Abstract
The ultimate goal of cytokinesis is to establish a membrane barrier between daughter cells. The fission yeast Schizosaccharomyces pombe utilizes an actomyosin-based division ring that is thought to provide physical force for the plasma membrane invagination. Ring constriction occurs concomitantly with the assembly of a division septum that is eventually cleaved. Membrane trafficking events such as targeting of secretory vesicles to the division site require a functional actomyosin ring suggesting that it serves as a spatial landmark. However, the extent of polarization of the secretion apparatus to the division site is presently unknown. We performed a survey of dynamics of several fluorophore-tagged proteins that served as markers for various compartments of the secretory pathway. These included markers for the endoplasmic reticulum, the COPII sites, and the early and late Golgi. The secretion machinery exhibited a marked polarization to the division site. Specifically, we observed an enrichment of the transitional endoplasmic reticulum (tER) accompanied by Golgi cisternae biogenesis. These processes required actomyosin ring assembly and the function of the EFC-domain protein Cdc15p. Cdc15p overexpression was sufficient to induce tER polarization in interphase. Thus, fission yeast polarizes its entire secretory machinery to the cell division site by utilizing molecular cues provided by the actomyosin ring.
INTRODUCTION
Cell division is the final event in the cell cycle that results in physical separation of two daughter cells. Despite being studied for more than a hundred years, the underlying molecular mechanisms and the cytological details of the process are still emerging. Various organisms and cell types have established multiple pathways to conduct cell division that are regulated at both signaling and structural levels (for review see Balasubramanian et al., 2004). In recent years, the fission yeast Schizosaccharomyces pombe has emerged as an attractive model for the study of cytokinesis because of its fully sequenced genome (Wood et al., 2002), a cell size convenient for cytological studies and the availability of a large set of conditional mutants compromised in various aspects of cell division (Chang et al., 1996; Balasubramanian et al., 1998).
On entry into mitosis, S. pombe assembles an actomyosin ring that is thought to drive a binary cell fission through constriction, similar to many other eukaryotes, including nematodes, insects, and vertebrates (for review see Hales et al., 1999). During early mitosis, actin is assembled into a ring structure through the action of several actin nucleating and bundling proteins and molecular motors. These include the formin Cdc12p (Chang et al., 1997), profilin Cdc3p (Balasubramanian et al., 1992), the extended Fer/CIP4 (EFC) domain protein Cdc15p (Fankhauser et al., 1995), and the myosin heavy chain Myo2p (Kitayama et al., 1997) together with its light chains Cdc4p (McCollum et al., 1995; Naqvi et al., 1999) and Rlc1p (Le Goff et al., 2000). It has been proposed that activation of the signaling cascade referred to as the septation initiation network (SIN) triggers actomyosin ring constriction upon nuclear division (for review see Krapp et al., 2004). Concomitantly with ring constriction, membrane material is inserted at the division site and the septum is synthesized (Jochova et al., 1991). Centripetal septum deposition and ring constriction seem to be tightly linked. Indeed, 1,3-β-glucan-synthase loss-of-function mutants that cannot form the septum are also incapable of ring constriction (Liu et al., 2000). As septum biogenesis proceeds, actin reorganizes to clusters of patches on both sides of the septum (Marks and Hyams, 1985). Secondary septa are then formed on either side of the primary one that is eventually dissolved resulting in cell separation (Humbel et al., 2001).
Formation and remodeling of the cell wall depend on action of enzymes such as the (1,3)-β-glucan-synthase and the endo-β-1,3-glucanase that are involved in organizing the (1,3)-β-glucan, the major constituent of the cell wall (Ribas et al., 1991; Martin-Cuadrado et al., 2003). These proteins have been shown to localize to the sites of active growth and their localization depends on secretion (Cortes et al., 2002; Martin-Cuadrado et al., 2003). Application of the drug brefeldin A (BFA), which inhibits the exchange factor for the small GTPase Arf, renders fission yeast cells incapable of localizing the (1,3)-β-glucan-synthase catalytic subunit Cps1p to the division site, presumably because of a block in secretion (Liu et al., 2002). Furthermore, the multiprotein complex involved in the late steps of the exocytic pathway named exocyst is essential for the proper localization of the endo-β-1,3-glucanase, Eng1p (Martin-Cuadrado et al., 2005). In fission yeast, the exocyst-mediated secretion is restricted to the cellular growth regions including cell tips and the cell division site (Wang et al., 2002).
In exocytosis, vesicles originating from the secretory pathway or endosomal recycling (Engstler et al., 2004) fuse with the plasma membrane. The delivery of newly synthesized proteins to the plasma membrane and cell surrounding depends almost exclusively on the function of the secretory pathway (for review see Mellman and Warren, 2000; Nickel, 2003). Proteins to be secreted are processed in the endoplasmic reticulum (ER) and the Golgi apparatus and then are sorted into the post-Golgi vesicles that are delivered to the plasma membrane (Alberts et al., 2002). Most published work on the involvement of protein secretion during polarity establishment has centered on targeting, localization, and delivery of post-Golgi vesicles. For example, it was shown that the early buds of the budding yeast Saccharomyces cerevisiae contain numerous secretory vesicles that are rarely observed in the mother cell (Preuss et al., 1992). Such polarized distribution depends on intact actin cables and type V myosins (Johnston et al., 1991; Govindan et al., 1995). However, less is known about the spatial distribution of the early secretory compartments. It was reported that the Golgi apparatus also localized close to the bud site in S. cerevisiae (Preuss et al., 1992). Similarly, Bevis et al., 2002 have suggested that the Golgi elements in Pichia pastoris might preferentially localize toward the budding site. During hyphal growth in Candida albicans, cells assemble the vesicle-rich Spitzenkörper structure at the tips of growing hyphae (Harris et al., 2005). The intimate association between Spitzenkörper behavior and hyphal morphogenesis suggests that the Spitzenkörper might function as a “Vesicle Supply Center,” producing the secretory vesicles (Bartnicki-Garcia et al., 1989). In fact, it was recently shown that most of the Golgi apparatus is indeed localized near the growing hyphal tips (Rida et al., 2006).
Considering the functional continuum of the secretory pathway (for review see Guo and Novick, 2004), we were interested in investigating the structural and spatial organization of the tER (transitional ER) and cis- and trans-Golgi during polarized tip growth and cell division, which represent major aspects of cellular polarity in fission yeast.
In this study we observed an enrichment of tER that was accompanied by the biogenesis of Golgi cisternae at the division site. These processes required assembly of a functional actomyosin ring and, in particular, the function of the EFC domain protein, Cdc15p. Interestingly, overexpression of Cdc15p in interphase was sufficient to induce the equatorial accumulation of the tER. Thus, fission yeast cells polarize the entire secretory machinery to the cell division site by utilizing molecular cues provided by the actomyosin ring.
MATERIALS AND METHODS
S. pombe Strains, Reagents, and Constructs
S. pombe strains used in this study and their genotypes are listed in Supplementary Table 1. Media for vegetative growth (EMM2 or YES) and genetic methods were as described in Moreno et al. (1991). Genetic crosses and sporulation were performed on YPD agar plates. The homologous recombination-based method was used to tag endogenous proteins with green fluorescent protein (GFP) or mCherry at their C termini. The candidate proteins were selected based on their homologies to proteins characterized in other yeast systems (accession numbers: SPBC1734.04 [Anp1p], SPAC27F1.07 [Ost1p], SPAC22F8.08 [Sec24p], SPBC36B7.03 [Sec63p], and SPAC30.01c [Sec72p]). Plasmids were constructed using standard molecular biology techniques. Latrunculin A (LatA), a drug that prevents actin polymerization, was purchased from Biomol International LP (Plymouth Meeting, PA). DNA fluorescent stain 4′,6-diamidino-2-phenylindole (DAPI) and the microtubule depolymerizing drug methyl-1-(butylcarbamoyl)-2-benzimidazole-carbamate (carbendazime; MBC) were obtained from Sigma-Aldrich (St. Louis, MO). The F-actin stain Alexa Fuor 593 phalloidin was obtained from Invitrogen (Karlsruhe, Germany).
Microscopy
Epifluorescence still images were collected using mercury lamp as an illumination source with appropriate sets of filters on a Zeiss Axiovert 200M (Plan Apochromat NA 1.4 objective, Göttingen, Germany) microscope equipped with CoolSnap camera (Photometrics, Tucson, AZ) and Uniblitz shutter driver (Photonics, Rochester, NY) under the control of Metamorph software package (Universal Imaging, Sunnyvale, CA). Typically, we acquired image stacks that consisted of nine sections of 0.5-μm spacing. Presented are the z-stack maximum projection images obtained by using Metamorph built-in module (Universal Imaging, West Chester, PA).
