Abstract
Objective
Because estrogen and testosterone affect transcription factors regulating mitochondrial function, we assessed the effects of gender on the metabolic response to dietary palmitic acid (PA) vs. oleic acid (OA) in subjects participating in a previously described trial.
Methods and Procedures
Adults (N = 43) were studied after following a baseline diet (PA = 8.4% kcal, OA = 13.1% kcal) and after undergoing one of two experimental diets: high PA (HI PA) (PA = 16.8%, OA = 16.4% kcal) (N = 21; 11 men) or high OA (HI OA) (PA = 1.7%, and OA = 31.4%) (N = 22; 11 men).
Results
Relative to baseline, the rate of fatty acid (FA) oxidation (% resting energy expenditure(REE)) (mean ± s.e.m.) increased in women on HI OA while decreasing on HI PA in the fed (+11.8 ± 5.6% vs. −6.3 ± 4.2%, P = 0.02) and fasting states (+13.4 ± 4.2% vs. −12.7 ± 6.9%, P = 0.047), but changes in men were not statistically significant. Daily energy expenditure changed only in men, increasing on HI OA and decreasing on HI PA (+66 ± 61 kcal/day or 1.2 ± 1.0 kcal/kg fat-free mass (FFM)/day vs. −266 ± 78 kcal/day or −4.2 ± 1.3 kcal/kg FFM/day, P = 0.004 and P = 0.007, respectively). Discussion: Increased dietary PA/OA caused decreased FA oxidation in women, in the fed and fasted states and decreased daily energy expenditure (DEE) in men.
INTRODUCTION
Oleic acid (OA) and palmitic acid (PA) are the most prevalent fatty acids (FA) in the diet and lipid storage pools (1,2). We recently showed that dietary PA and OA have different effects on FA oxidation and on accretion of body energy stores in response to energy intake; neither analysis of covariance nor two-way analysis of variance detected a statistically significant gender effect (3). However, menopause is associated with increased adiposity and decreased insulin sensitivity, and estrogen replacement can ameliorate these problems (4). Moreover, in rodents, estrogen and androgen have contrasting effects on the mRNA expression of transcription factors, which modify mitochondrial function, including FA oxidation (4–8). Therefore, using data previously collected during our trial (3), we sought to disprove our hypothesis (null) that men and women respond in a similar manner to dietary PA and OA.
METHODS AND PROCEDURES
Experimental subjects
Forty-three healthy, non-obese young adults, aged 21–34 years participated in a randomized, double-masked, controlled trial. The institutional review committees for human subjects approved the study.
Design
Since the design of this trial was previously reported in detail (3), the experimental plan will be briefly reported here. Each subject was studied twice for 28 days, first on a solid food, baseline diet (“preformula value”), and then on one of two experimental liquid formula diets (“postformula value”): a diet relatively high in PA (HI PA) or a diet low in PA and high in OA (HI OA). The compositions of the diets were reported previously (3). Compared to the baseline diet, HI PA resulted in a 100% increase in PA and a 25% increase in OA, as % kcal, and HI OA resulted in an 80% decrease in PA as % kcal and a 140% increase in OA.
Woman subjects were studied at the same phase of the cycle for both the baseline diet and the experimental diet. Ten of the twenty-one woman subjects were receiving oral contraceptives and not ovulating (N = 4, HI PA group).
After a 12-h overnight fast, at ~7:30 am, on day 27 of each diet period (solid and formula), blood was obtained by venipuncture for measurement of the serum concentration of total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, triacylglycerol, and calculated low density lipoprotein (LDL) cholesterol. On the 28th day of each diet period, after an evening meal at 6:00 pm, we assessed the overnight resting energy expenditure (REE) and substrate utilization using indirect calorimetry and urine urea N measurements (Vmax SPECTRA 29, Sensor Medics, Yorba Linda, CA) (9–12). Average values for the respiratory quotient (respiratory exchange ratio), rates of FA and carbohydrate oxidation, and REE were estimated for both the fed (5:20 pm to 1:20 am) and fasting states (1:20 am to 07:20 am) (9).
Changes in fat mass and fat-free mass (FFM) during the diets were assessed by dual-energy X-ray absorptiometry (Hologic Delphi QDR 4500A Bone Densitometer, Bedford, MA), and body energy and daily energy expenditure (DEE) were calculated as described previously (3).
