Abstract
In the spinal cord, developing motor neurons extend their axons into the periphery while their cell bodies remain within the motor columns in the spinal cord. Two recent papers show that this partitioning involves forward and reverse semaphorin-plexin signaling between motor neurons and neural crest boundary cap cells.
The vertebrate nervous system is subdivided into two main parts: the central nervous system (CNS) and the peripheral nervous system (PNS). Axons ensure connectivity between the CNS and the PNS but there is no mixing of cell bodies between the two. The interfaces between CNS and PNS compartments are the transitional zones in the spinal cord [1]. These are located at the motor exit point (MEP), where motor axons leave the cord in ventral roots, and at the dorsal root entry zone (DREZ), where the afferents of primary sensory dorsal root ganglion neurons enter the spinal cord. In embryonic rodents and birds, boundary cap cells (BCCs) reside at these segmental exit points [2,3]. BCCs are a small, transient population of cells that arise from ventrally migrating neural crest cells. BCC progeny at the DREZ emigrate to populate distal structures, where they differentiate into both glial and neuronal cells [4-6]. Elimination of BCCs at the MEP does not perturb motor axon outgrowth but results in motor neuron cell bodies migrating out of the spinal cord [7]. These observations led to the suggestion that BCCs regulate the migration of motor neuron cell bodies at the MEP. In two recent articles in Neural Development, Bron et al. [8] and Mauti et al. [9] address the molecular mechanisms underlying both the aggregation of BCCs at the DREZ and the MEP, and their gate-keeper functions at these interfaces. They report the involvement of classes of proteins that are also, perhaps unsurprisingly, known to be implicated in the patterning of neuronal circuits, namely the semaphorins and their receptors the plexins and neuropilins.
Semaphorin cues
Neuronal migration and axon guidance are directed largely by chemical cues in the cells' environment, which are detected by receptors on the migrating cell. Among the most important of these cues are the semaphorins, which are secreted, transmembrane or glycosyl-phosphotidyl-inositol-linked proteins with important roles in a variety of tissues. A common end point in semaphorin signaling is an alteration in the cytoskeleton arising from reorganization of actin filaments and the microtubule network. These effects occur primarily through binding of semaphorins to their receptors. The best-characterized receptors mediating semaphorin signaling are members of the neuropilin and plexin families of transmembrane proteins. In particular, the plexins, consisting of 10 members falling into four classes A to D, are thought to control many of the functional effects of semaphorins. There are eight classes of semaphorins, and except for Sema3E [10], the secreted class 3 semaphorins are unable to interact directly with plexins and use neuropilins as a ligand-binding component. In common with most other neural cue molecules, semaphorins are bifunctional, exerting repulsive or attractive signals. These dual activities intervene at different steps in the establishment of neural circuits, by regulating a variety of cellular events, including axon guidance, axon branching and neuronal migration (see [11-13] reviews). Although most work has focused on the secreted semaphorins, a few recent papers have described the ability of transmembrane semaphorins to behave as receptors on migrating neurons – resulting in reverse semaphorin-plexin signaling – and their consequent cell-autonomous action. The studies of Bron et al. [8] and Mauti et al. [9] now provide evidence for semaphorin-plexin signaling in regulating the positioning of motor neuron cell bodies and the function of BCCs, and suggest that both forward and reverse semaphorin-plexin signaling may be important in this process.
A previous study [7] proposed that BCCs secrete molecules exerting a repellent signal that confine motor neuron cell bodies to the spinal cord (Figure 1a). This repellent cue could be a semaphorin: in the chick embryos studied by Bron et al. [8] and Mauti et al. [9], the expression patterns of neuropilins and the plexin-A family in motor neurons and of classes 3 and 6 semaphorins are compatible with such a role. In their study, Bron et al. [8] focused on the MEP and used a combination of genetic approaches – RNA interference (RNAi) in the chick (delivered by electroporation in ovo) and phenotypic analysis of mutant mice lacking the various signaling proteins – to study the potential role of neuropilins, plexins and semaphorins in the positioning of motor neuron cell bodies. Targeted electroporation into the neural tube in chick embryos [14] showed that knock-down of Neuropilin 2 (Npn-2) but not Npn-1, and of PlexinA2 but not PlexinA1 or PlexinA4, leads to ectopic motor neurons in ventral spinal nerve roots. Those results indicated that the receptors Npn-2 and PlexinA2 are implicated in regulating the position of motor neuron cell bodies. This mechanism appears to be conserved in mammals, as in Npn-2 knockout mice there is also a mis-positioning of motor neuron cell bodies outside the spinal cord.
