Abstract
Genistein, a soy phytoestrogen, may improve vascular function but the mechanism of this effect is unclear. Endothelial-derived nitric oxide (NO) is a key regulator of vascular tone and atherogenesis. Previous studies have established that estrogen can act directly on vascular endothelial cells to enhance NO synthesis through genomic stimulation of endothelial nitric oxide synthase (eNOS) expression. However, it is unknown whether genistein has a similar effect. We therefore investigated whether genistein directly regulates NO synthesis in primary human aortic endothelial cells (HAEC) and human umbilical vein endothelial cells (HUVEC). Genistein, at physiologically achievable concentrations in individuals consuming soy products, enhanced the expression of eNOS and subsequently elevated NO synthesis in both HAEC and HUVEC, with 1–10 μmol/L genistein inducing the maximal effects. However, the effects of genistein on eNOS and NO were not mediated by activation of estrogen signaling or inhibition of typrosine kinases, two known biological actions of genistein. Genistein (1–10 μmol/L) increased eNOS gene expression (1.8–2.6-fold of control) and significantly increased eNOS promoter activity of the human eNOS gene in HAEC and HUVEC, suggesting that genistein activates eNOS transcription. Dietary supplementation of genistein to spontaneously hypertensive rats restored aortic eNOS levels, improved aortic wall thickness, and alleviated hypertension, confirming the biological relevance of the in vitro findings. Our data suggest that genistein has direct genomic effects on the vascular wall that are unrelated to its known actions, leading to increase in eNOS expression and NO synthesis, thereby improving hypertension.
Keywords: genistein, nitric oxide synthase, nitric oxide, endothelial cells, blood pressure, spontaneously hypertensive rats
Introduction
The prevalence of cardiac and other vascular diseases rises in aging population. It is also well recognized that the incidence of cardiovascular disease (CVD)3 is substantially increased in postmenopausal women due to the loss of estrogen. Experimental and clinical data support vascular protective effects of estrogen by various mechanisms (1). However, administration of estrogen is also associated with an increased incidence of heart disease which limits its therapeutic potential (2). In addition, the use of estrogen as a cardioprotective agent is further limited by carcinogenic effects in women and feminizing effects in men (3). Given the demonstrated risks of conventional estrogen therapy, a search for novel, cost-effective, alternative vasoactive agents for prevention of CVD is of major importance in the effort to decrease the burden of CVD morbidity.
The soy phytoestrogen genistein has drawn wide attention due to its potential healthy benefits in preventing chronic diseases such as CVD (4, 5), obesity (6, 7) and osteoporosis (8). Epidemiological studies show that genistein intake in American postmenopausal women is inversely associated with CVD risk factors (9, 10), supporting a beneficial role for genistein administration to aging individuals. Some human intervention studies suggest the beneficial effects of genistein on atherosclerosis (11), markers of cardiovascular risk (12, 13), vascular motor tone (14), vascular endothelial function (15), and systemic arterial compliance (16). Data from animals and in vitro studies also suggest a protective role of genistein in cardiovascular events (17, 18). However, the mechanism of genistein action in vasculature is still not clear, which hinders our further determining the physiological and pharmacological role of this nutraceutical compound in vascular function. Past studies have extensively explored its hypolipidemic (19), anti-oxidative (20, 21) and the estrogenic effects (22). While genistein may have both estrogen receptors (ER)- dependent and independent actions in vasculature, its average effect on plasma lipid profile is neutral (23). Interestingly, recent studies have shown that the beneficial effects of genistein on endothelial function in postmenopausal women can be blocked by NG-monomethyl-L-arginine, the inhibitor of endothelial nitric oxide synthase (eNOS) (24, 25). Moreover, genistein restores the nitric oxide (NO)-mediated vascular relaxation in ovariectomized (26) or chronically hypoxic (27) rats. Furthermore, long-term dietary supplementation of genistein elevates the plasma NO concentrations and reduces the plasma endothelin-1 levels in healthy postmenopausal women (15). Given the importance of NO in modulating vascular homeostasis, it is tempting to propose that genistein exerts vasculoprotective effects by regulating NO levels.
Previous studies have established a role for estrogen in the vascular endothelial cells (EC) to enhance NO synthesis through genomic stimulation of eNOS expression (28), and by ERs-mediated, non-genomic eNOS activation (29). We recently demonstrated that genistein acutely stimulates NO production by phosphorylation of eNOS via the cAMP/protein kinase A (PKA) cascade in EC (30, 31). However, it is unknown whether genistein has a similar genomic effect on eNOS. Studies have reported that administration of soy protein improves eNOS expression and subsequently reduces blood pressure in rats (32). However, other studies demonstrated that the beneficial effect of genistein on endothelial function is not through enhancing eNOS expression (33). Although genistein has been shown to enhance eNOS promoter activity in a transformed human EC (34), it is not clear whether genistein directly up-regulates eNOS expression in primary EC and thereby reduces blood pressure in vivo. In the present study, we tested whether genistein improves eNOS expression and subsequently increases NO synthesis in primary human aortic EC (HAEC) and in spontaneously hypertensive rats (SHR), and whether this is associated with a blood pressure-lowering effect of genistein.
Materials and Methods
Materials
Primary HAEC and endothelial growth factors were purchased from Cambrex Bioscience (Rockland, ME); primary human umbilical vein endothelial cells (HUVEC) were obtained from the Cardiovascular Research Cell Culture Core at the University of Iowa; competent cells for plasmid multiplication, M199 media, fetal bovine serum (FBS) and other cell culture supplements were obtained from Invitrogen (Carlsbad, CA); eNOS and β-actin monoclonal antibodies were purchased from Cell Signaling Technology (Beverly, MA); the superSignal chemiluminescence detection system was obtained from Pierce (Rockford, IL); nitrocellulose membranes, SYBR green supermix, cDNA synthesis and protein assay kits were from Bio-Rad (Hercules, CA); human eNOS promoter (−1193/+109) linked to a firefly luciferase reporter gene was kindly provided by Dr. William Sessa at Yale University; plasmid purification and RNeasy Mini kits were from Qiagen (Valencia, CA); primers were synthesized by Integrated DNA Technologies (Coralville, IA); transfection reagents were obtained from Targeting system (Santee, CA); dual luciferase reporter assay kits were obtained from Promega (Madison, WI); nitrite/nitrate fluorometric assay reagents were purchased from Cayman Chemical (Ann Arbor, MI); ICI182,780 was from Tocris (St. Louis, MO); genistein and daidzein were purchased from LC Laboratories (Woburn, MA) and Sigma (St. Louis, MO); 17β-estradiol (E2), protease and phosphatase inhibitor cocktails and other general chemicals were obtained from Sigma (St. Louis, MO). Stock solutions of genistein, daidzein or E2, at 20 mmol/L in dimethylsulfoxide (DMSO), were stored at −80°C before use.
Cell culture
HAEC were cultured in M199 medium containing 2% FBS and endothelial growth supplements-EGM2 and HUVEC were cultured in 20% FBS M199 medium at 37°C in a 5% CO2/95% air environment. The medium was changed every other day until the cells became confluent. HAEC and HUVEC were passaged by using 0.05% trypsin and passages 4–6 were used in all experiments.