In 39 subjects (N = 21, HI OA group and N = 18, in HI PA group), physical movement during the two phases of the study also was estimated using a uniaxial accelerometer (Computer Science and Applications, CSA, Model 71164, Manufacturing Technology, Fort Walton Beach, FL) (3,13).
Serum lipid concentrations
Serum was separated by low speed centrifugation at 1,500g, at 10 °C for 10 min. TC, triacylglycerol, and HDL were measured colorimetrically using the Vitros 950 analyzer and standard enzymatic procedures (University of Texas Medical Branch Clinical Laboratory).
Mathematical expressions and statistical analysis
For some analyses and data presentation (e.g., Figure 1), the rate of fat oxidation was expressed as a percentage of REE, in order to normalize this rate for differences in cellular mass as well as for possible gender differences in the relative mass of the more metabolically active organs (14,15).
Figure 1.
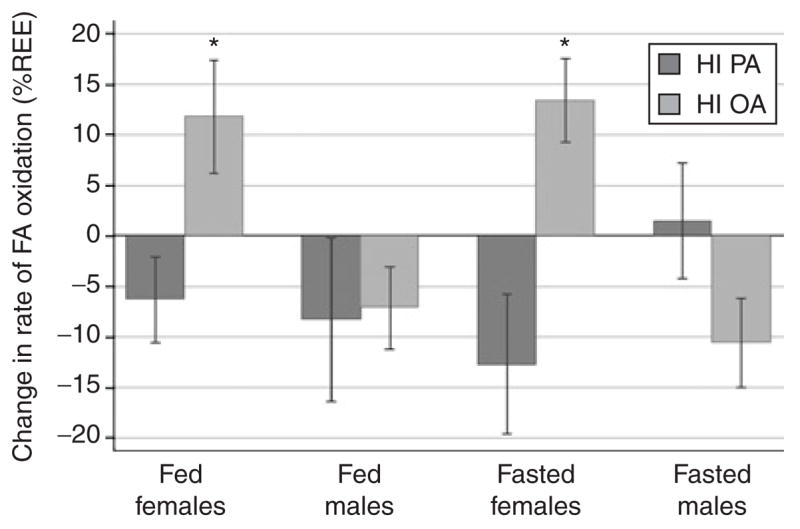
Gender differences in the change in the rate of fatty acid (FA) oxidation as percent of resting energy expenditure (REE) from a baseline, control diet fed to all subjects compared to diets containing a high percent of energy as palmitic acid (HI PA) or a low percent of energy as palmitic acid and a high percent of energy as oleic acid (HI OA). Asterisk denotes statistical difference (P < 0.05) between diet groups. Specific P values are as follows: women, fed state, P = 0.02; men, fed state, P = 0.083; women, fasting state, P = 0.047.
Results are expressed as mean ± s.e.m. The main approach to statistical analysis was an analysis of covariance of the change during the experimental formula diet using the respective preformula value as a covariate (SPSS Base 10.0, SPSS, Chicago, IL) (3).
RESULTS
Gender differences in body composition, fat oxidation, and energy expenditure after the control, baseline diet
BMI was significantly higher in men (25.2 ± 0.7) compared to women (22.4 ± 0.5) (P = 0.002). FFM was significantly (P < 0.001) higher and percent body fat was significantly lower in men (respectively 61.7 ± 1.4 kg FFM vs. 43.7 ± 1.0 kg FFM and 20.4 ± 1.5% body fat vs. 29.9 ± 1.1% body fat).
Men in the fed state exhibited a higher rate of FA oxidation expressed as a percentage of REE (39.6 ± 3.7% kcal vs. 20.1 ± 3.6% kcal) (P < 0.001) and a higher rate of FA oxidation in the fasting state of borderline significance (37.2 ± 3.2% kcal vs. 27.3 ± 4.5% kcal) (P = 0.076).
Men had a somewhat lower REE in the fed state compared to women, respectively 0.020 ± 0.0005 vs. 0.022 ± 0.0009 (kcal/kg FFM/min) (P = 0.071). REE in the fasting state was 8% lower in men compared to women (0.017 ± 0.0004 kcal/kg FFM/min vs. 0.019 ± 0.0007 kcal/kg FFM/min) (P = 0.016). There was no significant difference between men and women in physical activity (920 ± 68 accelerations/min vs. 998 ± 71 accelerations/min). DEE was 10% lower in men compared to women (48.6 ± 1.0 kcal/kg FFM/day vs. 54.1 ± 1.9 kcal/kg FFM/day) (P = 0.012) during the baseline diet.
