which is likely to be a long-term response of Q cells to ongoing starvation. These results provide a critical foundation for beginning to understand the differentiation of Q and NQ cells in yeast and the role of each of these cell types in species survival.
MATERIALS AND METHODS
See http://biology.unm.edu/biology/maggieww/Public_Html/aragon/Compendium.htm for all Supplemental Materials.
Strains
Yeast strains used in this study include S288c (MAT α gal2mal2) and the parental strain for the deletion set, BY4742 (MAT α his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0). In this study MATα yeast deletion strains (Winzeler et al., 1999) were used for 101 mutants whose mRNAs were previously identified as being abundant in SP cultures (Martinez et al., 2004). See the Supplemental Material for list of mutants.
Growth Conditions
Wild-type and parental strains were grown in YPD + A (1% yeast extract, 2% peptone, 2% d-glucose, and 0.04 mg/ml adenine) with aeration at 30°C to SP (7 d OD600 = 20–25). Yeast deletion strains (Winzeler et al., 1999) were grown in the same media with the addition of 200 μg/ml G-418 (Geneticin; Invitrogen, Carlsbad, CA). Cells in the exponential phase of growth were collected at OD600 = 1–2, after growth overnight.
Cell Separation and Harvest
Q and NQ cells were separated from SP cultures by using Percoll (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) density gradients as described previously (Allen et al., 2006). Briefly, 9 parts of Percoll was added to 1 part of 1.5 M NaCl and centrifuged at 19,240 × g for 15 min at 20°C to form a density gradient. For RNA isolations, yeast cells (200 OD600) were pelleted and resuspended in 1 ml 50 mM Tris buffer, pH 7.5. Cells were overlaid on the 25-ml Percoll gradient and centrifuged at 400 × g for 1 h at room temperature. Q and NQ fractions were removed with a pipette, and Percoll was removed by washing with 45 ml of 50 mM Tris buffer, pH 7.5. Cell pellets were frozen at −70°C. To obtain cells (OD600 = 10) for flow cytometry assays, gradients were formed as described above using 2-ml tubes. Cells were pelleted, resuspended in 100 μl of 50 mM Tris buffer, pH 7.5, overlaid on a 1.75-ml gradient, and centrifuged at 400 × g for 1 h. Percoll was removed by washing with 1.5 ml 50 mM Tris buffer, pH 7.5, and cells were then divided for flow assays.
Assays for Viability (FungaLight staining) and Reproductive Capacity (Colony-forming Units [CFUs])
For these assays, parental and mutant strains were grown to SP and separated into Q and NQ fractions as described above. To determine viability, cells were stained using the LIVE/DEAD FungaLight yeast viability kit (Invitrogen) according to the manufacturer's protocol. Cells from an exponential culture were used as a “live” control. A “killed” control was prepared by incubating cells from an SP culture in 70% isopropanol for 1 h at room temperature before staining. Unstained cells were used as an autofluorescence control, and these emissions were confined to the region between the upper and lower gated regions. All cell samples were diluted to 1 × 106 cells/ml in 500 μl of 50 mM Tris buffer, pH 7.5, stained, and incubated at 30°C for 30 min. For flow cytometry, 30,000 cells per sample were analyzed with a flow cytometer (FACScan; BD Biosciences, San Jose, CA) by using 488-nm excitation and collecting fluorescent emission with filters at 530/30 nm for FL-1 parameter and 585/42 nm for FL-2 parameter. CellQuest software (BD Biosciences) was used for data collection and analysis. Gates were established such that 99.9% of the emissions from the “live” control cells were contained in the lower gated area and 99.9% of the emissions from the “killed” control were contained in the upper gated area (see Supplemental Material). To determine reproductive capacity via CFUs, automated plating was performed using the MoFlo cell sorter (Dako Denmark, Glostrup, Denmark) as described previously (Allen et al., 2006).
RNA Isolation and Microarray Procedures
For microarray analysis, all strains were grown to SP, and Q and NQ fractions were separated as described above. Total RNA was isolated using a modified Gentra protocol, labeled, and hybridized as described previously (Aragon et al., 2006). To avoid confounding factors, all RNA preps, labeling, and hybridizations were carried out in random order and rerandomized for each of the three steps. Analysis of hybridized arrays was carried out using GenePix 6.0 and Acuity 4.0., as described previously (Aragon et al., 2006). VxOrd 1.58 was used to cluster by both gene expression and arrays, and VxInsight 2.165 was used for visualization (Davidson et al., 2001). Clustering was done using force-directed placement of 20 and 15 nearest neighbors, for cluster by gene and array, respectively, by using the t-statistic of Pearson's R (Werner-Washburne et al., 2002). Gene lists were then queried using Gene Ontology (GO) Term Finder. All microarrays analyzed in this study are available at Gene Expression Omnibus by using accession number GSE8624.
Reproducibility
Experimental reproducibility was determined using in-house generated MATLAB (Mathworks, Natick, MA) scripts (Slide Compare and Set Compare), as described previously (Martinez et al., 2004), and they are available by request. To determine biological reproducibility, five biological replicates were hybridized in duplicate for a total of 10 microarrays for both Q and NQ fractions from wild-type and parental strains. Correlation plots and histograms of the deviation were examined using Slide Compare, with the R2 value and proportional error estimate determined for each replicate. The R2 values were also visualized using Set Compare, by using an all-against-all plot (Martinez et al., 2004). Technical and biological samples were highly correlated for both the Q and NQ fractions (see Supplemental Material). Within Q and NQ fractions, Pearson's correlation coefficients were 0.97 for both Q and NQ cell fractions from the wild-type strain (see Supplemental Material) and 0.94 and 0.95 for Q and NQ fractions, respectively, from the parental strain (see Supplemental Material). In contrast, the correlation between Q and NQ fractions was 0.90 and 0.86 for the wild-type and parental strains, respectively (see Supplemental Material). Although still reasonably correlated, this difference was significant, with p < 1 × 10−30 for both wild-type and parental strains and allowed identification of hundreds of genes whose mRNA abundance is significantly different between Q and NQ cell fractions.
Statistical Ranking
A previously described statistical ranking method was used to determine the mRNAs whose abundance was significantly different in the Q versus NQ fractions (Allen et al., 2006). Briefly, the mean difference in mRNA abundance for each gene between the Q and NQ fraction in all replicate microarrays was determined. If the difference was positive, the mRNA was highly abundant in the Q fraction; if it was negative, the mRNA was more highly abundant in the NQ fraction. A t-distribution was calculated and only mRNAs with p value cutoffs of ≤1 × 10−5 were considered significantly different. Gene lists were queried using GO Term Finder and by manual curation.
Protease Treatment
Protease digests during RNA isolations were carried out as described previously (Aragon et al., 2006). Briefly, after bead beating samples were centrifuged at 13,000 × g for 3 min at 4°C to remove cell debris. Cell-free lysates were divided into two tubes and incubated on ice for 1 h with 14 mg/ml proteinase K (QIAGEN, Valencia, CA) in 10 mM Tris base, pH 7.5, or 10 mM Tris, pH 7.5, alone. To ensure mixing, samples were inverted every 10 min during the incubation. After incubation, the RNA isolation for both samples was completed as described previously (Aragon et al., 2006).
