Abstract
Histone acetyltransferases (HATs) and ATP-dependent chromatin remodeling factors (ADCRs) regulate transcription and recombination via alteration of local chromatin configuration. The ade6-M26 allele of Schizosaccharomyces pombe creates a meiotic recombination hotspot that requires a cAMP-responsive element (CRE)-like sequence M26, the Atf1/Pcr1 heterodimeric ATF/CREB transcription factor, the Gcn5 HAT, and the Snf22 SWI2/SNF2 family ADCR. Chromatin alteration occurs meiotically around M26, leading to the activation of meiotic recombination. We newly report the roles of other chromatin remodeling factors that function positively and negatively in chromatin alteration at M26: two CHD-1 family ADCRs (Hrp1 and Hrp3), a Spt-Ada-Gcn5 acetyltransferase component (Ada2), and a member of Moz-Ybf2/Sas3-Sas2-Tip60 family (Mst2). Ada2, Mst2, and Hrp3 are required for the full activation of chromatin changes around M26 and meiotic recombination. Acetylation of histone H3 around M26 is remarkably reduced in gcn5Δ, ada2Δ and snf22Δ, suggesting cooperative functions of these HAT complexes and Snf22. Conversely, Hrp1, another CHD-1 family ADCR, maintains repressive chromatin configuration at ade6-M26. Interestingly, transcriptional initiation site is shifted to a site around M26 from the original initiation sites, in couple with the histone acetylation and meiotic chromatin alteration induced around 3′ region of M26, suggesting a collaboration between these chromatin modulators and the transcriptional machinery to form accessible chromatin. These HATs and ADCRs are also required for the regulation of transcription and chromatin structure around M26 in response to osmotic stress. Thus, we propose that multiple chromatin modulators regulate chromatin structure reversibly and participate in the regulation of both meiotic recombination and stress-induced transcription around CRE-like sequences.
INTRODUCTION
Chromosomal DNA is compacted in chromatin structure, consisting of genomic DNA, histones, and other nonhistone proteins. The chromatin structure plays important roles in the expression and inheritance of genetic information in all eukaryotes. However, such chromatin compaction inhibits many DNA-related reactions, such as transcription, replication, DNA damage repair, and recombination, by preventing the access of transacting DNA-binding factors to the DNA substrates (Wolffe, 1997). Hence, such DNA-related reactions preferentially occur at nucleosome-free accessible chromatin regions, where the transacting factors can be easily recruited to the DNA substrates. Recent studies have revealed that posttranslational modification of histones (e.g., histone acetylation, methylation, and phosphorylation) and various chromatin remodeling complexes are required for the modulation of chromatin accessibility (Cosma et al., 1999; Krebs et al., 1999; Agalioti et al., 2000).
Homologous recombination contributes to increased genetic diversity during sexual reproduction and to proper segregation of homologous chromosomes in gametogenesis. Meiotic recombination is a highly regulated process initiated at defined chromosomal sites (recombination hotspots) that exhibit elevated levels of meiotic homologous recombination (Lichten and Goldman, 1995; Wahls, 1998; Petes, 2001; Nachman, 2002). In the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe, it has been demonstrated that meiotic transient DNA double-strand breaks (DSBs) at these hotspots initiate a homologous recombination reaction (Sun et al., 1989; Steiner et al., 2002). It has also been demonstrated that local chromatin configuration is involved in determining the timing and position of meiotic DSB formation. For S. cerevisiae and S. pombe, chromatin around the meiotic recombination hotspot often exhibits very high sensitivity to nucleases in vitro (Ohta et al., 1994; Wu and Lichten, 1994; Mizuno et al., 1997).
The ade6-M26 (hereafter referred as M26) locus in S. pombe is one of the most characterized meiotic recombination hotspots, which provides a good model system for studying the chromatin regulation during the activation of meiotic recombination, and even the activation of transcription coupled with the response to various extracellular stresses. The M26 allele is a single G/T transversion in the 5′ part of the ade6 coding region (Ponticelli et al., 1988; Szankasi et al., 1988). This mutation creates a nonsense codon and a cAMP-responsive element (CRE)-like heptanucleotide sequence. This heptamer acts as a binding site for the transcription factor Atf1/Pcr1, which is a heterodimer of Atf1 and Pcr1 (also called Mts1/Mts2 or Gad7/Pcr1). Both the subunits are required for hotspot activation (Wahls and Smith, 1994; Kon et al., 1997). The M26 mutation confers a meiosis-specific elevation of recombination of up to 20-fold, compared with control alleles, such as ade6-M375 (M375) (Gutz, 1971; Ponticelli et al., 1988; Schuchert et al., 1991), which also creates an identical nonsense mutation in the codon adjacent to that generated by M26. We have demonstrated that the local chromatin configuration around M26 is altered to have higher sensitivity to micrococcal nuclease (MNase) during early meiosis (Mizuno et al., 1997), suggesting that chromatin alteration is at least partly involved in the activation of recombination processes of M26. Furthermore, chromatin changes around M26 are coupled to an altered transcription of ade6-M26 in response to osmotic stress (Hirota et al., 2004). In addition, similar chromatin alteration coupled to transcriptional activation can be detected at natural M26-like sites that are often found in stress-responsive genes, such as cta3+ and fbp1+ (Hirota et al., 2004). Thus, chromatin alteration around M26 and natural M26-like sites is also important for transcriptional regulation in response to environmental stresses.
We have further indicated that full induction of this chromatin alteration is facilitated by a histone acetyltransferase (HAT), Gcn5, and a Swi2/Snf2-like ATP-dependent chromatin remodeling factor (ADCR), Snf22, but it is repressed by the global corepressors Tup11 and Tup12 (Hirota et al., 2003; Yamada et al., 2004). Deletion of the gcn5+ gene results in a partial loss of hotspot activity, whereas snf22+ deletion causes a much more severe reduction (Yamada et al., 2004). These observations suggest that the local chromatin alteration around M26 is vital for activation of the M26 recombination hotspot.
There are two major classes of chromatin modifying machineries. The first class consists of covalent modifications of histone amino termini such as acetylation and methylation. In histone acetylation, a HAT complex and deacetylase complex (HDAC) add and remove acetyl groups, respectively. Increased acetylation is usually associated with derepressed chromatin configuration (Grant et al., 1998). HAT enzymes can be divided into several broad groups based on their conserved sequence domains. Two large families are the Spt-Ada-Gcn5 acetyltransferase (SAGA) group, including Gcn5 and Ada2, and the Moz-Ybf2/Sas3-Sas2-Tip60 (MYST) group (Brown et al., 2000; Chen et al., 2001). The second class of chromatin-modifying machineries consists of ADCR complexes, which alter chromatin structure by changing the location of nucleosomes by using the energy from ATP-hydrolysis (Travers, 1999). The first ADCR to be identified was the yeast Swi2/Snf2 family protein, which contains helicase/ATPase motif as a catalytic domain and a bromodomain in its C terminus. ISWI family ADCR also has a helicase/ATPase domain but lacks a C-terminal bromodomain. A further class of ADCR implicated in chromatin remodeling is the CHD family of proteins, which contains double chromodomains in its N terminus (reviewed in Travers, 1999).
Here, we investigate the functions of Mst2, a homologue of S. cerevisiae Sas2p or Sas3p; Ada2, a homologue of S. cerevisiae Ada2p; in addition to Hrp1 and Hrp3, CHD1-like ADCRs having a chromodomain, in chromatin alteration around M26 during meiosis and stress response. We demonstrate that distinct classes of ADCRs regulate chromatin remodeling positively and negatively, giving new insight into reversible actions of chromatin remodeling at the M26 and CRE-related sites.
MATERIALS AND METHODS
Fission Yeast Strains, Genetic Methods, and Media
The S. pombe strains used in this study are listed in Table 1. The genetic procedures were carried out as described by Gutz et al. (1974). The strains were constructed by mating haploids on a sporulation medium (SPA) (Gutz et al., 1974), followed by tetrad dissection. To induce meiosis using diploid S. pombe strains, cells were cultured in MM medium (Isshiki et al., 1992) supplemented with NH4Cl as a nitrogen source to ∼1 × 107 cells/ml. These cells were harvested and washed with distilled H2O (dH2O) twice and then transferred to MM medium lacking nitrogen, to induce meiosis.
Table 1.
