Abstract
Background
Alterations in waveforms in the uterine artery are associated with the development of pre-eclampsia and intrauterine growth restriction. We investigated the predictive accuracy of all uterine artery Doppler indices for both conditions in the first and second trimesters.
Methods
We identified relevant studies through searches of MEDLINE, EMBASE, the Cochrane Library and Medion databases (all records to April 2006) and by checking bibliographies of identified studies and consulting with experts. Four of us independently selected studies, extracted data and assessed study validity. We performed a bivariable meta-analysis of sensitivity and specificity and calculated likelihood ratios.
Results
We identified 74 studies of pre-eclampsia (total 79 547 patients) and 61 studies of intrauterine growth restriction (total 41 131 patients). Uterine artery Doppler ultrasonography provided a more accurate prediction when performed in the second trimester than in the first-trimester. Most Doppler indices had poor predictive characteristics, but this varied with patient risk and outcome severity. An increased pulsatility index with notching was the best predictor of pre-eclampsia (positive likelihood ratio 21.0 among high-risk patients and 7.5 among low-risk patients). It was also the best predictor of overall (positive likelihood ratio 9.1) and severe (positive likelihood ratio 14.6) intrauterine growth restriction among low-risk patients.
Interpretation
Abnormal uterine artery waveforms are a better predictor of pre-eclampsia than of intrauterine growth restriction. A pulsatility index, alone or combined with notching, is the most predictive Doppler index. These indices should be used in clinical practice. Future research should also concentrate on combining uterine artery Doppler ultrasonography with other tests.
Pre-eclampsia and intrauterine growth restriction remain important causes of maternal and perinatal morbidity and mortality.1–3 Maternal complications of pre-eclampsia include coagulopathy, renal and liver failure, and stroke.1 Adults who were affected by intrauterine growth restriction in utero are at increased risk for cardiovascular disease, hypertension and type 2 diabetes.4,5
Pre-eclampsia and intrauterine growth restriction are characterized by abnormal placenta formation,6 which results in inadequate uteroplacental blood flow. This has led to the idea of using Doppler ultrasonography to assess the velocity of uterine artery blood flow as part of routine ultrasound screening.7 Low end-diastolic velocities and an early diastolic notch characterize the waveforms of uterine artery blood flow in women who are not pregnant or are in their first trimester. Persistence of a diastolic notch (beyond 24 weeks' gestation) or abnormal flow velocity ratios have been associated with inadequate trophoblast invasion.8
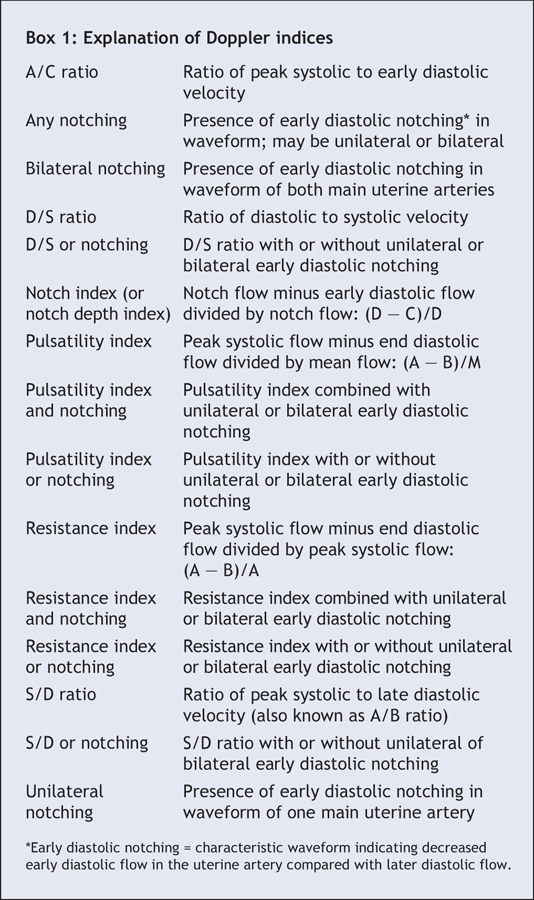
Accurate prediction of pre-eclampsia and intrauterine growth restriction is crucial to allow judicious allocation of resources for monitoring and preventive treatment to improve maternal and perinatal outcomes.9,10 However, studies investigating the predictive accuracy of uterine artery Doppler indices (Box 1) have revealed considerably varied results. Thus, it is questionable whether uterine artery Doppler ultrasonography should be used as a predictive test. We undertook this review to investigate the accuracy of all uterine artery Doppler indices in predicting pre-eclampsia and intrauterine growth restriction.
Box 1.
Methods
Literature search
We based our review on our previously published protocols.11,12 Experienced clinical librarians performed 2 electronic searches, without language restrictions, in the MEDLINE, EMBASE, Cochrane Library and Medion (www.mediondatabase.nl) databases for all records to April 2006 (details of the search strategies are provided in Appendix 1, available online at www.cmaj.ca/cgi/content/full/178/6/701/DC2). We also checked reference lists of identified articles and consulted with experts (Figure 1). We included studies that reported Doppler assessment of the main uterine arteries in the first and second trimester among pregnant women in any health care setting and at any level of risk for pre-eclampsia or intrauterine growth restriction. We included test-accuracy studies that allowed generation of 2 × 2 tables.
