Abstract
We studied the contribution of the main olfactory system to mate recognition and sexual behavior in female mice. Female mice received an intranasal irrigation of either a zinc sulfate (ZnSO4) solution to destroy the main olfactory epithelium (MOE) or saline (SAL) to serve as control. ZnSO4-treated female mice were no longer able to reliably distinguish between volatile as well as nonvolatile odors from an intact versus a castrated male. Furthermore, sexual behavior in mating tests with a sexually experienced male was significantly reduced in ZnSO4-treated female mice. Vomeronasal function did not seem to be affected by ZnSO4 treatment: nasal application of male urine induced similar levels of Fos protein in the mitral and granule cells of the accessory olfactory bulb (AOB) of ZnSO4 as well as SAL-treated female mice. Likewise, soybean agglutinin staining, which stains the axons of vomeronasal neurons projecting to the glomerular layer of the AOB was similar in ZnSO4-treated female mice compared to SAL-treated female mice. By contrast, a significant reduction of Fos in the main olfactory bulb was observed in ZnSO4-treated females in comparison to SAL-treated animals, confirming a substantial destruction of the MOE. These results show that the MOE is primarily involved in the detection and processing of odors that are used to localize and identify the sex and endocrine status of conspecifics. By contrast, both the main and accessory olfactory systems contribute to female sexual receptivity in female mice.
Keywords: main olfactory system, mice, sexual behavior, sex recognition, zinc sulfate
Introduction
Mice use odors to distinguish the sex, social and reproductive status, and kinship of individuals (Brown, 1979). In rodents, these odors are detected by two anatomically and functionally distinct groups of chemosensory receptors, the main olfactory epithelium (MOE) and the vomeronasal organ (VNO). The VNO, where the initial detection of odors takes place following direct contact with nonvolatile components of various body odorants such as skin secretions, urine, or scent marks (Wysocki et al., 1980; Halpern and Martinez-Marcos, 2003; Luo et al., 2003), detects primarily pheromones or pheromonal blends which have been shown to play a critical role in mediating neuroendocrine responses to conspecific odors, such as pregnancy block (Bruce, 1959) or puberty acceleration (Whitten, 1959). Many of these vomeronasal effects involve stereotyped, preprogrammed physiological or hormonal responses. In mice, several chemicals have been identified that elicit many of these responses, and these chemicals have been shown to stimulate vomeronasal receptor neurons at picomolar concentrations (Leinders-Zufall et al., 2000). The physiological and hormonal effects of vomeronasal stimulation are thought to be mediated by the vomeronasal projections to the accessory olfactory bulb (AOB) and from there on to limbic areas of the brain, including the medial and posteromedial cortical nuclei of the amygdala and the bed nucleus of the stria terminalis, as well as higher order projections to the hypothalamus (medial preoptic area). By contrast, the main olfactory system is envisioned as a general molecular analyzer that detects and differentiates among complex chemosignals by sensory neurons in the MOE. These MOE sensory neurons project to glomeruli in the main olfactory bulb (MOB). Different odors activate distinct clusters of glomeruli in the MOB (Sharp et al., 1975; Xu et al., 2000; Firestein, 2001) and as a consequence activate different groups of neurons in the olfactory cortex (Zou et al., 2001). It has been hypothesized that such maps of MOB glomerular activation serve as the first neural representation leading to the perceptual discrimination of odor quality and intensity (Xu et al., 2000).
In mice, olfaction plays a crucial role in mate recognition and consequently sexual behavior. Although the case is not unequivocal, evidence exists, suggesting that the main as opposed to the accessory olfactory system plays a more important role in mate recognition in female mice. Several studies (e.g., Lloyd-Thomas and Keverne, 1982; Keller et al., 2006) reported that VNO removal had no effect on the preference of female mice to approach male’s olfactory cues from an intact as opposed to a castrated male. Conversely, female mice in which the MOE was destroyed by intranasal application of zinc sulfate (ZnSO4) no longer preferred to approach soiled bedding from intact as opposed to castrated males (Lloyd-Thomas and Keverne, 1982). However, the ability to discriminate among complex chemosignals may not be linked exclusively to the main olfactory system. Removal of the VNO blocked the ability of congenic male odors to induce pregnancy block, whereas destruction of the MOE by either ZnSO4 (Lloyd-Thomas and Keverne, 1982) or by specifically killing MOE neurons in Olfactory marker protein-nitroreductase (OMP-ntr) mice (Ma et al., 2002) had no effect on pregnancy block. Likewise, it is not clear which olfactory system mediates sexual behavior in female mice. Early works (Thompson and Edwards, 1972; Edwards and Burge, 1973) suggested that the main olfactory system may mediate sexual receptivity since ZnSO4 destructions of the MOE attenuated lordosis behavior in estrogen-progesterone–treated female mice (Edwards and Burge, 1973). However, we recently showed that VNO removal completely abolished lordosis behavior in female mice (Keller et al., 2006) which suggests a critical role for the VNO in sexual behavior.
The present study was thus designed to further analyze the contribution of the main olfactory system to mate recognition and sexual behavior in female mice by assessing the effects of destroying the MOE on the expression of these behaviors. Thus, female mice treated with ZnSO4 or saline (SAL) were tested for olfactory investigation of various volatile and nonvolatile odors in a Y-maze and for the display of lordosis behavior when paired with a sexually experienced male. We also used the expression of c-fos and soybean agglutinin to confirm that ZnSO4 treatment destroyed only MOE and not VNO neurons.
