Abstract
Stress response pathways allow cells to sense and respond to environmental changes and adverse pathophysiological states. Pharmacological modulation of cellular stress pathways has implications in the treatment of human diseases, including neurodegenerative disorders, cardiovascular disease, and cancer. The quinone methide triterpene celastrol, derived from a traditional Chinese medicinal herb, has numerous pharmacological properties, and it is a potent activator of the mammalian heat shock transcription factor HSF1. However, its mode of action and spectrum of cellular targets are poorly understood. We show here that celastrol activates Hsf1 in Saccharomyces cerevisiae at a similar effective concentration seen in mammalian cells. Transcriptional profiling revealed that celastrol treatment induces a battery of oxidant defense genes in addition to heat shock genes. Celastrol activated the yeast Yap1 oxidant defense transcription factor via the carboxy-terminal redox center that responds to electrophilic compounds. Antioxidant response genes were likewise induced in mammalian cells, demonstrating that the activation of two major cell stress pathways by celastrol is conserved. We report that celastrol's biological effects, including inhibition of glucocorticoid receptor activity, can be blocked by the addition of excess free thiol, suggesting a chemical mechanism for biological activity based on modification of key reactive thiols by this natural product.
INTRODUCTION
The heat shock response (HSR) is an ancient and highly conserved cytoprotective mechanism. Production of heat shock proteins, including protein chaperones, is essential for the folding, repair, or triage of damaged proteins; thus, it serves to promote cellular viability under conditions that would otherwise induce apoptosis (McMillan et al., 1998; Christians et al., 2002). There is significant interest in the discovery and development of small molecules that modulate the HSR and parallel stress response pathways for therapeutic purposes (Morimoto and Santoro, 1998; Westerheide and Morimoto, 2005; Corson and Crews, 2007). The HSR is governed by the stress-inducible heat shock transcription factor HSF1, which plays a key regulatory role in response to environmental stress, development, and many pathophysiological conditions, including cancer, ischemia-reperfusion injury, diabetes, and aging (Morimoto and Santoro, 1998; Morano and Thiele, 1999a).
Significant progress has been made in identifying HSF1-modulating compounds such as triptolide and quercetin, inhibitors of HSF1, and activators such as the protein synthesis inhibitor puromycin, the proteasome inhibitor MG132, the nonsteroidal anti-inflammatory drugs salicylate and indomethacin, and the heat shock protein (Hsp)90 inhibitors radicicol and geldanamycin (Hightower, 1980; Jurivich et al., 1992; Lee et al., 1995; Nagai et al., 1995; Bagatell et al., 2000; Holmberg et al., 2000; Westerheide et al., 2006). A large-scale screen for novel pharmacologically active compounds that may be beneficial in treating the neurological manifestations of Huntington's disease and amyotrophic lateral sclerosis (ALS) identified the natural product celastrol, a triterpenoid compound isolated from the plant family Celastraceae (Abbott, 2002; Heemskerk et al., 2002). Root bark extracts from these plants are commonly used in traditional Chinese medicine for their anti-inflammatory properties, consistent with the recent identification of celastrol as an effective inhibitor of nuclear factor-κB (Corson and Crews, 2007; Sethi et al., 2007). In addition, celastrol reduces the neurotoxicity associated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)- and 3-nitropropionic acid-induced neurological degeneration in mice (Cleren et al., 2005). Some of these anti-neurodegenerative properties are likely attributable to activation of HSF1, because celastrol induces HSF1 DNA binding and hyperphosphorylation to promote heat shock gene expression and inhibits the function of the HSF1-repressor Hsp90 (Westerheide et al., 2004; Hieronymus et al., 2006). The chemical mechanism(s) by which celastrol produces these numerous physiological effects remains unknown.
Here, we report that celastrol activates Hsf1 in the yeast Saccharomyces cerevisiae with characteristics closely mirroring heat shock, including Hsf1 hyperphosphorylation, production of HSPs, and induction of tolerance to a lethal heat shock. Through genome-wide transcriptional profiling, we show that celastrol concomitantly elicits a previously unappreciated oxidant defense response in yeast. Consistently, celastrol treatment of RKO human colorectal carcinoma cells induces antioxidant genes in parallel with heat shock targets. The influence of celastrol on multiple cellular processes, including antioxidant response activation, heat shock response activation, and inhibition of glucocorticoid receptor maturation, is prevented by incubation with free thiols. Together, these findings indicate that celastrol simultaneously activates multiple stress pathways that ultimately impact cellular survival, and they highlight a potential mechanism through which celastrol carries out its intracellular effects via reacting with key thiols in proteins.
MATERIALS AND METHODS
Yeast Strains and Plasmids
Yeast strains used in this study were of the W303 (MATa ade2-1 trp1 can1-100 leu2-3112 his3-11,15 ura3) and BY4741 (MATa ura3Δ0 leu2Δ0 his3Δ1 met15Δ0) strain backgrounds. The YAP1-TAP (tandem affinity purification tag) strain and the gpx3Δ::kanMX4 deletion strain (gpx3Δ) were purchased from Open Biosystems (Huntsville, AL), and they are otherwise isogenic with BY4741. W303 HSF1-TAP was constructed through polymerase chain reaction (PCR) amplification of the TAP tag sequence and selectable TRP marker from a previously described plasmid construct (Puig et al., 1998). The resulting PCR product was transformed into W303 cells and Trp+ colonies screened by immunoblot for integration of the TAP-tag at the correct locus. The ability of the HSF1-TAP allele to confer normal levels of heat shock resistance was used as a diagnostic to confirm functionality of expressed Hsf1-TAP (data not shown). The HSE-lacZ reporter plasmid (HSE-lacZ) and the yeast AP-1 (Yap1) reporter pCEP12 (ARE-lacZ) have been described previously (Harshman et al., 1988; Santoro et al., 1998). Yeast were grown either in nonselective conditions in rich YPD (0.2% Bacto-peptone, 0.1% yeast extract, and 2% glucose) or synthetic complete (SC) media. To maintain the solubility of celastrol, experiments were performed as indicated with minimal media supplemented with 50 mM Tris-HCl, pH 7.5. Celastrol was dissolved as a 10 mM stock in dimethyl sulfoxide (DMSO). Unless otherwise indicated, experiments were performed with strains grown to logarithmic phase at 30°C. Dithiothreitol (DTT) inactivation of celastrol was achieved by premixing the two chemicals for 5 min before addition to cells. Plasmids p413GPD-rGR and pYRP-GRElacZ (URA3) were used as described to determine Hsp90 chaperoning of heterologously expressed rat glucocorticoid receptor (Morano et al., 1999).
Mammalian Cell Culture, RNA Isolation, and Reverse Transcription (RT)-PCR
RKO human colorectal carcinoma cells were grown in DMEM supplemented with 10% fetal bovine serum and antibiotics. Cells were treated at ∼60–80% confluence with varying concentrations of celastrol for 6 h. Total RNA was isolated from cells using an RNeasy extraction kit (QIAGEN, Valencia, CA) with on-column DNase I treatment according to the manufacturer's directions. RNA (1.0 μg) was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Primers for RT-PCR have been reported previously (West and Marnett, 2005). PCR products were amplified with Taq polymerase (Promega, Madison, WI) by using standard cycling conditions.