Animals and Diets
4-wk old male, spontaneously hypertensive rats (SHR) and Wistar-Kyoto rats (WKY) were purchased from Harlan Inc.(Indianapolis, IN). Rats were housed in a room maintained on a 12h light/dark cycle under constant temperature (22–25°C) with free access to food and water. The protocol of this study was reviewed and approved by the Institutional Animal Care and Use Committee At Virginia Polytechnic Institute and State University. After an initial acclimation period, SHR were randomly divided into 6 groups and were fed a basal soy-free AIN-76A diet (35) containing genistein at 0, 0.2, 0.5, or 2.0 g/kg diet for 19 wk. WKY were fed the basal AIN-76A diet for the same period. To determine whether genistein can improve established hypertension, adult SHR with overt hypertension (20 wk old) were randomly divided into 2 groups and fed either 0 or 2.0 g genistein/kg diet until their blood pressure was significantly lowered. Then, both groups of rats were fed the same basal diets for 6 wk.
Plasma genistein measurements
On the last day of the study, blood samples were drawn 30 min after food intake from the retrobulbar plexus through heparinized capillary tubes. Plasma was collected by centrifugation at 16,000 × g for 15 min. An aliquot of 250 μL serum per sample was used for extraction of genistein using a previously described method (36). Genistein in the extracted samples was determined by using the HPLC system (Waters2695) with a Luna Phenyl-hexyl column (5 μ C18 100 R) (36).
Blood pressure, heart rate, body weight, and food intake measurements
Every other week, rat blood pressure and heart rate were determined after a warming period using the Kent CODA 2 series computerized non-invasive blood pressure system (Kent Scientific, Litchfield, CT) as described (37). During these measurements, rats were under 0.8% isoflurane anesthesia, which had no effect on blood pressure as determined in our preliminary study. The digital values for the systolic, diastolic blood pressure and heart rate were recorded. Readings were taken for 20 cycles from each rat with the highest and the lowest values excluded. To minimize stress-induced variations in blood pressure, all measurements were taken by the same person in the same peaceful environment. Body weight and feed intake were recorded weekly throughout the study to determine whether genistein has any effect on these parameters.
Measurement of aortic wall thickness
The rats were killed using CO2 and segments of thoracic aorta were fixed in 10% neutral buffered formalin solution for 24 h. Aorta segments were then embedded in frozen embedding media, cut into 5μm section, and stained with Verhoeff’s Van Gieson, which specifically stains elastic tissue fibers. Stained sections were photographed by a computer-operated Olympus BH-2 photomicroscope. The wall thicknesses of aorta were measured using Image-pro plus system (Media Cyberretic, Inc.). Ten measurements were performed for each sample, and the average value was used as the thickness of the sample.
NO Measurement
To investigate the effect of genistein on NO release in vitro, confluent cells grown in 12-well plates were treated with genistein, vehicle (DMSO) or other agents in complete medium, over a range of concentrations and time points, as indicated in the figure legends. For assays focused on the effect of prolonged incubation with genistein, culture media were renewed in the third day from the initial treatment. In some experiments, cells were pretreated with ICI 182,780 (1 μmol/L), a highly specific inhibitor of ERs, for 30 min before addition of agonists. Following treatment, cells were adapted into Hank’s balanced salts solution (HBSS; 135 mmol/L NaCl, 1.2 mmol/L CaCl2, 1.2 mmol/L MgCl2 1.2, 5 mmol/L KOH, 10 mmol/L HEPES, 10 mmol/L glucose, pH 7.4) supplemented with L-arginine (0.1 mmol/L) for 30 min, followed by stimulation with 10 μmol/L A23187 for 30 min. Culture supernatants were then collected for NO assay as determined by measuring the sum concentration of NO2- and NO3- using a fluorometric assay kit following the manufacturer’s instructions. Briefly, cell supernatants were treated with NO3- reductase for 30 min at room temperature to reduce NO3- to NO2-, which then reacted with 2,3-diaminonaphthalene for 10 min to yield the fluorescent product 1(H)-naphthotriazole. Fluorescence was measured in a fluorescence microplate reader (Bio-Tek, Winooski, VT) with excitation and emission wavelengths of 365 and 450 nm, respectively. Fluorescence data were converted into concentrations based on standard curves constructed with NaNO3, normalized to protein concentration of the samples, and then expressed as folds of vehicle-treated controls.
Immunoblot analysis
Following experimental treatments, EC or aortic vessels from rats were harvested in lysis buffer and performed immunoblot analysis as previous described (30, 31). The tissues were sonicated (EC) or homogenized with a Rotor–stator homogenizer (aorta) and then centrifuged at 10,000 × g for 5 min. Protein levels of the extracts were measured using a Bio-Rad assay kit. Equal amounts of protein from cell extracts were subjected to immunoblot. Membranes were probed with antibody against eNOS. The immunoreactive proteins were detected by chemiluminescence. Nitrocellulose membranes were stripped and re-probed with β-actin. The protein bands were digitally imaged for densitometric quantitation with a software program (Gene tools, Synoptics Ltd. UK). eNOS protein level was normalized to β-actin expression from the same sample.
Quantitative real-time PCR analysis
Total RNA from genistein- or vehicle-treated HAEC was isolated using the RNeasy Mini Kit following the manufacturer’s protocol. Then, 0.5 μg of total RNA from each sample was reverse transcribed to cDNA using the iScript cDNA synthesis kit. eNOS was amplified on an iCycler IQ real-time quantitative PCR system using iQ SYBR Green supermix with β-actin as an internal control. A melting curve analysis was performed on each sample to verify that no non-specific products were synthesized. The reaction mixtures contained 100 nmol/L primers, 50 ng cDNA, and 12.5μL iQ SYBR Green supermix (0.2 mmol/L of each dNTP, 25 units/mL iTaq DNA polymerase, SYBR Green I, 10 nmol/L fluorescein, 3 mmol/L MgCl2, 50 mmol/L KCl, and 20 mmol/L Tris-HCl) as described previously (38). The primers used in quantitative real-time RT-PCR were eNOS (forward: 5′-GACATTGAGAG CAAAGGGCTGC-3′; reverse: 5′-CGGCTTGTCACCTCCTGG-3′), and β-actin (forward: 5′-CATGCCATCCTGCGTCTGGA-3′, reverse: 3′-CCGTGGCCATCTCTTGCTCG-5′) (39). The eNOS mRNA level was normalized to that of β-actin, and expressed as folds of control.
eNOS promoter activity assay
A reporter plasmid containing a human eNOS promoter (−1193/+109) linked to a firefly luciferase reporter gene (eNOS-Luc) was amplified with competent cells and purified using Qiagen’s Maxi kit according to the manufacturer’s instructions. For transient transfection of the plasmids, EC were grown in 24-well plates until 50–70% confluence. The cells were then co-transfected with 1.2 μg of eNOS-Luc and 0.5 ng of pRL reporter control plasmid per well using F-1 transfection reagent for 24 h according to the manufacturer’s protocol. The transfected cells were then treated with various concentrations of genistein or vehicle in phenol-red free M199 medium containing 2% FBS for 24 h. Treated cells were harvested in reporter lysis reagent. Luciferase activity, normalized to pRL activity in the cell extracts, was determined by using the dual luciferase reporter assay system as described (40).
Statistical analysis
Data was analyzed with one-way, or two-way ANOVA where designated, using the SAS® program. Data are expressed as the mean±SE. For the time course study, initial values (d1) from vehicle-treated cells were set as the control. Treatment and time point differences, as well as interaction between genistein and other agents if significant, were subjected to Tukey’s multiple comparison tests, where p < 0.05 was considered significant.
Results
Genistein enhances NO synthesis in HAEC
We first examined whether long-term exposure of genistein stimulates NO synthesis in HAEC. Genistein significantly stimulated NO synthesis following 5 d of incubation (Fig. 1A). The effect of genistein was concentration-dependent, with genistein concentrations of ≥ 1 μmol/L inducing significant NO production. The time-course study showed that genistein (5 μmol/L)-stimulated NO production was significantly increased after 3 d of exposure to genistein, with about 1.1 fold increase at 5 d compared to that at 1 d of incubation with genistein (Fig. 1B).