Materials and methods
Subjects
Adult (10–12 weeks), sexually naive female mice (n = 32) of the C57Bl6 inbred strain were obtained from a local breeding colony at the University of Liège. One week later, all females were ovariectomized under general anesthesia using a mixture of ketamine (80 mg/kg per mouse) and medetomidine (Domitor, Pfizer, 1 mg/kg per mouse). Mice received atipamezole (Antisedan, Pfizer, 4 mg/kg per mouse) subcutaneously (sc) at the end of the surgery in order to antagonize medetomidine-induced effects, thereby accelerating their recovery. During surgery, a Silastic capsule (inner diameter: 1.57 mm; outer diameter: 2.41 mm; length: 5 mm) filled with 17β-estradiol (diluted 1:1 with cholesterol) was implanted sc in the neck.
Subjects were housed alone in macrolon cages and were placed in a climate-controlled (light, temperature, and ventilation) animal housing unit (Iffa-Credo, L’Arbresle, France) on a reversed 12:12 h light:dark cycle. The air pressure in the housing units was higher than in the animal room, thereby avoiding the inflow of any odors from the room in which the housing unit was placed. Thus, care was taken that females were not exposed to any male-derived odors except when tested. Sawdust served as bedding and was not changed for at least 48 h before any behavioral test. Food and water were always available ad libitum.
All the procedures were conducted in accordance with the guidelines set forth by the National Institutes of Health Guiding Principles for the Care and Use of Research Animals and were approved by the Ethical Committee for Animal Use of the University of Liège.
ZnSO4 treatment
Before the onset of behavioral testing, mice received an intranasal application of 10% ZnSO4 or SAL solution under general anesthesia. Subjects were placed on their back, and each naris was injected with 8–10 μl of a sterile 10% ZnSO4 solution or SAL. Immediately after ZnSO4 irrigation, the mice were held with their head down for several seconds to minimize spread of the solution to the oral cavity.
Since regeneration of olfactory receptor cells in the MOE typically occurs within 7 days after ZnSO4 treatment (see McBride et al., 2003, for a review), all behavioral tests were completed within 1 week after intranasal application of ZnSO4. Peripheral anosmia was first assessed using habituation/dishabituation tests following ZnSO4 treatment. Undiluted urinary odors from intact males were used as odor stimulus. Only mice that no longer responded to the odor stimulus following ZnSO4 treatment were subsequently tested for odor preferences or sexual behavior. In order to obtain sufficient data, all females were retreated with ZnSO4 or SAL approximately 1 week following the first treatment and retested again for odor preferences or sexual behavior.
Behavioral procedures
Female mice were divided into two groups: one group, consisting of eight ZnSO4- and eight SAL-treated females, was tested a total of four times for odor preferences in the Y-maze, whereas the other group, again consisting of eight ZnSO4- and eight SAL-treated females, was tested a total of four times for sexual behavior with an intact male. Behavioral tests were always performed on days 2 and 5 following ZnSO4 treatment, and anosmia was reassessed by habituation/dishabituation tests on day 6 following ZnSO4 treatment. All behavioral tests were performed between 11:00 AM and 4:00 PM, during the dark phase of the light/dark cycle, and female subjects were injected sc with 500 μg progesterone 2–4 h before each behavioral test to induce behavioral estrus (Bakker et al., 2002).
Role of the MOE in discriminating the endocrine status among males
All olfactory discrimination tests were conducted in an enclosed, Plexiglas Y-maze (see Bakker et al., 2002, for a full description of the maze). When subjects were tested for mate recognition using volatile body odors as odor stimuli, removable opaque Plexiglas doors were placed at the distal end of each arm to separate the goal boxes from the rest of the maze. Volatile body odors were derived from anesthetized stimulus animals placed behind these doors. It should be noted that the top of each opaque door was perforated to allow air to flow from the goal boxes into the maze. However, these holes were placed above the “eye level” so that subjects could not see the stimulus animals. The level of anesthesia was checked regularly and adjusted—if necessary—between each trial. Also, stimulus animals were placed on a heating pad to prevent hypothermia. When subjects were tested for mate recognition using volatile urine odors, 30 μl of urine was pipetted onto a glass slide and placed behind the opaque Plexiglas doors. The time that the subject spent poking his nose in the holes of the door or actively sniffing the door was recorded. When subjects were tested for mate recognition using nonvolatile odors, the doors were removed to allow direct access to the odor stimuli which were placed in the back of each goal box. Soiled bedding was placed in bowls, whereas the different urine stimuli were pipetted onto glass slides. The time that the mouse spent investigating the stimulus in direct physical contact was recorded.
At the beginning of each test, the subject was placed in the start box with the door closed to adapt for 1 min. The test began when the door was removed and the subject could freely move around in the Y-maze. The time the subject spent investigating each odor stimulus was recorded with a stopwatch. Subjects were first tested for 5 min in the Y-maze with no stimulus animals in the goal boxes to adapt to the testing apparatus and to determine whether they would develop any side preferences. This test was conducted before animals were treated with either ZnSO4 or SAL. The maze was cleaned with 70% ethanol between trials. All Y-maze tests lasted 5 min and were separated by at least 3 days. For each test, cages were taken randomly out of the housing unit to prevent the same animals always being tested first or last.
Role of the MOE in detecting volatile odors
To determine whether female mice in which the MOE was destroyed were still capable of using volatile odors for mate recognition, ZnSO4 and SAL females were first tested for their ability to discriminate between volatile odor stimuli from an intact male versus a castrated male. Thus, subjects were offered the choice between volatile body odors (Test 1) followed by volatile urinary odors (Test 2) from a gonadally intact male versus those from a castrated male.
Role of the MOE in detecting nonvolatile odors
To determine whether female mice treated with ZnSO4 were capable of using nonvolatile odors for mate recognition, ZnSO4 and SAL females were tested for their ability to discriminate between various nonvolatile odor stimuli. Thus, subjects were first offered the choice between soiled bedding (Test 3) followed by urinary odors (Test 4) from a gonadally intact male versus those from a castrated male.
