Abstract
The small GTP-binding protein Arf6 regulates membrane remodeling at cell peripheries and plays crucial roles in higher orders of cellular functions including tumor invasion. Here we show that Fbx8, an F-box protein bearing the Sec7 domain, mediates ubiquitination of Arf6. This ubiquitination did not appear to be linked to immediate proteasomal degradation of Arf6, whereas Fbx8 knockdown caused hyperactivation of Arf6. Expression of Fbx8 protein was substantially lost in several breast tumor cell lines, in which Arf6 activity is pivotal for their invasion. Forced expression of Fbx8 in these cells suppressed their Arf6 activities and invasive activities, in which the F-box and Sec7 domains of Fbx8 are required. Together with the possible mechanism as to how Fbx8-mediated ubiquitination interferes with the functions of Arf6, we propose that Fbx8 provides a novel suppressive control of Arf6 activity through noncanonical ubiquitination. Our results indicate that dysfunction of Fbx8 expression may contribute to the invasiveness of some breast cancer cells.
INTRODUCTION
The Arf-family of small GTPases regulate membrane trafficking and remodeling (Donaldson, 2003; D'Souza-Schorey and Chavrier, 2006). There are six isoforms of Arf GTPases in mammals (Arf1-6), although Arf2 has been lost in humans. Arfs are classified into three classes by structural similarity: class I (Arf1-3), class II (Arf4 and 5), and class III (Arf6; Logsdon and Kahn, 2003). Class I and II Arfs primarily function at the Golgi and are involved in intracellular secretory processes (Volpicelli-Daley et al., 2005). On the other hand, Arf6 is the most divergent of the Arf isoforms, and primarily functions at cell peripheries by regulating endocytosis and recycling-back of plasma membrane components and several types of cell surface receptors (Donaldson, 2003). Several functions of Arf6 are closely linked to the functions of Rac, and Arf6 has been shown to play pivotal roles also in actin-cytoskeletal remodeling at the cell periphery (D'Souza-Schorey et al., 1997; Radhakrishna et al., 1999; Donaldson, 2003). Arf6 has moreover been shown to play crucial roles in higher order cellular functions, including Fcγ-receptor-mediated phagocytosis (Zhang et al., 1998), disassembly of E-cadherin–mediated epithelial cell–cell adhesions (Palacios et al., 2002), and tumor invasion (Sabe, 2003; Hashimoto et al., 2004a; Tague et al., 2004; D'Souza-Schorey, 2005).
Like other small GTPases, activities of Arf-family GTPases are primarily controlled by guanine nucleotide exchanging factors (GEFs) and GTPase-activating proteins (GAPs), through regulation of the guanine nucleotide binding states. On the other hand, ubiquitination has recently been shown to be involved in the cellular regulation of several small GTPases. Local levels of RhoA protein are regulated by Smurf-mediated polyubiquitination, which is coupled to proteasome-mediated degradation of RhoA (Wang et al., 2003). The active form of Rac1 has also been shown to undergo polyubiquitination, and this ubiquitination is also linked to its proteasomal degradation (Lynch et al., 2006). Ubiquitination of H-Ras also occurs, but primarily as the mono- and di-ubiquitinated forms (Jura et al., 2006). This ubiquitination does not lead to its immediate proteasomal degradation, but is closely related to the spatial sorting and signal transmission of intracellular H-Ras proteins. E3 components responsible for the ubiquitination of Rac1 and H-Ras have not been identified.
Members of F-box proteins act as subunits of the SCF (Skp1/Cul1/F-box) protein complexes of ubiquitin E3 ligases and primarily determine substrate specificity of ubiquitination through their direct interaction with substrates (Cardazo and Pagano, 2004). The SCF complexes include the common components of Cullin1 (Cul1) and Skp1, the latter of which directly binds to F-box proteins (Cardazo and Pagano, 2004). On the other hand, the Sec7 domain is known to generally encode the GEF domain for Arf-family GTPases (Jackson, 2003). The database of F-box proteins includes Fbx8, which contains an F-box domain and a putative Sec7 domain (Jin et al., 2004). Fbx8 was originally identified as a Skp1-binding protein by yeast two hybrid screening (Cenciarelli et al., 1999; Winston et al., 1999), and this binding has been subsequently confirmed biochemically (Winston et al., 1999). However, the property to bind to Skp1 is not enough to specify F-box proteins to be components of the E3 ligase (Cardazo and Pagano, 2004), and functions of Fbx8 remain unknown.
Here we show that Fbx8 is a component of the SCF complex and mediates ubiquitination of Arf6, whereas other Arf isoforms are not notably ubiquitinated by virtue of Fbx8. We found that Fbx8-mediated Arf6 ubiquitination is noncanonical, in other words not linked to its immediate proteasomal degradation, whereas Fbx8 itself is polyubiquitinated and appears to be immediately degraded proteasomally. Fbx8 mRNA is expressed widely in many tissues and cells (Ilyin et al., 2000), similar to Arf6 mRNA (Lebeda et al., 2003). However, expression of Fbx8 mRNA has been reported to be lost in some tumor cells, such as colon cancer cells and lung cancer cells, whereas it is expressed in normal colon and lung cells (Ilyin et al., 2000). We found that Fbx8 protein expression is lost in highly invasive breast cancer cells, including MDA-MB-231, whereas these cells express Fbx8 mRNA. On the other hand, normal mammary epithelial cells express both Fbx8 mRNA and protein. Arf6 is overexpressed in these highly invasive breast cancer cells, and Arf6 activity is essential for their invasive activities (Hashimoto et al., 2004a). We demonstrate that forced expression of Fbx8 in invasive breast cancer cells suppresses their Arf6 activities and also Matrigel invasion activities. Our results indicate that Fbx8 mediates the noncanonical ubiquitination of Arf6 and hence provides a novel mechanism of the suppressive control of Arf6 activity and function.
MATERIALS AND METHODS
Cell Culture and Reagents
Cos-7 cells were cultured in DMEM supplemented with 10% fetal calf serum (Hyclone, Logan, UT). NMuMG cells were cultured in DMEM supplemented with 10% fetal calf serum and 10 μg/ml insulin (Sigma, St. Louis, MO). Human breast cancer cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA). MDA-MB-231 cells were cultured in a 1:1 mixture of DMEM and RPMI 1640, supplemented with 10% fetal calf serum and 5% NuSerum (BD Bioscience, Franklin Lakes, NJ), and other cells were cultured according to the ATCC instructions. A primary preparation of human normal mammary epithelial cells (Cambrex, East Rutherford, NJ) was cultured according to the manufacturer's instructions. MG132 and Lactacystin were purchased from Calbiochem (San Diego, CA); and Epoxomycin, ZLLH [Z-Leu-Leu-H(aldehyde)] and E64 were from Peptide Institute (Osaka, Japan). Alexa Fluor647-conjugated phalloidin was from Invitrogen (Carlsbad, CA). Protein G- and glutathione-Sepharose beads were from GE Healthcare (Buckinghamshire, United Kingdom).
Antibodies
The rabbit polyclonal anti-Fbx8 antibody was raised against recombinant GST-Fbx8 protein, containing aa 1-67 of Fbx8 fused in frame to the carboxyl-terminus of glutathione S-transferase (GST), and affinity-purified using this GST-Fbx8 protein. The rabbit polyclonal antibody against human Arf6 was raised against aa 131-144 of human Arf6 and was affinity-purified using this peptide. For injecting into rabbits, this peptide was conjugated with keyhole limpet hemocyanin, via the addition of a cysteine to the amino terminus of the peptide. Antibodies against the following peptides and proteins were purchased from commercial sources: Arf6 (Santa Cruz Biotechnology, Santa Cruz, CA), ubiquitin (Covance, Berkley, CA), hemagglutinin (HA), Myc (clone 9E10) and enhanced green fluorescent protein (EGFP; Babco, Richmond, CA), FLAG and β-actin (Sigma), GST (Upstate Biotechnology, Lake Placid, NY, for immunoblotting, and Abcam, Cambridge, UK, for immunofluorescence), Crk (BD Biosciences, San Jose, CA), EGFR (Abcam, Cambridge, UK), and Arf1 (Abcam). Affinity-purified Cy2- or horseradish-conjugated donkey anti-rabbit IgG antibodies and affinity-purified Cy3- or horseradish-conjugated donkey anti-mouse IgG antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA).