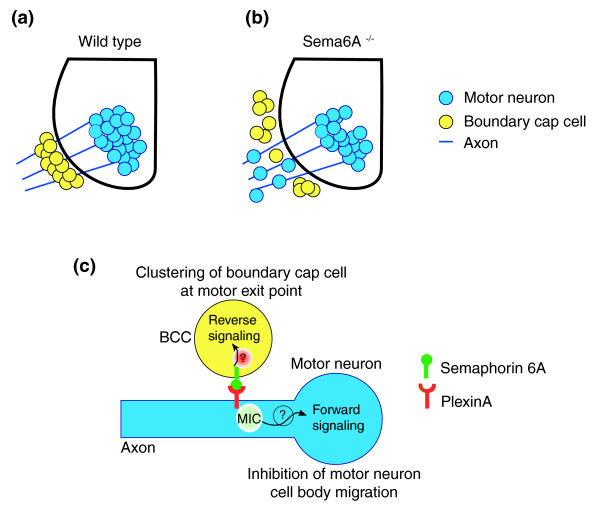
Figure 1.
A model of the proposed roles of Sema6A and PlexinA as gate-keepers at the motor exit point (MEP). (a) In wild-type mice, boundary cap cells (BCCs) express Semaphorin 6A (Sema6A) and motor neurons express members of the class A plexins. Motor neuron cell bodies are caged in the spinal cord. (b) Knock down of Sema6A (Sema6A-/-) leads to a lack of clustering of BCCs and ectopic migration of motor neuron cell bodies at the MEP. (c) Interactions between BCCs expressing Sema6A and motor neurons expressing PlexinA activate both reverse and forward signals to induce formation of BCC clusters and motor neuron caging, respectively. MIC, MICAL3, an intermediary of semaphorin signaling.
By tracing the localization of BCCs with specific markers, Bron et al. [8] found that these cells persist at the MEP in Npn-2 null mutant mice. This leads the authors to conclude that the phenotype of aberrant motor neuron migration is due to the absence of Npn-2 in motor neurons rather than to BCC mis-positioning. In addition, class 3 semaphorin ligands of Npn-2 are expressed in BCCs. However, genetic ablation of Sema3B in chick or mouse does not result in ectopic motor neuron positioning. Interestingly, transmembrane Sema6A is also expressed in BCCs, in both chick and mouse, and Bron et al. [8] find that loss of function of Sema6A in BCCs leads to ectopic motor neurons in ventral nerve roots, especially at the hindlimb level. Sema6A could therefore be the BCC 'stop signal' acting via PlexinA2 on motor neurons to cage the cell bodies.
In the second paper, Mauti et al. [9] focus on the involvement of semaphorin signaling in the formation of CNS/PNS interfaces in the chick embryo, and looked for a phenotype at both the MEP and the DREZ. They report a detailed expression pattern for Sema6A: it is expressed by neural crest cells destined to become BCCs before the appearance of markers detected only after the BCCs begin to aggregate. In this context, it should be remembered that an earlier study [7] showed that when grafted into crest-ablated embryos, neural crest cells preferentially migrate towards the presumptive MEP, suggesting that a chemoattractive signal might prefigure the BCCs. Interestingly, Mauti et al. [9] noticed that Sema6A downregulation, although not preventing BCC accumulation at the MEP and the DREZ, perturbed their clustering, a feature that may have been missed by Bron et al. [8]. Importantly, as also observed by Bron et al. [8], Sema6A knock down leads to motor neuron emigration through the MEPs (Figure 1b).
