Abstract
Although nerve growth factor (NGF) promotes survival of neurons, tumor necrosis factor α (TNF-α) contributes to cell death triggered by NGF depletion, through TNF-α receptor (TNFR) 1. In contrast to this effect, TNF-α can promote neural cell survival via TNF-α receptor TNFR2. Although these findings demonstrate pivotal roles of TNF-α and NGF in cell fate decisions, cross-talk between these signaling pathways has not been clarified. We find that NGF can induce TNF-α synthesis through the nuclear factor-κB transcription factor. This provides a new basis for examining the cross-talk between NGF and TNF-α. Inhibition of TNFR2 shows opposite effects on two downstream kinases of NGF, extracellular signal-regulated kinase (Erk) and Akt. It increases Erk activation by NGF, and this increased activation induces differentiation of neuroblastoma cell lines. Reciprocally, inhibition of TNFR2 decreases Akt activation by NGF. Consistent with an essential role of Akt in survival signaling, inhibition of TNF-α signaling decreases NGF-dependent survival of neurons from rat dorsal root ganglia. Thus, NGF and NGF-induced TNF-α cooperate to activate Akt, promoting survival of normal neural cells. However, the NGF-induced TNF-α suppresses Erk activation by NGF, blocking NGF-induced differentiation of neuroblastoma cells. TNFR2 signaling could be a novel target to modulate cell responses to NGF.
INTRODUCTION
Nerve growth factor (NGF) is a neurotrophin that can induce at least two major responses in neural cells (Huang and Reichardt, 2001, 2003). NGF induces differentiation of rat pheochromocytoma PC-12 cells into terminally differentiated neuron-like cells in vitro (Greene and Tischler, 1976). Activation of the mitogen-activated protein kinase kinase (MEK)–extracellular signal-regulated kinase (Erk) pathway is responsible for the differentiation of PC-12 cells (Qui and Green, 1992). However, it is uncertain whether NGF can contribute to neural cell differentiation under normal conditions of development. First, mice lacking the NGF receptor gene, TrkA, still show normal differentiation of neurons, although the differentiated neurons in these mice contain lower amounts of acetylcholine and gradually disappeared (Fagan et al., 1997; Schober et al., 1997). Second, expression of NGF receptors starts at the late phase of neural differentiation (Wyatt and Davies, 1993, 1995; v Holst et al., 1997). These reports suggest that NGF might not contribute to early neural cell differentiation during normal development.
Furthermore, NGF promotes survival and maturation of several types of neurons, including sympathetic and sensory neurons in the peripheral nervous system, and cholinergic neurons of both the basal forebrain and the striatum in the CNS (Huang and Reichardt, 2001, 2003). NGF is secreted from target tissues during development, and neurons extend neurites in the direction of the secreted NGF. Although excessive numbers of neurons are produced during development, only neurons that obtain a sufficient level of NGF can survive and interact with the target tissues (Fagan et al., 1997; Huang and Reichardt, 2001, 2003; Sofroniew et al., 2001). Activation of the phosphatidylinositol 3-kinase–Akt signaling pathway by NGF contributes to this NGF-dependent survival (Datta et al., 1999).
However, neurons that fail to obtain a sufficient level of NGF during development die through an apoptotic pathway (Fagan et al., 1997; Huang and Reichardt, 2001, 2003; Sofroniew et al., 2001). Tumor necrosis factor α (TNF-α) is a cytokine that contributes to this neural cell death (Barker et al., 2001). Barker et al. (2001) show that TNF-α is expressed in neurons that depend upon NGF for their survival. The endogenous TNF-α does not kill neurons in the presence of NGF. However, when NGF is withdrawn, the endogenous TNF-α shows cytotoxic effects on neurons through TNF-α receptor (TNFR) 1.
Contrary to the cytotoxic effects of TNF-α through TNFR1, there is substantial evidence showing that TNF-α can promote neural cell survival through another TNF-α receptor, TNFR2. Knockdown of TNFR2 sensitizes a neuroblastoma cell line to cell death initiated by β-amyloid (Shen et al., 1997). Hippocampal neurons isolated from TNFR2-deficient mice are more sensitive to TNF-α-induced cell death than the neurons from normal mice (Yang et al., 2002). Furthermore, TNFR2 signaling is required for both survival of retinal neurons from ischemia-reperfusion (Fontaine et al., 2002) and survival of cortical neurons from glutamate-induced excitotoxicity (Marchetti et al., 2004). In addition to the NGF-dependent survival of neurons, activation of Akt is associated with TNFR2-mediated survival of neurons (Fontaine et al., 2002; Marchetti et al., 2004). These results indicate that TNF-α could contribute to survival of neurons through TNFR2 at least under some conditions, although TNF-α shows cytotoxic effects on neurons through TNFR1 in the absence of NGF (Barker et al., 2001). Effects of endogenous TNF-α on neurons in the presence of NGF are still unknown.
We find that NGF induces TNF-α synthesis in both neuroblastoma cells and normal neural cells in vitro. This finding provides a new basis for examining the effects of endogenous TNF-α on NGF signaling. Our data indicate that endogenous TNF-α inhibits NGF-dependent differentiation, but it supports NGF-dependent survival.
MATERIALS AND METHODS
Materials
NGF, murine TNF-α, and human TNF-α were purchased from Merck Biosciences (Darmstadt, Germany). Small interfering RNA (siRNA) against TNFR2 (GGGUGAUAAAUUGUUGAUA) was purchased from Ambion (Austin, TX). Neutralizing anti-human TNF-α monoclonal antibody (mAb), anti-rat TNF-α mAb, anti-human TNFR2 mAb, and anti-human TNFR1 mAb were purchased from R&D Systems Europe (Abingdon, Oxfordshire, United Kingdom). Anti-β-tubulin III was purchased from Sigma Chemical (Poole, Dorset, United Kingdom). The 80M2 anti-TNFR2 mAb was purchased from Hycult Biotechnology (Uden, The Netherlands). Anti-phospho-specific p140trkA, anti-Erk1/2, anti-phospho-specific Erk1/2, and anti-phospho-specific Akt were purchased from Cell Signaling Technology (Danvers, MA). Antibody against Akt and antibody against p140trkA were purchased from Millipore (Billerica, MA). Antibody against growth-associated protein-43 (GAP-43) was purchased from Merck Biosciences. SH-SY5Y, BE(2)-C PC-12, and HCN-1A cell lines were purchased from American Type Culture Collection (Manassas, VA). Neurons from postnatal day 5 rat dorsal root ganglia, primary neuron basal media, l-glutamine, and NSF-1 supplement were purchased from Lonza Verviers (Verviers, Belgium). DMEM, Ham's F-12 medium, and Alexa Fluor 488-labeled phalloidin were purchased from Invitrogen (Paisley, United Kingdom). Nuclear factor (NF)-κB activation inhibitor 6-amino-4-(4-phenoxyphenylamino) quinazoline and Akt inhibitor, 1-(l)-6-hydroxymethyl-chiro-inositol-2-(R)-2-O-methyl-3-O-octadecylcarbonate were purchased from Merck Biosciences. MEK inhibitor 1, 4-diamino-2, 3-dicyano-1, 4-bis[2-aminophenylthio]butadiene was purchased from Cell Signaling Technology.
Cell Culture
SH-SY5Y, BE(2)-C, and HCN-1A cells were cultured in DMEM/Ham's F-12 containing 10% fetal bovine serum (FBS). These neural cells were treated with 10 ng/ml NGF. In some experiments, cells were pretreated for 1 h with the neutralizing antibody against TNF-α or TNFR2 (2 ng/ml), 80M2 mAb against TNFR2, the Akt inhibitor (5 μM), the MEK inhibitor (10 μM), the NF-κB activation inhibitor (1 μM), mouse TNF-α (0.5 ng/ml), or human TNF-α (0.5 ng/ml), as indicated in the figures. After addition of the neurotrophins, cells were cultured for 24 h (for TNF-α expression) or 48 h (for neural differentiation).