FIGURE 1.
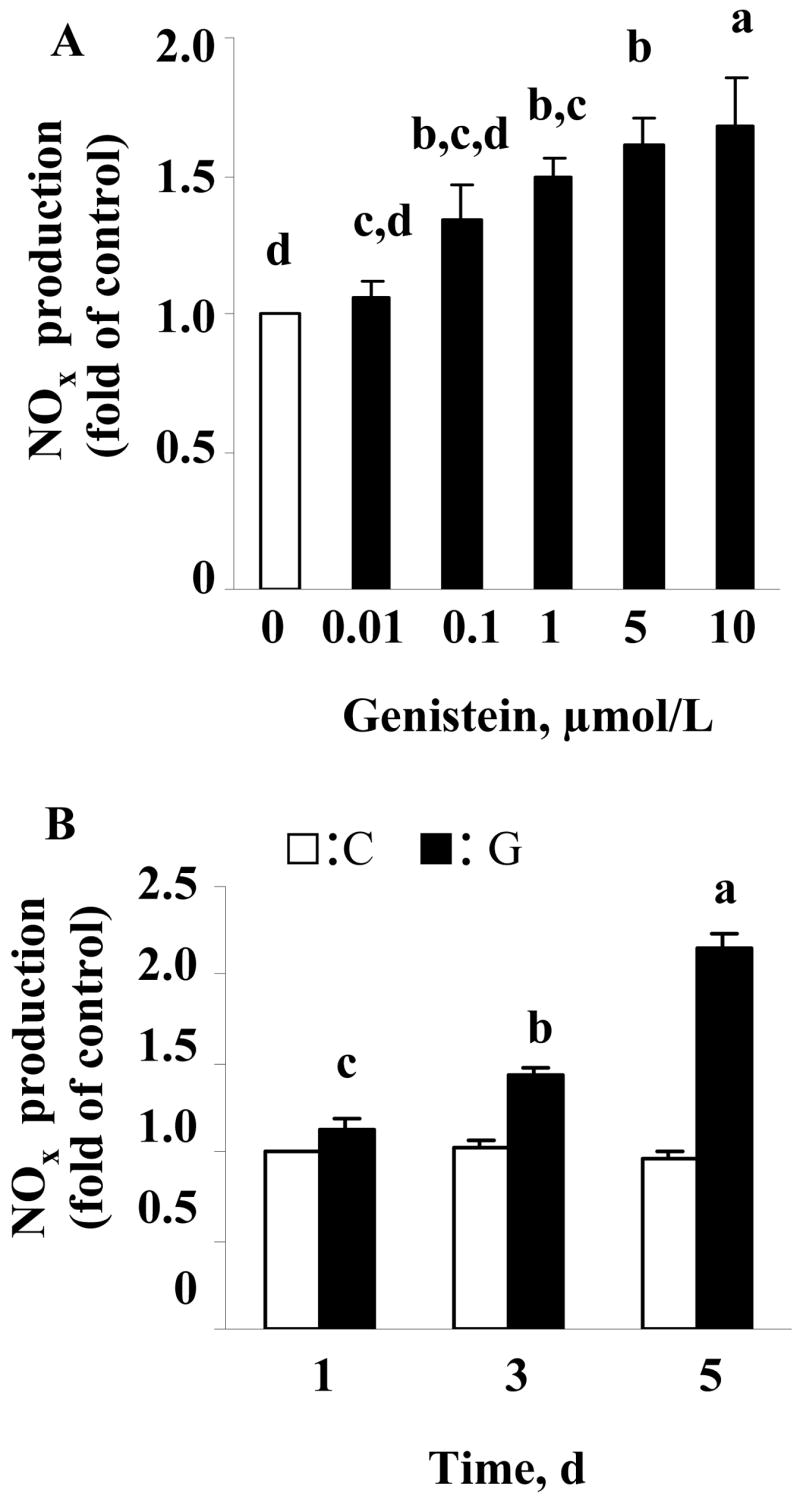
NO production in confluent HAEC incubated with various concentrations (0.01–10 μmol/L) of genistein or vehicle (DMSO) for 5 d (A), or with 5 μmol/L genistein for different times (B). Nitrite/nitrate (NOx) secreted was measured and at baseline was 0.074±0.003 μmol/L. Values are mean±SE from four separate experiments and expressed as fold of the control. Bars without a common letter differ, P<0.05.
Genistein-induced NO production is independent of ER and protein tyrosine kinase (PTK)
Genistein has weak estrogenic effects in some tissues by binding to ER (41). In addition, previous studies have shown that E2 also can stimulate NO production in human EC (28). However, incubation of the cells with excess amounts of the ER antagonist ICI 182,780 did not block genistein-induced NO release (Table 1). The activity of ICI 182,780 used in this study was validated through blocking the cytoprotective effect of E2 in our recent study (40). In addition, while genistein enhanced NO synthesis as expected, chronic exposure of EC to E2 (10 nmol/L) did not stimulate NO production in HAEC (Table 1). These results suggest that the effect of genistein on NO production in EC is independent of the estrogen signaling mechanism.
TABLE 1.
Genistein-stimulated NO production is independent of ER and PTK in HAEC 1
| Treatments
|
2-Way ANOVA, P values
|
||||||
|---|---|---|---|---|---|---|---|
| C | G | X2 | G+X | G | X | G+X | |
| I | 1±0.0b | 1.80±0.11a | 1.14±0.03b | 1.72±0.14a | 0.0003 | 0.5673 | 0.0004 |
| E2 | 1±0.0 b | 1.77±0.17 a | 0.95±0.08 b | 2.31±0.21 a | 0.0021 | 0.995 | 0.0001 |
| D | 1±0.0 b | 1.73±0.16 a | 1.67±0.18 a | 1.74±0.08 a | 0.0016 | 0.0009 | 0.0009 |
NO production in confluent HAEC stimulated with vehicle (C) or genistein (G, 5 μmol/L) in the presence or absence of ICI 182,780 (I, 1 μmol/L), 17β-estrodial (E2, 10 nmol/L), or daidzein (D, 5 μmol/L) for 5 d. Values are mean±SE from four separate experiments and expressed as fold of the control. Means without a common letter differ, P<0.05.
X=I, E2 or D.
To evaluate whether genistein enhances NO production through inhibition of PTK, we compared the effect of genistein with that of daidzein, an analogue of genistein that is inactive for PTK inhibition, on NO production. Daidzein was as potent as genistein in stimulation of NO production (Table 1). However, there was no additive effect between genistein and daidzein, suggesting that two molecules may act through the same mechanisms in stimulation of NO production.
Genistein enhances eNOS protein through up-regulating mRNA transcription in HAEC
Genistein increased eNOS protein levels, with 1 μmol/L genistein inducing a significant effect, although the maximal effect of genistein on eNOS protein expression was achieved at 10 μmol/L concentration (1.5 fold of control) (Fig. 2A). These results are consistent with the effect of genistein on NO production (Fig. 1A), suggesting that the elevated NO production by genistein may be attributable to an increase in eNOS protein expression. To investigate whether genistein elevates eNOS protein level via a transcriptional mechanism, we first tested whether genistein had an effect on eNOS mRNA expression in HAEC by using quantitative real-time PCR. Exposure of HAEC to various concentrations of genistein for 5 d, the same duration used to study genistein-induced eNOS protein expression and NO production, increased eNOS mRNA levels to 2.6 fold of control at 10 μmol/L genistein (Fig. 2B), consistent with its effect on eNOS protein expression and NO production. This result suggests that genistein may regulate eNOS expression at the transcriptional level. To confirm this, HAEC were transfected with a human eNOS promoter-driven reporter gene, followed by stimulation with genistein. Genistein significantly elevated human eNOS promoter activity to about 1.8-fold of control at 10 μmol/L (Fig. 2C), consistent with its effect on eNOS expression and NO synthesis. However, E2 (10 nmol/L), which failed to enhance NO production, also had no effect on the eNOS promoter activity in HAEC (data not shown).