cDNAs
Mouse Arf isoform cDNAs, each fused in frame to the amino-terminus of FLAG-tag, were ligated into HindIII-XbaI sites of pcDNA3. Site-directed mutations were introduced using PCR. PCR-amplified full-length mouse Fbx8 cDNA, its ΔSec7 mutant, in which the carboxy-terminal 133-320 aa were deleted, and its ΔFbox mutant, in which the amino-terminal 1-113 aa were deleted, were each cloned into BamHI-NotI sites of pEBG (encoding GST) to be fused in frame to the carboxy-terminus of GST. Human Cul1 cDNA, fused in frame to the carboxy-terminus of Myc-tag, was ligated into Asp718I-NotI sites of pcDNA3.1. Human Skp1 cDNA, fused in frame to the carboxy-terminus of FLAG-tag, was ligated into NheI-XhoI sites of pcDNA3.1. pMT123, encoding HA-tagged ubiquitin, was a gift from M. Treier. PCR-amplified cDNA fragments of full-length Fbx8, the Sec7 domain of Fbx8 (aa 114-320), GGA3 (aa 1-226), the Sec7 domain of human ARNO (aa 72-252), and the zinc-finger domain of human AMAP1 (aa 439-562) were each cloned into BamHI-EcoRI sites of pGEX. EFA6 cDNA, encompassing the entire coding region, was subcloned into pET3a vector, as described previously (Chavrier and Franco, 2001).
cDNA Transfection
For the coprecipitation assay, 2.0 × 105 Cos-7 cells were transfected with 1.0 μg pcDNA3.1/Myc-Cul1 and 1.5 μg pEBG/Fbx8, together with or without 1.0 μg pcDNA3.1/FLAG-Skp1, using Fugene6 (Roche, Indianapolis, IN) according to the manufacturer's instructions. When necessary, empty pEBG vector (0.5 μg) or sonicated calf thymus DNA was used instead of pEBG/Fbx8.
For the ubiquitination assays, 2.0 × 105 Cos-7 cells were transfected with 0.5 μg pcDNA/Arf-FLAG, 0.5 μg pMT123 (encoding HA-ubiquitin), and 1.5 μg of pEBG/Fbx8, pEBG/Fbx8ΔFbox, or pEBG/Fbx8ΔSec7, using Fugene6 according to the manufacturer's instructions. Cells were incubated for 24 h before analyses unless otherwise indicated. When necessary, empty pEBG vector (0.5 μg) or sonicated calf thymus DNA was used instead of pEBG/Fbx8 or pMT123.
For the Matrigel invasion assays, 5.0 × 105 MDA-MB-231 cells were transfected with 6.0 μg of pEBG/Fbx8, pEBG/Fbx8ΔSec7, or pEBG/Fbx8ΔFbox; or 2.0 μg of pEBG plus 4.0 μg of pcDNA3 using TransIT-LT1 (Mirus, Madison, WI) according to the manufacturer's instructions. Lipofectamine LTX (Invitrogen) was used for transfection of Hs578T and MDA-MB-435s cells. Two micrograms of pEGFP-C1 were simultaneously transfected to identify transfection-positive cells. To examine the effects of coexpression of GST-Fbx8 with Arf6-FLAG or Arf6 3/7/174R-FLAG, cells were first stably transfected with pcDNA/Arf6-FLAG or pcDNAArf6 3/7/174R-FLAG, together with pBabe puro (Morgenstern and Land, 1990), and selected for 1 wk using puromycin (1 μg/ml). These cells were then further transiently transfected with pEBG/Fbx8 and pEGFP-C1 before analysis.
Small Interfering RNA
Duplex oligonucleotides for mouse Fbx8 silencing, 5′-AACUGACCUUUGCUUGGCUUCUU-3′ and 5′-GAAGCCAAGCAAAGGUCAGUUUU-3′, were chemically synthesized and purified by Sigma-PROLIGO (Boulder, CO). The small interfering RNA (siRNA) duplex for Arf6 silencing has been described previously (Hashimoto et al., 2004a). An siRNA duplex with an irrelevant sequence (siCONTROL, RISC-free siRNA1; Dharmacon, Lafayette, CO) was used as a control. NMuMG cells were transfected with 20 nM oligonucleotide duplexes using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions and incubated for 24 h before analysis, unless otherwise described.
Immunoblotting
Cells were lysed with RIPA buffer, as described previously (Hashimoto et al., 2004a), supplemented with 200 μM of iodoacetamide and 800 μM of N-ethylmaleimide (Sigma), unless otherwise indicated. For in vitro protein-binding analyses, cells were lysed with 1% NP-40 buffer (Hashimoto et al., 2004a) supplemented with 200 μM of iodoacetamide and 800 μM of N-ethylmaleimide. For detection of endogenous ubiquitination of Arf6, cells were lysed with 1% SDS, boiled for 10 min, then reconstituted into RIPA buffer, and subjected to immunoprecipitation using a polyclonal anti-Arf6 antibody. GST-fusion proteins were precipitated using glutathione-Sepharose beads. Primary antibodies were precipitated using protein G-Sepharose beads. Subcellular fractionation was performed using a detergent-free hypotonic buffer (Mazaki et al., 2001), supplemented with 200 μM of iodoacetamide and 800 μM of N-ethylmaleimide. SDS-PAGE, immunoblotting analysis, and protein detection by enzyme-linked chemiluminescence were performed as described previously (Hashimoto et al., 2004a). Prestained protein molecular size markers (Nacalai tesque, Kyoto, Japan) were used. Each experiment was performed at least three times, and representative figures are shown.
In Vitro GEF Assay
The GEF assay was performed as previously described (Chavrier and Franco, 2001), in which 1 μM recombinant Arf6 protein was incubated with 300 nM GST-Fbx8, 60 nM GST-Fbx8 Sec7, or 200 nM EFA6 in a reaction buffer (50 mM HEPES-NaOH, pH 7.5, 1 mM MgCl2, 100 mM KCl, 1 mM dithiothreitol, and 1.5 mg/ml azolectin vesicles) in the presence of 10 μM [35S]GTP-γ-S (∼1250 Ci/mmol; NEN-Perkin Elmer-Cetus, Wellesley, MA); and radioactivities associated with Arf6 were measured by trapping them with nitrocellulose membranes (Schleicher & Schuell, Keene, NH).
Immunofluorescent Microscopy
Cells were fixed in 4% paraformaldehyde in phosphate-buffered saline, as described previously (Mazaki et al., 2001), and then permeabilized by 0.2% saponin in phosphate-buffered saline and labeled with mouse monoclonal anti-Arf6 antibody, coupled with Cy3-conjugated anti-mouse IgG, and rabbit polyclonal anti-GST antibody, coupled with Cy2-conjugated anti-rabbit IgG. Fluorescence microscopy was performed using a confocal laser-scanning microscope (LSM 510, Carl Zeiss, Thornwood, NY) and the associated software.
Arf Activities
Activities of cellular Arf GTPases were measured by pulling down their GTP-bound forms using GST-GGA (Golgi-localizing, γ-adaptin ear homology domain, Arf-binding protein) from each cell lysate (300 μg) and immunoblotting precipitated Arf GTPases with their antibodies, as previously described (Luton et al., 2004). Each experiment was performed at least three times, and representative figures are shown.
RT-PCR
Extraction of total RNA and subsequent cDNA synthesis were performed as described previously (Hashimoto et al., 2004a). PCR amplification was performed as follows: 95°C for 9 min followed by 35 cycles of 95°C for 30 s, 60°C for 1 min, and 72°C for 1 min. Primers for Fbx8 cDNA were, 5′-TATGTCCAAGGGTATCCTAGA-3′ and 5′-TTATGCAGCCACATGGCCAATAAG-3′. Primers for β-actin cDNA (Takara Shuzo, Kyoto, Japan) were used as a control.
Matrigel Invasion Assay
Matrigel invasion assay was performed using Biocoat Matrigel chambers (BD Bioscience), as described previously (Hashimoto et al., 2004a). Briefly, 1 × 105 cells were seeded on the upper wells of 24-well chambers in the absence of serum, in which the lower wells were filled with conditioned medium of NIH 3T3 cells cultured for 24 h in the absence of serum. After incubation for 6 h, cells migrated out onto the lower surface of the membrane through Matrigel and were fixed in 4% paraformaldehyde, and EGFP-positive cells (hence positive for the cDNA transfection) were then counted by detecting their autofluorescence, using an LSM 510 laser scanning microscope. Percent cell invasion was calculated by dividing the number of transfection-positive cells transmigrated into the lower wells by the number of transfection-positive cells initially applied onto the upper wells.