For differentiation of HCN-1A, HCN-1A cells were cultured sequentially in DMEM/Ham's F-12 containing 5% FBS, 25 ng/ml NGF, 0.5 mM dibutyryl cyclic AMP (dbcAMP), and 0.5 mg/ml 3-isobutyl-1-methylxanthine (IBMX) for 3 d and in DMEM/Ham's F-12 containing 2% FBS, 25 ng/ml NGF, 0.5 mM dbcAMP, and 0.5 mg/ml IBMX for another 3 d. After the 6-d culture, cells were washed with DMEM/Ham's F-12 medium without serum, and they were used for cell survival assay. PC-12 cells were cultured in DMEM containing 5% FBS and 10% horse serum.
Neurons from postnatal day 5 rat dorsal root ganglia (DRG) were seeded (5000 cells/well) into 96-well plates coated with poly-d-lysine and laminin and cultured in primary neuron basal media containing 2 mM l-glutamine, 1% NSF-1 supplement, 17.5 μg/ml uridine, and 7.5 μg/ml 5-fluoro-2-deoxyuridine without serum according to the manufacturer's recommendation. Medium was changed every 4 d. On day 10, cells were washed with primary neuron basal media without NSF-1 supplement and incubated in medium containing 2 mM l-glutamine with or without NGF (25 ng/ml) and the neutralizing antibody against rat TNF-α (5 ng/ml).
siRNA Transfection
To transfect double-stranded siRNA, siImporter (Millipore) was used. Transfection was performed according to the manufacturer's suggestions.
Immunoblotting
Cells were washed with phosphate-buffered saline (PBS) twice and resuspended in buffer containing 20 mM Tris-HCl, pH 8.0, 500 mM NaCl, 1 mM EDTA, 0.5% NP-40, 2 mM dithiothreitol, and phosphatase inhibitor cocktail (Sigma Chemical). After incubation for 15 min on ice, samples were centrifuged at 14,000 × g for 20 min at 4°C. The supernatants containing 40 μg of proteins were analyzed by SDS-polyacrylamide gel electrophoresis and blotted to poly(vinylidene difluoride) (PVDF) membrane. The PVDF membrane was incubated in blocking buffer containing 5% skimmed milk, 0.2% NP-40 in PBS for 1 h, first antibody diluted in the blocking buffer as indicated in figures, and appropriate secondary antibody labeled with horseradish peroxidase. Bound antibodies were visualized with enhanced chemiluminescence (ECL) or ECL Plus kits (GE Healthcare, Chalfont St. Giles, United Kingdom).
Immunostaining
Cells were washed twice with PBS and incubated with 2% paraformaldehyde in PBS at room temperature for 30 min. Then, they were incubated with 2% paraformaldehyde and 0.5% Triton X-100 in PBS at room temperature for 30 min. The fixed cells were washed with PBS and incubated with 3% bovine serum albumin (BSA) and 0.5% Triton X-100 in PBS at room temperature for 30 min and with anti-β-tubulin III mouse mAb, 3% BSA, and 0.5% Triton X-100 in PBS at 37°C for 2 h. After washing with PBS, cells were further incubated with appropriate secondary antibody labeled with fluorescein isothiocyanate (FITC), 3% BSA, and 0.5% Triton X-100 in PBS at 37°C for 30 min. After washing with PBS for three times, cells were mounted with VECTASHIELD mounting medium with 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Peterborough, United Kingdom), and then they were observed with confocal laser-scanning microscopy.
mRNA Isolation, cDNA Synthesis, and Quantitative Polymerase Chain Reaction (PCR)
mRNA was purified from cells treated with or without 10 ng/ml NGF for 24 h with MicropolyA mRNA isolation kit (Ambion). Purified mRNA was incubated with DNase I with the DNA-free kit (Ambion) to eliminate contamination of genomic DNA. DNase-treated mRNA fractions containing 60 ng of mRNA were used for cDNA synthesis. To synthesize cDNA, oligo(dT) and Superscript II (Invitrogen) were used. SYBRA Green Quantitative PCR kit (Invitrogen) and Rotor-Gene RG-3000 (Corbett Research, Westburg, The Netherlands) were used to perform quantitative PCR of TNF-α cDNA or NGF cDNA. In parallel to the quantitative PCR of TNF-α cDNA, quantitative PCR of ribosomal protein L11 (RPL11) cDNA was also performed as an internal standard of the amount of cDNA used as a template for PCR. The amount of TNF-α cDNA or NGF cDNA was normalized to the amount of RPL11 cDNA.
Measurement of NF-κB Activity
SH-SY5Y cells were transfected with pNFkB-SEAP vector (Takara, Gennevilliers, France). In this vector, a promoter regulated by NF-κB is followed by the secreted alkaline phosphatase (SEAP) reporter gene. After 24 h from the transfection, cells were split into 24-well plates and cultured for 48 h. NGF, the Akt inhibitor, or the MEK inhibitor was added to the culture medium. For cotreatment of inhibitors and NGF, inhibitors were added 1 h before addition of NGF. Twenty-four hours after addition of the reagents, culture medium was taken for SEAP assay. The SEAP assay was performed with a Great EscAPe SEAP assay kit (Takara).
5-Bromo-2′-deoxyuridine (BrdU) Incorporation
After transfection of siRNA against TNFR2, cells were incubated with 10 ng/ml NGF for 48 h. Then, the cells were further incubated with 1 μM BrdU for another 24 h. Cells were washed with PBS and fixed with 2% paraformaldehyde in PBS for 30 min at 4°C. After washing with PBS, cells were incubated with DNase I for 37°C for 1 h. Then, the incorporated BrdU was detected with rat anti-BrdU antibody (Harlan Sera-Lab, Loughborough, Leicestershire, United Kingdom) and visualized with Texas Red-labeled anti-rat immunoglobulin (Ig)G (GE Healthcare).
Phalloidin Staining
Cells were fixed as described in Immunostaining. The fixed cells were washed with PBS and incubated with 3% BSA and 0.5% Triton X-100 in PBS at room temperature for 1 h and with 1 U/ml Alexa Fluor 488-labeled phalloidin, 3% BSA, and 0.5% Triton X-100 in PBS at 37°C for 1 h. After washing with PBS three times, the cells were mounted with VECTASHIELD mounting medium with DAPI, and they were observed with confocal laser-scanning microscopy.
Cell Viability Assay
To assess cell viability, 3000 HCN-1A cells were seeded in 96-well plates. After 12 h, cells were washed extensively with serum-free DMEM/Ham's F-12 and cultured in serum-free DMEM/Ham's F-12 without (control) or with NGF, in the presence or absence of the neutralizing antibodies. After 24-h incubation, medium was changed to DMEM/Ham's F-12 containing 10% serum, and cells were cultured for another 24 h. 4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) reagent (Roche Diagnostics, Mannheim, Germany) was added directly to the culture medium, and cells were incubated for 2 h. For primary cultured neurons prepared from rat dorsal root ganglia, cells were seeded and cultured in 96 well plates as described in Cell Culture. On day 10, the neurons were washed and incubated for 24 h without (control) or with NGF in the presence or absence of neutralizing anti-TNF-α. WST-1 reagent was added directly to the culture medium and incubated for 4 h. Measurement of cleavage of WST-1 was performed following the manufacturer's recommendation, with a Fusion universal microplate analyzer (PerkinElmer Life and Analytical Sciences, Boston, MA).