FIGURE 2.
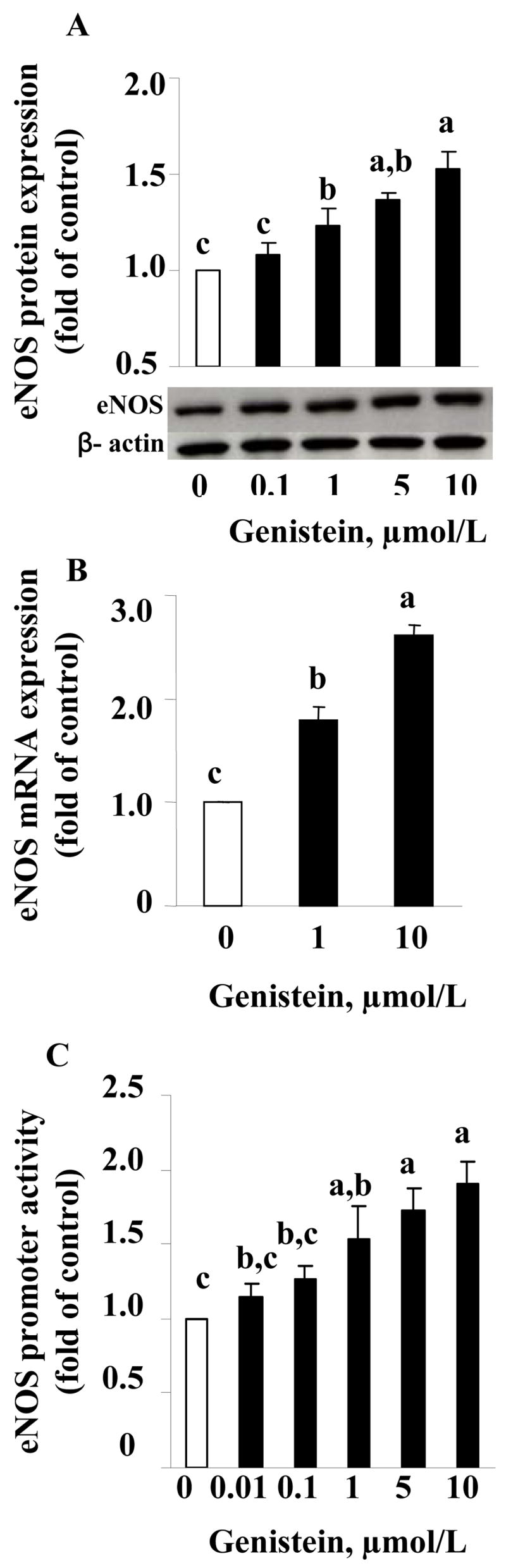
A. eNOS protein (A) or mRNA (B) expression normalized to β-actin content in HAEC treated with various concentrations of genistein or vehicle for 5 d; C. eNOS promoter activity in transfected HAEC stimulated with genistein or vehicle for 24 h. Values are mean±SE from three separate experiments and expressed as fold of the control. Means without a common letter differ, P<0.05.
Genistein increases NO production, eNOS protein expression and promoter activity in HUVEC
To determine whether genistein has a similar effect on another type of EC, we performed this study with HUVEC. The results demonstrated that genistein as low as 10 nmol/L induced NO production (Fig. 3A) and eNOS expression (Fig. 3B) in HUVEC, with a maximal effect at 1–10 μmol/L genistein. We further transfected the eNOS promoter-driven luciferase gene constructs in HUVEC. Genistein stimulated the eNOS promoter activity with a maximal effect at 1–10 μmol/L in HUVEC (Fig. 3C), confirming a transcriptional effect of genistein in HAEC.
FIGURE 3.
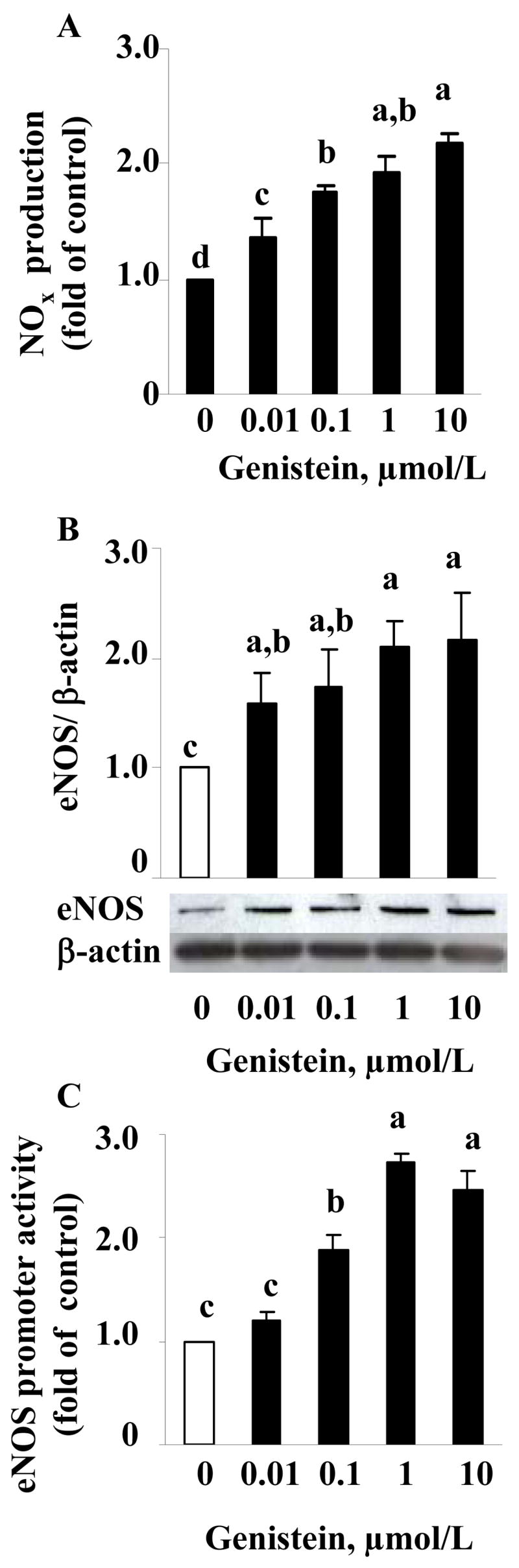
NO production in the supernatants (A) and eNOS protein expression normalized to β-actin content (B) in HUVEC treated with genistein or vehicle for 48 h. C. eNOS promoter activity in transfected HUVEC stimulated with genistein or vehicle for 24h. Values are mean±SE from four separate experiments and expressed as fold of the control. Means without a common letter differ, P<0.05.