RESULTS
Fbx8 Is a Component of the SCF Complex and Mediates Ubiquitination of Arf6
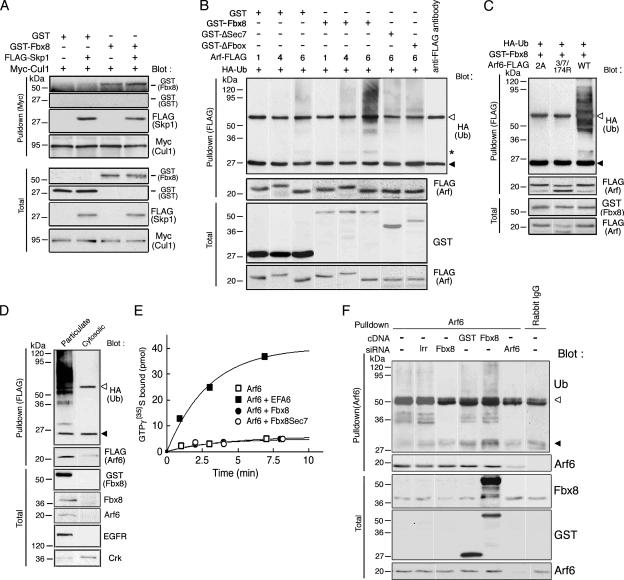
We first examined whether Fbx8 makes a complex with Cul1, through its binding to Skp1. We expressed GST-tagged Fbx8 together with FLAG-tagged Skp1 and Myc-tagged Cul1 in Cos-7 cells and found that Myc-Cul1 is coprecipitated with GST-Fbx8 in the presence of FLAG-Skp1 (Figure 1A). Because Cos-7 cells express Skp1 endogenously, a notable amount of GST-Fbx8, but not GST alone, was coprecipitated with Myc-Cul1 even in the absence of FLAG-Skp1 (Figure 1A). Fbx8 possesses the Sec7 domain and may interact with Arf-family GTPases. We then examined whether Fbx8 mediates ubiquitination of Arf-family GTPases. For this, we used Arf1, Arf4, and Arf6 as representatives of each class of Arf isoforms. Cos-7 cells were transfected with plasmids encoding FLAG-tagged Arf isoforms and HA-tagged ubiquitin, with or without GST-Fbx8. Arf-FLAG proteins were then immunoprecipitated with an anti-FLAG antibody and analyzed for their ubiquitination. Dense ubiquitination of Arf6-FLAG occurred in the presence of GST-Fbx8, which was observed as slow-migrating smear bands of immunoprecipitated Arf6-FLAG reactive with an anti-HA antibody (Figure 1B). Mutants of Fbx8 with a deletion in the Sec7 domain (ΔSec7) or the F-box domain (ΔFbox) did not induce ubiquitination of Arf6-FLAG (Figure 1B). A faint ∼30-kDa band was also detected as a ubiquitinated product of Arf6-FLAG (Figure 1B, indicated by an asterisk), which likely represents the intermediate, monoubiquitinated form of Arf6-FLAG. On the other hand, Arf1-FLAG and Arf4-FLAG did not undergo such Fbx8-mediated dense ubiquitination, whereas very weak ubiquitination of Arf4-FLAG was observed in the presence of GST-Fbx8 (Figure 1B). We also confirmed that the smear bands of the immunoprecipitated Arf6-FLAG, reactive with an anti-HA antibody, do not represent simple contamination of HA-ubiquitin in these immunoprecipitants: we boiled the anti-FLAG immunoprecipitants in 1% SDS, and then reprecipitated them with an anti-FLAG antibody after be reconstituting into RIPA buffer (Supplementary Figure S1A). Moreover, we found that the Fbx8 Sec7 domain directly interacts with Arf6 in vitro (Supplementary Figure S1B).
Figure 1.
Ubiquitination of Arf6 by Fbx8. (A) Fbx8 makes a complex with Cul1. Cos-7 cell lysates (300 μg), expressing GST-Fbx8, FLAG-Skp1, Myc-Cul1 or GST, as indicated, were subjected to immunoprecipitation with an anti-Myc antibody and analyzed for the coimmunoprecipitation of FLAG-Skp1 and GST-Fbx8 by immunoblotting, as indicated. (B–D) Arf6 ubiquitination in reconstituted systems. Cos-7 cells were transfected with FLAG-Arf isoforms (Arf1, 4, or 6) and HA-ubiquitin together with GST-Fbx8, GST-ΔSec7, GST-ΔFbox, or GST, as indicated (B); or with wild type (WT), or the 2A or 3/7/174R mutants of Arf6-FLAG, together with HA-ubiquitin and GST-Fbx8, as indicated (C). Cos-7 cells, expressing Arf6-FLAG, GST-Fbx8, and HA-ubiquitin, were subjected to fractionation into particulate and cytosolic fractions (D). Ubiquitination of FLAG-Arfs was assessed by their immunoprecipitation from each cell lysate (300 μg) with an anti-FLAG antibody and blotting with an anti-HA antibody. In B, another control included an anti-FLAG antibody per se, without cell lysate (anti-FLAG antibody). In D, blots by an anti-EGFR and an anti-Crk antibody are included as controls for the fractionation. (E) Lack of GEF activity of Fbx8 against Arf6. GEF activities of recombinant GST-Fbx8 (●) and GST-Fbx8 Sec7 (○) were assessed in vitro using recombinant Arf6 as a substrate in the presence of [35S]GTP-γ-S, and the amount of radioactivity bound to Arf6 after incubation for the indicated times were measured. Controls include incubation with buffer alone (□), or with EFA6 (■). (F) Endogenous ubiquitination of Arf6 by Fbx8. Endogenous Arf6 was immunoprecipitated from once boiled Cos-7 cell lysates (2 mg) using a rabbit polyclonal anti-Arf6 antibody and then subjected to immunoblot analysis using a mouse anti-ubiquitin antibody or a mouse anti-Arf6 antibody, as indicated. Rabbit preimmune serum (Rabbit IgG) was also used as a control. Cos-7 cells, transfected with GST cDNA, GST-Fbx8 cDNA, Fbx8 siRNA duplex, Arf6 siRNA duplex, or control irrelevant RNA duplex (Irr), were also used as controls, as indicated. Blots of total cell lysates (20 μg, Total) by antibodies as indicated are also shown in A–D and F. ◁, IgG heavy chain; ◀, IgG light chain in B–D and F. Asterisk in B indicates mono-ubiquitinated Arf6-FLAG.
Arf6 is myristoylated at the N-terminus to bind to cellular membranes, a binding that is necessary for its function (D'Souza-Schorey and Stahl, 1995). To assess whether the mature, myristoylated form of Arf6 is ubiquitinated by Fbx8, we made a myristoylation-negative mutant of Arf6-FLAG, in which Gly2 was changed to Ala (2A) and found that this mutant is not ubiquitinated by GST-Fbx8 (Figure 1C). We also found that GST-Fbx8, as well as endogenous Fbx8, predominantly exists in the particulate fraction (Figure 1D). Consistently, cellular fractionation revealed that ubiquitination of Arf6-FLAG occurs predominantly in the particulate fraction, but not in the cytosolic fraction (Figure 1D).
Ubiquitination generally occurs at lysines (Hershko and Ciechanover, 1992). Arf1 and Arf6 share high sequence similarity, and there are three lysines, Lys3, 7 and 174, unique to Arf6. Because Fbx8 does not recognize Arf1 as a ubiquitination substrate, we made an Arf6 mutant in which these three lysines were simultaneously changed to arginines (3/7/174R). This mutant of Arf6-FLAG always appeared as a doublet for unknown reasons, which could be detected both the anti-Arf6 and the anti-FLAG antibody immunoblot. This mutant of Arf6-FLAG did not undergo Fbx8-mediated ubiquitination (Figure 1C). These results further support the specificity of Fbx8 toward Arf6 and suggest that these three lysines of Arf6 are candidate sites for Fbx8-mediated ubiquitination (also see below). It is difficult to clearly distinguish whether high-molecular-weight smears bands of ubiquitination represent polyubiquitination of the target protein or its mono-ubiquitination at multiple sites (Haglund et al., 2003). Given that these three lysines are possible sites of Arf6 ubiquitination and that the smear bands of Arf6 ubiquitination are very high in molecular weight, larger than 70–90 kDa in appearance, it is likely that Arf6 is mostly polyubiquitinated.