For measurement of lactose dehydrogenase (LDH) activity, Cytotoxicity Detection kit Plus (Roche Diagnostics) was used. Cells were cultured in 96-well plates and treated as described above. For measurement of total LDH activity, 5 μl of lysis buffer was added into the well containing cells cultured with NGF alone and incubated for 15 min to release total LDH activity from cells. For measurement of LDH activity released into the culture medium from dead cells, 5 μl of PBS was added instead of lysis buffer. Fifty microliters of culture medium was taken and mixed with 50 μl of reaction mixture in a new 96-well plate and then incubated for 20 min at room temperature. Measurement of LDH activity was performed following the manufacturer's recommendation, with a Fusion universal microplate analyzer (PerkinElmer Life and Analytical Sciences).
Flow Cytometry
Forty-eight hours after siRNA transfection, cells were harvested by trypsinization and washed with DMEM/F12 containing 10% serum and PBS sequentially. The washed cells were resuspended in 2% paraformaldehyde in PBS and incubated at room temperature for 30 min. The fixed cells were incubated with anti-TNFR2 mouse mAb and 3% BSA in PBS at 37°C for 2 h. After washing with PBS, cells were further incubated with FITC-labeled anti-mouse IgG antibody and 3% BSA in PBS at 37°C for 30 min. After washing with PBS, cells were resuspended in PBS and analyzed with a flow cytometer.
RESULTS
NGF Induces TNF-α in SH-SY5Y Human Neuroblastoma Cells
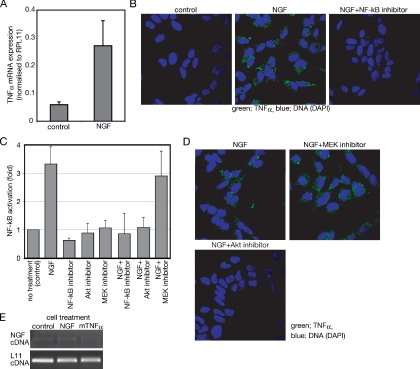
SH-SY5Y is a human neuroblastoma cell line that cannot respond to NGF by neural differentiation (Lavenius et al., 1995; Poluha et al., 1995). Figure 1A shows that SH-SY5Y cells treated with NGF contain ∼5 times more TNF-α mRNA than untreated cells. Immunofluorescence assay shows the increase of TNF-α protein after treatment with NGF (Figure 1B). Because NF-κB is a transcription factor that controls TNF-α expression (Collart et al., 1990), effects of an NF-κB inhibitor, 6-amino-4-(4-phenoxyphenylamino) quinazoline, were examined. NGF-dependent expression of TNF-α is blocked by the NF-κB inhibitor (Figure 1B, left). These results indicate that NGF induces de novo synthesis of TNF-α and that NF-κB is implicated in the TNF-α synthesis.
Figure 1.
NGF induces TNF-α expression in SH-SY5Y neuroblastoma cells. (A) Semiquantitative analysis of TNF-α mRNA prepared from cells incubated with or without (control) NGF for 24 h. Expression level of TNF-α is shown relative to RPL11 expression. The data represent the mean of three experiments. Error bars indicate SE; p < 0.001. (B) Effect of the NF-κB inhibitor on the TNF-α expression induced by NGF. Cells cultured on chambered glass slides were treated for 24 h with or without (control) NGF in the presence or absence of the NF-κB inhibitors. After fixation, TNF-α was detected with anti-TNF-α antibody and visualized by FITC-labeled secondary antibody. (C) Measurement of NF-κB activity after NGF treatment. After transfection of pNFkB SEAP vector, SH-SY5Y cells were treated as described in Materials and Methods section. Activation of NF-κB was measured as enzymatic activity of SEAP in each culture media. The NF-κB activities are shown relative to the NF-κB activity of untreated samples. The data represent the mean of three experiments. Error bars indicate SE. (D) Effect of the inhibitors on the TNF-α expression induced by NGF. Cells were treated as described in B. Either the Akt inhibitor or the MEK inhibitor was used instead of the NF-κB inhibitor. (E) PCR of NGF and L11 ribosomal protein cDNA prepared from cells treated with the indicated reagents for 24 h.
A reporter gene assay indicates that SH-SY5Y cells show around a threefold increase of NF-κB activity after NGF treatment (Figure 1C). To examine which downstream signaling pathways of NGF were responsible for the NF-κB activation, major downstream kinases of NGF signaling, Akt and MEK, were inhibited by known specific inhibitors, 1-(l)-6-hydroxymethyl-chiro-inositol-2-(R)-2-O-methyl-3-O-octadecylcarbonate for Akt, and 1,4-diamino-2,3-dicyano-1, 4-bis[2-aminophenylthio]butadiene for MEK. The NF-κB activation by NGF is blocked by the Akt inhibitor but not by the MEK inhibitor (Figure 1C). Figure 1D shows that the Akt inhibitor, but not the MEK inhibitor, blocks NGF-induced TNF-α expression, consistent with the involvement of NF-κB in the NGF-induced TNF-α expression. Together, these results show that NGF activates TNF-α expression through Akt and NF-κB.
Because TNF-α is known to induce NGF expression in fibroblasts (Manni et al., 2003) and astrocytes (Gadient et al., 1990; Kuno et al., 2006), expression of NGF was examined after treatment with either NGF or TNF-α. Expression of NGF in SH-SY5Y cells is faint and neither NGF nor TNF-α enhances the NGF expression in SH-SY5Y cells (Figure 1E). This indicates that neither endogenous TNF-α nor exogenously added TNF-α can induce NGF expression in SH-SY5Y cells. This difference in response could be due to the difference in cell type.
TNF-α Blocks NGF-induced Differentiation of SH-SY5Y Neuroblastoma Cells
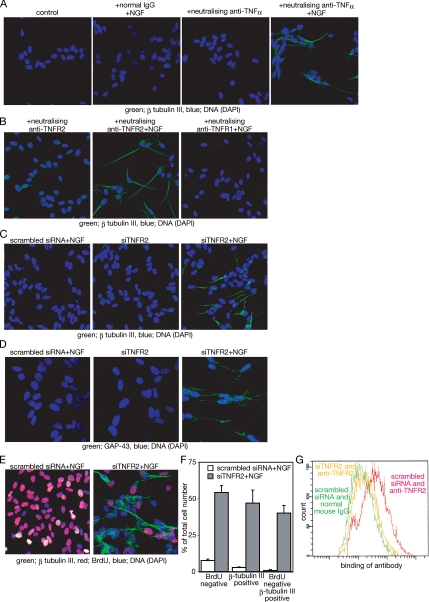
To examine the effect of TNF-α, TNF-α signaling was inhibited with a neutralizing antibody against TNF-α. Consistent with a previous report (Lavenius et al., 1995), neither NGF with normal mouse IgG, nor the antibody against TNF-α alone induces neuron-specific tubulin, β-tubulin III, which is a marker of neural differentiation. Outgrowth of neurite-like structures and expression of β-tubulin III are observed when the cells are simultaneously treated with NGF and the neutralizing antibody against TNF-α (Figure 2A).
Figure 2.