In summary, prior to the experimental diets, men exhibited higher rates of FA oxidation and lower rates of resting and daily energy expenditure.
Effects of dietary FA intake on FA oxidation in women and men
Fed state
For both women and men, there were no differences between diet groups in fat intake, either at the end of the baseline diet or at the end of the formula diet. In women, FA oxidation, expressed as mg/kg FFM/min, tended to increase during the HI OA diet (+0.253 ± 0.154) and decrease during the HI PA diet (−0.089 ± 0.102) (P = 0.059). When the change in the fed state was expressed as a % of REE, FA oxidation in women was significantly different (P = 0.02) with the rate of FA oxidation decreasing in the HI PA group (−6.3 ± 4.2%) and increasing in the HI OA group (+11.8 ± 5.6%) (Figure 1).
In men, there was no effect of diet on the change in FA oxidation (mg/kg FFM/min), but there was a trend for a difference in the change in FA oxidation, expressed as % of REE between the HI PA (−8.3 ± 8.1%) and HI OA groups (−7.1 ± 4.1%) (P = 0.083), although there was a decrease in both dietary groups (Figure 1).
Fasting state
In women, FA oxidation, expressed as mg/kg FFM/min, tended to increase during the HI OA diet (+0.259 ± 0.101) and decrease during the HI PA diet (−0.234 ± 0.155) (P = 0.068); expressed as % REE, the fasting rate of FA oxidation decreased in the HI PA group and increased in the HI OA group (P = 0.047) (Figure 1). There were no diet group effects in men for FA oxidation expressed as mg/kg FFM/min or as % of REE (Figure 1).
Comparison of changes in FA oxidation in women and men on each diet
We also examined whether the genders responded differently to each diet. Ingesting the HI OA diet caused an increased rate of FA oxidation (% REE) in women in both the fed and fasting states, compared to the baseline diet (+67 and +72% increases, respectively); in men, however, ingesting the HI OA diet decreased FA oxidation (−16 and −24%, respectively in the fed and fasted states) (effect of gender on change in FA oxidation in the fed and fasted state, respectively, P = 0.013 and P = 0.001). In contrast, changes in FA oxidation in response to the high PA diet were not significantly different between men and women.
Effects of dietary FA intake on body composition, physical activity, energy intake, and energy expenditure in women and men
In both women and men, the diet group did not affect the changes in BMI, fat mass, FFM, percent body fat, or body energy. There was no significant difference in the change in physical activity during the experimental diets, for either the women (HI PA vs. HI OA) (−86 ± 31 accelerations/min vs. −132 ± 101 accelerations/min) or men (21 ± 65 vs. 1 ± 143).
There was no significant change in energy intake during the formula diet for either the women or men. In women, REE (kcal/kg FFM/min) during the fed state increased slightly in the subjects on the HI PA diet (+0.0026 ± 0.0009) while remaining unchanged in those on the HI OA diet (0.0000 ± 0.0008) (P = 0.045). However, there was no significant difference in the change in REE in the fasting state. In men, during the formula diet, there was no significant change in energy intake or REE during the fed or fasting states.
In women, the change in DEE (expressed as kcal/day or as kcal/kg FFM/day) was not significantly different between the dietary groups (Figure 2). In contrast, in men, there were significant differences in the change in DEE, expressed as kcal/day or kcal kg FFM/year, with those on the HI PA diet exhibiting a decrease and those in the HI OA group exhibiting an increase (respectively −266 ± 78 kcal/day vs. +66 ± 61 kcal/day; P = 0.004; and −4.2 ± 1.3 kcal/kg FFM/day vs. +1.2 ± 1.0 kcal/kg FFM/day; P = 0.007) (Figure 2).
Figure 2.