Preparation of odor stimuli
Urine was collected from 10 gonadally intact C57Bl6 males, which were either left gonadally intact or were castrated under general anesthesia at least 2 weeks prior to urine sampling. Urine was collected by holding the mouse by the scruff of the neck over a funnel, taking care that no fecal contamination of the urine occurred. Same urine stimulus samples were pooled and subsequently aliquoted in 500 μl eppendorf and stored at −80°C until use. For soiled bedding, groups of gonadally intact males (n = 5) or castrated males (n = 5) were placed in clean cages containing fresh sawdust. Bedding was collected 10 h later. All beddings were stored in plastic freezer bags at −80°C prior to being used in the experiment.
Role of the MOE in sexual behavior
The sexual receptivity of ZnSO4 (n = 8) and SAL (n = 8) females was assessed by quantifying the ratio of the number of lordosis responses to the number of mounts received from a stimulus male (lordosis quotient). All lordosis tests were conducted in a Plexiglas aquarium (35 cm long ×25 cm high × 19 cm wide) whose floor was covered with fresh sawdust. At the beginning of each test, a sexually experienced male of the NMRI strain was placed alone in the aquarium and allowed to adapt for 15 min. Subsequently, an experimental female was placed in the aquarium, and the lordosis responses of the female to the mounts of the stimulus male were recorded. The test lasted until the female had received 20 mounts or 15 min had elapsed.
Determine whether MOE destruction affects anxiety
Since olfactory bulbectomy is commonly used as a model of depression in rodents (Kelly et al., 1997; Song and Leonard, 2005), we determined whether the behavioral changes observed in the Y-maze or during the sexual interactions with the male might be due to any changes in subjects’ state of anxiety. Therefore, ZnSO4 and SAL animals were tested for their behavior in the elevated plus maze. Female mice were brought into the test room at least 1 h before the onset of behavioral testing and remained in the same room throughout the test. The maze consisted of four arms (each arm 30 cm long ×15 cm high ×8 cm wide), two open and two closed arms formed a cross, which were raised 80 cm above the floor. At the beginning of the test, each mouse was placed in the center area, and subsequently the time spent in the center, open, and closed arms was recorded for 5 min. In addition, the number of entries into either the open or closed arms was registered. Behavioral variables were recorded using a stopwatch. It was considered that the mouse was in the open (or closed) arm when its four legs were not in the center area. Females were tested individually under normal white lighting in a random order. The maze was cleaned with 70% ethanol to eliminate odors after each test.
Assessment of the specificity of the destruction of the MOE
Habituation/dishabituation tests
To assess whether animals which were treated with ZnSO4 were anosmic, habituation/dishabituation tests were conducted using male volatile urinary odors as odor stimulus on days 1 and 6 following ZnSO4 treatment. Animals were tested in their home cages as described by Baum and Keverne (2002) and Pierman et al. (2006). The stainless steel cage top containing their food and water was removed and replaced with a clean top. Odor stimuli were presented by pipetting 30 μl of male urine onto a piece of filter paper that was glued to a plastic weighing boat (4.3 × 4.3 cm), which was then placed in the food hopper so that volatile odors from the stimulus were available at body level. Subjects were unable to make physical contact with the filter paper using either their snout or paws. Each test was constituted of a sequence of three water presentations followed by three odor presentations. The duration of investigation of the odor stimuli was recorded using a stopwatch; any significant increase of olfactory investigation (dishabituation) when being exposed to the odorant stimulus was considered as the subject detecting the odor. Only animals that did not show any significant dishabituation responses when presented with male urine were subsequently tested for odor preferences or sexual behavior.
Histological assessment of the specificity of MOE destruction
When behavioral testing was completed, the specificity of the ZnSO4 destruction was assessed by histological procedures. Thus, we determined whether or not the ZnSO4 infusions had damaged VNO sensory neurons functioning and thus disrupted function of the accessory olfactory system. Therefore, the brains of several ZnSO4- or SAL-treated females were processed for soybean agglutinin and Fos immunocytochemistry. Thus, ZnSO4 and SAL females were stimulated by direct application of 30 μl of either male urine (SAL: n = 4; ZnSO4: n = 6) or water (SAL: n = 4) onto the oronasal groove. Ninety minutes after stimulation, animals were anesthetized with ketamine/domitor and perfused transcardially with saline followed immediately by 4% cold paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) (pH = 7.4). Brains were removed and postfixed in 4% paraformaldehyde for 2 h. Then brains were cryoprotected in 30% sucrose/PBS solution and when sunken, frozen on dry ice and kept at −80°C. Thirty-micrometers sagital sections of the olfactory bulbs were cut on a Leica cryostat, and alternate sections were stained for soybean agglutinin conjugated with horse-radish peroxidase (SBA–HRP) or Fos. Sections were saved in antifreeze solution and maintained at −20°C for later immunocytochemistry.
In the present study, we did not analyze by means of histology, the extent of the MOE lesion following ZnSO4 treatment, since several studies have shown that it is difficult to predict the level of impairment in olfactory functioning from the size of the MOE lesion. For instance, Youngentob et al. (1997) showed that rats which were exposed to methyl bromide gas, which resulted in an almost complete lesion of the MOE (about 2–5% of the MOE was still present), displayed almost normal olfactory sensitivity as determined by olfactometry. There results confirmed an earlier study that suggested that mice are able to detect odors even though only about 5–10% of their olfactory epithelium is intact (Harding et al., 1978). Finally, both Lu and Slotnick (1998) and Setzer and Slotnick (1998) reported that rats with a severe reduction in the afferent connections between the MOE and the olfactory bulb were still able to detect a wide variety of odors. Therefore, we believe that a better method to evaluate the efficacy of ZnSO4 treatment in destructing the MOE is to determine whether stimulation with an odor can induce a functional response in the olfactory bulb since this structure is situated downstream in the processing of olfactory information. Thus, the fact that the MOB was not activated following stimulation with male urine strongly suggests that the MOE was completely destroyed by the ZnSO4 treatment.