Scanning confocal microscopy was performed on a Leica DMI6000B microscope (HCX Plan NA = 1.35 objective) equipped with SP5 confocal system (Leica Microsystems, Mannheim, Germany) controlled by the proprietary software package. Z-stack images were taken with 0.5-μm spacing and reconstructed in three dimensions using the projection module. Imaging was performed on S. pombe cells placed in sealed growth chambers containing 2% agarose YES medium.
Time-lapse fluorescent microscopy images were generated on a Zeiss Axiovert 200M (Plan Apochromat NA 1.4 objective) microscope equipped with UltraView RS-3 confocal system: CSU21 confocal optical scanner, 12 bit digital cooled Hamamatsu Orca-ER camera (OPELCO, Sterling, VA) and krypton-argon triple line laser illumination source (488, 568, and 647 nm) under the control of UltraView software package (PerkinElmer, Boston, MA). Typically, we acquired image stacks that consisted of nine 0.5-μm–spaced sections. Presented are the z-stack maximum projection images obtained by using ImageJ software package (http://rsb.info.nih.gov/ij/; National Institutes of Health, Bethesda, MD). Imaging was performed on S. pombe cells placed in sealed growth chambers containing 2% agarose YES medium.
Image Analysis
Single-cell maximum projection images obtained by epifluorescence microscopy were analyzed using customized CellProfiler image analysis software (Carpenter et al., 2006). CellProfiler object processing modules were used to identify S. pombe cells. Longitudinal cell axes were approximated by linear regression of cell boundaries. Images were rotated by the angle of inclination of the cell axes to the x-axis, thus orienting cells horizontally. Next, images were cropped to cell boundaries. Using nearest-neighbor interpolation, image width was resized to the nearest multiple of 20 pixels and image height to the nearest multiple of 10 pixels. The average intensity of an image field comprising one-twentieth of the width and one-tenth of the height of this image was represented as a single pixel, thus resulting in a 20 × 10-pixel image. Intensities were normalized and averaged over 50 images. Furthermore, we calculated intensities along longitudinal cell axes from mean column intensities of the 20 × 10 images. As above, these were averaged over 50 images and graphed. We determined the statistical significance of differences in fluorescence levels between two axial positions using the Kolmogorov-Smirnov test to calculate the p values. The critical p value (p = 0.05) was adjusted for multiple comparisons using Bonferroni correction.
Three-dimensional reconstructions were performed using Volocity software package (Perkin Elmer-Cetus, Waltham, MA).
Integrated intensity measurements were performed on maximum projection images obtained by time-lapse spinning disk confocal microscopy using the Metamorph built-in module. The measurements were performed over three equal areas corresponding to cell tips and the cell middle. The fluorescence intensities were adjusted for bleaching using interphase cells fluorescence intensities (n = 4 cells), assuming that the marker protein levels did not change during this time. Obtained values are presented as a time sequence of values relative to average intensity at cell tips and the moving average (n = 3) time sequence.
RESULTS
Organization of the Early Secretory Pathway in S. pombe
To investigate the organization of the early secretory compartments including the COPII-positive compartments and both cis- and trans-Golgi, we constructed S. pombe strains expressing a number of distinct marker proteins C-terminally tagged with GFP or mCherry at their genomic loci. The candidate proteins were selected based on their homologies to known secretory pathway markers reported in other yeast (see Materials and Methods). Tagging of these proteins did not adversely affect their essential functions judging by the normal cell morphology and division patterns.
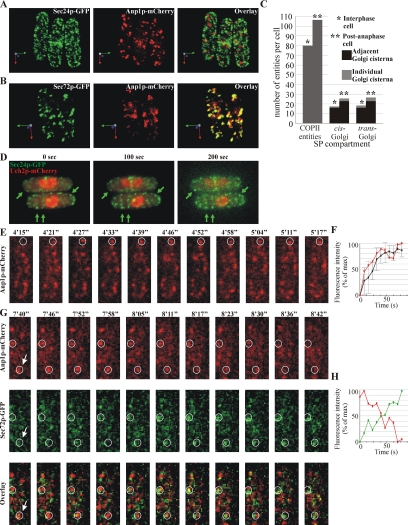
The general ER markers such as the component of the protein translocation complex Sec63p-GFP (Deshaies et al., 1991) and the predicted oligosaccharide transferase Ost1p-GFP (Silberstein et al., 1995) exhibited both cortical and nuclear envelope (NE) localization (Supplementary Figure 1A), consistent with previously published work (Pidoux and Armstrong, 1993; Broughton et al., 1997). The COPII vesicle coat protein Sec24p-GFP (Barlowe et al., 1994) localized to punctate structures (Figure 1A, left panel) of various sizes. We observed 80 ± 9 such entities in interphase cells and 106 ± 11 of those in dividing cells (n = 10 cells; Figure 1C). To explore the organization of COPII structures and cis-Golgi with respect to each other, we simultaneously observed the localization of Sec24p-GFP and the component of the Golgi mannosyltransferase complex Anp1p-mCherry (Jungmann and Munro, 1998) that served as a cis-Golgi marker protein (Figure 1A, center panel). Because of the large number of the COPII structures, it was difficult to determine the fraction of adjacent COPII-positive membranes and cis-Golgi compartments, although we did observe such instances (Figure 1A, right panel)
Figure 1.
Characterization of the early the secretory pathway in S. pombe. (A) 3D reconstruction of scanning confocal microscopy images of S. pombe cells expressing COPII vesicle marker Sec24p-GFP (green, left panel) and cis-Golgi marker Anp1p-mCherry (red, center panel). Numerous punctate COPII entities and multiple separate cis-Golgi cisternae could be visualized, some of which were adjacent or partially overlapping with each other (overlay, right panel). Arrows indicate the spatial orientation of objects in respect to x, y, and z-axis (green, red, and blue, respectively). (B) 3D reconstruction of scanning confocal microscopy images of S. pombe cells expressing cis-Golgi marker Anp1p-mCherry (red, center panel) and trans-Golgi marker Sec72p-GFP (green, left panel). The trans-Golgi localized as multiple separate cisternae that were in most cases partially overlapping or adjacent to the cis-Golgi cisternae (overlay, right panel). Arrows indicate the spatial orientation of objects in respect to x, y, and z-axis (green, red, and blue, respectively). (C) Quantification of the number of individual COPII entities and individual and adjacent cis- and trans-Golgi cisternae in fission yeast interphase and post-anaphase cells as visualized by scanning confocal imaging of Sec24p-GFP, Anp1p-mCherry, and Sec72p-GFP, respectively (n = 10 cells per compartment). SP, secretory pathway. (D) Representative frames from Supplementary Movie 1. Shown is the single focus plane images obtained by time-lapse epifluorescent microscopy of cells expressing Sec24p-GFP and Uch2p-mCherry. The arrows indicate COPII-positive membranes that persisted throughout the course of the experiment. (E) Representative frames corresponding to Supplementary Movie 2. Shown is the maximum projection image of the z-stack obtained by time-lapse spinning disk confocal imaging. Numbers refer to the time, in minutes (′) and seconds (″). The encircled area indicates the site of cis-Golgi cisterna (Anp1p-mCherry, red) biogenesis as visualized by time-lapse spinning disk confocal imaging. (F) Quantification of Anp1p-mCherry fluorescence intensity (red line) associated with cis-Golgi cisternae biogenesis event indicated in E and the fluorescence profile averaged over six biogenesis events (black line). The horizontal axis indicates time in seconds, and the vertical axis indicates fluorescence intensity as percentage of the maximal intensity observed. Error bars, SD. (G) Representative frames corresponding to Supplementary Movie 3. Shown is the single focus plane image sampled from the z-stack obtained by time-lapse spinning disk confocal imaging. Numbers refer to the time, in minutes (′) and seconds (″). Encircled areas indicate the single Golgi cisternae maturing from a cis-Golgi (Anp1p-mCherry, red) to trans-Golgi identity (Sec72p-GFP, green). (H) Quantification of intensity of Anp1p-mCherry (red) and Sec72p-GFP (green) fluorescence associated with a Golgi cisterna maturation event indicated in (G, arrow). Data are graphed as in F.
We further analyzed the relative distribution of the cis-Golgi marker Anp1p-mCherry (Figure 1B, center panel) and the Arf GEF Sec72p-GFP (Figure 1B, left panel), a homologue of S. cerevisiae Sec7p (Franzusoff et al., 1991) that localizes to the late Golgi compartments in budding yeast. We found that these proteins localized to several punctate structures (18 ± 3 cis- and 22 ± 3 trans-Golgi compartments during interphase and 23 ± 3 cis- and 26 ± 2 trans-Golgi compartments in dividing cells, n = 10 cells). These two markers often partially overlapped or were found adjacent to each other, consistent with their localization to distinct cisternae within Golgi stacks (Figure 1, B, right panel, and C). We also observed instances of several cis-Golgi cisternae adjacent to a single trans-Golgi structure. Individual cisternae were present, albeit at a lower frequency (∼10% of early and ∼13% of late Golgi compartments, n = 10 cells). Thus, the Golgi apparatus in S. pombe cells exists as multiple entities comprising mainly stacks of few cisternae and some individual cisternae.