Reporter Assays
Cells harboring the lacZ transcriptional reporter fusions were harvested by centrifugation and resuspended in selective media supplemented with 50 mM Tris-HCl, pH 7.5. Cells were treated with 20 μM celastrol or DMSO for 1 h at 30°C with continuous shaking and harvested by centrifugation. Cell pellets frozen immediately in dry ice. β-Galactosidase activity was determined as described previously (Morano et al., 1999). Heat shock experiments using a β-galactosidase reporter were done at 37°C because 39°C was found to diminish enzyme activity. Reporter assays for constructs containing individual transcription factor binding sites were performed as described previously using RKO cells (West and Marnett, 2005). Luciferase reporter assays using the stable HeLa-hsp70.1pr-luc cell line were performed as described previously (Westerheide et al., 2004).
Protein Analysis
Protein extracts were prepared using an alkaline lysis procedure as described previously (Ooi et al., 1996). To analyze formation of the transient Yap1–glutathione peroxidase 3 (Gpx3) complex, cells were immediately precipitated with ice-cold 20% trichloroacetic acid after treatment. Cell extracts were prepared by glass-bead lysis in nonreducing sample buffer and left untreated or reduced with 10 mM DTT before SDS-polyacrylamide gel electrophoresis (PAGE). Immunoblotting procedures were performed as described previously (Morano and Thiele, 1999b). Polyclonal antisera that recognizes both Ssa3 and Ssa4 was a kind gift from E. Craig (University of Wisconsin, Madison, WI). Antibodies against phosphoglycerate kinase (PGK) and TAP tag (protein A epitope) were obtained from Invitrogen (Carlsbad, CA) and Sigma-Aldrich (St. Louis, MO), respectively.
Heat Shock Sensitivity Assay
To assay celastrol-induced thermotolerance, BY4741 cells were resuspended in SC media supplemented with 50 mM Tris-HCl, pH 7.5, and subsequently treated with no reagent, 20 μM celastrol, or DMSO for 1 h at 30°C. After the treatment period, cells were diluted to an OD600 of 0.1 in sterile 0.2-ml PCR tubes in 100 μl. The diluted cells were heat shocked at 47°C in a thermocycler for 0, 5, 10, 15, and 20 min. The aliquots were spotted in equal volume on solid SC medium and incubated for 2 d at 30°C.
Transcriptional Profiling
Genome-wide transcriptional profiling was performed with logarithmic BY4741 cells harboring the HSE-lacZ reporter. The reporter plasmid was included in this experiment as a means to ensure adequate celastrol- and heat shock-mediated induction. Cells used for the heat shock experiment were divided into two equal volumes and either shifted to 39°C for 30 min or left at 30°C for the duration of the heat shock. Cells used for the celastrol/DMSO experiment were harvested by centrifugation and resuspended in minimal media supplemented with 50 mM Tris-HCl, pH 7.5. Cells were treated with 10 μM celastrol or an equal volume of DMSO and incubated for 1 h at 30°C. After heat shock and celastrol treatment, cells were harvested by centrifugation, and total RNA was isolated using an acid phenol-glass bead extraction procedure (Santoro, et al., 1998). All RNA-labeling and DNA microarray manipulations were performed by the University of Texas Southwestern Medical Center at Dallas-Microarray Core Facility (http://microarray.swmed.edu). The array consisted of duplicate sets of 6219 yeast genes printed on glass slides, and hybridization values were obtained for each spot by using GenePix software. The mean intensity for each gene was obtained in duplicate from each of the replicated experiments, and a compound mean of signal intensity was calculated from four independent spots. The compound mean induction ratios are represented in Figure 2 after log2 transformation. Genes shown in Table 1 are representative members of distinct gene groupings whose transcript levels were induced twofold or greater in response to either heat shock or celastrol treatment. The median induction ratios for the described gene families were calculated in response to heat shock or celastrol treatment, and a list of specific genes with respective individual changes in gene expression is provided in Supplemental Table 1. Primary data sets are accessible through the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo; accession no. GSE5608).
Figure 2.
Celastrol simultaneously activates transcription of heat shock and antioxidant genes in yeast. Parallel cultures of wild-type yeast were left untreated (DMSO) or treated with 10 μM celastrol for 1 h, or they were subjected to a 39°C heat shock for 30 min. RNA was isolated and processed for transcriptional profiling by DNA microarray as described in Materials and Methods. Mean induction ratios for each treatment were calculated for each gene, transformed to log2 and plotted. Selected genes exhibiting significant induction in both treatments are labeled.
Table 1.
Comparison of heat shock and celastrol-induced gene expression
| Gene familya | Median induction ratiob |
|
|---|---|---|
| Heat shock (1.0) | Celastrol (0.9) | |
| Xenobiotic metabolism and clearance | ||
| Glutathione metabolic enzymes | 2.2 | 5.7 |
| ECM4, GLO1, GLO4, GLR1, GSH1, GTT2, YJL068C | ||
| Aryl-alcohol dehydrogenases | 1.0 | 5.6 |
| AAD3, AAD4, AAD6, AAD14, AAD15, AAD16 | ||
| Other putative xenobiotic oxidoreductases | 1.9 | 9.1 |
| ADH5, ADH6, ALD4, ALD6, BDH2, FRE7, GRE2, GOR1, OYE2, OYE3, YDL124W, YGL157W, YHB1, YML131S, YNL134C, YPR1, ZTA1 | ||
| Export pumps | 1.4 | 5.8 |
| ATR1, FLR1, PDR5, PDR10, PDR15, PDR16, SNQ2, YCF1, YOR1 | ||
| Protein folding, processing, and turnover | ||
| Chaperones and co-chaperones | 6.2 | 4.9 |
| CPR1, CPR6, HSP26, HSP31, HSC82, HSP82, HSP104, PDI1, PHB1, PHB2, SBA1, SGT1, SSA1, SSA2, SSA4, SSE1 | ||
| Protein reductants | 1.2 | 3.7 |
| AHP1, MXR1, TRX2 | ||
| Regulators of protein turnover/proteasome subunits | 2.0 | 3.1 |
| BLM10, CDC48, DDI1, DSK2, ECM29, LAP4, NPL4, OUT1, PIM1, PRD1, PRE2, PRE3, PRE4, PRE5, PRE6, PRE7, PRE10, PUP1, PUP2, PUP3, RPN1, RPN2, RPN3, RPN5, RPN6, RPN7, RPN8, RPN9, RPN11, RPN12, RPN13, RPT1, RPT3, RPT5, RPT6, SCL1, SHP1, UBA1, UBC5, UBI4, UBP6, UBX5, UFD1, UMP1, YME1 | ||
a Genes selected based on induction ratio ≥2.0.
b Global induction medians shown for each treatment in parentheses.