RESULTS
Identification of Genes Required for Viability and Reproduction of Q and NQ Cells
Q and NQ fractions from 101 mutants in genes whose mRNA was identified as induced in SP (Martinez et al., 2004) were tested for viability, by using FungaLight staining (Invitrogen), and reproductive capacity, by using CFUs. We had previously demonstrated that at 7 d postinoculation, ∼50% of the viable NQ cells were unable to reproduce (Allen et al., 2006). We identified five categories of mutants that exhibited defects in viability and/or reproductive capacity the cell fractions (Table 1). Parental (BY4742) strains, on which percentage increase or decrease were based, showed 92 ± 2.2 and 87 ± 3.4% viability of Q and NQ cells, respectively, and 87 ± 3.4 and 38 ± 3.1% reproductive capacity (CFUs) for Q and NQ cells, respectively. The ability of less than half (44%) of the viable parental NQ cells to reproduce was consistent with our previous findings in wild-type cells (S288c) (Allen et al., 2006).
Table 1.
Change in viability and reproductive capacity of mutants from the parental strain
| Group | Strain | Q viability, % | Q CFU, % | NQ viability | NQ CFU, % | Functiona |
|---|---|---|---|---|---|---|
| 1 | ETR1b | NCc | ↓ 33 ± 1.4 | NC | NC | Thioester reductased |
| 2 | ATP2b | NC | ↓ 87 ± 4.5 | NC | ↓ 92 ± 7.8 | ATP synthesisd |
| FMP45b | ↓ 34 ± 20.2 | ↓ 42 ± 21.5 | Membrane proteind | |||
| QCR7b | ↓ 71 ± 21.1 | ↓ 59 ± 24.7 | Cytochrome-c reductased | |||
| ARD1b | ↓ 29 ± 6.6 | ↓ 46 ± 23.3 | Acetyltransferase | |||
| NAT1b | ↓ 24 ± 5.3 | ↓ 29 ± 2.0 | Acetyltransferase | |||
| 3 | POR1b | NC | NC | NC | ↓ 93 ± 5.5 | Mitochondrial porind |
| DDR2 | ↓ 28 ± 6.3 | Stress response | ||||
| 4 | ICL1b | NC | NC | NC | ↑ 80 ± 45.5 | Glyoxylate cycle |
| SPG3 | ↑ 116 ± 57.3 | Unknown | ||||
| 5 | DOA4pb | ↓ 48 ± 36.2 | ↓ 95 ± 4.4 | ↓ 32% ± 2.2 | ↓ 91 ± 2.4 | Ubiquitination |
a Saccharomyces Genome Database.
b Reduced Q cell production.
c NC, no change.
d Mitochondrial.
One mutant, etr1, exhibited a significant loss in Q cell reproductive capacity (Table 1, group 1). etr1 mutants lack a mitochondrial thioester reductase that likely functions in fatty acid synthesis (Torkko et al., 2003). In addition to reduced Q cell reproductive capacity, etr1 strains also showed a marked decrease in the typical 1:1 Q:NQ cell abundance (see Supplemental Material). The role of Etr1p in Q cells is consistent with our finding that mRNAs involved in fatty acid and membrane metabolism are abundant in Q cells (Allen et al., 2006).
Five mutants exhibited loss of reproductive capacity in both Q and NQ cells (Table 1, group 2). These included strains carrying deletions in the mitochondrial gene QCR7 encoding subunit 7 of the ubiquinol cytochrome-c reductase complex (De Haan M. et al., 1984); NAT1 and ARD1, encoding a subunits of N-terminal acetyltransferase, previously reported to be required for “viability” in SP (Park and Szostak, 1992); and FMP45, which encodes a highly conserved, cell cortex protein (MIPS) that has been shown to affect sphingolipid biosynthesis and sporulation (Young et al., 2002).
Mutants lacking ATP2, encoding the β-subunit in the F1 portion of mitochondrial F1F0 ATP synthase (Devenish et al., 2000), also showed reproductive defects. To determine whether other F0F1-ATPase components exhibit this phenotype, we also examined atp1, atp17, and atp18 mutants. Although atp1 and atp2 mutants frequently exhibited a similar effect on both Q and NQ cells, all of the atp mutants exhibited a high degree of variability in terms of their effects on reproductive capacity and the changes over time (data not shown). This suggests to us that these strains are capable of accumulating suppressors, possibly in an epigenetic manner. For example, atp17 strains from the deletion set typically produced both small and large colonies. When restreaked, cells from small colonies produced both large and small colonies. We conclude from this result that, although ATPase mutants have an effect on the reproductive capacity of Q and NQ cells, there are additional, possibly epigenetic mechanisms that promote a high degree of variability in these cells after glucose starvation.
All of the strains identified in group 2 affected the production Q cells, i.e., whether a band was visible, although for some, the effect is variable. We previously reported that atp1 and qcr7 mutants did not form a lower band (Allen et al., 2006); however, these and atp2 strains usually form a smear of cells that extends from the upper, NQ band to where Q cells typically band. These intermediate cells were evaluated. atp 17 and atp18 strains, which did not reduce reproductive capacity of either cell type also showed a variable reduction in the Q cell fraction (see Supplemental Material).
Two groups of mutants affected the reproductive capacity of NQ cells only (Table 1, groups 3 and 4). One group was composed of two mutants that exhibited reduced NQ cell replicative capacity, and the second group of two mutants exhibited increased reproductive capacity. In the first group were strains carrying deletions in POR1, encoding mitochondrial porin (Lee et al., 1998); and DDR2, encoding a Saccharomyces-specific stress-response protein of unknown function (Kobayashi et al., 1996). Mutants that resulted in an increase in NQ reproductive capacity carried deletions in ICL1, encoding isocitrate lyase, a key enzyme in the glyoxylate cycle and required for growth on ethanol (Fernandez et al., 1992), and SPG3, encoding a protein of unknown function. There were several other mutants that were not included in this table because they were highly variable in their ability to reduce or increase NQ cell reproductive capacity (see Supplemental Material). We hypothesize that induction of proteins involved in DNA transposition and recombination in NQ cells may make this cell fraction phenotypically more variable (Allen et al., 2006). We conclude from the identification of mutants that had an increase in the reproductive capacity of NQ cells (Table 1, group 4) that the loss of reproductive capacity in NQ cells at 7 d is genetically regulated (Allen et al., 2006).
Viability and reproductive capacity of both Q and NQ cells was reduced in doa4p strains, lacking ubiquitin isopeptidase, required for ubiquitin recycling (Amerik et al., 2000). DOA4 was previously shown to be required for maintenance of reproductive capacity in SP cultures (Swaminathan et al., 1999). To determine whether this was due to a decrease in proteasome function or the concentration of free ubiquitin, we examined mutants lacking UBI4, encoding polyubiquitin and known to be required for survival in SP (Finley et al., 1987), and UMP1 and RPN10, encoding proteasome proteins. ubi4 mutants exhibited a reduction in reproductive capacity of NQ cells, similar to the observation with doa4 mutants, whereas rpn10 and ump1 mutants showed a slight increase in reproductive capacity of NQ cells and no effect on Q cell reproduction (see Supplemental Material). Examination of these mutants grown on solid medium revealed that ubi4 and doa4 mutants produce a relatively high number of elongated and, sometimes, branched cells, whereas ump1 and rpn10 mutants do not (see Supplemental Material). We conclude from these results that ubi4 and doa4 deletions have similar effects on NQ and Q cells; thus, free ubiquitin, not proteasome function, is important for survival and reproduction of Q and NQ cells.