S. pombe strains used in this study
| Strain | Genotype |
|---|---|
| K26 | h−ade6-469 leu1-32 |
| K28 | h+ade6-M375 his5-303 |
| K31 | h+ade6-M26 his5-303 |
| K131 | h−ade6-M26 leu1-32 |
| K175 | h+ade6-M26 his5-303 ura4-D18 |
| K176 | h−ade6-M26 leu1-32 ura4-D18 |
| TY9 | h+ade6-M26 gcn5::ura4+uar4-D18 his5-303 |
| TY11 | h+ade6-M375 gcn5::ura4+uar4-D18 his5-303 |
| TY13 | h−ade6-469 gcn5::ura4+uar4-D18 his5-303 |
| PKH267 | h+ade6-M26 snf22::ura4+uar4-D18 his5-303 |
| PKH269 | h+ade6-M375 snf22::ura4+uar4-D18 his5-303 |
| PKH272 | h−ade6-469 snf22::ura4+uar4-D18 leu1-32 |
| PKH279 | h+ade6-M26 hrp1::ura4+uar4-D18 his5-303 |
| PKH281 | h+ade6-M375 hrp1::ura4+uar4-D18 his5-303 |
| PKH284 | h−ade6-469 hrp1::ura4+uar4-D18 leu1-32 |
| PKH285 | h+ade6-M26 hrp3::ura4+uar4-D18 his5-303 |
| PKH293 | h+ade6-M26 ada2::ura4+uar4-D18 leu1-32 |
| PKH296 | h+ade6-M375 ada2::ura4+uar4-D18 leu1-32 |
| PKH298 | h−ade6-469 ada2::ura4+uar4-D18 leu1-32 |
| PKH305 | h−ade6-469 hrp3::ura4+uar4-D18 leu1-32 |
| PKH307 | h+ade6-M375 hrp3::ura4+uar4-D18 his3-D1 |
| PKH314 | h+ade6-469 gcn5::ura4+ada2::ura4+uar4-D18 his5-303 leu1-32 |
| PKH316 | h−ade6-469 snf22::ura4+hrp3::ura4+uar4-D18 his5-303 leu1-32 |
| PKH318 | h−ade6-469 gcn5::ura4+hrp3::ura4+uar4-D18 his5-303leu1-32 |
| PKH322 | h−ade6-M26 gcn5::ura4+ada2::ura4+uar4-D18 his5-303 |
| PKH323 | h−ade6-M375 gcn5::ura4+ada2::ura4+uar4-D18 leu1-32 |
| PKH324 | h+ade6-M26 snf22::ura4+hrp3::ura4+uar4-D18 his5-303 |
| PKH326 | h+ade6-M375 snf22::ura4+hrp3::ura4+uar4-D18 his5-303 |
| PKH327 | h+ade6-M26 gcn5::ura4+hrp3::ura4+uar4-D18 his5-303 |
| PKH329 | h+ade6-M375 gcn5::ura4+hrp3::ura4+uar4-D18 his5-303 |
| PKH415 | h−ade6-M26 mst2::ura4+leu1-32 ura4-D18 |
| PKH422 | h−ade6-M375 mst2::ura4+leu1-32 ura4-D18 |
| PKH424 | h+ade6-M469 mst2::ura4+ura4-D18 his5-303 |
| PKH442 | h−ade6-M375 gcn5::ura4+mst2::ura4+ura4-D18 |
| PKH444 | h+ade6-469 gcn5::ura4+mst2::ura4+ura4-D18 his5-303 |
| PKH448 | h−ade6-M26 gcn5::ura4+mst2::ura4+ura4-D18 leu1-32 |
| D20 | h+/h−ade6-M26/ade6-M26 leu1-32/+ his5-303/+ |
| D51 | h+/h−ade6-M26/ade6-M26 hrp1::ura4+/hrp1::ura4+leu1-32/+ his5-303/+ ura4-D18/ura4-D18 |
| D52 | h+/h−ade6-M26/ade6-M26 ada2::ura4+/ada2::ura4+leu1-32/+ his5-303/+ ura4-D18/ura4-D18 |
| D53 | h+/h−ade6-M26/ade6-M26 hrp3::ura4+/hrp3::ura4+leu1-32/+ his5-303/+ ura4-D18/ura4-D18 |
| D72 | h+/h−ade6-M26/ade6-M26 mst2::ura4+/mst2::ura4+leu1-32/+ his5-303/+ ura4-D18/ura4-D18 |
| DTY9 | h+/h−ade6-M26/ade6-M26 gcn5::ura4+/gcn5::ura4+leu1-32/+ his5-303/+ ura4-D18/ura4-D18 |
| D13A1B5 | h+/h−ade6-M26/ade6-M26 snf22::ura4+/snf22::ura4+leu1-32/+ his5-303/+ ura4-D18/ura4-D18 |
| ELD203 | h+/h−ade6-M375/ade6-M375 leu3-155/+ ura1-61/+ |
| ELD205 | h+/h−ade6-M26/ade6-M26 leu3-155/+ ura1-61/+ |
Disruption of the hrp1+, hrp3+, ada2+, and mst2+ Genes
A BLAST search using the amino acid sequences of the S. cerevisiae Ada2p against all translated open reading frames (ORFs) in the S. pombe genome enabled us to identify an SPCC24B10.08c encoding protein that exhibits strong homology to Ada2p. The ClaI-Aor51HI fragment (0.9 kb) was eliminated from the cloned ada2+ sequence and replaced by the ura4+ gene. The EcoRI fragment carrying ada2::ura4+ was transformed into wild-type strain K175, and gene targeting was confirmed by polymerase chain reaction (PCR), by using an appropriate set of primers. The ORFs encoding the protein, consisting of a SWI/SNF ATPase-helicase domain and chromodomain, hrp1+ and hrp3+, were reported in a previous study (Yoo et al., 2000; Zaman et al., 2001). The ORF encoding Mst2 had also been reported in a previous study (Gomez et al., 2005). To make the strains lacking each ORF, 0.5 kbp of flanking sequence of hrp1+, hrp3+, and mst2+ was cloned and ligated with the ura4+ gene to construct hrp1::ura4+, hrp3::ura4+, and mst2::ura4+, which were transformed to make the disruptants.
Determination of Recombination Rate
Each strain was grown on a yeast extract plate (YE; Moreno et al., 1991) supplemented with 100 mg/l adenine at 30°C. Equal volumes of each strain were mixed in 50 μl of 10 mg/ml leucine solution. Suspensions were spotted on SPA plates and incubated at 30°C for 3 d. After incubation, sporulating cells were suspended in 700 μl of 0.5% glusulase and agitated at 30°C for 30 min. After incubation, 300 μl of ethanol was added to each sample and incubated at room temperature for 5 min to kill remaining cells completely. Suspensions were centrifuged and pellets were washed with dH2O. An appropriate number of spores was spread onto SD (Sherman et al., 1986) lacking adenine and YE containing adenine. Recombination rates around M26 were calculated as follows: colony number on SD plates lacking adenine/colony number on YE plates containing adenine.
Northern Blot Analysis
Total RNA was prepared from S. pombe cells according to the method described previously (Elder et al., 1983). For the Northern blot analysis, 10 μg of total RNA was denatured with formamide, separated on 1.5% agarose gels containing formaldehyde (Sambrook et al., 1989), and then blotted on a charged Nylon membrane (BioDyne B membrane; Pall, East Hills, NY). The probe to detect the ade6 transcript was prepared from a DNA fragment as described by Grimm et al. (1991).
Chromatin Analysis and Chromatin Immunoprecipitation
Analysis of chromatin structure by indirect end-labeling was performed according to the method of Mizuno et al. (1997). The DNA samples were digested with XhoI followed by Southern analysis by using the probe as described in the same study (Mizuno et al., 1997). Chromatin immunoprecipitation was performed according to the method of Yamada et al. (2004), with slight modifications as described below. Fifty milliliters of culture was incubated with 1.4 ml of 37% formaldehyde solution for 20 min at room temperature, and then 2.5 ml of 2.5 M glycine was added and incubated for 5 min. After centrifugation, collected cells were washed twice with cold Tris-buffered saline buffer (150 mM NaCl and 20 mM Tris-HCl, pH 7.5). The cells were mixed with 400 μl of lysis 500 buffer (0.1% Na-deoxycholate, 1 mM EDTA, 50 mM HEPES-KOH, pH 7.5, 500 mM NaCl, and 1% Triton X-100) for analysis in Figure 3, and 0.6 ml of zirconia beads were added. Alternatively, lysis 140 buffer (0.1% Na-deoxycholate, 1 mM EDTA, 50 mM HEPES-KOH, pH 7.5, 140 mM NaCl, and 1% Triton X-100) was used for analysis in Figure 4. After disruption of the cells using a multi-beads shocker (Yasuikikai, Osaka, Japan), the suspension was sonicated five times for 30 s each (6 times for Figure 4) to shear chromosomal DNA into ∼500 base pairs fragment (DNA was sheared under 500 bp for analysis in Figure 4), and centrifuged at 4°C. The supernatant was collected as a whole-cell extract. The proper amount of antibody, in accordance with the specifications provided by the manufacturer, and 40 μl of DYNA-protein A beads (Dynal Biotech, Oslo, Norway) were mixed at 4°C overnight to conjugate antibody and beads, it was then washed twice with phosphate-buffered saline (PBS) (138 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4) containing 0.1% BSA. Finally, 300 μl of whole-cell extract was mixed with pretreated beads and allowed to immunoprecipitate at 4°C overnight. The precipitates were washed twice with lysis 500 buffer (with lysis 140 for Figure 4), once with lysis 140 buffer, and further washed once with wash buffer (0.5% Na-deoxycholate, 1 mM EDTA, 250 mM LiCl, 0.5% NP-40, and 10 mM Tris-HCl, pH 8.0) followed by once with Tris-EDTA (TE) (10 mM Tris-HCl, pH 8.0, and 1 mM EDTA). The well-washed precipitates were mixed with 150 μl of elution buffer (10 mM EDTA, 1% SDS, and 50 mM Tris-HCl, pH 8.0) and allowed to elute the immunoprecipitated protein–DNA complexes at 65°C for 15 min (immunoprecipitation [IP] sample). The IP sample or 30 μl of whole-cell extract was mixed with 250 μl or 370 μl of 1% SDS containing TE buffer, 60 mg of proteinase K (Merck, Darmstadt, Germany) was added and incubated at 37°C for 8 h. After incubation, the temperature was shifted to 65°C, and the sample was further incubated overnight. After incubation, DNA was phenol/chloroform extracted from each of the samples and slot-blotted to a charged Nylon membrane (BioDyne B membrane; Pall), followed by Southern blot analysis to quantify DNA content. The probes were amplified from S. pombe genomic DNA by PCR with the following primer sets: ade6-1 (ACCAAACATCCACGGGGTCA and GTCTATTAAAAGTCGTCCAT), ade6-2 (CGTATTCTGCACTTGGTTCG and TTGTGAAATGCAACTTTAAA), ade6-3 (TGCTTGGAAATGTAACGATG and ACCTCCAAGGATCCCTACAA), ade6-4 (GGTCAATTGGGCCGAATGAT and GTGTTAATATGCTCAATTTC), and ade6-5 (TGATGCCTTGGCAGCCGTTA and TACTTTTCAGTACAAAAGGA). The probe used in Figure 3 was described in Yamada et al. (2004).