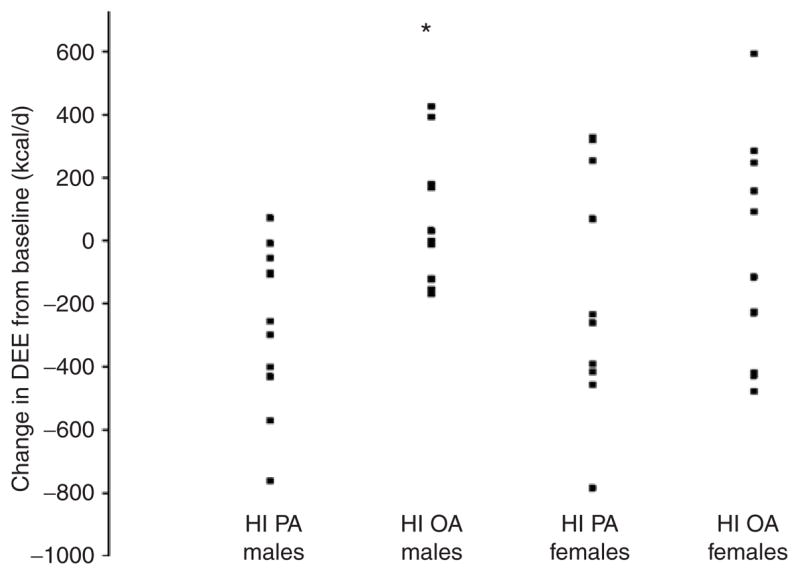
Individual changes in daily energy expenditure (DEE) in men and women during the experimental diets: HI PA (high percent of energy as palmitic acid) or HI OA (low percent of energy as palmitic acid and a high percent of energy as oleic acid). Asterisk denotes men, HI PA vs. HI OA, P = 0.004.
Effects of dietary FA intake on serum lipid concentrations
There were no differences between the groups preformula. For the entire study group (men and women), the serum concentration of TC, HDL, LDL, and LDL/HDL ratio all appeared to fall, on average, in both diet groups during the formula diets. The fall in serum TC was significantly greater in the HI OA group vs. HI PA: −1.01 ± 0.12 mmol/l vs. −0.53 ± 0.12 mmol/l (P < 0.001). The LDL concentration fell significantly more in the HI OA group: −0.88 ± 0.09 mmol/l vs. −0.62 ± 0.16 mmol/l (P = 0.001), as did the LDL/HDL ratio (−0.51 ± 0.08 vs. −0.29 ± 0.10; P = 0.012).
In women, none of these changes reached statistical significance, although there were trends towards greater decreases on the HI OA formula for TC (HI OA vs. HI PA, −0.99 ± 0.16 mmol/l vs. −0.55 ± 0. 21 mmol/l; P = 0.090) and HDL (P = 0.070). In men, the fall in serum TC in the HI OA group was significantly greater in the HI OA group as compared to the HI PA group: −1.04 ± 0.18 mmol/l vs. −0.52 ± 0.13 mmol/l (P = 0.001), and very similar to the magnitude of change in each group among the women. Otherwise, in men, only the change in the LDL/HDL ratio was statistically significant between groups (−0.67 ± 0.09 vs. −0.35 ± 0.17; P= 0.011); in this case, the 91% greater decrease in the HI OA group was almost double the non-significant (P = 0.78), 52% fall in women.
DISCUSSION
In this study, after following an identical control diet for 28 days, men exhibited higher rates of FA oxidation and lower rates of resting and daily energy expenditure. However, the high PA diet in contrast to the low PA/high OA diet, had more pronounced effects on FA oxidation in women, compared to men. By contrast, we observed that DEE was affected by diet only in the men and represented an average, diet group difference of 332 kcal/day but with considerable inter-individual variation. Since neither REE nor physical activity differentially changed during the experimental diets, we infer that non-resting expenditure, and more specifically, the energy cost of physical activity, was altered by the diets in men. We acknowledge that our measurements overnight included the hours after the evening meal; these measurements were appropriately affected by physical activity earlier in the day but would also have been affected by the subjects staying in bed continuously after the evening meal. While sedentary behavior after the evening meal may not be unusual in American culture, it is true that bed-rest and/or sleep may alter physiology that could change, in undetermined ways, the response to the two diets. For example, exercise/muscle contraction alters muscle lipid metabolism (16,17). The fasting measurements, made during the ordinary sleeping hours, are perhaps representative of metabolism during the typical overnight period when many people sleep, although minor interruptions of sleep, when the canopy is placed over the subject, may create some aberrations in the typical sleep process of young adults. Also, there are theoretical problems in making comparisons between measurements taken by others during the fed vs. the fasting state using a different protocol of making an initial fasting measurement at the end of the sleeping period followed by a series of postbreakfast measurements. All subjects were similarly studied in both the “fed” and ‘fasting” states.