In vivo effects of genistein
To confirm in vivo the importance of the genomic effects of genistein on eNOS, we tested whether dietary supplementation of genistein can improve eNOS expression and reduce blood pressure in SHR, a widely used hypertension animal model, given that the eNOS/NO signaling is critical for maintaining vascular tone. As expected, dietary supplementation of genistein significantly elevated plasma genistein levels. Under our experimental conditions, plasma genistein levels in rats fed 0, 0.2, 0.5, 2.0 g/kg diet of genistein were 0, 1.20±0.03, 1.90±0.20, 5.05±0.49 μmol/L, respectively, which overlap the concentrations used in our in vitro studies and attainable plasma levels in humans (0.74–6.0 μmol/L) following consumption of soy products or isoflavones as dietary supplements (42, 43). Genistein treatment significantly reduced both the elevated systolic and diastolic blood pressures in SHR (Table 2), whereas heart rate was not altered by dietary supplementation of genistein (data not shown). In addition, we found that dietary supplementation of genistein for 6 wk lowered blood pressure in adult SHR after the onset of hypertension. Impressively, this blood pressure-lowering effect of genistein was still significant at 6 wk after genistein withdrawal from the diet (Fig. 4 A). Genistein had no effect on body weight and food intake throughout the experimental period (data no shown). Furthermore, we found that aortic wall thickness was significantly greater in SHR than in WKY (Fig. 4 B), confirming previous study showing that the higher blood pressure is associated with the increased aortic wall thickness (44). However, genistein administration significantly decreased aortic wall thickness in SHR (Fig. 4 B). Previous studies have reported that eNOS protein expression was significantly reduced in SHR which led to hypertension in these animals (45, 46). To examine whether genistein has an effect on eNOS in these animals, as a possible explanation of its blood pressure-lowering effect, we measured the eNOS protein expression in aortic vessels by Western blotting. Our results showed that dietary intake of genistein restored eNOS protein content in the vasculature of SHR, with doses of 0.5–2.0 g/kg diet inducing eNOS expression similar to that in WKY (Fig. 4 C), suggesting that genistein administration likely reduces hypertension via a modulation of eNOS expression.
TABLE 2.
Dietary supplementation of genistein lowered blood pressure in SHR1
| WKY
|
SHR
|
||||
|---|---|---|---|---|---|
| Genistein, g/kg | 0 | 0 | 0.2 | 0.5 | 2 |
| Systolic, mmHg | 146.4±4.7c | 217.1±3.9a | 200.8±3.2b | 196.0±5.6b | 188.1±3.9b |
| Diastolic, mmHg | 97.6±2.6d | 155.1±3.3a | 149.8±2.4a,b | 142.4±2.7b,c | 140.4±4.0c |
Blood pressure in rats fed a basal or genistein diet for 19 wk. Values are means ± SE, n=8 rats. Means without a common letter differ. P<0.05.
FIGURE 4.
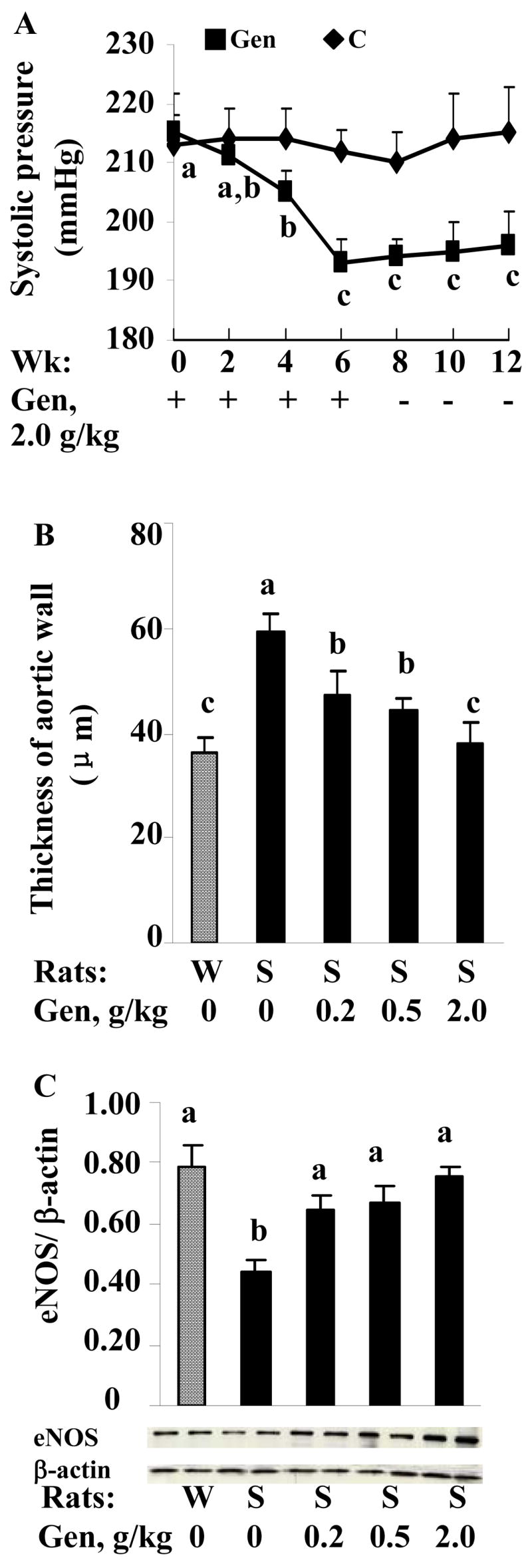
A. Systolic blood pressure in adult SHR fed a basal or genistein (Gen) diet for 6 wk followed by a genistein-free diet for additional 6 wk. Aortic wall thickness (B) and eNOS protein normalized to β-actin content (C) in WKY (W) and SHR (S) fed a basal or genistein (Gen) diet for 19 wk. Data are mean±SE (n=8 rats). Values without a common letter differ, P<0.05.
Discussion
Vascular EC, which not only serve as a biological barrier separating circulating blood and peripheral tissues, but also secrete various vasoactive substances, play a pivotal role in maintaining normal vascular function. Therefore, a major goal of our study was to determine whether genistein has a direct effect on vascular EC and thereby provide the molecular mechanisms by which genistein exerts some beneficial effects on the vasculature. We have demonstrated that, genistein, at physiologically achievable concentrations, activates eNOS transcription, leading to eNOS synthesis and NO production in human primary vascular EC. We further showed that this genistein effect on eNOS is present in vivo, confirming the biological relevance of the in vitro findings. Endothelium-derived NO is not only a potent vasodilator but also possesses anti-inflammatory (47), anti-atherogenic (48), anti-thrombotic (49), and anti-apoptotic (50) properties. Consistent with the key role of NO in vascular function, dietary administration of genistein lowered blood pressure in hypertensive rats. Recent studies reported that postmenopausal women taking genistein for 6 months have increased plasma levels of nitrate and nitrite, the stable metabolites of NO, and enhanced flow-mediated vasodilation in the forearm (51). Our finding that genistein directly targets EC to regulate eNOS is therefore important, since it may provide a molecular explanation for some vascular protective effects observed in animal and human studies (32, 51).