Figure 3.
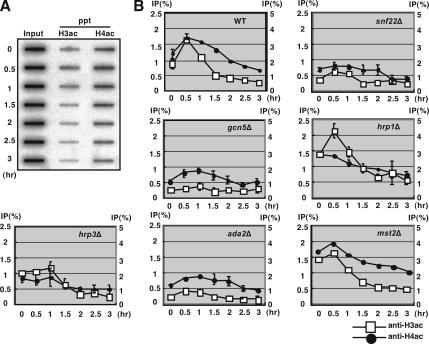
Hyperacetylation of histone H3 around the M26 mutation site was affected in the gcn5Δ, snf22Δ, and ada2Δ strains. (A) The diploid wild-type strain was cultured to induce meiosis, as described in Figure 1. DNA of the input and ChIP samples were quantified by a slot blotting followed by hybridization with the M26 probe. The time after meiotic induction is indicated on the left side of the panel. (B) Quantified data of acetylated histones H3 and H4 in wild-type, gcn5Δ, snf22Δ, hrp1Δ, hrp3Δ, ada2Δ, and mst2Δ strains. Vertical and horizontal axes represent ChIP efficiency (percentage) (left, histone H3ac; right, histone H4ac) and hours after meiotic induction. Squares and filled circles indicate data for acetylated histones H3 and H4, respectively. All experiments except for mst2Δ were repeated independently two times. Error bars represent SD.
Figure 4.
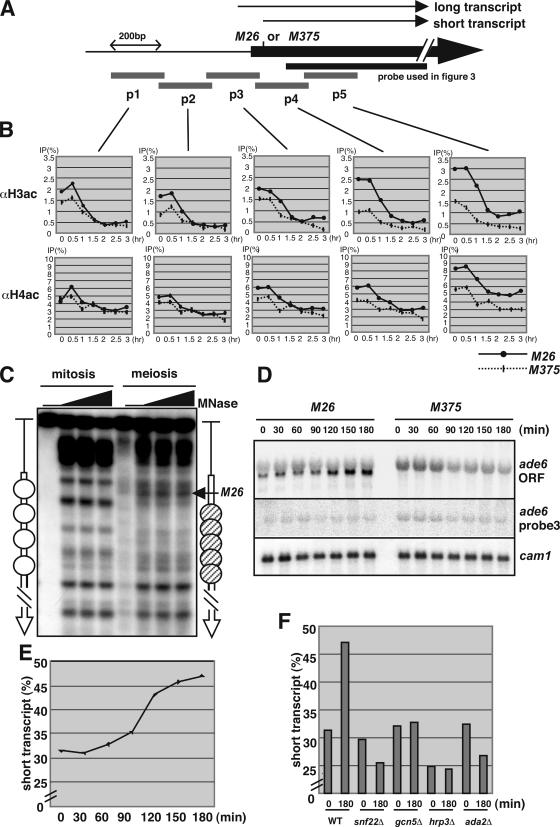
M26 specific hyperacetylation of histones H3 and H4, and transcriptional activation. (A) Schematics of the probes used to quantify the acetylation of histones H3 and H4 around the ade6 locus. The scale represents 200 bp. Arrows represent the initiation sites of long and short transcript. (B) Quantified data for acetylated histones H3 and H4 in M26 (bold line) and M375 (dotted line) strains. Vertical and horizontal axes represent ChIP efficiency (percentage) and hours after meiotic induction. (C) Meiotic chromatin remodeling is induced around 3′ region of M26 mutation point. Wild-type (D20) cells were cultured in MM + N (lanes; mitosis) and transferred to MM − N and cultured further for 4 h (lanes; meiosis). Open and hatched ovals represent phase and randomly positioned nucleosomes, respectively. (D) The initiation site of the ade6 transcript is shifted to the 3′ region in the M26 strain. The diploid M26 and M375 strains were cultured to induce meiosis, as described in Figure 1. RNA was isolated and analyzed by Northern blotting using the ade6-ORF probe, probe 3 in Figure 4A, and cam1+ as a loading control (Takeda and Yamamoto, 1987). (E) The intensity of the band corresponding to the short transcript and whole transcript was quantified, and relative band intensity (short transcript/whole transcript percentage) was calculated. (F) The increase of short transcript of ade6 during meiosis requires Snf22, Gcn5, Hrp3, and Ada2. The diploid wild-type, snf22Δ, gcn5Δ, hrp3Δ, and ada2Δ strains were cultured to induce meiosis, as described in Figure 1, and transcript of ade6 was detected as Figure 4D. The relative intensity (short transcript/whole transcript percentage) was calculated as Figure 4E.
RESULTS
Hrp3, Ada2, and Mst2 Are Required for Full Activation of Chromatin Alteration at M26 in Meiosis, whereas Hrp1 Is Required for Its Suppression
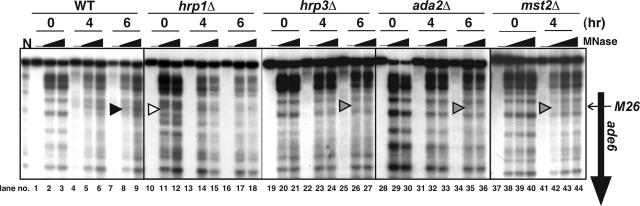
To further understand the molecular mechanisms of chromatin alteration at M26, we investigated ada2+ (a ScADA2 homologue in S. pombe), mst2+ (a homologue of ScSAS2 and 3 in S. pombe), and hrp1+, hrp3+ (S. pombe CHD1-like ADCRs). We analyzed the chromatin structure around M26 in the ada2Δ, mst2Δ, hrp1Δ, and hrp3Δ strains during the course of meiosis. The appearance of periodical MNase-cleavage patterns with ∼150 base pairs at regular intervals around the M26 site indicates that nucleosomes are positioned in the ade6 ORF under vegetative and premeiotic conditions. During meiosis, such cleavage patterns become rearranged, and an intense band occurs at the M26 mutation site in the wild type (Figure 1, arrowhead in the wild-type lanes 5–6, 8–9). In contrast, such chromatin alteration around M26 did not occur during meiosis in the ada2Δ, mst2Δ, and hrp3Δ strains as demonstrated previously in the gcn5Δ and snf22Δ strains (Yamada et al., 2004). Instead, a weak band occurred around M26 meiotically (Figure 1, gray arrowheads in the ada2Δ, mst2Δ, and hrp3Δ, lanes 35–36, 42–44, and 26–27, respectively). Interestingly, in the hrp1Δ strain, MNase-cleavage patterns seemed to be like the meiotic type that exhibited an intense band at the M26 site, even at the premeiotic time point (Figure 1, open arrowhead in hrp1Δ, lanes 11–12). These results suggest that Ada2, Mst2, and Hrp3 are required for the full activation of meiotic chromatin alteration at M26, whereas Hrp1 is required for the suppression of chromatin changes before the onset of meiosis.
Figure 1.