Yap1 Subcellular Localization
To assess the role of the Yap1 carboxy-terminal redox center in celastrol activation, a previously generated synthetic construct that includes a simian virus 40 (SV40) nuclear localization sequence and green fluorescent protein (GFP) fused to the Yap1 redox regulatory domain was used (Yap1-RDGFP, kindly provided by M. Wood, University of California, Davis) (Wood et al., 2004). To examine involvement of Cys residues in Yap1 nuclear accumulation, site-directed mutagenesis was performed using the QuikChange protocol (Stratagene, La Jolla, CA) to make the following replacements: C598A, C620A, and C629T. To obtain the C598,620A;C629T triple mutant, multiple rounds of site-directed mutagenesis were performed. All plasmids were sequenced to confirm mutagenesis. Plasmids were transformed into BY4741 and grown on uracil-deficient media. Experiments were conducted on cells in log phase that were resuspended in uracil-deficient liquid medium buffered with 50 mM Tris, pH 7.4. Incubations were carried out for 5 min with 300 μM H2O2 or 20 min for 10 μM celastrol and 0.1% DMSO. Cells were imaged using an Axiovert microscope (Carl Zeiss, Oberkochen, Germany).
RESULTS
Celastrol Activates Hsf1 in Yeast and Confers Tolerance to Thermal Stress
To understand the general mechanisms of cytoprotection afforded by celastrol, we addressed whether the heat shock response controlled by Hsf1 in S. cerevisiae is responsive to celastrol treatment. Yeast Hsf1, like mammalian HSF1, specifically recognizes multimeric “nGAAn” repeat sequences termed heat shock elements (HSEs) in promoters of target genes (Sorger and Pelham, 1987). The wild-type yeast strain BY4741 carrying an Hsf1-responsive HSE-lacZ reporter was treated with concentrations of celastrol ranging from 0 to 40 μM for a 1-h time period, and a dose-response curve was generated based on the resulting activity of the reporter construct (Figure 1A). The EC50 obtained from this assay was 7.5 μM, similar to the EC50 of 3.0 μM observed in mammalian cells (Westerheide et al., 2004). A reporter fusion construct bearing multiple mutations in the HSE was not induced by celastrol treatment, confirming that reporter activation required a functional HSE (data not shown) (Santoro et al., 1998). Induction of the HSE-lacZ reporter by 20 μM celastrol was ∼40% of the level achieved by a standard heat shock (30 min at 37°C), suggesting that celastrol is less potent than heat shock for reporter activation with these experimental conditions (Figure 1B).
Figure 1.
Celastrol activates yeast Hsf1 and induces thermotolerance. (A) Wild-type (BY4741) cells containing an HSE-lacZ reporter were treated with concentrations of celastrol ranging from 0 to 40 μM for 1 h, and β-galactosidase activity was determined as described in Materials and Methods. (B) The same cells as in A were grown at control temperature (30°C, −), heat shocked at 37°C for 1 h (HS), or exposed to DMSO or celastrol (cel, 20 μM), and β-galactosidase activities were determined. (C) W303 HSF1-TAP cells exposed to normal growth temperatures (30°C), heat shock (39°C), DMSO, or 10 μM celastrol were harvested at the indicated time points. Protein extracts were analyzed by SDS-PAGE and immunoblot analysis and Hsf1-TAP detected using antibodies directed against the protein A epitope. Extract prepared from W303 cells grown under normal growth conditions was used as a negative control (−). (D) Wild-type (BY4741) cells were exposed to 30°C, 39°C, DMSO, or 20 μM celastrol for 1 h, and protein extracts were analyzed by SDS-PAGE and immunoblot. Ssa3/4 levels were detected using polyclonal antibodies that detect both heat shock-inducible isoforms of the yeast Hsp70. PGK levels were determined as a loading control. (E) Wild-type (BY4741) cells were treated for 1 h with either no reagent, DMSO, or 10 μM celastrol for 1 h before heat shock at 47°C for the indicated times as described in Materials and Methods. Cells were spotted in equal volume on SC medium, and viability was assayed after growth at 30°C for 2 d.
Celastrol stimulates mammalian HSF1 DNA binding and hyperphosphorylation in mammalian cells, events required for activation of the heat shock response (Westerheide et al., 2004). Both of these events occur with kinetics similar to heat shock. Because yeast Hsf1 is constitutively bound to many HSE-containing promoters in the absence of stress, we chose to examine whether celastrol-mediated activation of Hsf1 produced changes in phosphorylation status similar to that observed in response to heat shock (Sorger and Pelham, 1988). To test this, a wild-type strain (W303) containing a genomic-TAP tagged allele of HSF1 was constructed to allow visualization of the altered mobility induced by phosphorylation. Hsf1-TAP cells were exposed to heat shock (HS), celastrol, or DMSO treatment, and cells were harvested before treatment, 15 min after treatment, and at the completion of the 1-h time course. Hsf1-TAP mobility was examined by immunoblot analysis. As shown in Figure 1C, celastrol induced the formation of a hyperphosphorylated form of Hsf1-TAP within 15 min that is comparable with heat shock (Sorger and Pelham, 1988; Liu and Thiele, 1996). Treatment with DMSO alone did not result in a detectable change in phosphorylation status. Restoration of basal phosphorylation levels was observed within 1 h in response to both treatments, suggesting that heat shock and celastrol exhibit similar features of attenuation.
The transcriptional activation of Hsf1-regulated genes in response to heat shock results in increased levels of protein chaperones essential for maintaining cellular viability during exposure to elevated temperature. Yeast cells exposed to a short period of sublethal elevated temperature (37°C) before exposure to lethal heat shock temperatures (>42°C) are more thermotolerant than untreated cells based in large part on the action of Hsf1 (Nieto-Sotelo et al., 1990; Sorger, 1990). We therefore assessed levels of two prominent heat shock-inducible cytosolic Hsp70 chaperones encoded by the SSA3 and SSA4 genes (Boorstein and Craig, 1990). Wild-type cells were untreated or exposed to heat shock, DMSO, or celastrol treatment for 1 h, and protein extracts were analyzed by immunoblot. As shown in Figure 1D, Ssa3/4 levels were low under control conditions and induced by celastrol, albeit to a lesser extent than heat shock (i.e., celastrol-induced levels of Ssa3/4 were ∼65% of heat shock-induced levels as determined by densitometry). Because celastrol treatment induced HSP expression in yeast, we reasoned that treatment before lethal heat shock would significantly increase resistance to thermal stress. To test this hypothesis, wild-type cells (BY4741) were left untreated, treated with DMSO, or treated with celastrol for 1 h at 30°C and subsequently exposed to a 47°C heat shock for 0, 5, 10, 15, or 20 min. Equivalent cell numbers from each culture were spotted onto solid media, and they were subsequently assayed for viability after growth at 30°C for 2 d (Figure 1E). Although cells receiving no treatment or DMSO exhibited a significant reduction in viability (at least 10-fold by colony formation) at the 15-min time period, celastrol treatment before heat shock resulted in sustained viability after incubation at 47°C for 20 min. Together, these data indicate that yeast Hsf1, like mammalian HSF1, is activated by celastrol to increase stress resistance and suggest a conserved mechanism of celastrol-mediated activation among diverse eukaryotes.