AOB morphology using soybean agglutinin
SBA–HRP stains the axons of VNO neurons that project to the glomerular layer of the AOB and serves as a useful marker for the presence of intact VNO neurons in mice and rats (Key and Giorgi, 1986; C.J. Wysocki and L.M. Wysocki, 1995). After surgical removal of the VNO, the lack of SBA staining in the AOB provides evidence that the VNO was successfully removed. In this study, SBA–HRP staining was performed to ensure that ZnSO4 treatment did not affect the integrity of the VNO. Sagital sections of the olfactory bulbs were first incubated in 3% normal goat serum (NGS)/1% H2O2/PBS for 2 h, followed by washes in 0.1 PBS. Sections were then incubated in SBA–HRP (15 μg/ml; Sigma, Bornem, Belgium) for 40 min at room temperature, followed by washes in PBS. Sections were reacted with nickel-3,3′-diaminobenzidine (DAB) for 5 min and then mounted onto gelatin-coated slides and coverslipped using permount.
Functional assessment of the MOB and AOB using the expression of c-fos
Every fourth section was processed for Fos immunoreactivity as previously described (Halem et al., 2001; Pankevich et al., 2004). All incubations were done at room temperature and all washes in Tris-buffered saline (TBS) or PBS. Briefly, sections were preincubated for 3 h in 7.5% NGS in TBS containing 0.1% Triton X-100 (TBST). Then sections were incubated overnight with a rabbit polyclonal anti–c-fos antibody (Santa Cruz SC-52; 1:3000 in TBST/2% NGS) and then incubated for 1 h in a goat anti-rabbit biotinylated antibody (Dako Cytomation, Glostrup, Denmark; 1:200 in TBST/2% NGS). To eliminate endogenous peroxidase activity, sections were incubated for 30 min in PBS containing H2O2 at a final concentration of 3%. Sections were then incubated for 45 min in avidin–biotin complex (Vector Laboratories, Burlingame, CA, USA) and reacted for 5 min with DAB containing nickel chloride (Vector Laboratories; prepared according to the manufacturer’s recommendations). Then sections were washed, mounted onto gelatin-coated slices, dried, dehydrated through graded alcohol, cleared in toluene, and coverslipped using permount. Numbers of Fos-immunoreactive cells were quantified throughout the mitral and granular cell layers of the AOB and MOB, using a microscope with a camera lucida attachment.
Statistical analysis
All data were analyzed using repeated measures analysis of variance (ANOVA). When appropriate, all ANOVAs were followed by Tukey highest signification difference post hoc comparisons adapted for repeated measures ANOVA. Only significant (P < 0.05) effects detected by the ANOVAs are mentioned in detail in the Results.
Results
Role of the MOE in detecting volatile odors
SAL-infused females showed a strong preference to investigate intact male– over castrated male–derived volatile odor stimuli (Figure 1A,B). By contrast, ZnSO4-treated females failed to show an odor preference and showed less olfactory investigation of any of the odor stimuli, indicating that they could not detect the odors. This was confirmed by two-way ANOVA with treatment as independent factor and odor stimulus as repeated factor showing for both volatile body and urinary odors a significant effect of ZnSO4 treatment (body odors: F(1,14) = 12.4, P = 0.003; urinary odors: F(1,14) = 11.0, P = 0.005), of odor stimulus (body odors: F(1,14) = 7.2, P = 0.017; urinary odors: F(1,14) = 4.8, P = 0.046), and a significant interaction between these two factors (body odors: F(1,14) = 7.2, P = 0.017; urinary odors: F(1,14) = 8.6, P = 0.010). By contrast, the number of visits to each arm was not affected by ZnSO4 treatment (Table 1), indicating that ZnSO4 females continued to explore the Y-maze.
Figure 1.
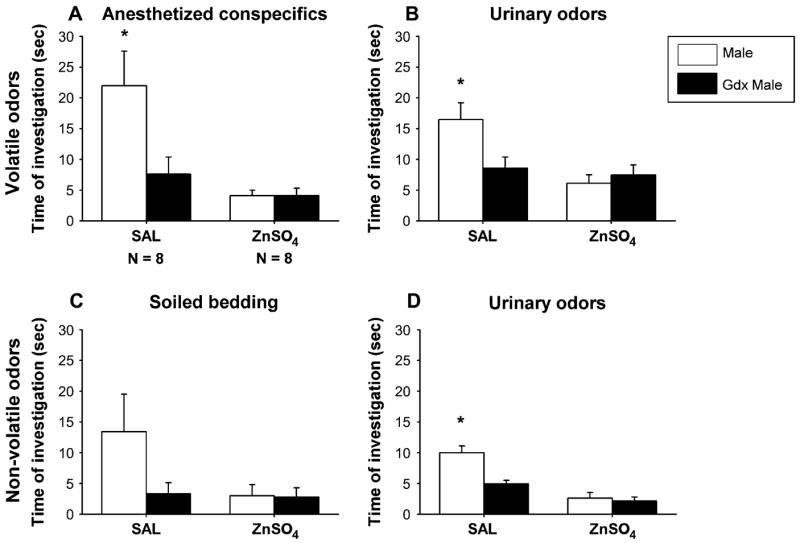
The mean (±SEM) amount of time that female mice spent investigating volatile (A, B) and nonvolatile (C, D) odor stimuli derived from intact male versus castrated (gdx) male in a Y-maze. Females had received either intranasal irrigation with zinc sulfate to destroy the MOE (ZnSO4) or saline to serve as control (SAL), prior to behavioral testing. *P < 0.05, post hoc comparisons between time spent investigating intact male versus castrated male odor by SAL females.