Hrp3 and Ada2 are required for meiotic chromatin remodeling around ade6-M26, whereas Hrp1 functions to suppress the alteration of chromatin structure. Diploid strains D20 (ade6-M26), D51 (ade6-M26, hrp1Δ), D53 (ade6-M26, hrp3Δ), D52 (ade6-M26, ada2Δ), and D72 (ade6-M26, mst2Δ) were cultured in MM + N medium (lanes 0 h). Cells were transferred to MM − N medium and cultured further for 4 h (lanes 4 h) or 6 h (lanes 6 h). Chromatin isolated from the cells was digested with MNase and analyzed as described previously (Mizuno et al., 1997). The vertical and horizontal arrows indicate the ade6 ORF and position of the M26 mutation, respectively. The arrowheads represent altered or unaltered chromatin configuration around M26 in the wild-type (filled), hrp1Δ (open), hrp3Δ, ada2Δ, and mst2Δ (gray) strains, respectively. Lane N indicates MNase digestion of naked DNA.
Diploids of the mst2Δ strain could undergo meiosis, but producing abnormal asci in elevated frequency, as shown previously by Gomez et al. (2005). We observed meiotic progression in ada2Δ and hrp3Δ strain in the condition of synchronous meiosis by using pat1-114 mutation (Iino and Yamamoto, 1985; McLeod and Beach, 1986), and we confirmed that ada2Δ and hrp3Δ strain, like gcn5Δ strain could undergo meiosis and induce meiotic DSBs formation proficiently (Supplemental Figure S1, A and B). Expression of the meiosis-specific rec genes rec6+, rec7+, rec8+, and rec12+ was activated, except for a slight reduction and their relative persistence in later timing (Supplemental Figure S1C). Moreover, we examined meiotic progression by monitoring meiotic nuclear divisions of nitrogen-starved diploids, and we verified that ada2Δ and hrp3Δ could undergo meiosis at kinetics similar to wild type (Supplemental Figure S1, D and E).
During the zygotic meiosis, regular asci containing four spores were efficiently formed in the snf22Δ mutant after a prolonged incubation (Supplemental Figure S1E), although meiotic nuclear division was seemingly defective in the snf22Δ (Supplemental Figure S1D). In addition, it should be noted that premeiotic DNA synthesis, meiotic DSB formation and induction of meiotic genes were only partially affected in the snf22Δ mutant (Supplemental Figure S1, A–C). Hence, snf22Δ may cause a severe delay rather than entire defects of meiotic progression. Importantly, at the same timing of early meiosis, we could not detect chromatin alteration at M26. This tendency was similarly observed, as we analyzed DSB formation and M26 chromatin structure in a simultaneous meiotic culture of pat1+ rad50S diploid strains (Supplemental Figure S1, F and G): little or no chromatin remodeling was observed in the snf22Δ mutant even at 8 h of meiotic culture, which was confirmed by the quantification of MNase sensitivity at M26 and a control site (Supplemental Figure S1G). The ratio of sensitivity (M26/control site) meiotically increased from 0.58 to 1.39 in wild type, whereas very little change of the ratio could be detected in the snf22Δ (0.63 vs. 0.69). In contrast, at the same meiotic time points (8 h), genome-wide DSB formation was almost fully detectable (Figure 1). Therefore, we consider that partial defects of early meiotic events in snf22Δ might not be the primary reason for the loss of the M26 chromatin remodeling in snf22Δ mutant. It is likely that a substantial portion of the mutant cells can undergo early meiotic events almost normally, but they cannot remodel chromatin structure specifically around M26, as in gcn5Δ (Yamada et al., 2004).
Effects of hrp1+, hrp3+, ada2 +, and mst2+ Deletion on Meiotic Recombination at M26
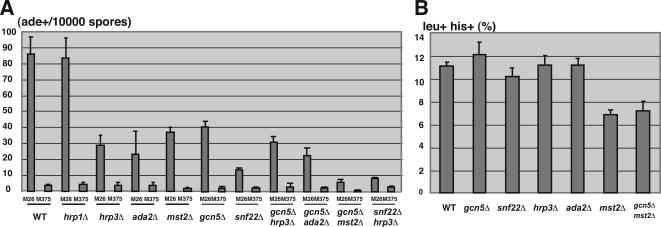
The present results raise the possibility that Ada2, Mst2, Hrp1, and Hrp3 play important roles in the regulation of meiotic recombination at M26 hotspots through chromatin modulation, because chromatin regulation has been shown to be important for the activation of recombination and transcription (Nicolas, 1998; Petes, 2001). To test this idea, we measured the recombination rates at the ade6 locus in the ada2Δ, mst2Δ, hrp1Δ, and hrp3Δ strains by using a tester allele, ade6-469. The recombination rates of M26 in the wild-type and hrp1Δ strains were almost indistinguishable, whereas considerable reductions in M26 recombination rates were observed in the ada2Δ, mst2Δ, and hrp3Δ strains (Figure 2A). The recombination rates of the control allele, M375, which does not exhibit hotspot activity, were not affected significantly in the ada2Δ, hrp1Δ, and hrp3Δ strains, whereas a more severe reduction was observed in the mst2Δ strain (WT, 3.6 ± 0.6 ade+/104 spores, n = 7; mst2Δ, 1.6 ± 0.26 ade+/104 spores, n = 3 [∼2.3-fold reduction]; Figure 2A). The recombination frequency in M26 and M375 was reduced by almost the same degree in the mst2Δ strain (WT, 86.1 ± 10.6 ade+/104 spores, n = 7; mst2Δ, 36.7 ± 2.5 ade+/104 spores, n = 3 [∼2.3-fold reduction]; Figure 2A). These results suggest that Ada2 and Hrp3 are required for the activation of meiotic recombination in an M26 hotspot-dependent manner, as demonstrated previously in the gcn5Δ and snf22Δ strains (Yamada et al., 2004). These also suggest that Mst2 is involved in the global recombination.
Figure 2.
The impact of hrp1+, hrp3+, ada2+, and mst2+ deletions on meiotic recombination frequency and meiotic DSB formation and genetic interactions between these proteins and Gcn5 or Snf22. (A) Recombination rates at M26 or M375 control allele (indicated as Ade+/104 spores) were examined as described in Materials and Methods. All crosses were repeated independently at least three times. Error bars represent SD. (B) Recombination between leu1-32 and his5-303 (indicated as leu+/his+ [percentage]) was measured as described in Materials and Methods. All crosses were repeated independently two or three times. Error bars represent SD.
We also examined the recombination rates between leu1 and his5 loci (intergenic recombination) in hrp1Δ, hrp3Δ, ada2Δ, and mst2Δ strains. A less severe but significant reduction was observed only in the mst2Δ strain, consistent with its severe defects in recombination frequency observed in M375 control allele (WT, 11.1 ± 0.35%, n = 2; mst2Δ, 6.9 ± 0.42%, n = 2 [∼1.6-fold reduction]; Figure 2B). More importantly, the recombination rates between leu1-32 and his5-303 in ada2Δ, hrp1Δ, and hrp3Δ strains were very similar to those observed in the wild-type strain (Figure 2B). Taken together, we concluded that the ada2Δ and hrp3Δ mutant effects, like the snf22Δ and gcn5Δ effects, are specific for the recombination at the M26 hotspot.
We further tested genetic interactions between ada2Δ or mst2Δ or hrp3Δ and gcn5Δ (or snf22Δ) mutations. The cells lacking gcn5+ showed a partial reduction in recombination rate (∼50%, Figure 2A; Yamada et al., 2004). However, the double deletions of the gcn5+ and ada2+ or hrp3+ genes did not show a further decrease in M26 recombination frequency, indicating that both Ada2 and Hrp3 function along the same pathway as Gcn5 (Figure 2A). Interestingly, the double deletions of the gcn5+ and mst2+ resulted in drastic reduction of recombination frequency in M26, indicating that Mst2 and Gcn5 participate in distinct pathways (Figure 2A). Furthermore, a snf22+ deleted strain showed a severe reduction in M26 recombination frequency (Figure 2A; Yamada et al., 2004). The ada2Δ/snf22Δ double-deleted strain exhibited a completely sterile phenotype; hence, we could not analyze the recombination rate in this strain. The deletion of the hrp3+ gene in addition to the snf22+ deletion showed slight or little reduction in the recombination rate at M26 (Figure 2A). This result suggests that Hrp3 and Snf22 generally function along the same pathway, but it is also possible that Hrp3 has some roles partly distinct from Snf22.