During this analysis, we noticed that, starting with nonpetite, single colonies from both wild type and mutants, NQ but not Q cell fractions harvested at 7 d postinoculation were more likely to contain cells that produced petite colonies. For parental, BY4742, 6 ± 2.7% of cells in Q cell fractions produced petite colonies, whereas 38 ± 15.9% of the cells in NQ fractions produced petite colonies (n = 10; 432 cells each). For wild type, S288c, the numbers were similar: Q and NQ fractions produced 8 ± 4.1 and 45 ± 18.7% petite colonies, respectively (n = 4; 432 cells each).
During our screen, we identified 13 mutants that showed increased or decreased petite formation (Table 2). Petite formation was selective, in that the production of petites in either Q or NQ cell fractions but not both (Table 2), and most mutants affected petite formation in the NQ fraction. Interestingly, some mutants that affected petite formation, such as gpg1, jen1, om14, gpd1, and mdh1, had no effect on viability or reproductive capacity, whereas the increase in NQ reproductive capacity shown by the spg3 mutant was due almost completely to the increased reproductive capacity of petite colonies. We conclude from these results that viability, reproductive capacity, and production of petites are independent phenotypes. However, these phenotypes can interact, for example, when NQ cell reproliferation increases as a result of increases in the ability of petite cells to reproduce or a decrease in apoptosis. Finally, the pleiotropic effects of different mitochondrial mutants again underscore the complexity of mitochondrial function during this phase of the yeast life cycle. We conclude from these results that Q and NQ cells have differentiated significantly but that, for both cell types, reproductive capacity is much more sensitive to perturbation than viability.
Table 2.
Change in petite colonies of mutants from the parental strain
| Group | Strain | Q petite, % | NQ petite, % | Functiona |
|---|---|---|---|---|
| 1 | ATP1b | NC | ↓ 99 ± 3.3 | ATP synthesisc |
| DOA4b | ↓ 98 ± 8.9 | Ubiquitination | ||
| POR1 | ↓ 97 ± 1.9 | Mitochondrial porinc | ||
| XBP1 | ↓ 95 ± 6.9 | Transcriptional repressor | ||
| JEN1 | ↓ 95 ± 2.2 | Lactate transporter | ||
| OM14 | ↓ 92 ± 9.9 | Mitochondrial outer membrane proteinc | ||
| GPD1 | ↓ 92 ± 4.1 | Dehydrogenase | ||
| MDH1 | ↓ 89 ± 3.5 | Dehydrogenasec | ||
| 2 | ARD1 | ↑ 1074 ± 303.0 | NCd | Acetyltransferase |
| GPG1 | ↑ 157 ± 32.0 | Signal transduction | ||
| GTT1 | ↑ 442 ± 202.0 | Glutathione transferasee | ||
| YLR312C | ↑ 294 ± 9.6 | Unknown | ||
| 3 | SPG3 | NC | ↑ 110 ± 9.9 | Unknown |
a Saccharomyces Genome Database.
b atp2 and ubi4 mutants, analyzed separately, exhibit the same phenotype.
c Mitochondrial.
d NC, no change.
e Endoplasmic reticulum.
Compendium Microarray Analysis
We were interested in identifying a large number of genes whose mRNA abundance distinguishes Q from NQ cells. Previous analysis of mRNA abundance, by using statistical ranking of data from 12 microarrays, revealed slightly >300 transcripts that distinguished Q from NQ cells (Allen et al., 2006). To increase our resolution of the differences between Q and NQ cells, we analyzed a total 398 microarrays of isolated cell fractions from wild type (S288c), auxotrophic parental (BY4742), and 101 deletion mutants (Winzeler et al., 1999) (358 arrays, including a replicate set of 98 deletion mutants).
Comparison of Q and NQ Cell Fractions
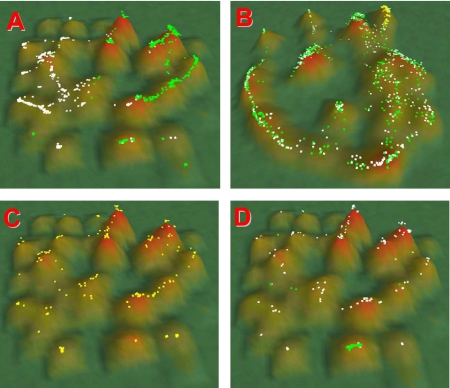
Using the yeast deletion strain data set (358 arrays), 1301 mRNAs were identified (p < 10−5) that distinguished Q from NQ cells (683/618 genes for Q/NQ fractions, respectively) (see Supplemental Material). Cluster analysis using VxInsight confirmed that the mRNAs from each group formed distinct domains in the topography (Figure 1A). As observed in previous clustering analysis (Werner-Washburne et al., 2002), genes encoding ribosomal proteins formed a tight cluster (Figure 1D, green dots). To determine whether Q cells exhibited a G1-like expression pattern, i.e., that the observed clustering in the Q/NQ topography was similar to that from published cell cycle data (Spellman et al., 1998; Werner-Washburne et al., 2002), we determined the cluster location of Q- and NQ-specific genes in the cell cycle topography (Figure 1B). It is clear that both Q- and NQ-specific genes are nonrandomly distributed over the entire topography. To determine whether G1-regulated genes were clustered in the Q/NQ VxInsight topography, we selected G1-regulated genes (Figure 1B, yellow dots) and we evaluated their distribution as a function of Q/NQ clusters (Figure 1C). Because G1-regulated genes do not cluster in the Q/NQ topography, we concluded that Q cells are not in a G1 arrest. This is consistent with our previous hypothesis that Q cells are in a G0 state (Allen et al., 2006). The clustering of G1-regulated genes with both Q and NQ genes (Figure 1D) further suggests that these genes may affect Q and NQ cells differently; thus, reevaluation of these mutants could provide important insight into the differentiation of Q and NQ cells.
Figure 1.
Distribution of gene sets in VxInsight Q/NQ gene-expression and cell cycle topographies (Spellman et al., 1998). Clustering is described in Materials and Methods. Hill height is a function of the number of genes in that cluster. (A) Clustering of gene expression for 178 arrays from Q and NQ fractions. Green dots, genes whose mRNAs are significantly increased in Q fractions; white dots, genes that are significantly increased in NQ fractions. (B) Localization of Q (green dots) and NQ (white dots) from A on the gene expression topography from Spellman's cell cycle data set. Yellow spots in upper right are G1-regulated genes. (C) Distribution of G1-regulated genes from B as a function of Q/NQ gene expression topography. (D) Green dots, ribosomal hill; white dots, distribution of the top 200 aging genes (Powers et al., 2006) as a function of the Q/NQ gene-expression topography.
We examined the gene lists from NQ and Q cells by using GO, and we found significant differences between the two cell fractions. For NQ cells, categories of Ty element transposition and DNA recombination were significantly increased (p ≤ 10−5) (Table 3). The identification of mRNAs encoding proteins involved in DNA recombination is consistent with our hypothesis that futile or nonproductive recombination events in these cells eventually results in apoptosis (Allen et al., 2006). In contrast, Q cell-abundant mRNAs were remarkable for the large number of significant GO process categories (Table 3). The most significant GO categories included exocytosis, membrane organization and biogenesis, and vesicle-mediated transport. The latter category was paradoxical, because one of the characteristics of these cells is the apparent absence of endoplasmic reticulum and Golgi (Allen et al., 2006). mRNAs encoding proteins involved in fatty acid oxidation and response to oxidative stress were consistent with the enhanced stress resistance of these cells and the likelihood that these cells survive long periods via lipid catabolism. Finally, signal transduction was a significant GO category in Q but not NQ cells in this analysis.