We investigated the dynamics of the early secretory pathway compartments using time-lapse microscopy. By performing epifluorescence microscopy of single planes acquired every 5 s in cells expressing Sec24p-GFP and the nuclear envelope marker Uch2p-mCherry (Li et al., 2000), we found that COPII entities were stable for periods extending 5 min during both cell growth and division (Figure 1D and Supplementary Movie 1). Considering that the protein coat is rapidly disassembled after COPII vesicles have budded (Antonny et al., 2001), we concluded that in fission yeast Sec24p-GFP marks the sites of COPII vesicles production, the tER (for review see Mancias and Goldberg, 2005). We were not able to determine the average tER site lifespan because of technical limitations and the large number of these sites.
Using the spinning disk confocal time-lapse microscopy, we observed instances of early Golgi biogenesis without visible contribution from pre-existing cisternae, as judged by Anp1p-mCherry dynamics (Figure 1, E and F, and Supplementary Movie 2). Moreover, we observed instances of cisternal identity change when Anp1p-mCherry positive structures became predominantly occupied by Sec72p-GFP (Figure 1G and Supplementary Movie 3), in concordance with the Golgi cisternal progression model (for review see Malhotra and Mayor, 2006). The quantification of the fluorescence signals associated with single cisternae maturation (Figure 1H) suggested that this conversion occurred with the fluorescence ratio doubling time of ∼30 ± 7 s (n = 12 maturation events).
Early Secretory Pathway Compartments Accumulate at the Division Site
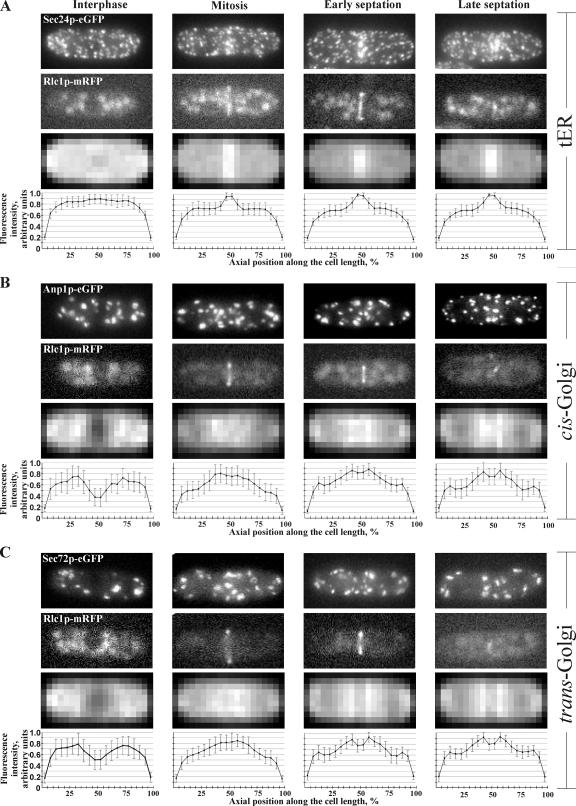
Although both tER and Golgi compartments exhibited a seemingly random distribution in interphase cells, they were clearly polarized during cell division (Figure 1, A and B). To explore this matter further, we performed epifluorescence microscopy analyses of live fission yeast cells expressing GFP-tagged secretory pathway markers together with the actomyosin ring marker Rlc1p fused to monomeric red fluorescent protein (mRFP) (Le Goff et al., 2000). We confirmed that both tER and Golgi apparatus did not exhibit a clearly polarized distribution of the marker proteins during interphase (Figure 2). However, pronounced recruitment of secretory compartments to the cell division site became noticeable at the time of actomyosin ring formation. Notably, although the tER exhibited the highest levels of accumulation at this time (Figure 2A), the Golgi apparatus appeared only moderately polarized toward the future division site (Figure 2, B and C). As cells progressed through septation, all marker proteins attained maximum polarization levels. It should be noted that tER showed a fairly narrow region of intense accumulation, ∼10% of mother cell length, whereas the Golgi marker proteins were enriched in the medial 30% of mother cell length. Thus, for further analyses cells exhibiting such accumulation of both tER and Golgi markers were considered polarized. Because polarization of the tER could be consequential to accumulation of the entire ER in the vicinity of the division site, we investigated the spatial distribution of general ER marker Ost1p-GFP throughout the cell cycle (Supplementary Figure 1A). We did not observe a pronounced accumulation of ER in the central region of dividing cells. We confirmed that the tER polarization did not reflect the general ER localization by performing epifluorescence microscopy on cells simultaneously expressing Sec24p-GFP and Ost1p-mCherry (Supplementary Figure 1B). Quantification of the fluorescence levels of tER and Golgi marker proteins suggested that their accumulation at the site of division was statistically significant (Supplementary Figure 2, n = 50 cells per marker per cell cycle stage; see Materials and Methods for details).
Figure 2.
Spatial distribution of the early secretory pathway throughout the cell cycle. (A) tER localization marked by Sec24p-GFP (top row of images) in interphase (first column), mitosis (second column), and early and late septation (third and fourth column). The cell cycle stage was deduced by the morphology of the actomyosin ring marker Rlc1p-mRFP (second row from the top) and the DIC image (not shown). Shown is the maximum projection image of the z-stack obtained by epifluorescence imaging. Epifluorescence maximum projection images of z-stacks of 50 individual cells at the same stage of the cell cycle expressing Sec24p-GFP were standardized and compared with produce an averaged image (third row from the top, for details see Materials and Methods). The horizontal axis indicates position along the cell axis in percentages of cell length and the vertical axis indicates fluorescence intensity in arbitrary units. (B) cis-Golgi localization marked by Anp1p-GFP; imaging and analysis as in A. (C) trans-Golgi localization marked by Sec72p-GFP; imaging and analysis as in A.
Actomyosin Ring Assembly Is Required for Secretory Machinery Recruitment to the Division Site
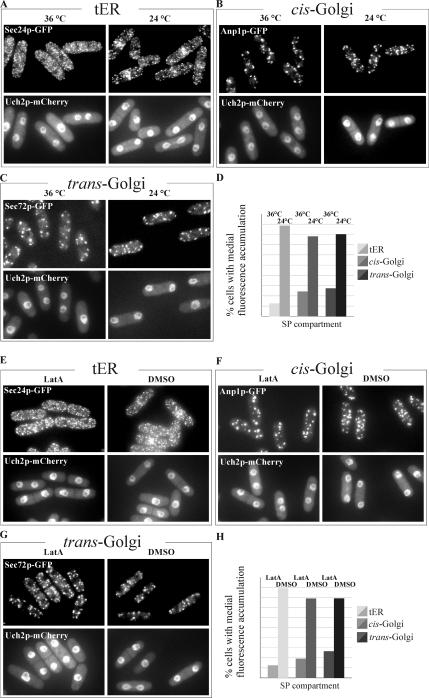
Given that we observed the initial medial accumulation of both tER and Golgi compartments occurring concomitantly with actomyosin ring formation (Figure 2), we hypothesized that the ring itself might serve as a determinant for the early secretory pathway polarization. To explore this question, we introduced the fluorescent markers of the secretory pathway into the cdc12-112 temperature-sensitive genetic background. The essential S. pombe formin Cdc12p plays a crucial role in actomyosin ring formation, and fission yeast cells are incapable of ring assembly when Cdc12p function is compromised (Chang et al., 1997). When grown at the restrictive temperature of 36°C, cdc12-112 mutant cells are not capable of cytokinesis but undergo the nuclear division (Arai and Mabuchi, 2002). We synchronized exponentially growing cell cultures in early G2 phase of the cell cycle by elutriation. Immediately upon elutriation cells were released into a fresh medium and grown at either permissive or restrictive temperatures. As judged by visualization of the Uch2p-mCherry, anaphase cell population peaked at ∼75 and 90 min after elutriation for cultures grown at 24 and 36°C, respectively. Samples were taken, fixed, and stained for actin and DNA to confirm that cells were unable to form rings at the restrictive temperature (data not shown). By analyzing the spatial distribution of the secretory pathway marker proteins, we noticed a strikingly reduced medial accumulation of tER and Golgi cisternae (Figure 3, A–D). Under permissive conditions ∼90 and ∼80% of cells exhibited tER and Golgi polarization, whereas only ∼10 and ∼25% of cells, respectively, polarized these compartments under restrictive conditions. We confirmed that the actomyosin ring structure was important for polarization of the early secretory compartments by examining the spatial distribution of the tER and Golgi compartments in cells with compromised function of the myosin light-chain Cdc4p (McCollum et al., 1995, Supplementary Figure 3). Thus, the establishment of early secretory pathway polarization during mitosis depends on actomyosin ring formation.