A discrepancy between the two studies, which we shall return to later, concerns the identity of the molecule that appears to interact with Sema6A. The results of Bron et al. [8] point to PlexinA2, whereas those of Mauti et al. [9] suggest PlexinA1. Mauti et al. [9] propose that BCC clustering is a primary and important event in restricting the migration of motor neuron cell bodies. In their scenario, Sema6A on the BCCs works as a receptor to allow clustering, whereas PlexinA1 expressed by motor neurons is acting in a non-autonomous manner to trigger signaling in the BCCs. In support of this idea, Sema6A does not make homophilic interactions and BCCs do not express PlexinA1. Also, unlike dorsal root ganglion neurons, cultured motor neurons are not repelled by cells expressing Sema6A. Finally, overexpression of ectodomain or full-length Sema6A in motor neurons apparently blocks PlexinA1-Sema6A interaction. This last experiment is open to alternative interpretations, however, as coexpression by a given cell of both a ligand and its receptors can lead to different outcomes. For example, Moret et al. [15] showed that coexpression by motor neurons of secreted Sema3A and its receptor Npn-1 desensitizes the neuronal response to Sema3A. In contrast, ephrins and their receptors (Ephs) coexpressed in the same cells do not interact in cis but segregate into separate membrane domains, which allows them to function as independent cues [16].
Sema6A reverse signaling
Whereas the cell-autonomous and non-autonomous mode of action of Ephs and ephrins is well established in several embryogenic processes [17], a cell-autonomous function for transmembrane semaphorins is poorly documented so far. One example is the role of class 6 semaphorins in the developing cardiac system, where Sema6D-PlexinA1 forward and reverse signaling are required for the proper development of the cardiac ventricle in chick embryos [18-20]. Reverse signaling by Sema6D results in the phosphorylation of the protein Enable, a regulator of actin dynamics [18,20]. Interestingly, Sema6A is also known to associate with proteins of the Enable family [21]. By analogy with Sema6D, Sema6A is potentially able to function cell autonomously. Nevertheless, the function of Sema6A as a ligand is better documented [22], and whether transmembrane semaphorins could act as signaling receptors during CNS development remains to be properly established.
In the present context, if Sema6A reverse signaling is at play in BCCs, with PlexinA1 in motor neurons in the role of a ligand, one would expect that expression of the PlexinA1 extracellular domain alone in motor neurons should rescue defective BCC clustering induced by suppression of PlexinA1, but not that resulting from defective Sema6A expression. Furthermore, overexpression of the Sema6A extracellular domain in BCCs should not rescue the clustering phenotype observed in the absence of Sema6A [9]. According to the hypothesis of Bron et al. [8], the Sema6A extracellular domain should, however, be able to rescue the ectopic motor neuron cell body phenotype (Figure 1c).
Sema6A interactions
The two papers raise several other important questions. One is a discrepancy between the identity of the plexin able to interact with Sema6A. Bron et al. [8] show that knock-down of PlexinA2 but not PlexinA1 leads to motor neuron emigration at the MEP, whereas Mauti et al. [9] show the reverse. It is difficult to proffer an explanation for this difference, as both groups performed convincing controls – the chosen targeted sequences downregulating the correct gene and not other plexins. The techniques used do differ, however. Bron et al. [8] specifically target either neural crest or neural tube, and use plasmids coexpressing short hairpin RNA (shRNA) and enhanced green fluorescent protein (EGFP) [14]. Mauti et al. [9] target both neural crest and neural tube, and co-electroporate a mixture of double-stranded RNA (dsRNA) and a vector encoding EGFP [23]. Because Bron et al. [8] used shRNA and Mauti et al. [9] the sequences targeted in plexin mRNA are different. The discrepancies in plexin identification could thus be due to differences in the efficiency of knock-down. If antibodies were available, measurements of protein levels could be helpful. Analysis of plexin null mouse phenotypes might also be informative but awaits the analysis of the phenotype of PlexinA2 mutant mice. In PlexinA1 knockout mice, proprioceptive sensory axons are misplaced in the dorsal spinal cord [24]. This effect is most probably mediated by Sema6C and Sema6D, and no ectopic motor neuron phenotype or BCC mis-positioning has been reported in this mutant.