Celastrol Induces Both Heat Shock- and Oxidant-responsive Gene Expression
Our results indicate that celastrol modulates Hsf1 activity in a manner similar to heat shock. We next sought to identify other responses regulated by celastrol to understand more fully its cytoprotective properties. We addressed this by comparing global transcriptional profiles of yeast cells exposed to heat shock or celastrol. Whole genome transcript analysis was performed using RNA isolated from wild-type cells left untreated, exposed to heat shock, DMSO, or 10 μM celastrol. Transcript induction ratios for each gene were averaged over replicate experiments and plotted as fold induction with celastrol versus fold induction with heat shock. Strongly induced genes are indicated in Figure 2, and a more comprehensive list of gene families induced by celastrol is in Table 1. The induced genes fall into two major groupings: 1) xenobiotic metabolism/clearance and 2) protein folding, processing, and turnover. Celastrol and heat shock commonly induce a subset of genes that primarily encode protein chaperones. As calculated in Table 1, a set of sixteen known Hsf1 targets exhibited a median induction ratio of 6.2-fold by heat shock, whereas celastrol treatment resulted in a 4.9-fold increase (Hahn et al., 2004). We also noted a significant increase in levels of genes encoding proteasome components in the array, consistent with a previous report (Hahn et al., 2006). In addition, celastrol increased levels of genes with cellular roles in xenobiotic metabolism (Figure 2 and Table 1). Many of these genes, including those encoding protein reductants such as TRX2, glutathione-metabolic enzymes such as GSH1, aryl-alcohol dehydrogenases (AADs), and multidrug resistance pumps, are not known targets of Hsf1, but they are targets of the transcription factor Yap1 (Jamieson et al., 1994; Gasch et al., 2000; Gasch and Werner-Washburne, 2002).
Celastrol Activates the Yap1 Transcription Factor through Its Carboxy-terminal Domain
Because our array studies suggested that celastrol activates Yap1 and the mechanism of activation of this transcription factor is known, we used Yap1 as a model to further dissect celastrol's biological effects. Yap1 contains two cysteine-rich domains clustered into two distinct redox centers (n-CRD and c-CRD; Figure 3A), and it is responsive to a variety of thiol-modifying molecules, including oxidants and electrophiles (Coleman et al., 1999; Kuge et al., 2001; Delaunay et al., 2002; Azevedo et al., 2003; Maeta et al., 2004). Specifically, Yap1 activation by H2O2 requires the thiol peroxidase Gpx3/Orp1 (Delaunay et al., 2002), and it involves formation of two disulfide bonds between cysteines 303–598 and 310–629 (Delaunay et al., 2000; Wood et al., 2003; Okazaki et al., 2007). Therefore, we tested whether Yap1 was activated by celastrol treatment and whether this activation required Gpx3 by using a transcriptional lacZ reporter containing a Yap1 response element (Harshman et al., 1988). Wild-type (BY4741) and gpx3Δ cells containing the reporter (ARE-lacZ) were left untreated or treated for 1 h with DMSO, 300 μM H2O2, or 10 μM celastrol, and they were subsequently assayed for β-galactosidase activity. As shown in Figure 3B, celastrol treatment resulted in approximately six-fold activation of the reporter, and this induction occurred independently of Gpx3. In contrast, H2O2 induced the reporter in wild-type cells, whereas no induction was observed in the gpx3Δ mutant.
Figure 3.
Celastrol activates the yeast oxidant-responsive transcription factor Yap1 through the carboxy-terminal domain. (A) Schematic of Yap1 cysteine residues involved in oxidation and alkylation. (B) Wild-type (BY4741) and gpx3Δ cells containing the Yap1 reporter (pCEP12) were treated with either no reagent, 300 μM H2O2, DMSO, or 10 μM celastrol. Harvested cells were subsequently assayed for β-galactosidase activity as described previously. (C) Cells expressing a TAP-tagged allele of YAP1 from the endogenous genomic locus were grown to logarithmic phase and treated with DMSO, 300 μM H2O2, or 10 μM celastrol for 10 min, followed by trichloroacetic acid precipitation. Cell extracts were prepared by glass-bead lysis in nonreducing sample buffer and either left untreated (−DTT) or reduced with 10 mM DTT (+DTT) before SDS-PAGE. Yap1 migration was detected using anti-protein A antibody. The locations of the Yap1-TAP fusion and the DTT-sensitive Yap1-TAP·Gpx3 heterodimer are indicated. (D) Cells expressing either wild-type or mutant GFP-Yap1 regulatory domain fusion proteins were treated with DMSO, 300 μM H2O2, or 10 μM celastrol to monitor GFP subcellular localization as described in Materials and Methods. Cartoon illustrates diffuse Yap1-GFP fluorescence before modification of the c-CRD (by either pathway), and nuclear localization after treatment. The yeast vacuole is indicated by a dark gray circle in the cartoon. Results are representative of three independent experiments.
The transient Yap1-Gpx3 complex induced by H2O2 can be detected by nonreducing SDS-PAGE (Delaunay et al., 2002). Based on the stimulation of Yap1 reporter activity by celastrol in the absence of Gpx3, we predicted that celastrol treatment would not induce formation of intermolecular disulfides. Cells expressing an integrated TAP-tagged allele of Yap1 were treated with DMSO, 300 μM H2O2 or 10 μM celastrol for 10 min, followed by protein extraction in nonreducing sample buffer. H2O2 induced formation of a slower-migrating species that disappeared upon disulfide reduction, as previously shown (Figure 3C). In contrast, treatment with celastrol or DMSO did not induce formation of the Yap1–Gpx3 complex. These data suggest that celastrol, unlike H2O2, exhibits the profile of other thiol-reactive compounds that modify the carboxy-terminal Gpx3-independent redox center without formation of interdomain disulfide bonds.
On modification of cysteine residues within the CRDs of Yap1, nuclear export is impaired, causing its nuclear retention to facilitate target gene expression (Kuge et al., 1998; Yan et al., 1998). We used a previously generated minimal Yap1 regulatory domain construct bearing the n-CRD, c-CRD, GFP, and the SV40 nuclear localization sequence (Yap1-RDGFP) to monitor changes in Yap1 subcellular localization in response to H2O2 and celastrol (Wood et al., 2004). On treatment of yeast expressing the wild-type fusion protein, nuclear accumulation was observed for both celastrol and H2O2, as shown in Figure 3D. However, upon mutation of C598 to alanine and C629 to threonine, nuclear accumulation was observed only for celastrol, consistent with the inability to form either of the two-domain disulfides. In contrast, mutation of C620 in Yap1 allowed H2O2-mediated nuclear accumulation but substantially reduced the amount of nuclear accumulation observed with celastrol. The Yap1 reporter containing all three carboxy-terminal cysteine mutations (AAT) failed to respond to either celastrol or H2O2. These results, when considered with the observed independence of Gpx3, indicate that celastrol induces Yap1 through a disulfide-independent mechanism, perhaps through direct alkylation of one or more cysteine residues within and adjacent to the nuclear export sequence located in the c-CRD.