Inhibition of TNF-α signaling through TNFR2 sensitizes SH-SY5Y neuroblastoma cells to NGF-dependent differentiation. (A) Expression of β-tubulin III after treatment with the indicated reagents for 48 h. As a control, untreated cells are shown. (B) Expression of β-tubulin III in cells treated with neutralizing anti-TNFR2 or anti-TNFR1 in the presence or absence of NGF. Cells were incubated with the indicated reagents for 48 h, and then they were fixed. (C) Effect of siRNA against TNFR2 on the NGF-induced β-tubulin III expression. Twenty-four hours after siRNA transfection, cells were incubated with NGF for a further 48 h, and then they were fixed. (D) Expression of GAP-43 after treatment of NGF and transfection of TNFR2 siRNA. Cells were treated as described in C. Fixed cells were incubated with anti-GAP-43 antibody. (E) BrdU incorporation of SH-SY5Y cells after siRNA transfection and NGF treatment. Cells were treated as described in C. Forty-eight hours after NGF addition, cells were cultured with BrdU for another 24 h, and then they were fixed. Fixed cells were treated as described in Materials and Methods. (F) Quantitation of differentiated cells after treatment with NGF and siRNA against TNFR2. The number of BrdU-negative, β-tubulin III-positive and BrdU-negative but β-tubulin III-positive cells, respectively, was counted manually. The total number of cells was also counted using DAPI. The proportion of these cells is indicated as percentage of the total number of cells. More than 500 DAPI-positive cells were examined in each sample. The same experiment was performed using cells treated with neither NGF nor siRNA against TNFR2 as a control. Each experiment was repeated three times, and an average of these experiments is shown. Error bars indicate SE between experiments. p < 0.0001 (G) Binding of anti-TNFR2 antibody to SH-SY5Y cells. Cells transfected with siRNA against TNFR2 (yellow) or scrambled siRNA (red and green) were incubated for 48 h, and then they were harvested by trypsinization. Cells were incubated with either normal mouse IgG (green) or anti-TNFR2 mouse mAb (yellow and red). Bound antibodies were detected by FITC-labeled anti-mouse IgG antibody.
Similarly, neutralizing antibody against TNFR2, which can inhibit the binding of TNF-α to this receptor, also allows NGF to induce outgrowth of neurite-like structures and expression of β-tubulin III (Figure 2B). Because SH-SY5Y cells express both TNFR1 and TNFR2 (Shen et al., 1997), effects of a neutralizing antibody against TNFR1 were examined. The combination of NGF and a neutralizing antibody against TNFR1 fails to show either outgrowth of neurite-like structures or expression of β-tubulin III (Figure 2B). NGF-dependent neural differentiation is also observed when TNFR2 is knocked down with siRNA (Figure 2C). Knockdown of TNFR1, instead of TNFR2, fails to show these effects on SH-SY5Y cells treated with NGF, consistent with results shown in Figure 2B (data not shown). In addition to β-tubulin III, TNFR2 knockdown cells respond to NGF by expressing GAP-43, another indicator of neural differentiation (Figure 2D). However, expression of glial fibrillary acidic protein, a marker of glial cells, is not detected in the TNFR2-knockdown cells even after NGF treatment (data not shown). These changes are coincident with cell cycle arrest. As shown in Figure 2, E and F, >50% of cells failed to incorporate BrdU and ∼50% of cells express β-tubulin III after cotreatment with NGF and siRNA against TNFR2. Although some cells expressing β-tubulin III still incorporated BrdU, the majority failed to incorporate BrdU after cotreatment with NGF and siRNA against TNFR2. Neither cells treated with NGF and scrambled siRNA nor cells treated with siRNA against TNFR2 alone show a significant increase of BrdU-negative cells (data not shown). The efficiency of siRNA against TNFR2 was confirmed by the binding of a specific antibody against TNFR2 to SH-SY5Y cells. In siRNA-transfected cells, binding of the antibody is decreased, and it is similar to the binding of negative control cells (Figure 2G), indicating that the siRNA is efficient in inhibiting TNFR2 signaling. These results indicate that NGF can induce neural differentiation of SH-SY5Y. However, this ability is blocked by TNFR2 signaling.
NGF Induces TNF-α in Both BE(2)-C Neuroblastoma Cells and HCN-1A–nontransformed Neural Cells, but Not in PC-12 Pheochromocytoma Cells
TNF-α induction by NGF is not limited to SH-SY5Y cells. We examined three types of neural cells: BE(2)-C neuroblastoma cells, HCN-1A–nontransformed neural cells and PC-12 pheochromocytoma cells. Neuroblastoma is a malignancy derived from the sympathetic nervous system (Hoehner et al., 1996), and amplification of the MYCN gene is correlated with poor prognosis of this cancer (Grimmer and Weiss, 2006). The MYCN gene is not amplified in SH-SY5Y cells (Smith et al., 2004), and SH-SY5Y cells show weak tumorigenicity in athymic mice (Walton et al., 2004). The BE(2)-C cell line is a subclone of another human neuroblastoma cell line SK-N-BE(2) that contains 85 times more NMYC gene than normal cells (Smith et al., 2004). BE(2)-C also shows much higher tumorigenicity in athymic mice than SH-SY5Y (Walton et al., 2004). HCN-1A is a nontransformed human neural cell line and it shows some neural properties consistent with pluripotent, immature cells of neural origin (Zhang et al., 1994). On cotreatment with NGF, dbcAMP, and IBMX, but not NGF alone, HCN-1A can be induced toward terminal differentiation and to show outgrowth of neurites (Zhang et al., 1994). PC-12 is derived from a rat pheochromocytoma, and it can differentiate into neuron-like cells in response to NGF alone (Greene and Tischler, 1976), in contrast to other cell lines used in this article, SH-SY5Y, BE(2)-C, and HCN-1A.
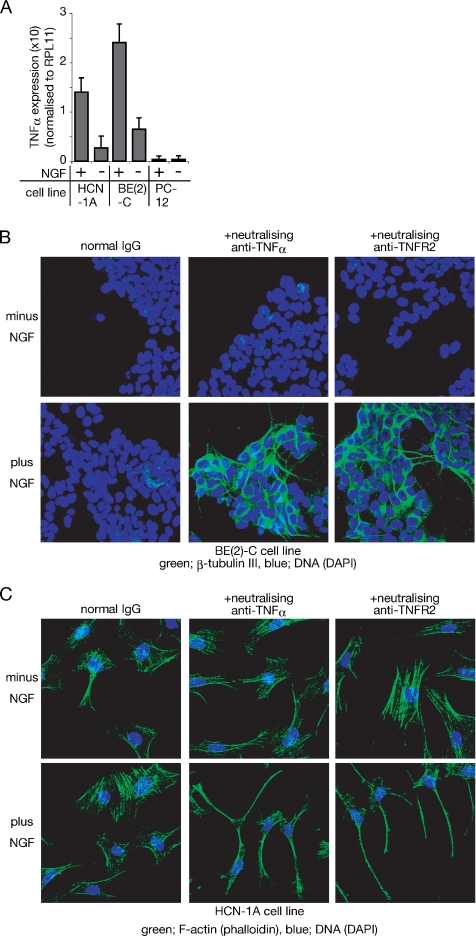
As shown in Figure 3A, both BE(2)-C and HCN-1A show an increase of TNF-α expression after treatment with NGF. However, NGF fails to induce TNF-α in PC-12 cells (Figure 3A). Thus, TNF-α induction by NGF seems to be coincident with inability to differentiate in response to NGF alone.
Figure 3.
HCN-1A and BE(2)-C, but not PC-12 cells, express TNF-α in response to NGF, thus inhibiting neural differentiation of these cells. (A) Semiquantitative analysis of TNF-α cDNA prepared from cells incubated with or without NGF for 24 h. Expression level of TNF-α is shown relative to RPL11 expression. The data represent the mean of three experiments. Error bars indicate SE; p < 0.001 in both BE(2)-C and HCN-1A and p > 0.05 in PC-12. (B) β-Tubulin III expression of BE(2)-C cells after incubation with the indicated reagents for 48 h. BE(2)-C cells cultured on collagen-coated dishes were incubated with the indicated reagents for 48 h, and expression of β-tubulin III was detected by anti-β-tubulin III antibody. Binding of the antibody was visualized with FITC-labeled secondary antibody. (C) Phalloidin staining of HCN-1A cells after incubation with the indicated reagents for 48 h. Cells were treated with or without NGF in the presence of either rabbit normal IgG (control), anti-TNF-α, or anti-TNFR2 for 48 h. Fixed cells were incubated with Alexa Fluor 488-labeled phalloidin.