Sec7 domains generally exhibit GEF activities for Arf GTPases. We next examined whether Fbx8 acts as a GEF for Arf6. Biochemical measurements showed no appreciable GEF activity of Fbx8, as well as its Sec7 domain alone toward Arf6, under conditions in which EFA6 exhibited robust GEF activity toward Arf6 (Chavrier and Franco, 2001; Figure 1E).
Cos-7 cells express Arf6 and Fbx8 endogenously (see Figure 1D). We then sought to obtain evidence supporting that endogenous Arf6 is ubiquitinated by endogenous Fbx8. We found that immunoprecipitation of ubiquitinated Arf6 is very difficult by using commercially available anti-Arf6 antibodies (data not shown). Comparison of the primary amino acid sequences among Arf isoforms revealed that amino acids (aa) 131-144 of human Arf6 is most divergent from the other human Arf isoforms. We therefore generated a rabbit polyclonal antibody against a synthetic peptide of this aa 131-144 sequence, which was affinity-purified before use, and confirmed the specificity of this polyclonal antibody against Arf6, but not other Arf isoforms (Supplementary Figure S2). We then found that this polyclonal antibody can precipitate endogenous Arf6, which was once boiled in 1% SDS and reconstituted into RIPA buffer. Using this polyclonal antibody, we immunoprecipitated endogenous Arf6 from Cos-7 cell lysates, which were once boiled and reconstituted into RIPA buffer. Reblotting of these anti-Arf6 immunoprecipitants by a mouse anti-ubiquitin antibody revealed high-molecular-weight smear bands (Figure 1F). Smear bands of Arf6 ubiqutination with high molecular weights (>70–90 kDa in appearance) are most likely to represent polyubiquitination. We also observed ubiquitination bands at about 35–40 kDa, although it is unknown whether they represent monoubiquitination of Arf6 at multiple sites or intermediates of Arf6 polyubiquitination (Figure 1F). All of these bands, reactive with an anti-ubiquitin antibody, almost disappeared when cells were treated with Fbx8 siRNA, whereas they were increased when cells were transfected with GST-Fbx8 (Figure 1F). We also confirmed that such smear bands of anti-Arf6 immunoprecipitants substantially disappeared when cells were treated with Arf6 siRNA (Figure 1F). These results and the results described above collectively indicate that Fbx8 mediates the ubiquitination of Arf6.
Limited Colocalization of Fbx8 with Arf6 at Cell Peripheries
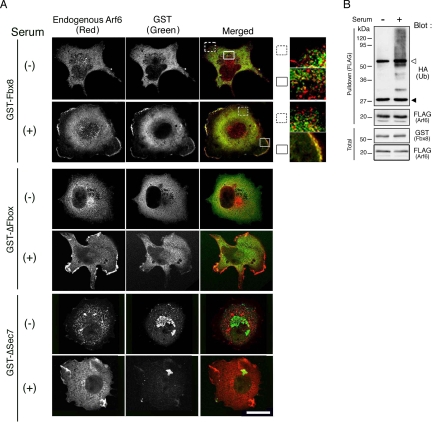
To determine which populations of Arf6 proteins are potential targets for Fbx8, we next examined the subcellular colocalization of Fbx8 with Arf6. For this, we again used Cos-7 cells expressing GST-Fbx8, because endogenous expression of Fbx8 in Cos-7 cells was too low to be detected clearly by immunolabeling. The inactive form of Arf6, as seen in serum-starved cells, is mostly localized to the cytoplasm (Peters et al., 1995; D'Souza-Schorey et al., 1995; Radhakrishna et al., 1996, 1997; Hashimoto et al., 2004b). We found that GST-Fbx8 is also predominantly localized to the cytoplasm in serum-starved cells (Figure 2A). However, GST-Fbx8 and Arf6 did not colocalize well with each other in the cytoplasm in these serum-starved cells, although their very minor colocalization in the cytoplasm may occur (Figure 2A). Consistently, Fbx8-mediated ubiquitination of Arf6 did not occur efficiently in these serum-starved cells, compared with that in cells cultured in the presence of serum (Figure 2B). This inefficiency of Arf6 ubiquitination is unlikely to be due to a general inefficiency of protein ubiquitination in these serum-starved cells, because it has been shown that protein ubiquitination can occur in such serum-starved cells (de Melker et al., 2004). We also confirmed that EGF-induced ubiquitination of EGF receptors occurs efficiently in these serum-starved cells (data not shown).
Figure 2.
Colocalization of Fbx8 with Arf6 at cell peripheries. (A) Cos-7 cells were transfected with GST-Fbx8, GST-ΔSec7, or GST-ΔFbox. Twenty-four hours after transfection, cells were either starved for serum or continued to be cultured in the presence of serum for another 20 h, as indicated. Cells were then fixed and labeled with a mouse anti-Arf6 antibody and a rabbit anti-GST antibody coupled with Cy3-conjugated anti-mouse IgG and Cy2-conjugated anti-rabbit IgG polyclonal antibodies, respectively. Merged images are shown as an overlay of Arf6 (red) and GST-Fbx8 proteins (green). The boxed regions are shown in higher magnification to the right. Scale bar, 20 μm. More than 20 cells were observed in each of three independent experiments, and representative images are shown. (B) Cos-7 cells, transfected with GST-Fbx8, Arf6-FLAG, and HA-ubiquitin, were either serum-starved or cultured with serum, as in A; ubiquitination of Arf6-FLAG was examined as in Figure 1B. Blots of 20 μg of total cell lysates are also shown (Total). ◁, IgG heavy chain; ◀, IgG light chain.
A significant fraction of Arf6 is activated and recruited to the plasma membrane, when cells are cultured in the presence of serum (Peters et al., 1995; D'Souza-Schorey et al., 1995; Radhakrishna et al., 1996, 1997). We found that a significant fraction of GST-Fbx8 is also recruited to the plasma membrane in cells cultured in the presence of serum and is well colocalized with Arf6 at the cell peripheries (Figure 2A). Deletion of the Sec7 domain and the F-box domain of Fbx8 caused the loss of the plasma membrane localization of Fbx8 (Figure 2A). However, it should be noted that not all Arf6 proteins recruited to the cell peripheries are colocalized with GST-Fbx8. It should also be noted that the majority of GST-Fbx8, as well as the majority of Arf6, still remained in the cytoplasm even in these cells cultured in the presence of serum, and they were not well colocalized with each other in the cytoplasm (Figure 2A). Together with the above observations, these results collectively suggest that only a small fraction of cellular Arf6 proteins can be targeted by Fbx8. These results also imply that targeting of Arf6 by Fbx8 occurs primarily after both Arf6 and Fbx8 are recruited to the plasma membrane, whereas not all Arf6 proteins recruited to the plasma membrane are targeted by Fbx8.
Noncanonical Nature of the Fbx8-mediated Ubiquitination of Arf6
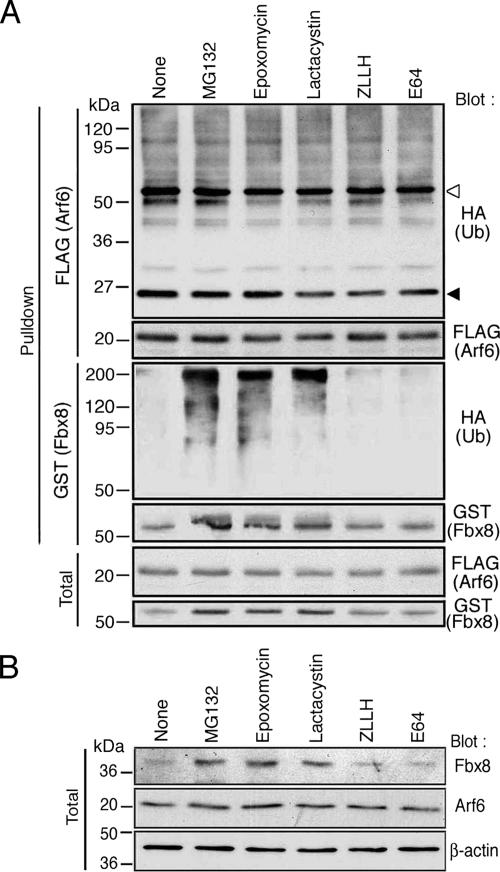
Protein ubiquitination was originally identified as a signal for proteasome-mediated rapid degradation (Hershko and Ciechanover, 1992). Recently, a number of reports have described noncanonical types of protein ubiquitination, which are not immediately coupled to proteasome-mediated degradation (Sun and Chen, 2004). We then investigated the nature of Fbx8-mediated Arf6 ubiquitination, by using proteasome inhibitors. Because ubiquitination of endogenous Arf6 by endogenous Fbx8 can only be detected very faintly, we again used a reconstitution system using Cos-7 cells. Cos-7 cells expressing Arf6-FLAG, GST-Fbx8, and HA-ubiquitin were treated with MG132, Epoxomycin, Lactacystin, and an inactive derivative of MG132, ZLLH. We also used a calpain inhibitor, E64, as another control. None of these compounds notably increased the amounts of ubiquitinated Arf6-FLAG (Figure 3A). Amounts of the nonubiquitinated form of Arf6-FLAG were also not notably changed by these compounds (Figure 3A). We also examined the effects of these inhibitors in cDNA-untransfected Cos-7 cells and found that the amounts of endogenous Arf6 in untransfected Cos-7 cells are also not notably changed by these treatments (Figure 3B). These results suggest that Fbx8-mediated ubiquitination of Arf6 may not be immediately linked to proteasomal degradation of Arf6.