Induction of Multiple Cytoprotective Genes by Celastrol in Mammalian Cells
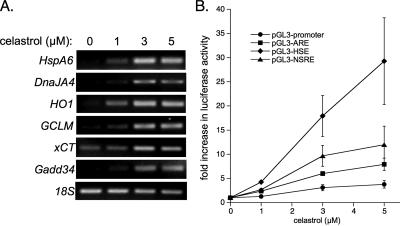
Our microarray results in yeast led us to hypothesize that celastrol induces multiple genes involved in providing protection against oxidative injury, in addition to activating a potent heat shock response. To determine whether celastrol simultaneously activates additional mammalian cytoprotective responses similar to that seen in S. cerevisiae, we examined a number of heat shock response- and antioxidant response-regulated transcripts. The latter response is controlled by the action of two transcription factors, NF-E2-related factor-2 (Nrf2) and activating transcription factor 4 (Atf4) (He et al., 2001; Harding et al., 2003; Nguyen et al., 2003). Human colorectal carcinoma (RKO) cells were exposed to increasing doses of celastrol for 6 h to monitor changes in HSF1, Nrf2, and/or Atf4 targets. A dose-dependent induction was observed in the heat shock genes HspA6 and DnaJA4, heme oxygenase-1 (HO1), the regulatory subunit of glutamate-cysteine ligase (GCLM), a cystine transporter subunit (xCT), and the growth arrest- and DNA damage-inducible gene 34 (Gadd34) (Figure 4A) (Fornace et al., 1989; Leung et al., 1990; He et al., 2001; Sasaki et al., 2002; Levonen et al., 2004).
Figure 4.
Celastrol activates the mammalian antioxidant response. (A) RNA was isolated from RKO cells treated with the indicated doses of celastrol or DMSO vehicle and reverse-transcribed as described in Materials and Methods. PCR reactions were performed on cDNAs for the indicated transcripts. 18S RNA levels were assayed to determine equal loading. Similar results were obtained in three independent experiments. (B) RKO cells were transfected with luciferase constructs for 20–24 h. Cells were treated with the indicated concentrations of celastrol for 8 h, lysed, assayed for firefly luciferase activity, and normalized for transfection efficiency with Renilla luciferase activity. Results (mean ± SD) are from triplicate cell treatments and are representative of two independent experiments.
To determine whether HSF1, Nrf2, and Atf4 were activated by celastrol, we used a previously generated reporter library consisting of binding sites for these transcription factors (i.e., HSE, antioxidant response element [ARE], and nutrient-sensing response element [NSRE], respectively) cloned upstream of a minimal promoter to regulate luciferase expression (West and Marnett, 2005). On treatment of RKO cells transiently transfected with these plasmids, we found that celastrol induced reporter expression above control vector (pGL3-promoter) for each transcription factor (Figure 4B). Together, these results indicate that celastrol induces both oxidant defense and heat shock responses in mammalian cells as in yeast, and they suggest that celastrol promotes simultaneous activation of HSF1, Nrf2, and Atf4.
Biological Effects of Celastrol Are Inhibited by Free Thiols
Our results suggest that celastrol possesses the signaling profile of a thiol-modifying molecule (Gasch et al., 2000; Causton et al., 2001). If celastrol acts intracellularly via a thiol-reactive mechanism, then excess free thiols would prevent target modification and thereby decrease its pharmacological potency. We therefore treated yeast cells containing either the HSE-lacZ (Hsf1) or ARE-lacZ (Yap1) reporters with 10 μM celastrol in the presence or absence of the reducing agent DTT at 50 μM. The results reveal that DTT significantly blocked celastrol-mediated activation of both responses in our assays, whereas DTT alone had no effect (Figure 5A). We extended these results by investigating the ability of DTT to prevent celastrol-mediated activation of HSF in HeLa cells stably transfected with a hsp70.1-luciferase reporter (Westerheide et al., 2004). This cell line was treated with 5 μM celastrol that was incubated with a range of DTT concentrations between 0 and 250 μM. We observed a dose-dependent inhibition of luciferase induction with increasing DTT concentration, with 50% inhibition at approximately 10-fold excess of DTT (Figure 5B). Likewise, induction of heat shock response- and antioxidant response-inducible transcripts in RKO cells was decreased upon incubation of celastrol with 250 μM DTT (Figure 5C). These data indicate that DTT blocks activation of two distinct stress pathways by celastrol in both mammalian and yeast cells, and they are consistent with a model wherein celastrol interacts with reactive thiols to regulate both the heat shock and antioxidant responses.
Figure 5.
Biological effects of celastrol are blocked by free thiol. (A) The effects of DTT on celastrol-mediated induction of Hsf1 and Yap1 in yeast were assessed by treating BY4741 cells bearing the HSE-lacZ plasmid or the ARE-lacZ plasmid (pCEP12) with celastrol alone (10 μM), celastrol (10 μM) that had been preincubated with DTT (50 μM) for 5 min, or the indicated controls for 1 h, followed by β-galactosidase assay. Results shown are the mean with SD from three independent experiments. (B) The efficacy of DTT inhibition of celastrol activation of mammalian HSF was tested in HeLa cells by using a firefly luciferase reporter fused to a hsp70.1 promoter fragment. Celastrol (5 μM) was mixed with the indicated concentrations of DTT for 30 min before cell treatment for 8 h, followed by luciferase assay. (C) Heat shock response- and antioxidant response-inducible genes were tested for the effects of DTT on celastrol in RKO cells. Celastrol (3 μM) was incubated with 250 μM DTT for 1 h before cell treatment. RKO cells were incubated under the indicated conditions for 6 h before RNA isolation and transcript analysis. Results are representative of two independent experiments. (D) BY4741 cells transformed with the glucocorticoid receptor assay system (p413GPD-rGR and pYRP-GRElacZ) were treated as described in A, followed by treatment with 10 μM DOC or vehicle alone for 1.5 h. β-Galactosidase activity was determined as described in Materials and Methods.
Recently, celastrol was identified for its ability to inhibit nuclear hormone receptor maturation and function, a process that requires the molecular chaperone Hsp90 (Hieronymus et al., 2006). We tested whether celastrol acts as an inhibitor of nuclear hormone receptor transactivation by using an assay system that consists of the rat glucocorticoid receptor (GR) coupled with a plasmid bearing a glucocorticoid response element driving β-galactosidase (GRE-lacZ) (Picard, 1990). Treatment of yeast containing the plasmids with deoxycorticosterone (DOC, a synthetic activator of GR) for 1.5 h resulted in a six-fold induction in cells treated with DMSO only (Figure 5D). In contrast, pretreatment for 1 h with 10 μM celastrol before DOC exposure blocked hormone-dependent induction. This effect was not observed when cells were treated with celastrol and a fivefold excess of DTT. Together, these findings suggest an explanation for the mode of action for celastrol in yeast and human cells based on modification of key reactive thiols.
DISCUSSION
In this study, we sought to elucidate the mechanism(s) through which the triterpenoid celastrol affords cytoprotection in response to stress. The findings that celastrol activates Hsf1, promotes molecular chaperone expression, and increases viability upon lethal heat shock in yeast and mammalian cells suggest a highly conserved biochemical mechanism (Figure 1). Moreover, celastrol potently induces genes involved in xenobiotic detoxification in yeast and mammalian cells as part of the antioxidant response (Figures 2 and 4 and Table 1). This response in S. cerevisiae is regulated by the transcription factor Yap1, a protein that undergoes nuclear accumulation and promotes target gene expression after modification of specific cysteine residues in its regulatory domain (Figure 3). Consistently, modulation of the antioxidant response, the heat shock response, and nuclear hormone receptor function, was prevented by incubation of celastrol with free thiols (Figure 5), suggesting a thiol-reactive mechanism for this compound.