Table 3.
GO processesa terms strongly associated with Q or NQ cells (p ≤ 10−5)
| Go Term | NQ cells |
|
|---|---|---|
| Gene no. | p value | |
| Ty element transposition | 56 | 4.19−29 |
| DNA transposition | 57 | 3.01−27 |
| DNA recombination | 63 | 1.94−19 |
| DNA metabolism | 81 | 2.48−06 |
| Q cells |
||
|---|---|---|
| Vesicle-mediated transport | 63 | 1.29−09 |
| Vesicle docking during exocytosis | 9 | 2.77−06 |
| Oxygen and reactive oxygen species metabolism | 18 | 9.11−06 |
| Vesicle docking | 9 | 9.41−06 |
| Fatty acid oxidation | 8 | 1.22−05 |
| Exocytosis | 14 | 1.55−05 |
| Membrane organization and biogenesis | 24 | 2.05−05 |
| Response to oxidative stress | 17 | 2.07−05 |
| Membrane fusion | 17 | 7.46−05 |
| Lipid metabolism | 41 | 9.20−05 |
| Cellular lipid metabolism | 39 | 9.55−05 |
| Signal transduction | 33 | 9.67−05 |
a Gene Ontology Saccharomyces Genome Database.
Analysis of mRNAs Encoding Signal Transduction Proteins in Q and NQ Cells
Conserved signal transduction pathways, including protein kinase (PK) A, target-of-rapamycin (TOR), PKC, SNF1, and PHO85 are known to be required for the transition from fermentative to respiratory growth at the diauxic shift and entry into SP (Herman, 2002; Gray et al., 2004). The SNF1 pathway must be activated and PKC transiently activated during the diauxic shift, whereas inhibition of PKA and TOR is required for this process (Gray et al., 2004).
Of the seven signaling-related genes whose mRNAs are abundant in NQ cells (p < 10−5), at least four are involved in the cell cycle or growth (Table 4). These include Sit4p, which up-regulates CLN1 and CLN2 expression (Fernandez-Sarabia et al., 1992); Ste20p, involved in pseudohyphal growth under conditions of nitrogen deficiency (Hohmann, 2002); HSL1, a kinase required for the degradation of Swe1p, an inhibitor of Cdc28p (McMillan et al., 1999); and Kic1p, a kinase required for cell wall morphogenesis and integrity (Sullivan et al., 1998). Hog1p, required for osmotolerance during exponential growth (Hohmann, 2002), is the only primary signaling kinase whose mRNA is abundant in NQ cells. These data are consistent with the hypothesis that NQ cells are unable to arrest growth and, as a result, they may attempt to maintain a physiological state closer to growing cells in nonstarvation medium.
Table 4.
Nonquiescent fraction signaling pathway genesa
| Gene | Pathway | Conservationb | Functional annotationb |
|---|---|---|---|
| HOG1 | HOG | HCc | Regulates osmoresponsive genes |
| HSL1 | HOG | HC | Relays nutrient conditions to Cdc28p, negative regulator of SWE1 |
| STE20 | HOG | HC | Involved in invasive and pseudohyphal growth pathways |
| KIC1 | PKA | HC | Serine/threonine protein kinase involved in cell separation |
| YGK3 | PKA | HC | Phosphorylates Bcy1p |
| SKM1 | PKA | HC | Acts on Cdc42p in the pseudohyphal/invasive growth pathways |
| SIT4 | TOR | HC | Involved in mitotic cell cycle regulation |
a Statistical ranking analysis.
b Proteome database.
c Highly conserved.
In contrast, 31 mRNAs abundant in Q cells encode proteins involved in every major signaling pathway (p < 10−5) (Table 5). Strikingly, these included the central protein kinases for TOR, PKA, and SNF1 pathways, including TPK1 and TPK2, encoding two of three catalytic subunits of PKA (Toda et al., 1987b), and TOR1 and TOR2, encoding the Tor kinases, positive regulators of ribosome biogenesis, cell cycle, and other growth-related processes (Raught et al., 2001). mRNA encoding Pho85p, a cyclin-dependent kinase required for proper gene regulation at the diauxic shift (Nishizawa et al., 2004) accumulated in Q cells, but it was present in <80% of our arrays (see Materials and Methods). These results suggest that Q cells have the capacity to respond rapidly to environmental stimuli.
Table 5.
Signal transduction genes associated with the quiescent fractiona
| Gene | Pathway | Conservationb | Annotationb |
|---|---|---|---|
| PTP3 | HOG | HCc | Negative regulator of HOG pathway |
| RCK2 | HOG | HC | Activated by Hog1p and activates elongation factor EF-2 |
| SSK1 | HOG | Yeast only | Involved with osmosensing |
| STE11 | HOG | HC | Pheromone response, pseudohyphal and invasive growth pathways |
| STE50 | HOG | Yeast only | Mating response, invasive/filamentous growth, and osmotolerance |
| VPS36 | HOG | Yeast only | Part of the ESCRT-II complex |
| FMP48 | HOG | HC | Serine/threonine protein kinase of unknown function interacts with HOG1 |
| PRR2 | HOG | HC | Mitogen-activated protein (MAPK) kinase signaling in the pheromone response pathway |
| CDC55 | HOG | HC | Required for DNA replication and spore production |
| MSG5 | HOG | HC | Inactivates Fus3p |
| CKA1 | PHO | HC | PHO pathway transporting inorganic phosphate |
| TPK1 | PKA | HC | Catalytic subunit of cAMP-dependent protein kinase BCY1 |
| TPK2 | PKA | HC | Activates filamentous growth, regulates unipolar budding and invasive growth |
| BCY1 | PKA | HC | PKA regulatory subunit |
| BMH2 | PKA | HC | RAS/MAPK cascade signaling during pseudohyphal development |
| CYR1 | PKA | HC | Adenylate cyclase |
| IRA1 | PKA | HC | Negatively regulates GTP-Ras2p basal level with Ira2p |
| PKH3 | PKA | HC | Member of the PKA-related protein kinases |
| RIM15 | PKA | HC | Positive regulator of entry into SP |
| YAK1 | PKA | HC | Negative regulator of cell growth in opposition to PKA |
| LSP1 | PKC | Yeast only | Down regulates PKC pathway |
| MKK2 | PKC | HC | Mitogen-activated kinase kinase involved in PKC signaling |
| RHO1 | PKC | HC | Required to activate PKC pathway |
| TUS1 | PKC | HC | Regulates Rhop1 activity |
| GAL83 | SNF | HC | Activates Snf1p |
| SNF1 | SNF | HC | Response to glucose starvation and derepression of glucose-repressed genes |
| SAK1 | SNF | HC | Activates Snf1p |
| SIP1 | SNF | Yeast only | Proposed regulator of Snf1p |
| TOR1 | TOR | HC | Reg of replicative life span, translation initiation, starvation response, etc. |
| TOR2 | TOR | HC | Actin cytoskeleton organization |
| PPH21 | TOR | HC | Catalytic subunit of PP2A-1, interacts with Tap42p |
a Statistical ranking analysis.
b Proteome database.
c Highly conserved.