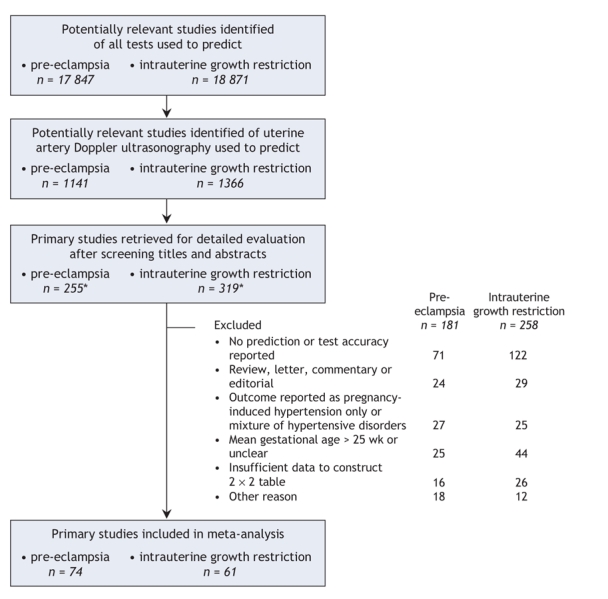
Figure 1: Identification of studies of uterine artery Doppler ultrasonography used to predict pre-eclampsia and intrauterine growth restriction, for inclusion in the meta-analysis. *Includes 6 studies on pre-eclampsia and 8 on intrauterine growth restriction that were added after manual search of bibliographies of selected articles.
Quality assessment and data abstraction
For pre-eclampsia, 4 of us (J.C., R.M., G.R. and B.M.) independently screened titles and abstracts of identified studies and, if relevant, the full-text articles for inclusion and data extraction.11 For intrauterine growth restriction, this work was carried out by 1 of us (R.M.), and 10% of the papers were examined by a second independent reviewer (B.M.).12 One reviewer (R.M.) assessed the studies for their methodological quality, using the QUADAS (quality assessment of diagnostic accuracy studies) criteria,13 and whether any preventive interventions had been used (see Appendix 2, available online at www.cmaj.ca/cgi/content/full/178/6/701/DC2 ). For multiple publications of the same data set, only the most recent or complete study was included. Disagreements were resolved by consensus or by a third reviewer.
Acceptable reference standards for pre-eclampsia were persistently high systolic (≥ 140 mm Hg) or diastolic (≥ 90 mm Hg) blood pressure and proteinuria (≥ 0.3 g of protein in 24-hour urine collection, or dipstick test result of ≥ 1+ [equivalent to 30 mg/dL in single urine sample]) of new onset after 20 weeks of gestation. We defined severe pre-eclampsia as a systolic blood pressure of 160 mm Hg or greater or a diastolic blood pressure of 110 mm Hg or greater plus a high level of proteinuria (≥ 2.0 g of protein in 24-hour urine collection or dipstick test result of ≥ 3+), or the development of pre-eclampsia before 34 weeks' gestation. We defined superimposed pre-eclampsia as the development of proteinuria (≥ 0.3 g of protein in 24-hour urine collection or dipstick test result ≥ 1+) after 20 weeks of gestation in patients with chronic hypertension.14
Acceptable reference standards for intrauterine growth restriction included birth weight below the 10th centile adjusted for gestational age and based on local population values. Severe intrauterine growth restriction was defined as birth weight below the fifth or third centile. We also assessed absolute birth-weight thresholds, neonatal ponderal index below the 10th centile, skin-fold thickness and ratio of mid-arm circumference to head circumference.15–19
Data analysis
We calculated the sensitivity and specificity for each study and pooled the results of studies with similar Doppler indices, outcomes and patient risk. We used a bivariable regression model that takes into account the (negative) correlation between sensitivity and specificity. This method has been extensively described elsewhere and has been recently recommended for meta-analyses of diagnostic tests.20,21 To estimate sensitivity and specificity with their 95% confidence intervals (CIs), we used the model with the smallest Akaike's information criterion (a measure of the goodness of fit of an estimated statistical model). This model best accounts for heterogeneity between studies. When several thresholds for a Doppler index were reported, we used the commonest. We derived likelihood ratios from the pooled sensitivities and specificities.22
We performed subgroup analyses as defined a priori: outcome (severe v. mild or overall), patient risk (low or risk not specified v. high) and gestational age at testing (before v. after 16 weeks). Sensitivity analyses were performed for application of preventive interventions (yes v. no or unclear) and high-quality studies. We considered studies to be of high quality if they met at least 4 of the following criteria: prospective design with consecutive recruitment, appropriate reference standard, adequate description of the index test, follow-up of more than 90% of patients and reporting preventive treatment.
Results
We included 83 studies in total in our review. Fifty-two of them had both pre-eclampsia and intrauterine growth restriction as outcomes. We included these 52 studies in the assessments of each of the 2 outcomes.
Pre-eclampsia
We included 74 studies (69 cohort studies, 3 randomized controlled trials and 2 case–control studies) in which uterine artery Doppler ultrasonography had been used to predict pre-eclampsia; from these studies we were able to construct 180 2 × 2 tables. Of the studies, 50 were prospective, 10 were retrospective, and in 14 this was unclear. Details of the studies are provided in Appendix 3 (available online at www.cmaj.ca/cgi/content/full/178/6/701/DC2). The total number of women was 79 547, of whom 2498 were found to have pre-eclampsia. Samples varied in size from 28 to 16 806 women. In most of the studies, Doppler ultrasonography was performed between 18 and 24 weeks' gestation during a routine prenatal scan; 7 articles reported Doppler results before 16 weeks' gestation. The median rate of pre-eclampsia was 4.9% (25th–75th centiles 2.2%–9.8%). Rates of pre-eclampsia varied from 0.4% to 18.7% among patients at low or unspecified risk and from 3.2% to 44.3% among high-risk patients.