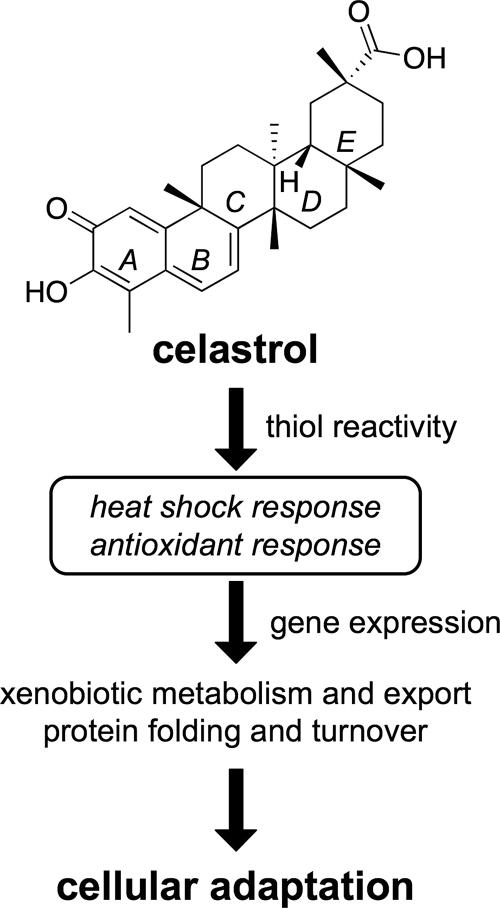
A variety of oxidants and electrophiles are potent inducers of the heat shock response and the antioxidant response (Morimoto and Santoro, 1998; Dinkova-Kostova et al., 2005). Although celastrol is not purported to be a direct protein oxidant, two predicted electrophilic centers reside within the A and B rings of celastrol (Figure 6), where nucleophilic amino acid residues (e.g., cysteine) in multiple target proteins may react to form covalent adducts (Huang et al., 1998; Yang et al., 2006). Structural analogs of celastrol with altered double bond arrangement in the A and B rings lack these reactive centers, and they are pharmacologically less potent as inhibitors of inflammatory signaling (He et al., 1998; Huang et al., 1998). Additionally, incubation of celastrol with excess thiols (e.g., DTT) results in its inactivation in multiple biological assays (Figure 5) (Lee et al., 2006). Therefore, these results suggest that the electrophilic character of the A and B rings of celastrol is responsible for altering signaling responses in both yeast and mammalian cells by modifying many proteins on cysteine residues.
Figure 6.
Model for celastrol-mediated cellular adaptation and cytoprotection. The rings of celastrol are identified by italic capital letters (A–E), and they are described in detail in Discussion. Celastrol treatment is shown to activate both heat shock and antioxidant pathways, resulting in increased expression of a number of cytoprotective genes.
The mechanism by which celastrol induces the heat shock response in yeast and mammalian cells remains a matter of investigation, and it may involve inactivation of several protein complexes involved in protein folding and turnover, specifically through targeting cysteine residues in one or more molecular chaperones and the proteasome. Inactivation of either the molecular chaperone machinery or the proteasome promotes Hsf1 activation and increases Hsp levels (Westerheide and Morimoto, 2005). In support of this reasoning, celastrol induces a gene expression profile similar to that reported for Hsp90 inhibitors in a chemical genetic screen (Hieronymus et al., 2006) and functionally inactivates GR, an Hsp90 client, in yeast (Figure 5D). These results suggest that celastrol has a pronounced influence on Hsp90 chaperone activity, although the exact biochemical mechanism of Hsp90 inhibition is unclear. Several proteomic studies have identified Hsp90 and other molecular chaperones (e.g., Hsp70, peptidylprolyl isomerases) as targets of cysteine-modifying agents, and these molecules inactivate both Hsp90 and Hsp70 in in vitro refolding assays (Carbone et al., 2004, 2005). Collectively, these findings suggest that multiple molecular chaperones may be inactivated by celastrol and related molecules. In addition, celastrol promotes accumulation of polyubiquitinated proteins and inhibits proteasome function, specifically by inactivating its chymotrypsin-like activity (Yang et al., 2006). It is possible that celastrol promotes Hsf1 activation by modifying cysteine residues in multiple proteins involved in protein folding and clearance.
To explore the regulation of oxidant defense genes by celastrol, we focused our efforts on Yap1 due to its direct regulation by cysteine-modifying agents. On exposure to either oxidants or electrophiles, Yap1 accumulates in the nucleus, where it promotes the expression of multiple genes involved in xenobiotic detoxification and export, including TRX2, PDR5, SNQ2, FLR1, YCF1, and ATR1 (Kuge and Jones, 1994; Wemmie et al., 1994; Miyahara et al., 1996; Alarco et al., 1997; Coleman et al., 1997; Kuge et al., 1997). All of these genes are induced strongly by celastrol in our microarray experiments (Table 1). Oxidants and electrophiles promote Yap1 nuclear retention and activity through modifying cysteines within or in proximity to the nuclear export sequence. With celastrol, we observed that Yap1 is activated in a GPX3-independent manner and that the C-terminal cysteine residues required for nuclear accumulation differ substantially from those involved in oxidation by H2O2 (Figure 3). Together, our results suggest that celastrol regulates Yap1 through direct adduction of one or more cysteine residues within the carboxy-terminal domain, rather than through oxidation.
In both yeast and mammalian cells, global gene expression profiling experiments reveal that celastrol induces two key transcriptional regulons (Figure 2) (Hieronymus et al., 2006). These genes are regulated by various transcription factors, including Hsf1 and Yap1 in S. cerevisiae and HSF1, Nrf2, and Atf4 in mammalian cells. As a result, we suggest a model wherein celastrol activates these transcription factors; promotes expression of genes involved in detoxification and export of reactive species, protein folding, and protein turnover; and affords protection against further environmental challenge (Figure 6). Because many of these genes carry out fundamental cytoprotective roles, induction of adaptive responses by celastrol and related molecules through modification of cysteines in target proteins represents a promising area of therapeutic exploration to prevent or ameliorate the effects of diverse conditions, including cardiovascular disease, cancer, and protein aggregation disorders.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Anwou Zhou from the University of Texas Southwestern Microarray Facility for excellent technical assistance, Dr. Ambro van Hoof for the TAP-tag plasmid, Dr. Elizabeth Craig for anti-Ssa3/4 antibody, and Dr. Scott Moye-Rowley for the ARE-lacZ reporter plasmid. We also thank Dr. Matthew Wood for providing the Yap1 regulatory domain reporter construct and the laboratory of Dr. Eric Weiss for assistance with the yeast imaging experiments. This work was supported by American Cancer Society Research Scholar Grant MBC-103134 and National Institute of General Medical Sciences grant GM-074696 (to K.A.M.) and National Institute of Neurological Disorders and Stroke grant NS-047331 (to R.B.S. and R.I.M.). J.D.W. was supported by National Institutes of Health National Research Service Award F32 GM-078965.
Abbreviations used:
- AAD
aryl-alcohol dehydrogenase
- ARE
antioxidant response element
- Atf4
activating transcription factor 4
- CRD
cysteine-rich domain
- DOC
deoxycorticosterone
- DMSO
dimethyl sulfoxide
- DTT
dithiothreitol
- GFP
green fluorescent protein
- Gpx3
glutathione peroxidase 3
- GR
glucocorticoid receptor
- HSE
heat shock element
- HSF1
heat shock transcription factor 1
- HSP
heat shock protein
- HSR
heat shock response
- Nrf2
NF-E2-related factor-2
- PGK
phosphoglycerate kinase
- TAP
tandem affinity purification
- Yap1
yeast AP-1.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-10-1004) on January 16, 2008.