The Level of Histone H3 Acetylation around M26 Is Decreased in snf22Δ, gcn5Δ, and ada2Δ
Hyperacetylation of histone H3 or H4 is often found in transcriptionally and recombinationally active chromosomal domains, such as transcription active loci and the gene conversion active chicken immunoglobulin locus (Brown et al., 2000; Seo et al., 2005). Hyperacetylation of histones is also observed around M26 during early meiosis. It requires the M26 sequence, its binding protein Atf1 and the Gcn5 HAT (Yamada et al., 2004). Thus, we investigated and compared the acetylation levels of histones H3 and H4 in the snf22Δ, gcn5Δ, hrp1Δ, hrp3Δ, ada2Δ, and mst2Δ strains. To examine the acetylation level of histones, we used chromatin immunoprecipitation (ChIP) analysis by using anti-acetylated histone H3 or H4, as described previously (Yamada et al., 2004). The DNA from the immunoprecipitated and input materials were transferred to slot blots and hybridized with a sequence of the ade6 locus (around the M26 hotspot as shown in Figure 4A) (Figure 3A). The acetylation levels of histone H3 and H4 around M26 were elevated at 0.5–1 h after nitrogen starvation in the wild-type cells. In the gcn5Δ, ada2Δ, and snf22Δ strains, however, histone H3 acetylation levels around M26 were not as significantly increased, and the histone H3 acetylation stayed at very low levels (Figure 3B). A less severe decrease in histone H4 acetylation was observed in the gcn5Δ and ada2Δ strains (Figure 3B). In contrast, the acetylation level of histone H4 is decreased in snf22Δ, suggesting that Snf22 plays some role in facilitating the acetylation of histones H3 and H4. Importantly, little increase in histone H3 acetylation was observed in the hrp3Δ strain (Figure 3B), which is consistent with its effects on recombination rate at M26 (Figure 2A). Moreover, the basal level of histone H3 acetylation in hrp1Δ, which had constitutively an open chromatin configuration around M26 (Figure 1), was significantly higher (∼1.5-fold) than wild-type levels. Furthermore, it should be noted that no decrease in the acetylation of histones H3 and H4 was observed in the mst2Δ strain (Figure 3B).
M26 Mediates Site-specific Acetylation of Histones H3 and H4 and Transcriptional Activation
The acetylation of histone H3 around M26 occurs in early meiosis, in which Gcn5-Ada2 and Snf22 are required (Figure 3B). However, the relation of histone acetylation and ATF/CREB transcription factor, Atf1, binding on M26 has not been fully elucidated. To address this issue, we investigated histone acetylation in the course of meiosis in M26 and M375 diploids more precisely. As illustrated in Figure 4A, for the quantitative analysis, the ade6 locus around the M26 or M375 site was divided into fragments of 200 base pairs, and the probes for each region were used to measure ChIP efficiency at those segments. To view the difference in acetylation level at high resolution, ChIP procedure was modified, as described in Materials and Methods. We found that the acetylation of histones H3 and H4 around ade6 promoter (probes 1, 2, and 3) was observed in the both M26 and M375 strains in early meiosis. Interestingly, strong acetylation of histone H3 was detected only in the M26 strain at the 3′ region of the M26 mutation site (M26/M375, >2.5-fold; Figure 4B). The acetylation of histone H4 was also observed in this region (probes 4 and 5) in the M26 strain rather than in M375, whereas the effect of M26 mutation was lower than that of histone H3 acetylation (M26/M375, <1.4-fold; Figure 4B). Consistent with this observation, the chromatin remodeling was observed around 3′ region of M26 mutation point in meiosis (Figure 4C).
Hyperacetylation of histones H3 and H4 and chromatin remodeling are assumed to be related to transcriptional activation. We investigated the transcript of the ade6 gene in the course of meiosis by Northern analysis. As shown in Figure 4D, a shorter version of the ade6 transcript was observed in the M26 strain; however, this short transcript was not seen in the M375 strain. Furthermore, the amount of the short transcript in the M26 strain was elevated in the course of meiosis (Figure 4E). To examine whether the short version of the ade6 transcript was initiated from 3′ region of the original initiation site, we hybridized probe 3 in Figure 4A on the same membrane. As shown in Figure 4D, only a long version of the ade6 transcript was detected by probe 3, indicating that the transcriptional initiation site was shifted from the original initiation site to the 3′ region of probe 3 in the short version of the transcript. Moreover, we determined the initiation site of the shorter transcript observed in ade6-M26 by rapid amplification of cDNA ends (RACE) analysis, as described in the Supplemental Material. Interestingly, the shorter transcript was initiated from 2 base pairs upstream of the M26 heptanucleotide sequence (Supplemental Figure S2). These results indicate that Atf1 binding on M26 provokes site-specific hyperacetylation of histones and the initiation of transcription followed by activation of recombination.
Furthermore, we examined whether the HATs and ADCRs required for the recombination in M26 are involved in the shift of the transcription initiation site of ade6 during meiosis. As shown in Figure 4F, the shorter ade6 transcript was detected in the diploid snf22Δ, gcn5Δ, hrp3Δ, and ada2Δ strain as in a diploid wild-type strain before meiosis, but the intensity of the shorter transcript did not increase in these mutants during meiosis (Figure 4F). These results indicate that these HATs and ADCRs are not required for the shift per se, whereas the transcriptional activation of shorter RNA is dependent on these HATs and ADCRs.
Cells Lacking hrp3+ or ada2+ Cannot Induce Chromatin Alteration at M26 in Response to Osmotic Stress
We previously demonstrated that chromatin alteration and transcriptional activation in ade6-M26 are induced in response to osmotic stress (Hirota et al., 2004). Therefore, we next examined the effects of deleting the snf22+, gcn5+, ada2+, hrp1+, and hrp3+ genes on chromatin structure around M26 under osmotic stress using haploid cells. In wild-type cells, we detected changes in MNase-sensitive band patterns. An intense band occurred at the M26 mutation site when cells were exposed to 1.2 M of sorbitol (Figure 5C, filled arrowhead, lanes 5, 6). Under such conditions, the size and amount of the ade6 transcripts became smaller and more abundant (∼sixfold increase; Figure 5A, filled arrowhead). In addition, we determined the initiation site of smaller RNA observed when cells were exposed to sorbitol (osmotic) stress by RACE analysis (data not shown). The shorter transcript was initiated from 48 base pairs downstream of M26 heptanucleotide sequence, indicating smaller RNA induced by osmotic stress is initiated from a close but distinct site from that of shorter meiotic RNA shown in Figure 4.
Figure 5.
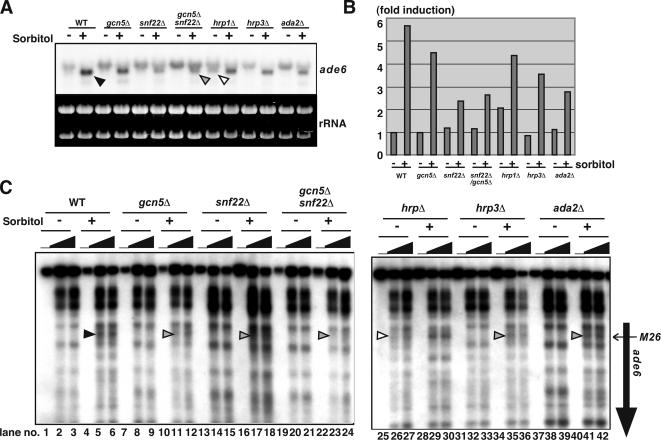
The chromatin regulation of ade6-M26 by the HATs and ADCRs is coupled to transcriptional activation in response to osmotic stress. (A) The cells of haploid strains (wild type, gcn5Δ, snf22Δ, gcn5Δ/snf22Δ, hrp1Δ, hrp3Δ, and ada2Δ) were cultured in YE to mid-log phase, transferred to YE containing 1.2 M sorbitol, and cultured further for 90 min. Total RNA was isolated from each culture and analyzed by Northern analysis. Arrowheads represent the shorter ade6-M26 transcript. (B) Quantification of the results in A. Vertical axis indicates -fold induction compared with the mRNA levels in the wild-type strain without the sorbitol treatment. (C) The crude nuclei were isolated from the simultaneous cultures indicated in Figure 5A. The chromatin structure was analyzed as described in Figure 1. The vertical and horizontal arrows indicate the ade6 ORF and position of the M26 mutation, respectively. Arrowheads represent the altered or partially altered chromatin configuration around M26 in the wild-type (filled), hrp1Δ (open), hrp3Δ, ada2Δ, and mst2Δ (gray) strains, respectively.
In the gcn5Δ, snf22Δ, ada2Δ, and hrp3Δ strains, alterations in chromatin structure at the ade6-M26 locus were reduced (Figure 5C, gray arrowhead, lanes 11–12, 17–18, 41–42, and 35–36, respectively). It should be noted that the impact of these mutations upon transcription in ade6-M26 (the shorter transcript, 1.3∼2.0-fold reduction compared with wild type) was much smaller than their effects on meiotic recombination at M26 (2.5∼10-fold reduction compared with wild type). Conversely, in the hrp1Δ strain, chromatin configuration around M26 was constitutively open to some extent, even without the treatment of sorbitol (Figure 5C, open arrowhead, lanes 26 and 27). In addition, consistent with the constitutive openness of the chromatin configuration at M26, a twofold induction of the short version of the ade6-M26 transcript was observed even in the absence of sorbitol (Figure 5, A and B, open arrowhead). Interestingly, the magnitude of the transcriptional induction was reduced in the hrp1Δ strain (sorbitol+/sorbitol−; WT ∼sixfold, hrp1Δ ∼twofold, Figure 5B), indicating that proper regulation of the chromatin structure is vital for effective induction of transcription. All mutants of snf22Δ, gcn5Δ, ada2Δ, hrp1Δ, and hrp3Δ conferred severe defects in chromatin alteration and transcriptional phase transition in ade6-M26 when cells were exposed to osmotic stress (Figure 5).