Table 1.
Number of entries into each arm of the Y-maze when subjects were provided with volatile (no access) or nonvolatile (access) odors from an intact male and those from a castrated male
| SAL
|
ZnSO4 |
|||||
|---|---|---|---|---|---|---|
| Male | Gdx male | Total | Male | Gdx male | Total | |
| Anesthetized conspecifics | 2.3 ± 0.3 | 1.7 ± 0.4 | 4.0 ± 0.5 | 2.9 ± 0.5 | 2.6 ± 0.6 | 5.1 ± 0.8 |
| Urine without access (volatile) | 2.7 ± 0.4 | 3.2 ± 0.8 | 5.9 ± 1.2 | 2.9 ± 0.3 | 2.1 ± 0.3 | 5.0 ± 0.6 |
| Soiled bedding | 2.1 ± 0.3 | 2.2 ± 0.5 | 4.3 ± 0.6 | 1.9 ± 0.5 | 2.3 ± 0.5 | 4.1 ± 0.9 |
| Urine with access (nonvolatile) | 1.9 ± 0.3 | 2.5 ± 0.4 | 4.4 ± 0.5 | 3.2 ± 0.6 | 2.5 ± 0.5 | 5.7 ± 0.7 |
Data are means ± SEMs.
Role of the MOE in detecting nonvolatile odors
As observed for volatile odors, SAL-treated females showed a strong preference to investigate intact male– over castrated male–derived nonvolatile urinary odors (Figure 1D). Again, no odor preference was present in ZnSO4-treated females. The latter females also spent less time investigating the odor stimuli, indicating that they probably did not detect the odors. Two-way ANOVA revealed a significant effect of the ZnSO4 treatment (F(1,14) = 55.7, P < 0.001), of the odor stimulus (F(1,14) = 11.6, P = 0.003), and a significant interaction between these two factors (F(1,14) = 10.7, P = 0.007). By contrast, ZnSO4-treated females continued to visit both arms of the Y-maze as indicated by the number of arm visits (Table 1). No statistical differences in arm visits were observed between ZnSO4- and SAL-treated females. When soiled bedding was used as odor stimulus, differences were not significant (Figure 1C) (effect of the ZnSO4 treatment: F(1,14) = 2.8, P = 0.113; effect of the odor stimulus: F(1,14) = 3.11, P = 0.099; and interaction: F(1,14) = 2.8, P = 0.113).
Role of MOE in sexual behavior
SAL females showed high levels of receptivity in all four tests (Figure 2). By contrast, sexual receptivity was clearly attenuated in ZnSO4 females. Two-way ANOVA with treatment as independent factor and tests as repeated factor on lordosis quotients showed a significant effect of ZnSO4 treatment (F(1,14) = 14.6, P = 0.002) and a marginally significant effect of repeated testing (F(3,42) = 2.7, P = 0.055) but no significant interaction. Lordosis quotients increased slightly over repeated testing in both female groups.
Figure 2.
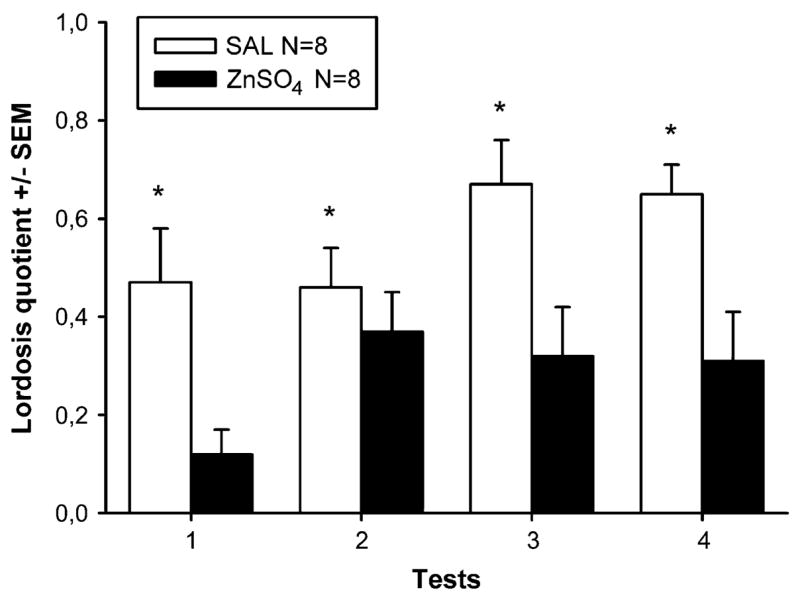
Mean (±SEM) lordosis quotients (%) of female mice which had received either intranasal irrigation with zinc sulfate to destroy the MOE (ZnSO4) or saline to serve as control (SAL). *P < 0.05, significant effect of ZnSO4 treatment (overall ANOVA).
Determine whether MOE destructions affect anxiety
No differences were observed between ZnSO4- and SAL-treated females in their behavior in the elevated plus maze, suggesting that the ZnSO4 destructions did not affect their state of anxiety. This was confirmed by two-way ANOVAs with treatment as independent factor and arm of the maze (open or closed) as repeated factor. Only an effect of arm of the maze was observed (F(2,32) = 13.2, P < 0.001). Post hoc analysis revealed that all females spent more time in the closed than in the open arm or in the center of the maze (closed arm—SAL-treated females: 155 ± 14 s, ZnSO4-treated females: 194 ± 32 s; center—SAL-treated females: 70 ± 8 s, ZnSO4-treated females: 29 ± 7 s; and open arm—SAL-treated females: 75 ±13 s, ZnSO4-treated females: 77 ± 33 s).