In fact, little is known about the identity of receptors for membrane bound semaphorins. BCCs do not express class A plexins, but the analysis of Sema6A-class A plexin binding provided by Mauti et al. [9] does not rule out the possibility of other receptors for Sema6A. Indeed, the best-characterized receptors for membrane-bound semaphorins, especially in the nervous system, are not plexins. Sema7A binds to a β1-integrin on olfactory neurons [25] and Sema5A binds to transmembrane heparin sulphate proteoglycan (HSPG) on axons [26]. It is also important to note that in the context of the semaphorin-plexin-neuropilin system, multiple combinations of ligands and receptors can cooperate in vivo. Multimolecular receptor complexes include, beside plexins and neuropilins, several kinds of modulators such as members of the immunoglobulin superfamily of cell adhesion molecules [27]. It has recently been shown that neuropilins themselves could exert a gating function in semaphorin-plexin signaling during the assembly of neuronal circuits [28]. A possibility would be that a similar mechanism is at play in the observations of Bron et al. [8], with Npn-2 working as a modulator of plexin signaling in this system. Indeed, these authors show that Npn-2 knock down leads to ectopic motor neuron cell bodies in ventral roots, whereas this phenotype could not be phenocopied by knock down of identified Npn-2 ligands. Nevertheless, the alternative possibility that PlexinA2 and Npn-2 signaling work in parallel cannot be excluded.
The phenotypes observed after knock-down of different semaphorins or plexins are less severe than those after complete BCC ablation [8]. This suggests a possible heterogeneity in the motor neurons, together with a complex interplay between ligands in the BCC environment and receptor components in the motor neurons. The severity of phenotypes varies along the antero-posterior axis, which is reminiscent of phenotypes described in zebrafish after PlexinA3 knock down [29]. Dorso-ventral differences were also noticed, as knock-down of Sema6B, Sema6D and PlexinA4 lead to defaults at the DREZ such as a failure of dorsal roots to form and segregate properly, but not at the MEP [9]. This would be consistent with recent, and still controversial, claims of differences in the roles of BCCs located at the MEP and the DREZ [30]. At the MEP, BCCs become associated with nerve root motor axons only after their exit from the CNS. By contrast, at the DREZ, BCC clusters prefigure the site of sensory axon entry to the spinal cord. Therefore, if BCCs are not properly positioned at the DREZ, axons would fail to enter correctly here. In contrast, axons would exit normally at the MEP, but the crucial interaction between the first motor axons and BCCs would be disrupted, leading to a failure of BCCs to cluster and a subsequent lack of motor neuron cell body caging.
How are cell-surface interactions linked with the establishment of neuronal polarity?
Establishment of polarity is an essential process during neuron migration and differentiation, and the signaling pathways leading to polarization of the cytoskeleton are topics of intense interest. The observations of Bron et al. [8] provide an impetus to undertake further experiments in this direction. They report that knock-down of MICAL3 (molecule interacting with CasL) leads to the most severe ectopic motor neuron phenotype in the chick ventral spinal cord. This is consistent with the convergence of several sets of signals on this intermediate of semaphorin signaling. The recently identified MICALs are a family of adaptor proteins containing multiple well conserved domains with known interactions with the cytoskeleton, in particular with microtubules, cytoskeletal adaptor proteins and other signaling proteins [31]. Indeed, a central component of the neuronal cytoskeletal structure is a microtubule array, with the centrosome or microtubule-organizing center lying at its hub. It has been hypothesized ([32], but see also [33]) that the centrosome acts as a link between microtubule-based pulling forces generated within the extending neuronal process and the network of microtubules that surrounds the nucleus. Forces generated in the leading process transmit through the centrosome to the nucleus and pull the nucleus forward [32]. In the case of CNS/PNS interfaces, a possibility would be that semaphorin extracellular cues are signaling to neurons to uncouple this process from axonal outgrowth. The analysis of spatio-temporal dynamics of the signals and polarity-regulating proteins will be required for a complete picture of the forces regulating the caging of migrating neuronal cell bodies.