Intrauterine growth restriction
We included 61 studies (57 cohort studies and 3 randomized controlled trials) in which uterine artery Doppler ultrasonography was used to predict intrauterine growth restriction; from these studies we were able to construct 168 2 × 2 tables. Of the studies, 48 were prospective, 10 were retrospective, and in 2 this was unclear. Details of the studies are provided in Appendix 4 (available online at www.cmaj.ca/cgi/content/full/178/6/701/DC2). The total number of women was 41 131, of whom 3723 had intrauterine growth restriction (as determined by birth weight below the 10th centile or less than 2500 g if several thresholds were reported). The samples varied in size from 28 to 7851 women. Ten articles reported Doppler results before 16 weeks' gestation. Most of the studies used birth weight below the 10th centile as the reference standard for intrauterine growth restriction. One study reported absolute birth-weight thresholds, and in 2 studies the threshold was unclear. Calculated rates of intrauterine growth restriction correlated poorly with thresholds (birth weight in centiles) based on local population values. The mean rates of intrauterine growth restriction were 9.6% among patients whose risk was not specified, 8.2% among low-risk patients and 20.7% among high-risk patients.
Quality assessment
For both pre-eclampsia and intrauterine growth restriction, over 70% of the studies met the following QUADAS quality-assessment criteria:13 avoidance of partial and differential verification, independent reference test, and blind assessment of index test (for the results of the quality assessment, see Appendix 5, available online at www.cmaj.ca/cgi/content/full/178/6/701/DC2). Studies scored poorly in terms of adequate descriptions of selection criteria and reference test, blind assessment of the reference test and availability of clinical data. Whether the study did or did not include the use of preventive treatment was reported in 17 of the studies of pre-eclampsia and 18 of the studies of intrauterine growth restriction. The randomized controlled trials that applied preventive treatment in a series of women after Doppler ultrasonography were analyzed together with the cohort studies. We excluded 2 case–control studies from the analyses to further enhance validity.