REFERENCES
- Abbott A. Neurologists strike gold in drug screen effort. Nature. 2002;417:109. doi: 10.1038/417109a. [DOI] [PubMed] [Google Scholar]
- Alarco A. M., Balan I., Talibi D., Mainville N., Raymond M. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J. Biol. Chem. 1997;272:19304–19313. doi: 10.1074/jbc.272.31.19304. [DOI] [PubMed] [Google Scholar]
- Azevedo D., Tacnet F., Delaunay A., Rodrigues-Pousada C., Toledano M. B. Two redox centers within Yap1 for H2O2 and thiol-reactive chemicals signaling. Free Radic. Biol. Med. 2003;35:889–900. doi: 10.1016/s0891-5849(03)00434-9. [DOI] [PubMed] [Google Scholar]
- Bagatell R., Paine-Murrieta G. D., Taylor C. W., Pulcini E. J., Akinaga S., Benjamin I. J., Whitesell L. Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin. Cancer Res. 2000;6:3312–3318. [PubMed] [Google Scholar]
- Boorstein W. R., Craig E. A. Transcriptional regulation of SSA3, an HSP70 gene from Saccharomyces cerevisiae. Mol. Cell. Biol. 1990;10:3262–3267. doi: 10.1128/mcb.10.6.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone D. L., Doorn J. A., Kiebler Z., Ickes B. R., Petersen D. R. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J. Pharmacol. Exp. Ther. 2005;315:8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- Carbone D. L., Doorn J. A., Kiebler Z., Sampey B. P., Petersen D. R. Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2004;17:1459–1467. doi: 10.1021/tx049838g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causton H. C., Ren B., Koh S. S., Harbison C. T., Kanin E., Jennings E. G., Lee T. I., True H. L., Lander E. S., Young R. A. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell. 2001;12:323–337. doi: 10.1091/mbc.12.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians E. S., Yan L. J., Benjamin I. J. Heat shock factor 1 and heat shock proteins: critical partners in protection against acute cell injury. Crit. Care Med. 2002;30:S43–S50. [PubMed] [Google Scholar]
- Cleren C., Calingasan N. Y., Chen J., Beal M. F. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J. Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- Coleman S. T., Epping E. A., Steggerda S. M., Moye-Rowley W. S. Yap1p activates gene transcription in an oxidant-specific fashion. Mol. Cell. Biol. 1999;19:8302–8313. doi: 10.1128/mcb.19.12.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman S. T., Tseng E., Moye-Rowley W. S. Saccharomyces cerevisiae basic region-leucine zipper protein regulatory networks converge at the ATR1 structural gene. J. Biol. Chem. 1997;272:23224–23230. doi: 10.1074/jbc.272.37.23224. [DOI] [PubMed] [Google Scholar]
- Corson T. W., Crews C. M. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay A., Isnard A. D., Toledano M. B. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 2000;19:5157–5166. doi: 10.1093/emboj/19.19.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay A., Pflieger D., Barrault M. B., Vinh J., Toledano M. B. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Holtzclaw W. D., Kensler T. W. The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol. Cell. Biol. 1989;9:4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch A. P., Spellman P. T., Kao C. M., Carmel-Harel O., Eisen M. B., Storz G., Botstein D., Brown P. O. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch A. P., Werner-Washburne M. The genomics of yeast responses to environmental stress and starvation. Funct. Integr. Genomics. 2002;2:181–192. doi: 10.1007/s10142-002-0058-2. [DOI] [PubMed] [Google Scholar]
- Hahn J. S., Hu Z., Thiele D. J., Iyer V. R. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell. Biol. 2004;24:5249–5256. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. S., Neef D. W., Thiele D. J. A stress regulatory network for co-ordinated activation of proteasome expression mediated by yeast heat shock transcription factor. Mol. Microbiol. 2006;60:240–251. doi: 10.1111/j.1365-2958.2006.05097.x. [DOI] [PubMed] [Google Scholar]
- Harding H. P., et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Harshman K. D., Moye-Rowley W. S., Parker C. S. Transcriptional activation by the SV40 AP-1 recognition element in yeast is mediated by a factor similar to AP-1 that is distinct from GCN4. Cell. 1988;53:321–330. doi: 10.1016/0092-8674(88)90393-5. [DOI] [PubMed] [Google Scholar]
- He C. H., Gong P., Hu B., Stewart D., Choi M. E., Choi A. M., Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- He W., Huang F. C., Gavai A., Chan W. K., Amato G., Yu K. T., Zilberstein A. Novel cytokine release inhibitors. Part III: truncated analogs of tripterine. Bioorg. Med. Chem. Lett. 1998;8:3659–3664. doi: 10.1016/s0960-894x(98)00671-4. [DOI] [PubMed] [Google Scholar]
- Heemskerk J., Tobin A. J., Bain L. J. Teaching old drugs new tricks. Meeting of the Neurodegeneration Drug Screening Consortium, 7–8 April 2002, Washington, DC, USA. Trends Neurosci. 2002;25:494–496. doi: 10.1016/s0166-2236(02)02236-1. [DOI] [PubMed] [Google Scholar]
- Hieronymus H., et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–330. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J. Cell Physiol. 1980;102:407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Holmberg C. I., Illman S. A., Kallio M., Mikhailov A., Sistonen L. Formation of nuclear HSF1 granules varies depending on stress stimuli. Cell Stress Chaperones. 2000;5:219–228. doi: 10.1379/1466-1268(2000)005<0219:fonhgv>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F. C., Chan W. K., Moriarty K. J., Zhang D. C., Chang M. N., He W., Yu K. T., Zilberstein A. Novel cytokine release inhibitors. Part I: triterpenes. Bioorg. Med. Chem. Lett. 1998;8:1883–1886. doi: 10.1016/s0960-894x(98)00331-x. [DOI] [PubMed] [Google Scholar]
- Jamieson D. J., Rivers S. L., Stephen D. W. Analysis of Saccharomyces cerevisiae proteins induced by peroxide and superoxide stress. Microbiology. 1994;140:3277–3283. doi: 10.1099/13500872-140-12-3277. [DOI] [PubMed] [Google Scholar]
- Jurivich D. A., Sistonen L., Kroes R. A., Morimoto R. Effect of sodium salicylate on human heat shock response. Science. 1992;255:1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- Kuge S., Arita M., Murayama A., Maeta K., Izawa S., Inoue Y., Nomoto A. Regulation of the yeast Yap1p nuclear export signal is mediated by redox signal-induced reversible disulfide bond formation. Mol. Cell. Biol. 2001;21:6139–6150. doi: 10.1128/MCB.21.18.6139-6150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S., Jones N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994;13:655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S., Jones N., Nomoto A. Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J. 1997;16:1710–1720. doi: 10.1093/emboj/16.7.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S., Toda T., Iizuka N., Nomoto A. Crm1 (XpoI) dependent nuclear export of the budding yeast transcription factor yAP-1 is sensitive to oxidative stress. Genes Cells. 1998;3:521–532. doi: 10.1046/j.1365-2443.1998.00209.x. [DOI] [PubMed] [Google Scholar]