Figure 3.
Effects of proteasomal inhibitors on Arf6 and Fbx8. (A) Cos-7 cells, expressing Arf6-FLAG, GST-Fbx8, and HA-ubiquitin were treated with MG132 (20 μM), Epoxomycin (2 μM), Lactacystin (27 μM), ZLLH (20 μM) and E64 (280 μM) for 5 h before lysis. The control included treatment with only DMSO, used as a solvent (None). Ubiquitination of Arf6-FLAG and GST-Fbx8 was assessed by their precipitation (Pulldown) from cell lysates (300 μg) using an anti-FLAG antibody and glutathione-beads, respectively, coupled with immunoblotting with an anti-HA antibody. Amounts of nonubiquitinated Arf6-FLAG and GST-Fbx8 are shown by an anti-FLAG and an anti-GST immunoblot, respectively. Total, 20 μg of cell lysates. ◁, IgG heavy chain; ◀, IgG light chain. (B) cDNA-untransfected cells were treated with chemical compounds as in A, and amounts of endogenous Fbx8 and Arf6 proteins were analyzed by an anti-Fbx8 and an anti-Arf6 immunoblot, respectively, using total cell lysates (20 μg). A β-actin immunoblot is included as a control.
Constitutive Ubiquitination and Proteasomal Degradation of Fbx8
In the same sets of cells as described above, we found that all three proteasome inhibitors, but not ZLLH or E64, cause the clear appearance of ubiquitinated forms of GST-Fbx8, which are detected as slow-migrating smear bands reactive with an anti-HA antibody (some were stacked at the top of the gel), whereas such ubiquitinated forms of GST-Fbx8 are otherwise almost undetectable (Figure 3A). Amounts of the nonubiquitinated form of GST-Fbx8 (63-kDa band) were also significantly increased by these proteasome inhibitors, but not by ZLLH or E64 (Figure 3A). GST protein itself did not undergo ubiquitination or stabilization under these conditions (data not shown). Moreover, amounts of endogenous, nonubiquitinated form of Fbx8 in untransfected Cos-7 cells were also significantly increased by these proteasome inhibitors, but not by other control compounds (Figure 3B). These results suggested that Fbx8 may be constitutively ubiquitinated and proteasomally degraded in Cos-7 cells.
Fbx8 Acts to Suppress Arf6 Activity
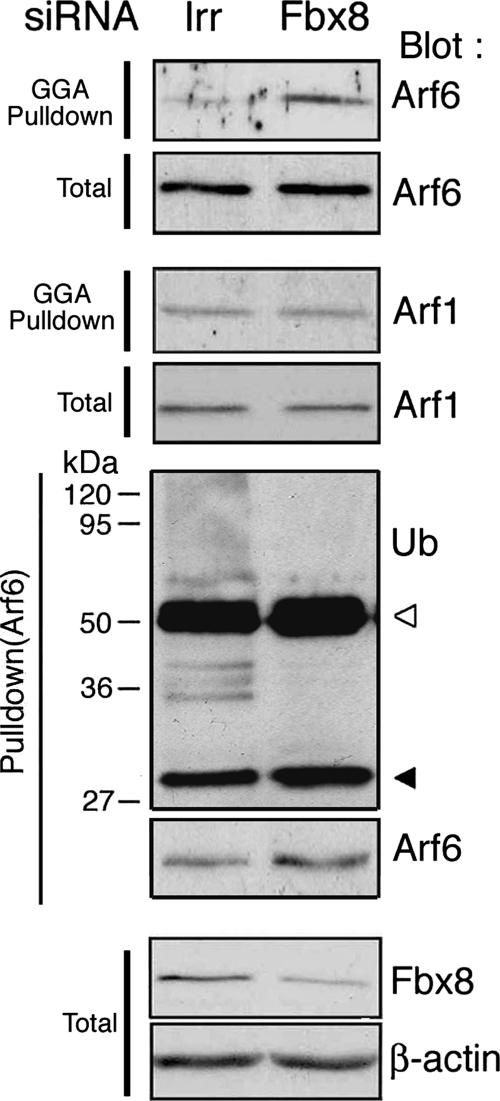
Given that Fbx8-induced Arf6 ubiquitination is noncanonical and not immediately linked to proteasomal degradation of Arf6, we then investigated the possible roles of this ubiquitination. For this, we examined the effects of Fbx8 knockdown on the activity of Arf6. Cos-7 cells express a low level of Arf6 protein (Supplementary Figure S3), and we found that it is difficult to detect clearly the possible changes of Arf6 activity in Cos-7 cells by biochemical methods, such as the GGA pulldown method (data not shown). On the other hand, NMuMG cells, a mouse normal mammary epithelial cell line, expressed a several fold higher amount of Arf6 protein endogenously than that in Cos-7 cells (Supplementary Figure S3). NMuMG cells also express Fbx8 protein (see Figure 4). We found that activities of endogenous Arf6 in NMuMG cells are significantly increased upon Fbx8 siRNA-treatment (Figure 4). Activity of endogenous Arf1, used as a control, was not notably changed by Fbx8 siRNA (Figure 4). Therefore, Fbx8 appears to suppress the activities of Arf6. However, it should be noted that protein levels of the endogenous, nonubiquitinated form of Arf6 are not notably increased (nor decreased) by Fbx8 siRNA-treatment (Figure 4). This is consistent with the above notion that only a small fraction of Arf6 may be targeted by Fbx8. In a separate experiment, we confirmed that endogenous Arf6 ubiquitination also occurs in NMuMG cells, a ubiquitination that disappears when endogenous Fbx8 is knocked down by Fbx8 siRNA-treatment, as in the case of Cos-7 cells (Figure 4). Similar to Cos-7 cells, the protein level of endogenous Fbx8, but not Arf6, was also increased in NMuMG cells when these cells were treated with proteasomal inhibitors (data not shown).
Figure 4.
Increased activity of endogenous Arf6 by Fbx8 siRNA treatment. NMuMG cells were transfected with an siRNA duplex specific for Fbx8 or with an irrelevant sequence (Irr). After culturing for 24 h, activities of Arf6 and Arf1 were measured by precipitating their active forms from cell lysates (300 μg) using GST-GGA (GGA pulldown) coupled with anti-Arf6 and anti-Arf1 immunoblots, respectively. Endogenous ubiquitination of Arf6 was also assessed by the same ubiquitination assay, using a rabbit polyclonal anti-Arf6 antibody as described in Figure 1F. Blots of 20 μg of total cell lysates are also shown (Total). Assays were performed three times, and a representative figure is shown.