These results led us to speculate that these genes might be involved in cellular responses to other environmental stresses. As shown in Supplemental Figure S3, deletion of snf22Δ, gcn5Δ, ada2Δ, hrp1Δ, and hrp3Δ results in various stress-sensitive phenotypes. For example, the snf22Δ, hrp1Δ, and hrp3Δ strains were all severely and weakly sensitive to cation (1 M KCl) and glucose starvation stresses, respectively (Supplemental Figure S3). Interestingly, these mutants showed little sensitivity to osmotic stress (1.2 M sorbitol). In addition, replication stress hydroxyurea (HU)-sensitive phenotypes were observed in the snf22Δ, gcn5Δ, hrp1Δ, and ada2Δ strains. Furthermore, the ada2Δ and gcn5Δ mutants exhibited weak sensitivity to UV and methylmethane sulfonate (Supplemental Figure S3).
To address the reasons for the partial sterility of snf22Δ and complete sterility of the snf22Δ/ada2Δ double mutant, we examined their ability to cause G1 phase arrest in response to nitrogen starvation. This is because the G1 phase serves as an exit for cell differentiation, such as mating and meiotic induction processes (Yamamoto et al., 1997). Wild type and ada2Δ cells could accumulate in the 1C population in response to nitrogen starvation, indicating that these strains can arrest the cell cycle in G1 phase (Supplemental Figure S4). In contrast, snf22Δ cells exhibited only partial accumulation at 1C, indicating that snf22Δ has a defect in G1 phase arrest in response to nitrogen starvation. Furthermore, this defect of snf22Δ in cell-cycle arrest was strengthened in the snf22Δ/ada2Δ double mutant (Supplemental Figure S4). Alternatively, it is also possible that Snf22 and Ada2 is involved in the induction of some genes required for mating, because a significant amount of cells arrested in G1 phase in snf22Δ/ada2Δ double mutant, whereas this mutant shows complete sterile phenotype.
These results suggest that Snf22, Gcn5, Hrp1, Hrp3, and Ada2 are pivotal for appropriate cellular response to various environmental stresses, possibly via chromatin regulation, as shown in ade6-M26.
DISCUSSION
This study on fission yeast, demonstrates the in vivo function of Ada2 (a putative S. pombe SAGA component), Mst2 (a MYST family HAT), and Hrp1, Hrp3 (ADCRs having a chromodomain) in chromatin remodeling processes at M26, which provides a good model system for studying chromatin regulation around CRE-related cis-acting DNA sequences. Ada2, Hrp1, and Hrp3 play essential roles in chromatin remodeling, activation of meiotic recombination, and transcriptional response to osmotic stress at M26. Additionally, Mst2 is involved in global recombination possibly via the chromatin remodeling process. Moreover, Hrp1, Hrp3, and Snf22—three distinct entities of ADCR—have redundant and antagonizing functions in regulating chromatin alteration at M26; Hrp3 and Snf22 function to form an open chromatin configuration, whereas Hrp1 plays a suppressive role.
HAT and ADCR Components Function Together in Chromatin Modification and Recombination Activation at M26
These results indicate that chromatin alteration at M26 is controlled by multiple chromatin modulators. Both Ada2 and Gcn5 are shown to be equally needed for hyperacetylation of histone H3 around M26, and for meiotic recombination enhancement and chromatin changes around M26 (Figures 1–3). In addition, the ada2Δ/gcn5Δ double deletion confers defects in M26 recombination hotspot activation during meiosis at comparable levels to the ada2Δ mutants. This result, together with the cooperative action of their S. cerevisiae counterparts in the SAGA complex in specific transcriptional responses (Grant et al., 1998), suggests that they function in concert in a SAGA-like complex to regulate chromatin configuration and recombination competency at M26.
Interestingly, Mst2 is required for efficient recombination in the ade6 locus (both of M26 and M375) but not for acetylation of histones H3 and H4. This suggests its involvement possibly in the formation of stable remodeling complexes, besides its function as a HAT in the recombination. The recombination frequency in the leu1–his5 interval is less severely affected than in the ade6 locus of the mst2Δ mutant. It has previously been shown that recombination frequency in the his4–lys4 interval is not decreased in the mst2Δ strain (Gomez et al., 2005). This suggests that the impact of the lack of Mst2 on recombination depends on the locus observed, possibly because of the context of the local chromatin structure.
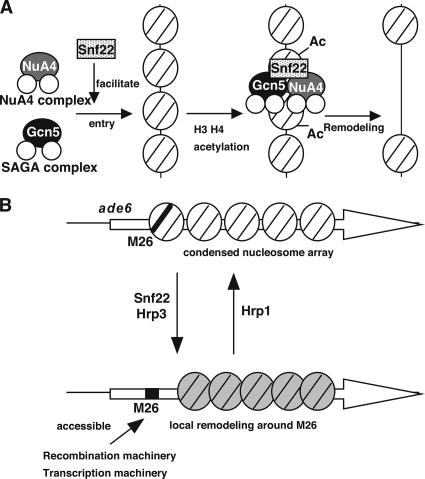
Gcn5 and Snf22 are both required for the complete chromatin changes around M26. We previously demonstrated that the double deletion of snf22+ and gcn5+ genes results in snf22Δ like defects in chromatin remodeling and meiotic recombination enhancement at M26 (Yamada et al., 2004). We thus hypothesized that Snf22 ADCR and Gcn5 HAT play roles in the same pathway, and that Gcn5 HAT may function at earlier stages than Snf22 to facilitate the specific entry of ADCR to the M26 sites. However, unexpectedly, the Gcn5-dependent hyperacetylation of histone H3 around M26 is highly dependent on Snf22 (Figure 3), suggesting that they may act in an interdependent manner. This can be explained by the idea that Snf22 facilitates Gcn5 entry to the M26 site by forming a HAT-ADCR loading complex (Figure 6A).
Figure 6.
(A) Cooperative behavior of HATs-ADCRs for the modification and remodeling of chromatin structure around M26. (B) Reversible regulation of M26/CRE-dependent chromatin remodeling by chromatin modifying machineries. The model postulates that distinct remodeling complexes are required for nucleosome disruption to recruit either recombination complexes or transcription complexes. In addition, Hrp1 is involved in the suppression of chromatin remodeling at M26, thereby providing reversible regulation of the M26/CRE-dependent chromatin configuration.
Given the interdependent relation between Gcn5 and Snf22, the next question is why the gcn5Δ single mutant has only partial defects in meiotic chromatin alteration and recombination activation at M26. In addition, deletion of Ada2 or Gcn5 HAT cause only partial reduction in M26-dependent hyperacetylation of histone H4 (Figure 3; Yamada et al., 2004). Most simply, in comparison with Gcn5 HAT, Snf22 has a more general role in loading of SAGA and NuA4 HAT complexes, presumably via recruitment of Tra1-like protein containing HAT complexes (Allard et al., 1999). This notion is consistent with the observation that the acetylation of histone H4 severely decreases in snf22Δ. Alternative rationale is that other types of HATs are responsible for the hyperacetylation of histone H4 and function redundantly in chromatin modulation with Ada2 and Gcn5, which are mainly involved in H3 hyperacetylation. In the budding yeast, nucleosomal acetyltransferase of histone H4 (NuA4), a multiprotein HAT complex that preferentially acetylates histone H4, has been purified and shown to contain the MYST-family HAT Esa1 as the catalytic subunit (Allard et al., 1999). The fission yeast ESA1 homologue mst1+ is cloned, and it was shown to be essential for viability (Gomez et al., 2005). Therefore, for the investigation of the genetic relation between mst1+ and gcn5+ (or ada2+) HAT, we need to newly isolate conditional mutants of mst1+ in the future work.
The Reversible Control Mechanism for Chromatin Remodeling
Many ADCRs, such as ISWI and SWI/SNF, are assumed to primarily increase DNA accessibility in chromatin by disrupting local nucleosomal arrays (reviewed in Travers, 1999). However, the present data demonstrate that one of the CHD-1 like ADCRs, Hrp1, plays a role in suppressing chromatin opening, whereas Snf22 and Hrp3 ADCRs act to establish an open chromatin configuration at M26. This indicates that chromatin regulation by ADCRs is reversible and that the status of local chromatin structure is controlled by dynamic shifts of equilibrium between inverse reactions by distinct types of ADCRs (Figure 6B). This is supported by previous reports that human Mi2, a chromodomain ADCR, has been copurified with HDAC1 and 2 in the NuRD complex (Tong et al., 1998; Zhang et al., 1998), which seem to form a repressive (inaccessible) chromatin configuration. An alternative but nonexclusive possibility is that Hrp1 represses Snf22 and Gcn5-dependent chromatin remodeling under certain conditions, such as during vegetative growth. Our preliminary experiments indicate that double or triple mutants of hrp1Δ/snf22Δ, hrp1Δ/gcn5Δ, and hrp1Δ/gcn5Δ/snf22Δ confer defects in meiotic chromatin alteration at M26, suggesting that Gcn5 and Snf22 are essential in the Hrp1-dependent constitutive disclosure of chromosomal DNA at M26 (Mizuno, unpublished observation).