Diagnostic test characteristics
For both pre-eclampsia and intrauterine growth restriction, Doppler testing was less accurate in the first trimester than in the second trimester. In this section, we noted the test characteristics that best predicted the development of pre-eclampsia and intrauterine growth restriction (largest positive likelihood ratio). Test characteristics that best predicted the absence of pre-eclampsia and intrauterine growth restriction were those with the smallest negative likelihood ratio (Table 1, Table 2, Table 3, Table 4).
Table 1
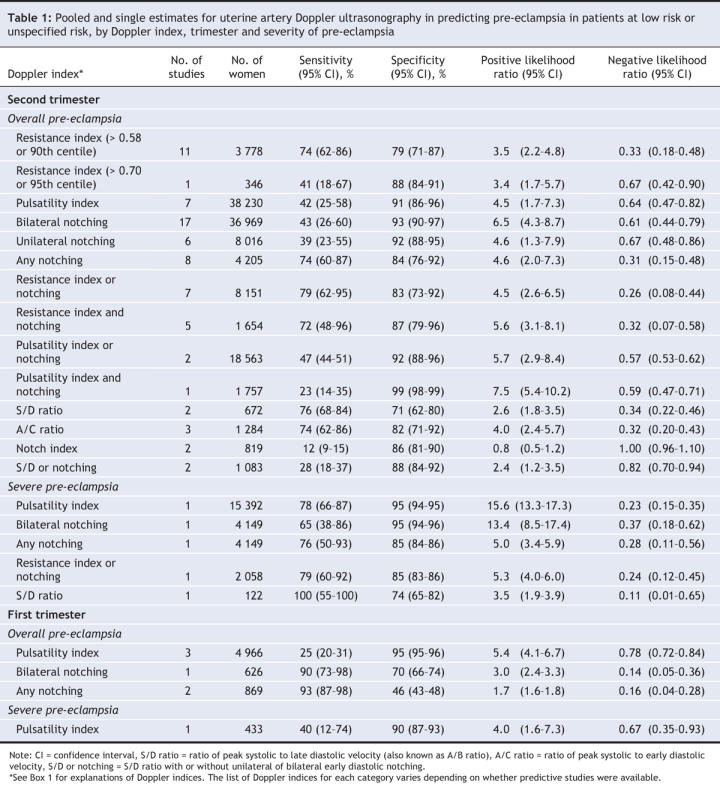
Table 2
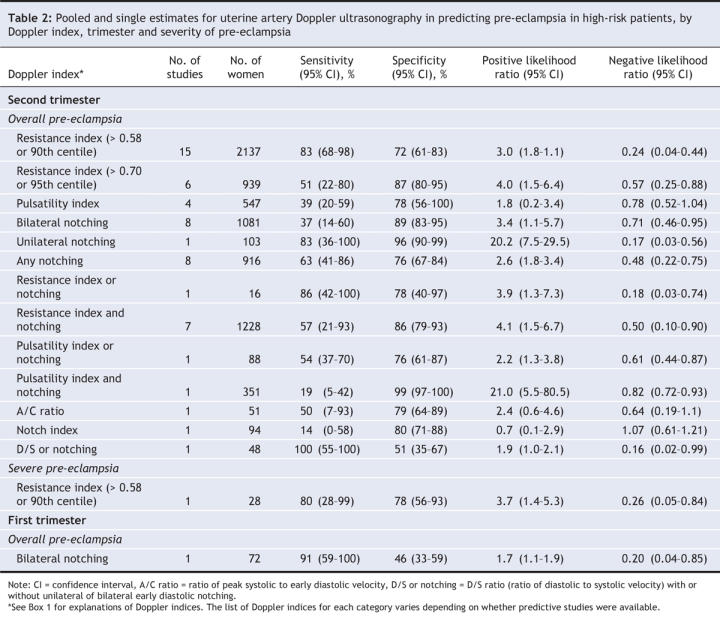
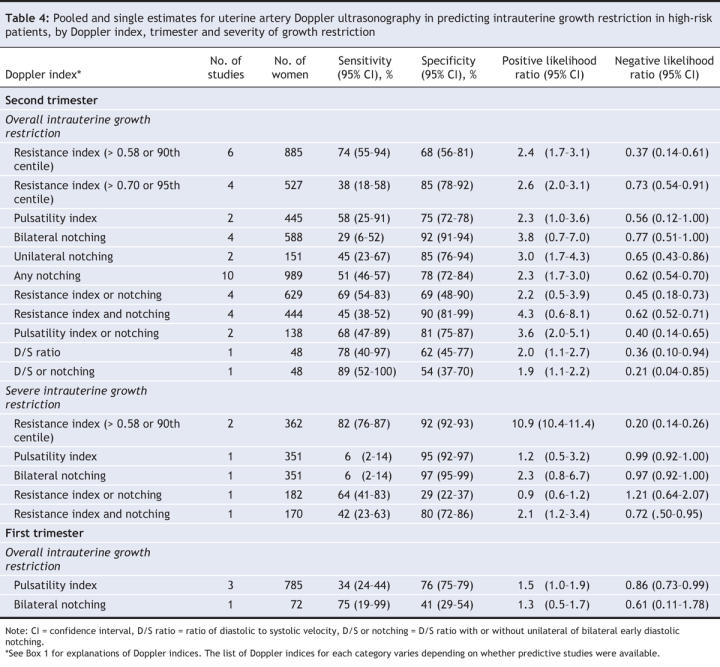
Table 3
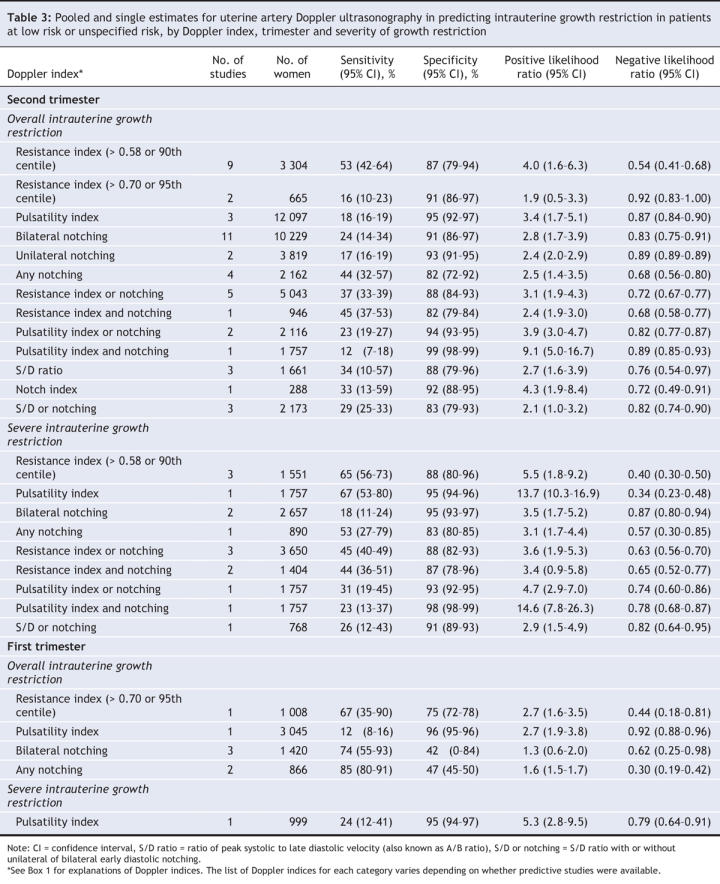
Table 4
Pre-eclampsia in low-risk patients was best predicted by an increased pulsatility index with diastolic notching in the second trimester (> 16 weeks) (positive likelihood ratio 7.5, 95% CI 5.4–10.2; negative likelihood ratio 0.59, 95% CI 0.47–0.71) (Table 1, Figure 2). Severe pre-eclampsia in low-risk patients was best predicted in the second trimester by an increased pulsatility index (positive likelihood ratio 15.6, 95% CI 13.3–17.3; negative likelihood ratio 0.23, 95% CI 0.15–0.35) and bilateral notching (positive likelihood ratio 13.4, 95% CI 8.5–17.4); negative likelihood ratio 0.4 (95% CI 0.2–0.6) (Figure 3). Among high-risk patients, pre-eclampsia was best predicted in the second trimester by unilateral notching (positive likelihood ratio 20.2, 95% CI 7.5–29.5; negative likelihood ratio 0.17, 95% CI 0.03–0.56) and an increased pulsatility index with notching (positive likelihood ratio 21.0, 95% CI 5.5–80.5; negative likelihood ratio 0.82, 95% CI 0.72–0.93) (Table 2, Figure 2). Doppler assessment to predict severe pre-eclampsia in high-risk patients showed low diagnostic characteristics (positive likelihood ratio 3.7) (Table 2, Figure 3). Many estimates were based on single studies or small numbers of women.