Fbx8-mediated Arf6 Ubiquitination Interferes with the Interaction of Modified Arf6 with Its Activator and Effector
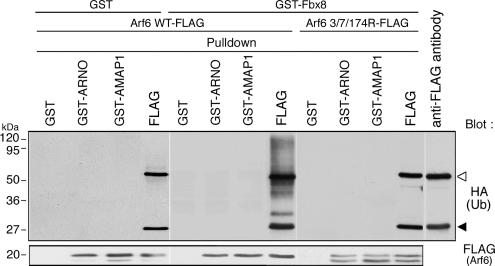
To probe the possible mechanism as to how Fbx8-mediated ubiquitination of Arf6 blocks its activation or function, we next examined whether this ubiquitination affects the interaction of Arf6 with its auxiliary proteins. Several GEF domains have been shown to bind stably to their cognate small GTPases in their GDP-bound forms in vitro, if GTP is not present (Hart et al., 1994; Hussain et al., 2001). ARNO (ADP ribosylation factor nucleotide binding site opener) is a GEF for Arf GTPases including Arf6 (Frank et al., 1998). The nonubiquitinated form of Arf6-FLAG was pulled down in vitro by the Sec7 domain of ARNO, fused to GST, in the absence of GTP, whereas such pull down was undetectable with the ubiquitinated forms of Arf6-FLAG (Figure 5). On the other hand, we have shown previously that AMAP1 (multidomain Arf GAP protein) binds stably to the GTP-bound form of Arf6 via its ArfGAP domain and acts as an effector for GTP-Arf6 in tumor invasion, but not as a GAP enzyme for Arf6 (Onodera et al., 2005). The ubiquitinated forms of Arf6-FLAG were not found to bind to the ArfGAP domain of AMAP1, fused to GST, under conditions in which a significant amount of nonubiquitinated Arf6-FLAG was coprecipitated with this domain (Figure 5). In these assays, GST-Fbx8, used for the induction of Arf6-FLAG ubiquitination, is still present in the cell lysates. Levels of the nonubiquitinated Arf6 protein, pulled down with ARNO or AMAP1 probes, were not notably changed either in the presence of GST-Fbx8 or GST alone (Figure 5). Therefore, GST-Fbx8 was unlikely to prevent binding of Arf6 to ARNO and AMAP1 probes. These results suggest that Fbx8-mediated ubiquitination of Arf6 has the potential to interfere with the interactions of Arf6 with its activators and effectors. Lys3, 7, and 174, which are putative ubiquitination sites of Arf6, are not located within the subdomains of Arf6 directly involved in its interaction with the Sec7 domain or the ArfGAP domain, whereas they are all located within a close proximity in the three-dimensional structure of Arf6 (Pasqualato et al., 2001). We also confirmed that ubiquitination deficient mutant Arf6, 3/73174R-FLAG, is able to bind to the Sec7 domain of ARNO and the ArfGAP domain of AMAP1 (Figure 5). These interferences by ubiquitination may be due to steric hindrance.
Figure 5.
Interference of the interactions of Arf6 with ARNO and AMAP1 by ubiquitination. Cos-7 cells were transfected with GST alone or GST-Fbx8, Arf6-FLAG, or Arf6 3/7/174R-FLAG and HA-ubiquitin. Twenty-four hours after transfection, cell lysates (300 μg) were prepared and incubated with 5 μg of GST-fusion proteins of the ARNO Sec7 domain (GST-ARNO) and the AMAP1 ArfGAP domain (GST-AMAP1), or GST alone, each coupled with glutathione-Sepharose beads, as indicated. Proteins pulled down with these glutathione beads were analyzed using an anti-HA antibody and an anti-FLAG antibody. As a control, immunoprecipitation of Arf6-FLAG with an anti-FLAG antibody from the same cell lysates (80 μg) was included (FLAG). Another control included an anti-FLAG antibody per se, without cell lysate (anti-FLAG antibody). ◁, IgG heavy chain; ◀, IgG light chain.
Loss of Fbx8 Expression in Invasive Breast Cancer Cells Contributes to Arf6 Activation and Invasiveness
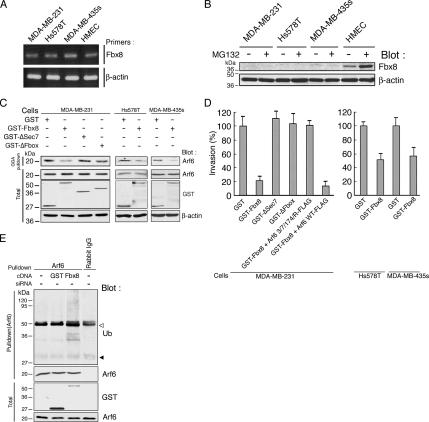
Fbx8 mRNA expression has been reported to be lost in several tumor cells, such as colorectal carcinoma SW480 cells and lung carcinoma A549 cells, whereas Fbx8 mRNA is expressed in normal colon and lung (Ilyin et al., 2000). We have shown previously that Arf6 is overexpressed in highly invasive breast cancer cells such as MDA-MB-231, Hs578T, and MDA-MB-435s; and its activity is essential for their invasive activities (Hashimoto et al., 2004a). We then examined the expression of Fbx8 in these breast cancer cells as well as in a primary culture of human normal mammary epithelial cells (HMECs). We found that all of these breast cancer cells expressed almost comparable levels of Fbx8 mRNA to that of HMECs (Figure 6A). However, although protein expression of Fbx8 was detectable in HMECs, Fbx8 protein was undetectable in these breast cancer cells (Figure 6B). Moreover, although Fbx8 protein level was increased in HMECs upon MG132 treatment, protein expression of Fbx8 was still undetectable in all of these breast cancer cells even after MG132 treatment (Figure 6B).
Figure 6.
Lack of Fbx8 protein expression in invasive breast cancer cells. (A) Expression of Fbx8 mRNA in breast cancer cells and HMECs. Expression of β-actin mRNA is included as a control. (B) Expression of Fbx8 protein. Cells were treated with or without 20 μM MG132 for 5 h before analysis. Twenty micrograms of each cell lysate were subjected to immunoblotting with an anti-Fbx8 antibody. Reblotting of filters with an anti-β-actin antibody is included as a control. (C and D) Forced expression of Fbx8 suppresses Arf6 activity and Matrigel invasion. Cells were transfected with GST-Fbx8 or GST alone, as indicated. MDA-MB-231 cells were further transfected with GST-ΔSec7, GST-ΔFbox, Arf6 3/7/174R-FLAG, or Arf6 3/7/174R-FLAG plus GST-Fbx8, as indicated. After culturing for 30 h, cells were lysed and subjected to GST-GGA pulldown (GGA pulldown) to measure endogenous Arf6 activities (C), or were subjected to the Matrigel invasion assay (D). In C, blots of 50 μg of total cell lysates are also shown (Total), in which an anti-β-actin antibody blot is included as a control. In D, pEGFP-C1 was simultaneously transfected to identify transfection-positive cells, and percentages of transfection-positive cells that transmigrated through Matrigel were calculated by normalizing the values obtained for the GST transfected cells as 100%. Data are from three independent experiments in each of which more than 80 cells were scored. Error bars, means ± SEM (E) Ubiquitination of endogenous Arf6 in MDA-MB-231 cells untransfected or transfected with GST-Fbx8 or GST alone was analyzed by immunoblotting as in Figure 1F. Rabbit preimmune serum (Rabbit IgG) was used as a control. ◁, IgG heavy chain; ◀, IgG light chain.
We finally examined whether forced expression of Fbx8 suppresses Arf6 activity of breast cancer cells and suppresses their invasive activities. We found that expression of GST-Fbx8 in all of these breast cancer cells suppresses their Arf6 activities and Matrigel invasion activities (Figure 6, C and D). Using MDA-MB-231 cells, we moreover confirmed that both the ΔSec7 and ΔFbox mutants of Fbx8 do not suppress their Arf6 activity and Matrigel invasion activity (Figure 6, C and D) and that Arf6 ubiquitination is evoked when cells are transfected with GST-Fbx8 (Figure 6E). We furthermore confirmed that coexpression of the 3/7/174R mutant of Arf6, together with GST-Fbx8, can restore the invasion activity of MDA-MB-231 cells, whereas wild-type Arf6 cannot (Figure 6D). Expression of each protein in these experiments, including EGFP used as a marker for the transfection, was assessed by immnoblotting (Supplementary Figure S4). These results indicate that Fbx8 has the potential to suppress Arf6 activity that is involved in invasion, most likely via its activity to ubiquitinate Arf6, and suggest that impairment of Fbx8 protein expression may contribute to the acquisition of invasive phenotypes of some breast cancer cells.
DISCUSSION
In this article, we provide new insight into the cellular regulation of Arf6. Our results indicate that Fbx8 mediates the ubiquitination of Arf6 and acts to suppress Arf6 activity and function. Several small GTPases have been shown to undergo ubiquitination, as described earlier. RhoA and Rac1 are polyubiquitinated, which is linked to their immediate proteasomal degradation, whereas H-Ras has been shown to be mono- and di-ubiquitinated and is not degraded immediately. We show that ubiquitination of Arf6 occurs predominantly as polymer forms and yet it does not appear to be immediately linked to its proteasomal degradation. Therefore, the mechanism by which ubiquitination regulates the activity of Arf6 appears to be novel and distinct from those known for other small GTPases.