Genistein is considered as a specific ERβ agonist since it binds to ERβ with an affinity comparable to that of E2 but has a considerably lower affinity for ERα (52). Studies showed that E2 may regulate the transcription of eNOS in an ER-dependent manner in these cells (53, 54). However, our data indicate that genistein regulation of eNOS and NO was independent of ERs. First, the specific ER antagonist ICI 182,780 did not inhibit the effect of genistein on eNOS activation. Second, while E2 potentiated the effect of genistein on NO production, it had no effect on NO and eNOS promoter activity in HAEC. Third, daidzein, an analogue of genistein that is essentially lack of affinity for ERs (52), also induced an increase in NO production similar to that caused by genistein in HAEC. Thus, the transcriptional effect of genistein on eNOS is independent of this classical estrogen signaling mechanism. In line with our finding, a recent study showed that the effect of genistein on eNOS promoter activity is not mediated through ERs in transformed vascular cells (34). In addition, accumulating evidence indicates that genistein exerts various vascular effects that are ER-independent (31, 55). While both ERs are present in vascular EC, the role for ERβ in vascular function remains to be investigated. Some studies indicated that the effect of E2 on NO is mediated through ERα but not ERβ (56), providing a possible explanation for an ER-independent effect of genistein on NO, given that genistein only has about 4% affinity to ERα compared with E2 (52). Recently, an estrogen-related receptor α1 (ERRα1), a member of the steroid/thyroid hormone receptor superfamily expressed in EC, was reported to up-regulate eNOS promoter and protein expression in EC that was not related to ERs (57). Interestingly, this ERRα1-mediated eNOS expression pattern is similar to that observed in genistein-treated EC. It is therefore compelling to investigate whether genistein regulates eNOS through this estrogen-related signaling pathway.
Previous studies established that phosphoinositol-3-kinase/Akt (PI3K/Akt) and ERK-mitogen activated protein kinases (ERK/MPAK)-mediated pathways are two important signaling cascades mediating eNOS activation by many stimuli in vascular EC (58, 59). However, activation of these signaling pathways only leads to acute eNOS activation without an increase in protein expression, suggesting that genistein-induced eNOS expression is unlikely related to PI3K/Akt or ERK/MAPK activity. Indeed, pharmacological inhibition of these pathways had no effect on genistein-stimulated eNOS and NO (data not shown). Cyclic AMP responsive element (CRE) sites are present within neuronal NO synthase (nNOS), which regulate nNOS gene expression through binding with CRE binding protein (CREB) (60). A recent study reported that eNOS also contains CRE sites through which the cAMP signaling regulates eNOS transcription (61). We recently found that genistein directly activates the cAMP signaling system and regulates CRE-mediated gene expression in primary vascular EC (31). Our unpublished results showed that genistein dose-dependently increased CREB phosphorylation in HAEC, which is required to activate transcription of target genes, and this effect was abolished by H89, an inhibitor of PKA. Thus, it is conceivable that genistein may, at least in part, up-regulate eNOS expression via activation of cAMP signaling, which is an ongoing area of investigation in this laboratory.
We have shown that dietary administration of genistein reduced the thickness of the wall of the aorta and improved arterial blood pressure in SHR, a widely used animal model for the study of human hypertension, as these rats spontaneously develop the metabolic features similar to the pathogenesis of human hypertension (62). Our study also showed that genistein had no effect on heart rate, food intake and body weight, suggesting that the beneficial effect on blood pressure is not due to alteration of these parameters. Our further animal studies demonstrated that genistein also can improve blood pressure in adult SHR with well-developed hypertension, suggesting a possibly therapeutic potential of genistein for hypertension. Remarkably, after 6 weeks of genistein withdrawal, the blood pressure in genistein-fed SHR was still significantly lower than that in control SHR. Previous studies demonstrated that eNOS expression is reduced in SHR compared to that of normal rats (45, 63) which was further confirmed in this study. However, dietary supplementation of genistein restored eNOS levels in aortic vessels isolated from these rats, suggesting that the reduced eNOS expression contributes to the increased blood pressure in SHR, given the important role of eNOS in regulating vascular homeostasis. These outcomes are consistent with previous studies showing that genistein increases eNOS in rat aorta, liver (32) and heart (64). While it is presently unknown how genistein affects in vivo eNOS expression, the evidence from our in vitro study suggests that genistein may induce eNOS protein expression by directly targeting the vascular wall.
Progressive arterial hypertrophy is an important component of vasculature adaptation to the elevated arterial pressure. It has been found in the present study that the thickness of arterial wall is significantly greater in SHR than in WKY, consistent with previous observations (44). However, genistein administration significantly decreased aortic wall thickness in SHR. Recent studies showed that genistein inhibits the proliferation of vascular smooth muscle cells (VSMC) isolated from SHR, suggesting that genistein may have a direct effect on VSMC in vessel wall, though this effect was obtained only at pharmacological doses of genistein (65). It has been established that eNOS-derived NO inhibits VSMC cell growth (66), and our in vitro and in vivo data indicated that genistein has a direct genomic effect on eNOS expression, it is therefore intriguing to speculate that a secondary action whereby genistein enhances eNOS may contribute to the overall inhibitory effect of genistein on VSMC growth, and thereby improves blood pressure. This aspect however, needs further investigation.
In summary, this study demonstrates for the first time to our knowledge, that genistein can enhance eNOS gene transcription and protein synthesis in primary human vascular EC, leading to NO production. Dietary genistein administration stimulated eNOS expression, improved vessel wall thickening, and alleviated hypertension in SHR, confirming the biological relevance of the in vitro findings. These findings potentially provide a basic mechanism underlying the physiological effects of genistein in the vasculature.
Acknowledgments
We thank Kathy Reynolds, Janet Rinehart and Wei Zhen for their excellent technical assistance.
Footnotes
Supported by grants from the American Heart Association Mid-Atlantic Affiliate (to D. Liu), the National Center for Complementary and Alternative Medicine of the National Institute of Health (R21AT002739 to D. Liu) and Diabetes Research and Education Foundation (to D. Liu), and an ASPIRES award (to D. Liu) and the John Lee Pratt Fellowship (H. Si) from Virginia Polytechnic Institute and State University.
Author disclosures: D.Liu and H.Si, no conflicts of interest.
Abbreviations used: CRE, cAMP-responsive element; CREB, cAMP-responsive element binding protein; CVD, cardiovascular disease; DMSO, dimethylsulfoxide; E2, 17β-estradiol; EC, endothelial cells; eNOS, endothelial nitric oxide synthase; ERK/MAPK, ERK-mitogen activated protein kinase; ERRα1, estrogen-related receptor α1; ER, estrogen receptors; FBS, fetal bovine serum; HAEC, human aortic endothelial cells; HBSS, Hank’s balanced salts solution; HUVEC, human umbilical vein endothelial cells; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; PI3K/AKT, phosphoinositol-3 kinase/AKT; PKA, protein kinase A; PTK, protein tyrosine kinase; SHR, spontaneously hypertensive rats; VSMC, vascular smooth muscle cells; WKY, Wistar-Kyoto rats.