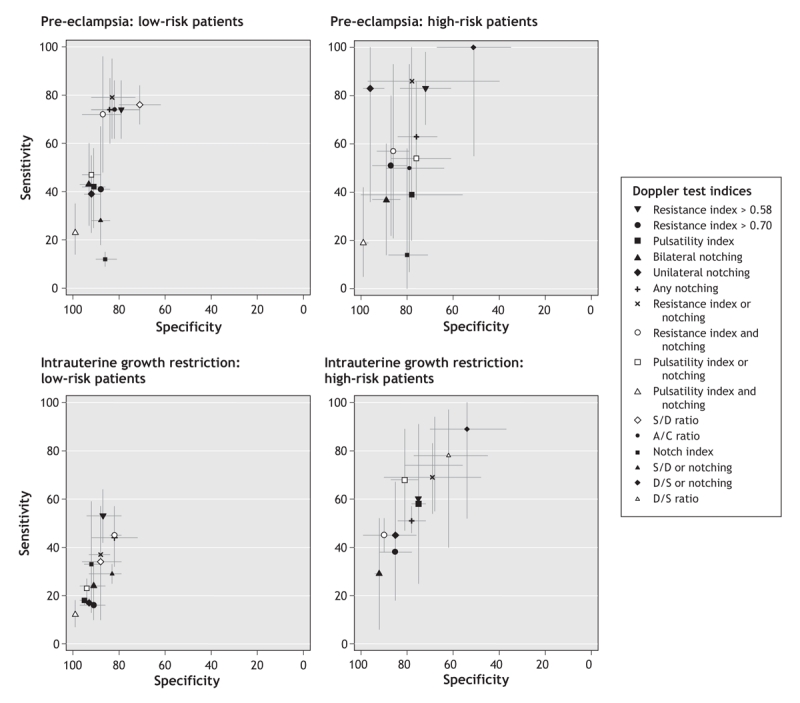
Figure 2: Plots of receiver operating characteristics showing pooled and single accuracy estimates, with 95% confidence intervals, for uterine artery Doppler indices to predict pre-eclampsia and intrauterine growth restriction in the second trimester according to patient risk. Note: the x axis shows reversed specificity. The closer the index values are to the upper left corner of each graph, the greater the accuracy of that index. The test index that best predicted the development of pre-eclampsia (highest positive likelihood ratio) in low-and high-risk patients was an increased pulsatility index with notching. This index was also the best predictor of intrauterine growth restriction in low-risk patients. For intrauterine growth restriction in high-risk patients, the Doppler indices showed low predictive value. (The thresholds for the Doppler indices reported in the studies we reviewed are provided in Appendices 3 and 4 [available online at www.cmaj.ca/cgi/content/full/178/6/701/DC2].)
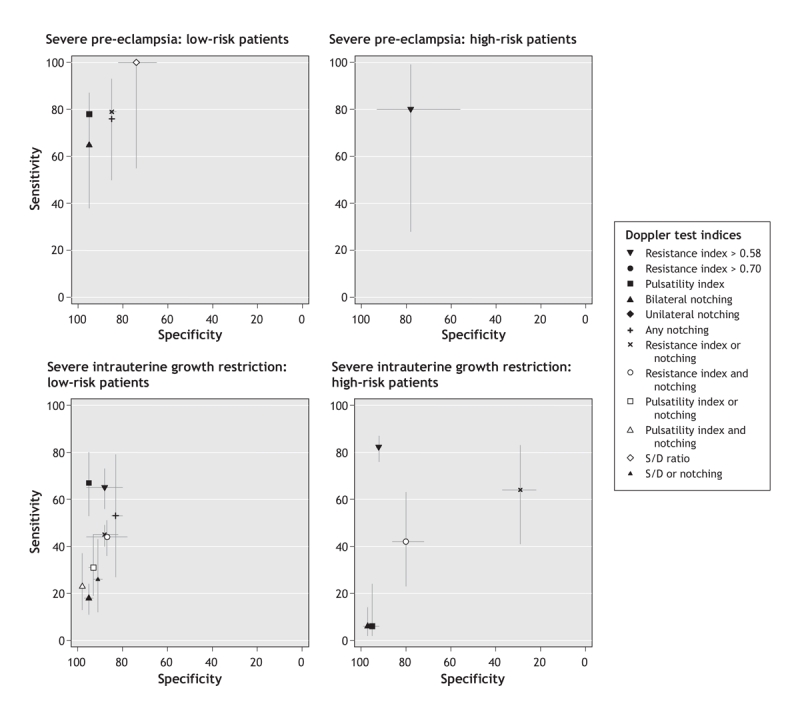
Figure 3: Plots of receiver operating characteristics showing pooled and single accuracy estimates, with 95% confidence intervals, for uterine artery Doppler indices to predict severe pre-eclampsia and severe intrauterine growth restriction in the first and second trimester. Note: the x axis shows reversed specificity. The closer the index values are to the upper left corner of each graph, the greater the accuracy of that index. In low-risk patients, an increased pulsatility index was the test characteristic that best predicted the development of severe pre-eclampsia or severe intrauterine growth restriction (highest positive likelihood ratio). In high-risk patients, the best predictor of each outcome was an increased resistance index > 0.58. (The thresholds for the Doppler indices reported in the studies we reviewed are provided in Appendices 3 and 4 [available online at www.cmaj.ca/cgi/content/full/178/6/701/DC2].)