Figure 3.
Actomyosin ring assembly is required for secretory pathway recruitment to the division site and maintenance of the polarized state requires an intact actin cytoskeleton. (A) Temperature-sensitive cdc12-112 mutant cells expressing Sec24p-GFP (top row) and the nuclear marker Uch2p-mCherry (bottom row) were imaged upon entry into anaphase after previously being synchronized in G2 by elutriation, transferred into fresh medium, and allowed to grow at 36°C (left column) and 24°C (right column). Shown is the maximum projection image of the z-stack obtained by epifluorescence imaging. (B) Temperature-sensitive cdc12-112 mutant cells expressing Anp1p-GFP (top row) and the nuclear marker Uch2p-mCherry (bottom row) were treated as in A. (C) Temperature-sensitive cdc12-112 mutant cells expressing Sec72p-GFP (top row) and the nuclear marker Uch2p-mCherry (bottom row) were treated as in A. (D) Quantitation of Sec24p-GFP, Anp1p-GFP and Sec72p-GFP polarization under experimental conditions described in A (n = 250 cells per sample). (E) Cells expressing Sec24p-GFP (top row) and Uch2p-mCherry (bottom row) were treated with 10 μM LatA (left column) or DMSO (right column) for 30 min. Shown is the maximum projection image of the z-stack obtained by epifluorescence imaging. (F) Cells expressing Anp1p-GFP (top row) and Uch2p-mCherry (bottom row) were treated as in E. (G) Cells expressing Sec72p-GFP (top row) and Uch2p-mCherry (bottom row) were treated as in E. (H) Graph quantifying the proportion of cells exhibiting Sec24p-GFP, Anp1p-GFP and Sec72p-GFP polarization under experimental conditions described in E (n = 250 cells per sample).
Maintenance of a Polarized State of the Early Secretory Pathway Compartments Requires an Intact Actin Cytoskeleton
Our observations indicated that the establishment of the polarized distribution of the early secretory pathway compartments required actomyosin ring assembly. However, the polarized state persisted throughout septation at the time when the ring is disassembled. Thus it was possible that other factors could be used for maintenance of the polarized tER and Golgi distribution. Because actin patches localize at the site of septation after ring constriction, we probed the importance of an intact actin cytoskeleton for this phenomenon. To this end, we analyzed the behavior of the early secretory pathway marker proteins in asynchronously growing cell cultures treated with latrunculin A (LatA), a drug that prevents actin polymerization. LatA at 10 μM has been shown to cause a rapid depolymerization of the actin cytoskeleton in S. pombe (Karagiannis et al., 2005), and we confirmed that both division rings and interphase actin structures were eliminated by staining the fixed cells samples with phalloidin and DAPI (Supplementary Figure 4). We observed that cells treated with LatA for 15 min underwent a significant decrease in polarization of all secretory pathway compartments (Figure 3, E–H). The binucleate mitotic cells treated with LatA did not show any degree of either tER or Golgi accumulation, likely because of their inability to assemble the actomyosin ring. Although some septating cells exhibited polarization 15 min after drug application, 30 min of LatA treatment completely abolished the medial accumulation of the secretory pathway compartments. These data confirmed that the actomyosin ring was essential for the establishment of tER and Golgi compartments polarization and suggested that an intact actin cytoskeleton was required for its maintenance during septation.
The Septation Initiation Network Is Required for Maintenance But Not for the Initial Recruitment of the Early Secretory Pathway Compartments at the Division Site
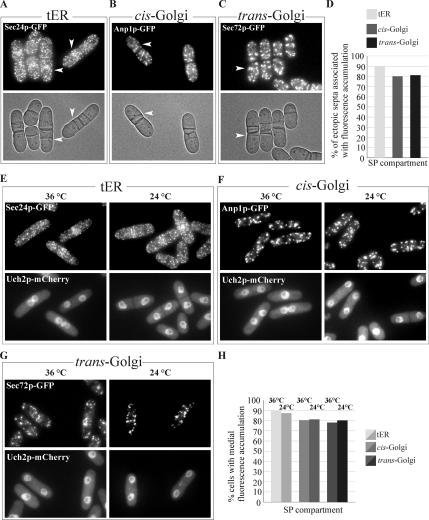
To determine whether accumulation of the secretory compartments to the division site depended on the cell cycle stage, we analyzed the behavior of both the tER and the Golgi apparatus in the cdc16-116 temperature-sensitive genetic background. Cdc16p is a module of the two-component GTPase-activating protein for the small GTPase Spg1p and thus serves as a negative regulator of the SIN pathway (Furge et al., 1998). Loss-of-function of Cdc16p untimely activates the SIN pathway resulting in successive rounds of septum depositions at any stage of the cell cycle (Minet et al., 1979). Asynchronously growing cdc16-116 cells expressing GFP fusions with the early secretory pathway markers were shifted to the restrictive temperature of 36°C and imaged 1 h after the temperature shift-up. We found that Sec24p-GFP, Anp1p-GFP, and Sec72p-GFP, which represent tER and early and late Golgi compartments, respectively, accumulated in the vicinity of most ectopic septa (Figure 4, A–C). Considering that some cells simultaneously exhibited more than one ectopic septum, we quantified the number of ectopic septa that recruited the secretory compartments rather than the proportion of cells showing such accumulation (Figure 4D). We concluded that early secretory pathway accumulation spatially and temporally coincided with contractile ring and septum formation independently of the cell cycle.
Figure 4.
The Septation Initiation Network (SIN) is required for maintenance but not for the initial recruitment of the early secretory pathway compartments at the division site, yet hyperactivation of the SIN pathway results in cell cycle–independent formation of ectopic septa that were accompanied by the tER and cis- and trans-Golgi recruitment to its vicinity. (A) Epifluorescence and DIC images (top and bottom, respectively) of temperature-sensitive cdc16-116 mutant cells expressing Sec24p-GFP grown at 36°C. Shown is the maximum projection image of the z-stack obtained by epifluorescence imaging. (B) Epifluorescence and DIC images (top and left column, respectively) of temperature-sensitive cdc16-116 mutant cells expressing Anp1p-GFP grown at 36°C. (C) Epifluorescence and DIC images (top and left column, respectively) of temperature-sensitive cdc16-116 mutant cells expressing Sec72p-GFP grown at 36°C. (D) Quantitation of Sec24p-GFP, Anp1p-GFP, and Sec72p-GFP polarization under experimental conditions described in A above (n = 250 ectopic septa per sample). (E) Temperature-sensitive sid2-250 mutant cells expressing Sec24p-GFP (top row) and the nuclear marker Uch2p-mCherry (bottom row) were imaged upon entry into anaphase after previously being synchronized in G2 by elutriation, transferred into fresh medium, and allowed to grow at 36 (left column) and 24°C (right column). Shown is the maximum projection image of the z-stack obtained by epifluorescence imaging. (F) Temperature-sensitive sid2-250 mutant cells expressing Anp1p-GFP (top row) and the nuclear marker Uch2p-mCherry (bottom row) were treated as in E. (G) Temperature-sensitive sid2-250 mutant cells expressing Sec72p-GFP (top row) and the nuclear marker Uch2p-mCherry (bottom row) were treated as in E. (H) Graph quantifying the proportion of cells exhibiting Sec24p-GFP, Anp1p-GFP, and Sec72p-GFP polarization under experimental conditions described in E (n = 250 cells per sample).
To explore the possibility that the SIN pathway was directly responsible for the polarization of the early secretory compartments, we observed the spatial distribution of the tER and Golgi marker proteins in the sid2-250 temperature-sensitive mutant background. Sid2p is the downstream effector kinase of SIN signaling (Balasubramanian et al., 1998; Sparks et al., 1999), and loss of its activity leads to septation failure. Using elutriation, we synchronized cells in the manner described above and observed the localization of Sec24p-GFP, Anp1p-GFP, and Sec72p-GFP in anaphase cells (Figure 4, E, F, and G, respectively) also expressing the nuclear envelope marker, Uch2p-mCherry, at both 24 and 36°C. As indicated by the marker proteins, in mitotic cells all three compartments, including the tER, cis-Golgi, and trans-Golgi, accumulated medially regardless of whether or not the SIN function was compromised (Figure 4H). It should be noted that medial accumulation of the tER and Golgi compartments did decrease upon longer incubation at the restrictive temperature. This might be attributed to the fact that SIN functions in maintaining the actomyosin ring throughout anaphase and cytokinesis (Balasubramanian et al., 1998; Sparks et al., 1999) and therefore is essential for ensuring the continuous localization of the secretory compartments to the site of septation. To confirm that the SIN pathway activity was not essential for the initial secretory machinery recruitment but was required for the maintenance of its polarization state, we performed the time-lapse imaging of sid2-250 mutant cells expressing GFP-tagged secretory pathway marker proteins and Uch2p-mCherry, growing either at the permissive temperature of 24°C or after a 1-h shift to the restrictive temperature of 36°C (Supplementary Figure 5). We found that anaphase cells (as indicated by the nuclear envelope marker Uch2p-mCherry) incubated at both 24 and 36°C exhibited initial medial accumulation of the secretory compartments. Although this accumulation persisted in cells grown at 24°C (7/8, 5/6, and 5/5 cells for tER, cis-Golgi, and trans-Golgi, respectively), it was abolished at 36°C (only 2/8, 0/4, and 0/4 cells exhibited some residual accumulation in case of tER, cis-Golgi, and trans-Golgi, respectively). In conclusion, our findings implied that the medial recruitment of the early secretory pathway was SIN independent, whereas its maintenance did rely on SIN regulated cellular processes.