- Lee B. S., Chen J., Angelidis C., Jurivich D. A., Morimoto R. I. Pharmacological modulation of heat shock factor 1 by antiinflammatory drugs results in protection against stress-induced cellular damage. Proc. Natl. Acad. Sci. USA. 1995;92:7207–7211. doi: 10.1073/pnas.92.16.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Koo T. H., Yoon H., Jung H. S., Jin H. Z., Lee K., Hong Y. S., Lee J. J. Inhibition of NF-κB activation through targeting I κB kinase by celastrol, a quinone methide triterpenoid. Biochem. Pharmacol. 2006;72:1311–1321. doi: 10.1016/j.bcp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Leung T. K., Rajendran M. Y., Monfries C., Hall C., Lim L. The human heat-shock protein family. Expression of a novel heat-inducible HSP70 (HSP70B') and isolation of its cDNA and genomic DNA. Biochem. J. 1990;267:125–132. doi: 10.1042/bj2670125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levonen A. L., Landar A., Ramachandran A., Ceaser E. K., Dickinson D. A., Zanoni G., Morrow J. D., Darley-Usmar V. M. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.-D., Thiele D. J. Oxidative stress induces heat shock factor phosphorylation and HSF-dependent activation of yeast metallothionein gene transcription. Genes Dev. 1996;10:592–603. doi: 10.1101/gad.10.5.592. [DOI] [PubMed] [Google Scholar]
- Maeta K., Izawa S., Okazaki S., Kuge S., Inoue Y. Activity of the Yap1 transcription factor in Saccharomyces cerevisiae is modulated by methylglyoxal, a metabolite derived from glycolysis. Mol. Cell. Biol. 2004;24:8753–8764. doi: 10.1128/MCB.24.19.8753-8764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan D. R., Xiao X., Shao L., Graves K., Benjamin I. J. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J. Biol. Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- Miyahara K., Hirata D., Miyakawa T. yAP-1- and yAP-2-mediated, heat shock-induced transcriptional activation of the multidrug resistance ABC transporter genes in Saccharomyces cerevisiae. Curr. Genet. 1996;29:103–105. doi: 10.1007/BF02221572. [DOI] [PubMed] [Google Scholar]
- Morano K. A., Santoro N., Koch K. A., Thiele D. J. A trans-activation domain in yeast heat shock transcription factor is essential for cell cycle progression during stress. Mol. Cell. Biol. 1999;19:402–411. doi: 10.1128/mcb.19.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano K. A., Thiele D. J. Heat shock factor function and regulation in response to cellular stress, growth and differentiation signals. Gene Expr. 1999a;7:271–282. [PMC free article] [PubMed] [Google Scholar]
- Morano K. A., Thiele D. J. The Sch9 protein kinase regulates Hsp90 chaperone complex signal transduction activity in vivo. EMBO J. 1999b;18:5953–5962. doi: 10.1093/emboj/18.21.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R. I., Santoro M. G. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat. Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- Nagai N., Nakai A., Nagata K. Quercetin suppresses heat shock response by down regulation of HSF1. Biochem. Biophys. Res. Commun. 1995;208:1099–1105. doi: 10.1006/bbrc.1995.1447. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Sherratt P. J., Pickett C. B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- Nieto-Sotelo J., Wiederrecht G., Okuda A., Parker C. S. The yeast heat shock transcription factor contains a transcriptional activation domain whose activity is repressed under nonshock conditions. Cell. 1990;62:807–817. doi: 10.1016/0092-8674(90)90124-w. [DOI] [PubMed] [Google Scholar]
- Okazaki S., Tachibana T., Naganuma A., Mano N., Kuge S. Multistep disulfide bond formation in Yap1 is required for sensing and transduction of H2O2 stress signal. Mol. Cell. 2007;27:675–688. doi: 10.1016/j.molcel.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Ooi C. E., Rabinovich E., Dancis A., Bonifacino J. S., Klausner R. D. Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J. 1996;15:3515–3523. [PMC free article] [PubMed] [Google Scholar]
- Puig O., Rutz B., Luukkonen B. G., Kandels-Lewis S., Bragado-Nilsson E., Seraphin B. New constructs and strategies for efficient PCR-based gene manipulations in yeast. Yeast. 1998;14:1139–1146. doi: 10.1002/(SICI)1097-0061(19980915)14:12<1139::AID-YEA306>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Santoro N., Johansson N., Thiele D. J. Heat shock element architecture is an important determinant in the temperature and transactivation domain requirements for heat shock transcription factor. Mol. Cell. Biol. 1998;18:6340–6352. doi: 10.1128/mcb.18.11.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Sato H., Kuriyama-Matsumura K., Sato K., Maebara K., Wang H., Tamba M., Itoh K., Yamamoto M., Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J. Biol. Chem. 2002;277:44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- Sethi G., Ahn K. S., Pandey M. K., Aggarwal B. B. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-κB-regulated gene products and TAK1-mediated NF-κB activation. Blood. 2007;109:2727–2735. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- Sorger P. K. Yeast heat shock factor contains separable transient and sustained response transcriptional activators. Cell. 1990;62:793–805. doi: 10.1016/0092-8674(90)90123-v. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J. 1987;6:3035–3041. doi: 10.1002/j.1460-2075.1987.tb02609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H.R.B. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988;54:855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Wemmie J. A., Szczypka M. S., Thiele D. J., Moye-Rowley W. S. Cadmium tolerance mediated by the yeast AP-1 protein requires the presence of an ATP-binding cassette transporter-encoding gene, YCF1. J. Biol. Chem. 1994;269:32592–32597. [PubMed] [Google Scholar]
- West J. D., Marnett L. J. Alterations in gene expression induced by the lipid peroxidation product, 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005;18:1642–1653. doi: 10.1021/tx050211n. [DOI] [PubMed] [Google Scholar]
- Westerheide S. D., Bosman J. D., Mbadugha B. N., Kawahara T. L., Matsumoto G., Kim S., Gu W., Devlin J. P., Silverman R. B., Morimoto R. I. Celastrols as inducers of the heat shock response and cytoprotection. J. Biol. Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- Westerheide S. D., Kawahara T. L., Orton K., Morimoto R. I. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J. Biol. Chem. 2006;281:9616–9622. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- Westerheide S. D., Morimoto R. I. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J. Biol. Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- Wood M. J., Andrade E. C., Storz G. The redox domain of the Yap1p transcription factor contains two disulfide bonds. Biochemistry. 2003;42:11982–11991. doi: 10.1021/bi035003d. [DOI] [PubMed] [Google Scholar]
- Wood M. J., Storz G., Tjandra N. Structural basis for redox regulation of Yap1 transcription factor localization. Nature. 2004;430:917–921. doi: 10.1038/nature02790. [DOI] [PubMed] [Google Scholar]
- Yan C., Lee L. H., Davis L. I. Crm1p mediates regulated nuclear export of a yeast AP-1-like transcription factor. EMBO J. 1998;17:7416–7429. doi: 10.1093/emboj/17.24.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Chen D., Cui Q. C., Yuan X., Dou Q. P. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.