Regulation of these signal transduction pathways is critical for survival. To evaluate the potential for Q cells to activate or repress signaling, we examined mRNAs for other proteins in these pathways. Snf1p needs to be activated for cells to transit the diauxic shift, and, consistent with Snf1p activation, mRNAs encoding Snf1p activators Gal83p and Sak1p (Vincent and Carlson, 1998) were abundant in Q cells. In contrast, for the progrowth PKA pathway, mRNAs were abundant that encode negative regulators or antagonists, including Rim15p kinase, required for survival after glucose exhaustion and requiring down-regulation of PKA, TOR, and Pho85p for activation (Cameroni et al., 2004; Gray et al., 2004; Wanke et al., 2005); Yak1p, a negative regulator of PKA required for the transient growth arrest after glucose exhaustion (Garrett et al., 1991; Moriya et al., 2001); and Bcy1p, the evolutionarily conserved regulatory subunit of PKA (Toda et al., 1987a). Similarly, for the TOR pathway, PPH22 mRNA was abundant, encoding protein phosphatase 2A, an antagonist of TOR activity. These results suggest that signal-transduction pathways in Q cells are highly regulated; thus, Q cells are poised for long-term survival of starvation and rapid response when conditions become favorable for growth.
mRNAs Released after Protease Treatment Are Found Predominately in the Q Fraction
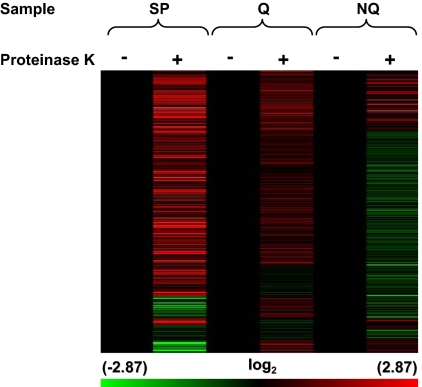
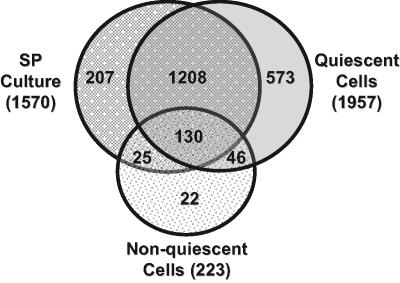
We previously demonstrated the presence of thousands of mRNAs released after protease treatment during RNA isolation in wild-type cells in unfractionated SP cultures and that these mRNAs can be released in a stress-specific manner (Aragon et al., 2006). To determine whether Q or NQ cell fractions preferentially contain protease-released mRNAs, lysates from Q and NQ cell fractions and unfractionated cells from parental SP cultures were incubated with or without proteinase K (Figure 2). Cells from SP culture contained 1570 protease-released transcripts that increased 1.5- to 128-fold. Lysates from Q and NQ cell fractions exhibited similar increases in 1957 and 223 transcripts, respectively. Thus, there were almost 9 times more protease-released mRNAs in Q as in NQ cells. The overlap between protease-released mRNAs present in both SP cells and Q cells was 85% (p < 1 × 10−50) and between SP and NQ cells was 70% (p < 1 × 10−40) (Figure 3). We hypothesize that the protease-released mRNAs found in Q or NQ cells but not in cells from SP cultures are likely to be mRNAs that are relatively abundant in the other cell fraction so they do not increase significantly in protease-treated lysates from SP cultures.
Figure 2.
mRNA abundance in Q and NQ samples treated with or without proteinase K. Unsupervised hierarchical clustering (Pearson's centered, average-linkage) was used to cluster ∼2400 transcripts. Samples include, unseparated SP culture (SP) and Q and NQ cell fractions. Cell lysates from samples were incubated with buffer alone (−) or proteinase K (+). Before clustering, treated samples were normalized to untreated samples (black lanes). The color scale at the bottom indicates the log2 values for changes in mRNA abundance.
Figure 3.
Venn diagram of transcripts that increased after proteinase K treatment of cell lysates. Transcripts were evaluated that had a ≥1.5-fold increase in abundance after proteinase K treatment of SP culture and Q and NQ cell fraction lysates. The transcripts that were evaluated were required to be present in 80% of the data points.
Protease-released mRNAs in Q cells included almost half of the mRNAs identified as abundant in NQ cells (299/618), whereas only 26 of 683 Q mRNAs were found among protease-released mRNAs from NQ cells. We conclude from these results that during differentiation of Q and NQ cells, Q cells selectively sequester the mRNAs that can be released in a stress-specific manner (Aragon et al., 2006). This makes Q cells uniquely capable of responding to environmental changes through the rapid release of groups of mRNAs.
We identified significant GO process groups for protease-released mRNAs from SP cultures and Q and NQ cells. Protease-released mRNAs in cells in SP culture encoded proteins involved in Ty element transposition and carbohydrate metabolism (Table 6). There were no significant GO categories in the protease-released NQ gene list (p < 10−5). The largest group was annotated as hypothetical open reading frames, again, consistent with the lack of information about these cells, and this stage of the yeast life cycle. In Q cells, the most significant groups of protease-released mRNAs (p < 10−5) encode proteins involved in Ty-element transposition and DNA recombination and a large number of HSP70-family genes (Table 5). Strikingly, these are the same mRNAs that are constitutively abundant in NQ cells (Table 3). This result suggests that Q cells are able not only to respond to progrowth signals but, should starvation continue for very long periods, they are also poised to enter apoptosis.
Table 6.
GO processesa associated with protease-treated cell lysates (p < 10−5)
| Go Term | Cells in stationary phase culture |
|
|---|---|---|
| Gene no. | p value | |
| Generation of precursor metabolites and energy | 92 | 9.64−13 |
| Main pathways of carbohydrate metabolism | 44 | 3.94−12 |
| Ty element transposition | 47 | 4.78−10 |
| DNA-mediated transposition | 49 | 7.30−10 |
| Energy derivation by oxidation of organic compounds | 74 | 9.35−10 |
| Cofactor metabolism | 62 | 2.04−09 |
| Coenzyme metabolism | 53 | 2.44−09 |
| Organic acid metabolism | 97 | 1.77−08 |
| Carboxylic acid metabolism | 97 | 1.77−08 |
| Carbohydrate metabolism | 76 | 1.10−07 |
| Acetyl-CoA metabolism | 17 | 1.59−07 |
| Tricarboxylic acid (TCA) cycle intermediate metabolism | 16 | 4.12−07 |
| Cellular carbohydrate metabolism | 69 | 5.03−07 |
| TCA cycle | 14 | 6.56−07 |
| Acetyl-CoA catabolism | 14 | 6.56−07 |
| Coenzyme catabolism | 14 | 2.75−06 |
| Cofactor catabolism | 14 | 5.23−06 |
| Pyruvate metabolism | 20 | 8.83−06 |
| Q cells |
||
|---|---|---|
| Ty element transposition | 74 | 2.21−28 |
| transposition | 75 | 5.54−27 |
| transposition, DNA-mediated | 75 | 5.54−27 |
| DNA recombination | 82 | 3.15−15 |
| DNA metabolism | 132 | 4.82−06 |
a Gene Ontology Saccharomyces Genome Database.