The EFC Domain Protein Cdc15p Is Required for tER Recruitment to the Division Site
Our results showed that the actomyosin ring is required for the tER and Golgi polarization during mitosis. Cdc15p is a component of the actomyosin ring and is the member of the EFC domain family characterized by an EFC domain at the N-terminus (Tsujita et al., 2006) and an SH3-domain at the C-terminus (Fankhauser et al., 1995). It has been suggested that Cdc15p functions downstream of SIN in regulating septum assembly (Marks et al., 1992; Wachtler et al., 2006). Interestingly, the EFC protein family members were shown to couple membrane deformation to the actin cytoskeleton (Tsujita et al., 2006), providing a potential link to COPII vesicle biogenesis (Lee et al., 2005). Thus, we set out to investigate whether Cdc15p functioned during establishment of the polarized state of tER and Golgi during cell division.
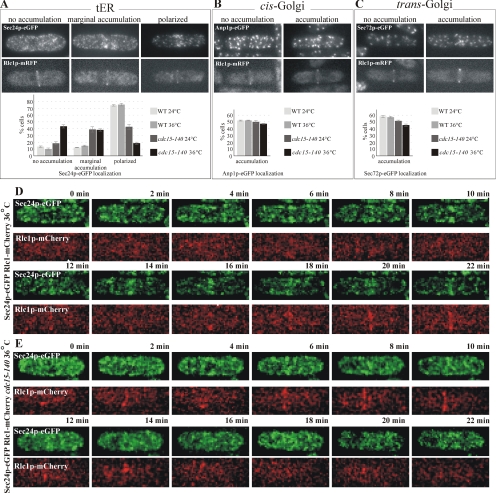
Although not essential for actomyosin ring formation in metaphase, Cdc15p is necessary for its maintenance upon SIN activation (Wachtler et al., 2006). Thus, to assess the specific contribution of Cdc15p independently from the actomyosin ring, we observed the spatial distribution of the secretory pathway proteins in wild-type and temperature-sensitive cdc15-140 mutant cells arrested in metaphase by overexpressing the spindle assembly checkpoint component Mad2p (He et al., 1997) under the nmt1 promoter (Maundrell, 1990). Briefly, Mad2p overexpression was induced for 20 h at the permissive temperature of 24°C followed by a temperature shift-up for 4 h at 36°C. Samples were taken from cell cultures and stained by phalloidin and DAPI, indicating that under the experimental conditions described ∼38% of cells contained actomyosin ring, with ∼85% of these appearing arrested in metaphase. We analyzed the distribution of the early secretory pathway markers in live cells exhibiting Rlc1p-mRFP rings. We observed a marginal difference in the spatial distribution of cis- and the trans-Golgi compartments between wild-type and cdc15-140 mutant cells at both 24 and 36°C, with ∼50 and 55% cells exhibiting polarization of early and late Golgi elements, respectively (Figure 5, B and C, respectively). On the other hand, the tER marker protein Sec24p-GFP exhibited a more complex distribution (Figure 5A). In wild-type cells, at both 24 and 36°C, ∼80% of cells displaying Rlc1p-mRFP ring exhibited a clear recruitment of Sec24p-GFP to the narrow medial band, whereas ∼10% of these cells showed some degree of accumulation in a broader, less-defined medial region (Figure 5A, center panel). Sec24p-GFP appeared nonpolarized in ∼10% of cells. We observed a striking reduction in the number of cells exhibiting clear polarization of the tER in cdc15-140 cells already at the permissive temperature of 24°C (Figure 5A, bottom panel), suggesting that under these circumstances the mutant Cdc15p protein might be partially inactivated already at 24°C. Among cdc15-140 cells grown at 24°C and displaying the Rlc1p-RFP, 40, 40, and 20% exhibited the polarized, marginally polarized, and unpolarized localization of Sec24p-GFP, respectively. On the temperature shift-up to 36°C, the distribution of cdc15-140 mutant cells with Rlc1p-mRFP rings showing polarized, marginally polarized and unpolarized Sec24p-GFP localization changed to ∼20, ∼40, and ∼40%, respectively. We concluded that Sec24p-GFP accumulation at the division site was impaired in metaphase-arrested cdc15-140 cells despite their ability to assemble actomyosin rings, implicating Cdc15p in tER polarization during cell division.
Figure 5.
The EFC domain protein Cdc15p was required for the recruitment of the tER to the division site. (A) pREP1-mad2+ cells coexpressing Sec24p-GFP and Rlc1p-mRFP in wild-type or cdc15-140 mutant background were grown for 20 h in the absence of thiamine to induce metaphase arrest by overproduction of Mad2p and additionally allowed to grow for 4 h at either 24 or 36°C. In each sample, cells displaying Rlc1p-mRFP rings (second row from the top) exhibited three distinct phenotypes of Sec24p-GFP localization (top row of images). Shown is the maximum projection image of the z-stack obtained by epifluorescence imaging. Quantitation of Sec24p-GFP localization profiles under experimental conditions described is presented in the graph (n = 250 cells per sample). (B) pREP1-mad2+ cells coexpressing Anp1p-GFP and Rlc1p-mRFP in wild-type or cdc15-140 mutant background were treated as in A. In each sample, cells displaying Rlc1p-mRFP rings (second row from the top) exhibited distinct phenotypes of Anp1p-GFP localization (top row of images). Shown is the maximum projection image of the z-stack obtained by epifluorescence imaging. Quantitation of Anp1p-GFP localization profiles under experimental conditions described are presented in the graph (n = 250 cells per sample). (C) pREP1-mad2+ cells coexpressing Sec72p-GFP and Rlc1p-mRFP in wild-type or cdc15-140 mutant background were treated as in A. In each sample, cells displaying Rlc1p-mRFP rings (second row from the top) exhibited distinct phenotypes of Sec72p-GFP localization (top row of images). Shown is the maximum projection image of the z-stack obtained by epifluorescence imaging. Quantitation of Sec72p-GFP localization profiles under experimental conditions described are presented in the graph (n = 250 cells per sample). (D) Wild-type cells expressing Sec24p-GFP (green) and Rlc1p-mCherry (red) were grown overnight at 24°C and shifted to 36°C for 1 h before imaging. Shown is the maximum projection image of the z-stack obtained by time-lapse spinning disk confocal imaging. (E) cdc15-140 mutant cells expressing Sec24p-GFP (green) and Rlc1p-mCherry (red) were grown overnight at 24°C and shifted to 36°C for 1 h before imaging. Shown is the maximum projection image of the z-stack obtained by time-lapse spinning disk confocal imaging.
To further explore the role of Cdc15p in polarization of the tER at the level of individual cells, we performed the time-lapse analyses of either wild-type or cdc15-140 cells expressing Sec24p-GFP and Rlc1p-mCherry grown overnight at 24°C and shifted to 36°C for 1 h (Figure 5, D and E). The wild-type cells entered mitosis and formed an actomyosin ring that persisted and eventually constricted. We observed a marked and persistent recruitment of tER occurring concomitantly with the actomyosin ring assembly (8/8 cells). The cdc15-140 cells could form actomyosin rings upon entry into mitosis, but these rings neither persisted nor constricted. Formation of the actomyosin ring in cdc15-140 cells did not induce pronounced Sec24p-GFP polarization (Figure 5E, 5/8 cells). Incomplete inactivation of Cdc15p temperature-sensitive protein could be a reason for the weak and transient Sec24p-GFP accumulation detected in the remaining cells.