Histological assessment of the specificity of the destruction of the MOE
AOB morphology using soybean agglutinin
Both ZnSO4- and SAL-treated females showed SBA–HRP labeling in every section containing the AOB (Figure 3). No gross anatomical differences could be observed in the morphology of the glomerular layer of the AOB or in the intensity of the labeling between ZnSO4- and SAL-treated females.
Figure 3.
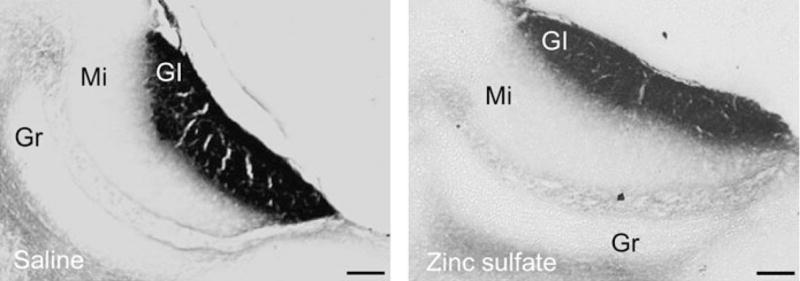
Representative photomicrographs showing sagital sections stained with SBA–HRP of the AOB of female mice which had either undergone ZnSO4 or SAL treatment. The presence of SBA–HRP staining in the glomerular layer of both groups of animals was taken as evidence of intact VNO function in ZnSO4-treated females. Gl, glomerular cell layer; Mi, mitral cell layer; Gr, granular cell layer. Scale bar: 100 μm.
Functional assessment of the MOB and AOB using the expression of c-fos
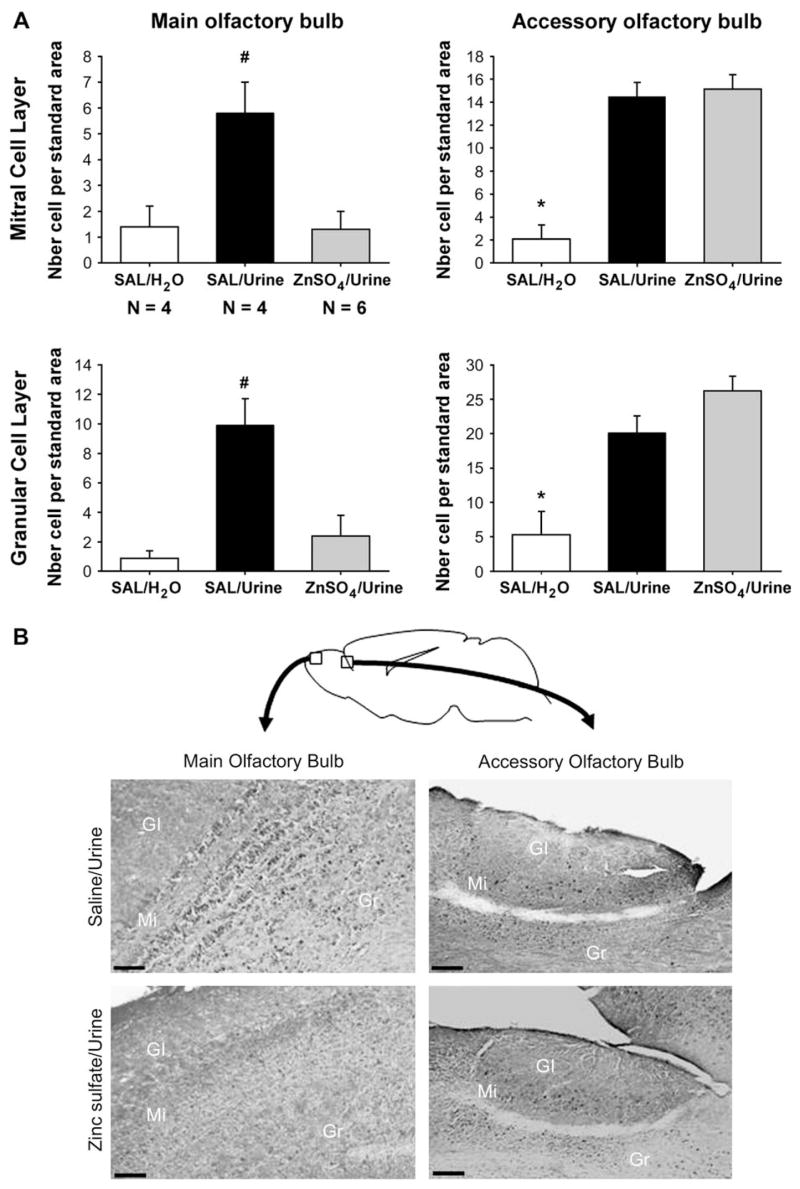
Nasal application of male urine significantly induced Fos protein in the mitral and granular cell layers of the AOB of both ZnSO4- and SAL-treated females (Figure 4). By contrast, only a significant urine-induced Fos was observed in the MOB of SAL-treated females and not of ZnSO4-treated females, thereby confirming a complete destruction of the MOE. One-way ANOVA on the number of Fos-immunoreactive cells in the AOB revealed a significant effect of odor exposure in the mitral (F(2,11) = 28.4, P < 0.001) and granular cell layers (F(2,11) = 17.0, P < 0.01) but no significant differences between urine-exposed ZnSO4- and SAL-treated females (Figure 4A). By contrast, one-way ANOVA on the number of Fos-immunoreactive cells in the MOB revealed significant differences between urine-exposed ZnSO4- and SAL-treated females in the mitral cell layer (F(2,11) = 7.6, P = 0.008) and granular cell layer (F(2,11) = 10.4, P = 0.003). In fact, ZnSO4-treated females exposed to male urine showed the same level of Fos activation as SAL-treated females exposed to water.