DISCUSSION
This study expands on our previous characterization of the distinctions between Q and NQ cells in yeast SP cultures (Allen et al., 2006) and reinforces the importance of studying specific cell types in a heterogeneous mix of cells and distinguishing viability/metabolic activity from reproductive capacity. The mutants identified here are the first known to selectively affect viability or reproductive capacity in Q and/or NQ cells and to demonstrate the significant physiological differentiation of these cell types. Mitochondrial function is important for both Q and NQ reproduction and petite formation and plays a complex role in the differentiation of these cells. The ability of deletions in mitochondrial genes, especially those in the oxidative phosphorylation or ATP synthesis pathways to cause different phenotypes, including inhibiting the formation of Q cells (Allen et al., 2006), reveals the importance and complexity of mitochondrial function during differentiation, consistent with the pleiotropic role of mitochondria in human health (Steinmetz et al., 2002; Barrientos, 2003). The finding that free ubiquitin and not proteasome function is important for survival and reproduction of both cell types may be relevant to other types of cells, including stem cells, where ubiquitin has also been shown to be important for regulation of differentiation (Naujokat and Saric, 2007).
Although nuclear genes have been identified that cause differences in the production of petite or respiratory incompetent yeast cells (Contamine and Picard, 2000), the cell type that produces petite cells has not previously been known. It has been suggested that petite formation (reduction of mitochondrial function) protects cells experiencing high levels of reactive oxygen species (ROS) (Davermann et al., 2002). Our data showing that NQ cells exhibit much higher levels of ROS within days after glucose exhaustion (Allen et al., 2006) is consistent with this hypothesis. However, our results also show that many mitochondrial mutants do not form petites, consistent with other studies showing mutants, including ATPase mutants, that cannot tolerate loss of mitochondrial DNA (Dunn et al., 2006).
The results presented in this study are relevant to chronological aging studies using SP yeast cultures (Longo et al., 1996; Ashrafi et al., 1999; Gershon and Gershon, 2000; Jakubowski et al., 2000; Chen et al., 2005). The identification of the NQ cell (mother cell) population as producing petite colonies is especially relevant to the question of genomic instability during aging (Laun et al., 2006). Because clustering analysis revealed that the top 200 aging genes were randomly distributed (Figure 1D), we hypothesize that genes differentially affecting either Q or NQ cells could contribute to an aging phenotype in cultures, either by extending the life of Q cells or allowing NQ cells to better protect Q cells or extend the life of a subpopulation of NQ cells. Our results suggest that more precise evaluation of phenotypes based on fractionated cells could provide important insights into chronological aging.
The identification of mRNAs encoding proteins in “progrowth” signaling pathways in Q cells is consistent with previous work showing an eightfold increase in Bcy1p, the regulatory subunit of PKA, and Tpk1p, one of three PKA catalytic subunits, as cultures entered SP (Werner-Washburne et al., 1991). We hypothesized that regulation of these pathways was dynamic and that they were poised for rapid activation under favorable growth conditions (Peck et al., 1997). To our knowledge, similar experiments have not been carried out with TOR pathway components, but the role of TOR in mRNA turnover (P-bodies) and translation (De Virgilio and Loewith, 2006) suggest that this pathway is also dynamic at this time.
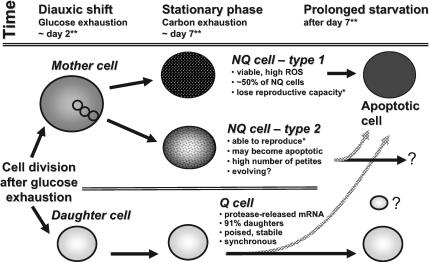
We summarize these results in our model (Figure 4). Quiescent daughter and nonquiescent cells differentiate after glucose exhaustion in cultures grown in rich, glucose-based medium (YPD). Mother cells continue to divide during the postdiauxic phase, producing unbudded, NQ daughter cells, and both budded and unbudded NQ cells rapidly lose their ability to divide (Allen et al., 2006). NQ cells are heterogeneous, with at least two types of NQ cells—those that enter apoptosis (type 1) and those that retain the capacity to reproduce and produce a high proportion of petite colonies (type 2). Q cells contain protease-released mRNA, in P-bodies or other RNA granules (Wickens and Goldstrohm, 2003; Parker and Sheth, 2007). The very tiny Q cell in Figure 4 represents cells that we see in some very old yeast cultures that are extremely small but seem to be yeast (Werner-Washburne, unpublished data). They are included to indicate that the complete story of the diversification of cell types in stationary-phase cultures is not yet completely understood.
Figure 4.
Model for cell differentiation in yeast grown in rich, glucose-based medium (YPD) after glucose and carbon starvation. Glucose exhaustion leads to the formation of daughter cells, which will become quiescent, and mother cells, 50% of which will become apoptotic by day 14 after inoculation. The NQ cells rapidly lose the ability to reproduce, and many of the NQ cells that can reproduce lose mitochondrial function, i.e., are petite. We hypothesize that this is a result of genomic instability in these cells. Q cells contain large P-bodies (Ray, unpublished) and the majority of protease-released mRNAs. In addition, based on abundant mRNAs, these cells are also poised to respond to a variety of environmental changes. Because a major group of protease-released mRNAs includes those encoding proteins involved in recombination and transposition, it seems likely that these cells are also poised to become apoptotic. We hypothesize that the differentiation of these cells, including the group of NQ cells that undergoes genomic rearrangements, is an evolutionarily conserved process that serves to provide both stability in the quiescent cells and innovation in the nonquiescent cells during times of nutrient limitation.
The differentiation of Q and NQ cell types in yeast begs the question of the relationship of this process to the production of quiescent cells in other eukaryotes. Localized or “protease-released” mRNAs are found in quiescent cells from higher eukaryotes, including neurons and stem cells (Kedersha and Anderson, 2002; Anderson and Kedersha, 2006). mRNAs encoding a variety of highly conserved and regulated signaling pathways are also highly abundant during stem-cell renewal and differentiation (Ramalho-Santos et al., 2002; Arbouzova and Zeidler, 2006) and stem cells, like Q cells, are poised to activate any number of signaling pathways (Molofsky et al., 2004; Moore and Lemischka, 2006). Regulation of the process of Q cell and NQ cell differentiation involves the same signaling pathways, including Pho85p and TOR, that regulate nutrient-regulated stem cell proliferation in C. elegans and Drosophila (Narbonne and Roy, 2006). Apoptosis observed in NQ cells is commonly observed during the asymmetrical cell divisions leading to neuronal and stem cell biogenesis (Yoshikawa, 2000; Cai et al., 2004; Lynch, 2004). Finally, the potential for Q cells to become apoptotic, based on the presence of protease-released mRNAs in Q cells, is thought to be the mechanism by which the body removes most stem cells with oncogenic mutations (Lynch, 2004). We hypothesize, therefore, that Q and NQ cells are analogous to undifferentiated stem cells and differentiated niche cells, respectively, and that the process of formation of these two cell types is related to biogenesis of stem cells in higher eukaryotes.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the laboratory and especially Dr. Steve Phillips for helpful discussions. This work was supported by National Institutes of Health (NIH) grant GM-67593 and National Science Foundation grant MCB-0092364 (to M.W.W.). A.D.A. was supported by a grant from NIH/Initiative for Maximizing Student Diversity (IMSD) grant GM-60201. S.R. was supported by a fellowship from the Program in Interdisciplinary Biological and Bio-medical Science funded by the Howard Hughes Medical Institute and the NIH/National Institute of Biomedical Imaging and Bioengineering. C.A. was supported by NIH grant GM-072351. R.J. was supported by NIH grant GM-075149. P.H.T. was supported by NIH/IMSD grant GM-060201. This work was funded in part by the U.S. Department of Energy's Genomics: GTL Program (http://www.doegenomestolife.org) under project, “Carbon Sequestration in Synechococcus sp.: From Molecular Machines to Hierarchical Modeling” (http://www.genomes-to-life.org). Sandia National Laboratories is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the U.S. Department of Energy under contract DE-ACO4-94AL85000. Data were generated in the Flow Cytometry Shared Resource Center supported by the University of New Mexico Health Sciences Center and the University of New Mexico Cancer Center.