Intrauterine growth restriction in low-risk patients was best predicted in the second trimester by an increased pulsatility index with notching (positive likelihood ratio 9.1, 95% CI 5.0–16.7; negative likelihood ratio 0.89, 95% CI 0.85–0.93) (Table 3, Figure 2). Severe intrauterine growth restriction in low-risk patients was best predicted in the second trimester by an increased pulsatility index (positive likelihood ratio 13.7, 95% CI 10.3–16.9; negative likelihood ratio 0.34, 95% CI 0.23–0.48) or an increased pulsatility index with notching (positive likelihood ratio 14.6, 95% CI 7.8–26.3; negative likelihood ratio 0.78, 95% CI 0.68–0.87) (Figure 3). Doppler assessment to predict intrauterine growth restriction in high-risk patients showed low diagnostic characteristics (Table 4, Figure 2). An increased resistance index (> 0.58 or > 90th centile) in the second trimester best predicted severe intrauterine growth restriction in high-risk patients (positive likelihood ratio 10.9, 95% CI 10.4–11.4; negative likelihood ratio 0.20, 95% CI 0.14–0.26).
Subgroup and sensitivity analyses
Our exclusion of studies that applied preventive treatment did not improve the accuracy of Doppler testing in predicting pre-eclampsia and intrauterine growth restriction. When we included only the high-quality studies in the analysis, we found that unilateral notching in the second trimester best predicted pre-eclampsia in patients at low risk or unspecified risk (positive likelihood ratio 12.5, 95% CI 5.1–20.0; negative likelihood ratio 0.45, 95% CI 0.09–0.80). In high-risk patients, an increased pulsatility index with notching remained the best predictor of pre-eclampsia in the high-quality studies. Results for intrauterine growth restriction showed low to moderate predictive value in both low-risk and high-risk patients. (Data from the subgroup and sensitivity analyses are available from the corresponding author.)
Interpretation
We evaluated the accuracy of 15 uterine artery Doppler indices in predicting pre-eclampsia and intrauterine growth restriction. Uterine artery Doppler ultrasonography predicts pre-eclampsia, the maternal consequence of placental disease, more confidently than intrauterine growth restriction. Specifically, an increased pulsatility index with notching in the second trimester best predicted overall pre-eclampsia in low-risk and high-risk patients. An increased pulsatility index or bilateral notching best predicted severe pre-eclampsia. An increased pulsatility index alone or in combination with notching best predicted severe intrauterine growth restriction in low-risk patients, whereas the best predictor in high-risk patients was an increased resistance index. Other Doppler indices showed low to moderate predictive value.
Previous reviews have reported that uterine artery Doppler ultrasonography has limited accuracy in predicting pre-eclampsia and intrauterine growth restriction.23–25 However, these reviews were restricted in terms of the thresholds and Doppler indices they reviewed or they reported independently pooled likelihood ratios, which is now discouraged.22 Two reviews reporting on pre-eclampsia, intrauterine growth restriction and perinatal death were limited to articles retrieved through MEDLINE: one review23 was based on a search of articles published before January 1997, and the other24 included patients with unspecified risk in articles up to 2001. A third review,25 on pre-eclampsia, has been criticized for its methodology.26 A fourth review9 concluded that Doppler assessment identifies high-risk women in whom acetylsalicylic acid therapy results in a significant reduction in pre-eclampsia. Since these reviews were published, substantial new evidence has emerged allowing for more robust and specific inferences for clinical practice.
The strength of our review was that we carried out extensive literature searches without language restrictions, assessed the quality of studies and their reporting and used recently developed statistical methods. However, our study had several limitations. First, our quality assessment was hindered by the lack of information in many studies, which is a common problem in diagnostic reviews. Poor study design and conduct can affect estimates of diagnostic accuracy,27,28 although it is not entirely clear how individual aspects of quality may affect accuracy and to what magnitude. Of many strategies applied to account for differences in quality, none has led systematically to less optimistic estimates than that of ignoring methodologic quality in meta-analyses of test accuracy studies.29,30 The reporting of details of how the index test was carried out and reference standards were applied was uniformly poor in the studies we reviewed. Second, definitions of pre-eclampsia have changed over time. Earlier definitions were also based on increases in blood pressure and edema. The measurement of blood pressure has been poorly reported, and the importance of recording diastolic blood pressure with Korotkoff phase V (which is more reliably recorded and reflects true diastolic blood pressure) has been underestimated.31–33 Third, for intrauterine growth restriction, there is still no agreement on the best definition of the condition at birth, nor on the best predictor of future infant and childhood morbidity and mortality for term infants. Although population-based birth-weight standards have been used most commonly, they do not distinguish between the small healthy infant and the compromised infant. Customized growth charts that are adjusted for sex, gestational age, parity, maternal weight and height, and ethnicity have been shown to improve the detection of infants at risk of stillbirth,34 whereas neonatal indices have been shown to identify the malnourished infant at risk of peripartum asphyxia35 and long-term neurologic sequelae.36 Unfortunately, these have rarely been used as outcome measures. Fourth, poor reporting of patient selection criteria may explain in part the great variation in rates of pre-eclampsia among so-called high-risk and low-risk patients. The term “high risk” can have multiple origins and varying rates. Large cohort studies (> 300 000 women) of populations without specified risk levels have shown pre-eclampsia rates ranging from 0.8% to 5.1%.37–39 This suggests that small studies of populations at low or unspecified risk may be prone to selection bias.