In conclusion, these two papers [8,9] demonstrate that the formation of CNS/PNS interfaces comprises a stepwise and complex set of cellular and molecular events in which semaphorin-plexin-neuropilin signaling is at play. They strongly suggest that Sema6A could be a cue used to perform several tasks. Although the signaling mechanisms remain to be confirmed, the data are consistent with clustering of BCC at the MEP and the DREZ controlled by attractive Sema6A reverse signaling [9] and forward signaling by Sema6A (and possibly other semaphorins) expressed by BCCs regulating the positioning of motor neuron cell bodies [8]. The latter is further supported by the observation that knockdown of MICAL3, a downstream component in semaphorin signaling, led to extensive mis-positioning of motor neurons in the PNS and opens the way to understanding how extracellular cues control the complex process of neuronal polarity.
Acknowledgements
Our group is supported by the CNRS, the Université de la Méditerran-née and by grants from the Institut pour la Régénération de la Moelle Epinière (IRME), Institut de Recherche contre le Cancer (INCA), Fédération pour la Recherche sur le Cerveau (FRC) and the French National Agency for Research (ANR).
References
- Fraher J, Dockery P, O'leary D, Mobarak M, Ramer M, Bishop T, Kozlova E, Priestley E, McMahon S, Aldskogius H. The dorsal root transitional zone model of CNS axon regeneration: morphological findings. J Anat. 2002;200:214. doi: 10.1046/j.1469-7580.2002.00037.x. [DOI] [PubMed] [Google Scholar]
- Golding JP, Cohen J. Border controls at the mammalian spinal cord: late-surviving neural crest boundary cap cells at dorsal root entry sites may regulate sensory afferent ingrowth and entry zone morphogenesis. Mol Cell Neurosci. 1997;9:381–396. doi: 10.1006/mcne.1997.0647. [DOI] [PubMed] [Google Scholar]
- Niederlander C, Lumsden A. Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor nerves. Development. 1996;122:2367–2374. doi: 10.1242/dev.122.8.2367. [DOI] [PubMed] [Google Scholar]
- Aquino JB, Hjerling-Leffler J, Koltzenburg M, Edlund T, Villar MJ, Ernfors P. In vitro and in vivo differentiation of boundary cap neural crest stem cells into mature Schwann cells. Exp Neurol. 2006;198:438–449. doi: 10.1016/j.expneurol.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Marmigère F, Heglind M, Cederberg A, Koltzenburg M, Enerbäck S, Ernfors P. The boundary cap: a source of neural crest stem cells that generate multiple sensory neuron subtypes. Development. 2005;132:2623–2632. doi: 10.1242/dev.01852. [DOI] [PubMed] [Google Scholar]
- Maro GS, Vermeren M, Voiculescu O, Melton L, Cohen J, Charnay P, Topilko P. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci. 2004;7:930–938. doi: 10.1038/nn1299. [DOI] [PubMed] [Google Scholar]
- Vermeren M, Maro GS, Bron R, McGonnell IM, Charnay P, Topilko P, Cohen J. Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron. 2003;37:403–415. doi: 10.1016/S0896-6273(02)01188-1. [DOI] [PubMed] [Google Scholar]
- Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J. Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural Develop. 2007;2:21. doi: 10.1186/1749-8104-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauti O, Domanitskaya E, Andermatt I, Sadhu R, Stoeckli ET. Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Develop. 2007;2:28. doi: 10.1186/1749-8104-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Mann F, Chauvet S, Rougon G. Semaphorins in development and adult brain: Implication for neurological diseases. Prog Neurobiol. 2007;82:57–79. doi: 10.1016/j.pneurobio.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron R, Eickholt BJ, Vermeren M, Fragale N, Cohen J. Functional knockdown of neuropilin-1 in the developing chick nervous system by siRNA hairpins phenocopies genetic ablation in the mouse. Dev Dyn. 2004;230:299–308. doi: 10.1002/dvdy.20043. [DOI] [PubMed] [Google Scholar]