Our results and results in the literature reveal that Fbx8 expression is impaired in some tumor cells. In the case of colon and lung cancer cells, mRNA expression of Fbx8 has been shown to be lost, as mentioned earlier. In the case of the invasive breast cancer cells that we examined, protein expression of Fbx8 is impaired, although these cells express Fbx8 mRNA. Therefore, the genetic alterations and mechanisms involved in the loss of Fbx8 expression appear to vary among different types of tumor cells. We demonstrated that forced expression of Fbx8 suppresses Arf6 activities and the invasive activities of these breast cancer cells. Therefore, loss of Fbx8 expression appears to contribute to the development of malignancy of some breast cancer cells. It will hence be interesting to analyze the expression of Fbx8, in its protein and mRNA, in clinical specimens of various human tumors.
Fbx8 appears to ubiquitinate only small, limited amounts of cellular Arf6 and is yet able to significantly suppress its activity. Our results show that only a limited fraction of cellular Arf6 colocalizes with Fbx8. Our results also show that knockdown of Fbx8 by siRNA does not notably increase the amounts of the nonubiquitinated form of Arf6, whereas activity of intracellular Arf6 is significantly increased in the Fbx8 siRNA-treated cells. Consistently, the amounts of nonubiquitinated Arf6 is not notably decreased even when Fbx8 is overexpressed and dense ubiquitination of Arf6 occurs, whereas Arf6 activity is substantially blocked under these conditions (see Figures 1B and 6C). Our analyses moreover show that Arf6 and Fbx8 become well colocalized with each other only after they are both recruited to the plasma membrane, whereas not all Arf6 proteins recruited to the plasma membrane are colocalized with Fbx8. The active form of Arf6 is predominantly localized to the plasma membrane, whereas the inactive form of Arf6 is predominantly localized to the cytoplasm, as mentioned earlier. On the other hand, Arf6 proteins recruited to the plasma membrane may not necessarily be in the active form. Therefore, it is conceivable that Fbx8 is able to target Arf6, when Fbx8 and Arf6 are both recruited to the plasma membrane and that such populations of Arf6, which are potential targets of Fbx8, may be in the active form or under processes to be activated. On the other hand, it is suggested that Arf6 can also be activated and function at cytoplasmic endomembranes (Brown et al., 2001). We have not yet analyzed in detail whether Fbx8 targets such a population of Arf6, which is activated and functions in the cytoplasm.
The important question that remains is, what are the biological contexts in which Fbx8-mediated suppressive control of Arf6 activity occurs. In general, substrate proteins are covalently modified, such as by phosphorylation and hydroxylation, to be recognized by their cognate E3 ligases (Glickman and Ciechanover 2002; Kaelin, 2005). It is also well known that several E3 ligases ubiquitinate immature or misfolded proteins for their degradation (Meusser et al., 2005). We showed that myristoylation of Arf6 is necessary for its ubiquitination by Fbx8, and it is unlikely that Fbx8 ubiquitinates premature or misfolded Arf6 molecules. On the other hand, Smurf1 recognizes the nucleotide-binding status of RhoA to ubiquitinate this small GTPase (Wang et al., 2003). We found, however, that both the GTP-bound form [Arf6 (Q67L)] and the GDP-bound form [Arf6 (T27N)] of Arf6 can be ubiquitinated almost equally when they are coexpressed with GST-Fbx8 in Cos-7 cells (Supplementary Figure S5). Recently, isomerization of proline residues of cyclinE molecules was suggested to provide a cue to be recognized by the E3 ligase SCFhcd4γ (van Drogon et al., 2006), whereas it is also known that cyclinE, which is freed from CDK2 and is not phosphorylated, is targeted by SCFSkp2 for ubiquitination (Nakayama et al., 2000). On the other hand, it has been reported that recognition of Smad1 by Smurf1 may be independent of the modification of Smad1 and simply mediated by their interaction through the WW domain of Smurf1 and the PPXY motif of Smad1 (Zhu et al., 1999). Ubiquitination of Aux/IAA, a transcription repressor, by virtue of an F-box protein, TIR, also appears to be independent of the modification of Aux/IAA (Dharmasiri et al., 2005). A cell-free system of protein ubiquitination in vitro is a powerful way to analyze the precise mechanism of ubiquitination. However, Arf6 is a membrane protein, and we showed that Fbx8-mediated Arf6 ubiquitination occurs in the membrane fraction. At this moment, it is well known that reconstitution in vitro of such protein ubiquitination that takes place at membranes is very difficult. Moreover, our results strongly suggest that Fbx8-mdiated Arf6 ubiquitination is noncanonical. Noncanonical ubiquitinations may utilize ubiquitin lysines other than Lys48 for their polymer formation. However, clear experimental demonstrations of such noncanonical utilization of ubiquitin lysines onto membrane proteins have so far been successful only for a limited number of cases (Geetha et al., 2005; Jura et al., 2006). In our case, we also tried in vain to determine which lysines of ubiquitin are utilized for Arf6 modification. The precise mechanisms by which Fbx8 recognizes Arf6 as its substrate for ubiquitination remain totally unknown and await to be determined.
We show that overexpression of Fbx8 increases the levels of Arf6 ubiquitination in Cos-7 cells. Therefore, cellular levels of Fbx8 protein may be one of the factors regulating Arf6 ubiquitination. On the other hand, our results indicate that Fbx8 is ubiquitinated and degraded proteasomally. Therefore, it will be important to clarify mechanism by which Fbx8 is ubiquitinated, together with the mechanism of regulation of this ubiquitination. Whether transcription of Fbx8 gene and levels of Fbx8 mRNA are regulatable and whether the expression of Fbx8 protein is regulated by mechanisms other than its ubiquitination and degradation, such as by translational control of Fbx8 mRNA, should also be investigated. Possible dysfunction of such mechanisms in human tumors also await to be analyzed.
In conclusion, we show that Arf6 has a novel mechanism for its suppressive control through Fbx8. Our results also imply that loss of Fbx8 expression may contribute to tumor malignancy. In the case of RhoA, its ubiquitination by Smurf1 has been implicated such as in cell polarity, motility, and epithelial-mesenchymal transdifferentiation (Wang et al., 2003) and also in the mesenchymal-type invasion of colon cancer cells (Sahai et al., 2007). Noncanonical ubiquitination of H-Ras has been shown to be involved in the endosomal association of modified H-Ras and hence in the regulation of Raf/MAP kinase signaling (Jura et al., 2006). Further understanding of the precise biological contexts and timings in which ubiquitination-mediated regulation of these small GTPases, including Arf6, occur besides their classical regulation by GEFs and GAPs. Also, precise mechanisms as to how these small GTPases become recognized by their cognate E3 ligases will unveil important biological processes in which these small GTPases are crucially involved, such as in cell growth and migration, tissue remodeling, and tumor progression. It may also be worth analyzing whether other Arf isoforms are ubiquitinated by some E3 ligases.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Manami Hiraishi, Tomoko Yoneda, and Yumiko Shibata for their technical assistance and Mayumi Iwahara and Mika Miyoshi for their secretarial work. We also thank Helena Akiko Popiel for her critical reading of the manuscript and Mathias Treier (EMBL, Germany) for pMT123. This work was supported in part by Grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations used:
- Arf6
ADP ribosylation factor 6
- GEF
guanine nucleotide exchange factor
- GAP
GTPase-activating protein
- SCF complex
Skp1/Cul1/F-box complex
- GGA
Golgi-localizing, γ-adaptin ear homology domain, Arf-binding protein
- ARNO
ADP ribosylation factor nucleotide binding site opener
- AMAP1
a multidomain Arf GAP protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-08-0763) on December 19, 2007.