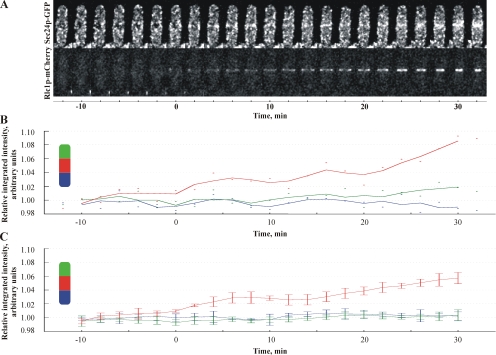
Cdc15p could be involved in targeting the preexisting COPII-positive membranes to the division site. Alternatively, Cdc15p might directly promote tER biogenesis in the vicinity of the actomyosin ring. We assessed whether the COPII structures in dividing cells exhibited the net centripetal movement toward the actomyosin ring by dual color image analyses of Sec24p-GFP and Rlc1p-mCherry (Figure 6A). Measurements of integrated fluorescence intensities of Sec24p-GFP in areas representing both cell tips and cell middle (see Materials and Methods) throughout cell division showed that COPII-positive structures accumulated at the division site concurrently with the actomyosin ring formation (Figure 6B, red line). At the same time, the fluorescence intensity of Sec24p-GFP did not change at cell tips (Figure 6B, green and blue lines; see also Figure 6C, n = 5 cells). Importantly, Sec24p-GFP accumulated at the division site in cells lacking both type V myosins, Myo4p and Myo5p (Motegi et al., 2004), suggesting that medial accumulation of tER did not require actin cable–directed transport (Supplementary Figure 6, 70% cells exhibited medial accumulation of tER compartments, n = 150 cells). Taken together, our data favor the model that Cdc15p could promote tER biogenesis at the division site.
Figure 6.
Polarization of tER does not depend on net centripetal movement of pre-exsisting COPII-positive membranes. (A) Shown is the maximum projection images of the z-stacks obtained by time-lapse spinning disk confocal imaging of cells expressing Sec24p-GFP (top row) and Rlc1p-mCherry (bottom row) grown at 24°C. (B) Quantitation of the relative integrated fluorescence intensities of Sec24p-GFP in tips (green, blue) and the center (red) of the cell presented in A. Dots indicate the measured values at distinct time points, and the graph lines represent the moving average values (for details see Material and Methods). (C) Quantitation of the relative integrated fluorescence intensities of Sec24p-GFP in tips (green, blue) and the center (red) of five cells. y-axis is scaled relative to average intensity at cell tips. The graph lines represent the moving average values and error bars correspond to the SE (for details see Material and Methods).
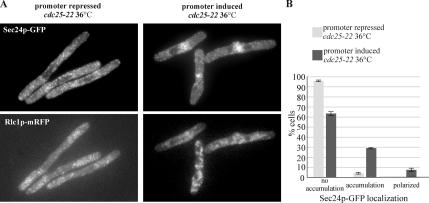
It has been shown that overexpression of Cdc15p causes actin relocalization to the equatorial region in interphase cells (Fankhauser et al., 1995). To assess whether this is sufficient for the early secretory pathway compartments polarization, we overexpressed Cdc15p under the nmt1 promoter in cells arrested in interphase by the use of cdc25-22 genetic background (Gould and Nurse, 1989). When we induced Cdc15p overexpression for a total of 16 h (12 h at 24°C and 4 h at 36°C), ∼35% of interphase-arrested cells relocalized actin to the cell middle, as judged by DAPI and phalloidin staining, whereas few of these formed an organized ring as judged by localization of Rlc1p-mRFP. We did not observe medial accumulation of either cis- and trans-Golgi compartments (data not shown). Interestingly, a total of 37 ± 4% interphase-arrested cells showed a marked accumulation of tER at the medial region, a number that corresponded to the percentage of cells that relocalized actin (Figure 7, A and B). Most cells exhibiting the tER polarization did not assemble the Rlc1p-mRFP–positive actomyosin ring structures (Figure 7A, bottom panel). Taken together, our experiments imply that the EFC domain protein Cdc15p is specifically required for the COP II vesicle polarization at the division site.
Figure 7.
Overexpression of Cdc15p is sufficient to induce medial tER accumulation in interphase. (A) cdc25–22 pREP1-cdc15+ cells expressing Sec24p-GFP (top row) and Rlc1mRFP (bottom row) were grown for 12 h at 24°C and for 4 h at 36° in the absence or presence of thiamine (left and right column, respectively). Shown is the maximum projection image of the z-stack obtained by epifluorescence imaging. (B) Quantitation of Sec24p-GFP polarization under experimental conditions described above (n = 250 cells per sample).
DISCUSSION
Here we have shown that in fission yeast the entire early secretory machinery becomes polarized during cell division in an actomyosin ring–dependent manner and relies on the EFC domain protein Cdc15p function.
Organization of the Early Secretory Pathway Compartments in Fission Yeast
As indicated by the tER marker Sec24p-GFP (Figure 1A; and Sec13p-GFP, our unpublished data), the tER of S. pombe is not organized as several discrete structures in P. pastoris but resemble the delocalized tER of budding yeast (Rossanese et al., 1999). On the other hand, the colocalization studies of two different Golgi compartments, marked by Anp1p and Sec72p (Figure 1, B and C), support the electron microscopy evidence (Johnson et al., 1973; Ayscough et al., 1993) of the Golgi apparatus in fission yeast being predominantly organized as adjacent stacks of few cisternae. In the view of Rossanese and colleagues, the difference in Golgi organization between two budding yeast species could arise from the difference in tER structure. Thus, the stable tER structure of P. pastoris would consecutively produce the novel cis-Golgi cisternae at a fixed location and maturation of these cisternae will give rise to a stacked Golgi apparatus structure. Absence of the long-lived, discrete tER sites in S. cerevisiae would cause the formation of novel Golgi cisternae at random locations, yielding dispersed Golgi entities. Our results suggest that in fission yeast a relatively disorganized and yet stable tER is present as multiple entities that are accompanied by considerably fewer coherent Golgi stacks (Figure 1, A–C). Such structural organization of the early secretory pathway might reflect the possible involvement of the Golgi matrix (for review see Barr and Short, 2003) or tether (Cai et al., 2007) proteins in maintaining attachment between Golgi cisternae, a function that was lost in S. cerevisiae but is present in fission yeast.
Recent advances in high-speed microscopy have allowed the study of Golgi biogenesis and maturation (for review see Hammond and Glick, 2000). Our data provide further evidence supporting the cisternal progression model of Golgi maturation (Figure 1G and Supplementary Movie 3). Time required for Golgi cisternae identity change in fission yeast (Figure 1F) was consistent with maturation dynamics reported previously in S. cerevisiae (Losev et al., 2006; Matsuura-Tokita et al., 2006). As judged by the dynamics of the early Golgi marker protein, Anp1p-mCherry, cis-Golgi cisternae could also arise de novo (Figure 1, E and F, and Supplementary Movie 2), but the relationship of these biogenesis events to tER was more difficult to establish because of the large number of Sec24p-GFP entities.
Polarization of the Secretory Pathway to the Division Site
The secretory pathway is a major player during cell polarization in many organisms including epithelial polarization in Drosophila embryos (Lecuit and Wieschaus, 2000), axon outgrowth in cultured rat embryonic neurons (Bradke and Dotti, 1997) and bud development in S. cerevisiae (for review see Finger and Novick, 1998). However, polarized secretion has been mainly studied with respect to the most downstream elements of the secretory pathway, such as the exocytotic machinery and the secretory vesicles. By observing the spatial distribution of the fluorescently tagged proteins specific to distinct early secretory pathway compartments during the cell cycle, we found that the tER and both the cis- and trans-Golgi established preferential localization to the division site. Surprisingly, although we did not observe the relocalization of the general ER to the site of septation as judged by Ost1p-GFP (Supplementary Figure 1), we found a marked accumulation of tER (Figure 2A). Thus it would be of interest to investigate the manner of tER distribution in other instances where Golgi accumulation at the growth sites was reported (Rida et al., 2006). Interestingly, the Golgi and the secretory vesicles accumulated at the sites of cell wall formation as judged by three-dimensional (3D) reconstruction of reverting S. pombe protoplasts (Osumi et al., 1998). The importance of ER recruitment has been recently reported in a study on the role of the gene jagunal in Drosophila oocyte development (Lee and Cooley, 2007). This study highlighted the importance of ER reorganization to the subcortical clusters as essential for increased exocytotic membrane traffic that is vital for oocyte development. However, in these cells concordant relocalization of the Golgi compartments was not investigated. We propose that although different organisms could undergo polarization of the secretory pathway at various stages of its biogenesis, the common feature of the examples available in the published literature could be a necessity for a rapid increase in cell surface area. It is conceivable that such intense events might require massive trafficking that could be achieved by concentrating the entire secretory machinery to the proximity of the secretion site, rather than relying on a long-range delivery of post-Golgi vesicles. Interestingly, fission yeast cells lacking functional exocyst components still form complete septa, although they are defective in septum cleavage (Wang et al., 2002).