Abbreviations used:
- NQ
nonquiescent
- Q
quiescent
- SP
stationary phase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0666) on January 16, 2008.
REFERENCES
- Abbott A. The root of the problem. Nature. 2006;442:742–743. doi: 10.1038/442742a. [DOI] [PubMed] [Google Scholar]
- Allen C., et al. Isolation of quiescent and non-quiescent cells from stationary-phase yeast cultures J. Cell Biol. 2006;174:89–100. doi: 10.1083/jcb.200604072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerik A. Y., Nowak J., Swaminathan S., Hochstrasser M. The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein-sorting and endocytic pathways. Mol. Biol. Cell. 2000;11:3365–3380. doi: 10.1091/mbc.11.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Kedersha N. RNA granules. J. Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon A. D., Quiñones G. A., Thomas E. V., Roy S., Werner-Washburne M. Release of extraction-resistant mRNA in stationary-phase S. cerevisiae produces a massive increase in transcript abundance in response to stress. Genome Biol. 2006;7:R9. doi: 10.1186/gb-2006-7-2-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbouzova N. I., Zeidler M. P. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Ashrafi K., Sinclair D., Gordon J. I., Guarente L. Passage through stationary phase advances replicative aging in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1999;96:9100–9105. doi: 10.1073/pnas.96.16.9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A. Yeast models of human mitochondrial diseases. Iubmb Life. 2003;55:83–95. doi: 10.1002/tbmb.718540876. [DOI] [PubMed] [Google Scholar]
- Cai J. L., Weiss M. L., Rao M. S. In search of “stemness”. Exp. Hematol. 2004;32:585–598. doi: 10.1016/j.exphem.2004.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameroni E., Hulo N., Roosen J., Winderickx J., De Virgilio C. The novel yeast PAS kinase RIM15 orchestrates G0-associated antioxidant defense mechanisms. Cell Cycle. 2004;3:462–468. [PubMed] [Google Scholar]
- Chen Q. H., Ding Q. X., Keller R. N. The stationary phase model of aging in yeast for the study of oxidative stress and age-related neurodegeneration. Biogerontology. 2005;6:1–13. doi: 10.1007/s10522-004-7379-6. [DOI] [PubMed] [Google Scholar]
- Contamine V., Picard M. Maintenance and integrity of the mitochondrial genome: a plethora of nuclear genes in the budding yeast. Microbiol. Mol. Biol. Rev. 2000;64:281–315. doi: 10.1128/mmbr.64.2.281-315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davermann D., Martinez M., McKoy J., Patel N., Averbeck D., Moore C. W. Impaired mitochondrial function protects against free radical-mediated cell death. Free Radic. Biol. Med. 2002;33:1209–1220. doi: 10.1016/s0891-5849(02)00984-x. [DOI] [PubMed] [Google Scholar]
- Davidson G. S., Wylie B. N., Boyack K. Cluster stability and the use of noise in interpretation of clustering. Proc. IEEE Inf. Visualization. 2001;2001:23–30. [Google Scholar]
- De Haan M, van Loon A. P, Kreike J, Vaessen R. T, L. A., G. The biosynthesis of the ubiquinol-cytochrome c reductase complex in yeast. DNA sequence analysis of the nuclear gene coding for the 14-kDa subunit. Eur. J. Biochem. 1984;138:169–177. doi: 10.1111/j.1432-1033.1984.tb07896.x. [DOI] [PubMed] [Google Scholar]
- De Virgilio C., Loewith R. Cell growth control: little eukaryotes make big contributions. Oncogene. 2006;25:6392–6415. doi: 10.1038/sj.onc.1209884. [DOI] [PubMed] [Google Scholar]
- Devenish R. J., Prescott M., Roucou X., Nagley P. Insights into ATP synthase assembly and function through the molecular genetic manipulation of subunits of the yeast mitochondrial enzyme complex. Biochim. Biophys. Acta. 2000;1458:428–442. doi: 10.1016/s0005-2728(00)00092-x. [DOI] [PubMed] [Google Scholar]
- Dunn C. D., Lee M. S., Spencer F. A., Jensen R. E. A genomewide screen for petite-negative yeast strains yields a new subunit of the i-AAA protease complex. Mol. Biol. Cell. 2006;17:213–226. doi: 10.1091/mbc.E05-06-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sarabia M. J., Sutton A., Zhong T., Arndt K. T. SIT4 protein phosphatase is required for the normal accumulation of SWI4, CLN1, CLN2, and HCS26 RNAs during late G1. Genes Dev. 1992;6:2417–2428. doi: 10.1101/gad.6.12a.2417. [DOI] [PubMed] [Google Scholar]
- Fernandez E., Moreno F., Rodicio R. The ICL1 gene from Saccharomyces cerevisiae. Eur. J. Biochem. 1992;204:983–990. doi: 10.1111/j.1432-1033.1992.tb16720.x. [DOI] [PubMed] [Google Scholar]
- Finley D., Özaynak E., Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high-temperatures, starvation, and other stresses. Cell. 1987;48:1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- Garrett S., Menold M. M., Broach J. R. The Saccharomyces cerevisiae YAK1 gene encodes a protein kinase that is induced by arrest early in the cell cycle. Mol. Cell. Biol. 1991;11:4045–4052. doi: 10.1128/mcb.11.8.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon H., Gershon D. The budding yeast; Saccharomyces cerevisiae; as a model for aging research: a critical review. Mech. Ageing Dev. 2000;120:1–22. doi: 10.1016/s0047-6374(00)00182-2. [DOI] [PubMed] [Google Scholar]
- Gray J. V., Petsko G. A., Johnston G. C., Ringe D., Singer R. A., Werner-Washburne M. “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2004;68:187–206. doi: 10.1128/MMBR.68.2.187-206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P. K. Stationary phase in Yeast. Curr. Opin. Microbiol. 2002;5:602–607. doi: 10.1016/s1369-5274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 2002;66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski W., Bilinski T., Bartosz G. Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic. Biol. Med. 2000;28:659–664. doi: 10.1016/s0891-5849(99)00266-x. [DOI] [PubMed] [Google Scholar]
- Kaeberlein T., Lewis K., Epstein S. S. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science. 2002;296:1127–1129. doi: 10.1126/science.1070633. [DOI] [PubMed] [Google Scholar]
- Kedersha N., Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 2002;30:963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- Kim C. F. B., Jackson E. L., Woolfenden A. E., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R. T., Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., McClanahan T. K., Simon J. R., Treger J. M., McEntee K. Structure and functional analysis of the multistress response gene DDR2 from Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1996;229:540–547. doi: 10.1006/bbrc.1996.1840. [DOI] [PubMed] [Google Scholar]
- Laun P., Rinnerthaler M., Bogengruber E., Heeren G., Breitenbach M. Yeast as a model for chronological and reproductive aging–a comparison. Exp. Gerontol. 2006;41:1208–1212. doi: 10.1016/j.exger.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Lee A. C., Xu X., Blachly-Dyson E., Forte M., Colombini M. The role of yeast VDAC genes on the permeability of the mitochondrial outer membrane. J. Membr. Biol. 1998;161:173–181. doi: 10.1007/s002329900324. [DOI] [PubMed] [Google Scholar]