Figure 4.
(A) Mean (±SEM) numbers of Fos-immunoreactive cells in the mitral and granular cell layers of both the MOB and AOB. Female mice which were either treated with zinc sulfate to destroy the MOE (ZnSO4)or SAL were either exposed to male urine or water placed directly on the nose. *P < 0.05, significantly different between SAL females exposed to water and SAL and ZnSO4 females exposed to male urine. #P < 0.05, significantly different between SAL females exposed to urine and SAL females exposed to water or ZnSO4 females exposed to male urine. (B) Representative sagital sections showing Fos-immunoreactive cells in the mitral and granular cell layers of the MOB and AOB. Female mice in which the MOE was destroyed by intranasal application of ZnSO4 did not show a significant induction of c-fos in both cell layers of the MOB after exposure to male urine. By contrast, they showed a similar Fos induction in both cell layers of the AOB. Scale bar: 100 μm.
Discussion
Role of the MOE in discriminating the endocrine status among males
The present results clearly show that destruction of the MOE by applying ZnSO4 rendered female mice anosmic and that animals remained anosmic for at least 6 days following ZnSO4 application. ZnSO4-treated female mice did not respond to volatile male urinary odors in habituation/dishabituation tests. Furthermore, when presented with various volatile odor stimuli in the Y-maze, they spent less time investigating volatile odors derived from either an intact or castrated male compared to SAL-infused controls. These lower levels of olfactory investigation were not due to the fact that the ZnSO4-treated females did not explore the Y-maze; indeed, no differences were observed in the number of visits to each arm. In addition, ZnSO4-treated females did not show any odor preferences, whereas SAL controls clearly preferred to investigate intact male odors. However, the absence of an odor preference does not provide ultimate proof that the ZnSO4-treated females are anosmic since control females also showed a low level of investigation toward gdx male cues. However, it shows at least that these females failed to detect testosterone-dependent male chemosignals. In this respect, it is interesting to note that Lin et al. (2005) recently showed that adding the volatile compound (methylthio)methanethiol (MTMT) to urine from castrated males restored its ability to attract female mice. In addition, it was successful in activating mitral cells in the MOB. This compound is normally found in male mouse urine, whereas it is almost undetectable in female urine, suggesting that its synthesis may depend on testosterone. Thus, females may use MTMT to determine the endocrine status of male conspecifics.
Remarkably, when subjects were given direct access to the urinary odor stimuli, ZnSO4-treated females still showed a reduced olfactory investigation in addition to no odor preferences. Again, these lower levels of olfactory investigation were clearly not due to the fact that the ZnSO4-treated females did not visit both arms containing the odor stimuli. We were concerned that intranasal irrigation with ZnSO4 leaked into the VNO, thereby partially lesioning it. Therefore, we used soybean agglutinin as well as Fos immunocytochemistry to assess VNO functioning in ZnSO4-treated females. Staining of the AOB using soybean agglutinin did not reveal any differences between ZnSO4 and SAL-treated females. ZnSO4-treated females, like SAL-treated females, showed a significant induction of c-fos, as indicated by the presence of Fos-immunoreactive cells, in both the mitral and granular cell layers of the AOB following nasal application of male urine, suggesting that VNO functioning was not affected by the ZnSO4 treatment. By contrast, no significant Fos induction was observed in the MOB of ZnSO4-treated females after exposure to male urine confirming a complete destruction of MOE functioning in these females. Thus, our results confirm the long held view that the main olfactory system is used to localize and identify the sex and endocrine status of conspecifics on the basis of their volatile odors (Powers et al., 1979; O’Connell and Meredith, 1984). In addition, our results confirm previous observations that mice use predominantly volatile odors for discrimination of conspecifics on the basis of their sex or endocrine status (Bakker et al., 2002; Pankevich et al., 2004; Keller et al., 2006). Thus, when provided with direct access to soiled bedding or urine samples, ZnSO4-treated females do not detect the volatile odors released from these odor sources and subsequently are not attracted to investigate them further. This is in line with early observations in OMP-ntr mice, where targeted destruction of the MOE made it impossible for female mice to locate male urine spots placed in their home cage (Ma et al., 2002).
Role of the MOE in sexual behavior
Destruction of the MOE with ZnSO4 reduced lordosis quotients by about 50%. These results confirm previous findings by Edwards and Burge (1973) who showed that peripheral anosmia induced by intranasal application of ZnSO4 solution attenuated lordosis in sexually experienced and hormone-primed female mice, although not to the same extent as following bulbectomy (Thompson and Edwards, 1972). This reduction in sexual receptivity may be directly related to the absence of any main olfactory inputs since sexual receptivity is determined by inputs from a range of different external, sensory as well as internal, hormonal signals. Thus, deprivation of one sensory input (in this case from the main olfactory system) may induce less activation of the brain centers regulating lordosis and as a consequence impairs lordosis behavior. Alternatively, this reduction in lordosis behavior may be indirectly due to a deficit in VNO signaling. We have previously shown that VNO-lesioned females fail to show lordosis behavior when paired with a sexually active male, suggesting that nonvolatile male odors are necessary to induce female sexual receptivity in this species (Keller et al., 2006). Thus, if the main olfactory system is not functional, the VNO never has a chance to be activated because the animal never locates the nonvolatile odor stimuli needed to activate it. To test this possibility, we will attempt in future experiments to counter-act the deficits in lordosis behavior in ZnSO4-treated females by pipetting male urine or lacrimal gland secretions directly onto their noses.