CREB/ATF-M26 Complex Provides Hyperacetylation of Histone H3 Followed by Initiation of Transcription and Recombination
The acetylation of histones H3 and H4 is enriched at the 3′ region of the M26 mutation site, whereas such hyperacetylation was not observed in the M375 control allele that lacks the binding site for Atf1 (Figure 4). The acetylation of histones H3 and H4 also occurs in the promoter region of the ade6 gene in both M26 and M375 alleles, indicating that site-specific hyperacetylation is provided by Atf1 binding on M26, and is pivotal for specific activation of recombination. Gcn5-Ada2 HAT and Snf22 is required for this hyperacetylation. These results suggest Atf1 recruits Gcn5-Ada2 HAT and Snf22 to acetylate histone in the targeted region. However, in the present ChIP condition, we cannot detect the binding of Gcn5 to M26 in the course of meiosis, which may be due to a transient interaction of Gcn5 to M26.
Interestingly, M26 strain produces a smaller transcript that is initiated from 2 base pairs upstream of the M26 site. In addition, the smaller transcript exists before induction of meiosis and increases during the course of meiosis, indicating that transcriptional initiation site is shifted to M26 and the transcription of shorter RNA is activated during meiosis (Figure 4, D and E). Furthermore, the HATs and ADCRs required for the recombination at M26 is also essential for the meiotic transcriptional activation of shorter RNA, but they are not required for the shift of initiation site before meiosis per se (Figure 4F). M26 dependent hyperacetylation of histone H3 and H4 and chromatin remodeling was observed at 3′ region of M26 site (Figure 4, B and C), implicating a possible role of RNA polymerase II (RNAPII) in chromatin remodeling process. In addition, the HATs and ADCRs were required for the hyperacetylation of histones and elevation of smaller transcript in meiosis. Thus, we speculate the possible model that RNAPII may function to induce chromatin remodeling at 3′ region o M26 site collaborating with the HATs and ADCRs during meiosis to gain access of proteins required for DSB formation. This model explains the reason why histone acetylation occurred in earlier stage before DSB formation. This model is also supported by previous studies; Orphanides and Reinberg had suggested that RNAPII elongates through the transcribed region with disrupting chromatin, cooperatively with HAT and other remodeling complexes (Orphanides and Reinberg, 2000). In addition, it had been reported the PCAF (HAT complex) binds specifically to the phosphorylated, elongating form of RNAPII (Cho et al., 1998). Further investigation might be necessary to clarify the role of RNAPII in chromatin regulation in M26.
Roles of CRE-dependent Chromatin Modifiers in Transcriptional Regulation
Conversely, it is obvious that the initiation site of ade6 transcript is also shifted under osmotic stress (Figure 5). It is important that the HATs and ADCRs reported here are also involved in the shift of the initiation site and transcriptional activation of ade6-M26 in response to osmotic stress (Figure 5). Indeed, the initiation site of the shorter transcript under osmotic stress is not identical, but very close to that of meiotic short RNA. This suggests that M26 transcriptions in meiosis and osmotic stress are regulated by generally common but slightly different mechanisms. In addition, the same HATs and ADCRs function in both mechanisms. Possibly, the regulation of chromatin structure by the HATs and ADCRs is pivotal to both osmotic response (the shift of the initiation site of ade6) and meiotic recombination activation at M26. Moreover, they may be required for proper adaptation against various environmental stresses, presumably by proper induction of appropriate genes via modulating the local chromatin configuration (Supplemental Figure S3). Alternatively, it is also possible that histone acetylation is pivotal for DNA repair process via chromatin regulation, because ada2Δ and gcn5Δ mutants exhibited DNA damage sensitive phenotype. The present result together with previous finding in S. cerevisiae support this possibility (Bird et al., 2002; Downs et al., 2004; van Attikum et al., 2004). Hence, these M26-dependent (in other words, CRE-dependent) chromatin modifiers have multiple roles in site-specific recombination activation and stress-induced transcriptional activation at M26 or CRE-related cis-acting DNA sequences. Because shortening of the ade6-M26 transcript is at least partly coupled with transcriptional activation and chromatin alteration around M26, those HATs and ADCRs are involved in the access of RNAPII or basic transcription factors at the level of chromatin structure. Recently, it has also been shown that CHD remodelers (Hrp1 and Hrp3) interacting with Nap1 histone chaperone participate in nucleosome disassembly at promoters, suggesting possible roles of these remodelers in the transcriptional regulation via chromatin modulation (Walfridsson et al., 2007). The combination of Tup1-like global corepressors, and some sequence-specific coregulators, such as Rst2 and Scr1 Zn-finger protein, probably governs the differential responses of chromatin structure in response to distinct stresses (Hirota et al., 2003; Hirota et al., 2004; Hirota et al., 2006).
The study suggests the redundant and antagonistic roles of these HATs and ADCRs in chromatin remodeling. These factors might play pivotal roles in regulating chromatin dynamics in the cellular response to environmental stresses. Further understanding of those factors might shed light on this essential mechanism of regulation.
Supplementary Material
ACKNOWLEDGMENTS
K.H. thanks all members of the Genetic System Regulation Laboratory and Cellular and Molecular Biology Laboratory in RIKEN for helpful discussions and T. Yamada for critical advice on ChIP analysis. We thank K. Yamada for critical reading of this manuscript. We also thank Y. Ichikawa and R. Nakazawa for DNA sequencing and Y. Sakuma for technical assistance. This work was supported by grants from the following sources: basic research from the Bio-oriented Technology Research Advancement Institution (to T.S. and K.O.); grants-in-aid for scientific research on priority areas from the Ministry of Education, Science, Culture and Sports, Japan (to K.O.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-04-0377) on January 16, 2008.
REFERENCES
- Agalioti T., Lomvardas S., Parekh B., Yie J., Maniatis T., Thanos D. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell. 2000;103:667–678. doi: 10.1016/s0092-8674(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Allard S., Utley R. T., Savard J., Clarke A., Grant P., Brandl C. J., Pillus L., Workman J. L., Cote J. NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J. 1999;18:5108–5119. doi: 10.1093/emboj/18.18.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. W., Yu D. Y., Pray-Grant M. G., Qiu Q., Harmon K. E., Megee P. C., Grant P. A., Smith M. M., Christman M. F. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature. 2002;419:411–415. doi: 10.1038/nature01035. [DOI] [PubMed] [Google Scholar]
- Brown C. E., Lechner T., Howe L., Workman J. L. The many HATs of transcription coactivators. Trends Biochem. Sci. 2000;25:15–19. doi: 10.1016/s0968-0004(99)01516-9. [DOI] [PubMed] [Google Scholar]
- Chen H., Tini M., Evans R. M. HATs on and beyond chromatin. Curr. Opin. Cell Biol. 2001;13:218–224. doi: 10.1016/s0955-0674(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Cho H., Orphanides G., Sun X., Yang X. J., Ogryzko V., Lees E., Nakatani Y., Reinberg D. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol. Cell. Biol. 1998;18:5355–5363. doi: 10.1128/mcb.18.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma M. P., Tanaka T., Nasmyth K. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell. 1999;97:299–311. doi: 10.1016/s0092-8674(00)80740-0. [DOI] [PubMed] [Google Scholar]
- Downs J. A., Allard S., Jobin-Robitaille O., Javaheri A., Auger A., Bouchard N., Kron S. J., Jackson S. P., Cote J. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol. Cell. 2004;16:979–990. doi: 10.1016/j.molcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Elder R. T., LOH E. Y., Davis R. W. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc. Natl. Acad. Sci. USA. 1983;80:2432–2436. doi: 10.1073/pnas.80.9.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez E. B., Espinosa J. M., Forsburg S. L. Schizosaccharomyces pombe mst2+ encodes a MYST family histone acetyltransferase that negatively regulates telomere silencing. Mol. Cell. Biol. 2005;25:8887–8903. doi: 10.1128/MCB.25.20.8887-8903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. A., Sterner D. E., Duggan L. J., Workman J. L., Berger S. L. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell Biol. 1998;8:193–197. doi: 10.1016/s0962-8924(98)01263-x. [DOI] [PubMed] [Google Scholar]