Both a Highly Malignant Neuroblastoma Cell Line, BE(2)-C, and a Nontransformed Neural Cell Line, HCN-1A, Also Differentiate in Response to Cotreatment with NGF and Inhibition of TNF-α Signaling
When BE(2)-C cells are treated with NGF and the neutralizing antibody against either TNF-α or TNFR2, they express β-tubulin III, and they show morphological changes (Figure 3B), as seen in SH-SY5Y (Figure 2). Thus, both the SH-SY5Y cell line and the BE(2)-C cell line increase the endogenous level of TNF-α after treatment with NGF, and they differentiate into neuron-like cells in response to NGF when TNF-α signaling is inhibited through TNFR2.
Figure 3C shows the morphological change of HCN-1A after the cotreatment of NGF and neutralizing antibodies against either TNF-α or TNFR2. After cotreatment for 48 h, cells show outgrowth of neurite-like structures (Figure 3C), without either dbcAMP or IBMX.
These results indicate that NGF induces TNF-α in the neuroblastoma cell lines SH-SY5Y and BE(2)-C and in the nontransformed neural cell line HCN-1A. In addition, the results indicate that TNF-α signaling through TNF2 blocks NGF-dependent differentiation of both types of cell.
Akt Is Required for TNF-α–mediated Inhibition of Neural Differentiation, and for Induction of TNF-α
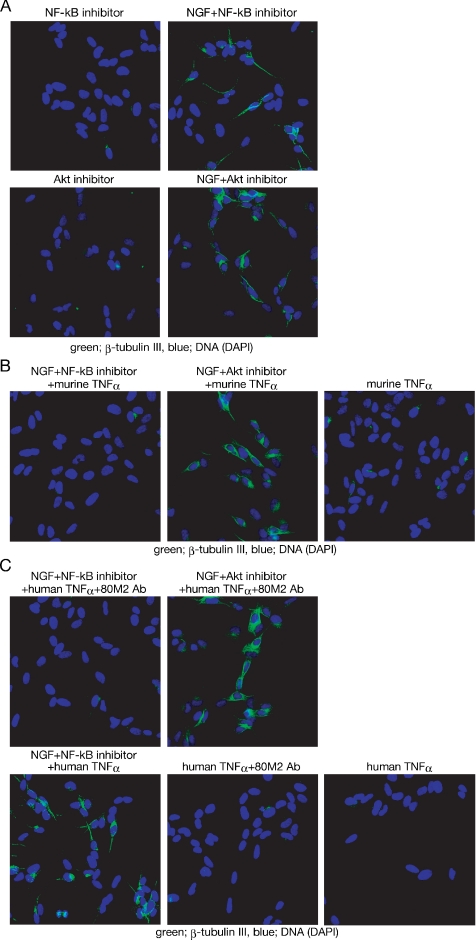
Because TNF-α–TNFR2 signaling inhibits neural differentiation of neuroblastoma cells (Figures 2 and 3), blockage of TNF-α synthesis should allow NGF to induce their neural differentiation. The Akt inhibitor and the NF-κB inhibitor, which can inhibit TNF-α induction by NGF (Figure 1, B and D), were examined to see whether they promote NGF-dependent differentiation of SH-SY5Y cells. As shown in Figure 4A, NGF can induce β-tubulin III expression when either the Akt inhibitor or the NF-κB inhibitor is provided, without direct inhibition of either TNF-α or TNFR2.
Figure 4.
Akt, but not NF-κB, is required for the inhibition of NGF-induced differentiation of SH-SY5Y cells. (A) Effect of inhibitors on NGF-induced β-tubulin III expression. SH-SY5Y cells were cultured with the indicated reagents for 48 h. Inhibitors were added to the culture medium 1 h before NGF addition. (B and C) Effect of exogenously added TNF-α on the expression of β-tubulin III. SH-SY5Y cells were incubated with murine TNF-α (B) or with human TNF-α, with or without the 80M2 mAb (C), in the presence of the Akt inhibitor or the NF-κB inhibitor. After 1-h incubation, NGF was added directly to the culture medium. After 48-h incubation with NGF, cells were fixed.
Next, we assessed the effects of exogenously applied TNF-α on the differentiation induced by the combination of NGF with either the Akt inhibitor or the NF-κB inhibitor. To activate TNFR2 efficiently, soluble murine TNF-α (Figure 4B) or a combination of soluble human TNF-α and the mAb against human TNFR2, 80M2 (Figure 4C), was used. Although soluble murine TNF-α can activate both TNFR1 and TNFR2, soluble human TNF-α efficiently activates TNFR1, but not TNFR2 (Grell et al., 1995). The mAb 80M2 can modify the agonist specificity of TNFR2, allowing soluble human TNF-α to activate TNFR2 (Grell et al., 1995). Soluble murine TNF-α inhibits the β-tubulin III expression induced by the combination of NGF with the NF-κB inhibitor (Figure 4B). However, soluble murine TNF-α has no effect on the β-tubulin III expression induced by the combination of NGF with the Akt inhibitor (Figure 4B). The combination of soluble human TNF-α and the 80M2 mAb, instead of murine-soluble TNF-α, also inhibits the β-tubulin III expression induced by the combination of NGF with the NF-κB inhibitor, but not the expression induced by the combination of NGF with the Akt inhibitor (Figure 4C). Soluble human TNF-α alone fails to inhibit the neural differentiation induced by the combination of NGF with the NF-κB inhibitor, indicating that TNFR2 signaling is required for inhibition of the differentiation. Together, these results suggest that, whereas NF-κB is required only for induction of TNF-α, Akt is required not only for induction of TNF-α but also for the inhibition of neural differentiation by TNF-α–TNFR2 signaling.
NGF Shows Sustained Activation of Erk1/2 and Decreased Activation of Akt When TNF-α Signaling Is Inhibited
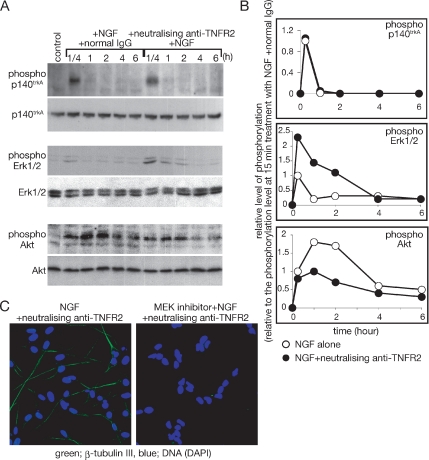
Figure 4 shows that Akt can act downstream of TNFR2. Therefore, we have examined the contribution of TNFR2 signaling to Akt activation after NGF treatment. Figure 5, A and B, show the time courses of Akt activation and Erk1/2 activation, after NGF treatment. Although NGF-induced autophosphorylation of an NGF-specific receptor p140trkA is not changed significantly with the neutralizing antibody against TNFR2, enhanced and prolonged activation of Erk1/2 by NGF is observed in the presence of the neutralizing antibody (Figure 5, A and B). Conversely, the NGF-induced phosphorylation of Akt is markedly decreased by the neutralizing antibody against TNFR2 (Figure 5, A and B). Thus, upon inhibition of TNFR2, NGF causes decreased activation of Erk1/2 and increased activation of Akt.
Figure 5.