REFERENCES
- Brown F. D., Rozelle A. L., Yin H. L., Balla T., Donaldson J. G. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardazo T., Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat. Rev. Mol. Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C., Chiaur D. S., Guardavaccaro D., Parks W., Vidal M., Pagano M. Identification of a family of human F-box proteins. Curr. Biol. 1999;9:1177–1179. doi: 10.1016/S0960-9822(00)80020-2. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Franco M. Expression, purification, and biochemical properties of EFA6, a Sec7 domain-containing guanine exchange factor for ADP-ribosylation factor6 (Arf6) Methods Enzymol. 2001;329:272–279. doi: 10.1016/s0076-6879(01)29088-0. [DOI] [PubMed] [Google Scholar]
- de Melker A., van der Horst G., Borst J. Ubiquitin ligase activity of c-Cbl guides the epidermal growth factor receptor into Clathrin-coated pits by two distinct modes of Eps15 recruitment. J. Biol. Chem. 2004;279:55465–55473. doi: 10.1074/jbc.M409765200. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N., Dharmasiri S., Estelle M. The F-box protein TIR is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G. Multiple roles for Arf6, Sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Stahl P. D. Myristoylation is required for the intracellular localization and endocytic function of ARF6. Exp. Cell Res. 1995;221:153–159. doi: 10.1006/excr.1995.1362. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Li G., Colombo M. L., Stahl P. D. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Boshans R. L., McDonough M., Stahl P. D., Aelst L. V. A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J. 1997;16:5445–5454. doi: 10.1093/emboj/16.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey C. Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol. 2005;15:19–26. doi: 10.1016/j.tcb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- Frank S., Upender S., Hanse S. H., Casanova J. E. ARNO is a guanine nucleotide exchange factor for ADP-ribosylation Factor 6. J. Biol. Chem. 1998;273:23–27. doi: 10.1074/jbc.273.1.23. [DOI] [PubMed] [Google Scholar]
- Geetha T., Jiang J., Wooten M. W. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol. Cell. 2005;20:301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Glickman M. H., Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Haglund K., Sigismund S., Polo S., Szymkiewicz I., Di Fiore P. P., Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- Hart M. J., Eva A., Zangrilli D., Aaronson S. A., Evans T., Cerione R. A., Zhenf Y. Cellular transformation and guanine nucleotide exchange activity are catalyzed by a common domain on the dbl oncogene product. J. Biol. Chem. 1994;269:62–65. [PubMed] [Google Scholar]
- Hashimoto S., Onodera Y., Hashimoto A., Tanaka M., Hamaguchi M., Yamada A., Sabe H. Requirement for Arf6 in breast cancer invasive activities. Proc. Natl. Acad. Sci. USA. 2004a;101:6647–6652. doi: 10.1073/pnas.0401753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Hashimoto A., Yamada A., Kojima C., Yamamoto H., Tsutsumi T., Higashi M., Mizoguchi A., Yagi R., Sabe H. A novel mode of action of an ArfGAP, AMAP2/PAG3/Papα, in Arf6 function. J. Biol. Chem. 2004b;279:37677–37684. doi: 10.1074/jbc.M404196200. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system for protein degradation. Annu. Rev. Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Hussain N. K., et al. Endocytic protein intersectin-1 regulates actin assembly via Cdc42 and N-WASP. Nat. Cell Biol. 2001;3:927–932. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- Ilyin G. P., Rialland M., Pigeon C., Guguen-Guillouzo C. cDNA cloning and expression of new members of the mammalian F-box protein family. Genomics. 2000;67:40–47. doi: 10.1006/geno.2000.6211. [DOI] [PubMed] [Google Scholar]
- Jackson C. L. The Sec7 family of Arf guanine nucleotide exchange factors. In: Kahn R. A., editor. Arf family GTPases. Dordrecht, the Netherlands: Kluwer Academic Publications; 2003. pp. 71–99. [Google Scholar]
- Jin J., Cardazo T., Lovering R. C., Eliedge S. J., Pagano M., Harper J. W. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–2580. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jura N., Scotto-Lavino E., Sobczyk A., Bar-Sagi D. Differential modification of Ras proteins by ubiquitination. Mol. Cell. 2006;21:679–687. doi: 10.1016/j.molcel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Kaelin W. G., Jr Proline hydroxylation and gene expression. Annu. Rev. Biochem. 2005;74:115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- Lebeda R. A., Johnson S. K., Stewart M. I., Haun R. S. Sequence, genomic organization, and expression of human ADP-ribosylation factor 6 (ARF6): a class III ARF. DNA Cell Biol. 2003;22:737–741. doi: 10.1089/104454903770946719. [DOI] [PubMed] [Google Scholar]
- Logsdon J. M., Kahn R. A. The Arf family tree. In: Kahn R. A., editor. Arf family GTPases. Dordrecht, the Netherlands: Kluwer Academic Publications; 2003. pp. 1–21. [Google Scholar]
- Luton F., Klein S., Chauvin J. P., Le Bivic A., Bourgoin S., Franco M., Chardin P. EFA6, exchange factor for ARF6, regulates the actin cytoskeleton and associated tight junction in response to E-cadherin engagement. Mol. Biol. Cell. 2004;15:1134–1145. doi: 10.1091/mbc.E03-10-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch E. A., Stall J., Schmidt G., Chavrier P., D'Souza-Schorey C. Proteasome-mediated degradation of Rac1-GTP during epithelial cell scattering. Mol. Biol. Cell. 2006;17:2236–2242. doi: 10.1091/mbc.E05-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaki Y., et al. An ADP-ribosylation factor GTPase-activating protein Git2-short/KIAA0148 is involved in subcellular localization of paxillin and actin cytoskeletal organization. Mol. Biol. Cell. 2001;12:645–662. doi: 10.1091/mbc.12.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusser B., Hirsch C., Jarosch E., Sommer T. ERAD: the long road to destruction. Nat. Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera Y., et al. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005;24:963–973. doi: 10.1038/sj.emboj.7600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios F., Schweitzer J. K., Boshans R. L., D'Souza-Schorey C. ARF6-GTP recruits Nm23–H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 2002;4:929–936. doi: 10.1038/ncb881. [DOI] [PubMed] [Google Scholar]
- Pasqualato S., Menetrey J., Franco M., Cherfils J. The structural GDP/GTP cycle of human Arf6. EMBO Rep. 2001;2:234–238. doi: 10.1093/embo-reports/kve043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters P. J., Hsu V. W., Ooi C. E., Finazzi D., Teal S. B., Oorschot V., Donaldson J. G., Klausner R. D. Overexpression of wild-type and mutant ARF1 and ARF 6, distinct perturbations of nonoverlapping membrane compartments. J. Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H., Klausner R. D., Donaldson J. G. Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J. Cell Biol. 1996;134:935–947. doi: 10.1083/jcb.134.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H., Donaldson J. G. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H., Al-Awar O., Khachikian Z., Donaldson J. G. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J. Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- Sabe H. Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion. J. Biochem. 2003;134:485–489. doi: 10.1093/jb/mvg181. [DOI] [PubMed] [Google Scholar]
- Sahai E., Garcia-Medina R., Pouyssegur J., Vial E. Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J. Cell Biol. 2007;176:35–42. doi: 10.1083/jcb.200605135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Chen Z. L. The novel functions of ubiquitination in signaling. Curr. Opin. Cell Biol. 2004;16:119–126. doi: 10.1016/j.ceb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Tague S. E., Muralidharan V., D'Souza-Schorey C. ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc. Natl. Acad. Sci. USA. 2004;101:9671–9676. doi: 10.1073/pnas.0403531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drogon F., Sangfelt O., Malyukova A., Matskova L., Yeh E., Means A. R., Reed S. I. Ubiquitylation of cyclin E requires the sequential function of SCF complexes containing distinct hCdc4 isoforms. Mol. Cell. 2006;23:37–48. doi: 10.1016/j.molcel.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley L. A., Li Y., Zhang C. J., Kahn R. A. Isoform-selective effects of the depletion of ADP-ribosylation factors 1–5 on membrane traffic. Mol. Biol. Cell. 2005;16:4495–4508. doi: 10.1091/mbc.E04-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Zhang Y., Ozdamar B., Ogunjimi A. A., Alexandrova E., Thomsen G. H., Wrana J. L. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- Winston J. T., Koepp D. M., Zhu C., Elledge S. J., Harper J. W. A family of mammalian F-box proteins. Curr. Biol. 1999;9:1180–1182. doi: 10.1016/S0960-9822(00)80021-4. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Cox D., Tseng C. C., Donaldson J. G., Greenberg S. A requirement for Arf6 in Fcγ receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 1998;273:19977–19981. doi: 10.1074/jbc.273.32.19977. [DOI] [PubMed] [Google Scholar]
- Zhu H., Kavsak, Abdollah P., S., Wrana J. L., Thomsen G. H. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.