- Grimm C., Schaer P., Munz P., Kohli J. The strong ADH1 promoter stimulates mitotic and meiotic recombination at the ADE6 gene of Schizosaccharomyces pombe. Mol. Cell. Biol. 1991;11:289–298. doi: 10.1128/mcb.11.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz H. Site specific induction of gene conversion in Schizosaccharomyces pombe. Genetics. 1971;69:317–337. doi: 10.1093/genetics/69.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz H., Heslot H., Leupold U., Loprieno N. Schizosaccharomyces pombe. In: King R. D., editor. Handbook of Genetics. Vol. 1. New York: Plenum; 1974. pp. 395–446. [Google Scholar]
- Hirota K., Hasemi T., Yamada T., Mizuno K. I., Hoffman C. S., Shibata T., Ohta K. Fission yeast global repressors regulate the specificity of chromatin alteration in response to distinct environmental stresses. Nucleic Acids Res. 2004;32:855–862. doi: 10.1093/nar/gkh251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K., Hoffman C. S., Ohta K. Reciprocal nuclear shuttling of two antagonizing Zn finger proteins modulates Tup family corepressor function to repress chromatin remodeling. Eukaryot. Cell. 2006;5:1980–1989. doi: 10.1128/EC.00272-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K., Hoffman C. S., Shibata T., Ohta K. Fission yeast Tup1-like repressors repress chromatin remodeling at the fbp1(+) promoter and the ade6–M26 recombination hotspot. Genetics. 2003;165:505–515. doi: 10.1093/genetics/165.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino Y., Yamamoto M. Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA. 1985;82:2447–2451. doi: 10.1073/pnas.82.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki T., Mochizuki N., Maeda T., Yamamoto M. Characterization of a fission yeast gene, gpa2, that encodes a G alpha subunit involved in the monitoring of nutrition. Genes Dev. 1992;6:2455–2462. doi: 10.1101/gad.6.12b.2455. [DOI] [PubMed] [Google Scholar]
- Kon N., Krawchuk M. D., Warren B. G., Smith G. R., Wahls W. P. Transcription factor Mts1/Mts2 (Atf1/Pcr1, Gad7/Pcr1) activates the M26 meiotic recombination hotspot in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA. 1997;94:13765–13770. doi: 10.1073/pnas.94.25.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs J. E., Kuo M. H., Allis C. D., Peterson C. L. Cell cycle-regulated histone acetylation required for expression of the yeast HO gene. Genes Dev. 1999;13:1412–1421. doi: 10.1101/gad.13.11.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten M., Goldman A. S. Meiotic recombination hotspots. Annu. Rev. Genet. 1995;29:423–444. doi: 10.1146/annurev.ge.29.120195.002231. [DOI] [PubMed] [Google Scholar]
- McLeod M., Beach D. Homology between the ran1+ gene of fission yeast and protein kinases. EMBO J. 1986;5:3665–3671. doi: 10.1002/j.1460-2075.1986.tb04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Emura Y., Baur M., Kohli J., Ohta K., Shibata T. The meiotic recombination hot spot created by the single-base substitution ade6–M26 results in remodeling of chromatin structure in fission yeast. Genes Dev. 1997;11:876–886. doi: 10.1101/gad.11.7.876. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nachman M. W. Variation in recombination rate across the genome: evidence and implications. Curr. Opin. Genet. Dev. 2002;12:657–663. doi: 10.1016/s0959-437x(02)00358-1. [DOI] [PubMed] [Google Scholar]
- Nicolas A. Relationship between transcription and initiation of meiotic recombination: toward chromatin accessibility. Proc. Natl. Acad. Sci. USA. 1998;95:87–89. doi: 10.1073/pnas.95.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K., Shibata T., Nicolas A. Changes in chromatin structure at recombination initiation sites during yeast meiosis. EMBO J. 1994;13:5754–5763. doi: 10.1002/j.1460-2075.1994.tb06913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanides G., Reinberg D. RNA polymerase II elongation through chromatin. Nature. 2000;407:471–475. doi: 10.1038/35035000. [DOI] [PubMed] [Google Scholar]
- Petes T. D. Meiotic recombination hot spots and cold spots. Nat. Rev. Genet. 2001;2:360–369. doi: 10.1038/35072078. [DOI] [PubMed] [Google Scholar]
- Ponticelli A. S., Sena E. P., Smith G. R. Genetic and physical analysis of the M26 recombination hotspot of Schizosaccharomyces pombe. Genetics. 1988;119:491–497. doi: 10.1093/genetics/119.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- Schuchert P., Langsford M., Kaslin E., Kohli J. A specific DNA sequence is required for high frequency of recombination in the ade6 gene of fission yeast. EMBO J. 1991;10:2157–2163. doi: 10.1002/j.1460-2075.1991.tb07750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H., Masuoka M., Murofushi H., Takeda S., Shibata T., Ohta K. Rapid generation of specific antibodies by enhanced homologous recombination. Nat. Biotechnol. 2005;23:731–735. doi: 10.1038/nbt1092. [DOI] [PubMed] [Google Scholar]
- Sherman F., Fink G., Hicks J. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1986. Methods in Yeast Genetics: Laboratory Course Manual. [Google Scholar]
- Steiner W. W., Schreckhise R. W., Smith G. R. Meiotic DNA breaks at the S. pombe recombination hot spot M26. Mol. Cell. 2002;9:847–855. doi: 10.1016/s1097-2765(02)00489-6. [DOI] [PubMed] [Google Scholar]
- Sun H., Treco D., Schultes N. P., Szostak J. W. Double-strand breaks at an initiation site for meiotic gene conversion. Nature. 1989;338:87–90. doi: 10.1038/338087a0. [DOI] [PubMed] [Google Scholar]
- Szankasi P., Heyer W. D., Schuchert P., Kohli J. DNA sequence analysis of the ade6 gene of Schizosaccharomyces pombe. Wild-type and mutant alleles including the recombination host spot allele ade6–M26. J. Mol. Biol. 1988;204:917–925. doi: 10.1016/0022-2836(88)90051-4. [DOI] [PubMed] [Google Scholar]
- Takeda T., Yamamoto M. Analysis and in vivo disruption of the gene coding for calmodulin in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA. 1987;84:3580–3584. doi: 10.1073/pnas.84.11.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J. K., Hassig C. A., Schnitzler G. R., Kingston R. E., Schreiber S. L. Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature. 1998;395:917–921. doi: 10.1038/27699. [DOI] [PubMed] [Google Scholar]
- Travers A. An engine for nucleosome remodeling. Cell. 1999;96:311–314. doi: 10.1016/s0092-8674(00)80543-7. [DOI] [PubMed] [Google Scholar]
- van Attikum H., Fritsch O., Hohn B., Gasser S. M. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell. 2004;119:777–788. doi: 10.1016/j.cell.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Wahls W. P. Meiotic recombination hotspots: shaping the genome and insights into hypervariable minisatellite DNA change. Curr. Top. Dev. Biol. 1998;37:37–75. doi: 10.1016/s0070-2153(08)60171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahls W. P., Smith G. R. A heteromeric protein that binds to a meiotic homologous recombination hot spot: correlation of binding and hot spot activity. Genes Dev. 1994;8:1693–1702. doi: 10.1101/gad.8.14.1693. [DOI] [PubMed] [Google Scholar]
- Walfridsson J., Khorosjutina O., Matikainen P., Gustafsson C. M., Ekwall K. A genome-wide role for CHD remodelling factors and Nap1 in nucleosome disassembly. EMBO J. 2007;26:2868–2879. doi: 10.1038/sj.emboj.7601728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. 3rd ed. San Diego, CA: Academic Press; 1997. Chromatin: Structure and Function. [Google Scholar]
- Wu T. C., Lichten M. Meiosis-induced double-strand break sites determined by yeast chromatin structure. Science. 1994;263:515–518. doi: 10.1126/science.8290959. [DOI] [PubMed] [Google Scholar]
- Yamada T., Mizuno K. I., Hirota K., Kon N., Wahls W. P., Hartsuiker E., Murofushi H., Shibata T., Ohta K. Roles of histone acetylation and chromatin remodeling factor in a meiotic recombination hotspot. EMBO J. 2004;23:1792–1803. doi: 10.1038/sj.emboj.7600138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Imai Y., Watanabe Y. Mating and Sporulation in Schizosaccharomyces pombe. In: Prigle J. R., Broach J. R., Jones E. W., editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor, NY: Cold Spring Habor Laboratory Press; 1997. pp. 1037–1106. [Google Scholar]
- Yoo E. J., Jin Y. H., Jang Y. K., Bjerling P., Tabish M., Hong S. H., Ekwall K., Park S. D. Fission yeast hrp1, a chromodomain ATPase, is required for proper chromosome segregation and its overexpression interferes with chromatin condensation. Nucleic Acids Res. 2000;28:2004–2011. doi: 10.1093/nar/28.9.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman Z., Ansari A. Z., Koh S. S., Young R., Ptashne M. Interaction of a transcriptional repressor with the RNA polymerase II holoenzyme plays a crucial role in repression. Proc. Natl. Acad. Sci. USA. 2001;98:2550–2554. doi: 10.1073/pnas.041611198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., LeRoy G., Seelig H. P., Lane W. S., Reinberg D. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell. 1998;95:279–289. doi: 10.1016/s0092-8674(00)81758-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.