Literature Cited
- 1.Scuteri A, Ferrucci L. Blood pressure, arterial function, structure, and aging: the role of hormonal replacement therapy in postmenopausal women. J Clin Hypertens (Greenwich) 2003;5:219–25. doi: 10.1111/j.1524-6175.2003.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. New Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 3.Dubey RK, Gillespie DG, Imthurn B, Rosselli M, Jackson EK, Keller PJ. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension. 1999;33:177–82. doi: 10.1161/01.hyp.33.1.177. [DOI] [PubMed] [Google Scholar]
- 4.Altavilla D, Crisafulli A, Marini H, Esposito M, D’Anna R, Corrado F, Bitto A, Squadrito F. Cardiovascular effects of the phytoestrogen genistein. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:179–86. doi: 10.2174/1568016043477297. [DOI] [PubMed] [Google Scholar]
- 5.Park D, Huang T, Frishman WH. Phytoestrogens as cardioprotective agents. Cardiol Rev. 2005;13:13–7. doi: 10.1097/01.crd.0000126084.68791.32. [DOI] [PubMed] [Google Scholar]
- 6.B HARP AWHAJ. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am J Physiol cell physiol. 2001;280:c807–c13. doi: 10.1152/ajpcell.2001.280.4.C807. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Sohn I, Lee YS, Lee YS. Hepatic gene expression profiles are altered by genistein supplementation in mice with diet-induced obesity. J Nutr. 2005;135:33–41. doi: 10.1093/jn/135.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr. 2003;78:593S–609S. doi: 10.1093/ajcn/78.3.593S. [DOI] [PubMed] [Google Scholar]
- 9.Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr. 2001;131:1202–6. doi: 10.1093/jn/131.4.1202. [DOI] [PubMed] [Google Scholar]
- 10.de Kleijn MJ, van der Schouw YT, Wilson PW, Adlercreutz H, Mazur W, Grobbee DE, Jacques PF. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study(1–4) J Nutr. 2001;131:1826–32. doi: 10.1093/jn/131.6.1826. [DOI] [PubMed] [Google Scholar]
- 11.Anthony MS, Clarkson TB, Williams JK. Effects of soy isoflavones on atherosclerosis: potential mechanisms. Am J Clin Nutr. 1998;68:1390S–3S. doi: 10.1093/ajcn/68.6.1390S. [DOI] [PubMed] [Google Scholar]
- 12.van der Schouw YT, de Kleijn MJ, Peeters PH, Grobbee DE. Phyto-oestrogens and cardiovascular disease risk. Nutr Metab Carciovas. 2000;10:154–67. [PubMed] [Google Scholar]
- 13.Wangen KE, Duncan AM, Xu X, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001;73:225–31. doi: 10.1093/ajcn/73.2.225. [DOI] [PubMed] [Google Scholar]
- 14.Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The Phytoestrogen Genistein Produces Acute Nitric Oxide-Dependent Dilation of Human Forearm Vasculature With Similar Potency to 17ss-Estradiol. Circulation. 2001;103:258–62. doi: 10.1161/01.cir.103.2.258. [DOI] [PubMed] [Google Scholar]
- 15.Squadrito F, Altavilla D, Crisafulli A, Saitta A, Cucinotta D, Morabito N, D’Anna R, Corrado F, Ruggeri P, Frisina N, Squadrito G. Effect of genistein on endothelial function in postmenopausal women: a randomized, double-blind, controlled study. Am J Med. 2003;114:470–6. doi: 10.1016/s0002-9343(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 16.Nestel PJ, Yamashita T, Sasahara T, Pomeroy S, Dart A, Komesaroff P, Owen A, Abbey M. Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler Thromb Vasc Biol. 1997;17:3392–8. doi: 10.1161/01.atv.17.12.3392. [DOI] [PubMed] [Google Scholar]
- 17.Anthony MS, Clarkson TB, Hughes CL, Jr, Morgan TM, Burke GL. Soybean isoflavones improve cardiovascular risk factors without affecting the reproductive system of peripubertal rhesus monkeys. J Nutr. 1996;126:43–50. doi: 10.1093/jn/126.1.43. [DOI] [PubMed] [Google Scholar]
- 18.Kondo K, Suzuki Y, Ikeda Y, Umemura K. Genistein, an isoflavone included in soy, inhibits thrombotic vessel occlusion in the mouse femoral artery and in vitro platelet aggregation. Eur J Pharmacol. 2002;455:53–7. doi: 10.1016/s0014-2999(02)02449-4. [DOI] [PubMed] [Google Scholar]
- 19.Cassidy A, Hooper L. Phytoestrogens and cardiovascular disease. J Br Menopause Soc. 2006;12:49–56. doi: 10.1258/136218006777525776. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- 21.Vega-Lopez S, Yeum KJ, Lecker JL, Ausman LM, Johnson EJ, Devaraj S, Jialal I, Lichtenstein AH. Plasma antioxidant capacity in response to diets high in soy or animal protein with or without isoflavones. Am J Clin Nutr. 2005;81:43–9. doi: 10.1093/ajcn/81.1.43. [DOI] [PubMed] [Google Scholar]
- 22.An J, Tzagarakis-Foster C, Scharschmidt TC, Lomri N, Leitman DC. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J Biol Chem. 2001;276:17808–14. doi: 10.1074/jbc.M100953200. [DOI] [PubMed] [Google Scholar]
- 23.Clarkson TB, Anthony MS, Williams JK, Honore EK, Cline JM. The potential of soybean phytoestrogens for postmenopausal hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:365–8. doi: 10.3181/00379727-217-44246. [DOI] [PubMed] [Google Scholar]
- 24.Colacurci N, Chiantera A, Fornaro F, de Novellis V, Manzella D, Arciello A, Chiantera V, Improta L, Paolisso G. Effects of soy isoflavones on endothelial function in healthy postmenopausal women. Menopause. 2005;12:299–307. doi: 10.1097/01.gme.0000147017.23173.5b. [DOI] [PubMed] [Google Scholar]
- 25.Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17beta-estradiol. Circulation. 2001;103:258–62. doi: 10.1161/01.cir.103.2.258. [DOI] [PubMed] [Google Scholar]
- 26.Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, Deodato B, Ferlito M, Campo GM, Bova A, Caputi AP. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc Res. 2000;45:454–62. doi: 10.1016/s0008-6363(99)00359-4. [DOI] [PubMed] [Google Scholar]
- 27.Karamsetty MR, Klinger JR, Hill NS. Phytoestrogens restore nitric oxide-mediated relaxation in isolated pulmonary arteries from chronically hypoxic rats. J Pharmacol Exp Ther. 2001;297:968–74. [PubMed] [Google Scholar]
- 28.MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, Shaul PW. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res. 1997;81:355–62. doi: 10.1161/01.res.81.3.355. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–6. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D, Homan LL, Dillon JS. Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinology. 2004;145:5532–9. doi: 10.1210/en.2004-0102. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Jiang H, Grange RW. Genistein activates the 3′,5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology. 2005;146:1312–20. doi: 10.1210/en.2004-1221. [DOI] [PubMed] [Google Scholar]
- 32.Mahn K, Borras C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, Vina J, Aaronson PI, Mann GE. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. Faseb J. 2005;19:1755–7. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- 33.Vera R, Sanchez M, Galisteo M, Villar IC, Jimenez R, Zarzuelo A, Perez-Vizcaino F, Duarte J. Chronic administration of genistein improves endothelial dysfunction in spontaneously hypertensive rats: involvement of eNOS, caveolin and calmodulin expression and NADPH oxidase activity. Clin Sci (Lond) 2007;112:183–91. doi: 10.1042/CS20060185. [DOI] [PubMed] [Google Scholar]
- 34.Rathel TR, Leikert JF, Vollmar AM, Dirsch VM. The soy isoflavone genistein induces a late but sustained activation of the endothelial nitric oxide-synthase system in vitro. Br J Pharmacol. 2005;144:394–9. doi: 10.1038/sj.bjp.0706075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.John GB. Second report of the ad hoc committee on standards for nutritional studies. J Nutr. 1980;110:1726. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 36.Thomas BF, Zeisel SH, Busby MG, Hill JM, Mitchell RA, Scheffler NM, Brown SS, Bloeden LT, Dix KJ, Jeffcoat AR. Quantitative analysis of the principle soy isoflavones genistein, daidzein and glycitein, and their primary conjugated metabolites in human plasma and urine using reversed-phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl. 2001;760:191–205. doi: 10.1016/s0378-4347(01)00269-9. [DOI] [PubMed] [Google Scholar]