Effect of TNFR2 signaling on NGF signaling. (A) Estimation of NGF-induced phosphorylation of p140trkA, Erk1/2, and Akt with immunoblotting. SH-SY5Y cells were cultured with NGF alone or NGF and the neutralizing antibody against TNFR2. At the indicated time points after addition of NGF, cells were harvested, and whole cell extracts were prepared. The levels of phosphorylated proteins and total proteins were detected by the indicated antibodies. (B) The intensity of the signals of phosphorylated proteins indicated in A was measured with the application ImageJ (version 1.32j, National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/). Phosphorylation levels of each time point were shown relative to the phosphorylation level at 15-min treatment with NGF alone. Experiments were repeated three times, and the average phosphorylation level at each time point was plotted. Open circles indicate results from cells treated with NGF alone. Closed circles indicate results from cells treated with the combination of NGF, and the neutralizing antibody against TNFR2. (C) Effect of the MEK inhibitor on the induction of neural differentiation by the combination of NGF and neutralizing anti-TNFR2. SH-SY5Y cells were cultured on a chambered slide glass and incubated with the indicated reagents for 48 h. After fixation of cells, β-tubulin III expression was detected by anti-β-tubulin III antibody.
The MEK–Erk1/2 Pathway Is Required for NGF-dependent Neural Differentiation
Because the MEK–Erk1/2 pathway is stimulated after treatment with the combination of NGF and neutralizing TNFR2 antibody, we have assessed the effects of the MEK inhibitors on neural differentiation in response to the NGF and antibody combination. As shown in Figure 5C, the MEK inhibitor inhibits the β-tubulin III expression induced by the combination of NGF and the neutralizing TNFR2 antibody, indicating that the differentiation is dependent upon the activation of Erk1/2.
Induction of TNF-α by NGF Contributes to NGF-dependent Survival of Rat DRG Neurons
In addition to the induction of differentiation, NGF has another important biological function, promotion of neural cell survival. Under physiological conditions, expression of NGF receptors starts at the late phase of neural differentiation, and NGF-dependent survival can be observed in differentiated neurons (Wyatt and Davies, 1993, 1995; v Holst et al., 1997). Because Akt is involved in the signaling for promotion of neural cell survival (Song et al., 2005), Figure 5, A and B, suggests that TNF-α signaling from TNFR2 could contribute to promotion of neural cell survival through Akt activation. Consistent with this possibility, signaling from TNFR2 can promote survival of several types of neurons (Renauld and Spengler, 2002; Sarkar and Sharma, 2002; Klassen et al., 2003).
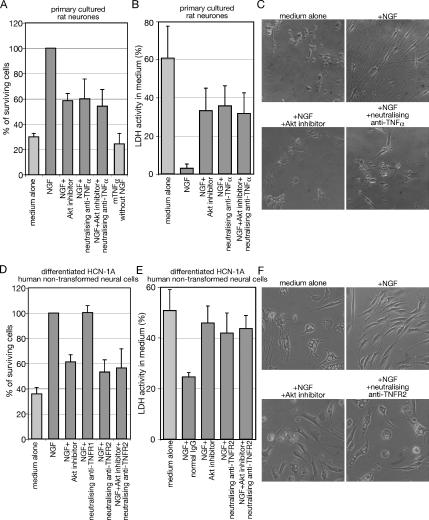
Neurons from postnatal day 5 rat DRG show NGF-dependent survival (Figure 6A). The survival is blocked partially by the Akt inhibitor (Figure 6A). A neutralizing antibody against TNF-α also reduces NGF-dependent survival of the neurons (p < 0.001). Cotreatment of the Akt inhibitor and the neutralizing antibody against TNFR2 did not show further increase of cell death (Figure 6A). These results suggest a contribution of endogenous TNF-α– to NGF-dependent survival. However, exogenously added TNF-α without NGF fails to support survival of neurons. This suggests an essential role for the cooperation between TNF-α and NGF signaling for survival of rat DRG neurons. Neither NGF without TNFR2 signaling nor TNF-α without NGF signaling is enough to promote neural cell survival efficiently.
Figure 6.
TNF-α signaling is essential for NGF-dependent survival. (A and B) Cell viability assay with WST-1. The primary cultured neurons dissociated from rat dorsal root ganglia were incubated with the indicated reagents for 48 h. (A) WST-1 reagent was added directly to the culture medium after the incubation, and cells were cultured for 4 h. (B) Fifty microliters of culture medium was taken and used for LDH assay as described in Materials and Methods. (C) Cell images of neurons after incubation with the indicated reagents are taken with phase-contrast microscopy. (D and E) Differentiated HCN-1A cells were cultured in serum-free DMEM containing the indicated reagent for 24 h. (D) WST-1 reagent was added directly to the culture medium after the incubation, and cells were cultured for 2 h. (E) Fifty microliters of culture medium was treated as mentioned in B. (F) Cell images of differentiated HCN-1A cells after incubation with the indicated reagent for 24 h are taken with phase-contrast microscopy. The data represent the mean of at least three experiments. Error bars indicate SE.
Mitochondrial function in live neurons is measured in Figure 6A to estimate survival of the cell population and these results are confirmed by LDH activity released from dead neurons into the culture media in Figure 6B. In the absence of NGF, LDH activity in the medium shows ∼70% of total LDH activity. Addition of NGF decreases the LDH activity to <10%, showing the survival-promoting activity of NGF. Consistent with Figure 6A, both the Akt inhibitor and the neutralizing antibody against TNF-α inhibit the survival promoting activity of NGF and cotreatment of these did not show further cell death. Figure 6C shows images of the DRG neurons after these treatments. In agreement with the cell death observed in Figure 6, A and B, cell debris is observed. Although some cells still survive after addition of either the Akt inhibitor or the neutralizing anti-TNF-α, the majority of the surviving cells lose neural cell shapes. These results indicate that TNF-α signaling contributes to NGF-dependent survival of rat DRG neurons.
TNFR2 Signaling Contributes to the NGF-dependent Survival of Differentiated, Nontransformed Human HCN-1A Cells
The receptor-specific neutralizing antibodies used in this study are against human TNF-α receptors, and they cannot block rat TNF-α receptors efficiently. To examine the effects of these antibodies on survival of differentiated neurons, we used HCN-1A–nontransformed human neural cells after induction of differentiation. The differentiated HCN-1A neural cells also show NGF-dependent survival when serum is withdrawn from the culture medium (Figure 6D). The Akt inhibitor partially suppresses the NGF-dependent survival (p < 0.001). The neutralizing antibody against TNFR2 also inhibits the NGF-dependent survival (p < 0.001) to a similar extent as the Akt inhibitor (p > 0.05). Cotreatment of the Akt inhibitor and the neutralizing antibody against TNFR2 did not show further decrease of cell survival (p > 0.05). The neutralizing antibody against TNFR1 fails to suppress the NGF-dependent survival (p > 0.05), unlike the antibody against TNFR2. The measurement of LDH activities in the media also shows the contribution of TNFR2 signaling to the NGF-dependent survival (Figure 6E). Figure 6F shows images of the differentiated HCN-1A cells after these treatments. In agreement with the cell death observed in Figure 6, D and E, the majority of cells show large vesicles when the cells were incubated without NGF. Addition of either the Akt inhibitor or the neutralizing anti-TNFR2 induces the vesicles in cells incubated with NGF. These results indicate that TNF-α signaling participates in NGF-dependent survival of both primary cultured rat neurons and differentiated HCN-1A–nontransformed human neural cells. Thus, induction of TNF-α by NGF has two different consequences. First, it inhibits differentiation of neuroblastoma cells or HCN-1A–nontransformed neural cells; and second, it promotes neuron survival in the presence of NGF.