Acknowledgments
This work was supported by Fonds National de la Recherche Scientifique (FNRS) (J.B.) and National Institute for Child Health and Human Development grant number HD044897 to J.B. and M.J.B. Julie Bakker is a research associate of the FNRS.
References
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav. 2002;41:213–219. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- Brown RE. Mammalian social odors. Adv Study Behav. 1979;10:107–161. [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Burge KG. Olfactory control of sexual behavior of male and female mice. Physiol Behav. 1973;11:867–872. doi: 10.1016/0031-9384(73)90282-5. [DOI] [PubMed] [Google Scholar]
- Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- Halem HA, Baum MJ, Cherry JA. Sex difference and steroid modulation of pheromone induced immediate early genes in the two zones of the mouse accessory olfactory system. J Neurosci. 2001;21:2474–2480. doi: 10.1523/JNEUROSCI.21-07-02474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Harding JW, Getchell TV, Margolis FL. Denervation of the primary olfactory pathway in mice, V. Long-term effect of intranasal ZnSO4 irrigation on behavior, biochemistry and morphology. Brain Res. 1978;140:271–285. doi: 10.1016/0006-8993(78)90460-2. [DOI] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Key B, Giorgi PP. Soybean agglutinin binding to the olfactory systems of the rat and mouse. Neurosci Lett. 1986;69:131–136. doi: 10.1016/0304-3940(86)90591-4. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;405:792–796. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- Lloyd-Thomas A, Keverne EB. Role of the brain and accessory olfactory system in the block to pregnancy in mice. Neuroscience. 1982;7:907–913. doi: 10.1016/0306-4522(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Lu XC, Slotnick B. Olfaction in rats with extensive lesions of the olfactory bulbs: implications for odor coding. Neuroscience. 1998;84:849–866. doi: 10.1016/s0306-4522(97)00520-4. [DOI] [PubMed] [Google Scholar]
- Luo M, Fee MS, Katz LC. Encoding pheromonal signal in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Ma D, Allen ND, Van Bergen YC, Jones CM, Baum MJ, Keverne EB, Brennan PA. Selective ablation of olfactory receptor neurons without functional impairment of vomeronasal receptor neurons in OMP-ntr transgenic mice. Eur J Neurosci. 2002;16:2317–2323. doi: 10.1046/j.1460-9568.2002.02303.x. [DOI] [PubMed] [Google Scholar]
- McBride K, Slotnick B, Margolis FL. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem Senses. 2003;28:659–670. doi: 10.1093/chemse/bjg053. [DOI] [PubMed] [Google Scholar]
- O’Connell RJ, Meredith M. Effects of volatile and nonvolatile chemical signals on male sex behaviors mediated by the main and accesory olfactory systems. Behav Neurosci. 1984;98:1083–1093. doi: 10.1037//0735-7044.98.6.1083. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierman S, Douhard Q, Balthazart J, Baum MJ, Bakker J. Attraction thresholds and sex discrimination of urinary odorants in male and female aromatase knockout (ArKO) mice. Horm Behav. 2006;49:96–104. doi: 10.1016/j.yhbeh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Powers JB, Fields RB, Winans SS. Olfactory and vomeronasal system participation in male hamsters’ attraction to female vaginal secretions. Physiol Behav. 1979;22:77–84. doi: 10.1016/0031-9384(79)90407-4. [DOI] [PubMed] [Google Scholar]
- Rajendren G, Dudley CA, Moss RL. Role of the vomeronasal organ in the male-induced enhancement of sexual receptivity in female rats. Neuroendocrinology. 1990;52:368–372. doi: 10.1159/000125619. [DOI] [PubMed] [Google Scholar]
- Setzer AK, Slotnick B. Odor detection in rats with 3-methylindole-induced reduction of sensory input. Physiol Behav. 1998;65:489–496. doi: 10.1016/s0031-9384(98)00186-3. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res. 1975;98:596–600. doi: 10.1016/0006-8993(75)90377-7. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomized rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Thompson ML, Edwards DA. Olfactory bulb removal impairs the hormonal induction of sexual receptivity in spayed female mice. Physiol Behav. 1972;8:1141–1146. doi: 10.1016/0031-9384(72)90210-7. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Occurrence of anoestrus in mice cage in groups. J Endocrinol. 1959;18:102–107. doi: 10.1677/joe.0.0180102. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Wellington JL, Beauchamp GK. Access of urinary nonvolatiles to the mammalian vomeronasal organ. Science. 1980;207:781–783. doi: 10.1126/science.7352288. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Wysocki LM. Surgical removal of the vomeronasal organ and its verification. In: Speilman AI, Brand JG, editors. Experimental Cell Biology of Taste and Olfaction. CRC Press; New York: 1995. pp. 49–57. [Google Scholar]
- Xu F, Kida I, Hyder F, Shulman RG. Assessment and discrimination of odor stimuli in rat olfactory bulb by dynamic functional MRI. Proc Natl Acad Sci USA. 2000;97:10601–10606. doi: 10.1073/pnas.180321397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Schwob JE, Sheehe PR, Youngentob LM. Odorant threshold following methyl bromide-induced lesions of the olfactory epithelium. Physiol Behav. 1997;62:1241–1252. doi: 10.1016/s0031-9384(97)00301-6. [DOI] [PubMed] [Google Scholar]
- Zou Z, Horowitz LF, Montmayeur JP, Snapper S, Buck LB. Genetic tracing reveals a stereotyped sensory map in the olfactory cortex. Nature. 2001;414:173–174. doi: 10.1038/35102506. [DOI] [PubMed] [Google Scholar]