These data may prove to be relevant to the prevention of obesity and/or insulin resistance and type 2 diabetes. In men, in whom changes in FA oxidation were less affected by diet than in women, we observed an effect on daily energy needs; a difference in the diets by 332 kcal/day corresponds to a difference of fat accretion of 13.5 kg/year of adipose tissue, if it is not compensated by (eventual) changes in energy intake or physical activity. Of course, these data on DEE especially are predicated on the veracity of the data on energy intake. While one could speculate that errors made in the estimation of energy intake might be incorrect or even gender-biased, it is not obvious why, for example, an unrecorded intake of energy outside of the study protocol would be so much isolated to the men in the HI PA group (3). Nevertheless, confirmation of the conclusion about DEE would require more informed data on energy intake (e.g., use of doubly labeled water).
The relative failure of complete FA oxidation in women fed the high PA diet could result in the accumulation of lipid compounds within the cytosol or mitochondria with resultant defective insulin signaling (18,19). Since mitochondrial function and especially FA oxidation per se may play a salutary role in preventing insulin resistance (18,20), these data may be relevant to assessing the possible differential risks faced by men and women for type 2 diabetes because of their dietary FA pattern. In rodents, estrogen increases the expression of peroxisome proliferator activated receptor δ (PPARδ) and PPARγ coactivator-1α (PGC-1α) and decreases the expression of PPARα; these transcription factors stimulate FA oxidation, via their down-stream targets (4–6). On the other hand, androgen may decrease PGC-1α (5) and increase or decrease PPARα expression (7,8). Thus, the differential changes in FA oxidation produced by these diets, especially in women, are potentially important because they might suggest the existence of a synergy between the metabolic effects of dietary FA (specifically OA) and the sex hormones (particularly estrogen). However, our study included neither muscle biopsies nor serum hormone measurements, and a further exploration of the significance of these findings would require measurements to be taken of the mRNA and protein expression in the skeletal muscle of these critical genes, and/or measurements of changes in cytosolic and mitochondrial lipids, which might be altered by changes in the oxidation of all FA or PA per se (16,19,21).
We also observed that dietary PA and OA affected non-resting energy expenditure, but only in men; again, we can look to possible molecular mechanisms as explanation. Both PPARδ and PGC-1α (which co-activates PPAR-δ) induce the formation of type I fibers in skeletal muscle, which has the net effect of increasing the efficiency of muscle contraction, that is, allowing more work/O2 consumption (16,22–24). So, enhanced PGC-1α and PPARδ expression per se might result in lower non-resting energy expenditure for the same amount of work (i.e., enhanced mechanical efficiency). Androgen, then, could, by decreasing PGC-1α expression (5), result in less efficient muscle contraction and greater non-resting energy expenditure. A high fat, lard-based diet (high saturated fat) caused lowered PGC-1α expression in rats (16). However, our data (Figure 2) suggest that the HI PA diet lowered DEE, rather than increasing it. Thus, suppression of PGC-1α expression by a combination of dietary PA and androgen cannot explain our results (5,16).
In summary, this study suggests that gender alters differentially the response to dietary FA. Women responded to a high oleate/low palmitate diet by markedly increasing FA oxidation. Men, on the other hand, responded to the HI OA and HI PA diets by more markedly increasing and decreasing DEE respectively, compared to an attenuated response in women.
Acknowledgments
We thank our subjects for their thoughtful participation in this study, the nursing and dietary staffs of both The Ohio State University GCRC and the UTMB GCRC, and Travis Solley and Mary Schmitz-Brown for technical assistance. This study was supported by NIH grant R01 DK55384, and NIH grants to the General Clinical Research Centers (GCRC) at the University of Texas Medical Branch at Galveston, The Ohio State University, and the University of Vermont (respectively, M01 RR 00073, M01 RR 00034, and M01 RR00109). The authors are grateful to Ross Products Division of Abbott Laboratories, for providing the experimental formula and to the body composition staff at the Shriners Hospital for Children (SHC grant no. 8760).