Both pre-eclampsia and intrauterine growth restriction have a relatively low prevalence. A clinically useful test for either would thus have to have a high positive likelihood ratio (> 10) and low negative likelihood ratio (< 0.10).40 Based on the results of our review, pulsatility index and bilateral notching are the most promising Doppler indices to meet these requirements and should be used in daily clinical practice, although the results vary according to patient risk. Doppler assessment is noninvasive and thus acceptable to patients. It is specialized, both in terms of the equipment required and the operator's expertise. In industrialized countries, Doppler assessment could be fairly easily performed at the time of a detailed anomaly scan; in developing countries, it would be difficult to introduce this test into routine antenatal practice. For mothers, being identified as at risk in an antenatal test can cause considerable anxiety. Currently, no pharmacologic treatment or management strategy has been shown to be effective in preventing pre-eclampsia or intrauterine growth restriction or ameliorating their complications. Research into treatment with acetylsalicylic acid, which is inexpensive and readily available, has shown a small preventive effect (relative risk 0.9 for both pre-eclampsia and intrauterine growth restriction)10 in the absence of any serious side effects. In this case, a false-negative test result is potentially more harmful than a false-positive test result. When looking at predictive test accuracy and test–treatment combinations in pre-eclampsia and intrauterine growth restriction, we should consider whether it is more harmful to classify a patient's results as false positive or as false negative.
It is imperative to differentiate between mild and severe disease, because early or severe pre-eclampsia is associated with increased maternal morbidity and mortality and pronounced risks for the fetus, such as severe intrauterine growth restriction.41,42 Future research should also concentrate on the use of combinations of tests — a diagnostic process that is used in clinical care and may improve the predictive accuracy of the tests to clinically important values.
@ See related article page 727
Supplementary Material
Acknowledgments
We thank Mariska Leeflang DVM (research fellow) and Hans Reitsma MD PhD, Department of Clinical Epidemiology and Biostatistics, for their help with the statistical analysis.
Footnotes
Une version française de ce résumé est disponible à l'adresse www.cmaj.ca/cgi/content/full/178/6/701/DC1
This article has been peer reviewed.
Contributors: Jeltsje Cnossen, Rachel Morris, Gerben ter Riet, Ben Mol, Joris van der Post, Arri Coomarasamy, Stephen Robson, Patrick Bindels, Jos Kleijnen and Khalid Khan made substantial contributions to the study conception and design. Jeltsje Cnossen, Rachel Morris, Gerben ter Riet, Ben Mol and Joris van der Post contributed to the acquisition, analysis and interpretation of data. Aeilko Zwinderman contributed to the analysis and interpretation of data. All of the authors drafted the manuscript or revised it critically for important intellectual content, and all gave final approval of the version to be published.
Jeltsje Cnossen is supported by a project grant (no. 01/64/04) under the Health Technology Assessment program of the National Health Service, United Kingdom. Rachel Morris is supported by a project grant (no. NBTF626/03) from Wellbeing of Women.
Competing interests: None declared.
Correspondence to: Dr. Jeltsje S. Cnossen, Department of General Practice, Academic Medical Center, Meibergdreef 15, 1100 DD, Amsterdam, The Netherlands; fax +31-20-5669186; j.s.cnossen@amc.uva.nl
REFERENCES
- 1.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005;365:785-99. [DOI] [PubMed]
- 2.Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066-74. [DOI] [PubMed]
- 3.Montan S, Sjoberg NO, Svenningsen N. Hypertension in pregnancy — fetal and infant outcome: a cohort study. Clin Exp Hypertens — Part B Hypertens Pregnancy 1987;6:337-48.
- 4.Rich-Edwards JW, Colditz GA, Stampfer MJ, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med 1999;130:278-84. [DOI] [PubMed]
- 5.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl 2004;93:26-33. [DOI] [PubMed]
- 6.Khong TY, De Wolf F, Robertson WB, et al. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986;93:1049-59. [DOI] [PubMed]
- 7.Steel SA, Pearce JM, Chamberlain G. Doppler ultrasound of the uteroplacental circulation as a screening test for severe pre-eclampsia with intra-uterine growth retardation. Eur J Obstet Gynecol Reprod Biol 1988;28:279-87. [DOI] [PubMed]
- 8.Bolte AC, Dekker GA. Uterine artery Doppler as screening tool for preeclampsia. In Wildschut HJ, Weiner CP, editors. When to screen in obstetrics and gynecology. Philadelphia: Saunders Elsevier; 2006. p. 408-19.