How could such polarization be achieved? One obvious solution is the recruitment of the secretion machinery by cytoskeletal elements. In S. cerevisiae, compromised function of actin cytoskeleton components abolishes polarized secretion (for review see Finger and Novick, 1998). In particular, polarized secretion at the bud requires intact actin cables and the type V myosin Myo2 (Govindan et al., 1995). In S. pombe, both actin cables (Feierbach and Chang, 2001) and the type V myosin Myo4p (Motegi et al., 2001) are necessary for the establishment of proper cell morphology. Thus, our results on the requirement of the actomyosin ring for the establishment and of an intact actin cytoskeleton for the maintenance of the polarized early secretory pathway are in agreement with the previously reported roles for the actin cytoskeleton in polarized secretion. Again, the tight coupling of ring formation and secretory pathway polarization could be necessary for fission yeast to timely prime the site for intense secretion that is associated with building a septum across the cylindrical cell.
The SIN pathway has been shown to be vital for targeted secretion because its inactivation hinders secretion of the septum material at the division site (Liu et al., 2002). Our data show that the early secretory pathway polarization occurs before SIN activation and does not require its function (Figure 4 and Supplementary Figure 5), therefore suggesting that recruitment of the early secretory machinery and post-Golgi secretion are differentially regulated. Thus it seems that SIN functions in activating most downstream secretion events, such as post-Golgi vesicle trafficking.
Our results are consistent with the possibility that the EFC domain protein Cdc15p might function as the actomyosin ring component recruiting the tER to the site of division. Lee et al. (2005) have shown that the COPII coat proteins alone were defective in forming COPII vesicles in vitro and required Sar1p function to vesiculate lipid membranes. They suggested that the formation of membrane curvature by components other than COPII vesicle proteins might be vital to the formation of the vesicles. They also suggested that factors such as the microtubule-driven membrane deformation characteristic of mammalian cells could generate curvature that could be subsequently acted upon by the cytosolic coat proteins. Cdc15p possesses an EFC domain that has been shown to induce membrane tubulation in other proteins (Tsujita et al., 2006). As we neither observed any net movement of COPII-positive membranes toward the division site (Figure 6) nor involvement of type V myosins (Supplementary Figure 6) in this phenomenon, an exciting possibility is that Cdc15p might be involved in generating membrane curvature and thus promote tER formation in the vicinity of the actomyosin ring. We found that Cdc15p was necessary and sufficient for the medial accumulation of the tER (Figures 5 and 7). Overexpression of the Cdc15p EFC domain alone or of Cdc15p lacking the EFC domain was not sufficient for the induction of tER recruitment to the equatorial region in interphase cells (our unpublished data). Cdc15p was not required for localization of Golgi cisternae, suggesting that the recruitment of tER and Golgi to the division site may occur through different mechanisms. Our experiments together with the published research suggest that actin structures could possibly act as a delivery or anchor system for Golgi apparatus.
In conclusion, our work identifies the fission yeast S. pombe as an interesting system for studying the phenomena of secretion and polarity establishment. We propose that the polarization of both tER and Golgi might fulfill the need for massive vesicle trafficking events occurring at the division site. It would be interesting to gain a better understanding of the role of Cdc15p and to identify additional molecular determinants of the process in future.
Supplementary Material
ACKNOWLEDGMENTS
We are most grateful to M. Balasubramanian and H. Wang (Temasek Life Sciences Laboratory) for sharing S. pombe strains and for valuable comments on the manuscript. We thank V. Wachtler and E. Makeyev for critical reading of the manuscript and R. Thadani for valuable discussions. X.-Z.T., a student of Raffles Junior College, Singapore, participated in the Research Attachment Programme (REAP) organized by the Ministry of Education (MOE), National University of Singapore (NUS), and Temasek Life Sciences Laboratory. This work has been supported by intramural funds from the Temasek Life Sciences Laboratory.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0663) on January 9, 2008.
REFERENCES
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. New York: Garland Science; 2002. Molecular Biology of the Cell. [Google Scholar]
- Antonny B., Madden D., Hamamoto S., Orci L., Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nat. Cell Biol. 2001;3:531–537. doi: 10.1038/35078500. [DOI] [PubMed] [Google Scholar]
- Arai R., Mabuchi I. F-actin ring formation and the role of F-actin cables in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 2002;115:887–898. doi: 10.1242/jcs.115.5.887. [DOI] [PubMed] [Google Scholar]
- Ayscough K., Hajibagheri N. M., Watson R., Warren G. Stacking of Golgi cisternae in Schizosaccharomyces pombe requires intact microtubules. J. Cell Sci. 1993;106(Pt 4):1227–1237. doi: 10.1242/jcs.106.4.1227. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., Bi E., Glotzer M. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 2004;14:R806–R818. doi: 10.1016/j.cub.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., Helfman D. M., Hemmingsen S. M. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature. 1992;360:84–87. doi: 10.1038/360084a0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., McCollum D., Chang L., Wong K. C., Naqvi N. I., He X., Sazer S., Gould K. L. Isolation and characterization of new fission yeast cytokinesis mutants. Genetics. 1998;149:1265–1275. doi: 10.1093/genetics/149.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M. F., Ravazzola M., Amherdt M., Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Barr F. A., Short B. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 2003;15:405–413. doi: 10.1016/s0955-0674(03)00054-1. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Hergert F., Gierz G. Computer simulation of fungal morphogenesis and the mathematical basis for hyphal (typ) growth. Protoplasma. 1989;153:46–57. [Google Scholar]
- Bevis B. J., Hammond A. T., Reinke C. A., Glick B. S. De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat. Cell Biol. 2002;4:750–756. doi: 10.1038/ncb852. [DOI] [PubMed] [Google Scholar]
- Bradke F., Dotti C. G. Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron. 1997;19:1175–1186. doi: 10.1016/s0896-6273(00)80410-9. [DOI] [PubMed] [Google Scholar]
- Broughton J., Swennen D., Wilkinson B. M., Joyet P., Gaillardin C., Stirling C. J. Cloning of SEC61 homologues from Schizosaccharomyces pombe and Yarrowia lipolytica reveals the extent of functional conservation within this core component of the ER translocation machinery. J. Cell Sci. 1997;110(Pt 21):2715–2727. doi: 10.1242/jcs.110.21.2715. [DOI] [PubMed] [Google Scholar]
- Cai H., Yu S., Menon S., Cai Y., Lazarova D., Fu C., Reinisch K., Hay J. C., Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]
- Carpenter A. E., et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Drubin D., Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Woollard A., Nurse P. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J. Cell Sci. 1996;109(Pt 1):131–142. doi: 10.1242/jcs.109.1.131. [DOI] [PubMed] [Google Scholar]
- Cortes J. C., Ishiguro J., Duran A., Ribas J. C. Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 2002;115:4081–4096. doi: 10.1242/jcs.00085. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Sanders S. L., Feldheim D. A., Schekman R. Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature. 1991;349:806–808. doi: 10.1038/349806a0. [DOI] [PubMed] [Google Scholar]
- Engstler M., Thilo L., Weise F., Grunfelder C. G., Schwarz H., Boshart M., Overath P. Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J. Cell Sci. 2004;117:1105–1115. doi: 10.1242/jcs.00938. [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Reymond A., Cerutti L., Utzig S., Hofmann K., Simanis V. The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell. 1995;82:435–444. doi: 10.1016/0092-8674(95)90432-8. [DOI] [PubMed] [Google Scholar]
- Feierbach B., Chang F. Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr. Biol. 2001;11:1656–1665. doi: 10.1016/s0960-9822(01)00525-5. [DOI] [PubMed] [Google Scholar]
- Finger F. P., Novick P. Spatial regulation of exocytosis: lessons from yeast. J. Cell Biol. 1998;142:609–612. doi: 10.1083/jcb.142.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff A., Redding K., Crosby J., Fuller R. S., Schekman R. Localization of components involved in protein transport and processing through the yeast Golgi apparatus. J. Cell Biol. 1991;112:27–37. doi: 10.1083/jcb.112.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furge K. A., Wong K., Armstrong J., Balasubramanian M., Albright C. F. Byr4 and Cdc16 form a two-component GTPase-activating protein for the Spg1 GTPase that controls septation in fission yeast. Curr. Biol. 1998;8:947–954. doi: 10.1016/s0960-9822(98)70394-x. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Govindan B., Bowser R., Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Novick P. The exocyst meets the translocon: a regulatory circuit for secretion and protein synthesis? Trends Cell Biol. 2004;14:61–63. doi: 10.1016/j.tcb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Hales K. G., Bi E., Wu J. Q., Adam J. C., Yu I. C., Pringle J. R. Cytokinesis: an emerging unified theory for eukaryotes? Curr. Opin. Cell Biol. 1999;11:717–725. doi: 10.1016/s0955-0674(99)00042-3. [DOI] [PubMed] [Google Scholar]