Footnotes
DISCLOSURE
The author declared no conflict of interest.
References
- 1.Berry EM. Dietary fatty acids in the management of diabetes mellitus. Am J Clin Nutr. 1997;66:S991–S997. doi: 10.1093/ajcn/66.4.991S. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Denke MA. Dietary influences on serum lipids and lipoproteins. J Lipid Res. 1990;31:1149–1172. [PubMed] [Google Scholar]
- 3.Kien CL, Bunn JY, Ugrasbul F. Increasing dietary palmitic acid decreases fat oxidation and daily energy expenditure. Am J Clin Nutr. 2005;82:320–326. doi: 10.1093/ajcn.82.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Eon TM, Souza SC, Aronovitz M, et al. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983–35991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh YC, Yang S, Choudhry MA, et al. PGC-1 upregulation via estrogen receptors: a common mechanism of salutary effects of estrogen and flutamide on heart function after trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2005;289:H2665–H2672. doi: 10.1152/ajpheart.00682.2005. [DOI] [PubMed] [Google Scholar]
- 6.Kamei Y, Suzuki M, Miyazaki H, et al. Ovariectomy in mice decreases lipid metabolism-related gene expression in adipose tissue and skeletal muscle with increased body fat. J Nutr SciVitaminol (Tokyo) 2005;51:110–117. doi: 10.3177/jnsv.51.110. [DOI] [PubMed] [Google Scholar]
- 7.Jalouli M, Carlsson L, Ameen C, et al. Sex difference in hepatic peroxisome proliferator-activated receptor alpha expression: influence of pituitary and gonadal hormones. Endocrinology. 2003;144:101–109. doi: 10.1210/en.2002-220630. [DOI] [PubMed] [Google Scholar]
- 8.Collett GP, Betts AM, Johnson MI, et al. Peroxisome proliferator-activated receptor alpha is an androgen-responsive gene in human prostate and is highly expressed in prostatic adenocarcinoma. Clin Cancer Res. 2000;6:3241–3248. [PubMed] [Google Scholar]
- 9.Roust LR, Hammel KD, Jensen MD. Effects of isoenergetic, low-fat diets on energy metabolism in lean and obese women. Am J Clin Nutr. 1994;60:470–475. doi: 10.1093/ajcn/60.4.470. [DOI] [PubMed] [Google Scholar]
- 10.Garrow JS. Energy Balance and Obesity in Man. North-Holland Publishing; Amsterdam, the Netherlands: 1974. [Google Scholar]
- 11.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 12.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melanson ELJ, Freedson PS. Validity of the Computer Science and Applications, Inc. (CSA) activity monitor. Med Sci Sports Exerc. 1995;27:934–940. [PubMed] [Google Scholar]
- 14.Holliday MA. Metabolic rate and organ size during growth from infancy to maturity and during late gestation and early infancy. Pediatrics. 1971;47:169–179. [PubMed] [Google Scholar]
- 15.Kien CL. Energy metabolism and requirements in disease. In: Walker WA, Watkins JB, editors. Nutrition in Pediatrics: Basic Science and Clinical Application. Little, Brown and Company; Boston/Toronto: 1985. pp. 87–121. [Google Scholar]
- 16.Koves TR, Li P, An J, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–98. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 17.Thyfault JP, Cree MG, Zheng D, et al. Contraction of insulin resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol. 2007;292:C729–C739. doi: 10.1152/ajpcell.00311.2006. [DOI] [PubMed] [Google Scholar]
- 18.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 20.Cortright RN, Sandhoff KM, Basilio JL, et al. Skeletal muscle fat oxidation is increased in African-American and white women after 10 days of endurance exercise training. Obesity (Silver Spring) 2006;14:1201–1210. doi: 10.1038/oby.2006.137. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt DE, Allred JB, Kien CL. Fractional oxidation of chylomicron-derived oleate is greater than that of palmitate in healthy adults fed frequent small meals. J Lipid Res. 1999;40:2322–2332. [PubMed] [Google Scholar]
- 22.Spiegelman BM, Heinrich R. Biological control through regulated transcriptional coactivators. Cell. 2004;119:157–67. doi: 10.1016/j.cell.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Wang YX, Zhang CL, Yu RT, et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]