- 9.Coomarasamy A, Papaioannou S, Gee H, et al. Aspirin for the prevention of preeclampsia in women with abnormal uterine artery Doppler: a meta-analysis. Obstet Gynecol 2001;98:861-6. [DOI] [PubMed]
- 10.Askie LM, Duley L, Henderson-Smart DJ, et al. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369:1791-8. [DOI] [PubMed]
- 11.Cnossen JS, van der Post JA, Mol BW, et al. Prediction of pre-eclampsia: a protocol for systematic reviews of test accuracy. BMC Pregnancy Childbirth 2006;6:29. [DOI] [PMC free article] [PubMed]
- 12.Morris RK, Khan KS, Coomarasamy A, et al. The value of predicting restriction of fetal growth and compromise of its wellbeing: systematic quantitative overviews (meta-analysis) of test accuracy literature. BMC Pregnancy Childbirth 2007;7:3. [DOI] [PMC free article] [PubMed]
- 13.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed]
- 14.Brown MA, Lindheimer MD, de Swiet M, et al. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 2001; 20:IX-XIV. [DOI] [PubMed]
- 15.McCowan LM, Harding JE, Stewart AW. Customized birthweight centiles predict SGA pregnancies with perinatal morbidity. BJOG 2005;112:1026-33. [DOI] [PubMed]
- 16.Owen P, Farrell T, Hardwick JC, et al. Relationship between customised birthweight centiles and neonatal anthropometric features of growth restriction. BJOG 2002;109:658-62. [DOI] [PubMed]
- 17.Fay RA, Dey PL, Saadie CM, et al. Ponderal index: a better definition of the “at risk” group with intrauterine growth problems than birth-weight for gestational age in term infants. Aust N Z J Obstet Gynaecol 1991;31:17-9. [DOI] [PubMed]
- 18.Tamim H, Beydoun H, Itani M, et al. Predicting neonatal outcomes: Birthweight, body mass index or ponderal index? J Perinat Med 2004;32:509-13. [DOI] [PubMed]
- 19.Excler JL, Sann L, Lasne Y, et al. Anthropometric assessment of nutritional status in newborn infants. Discriminative value of mid arm circumference and of skinfold thickness. Early Hum Dev 1985;11:169-78. [DOI] [PubMed]
- 20.Harbord RM, Deeks JJ, Egger M, et al. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. [DOI] [PubMed]
- 21.Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. [DOI] [PubMed]
- 22.Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Stat Med 2008;27:687-97. [DOI] [PubMed]
- 23.Chien PF, Arnott N, Gordon A, et al. How useful is uterine artery Doppler flow velocimetry in the prediction of pre-eclampsia, intrauterine growth retardation and perinatal death? An overview. BJOG 2000;107:196-208. [DOI] [PubMed]
- 24.Papageorghiou AT, Yu CK, Cicero S, et al. Second-trimester uterine artery Doppler screening in unselected populations: a review. J Matern Fetal Neonatal Med 2002;12:78-88. [DOI] [PubMed]
- 25.Conde-Agudelo A, Villar J, Lindheimer M. World health organization systematic review of screening tests for preeclampsia. Obstet Gynecol 2004;104:1367-91. [DOI] [PubMed]
- 26.Cnossen JS, Mol BW, van der Post JA, et al. World health organization systematic review of screening tests for preeclampsia. Obstet Gynecol 2005;105:1151-2. [DOI] [PubMed]
- 27.Lijmer JG, Mol BW, Heisterkamp S, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999;282:1061-6. [DOI] [PubMed]
- 28.Rutjes AW, Reitsma JB, Di Nisio M, et al. Evidence of bias and variation in diagnostic accuracy studies. CMAJ 2006;174:469-76. [DOI] [PMC free article] [PubMed]
- 29.Whiting P, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol 2005;5:19. [DOI] [PMC free article] [PubMed]
- 30.Leeflang M, Reitsma J, Scholten R, et al. Impact of adjustment for quality on results of metaanalyses of diagnostic accuracy. Clin Chem 2007;53:164-72. [DOI] [PubMed]
- 31.Brown MA, Reiter L, Smith B, et al. Measuring blood-pressure in pregnant women: a comparison of direct and indirect methods. Am J Obstet Gynecol 1994; 171:661-7. [DOI] [PubMed]
- 32.Brown MA, Hague WM, Higgins J, et al. The detection, investigation and management of hypertension in pregnancy: full consensus statement. Aust N Z J Obstet Gynaecol 2000;40:139-55. [DOI] [PubMed]
- 33.Shennan A, Gupta M, Halligan A, et al. Lack of reproducibility in pregnancy of Korotkoff phase IV as measured by mercury sphygmomanometry. Lancet 1996; 347: 139-42. [DOI] [PubMed]
- 34.Clausson B, Gardosi J, Francis A, et al. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG 2001;108:830-4. [DOI] [PubMed]
- 35.Scott KE, Usher R. Fetal malnutrition: its incidence, causes, and effects. Am J Obstet Gynecol 1966;94:951-63. [DOI] [PubMed]
- 36.Hill RM, Verniaud WM, Deter RL, et al. The effect of intrauterine malnutrition on the term infant. A 14-year progressive study. Acta Paediatr Scand 1984;73:482-7. [DOI] [PubMed]
- 37.Conde-Agudelo A, Althabe F, Belizan JM, et al. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol 1999; 181: 1026-35. [DOI] [PubMed]
- 38.Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 2001; 25: 1175-82. [DOI] [PubMed]
- 39.Sebire NJ, Jolly M, Harris J, et al. Is maternal underweight really a risk factor for adverse pregnancy outcome? A population-based study in London. BJOG 2001; 108: 61-6. [DOI] [PubMed]
- 40.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004;329:168-9. [DOI] [PMC free article] [PubMed]
- 41.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1-22. [PubMed]
- 42.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005;365:785-99. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