DISCUSSION
Although both NGF and TNF-α have pivotal roles in neural cell fate decisions, their signaling pathways have been studied independently, and cross-talk between them has not been examined in neural cells. This study shows that NGF can induce TNF-α expression in both neuroblastoma cells and nontransformed neural cells. The signaling pathway involving Akt and NF-κB, but not MEK, mediates the NGF-dependent TNF-α expression (Figure 1). We show that TNF-α induced by NGF cooperates with NGF itself for full activation of Akt, but it suppresses Erk activation by NGF (Figure 5, A and B). Thus, Akt acts not only in the induction of TNF-α but also in downstream of TNF-α signaling. We examined the effect of cooperation between NGF and TNF-α on the two major targets of NGF, differentiation of neural cell lines and survival of differentiated neurons. TNF-α induced by NGF contributes to NGF-dependent neural cell survival through Akt activation (Figure 6), but it inhibits NGF-induced differentiation of neuroblastoma cells through Akt activation (Figure 4) and Erk suppression (Figure 5). Thus, TNF-α induction by NGF has a pivotal role in NGF-dependent Akt activation and this controls two cellular responses to NGF. Akt activation by the cooperation of TNF-α and NGF promotes neuron survival and inhibits neuroblastoma differentiation.
Other groups have show that a high expression level of p140trkA in neuroblastoma cells is correlated with good prognosis (Kogner et al., 1993) and that overexpression of p140trkA by transfection directs neuroblastoma cells to NGF-dependent differentiation through Erk1/2 activation (Matsushima and Bogenmann, 1993, 1994; Lavenius et al., 1995; Poluha et al., 1995), without investigating the effects of inhibiting TNFR2 signaling. Therefore, the insensitivity of neuroblastoma cells to NGF-dependent differentiation was explained by insufficient expression of an NGF-specific receptor p140trkA.
We also find that Erk1/2 activation by NGF, without inhibition of TNFR2 signaling, is weak and transient (Figure 5), and that it is not strong enough to induce differentiation of neuroblastoma cells (Figure 2A). However, this study indicates that the weak activation of Erk1/2 by NGF is not due to poor expression of p140trkA but that it is due to inhibition by TNFR2 signaling. When the TNFR2 signaling pathway is inhibited, NGF can activate Erk1/2 strongly enough to induce neural differentiation of neuroblastoma cells without enhanced expression of p140trkA (Figure 5). Thus, this study shows that overexpression of p140trkA is not necessary to induce NGF-dependent differentiation of neuroblastoma cells when TNFR2 signaling is inhibited. The overexpression of p140trkA seems to overcome the inhibition of Erk1/2 activation by the TNFR2 signaling pathway. A combination of NGF and inhibitors of TNF-α signaling might provide a new approach to the treatment of neuroblastomas.
Consistent with the inhibitory effects of the TNF-α-TNFR2 signaling pathway on Erk activation and consequently on NGF-dependent differentiation, NGF-dependent expression of TNF-α is not observed in the rat pheochromocytoma cell line PC-12 (Figure 3A). The PC-12 cells show NGF-dependent differentiation (Greene and Tischler, 1976) in an Erk-dependent manner (Qui and Green, 1992), without either inhibition of TNF-α signaling or overexpression of p140trkA. The different effects of NGF on these cells could be explained by a single difference in NGF signaling between neuroblastoma and PC-12 cells. Although this study shows that NF-κB mediates NGF-dependent expression of TNF-α (Figure 1B), Furuno and Nakanishi (2006) show that NF-κB is not activated in PC-12 cells in response to NGF. NF-κB is a transcription factor that controls TNF-α expression (Collart et al., 1990). Therefore, PC-12 cells do not express TNF-α in response to NGF (Figure 3A), but they differentiate, after treatment with NGF alone. In contrast, this study shows that NGF induces TNF-α expression in neuroblastoma cells; therefore, it can induce their differentiation only when TNF-α signaling is blocked.
Several groups have independently reported the synthesis of TNF-α in neural cells, including multipotential neural stem cells and differentiated hippocampal neurons (Barker et al., 2001; Sarkar and Sharma, 2002; Renauld and Spengler, 2002; Klassen et al., 2003). Endogenous TNF-α in NGF-dependent neurons is reported to contribute to induction of neural cell death through TNFR1 after withdrawal of NGF (Barker et al., 2001). However, TNF-α is expressed not only in dying neurons but also in surviving neurons (Barker et al., 2001) and the function of endogenous TNF-α in surviving neurons has not been clarified. Other groups have reported endogenous TNF-α in other types of neurons, but could not show significant death of neurons that express TNF-α (Renauld and Spengler, 2002; Sarkar and Sharma, 2002; Klassen et al., 2003). This study shows a mechanism in which endogenous TNF-α can promote neural cell survival, rather than cell killing, but by signaling through TNFR2. Therefore, endogenous TNF-α can have two opposite effects through two different signaling receptors, TNFR1 and TNFR2. It seems that endogenous TNF-α activates TNFR2 preferentially in the presence of NGF, but it activates TNFR1 in the absence of NGF. Although further experiments are required to clarify the mechanism controlling the receptor preference of TNF-α, one possibility is that NGF-induced TNF-α activates both TNFR1 and TNFR2 but cooperation of NGF signaling and TNFR2 signaling antagonizes TNFR1-induced proapoptotic signals. In agreement with this, exogenously added TNF-α fails to induce sufficient neural cell death of cultured neurons in the presence of NGF (Barker et al., 2001), and signaling from TNFR2 can antagonize proapoptotic signals from TNFR1 (Yang et al., 2002). Therefore, NGF-induced TNF-α seems to be bifunctional; NGF-induced TNF-α has a major role in the NGF-dependent survival of neurons, but once NGF is withdrawn, the TNF-α contributes to neuron killing.
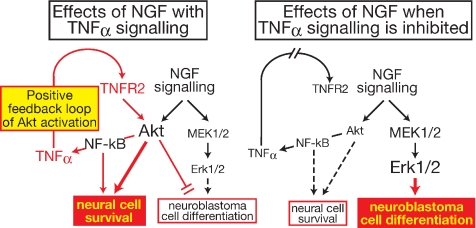
In summary, this study demonstrates that TNF-α signaling can modulate NGF signaling (Figure 7). NGF induces TNF-α through Akt and NF-κB. The TNF-α induced by NGF activates TNFR2, which causes further activation of Akt. Thus, TNFR2 signaling from TNF-α induced by NGF is a positive feedback activator of Akt. Cooperation of NGF signaling and TNF-α signaling is required for efficient survival of nontransformed neurons. Furthermore, Akt activation by this cooperation blocks neural differentiation of neuroblastoma cells, which provides a new explanation for insensitivity of neuroblastoma cells to NGF-dependent differentiation. Only when TNFR2 signaling is inhibited, NGF augments Erk1/2 activation. The increased activation of Erk1/2 is essential for differentiation of neuroblastoma cells. Thus, TNFR2 signaling lies downstream of NGF, and it has a crucial role in the determination of cell responses to NGF. This novel regulatory mechanism of NGF function could provide a platform for new therapeutic strategies for diseases related to neural cell survival and differentiation.
Figure 7.
Effect of endogenous TNF-α on the cell responses to NGF. Details are explained in Discussion.
ACKNOWLEDGMENTS
We thank Dr. Linda Ko Ferrigno for comments on this article. This work was supported by the Alzheimer's Research Trust, the Medical Research Council, and Cancer Research UK.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-06-0624) on December 19, 2007.