- Longo V. D., Gralla E. B., Valentine J. S. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae: mitochondrial production of toxic oxygen species in vivo. J. Biol. Chem. 1996;271:12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- Lynch M. D. The role of cellular senescence may be to prevent proliferation of neighboring cells within stem cell niches. Ann. N Y Acad. Sci. 2004;1019:191–194. doi: 10.1196/annals.1297.030. [DOI] [PubMed] [Google Scholar]
- Martinez M., Roy S., Archuletta A., Wentzell P., Santa Anna-Arriola S., Rodriguez A., Aragon A., Quinones G., Allen C., Werner-Washburne M. Genomic analysis of stationary-phase and exit in Saccharomyces cerevisiae: gene expression and identification of novel essential genes. Mol. Biol. Cell. 2004;15:5295–5305. doi: 10.1091/mbc.E03-11-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan J. N., Longtine M. S., Sia R. A. L., Theesfeld C. L., Bardes E. S. G., Pringle J. R., Lew D. J. The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol. Cell. Biol. 1999;19:6929–6939. doi: 10.1128/mcb.19.10.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A. V., Pardal R., Morrison S. J. Diverse mechanisms regulate stem cell self-renewal. Curr. Opin. Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Moore K. A., Lemischka I. R. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Moriya H., Shimuzu-Yshida Y., Omori A., Iwashita S., Katoh M., Sakai A. Yak1p, a DYRK family kinase, translocates to the nucleus and phosphorylates yeast Pop2p in response to a glucose signal. Genes Dev. 2001;15:1217–1228. doi: 10.1101/gad.884001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. J. Defining the requirements for immunological control of mycobacterial infections. Trends Microbiol. 1999;7:366–372. doi: 10.1016/s0966-842x(99)01567-x. [DOI] [PubMed] [Google Scholar]
- Narbonne P., Roy R. Regulation of germline stem cell proliferation downstream of nutrient sensing. Cell Div. 2006;1:29. doi: 10.1186/1747-1028-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokat C., Saric T. Concise review: role and function of the ubiquitin-proteasome system in mammalian stem and progenitor cells. Stem Cells. 2007;25:2408–2418. doi: 10.1634/stemcells.2007-0255. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Katou Y., Shirahige K., Toh-e A. Yeast Pho85 kinase is required for proper gene expression during the diauxic shift. Yeast. 2004;21:903–918. doi: 10.1002/yea.1138. [DOI] [PubMed] [Google Scholar]
- Park E.-C., Szostak J. W. ARD1 and NAT1 proteins form a complex that has N-terminal acetyltransferase activity. EMBO J. 1992;11:2087–2093. doi: 10.1002/j.1460-2075.1992.tb05267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., Sheth U. P bodies and the control of mRNA translation and degradation. Mol. Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Peck V., Fuge E., Padilla P., Gomez M., Werner-Washburne M. Yeast bcy1 mutants with stationary phase-specific defects. Curr. Genet. 1997;32:83–92. doi: 10.1007/s002940050251. [DOI] [PubMed] [Google Scholar]
- Powers R. W., Kaeberlein M., Caldwell S. D., Kennedy B. K., Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M., Yoon S., Matsuzaki Y., Mulligan R. C., Melton D. A. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Raught B., Gingras A. C., Sonenberg N. The target of rapamycin (TOR) proteins. Proc. Natl. Acad. Sci. USA. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman P. T., Sherlock G., Zhang M. Q., Iyer V. R., Anders K., Eisen M. B., Brown P. O., Botstein D., Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz L. M., et al. Systematic screen for human disease genes in yeast. Nature Genetics. 2002;31:400–404. doi: 10.1038/ng929. [DOI] [PubMed] [Google Scholar]
- Suda T., Arai F., Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26:426–433. doi: 10.1016/j.it.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Sullivan D. S., Biggins S., Rose M. D. The yeast centrin, Cdc31p, and the interacting protein kinase, Kic1p, are required for cell integrity. J. Cell Biol. 1998;143:751–765. doi: 10.1083/jcb.143.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S., Amerik A. Y., Hochstrasser M. The DOA4 deubiquitinating enzyme is required for ubiquitin homeostasis in yeast. Mol. Biol. Cell. 1999;10:2583–2594. doi: 10.1091/mbc.10.8.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Scott J. D., McMullen B., Hurwitz M., Krebs E. G., Wigler M. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 1987a;7:1371–1377. doi: 10.1128/mcb.7.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987b;50:277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Torkko J. M., Koivuranta K. T., Kastaniotis A. J., Airenne T. T., Glumoff T., Ilves M., Hartig A., Gurvitz A., Hiltunen J. K. Candida tropicalis expresses two mitochondrial 2-enoyl thioester reductases that are able to form both homodimers and heterodimers. J. Biol. Chem. 2003;278:41213–41220. doi: 10.1074/jbc.M307664200. [DOI] [PubMed] [Google Scholar]
- Vincent O., Carlson M. Sip4, a Snf1 kinase-dependent transcriptional activator, binds to the carbon source-responsive element of gluconeogenic genes. EMBO J. 1998;17:7002–7008. doi: 10.1093/emboj/17.23.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke V., Pedruzzi I., Cameroni E., Dubouloz F., De Virgilio C. Regulation of G0 entry by the Pho80-Pho85 cyclin-CDK complex. EMBO J. 2005;24:4271–4278. doi: 10.1038/sj.emboj.7600889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M., Brown D., Braun E. Bcy1, the regulatory subunit of cAMP-dependent protein kinase in yeast, is differentially modified in response to the physiological status of the cell. J. Biol. Chem. 1991;266:19704–19709. [PubMed] [Google Scholar]
- Werner-Washburne M., Wylie B., Boyack K., Fuge E., Galbraith J., Weber J., Davidson G. Comparative analysis of multiple genome-scale data sets. Genome Res. 2002;12:1564–1573. doi: 10.1101/gr.225402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Goldstrohm A. A place to die, a place to sleep. Science. 2003;300:753–755. doi: 10.1126/science.1084512. [DOI] [PubMed] [Google Scholar]
- Winzeler E. A., et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- Yang H., Ren Q., Zhang Z. Chromosome or chromatin condensation leads to meiosis or apoptosis in stationary yeast. FEMS. 2006;6:1254–1263. doi: 10.1111/j.1567-1364.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K. Cell cycle regulators in neural stem cells and postmitotic neurons. Neurosci. Res. 2000;37:1–14. doi: 10.1016/s0168-0102(00)00101-2. [DOI] [PubMed] [Google Scholar]
- Young M. E., Karpova T. S., Brügger B., Moschenross D. M., Wang G. K., Schneiter R., Wieland F. T., Cooper J. A. The Sur7p family defines novel cortical domains in Saccharomyces cerevisiae, affects sphingolipid metabolism, and is involved in sporulation. Mol. Cell. Biol. 2002;22:927–934. doi: 10.1128/MCB.22.3.927-934.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.