- Moret F, Renaudot C, Bozon M, Castellani V. Semaphorin and neuropilin co-expression in motoneurons sets axon sensitivity to environmental semaphorin sources during motor axon pathfinding. Development. 2007;134:4491–4501. doi: 10.1242/dev.011452. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell. 2005;121:127–139. doi: 10.1016/j.cell.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Egea J, Klein R. Bidirectional Ephephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Kikutani H. Semaphorin signaling during cardiac development. Adv Exp Med Biol. 2007;600:109–117. doi: 10.1007/978-0-387-70956-7_9. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Yabuki M, Harada K, Hori M, Kikutani H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat Cell Biol. 2004;6:1204–1211. doi: 10.1038/ncb1193. [DOI] [PubMed] [Google Scholar]
- Klostermann A, Lutz B, Gertler F, Behl C. The orthologous human and murine semaphorin 6A-1 proteins (SEMA6A-1/Sema6A-1) bind to the enabled/vasodilator-stimulated phosphoprotein-like protein (EVL) via a novel carboxyl-terminal zyxin-like domain. J Biol Chem. 2000;275:39647–39653. doi: 10.1074/jbc.M006316200. [DOI] [PubMed] [Google Scholar]
- Kerjan G, Dolan J, Haumaitre C, Schneider-Maunoury S, Fujisawa H, Mitchell KJ, Chédotal A. The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat Neurosci. 2005;8:1516–1524. doi: 10.1038/nn1555. [DOI] [PubMed] [Google Scholar]
- Pekarik V, Bourikas D, Miglino N, Joset P, Preiswerk S, Stoeckli ET. Screening for gene function in chicken embryo using RNAi and electroporation. Nat Biotechnol. 2003;21:93–96. doi: 10.1038/nbt770. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Han B, Mendelsohn M, Jessell TM. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron. 2006;52:775–788. doi: 10.1016/j.neuron.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Bechara A, Falk J, Moret F, Castellani V. Modulation of semaphorin signaling by Ig superfamily cell adhesion molecules. Adv Exp Med Biol. 2007;600:61–72. doi: 10.1007/978-0-387-70956-7_6. [DOI] [PubMed] [Google Scholar]
- Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, Segu L, Buhot MC, Jessell TM, Henderson CE, Mann F. Gating of Sema3E/PlexinD1 signaling by Neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56:807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner J, Reimer MM, Schweitzer J, Wendik B, Meyer D, Becker T, Becker CG. PlexinA3 restricts spinal exit points and branching of trunk motor nerves in embryonic zebrafish. J Neurosci. 2007;27:4978–4983. doi: 10.1523/JNEUROSCI.1132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraher JP, Dockery P, O'Donoghue O, Riedewald B, O'Leary D. Initial motor axon outgrowth from the developing central nervous system. J Anat. 2007;211:600–611. doi: 10.1111/j.1469-7580.2007.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk SM, Pasterkamp RJ. MICAL flavoprotein monooxygenases: structure, function and role in semaphorin signaling. Adv Exp Med Biol. 2007;600:38–51. doi: 10.1007/978-0-387-70956-7_4. [DOI] [PubMed] [Google Scholar]
- Higginbotham HR, Gleeson JG. The centrosome in neuronal development. Trends Neurosci. 2007;30:276–283. doi: 10.1016/j.tins.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Umeshima H, Hirano T, Kengaku M. Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc Natl Acad Sci USA. 2007;104:16182–16187. doi: 10.1073/pnas.0708047104. [DOI] [PMC free article] [PubMed] [Google Scholar]