REFERENCES
- Barker V., Middleton G., Davey F., Davies A. M. TNFalpha contributes to the death of NGF-dependent neurons during development. Nat. Neurosci. 2001;4:1194–1198. doi: 10.1038/nn755. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Baeuerle P., Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol. Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. R., Brunet A., Greenberg M. E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Fagan A. M., Garber M., Barbacid M., Silos-Santiago I., Holtzman D. M. A role for TrkA during maturation of striatal and basal forebrain cholinergic neurons in vivo. J. Neurosci. 1997;17:7644–7654. doi: 10.1523/JNEUROSCI.17-20-07644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine V., Mohand-Said S., Hanoteau N., Fuchs C., Pfizenmaier K., Eisel U. Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J. Neurosci. 2002;22:RC216. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno T., Nakanishi M. Neurotrophic factors increase tumor necrosis factor-alpha-induced nuclear translocation of NF-kappaB in rat PC12 cells. Neurosci. Lett. 2006;392:240–244. doi: 10.1016/j.neulet.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Gadient R. A., Cron K. C., Otten U. Interleukin-1 beta and tumor necrosis factor-alpha synergistically stimulate nerve growth factor (NGF) release from cultured rat astrocytes. Neurosci. Lett. 1990;117:335–340. doi: 10.1016/0304-3940(90)90687-5. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell M., et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Grimmer M. R., Weiss W. A. Childhood tumors of the nervous system as disorders of normal development. Curr. Opin. Pediatr. 2006;18:634–638. doi: 10.1097/MOP.0b013e32801080fe. [DOI] [PubMed] [Google Scholar]
- Hoehner J. C., Gestblom C., Hedborg F., Sandstedt B., Olsen L., Pahlman S. A developmental model of neuroblastoma: differentiating stroma-poor tumors' progress along an extra-adrenal chromaffin lineage. Lab. Invest. 1996;75:659–675. [PubMed] [Google Scholar]
- Huang E. J., Reichardt L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. J., Reichardt L. F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Klassen H. J., Imfeld K. L., Kirov I. I., Tai L., Gage F. H., Young M. J., Berman M. A. Expression of cytokines by multipotent neural progenitor cells. Cytokine. 2003;22:101–106. doi: 10.1016/s1043-4666(03)00120-0. [DOI] [PubMed] [Google Scholar]
- Kogner P., Barbany G., Dominici C., Castello M. A., Raschella G., Persson H. Coexpression of messenger RNA for TRK protooncogene and low affinity nerve growth factor receptor in neuroblastoma with favorable prognosis. Cancer Res. 1993;53:2044–2050. [PubMed] [Google Scholar]
- Kuno R., Yoshida Y., Nitta A., Nabeshima T., Wang J., Sonobe Y., Kawanokuchi J., Takeuchi H., Mizuno T., Suzumura A. The role of TNF-alpha and its receptors in the production of NGF and GDNF by astrocytes. Brain Res. 2006;1116:12–18. doi: 10.1016/j.brainres.2006.07.120. [DOI] [PubMed] [Google Scholar]
- Lavenius E., Gestblom C., Johansson I., Nanberg E., Pahlman S. Transfection of TRK-A into human neuroblastoma cells restores their ability to differentiate in response to nerve growth factor. Cell Growth Differ. 1995;6:727–736. [PubMed] [Google Scholar]
- Manni L., Lundeberg T., Fiorito S., Bonini S., Vigneti E., Aloe L. Nerve growth factor release by human synovial fibroblasts prior to and following exposure to tumor necrosis factor-alpha, interleukin-1 beta and cholecystokinin-8, the possible role of NGF in the inflammatory response. Clin. Exp. Rheumatol. 2003;21:617–624. [PubMed] [Google Scholar]
- Marchetti L., Klein M., Schlett K., Pfizenmaier K., Eisel U. L. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J. Biol. Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Matsushima H., Bogenmann E. Expression of trkA cDNA in neuroblastomas mediates differentiation in vitro and in vivo. Mol. Cell Biol. 1993;13:7447–7456. doi: 10.1128/mcb.13.12.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima H., Bogenmann E. NGF induces terminal differentiation in trkA expressing neuroblastoma cells in vitro and in vivo. Prog. Clin. Biol. Res. 1994;385:177–183. [PubMed] [Google Scholar]
- Poluha W., Poluha D. K., Ross A. H. TrkA neurogenic receptor regulates differentiation of neuroblastoma cells. Oncogene. 1995;10:185–189. [PubMed] [Google Scholar]
- Qui M. S., Green S. H. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron. 1992;9:705–717. doi: 10.1016/0896-6273(92)90033-a. [DOI] [PubMed] [Google Scholar]
- Renauld A. E., Spengler R. N. Tumor necrosis factor expressed by primary hippocampal neurons and SH-SY5Y cells is regulated by alpha(2)-adrenergic receptor activation. J. Neurosci. Res. 2002;67:264–274. doi: 10.1002/jnr.10101. [DOI] [PubMed] [Google Scholar]
- Sarkar S. A., Sharma R. P. Expression of selected apoptosis related genes, MIF, IGIF and TNF alpha, during retinoic acid-induced neural differentiation in murine embryonic stem cells. Cell Struct. Funct. 2002;27:99–107. doi: 10.1247/csf.27.99. [DOI] [PubMed] [Google Scholar]
- Schober A., Minichiello L., Keller M., Huber K., Layer P. G., Roig-Lopez J. L., Garcia-Arraras J. E., Klein R., Unsicker K. Reduced acetylcholinesterase (AChE) activity in adrenal medulla and loss of sympathetic preganglionic neurons in TrkA-deficient, but not TrkB-deficient, mice. J. Neurosci. 1997;17:891–903. doi: 10.1523/JNEUROSCI.17-03-00891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Li R., Shiosaki K. Inhibition of p75 tumor necrosis factor receptor by antisense oligonucleotides increases hypoxic injury and beta-amyloid toxicity in human neuronal cell line. J. Biol. Chem. 1997;272:3550–3553. [PubMed] [Google Scholar]
- Smith A. G., Popov N., Imreh M., Axelson H., Henriksson M. Expression and DNA-binding activity of MYCN/Max and Mnt/Max during induced differentiation of human neuroblastoma cells. J. Cell Biochem. 2004;92:1282–1295. doi: 10.1002/jcb.20121. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Howe C. L., Mobley W. C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Song G., Ouyang G., Bao S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell Mol. Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- v Holst A., Lefcort F., Rohrer H. TrkA expression levels of sympathetic neurons correlate with NGF-dependent survival during development and after treatment with retinoic acid. Eur. J. Neurosci. 1997;9:2169–2177. doi: 10.1111/j.1460-9568.1997.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Walton J. D., Kattan D. R., Thomas S. K., Spengler B. A., Guo H. F., Biedler J. L., Cheung N. K., Ross R. A. Characteristics of stem cells from human neuroblastoma cell lines and in tumors. Neoplasia. 2004;6:838–845. doi: 10.1593/neo.04310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt S., Davies A. M. Regulation of expression of mRNAs encoding the nerve growth factor receptors p75 and trkA in developing sensory neurons. Development. 1993;119:635–648. doi: 10.1242/dev.119.3.635. [DOI] [PubMed] [Google Scholar]
- Wyatt S., Davies A. M. Regulation of nerve growth factor receptor gene expression in sympathetic neurons during development. J. Cell Biol. 1995;130:1435–1446. doi: 10.1083/jcb.130.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Lindholm K., Konishi Y., Li R., Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J. Neurosci. 2002;22:3025–3032. doi: 10.1523/JNEUROSCI.22-08-03025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Drzewiecki G. J., Hom J. T., May P. C., Hyslop P. A. Human cortical neuronal (HCN) cell lines: a model for amyloid beta neurotoxicity. Neurosci. Lett. 1994;177:162–164. doi: 10.1016/0304-3940(94)90892-3. [DOI] [PubMed] [Google Scholar]