- Hammond A. T., Glick B. S. Raising the speed limits for 4D fluorescence microscopy. Traffic. 2000;1:935–940. [PubMed] [Google Scholar]
- Harris S. D., Read N. D., Roberson R. W., Shaw B., Seiler S., Plamann M., Momany M. Polarisome meets spitzenkorper: microscopy, genetics, and genomics converge. Eukaryot. Cell. 2005;4:225–229. doi: 10.1128/EC.4.2.225-229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Patterson T. E., Sazer S. The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc. Natl. Acad. Sci. USA. 1997;94:7965–7970. doi: 10.1073/pnas.94.15.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbel B. M., Konomi M., Takagi T., Kamasawa N., Ishijima S. A., Osumi M. In situ localization of beta-glucans in the cell wall of Schizosaccharomyces pombe. Yeast. 2001;18:433–444. doi: 10.1002/yea.694. [DOI] [PubMed] [Google Scholar]
- Jochova J., Rupes I., Streiblova E. F-actin contractile rings in protoplasts of the yeast Schizosaccharomyces. Cell Biol. Int. Rep. 1991;15:607–610. doi: 10.1016/0309-1651(91)90007-6. [DOI] [PubMed] [Google Scholar]
- Johnson B. F., Yoo B. Y., Calleja G. B. Cell division in yeasts: movement of organelles associated with cell plate growth of Schizosaccharomyces pombe. J. Bacteriol. 1973;115:358–366. doi: 10.1128/jb.115.1.358-366.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Prendergast J. A., Singer R. A. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J. Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann J., Munro S. Multi-protein complexes in the cis Golgi of Saccharomyces cerevisiae with alpha-1,6-mannosyltransferase activity. EMBO J. 1998;17:423–434. doi: 10.1093/emboj/17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis J., Bimbo A., Rajagopalan S., Liu J., Balasubramanian M. K. The nuclear kinase Lsk1p positively regulates the septation initiation network and promotes the successful completion of cytokinesis in response to perturbation of the actomyosin ring in Schizosaccharomyces pombe. Mol. Biol. Cell. 2005;16:358–371. doi: 10.1091/mbc.E04-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama C., Sugimoto A., Yamamoto M. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J. Cell Biol. 1997;137:1309–1319. doi: 10.1083/jcb.137.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A., Gulli M. P., Simanis V. SIN and the art of splitting the fission yeast cell. Curr. Biol. 2004;14:R722–730. doi: 10.1016/j.cub.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Le Goff X., Motegi F., Salimova E., Mabuchi I., Simanis V. The S. pombe rlc1 gene encodes a putative myosin regulatory light chain that binds the type II myosins myo3p and myo2p. J. Cell Sci. 2000;113(Pt 23):4157–4163. doi: 10.1242/jcs.113.23.4157. [DOI] [PubMed] [Google Scholar]
- Lecuit T., Wieschaus E. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J. Cell Biol. 2000;150:849–860. doi: 10.1083/jcb.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. C., Orci L., Hamamoto S., Futai E., Ravazzola M., Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Lee S., Cooley L. Jagunal is required for reorganizing the endoplasmic reticulum during Drosophila oogenesis. J. Cell Biol. 2007;176:941–952. doi: 10.1083/jcb.200701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Naqvi N. I., Yang H., Teo T. S. Identification of a 26S proteasome-associated UCH in fission yeast. Biochem. Biophys. Res. Commun. 2000;272:270–275. doi: 10.1006/bbrc.2000.2767. [DOI] [PubMed] [Google Scholar]
- Liu J., Tang X., Wang H., Oliferenko S., Balasubramanian M. K. The localization of the integral membrane protein Cps1p to the cell division site is dependent on the actomyosin ring and the septation-inducing network in Schizosaccharomyces pombe. Mol. Biol. Cell. 2002;13:989–1000. doi: 10.1091/mbc.01-12-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang H., Balasubramanian M. K. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. J. Cell Sci. 2000;113(Pt 7):1223–1230. doi: 10.1242/jcs.113.7.1223. [DOI] [PubMed] [Google Scholar]
- Losev E., Reinke C. A., Jellen J., Strongin D. E., Bevis B. J., Glick B. S. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- Malhotra V., Mayor S. Cell biology: the Golgi grows up. Nature. 2006;441:939–940. doi: 10.1038/441939a. [DOI] [PubMed] [Google Scholar]
- Mancias J. D., Goldberg J. Exiting the endoplasmic reticulum. Traffic. 2005;6:278–285. doi: 10.1111/j.1600-0854.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- Marks J., Fankhauser C., Simanis V. Genetic interactions in the control of septation in Schizosaccharomyces pombe. J. Cell Sci. 1992;101(Pt 4):801–808. doi: 10.1242/jcs.101.4.801. [DOI] [PubMed] [Google Scholar]
- Marks J., Hyams J. S. Localization of F-actin through the cell division cycle of Schizosaccharomyces pombe. Eur. J. Cell Biol. 1985;39:27–32. [Google Scholar]
- Martin-Cuadrado A. B., Duenas E., Sipiczki M., Vazquez de Aldana C. R., del Rey F. The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 2003;116:1689–1698. doi: 10.1242/jcs.00377. [DOI] [PubMed] [Google Scholar]
- Martin-Cuadrado A. B., Morrell J. L., Konomi M., An H., Petit C., Osumi M., Balasubramanian M., Gould K. L., Del Rey F., de Aldana C. R. Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol. Biol. Cell. 2005;16:4867–4881. doi: 10.1091/mbc.E04-12-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura-Tokita K., Takeuchi M., Ichihara A., Mikuriya K., Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J. Biol. Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- McCollum D., Balasubramanian M. K., Pelcher L. E., Hemmingsen S. M., Gould K. L. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J. Cell Biol. 1995;130:651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Warren G. The road taken: past and future foundations of membrane traffic. Cell. 2000;100:99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- Minet M., Nurse P., Thuriaux P., Mitchison J. M. Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe. J. Bacteriol. 1979;137:440–446. doi: 10.1128/jb.137.1.440-446.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Motegi F., Arai R., Mabuchi I. Identification of two type V myosins in fission yeast, one of which functions in polarized cell growth and moves rapidly in the cell. Mol. Biol. Cell. 2001;12:1367–1380. doi: 10.1091/mbc.12.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N. I., Eng K., Gould K. L., Balasubramanian M. K. Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. EMBO J. 1999;18:854–862. doi: 10.1093/emboj/18.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur. J. Biochem. 2003;270:2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- Osumi M., Sato M., Ishijima S. A., Konomi M., Takagi T., Yaguchi H. Dynamics of cell wall formation in fission yeast, Schizosaccharomyces pombe. Fungal. Genet. Biol. 1998;24:178–206. doi: 10.1006/fgbi.1998.1067. [DOI] [PubMed] [Google Scholar]
- Pidoux A. L., Armstrong J. The BiP protein and the endoplasmic reticulum of Schizosaccharomyces pombe: fate of the nuclear envelope during cell division. J. Cell Sci. 1993;105(Pt 4):1115–1120. doi: 10.1242/jcs.105.4.1115. [DOI] [PubMed] [Google Scholar]
- Preuss D., Mulholland J., Franzusoff A., Segev N., Botstein D. Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol. Biol. Cell. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas J. C., Diaz M., Duran A., Perez P. Isolation and characterization of Schizosaccharomyces pombe mutants defective in cell wall (1-3)beta-D-glucan. J. Bacteriol. 1991;173:3456–3462. doi: 10.1128/jb.173.11.3456-3462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rida P. C., Nishikawa A., Won G. Y., Dean N. Yeast-to-hyphal transition triggers formin-dependent Golgi localization to the growing tip in Candida albicans. Mol. Biol. Cell. 2006;17:4364–4378. doi: 10.1091/mbc.E06-02-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossanese O. W., Soderholm J., Bevis B. J., Sears I. B., O'Connor J., Williamson E. K., Glick B. S. Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J. Cell Biol. 1999;145:69–81. doi: 10.1083/jcb.145.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein S., Collins P. G., Kelleher D. J., Rapiejko P. J., Gilmore R. The alpha subunit of the Saccharomyces cerevisiae oligosaccharyltransferase complex is essential for vegetative growth of yeast and is homologous to mammalian ribophorin I. J. Cell Biol. 1995;128:525–536. doi: 10.1083/jcb.128.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks C. A., Morphew M., McCollum D. Sid2p, a spindle pole body kinase that regulates the onset of cytokinesis. J. Cell Biol. 1999;146:777–790. doi: 10.1083/jcb.146.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita K., Suetsugu S., Sasaki N., Furutani M., Oikawa T., Takenawa T. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J. Cell Biol. 2006;172:269–279. doi: 10.1083/jcb.200508091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtler V., Huang Y., Karagiannis J., Balasubramanian M. K. Cell cycle-dependent roles for the FCH-domain protein Cdc15p in formation of the actomyosin ring in Schizosaccharomyces pombe. Mol. Biol. Cell. 2006;17:3254–3266. doi: 10.1091/mbc.E05-11-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Tang X., Liu J., Trautmann S., Balasundaram D., McCollum D., Balasubramanian M. K. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell. 2002;13:515–529. doi: 10.1091/mbc.01-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood V., et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–880. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.