- 37.Turbino-Ribeiro SM, Silva ME, Chianca DA, Jr, De Paula H, Cardoso LM, Colombari E, Pedrosa ML. Iron overload in hypercholesterolemic rats affects iron homeostasis and serum lipids but not blood pressure. J Nutr. 2003;133:15–20. doi: 10.1093/jn/133.1.15. [DOI] [PubMed] [Google Scholar]
- 38.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–41. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 39.Qian X, Jin L, Hayden RT, Macon WR, Lloyd RV. Diagnosis of cat scratch disease with Bartonella henselae infection in formalin-fixed paraffin-embedded tissues by two different PCR assays. Diagn Mol Pathol. 2005;14:146–51. doi: 10.1097/01.pas.0000176771.64165.fb. [DOI] [PubMed] [Google Scholar]
- 40.Liu D, Si H, Reynolds KA, Zhen W, Jia Z, Dillon JS. Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Galphai protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endocrinology. 2007;148:3068–76. doi: 10.1210/en.2006-1378. [DOI] [PubMed] [Google Scholar]
- 41.Kim H, Peterson TG, Barnes S. Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor beta signaling pathways. Am J Clin Nutr. 1998;68:1418S–25S. doi: 10.1093/ajcn/68.6.1418S. [DOI] [PubMed] [Google Scholar]
- 42.Adlercreutz CH, Goldin BR, Gorbach SL, Hockerstedt KA, Watanabe S, Hamalainen EK, Markkanen MH, Makela TH, Wahala KT, Adlercreutz T. Soybean phytoestrogen intake and cancer risk. J Nutr. 1995;125:757S–70S. doi: 10.1093/jn/125.3_Suppl.757S. [DOI] [PubMed] [Google Scholar]
- 43.Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S. Bioavailability of soybean isoflavones depends upon gut microflora in women. J Nutr. 1995;125:2307–15. doi: 10.1093/jn/125.9.2307. [DOI] [PubMed] [Google Scholar]
- 44.Kitayama J, Kitazono T, Ooboshi H, Ago T, Ohgami T, Fujishima M, Ibayashi S. Chronic administration of a tyrosine kinase inhibitor restores functional and morphological changes of the basilar artery during chronic hypertension. J Hypertens. 2002;20:2205–11. doi: 10.1097/00004872-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 45.Chou TC, Yen MH, Li CY, Ding YA. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–8. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- 46.Safar M, Chamiot-Clerc P, Dagher G, Renaud JF. Pulse pressure, endothelium function, and arterial stiffness in spontaneously hypertensive rats. Hypertension. 2001;38:1416–21. doi: 10.1161/hy1201.096538. [DOI] [PubMed] [Google Scholar]
- 47.Higuchi H, Granger DN, Saito H, Kurose I. Assay of antioxidant and antiinflammatory activity of nitric oxide in vivo. Methods Enzymol. 1999;301:424–36. doi: 10.1016/s0076-6879(99)01106-4. [DOI] [PubMed] [Google Scholar]
- 48.Iturry-Yamamoto G, Alves AA, Picon PD. Antiatherogenic effects of endothelium-derived relaxing factor (nitric oxide) Arq Bras Cardiol. 1997;69:349–57. doi: 10.1590/s0066-782x1997001100010. [DOI] [PubMed] [Google Scholar]
- 49.Asakura H, Okudaira M, Ontachi Y, Mizutani T, Omote M, Yoshida T, Kaneda M, Yamazaki M, Morishita E, Takami A, Miyamoto K, Nakao S. Antithrombotic role of nitric oxide in rats under physiological conditions. Thromb Haemost. 2004;91:71–5. doi: 10.1160/TH03-05-0292. [DOI] [PubMed] [Google Scholar]
- 50.Li DY, Tao L, Liu H, Christopher TA, Lopez BL, Ma XL. Role of ERK1/2 in the anti-apoptotic and cardioprotective effects of nitric oxide after myocardial ischemia and reperfusion. Apoptosis. 2006;11:923–30. doi: 10.1007/s10495-006-6305-6. [DOI] [PubMed] [Google Scholar]
- 51.Squadrito F, Altavilla D, Morabito N, Crisafulli A, D’Anna R, Corrado F, Ruggeri P, Campo GM, Calapai G, Caputi AP, Squadrito G. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163:339–47. doi: 10.1016/s0021-9150(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 52.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 53.Sieck GC. Genome and hormones: an integrated approach to gender differences in physiology. J Appl Physiol. 2001;91:1485–6. doi: 10.1152/jappl.2001.91.4.1485. [DOI] [PubMed] [Google Scholar]
- 54.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–86. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 55.Vera R, Galisteo M, Villar IC, Sanchez M, Zarzuelo A, Perez-Vizcaino F, Duarte J. Soy isoflavones improve endothelial function in spontaneously hypertensive rats in an estrogen-independent manner: role of nitric-oxide synthase, superoxide, and cyclooxygenase metabolites. J Pharmacol Exp Ther. 2005;314:1300–9. doi: 10.1124/jpet.105.085530. [DOI] [PubMed] [Google Scholar]
- 56.Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, Chambon P, Bayard F, Arnal JF. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ Res. 2002;90:413–9. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- 57.Sumi D, Ignarro LJ. Estrogen-related receptor alpha 1 up-regulates endothelial nitric oxide synthase expression. Proc Natl Acad Sci U S A. 2003;100:14451–6. doi: 10.1073/pnas.2235590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernier SG, Haldar S, Michel T. Bradykinin-regulated interactions of the mitogen-activated protein kinase pathway with the endothelial nitric-oxide synthase. J Biol Chem. 2000;275:30707–15. doi: 10.1074/jbc.M005116200. [DOI] [PubMed] [Google Scholar]
- 59.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem. 2001;276:12420–6. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- 60.Sasaki M, Gonzalez-Zulueta M, Huang H, Herring WJ, Ahn S, Ginty DD, Dawson VL, Dawson TM. Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proc Natl Acad Sci U S A. 2000;97:8617–22. doi: 10.1073/pnas.97.15.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niwano K, Arai M, Tomaru K, Uchiyama T, Ohyama Y, Kurabayashi M. Transcriptional stimulation of the eNOS gene by the stable prostacyclin analogue beraprost is mediated through cAMP-responsive element in vascular endothelial cells: close link between PGI2 signal and NO pathways. Circ Res. 2003;93:523–30. doi: 10.1161/01.RES.0000091336.55487.F7. [DOI] [PubMed] [Google Scholar]
- 62.Trippodo NC, Frohlich ED. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ Res. 1981;48:309–19. doi: 10.1161/01.res.48.3.309. [DOI] [PubMed] [Google Scholar]
- 63.Zecchin HG, Bezerra RM, Carvalheira JB, Carvalho-Filho MA, Metze K, Franchini KG, Saad MJ. Insulin signalling pathways in aorta and muscle from two animal models of insulin resistance--the obese middle-aged and the spontaneously hypertensive rats. Diabetologia. 2003;46:479–91. doi: 10.1007/s00125-003-1073-0. [DOI] [PubMed] [Google Scholar]
- 64.Tang YB, Wang QL, Zhu BY, Huang HL, Liao DF. Phytoestrogen genistein supplementation increases eNOS and decreases caveolin-1 expression in ovariectomized rat hearts. Sheng Li Xue Bao. 2005;57:373–8. [PubMed] [Google Scholar]
- 65.Pan W, Ikeda K, Takebe M, Yamori Y. Genistein, daidzein and glycitein inhibit growth and DNA synthesis of aortic smooth muscle cells from stroke-prone spontaneously hypertensive rats. J Nutr. 2001;131:1154–8. doi: 10.1093/jn/131.4.1154. [DOI] [PubMed] [Google Scholar]
- 66.von der Leyen HE, Gibbons GH, Morishita R, Lewis NP, Zhang L, Nakajima M, Kaneda Y, Cooke JP, Dzau VJ. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. Proc Natl Acad Sci U S A. 1995;92:1137–41. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]


