Abstract
Previous research showed that ferrets of both sexes rely on the perception of conspecifics’ body odors to identify and motivate approach towards opposite-sex mating partners, and exposure to male body odors stimulated Fos expression in an olfactory projection circuit of female, but not male, ferrets that terminates in the ventromedial hypothalamic nucleus (VMH). We asked whether the female-typical preference of ferrets to approach male as opposed to female body odors in Y-maze tests would be disrupted by VMH lesions. Sexually experienced female ferrets were ovo-hysterectomized prior to receiving bilateral electrolytic lesions of the VMH, the preoptic area/anterior hypothalamus (POA/AH) or a sham operation. Subsequently, while receiving estradiol benzoate, females that received either complete or partial bilateral lesions of the VMH approached volatile odors from an anesthetized male on significantly fewer trials than females given POA/AH lesions or a sham operation. Both groups of ferrets with VMH lesion damage reliably discriminated between volatile anal scents as well as urinary odors from the 2 sexes in home cage habituation/dishabituation tests, suggesting that their odor-based sex discrimination remained intact. Females with complete bilateral VMH lesions showed significantly lower acceptance of neck gripping from a stimulus male (receptivity) and more aggression towards the male than all other groups of female subjects. Estrogen-sensitive neurons in the VMH appear to play a central role in female-typical neural processing of odor inputs leading to a preference to seek out a male sex partner, in addition to facilitating females’ sexual receptivity.
Keywords: Pheromone, Olfaction, Preoptic area, Anterior hypothalamus, Sexual behavior
Introduction
Numerous studies (reviewed in Pankevich et al., 2004) suggest that sex discrimination and resultant heterosexual mate recognition in many non-primate mammals rely on the detection and processing of body odors by the main olfactory system. For example, in the ferret, the induction of anosmia by occlusion of the main olfactory epithelium eliminated the preference of both male and female subjects to approach tethered opposite-sex conspecifics in Y-maze tests (Kelliher and Baum, 2001). Anosmic ferrets of both sexes showed normal mating behavior when they were placed in close proximity with a conspecific, suggesting that olfactory cues are only required to identify mating partners at a distance. In another study (Woodley and Baum, 2004), exposing male and female ferrets to volatile anal scent gland odorants resulted in a differential activation (indexed by Fos expression in periglomerular cells) of glomeruli in the main olfactory bulb, but not in the accessory olfactory bulb, which receives inputs from the vomeronasal organ (VNO). Surgical removal of the VNO failed to disrupt the preference of female ferrets to approach odors emitted from anesthetized male vs female ferrets in Y-maze tests (Woodley et al., 2004), further suggesting that mate recognition in this species relies on the processing of body odors via the main as opposed to the accessory olfactory system. A recent study using mice (Choi et al., 2005) suggests that the transcription factor Lhx6 delineates a neural circuit that conveys olfactory inputs of reproductive significance from the posterior dorsal medial amygdala to the ventromedial hypothalamic nucleus (VMH). Evidence of such an olfactory projection was previously obtained in estrous female ferrets (Wersinger and Baum, 1997) in which exposure to soiled male bedding augmented Fos expression in the main olfactory bulb as well as in several forebrain sites including the medial amygdala (MA), the bed nucleus of the stria terminalis (BNST), the medial preoptic area (MPOA) and the lateral border of the VMH. A similar profile of Fos activation in these forebrain regions was subsequently observed (Kelliher et al., 1998) in ovariectomized, testosterone-primed female ferrets following exposure to odors from soiled male bedding. By contrast, these same male odors activated Fos expression in the main olfactory bulb, MA and BNST, but not in the MPOA or VMH, of castrated male ferrets. Interestingly, exposure to odors from soiled female bedding failed to stimulate Fos expression in the VMH of gonadectomized, testosterone-primed ferrets of either sex (Kelliher et al., 1998), raising the possibility that neurons in this structure are selectively activated in the female in response to male body odorants. Ovariectomized ferrets are more likely to approach male vs female body odorants when they are treated with estradiol as opposed to no hormone (Chang et al., 2000; Kelliher and Baum, 2002), and in ferrets, as in many other species, VMH neurons selectively bind estradiol (Baum et al., 1986). This profile of results led us to ask whether bilateral lesioning of the VMH would disrupt the preference of ovariectomized, estradiol-primed female ferrets to approach male vs female derived odor stimuli or tethered stimulus ferrets in Y-maze tests. In order to control for possible non-specific effects of brain lesions on females’ odor preference, we also compared the ability of bilateral lesions centered in the medial preoptic area/anterior hypothalamus (MPOA/AH) to affect females’ odor and partner preference.
After observing that either partial or complete bilateral lesions of the VMH significantly reduced ferrets’ preference to approach male odors or a tethered male stimulus ferret in Y-maze tests, we asked whether this reflected an inability of these lesioned females to perceive the difference between male and female body odorants as opposed to a reduction in their motivation to approach male body odors. We have previously used home cage habituation/dishabituation tests to show that female ferrets readily discriminate between anal scents (Woodley and Baum, 2003) and urinary odors (Woodley et al., 2004) derived from male vs female conspecifics. We therefore administered the same tests to the different groups of lesioned and sham-operated female ferrets included in the current experiment in order to assess their olfactory discrimination capacity.
There are numerous reports of reduced sexual receptivity (lordosis) in female rodents including rat (Clark et al., 1981; Mathews and Edwards, 1977; Pfaff and Sakuma, 1979) and hamster (Malsbury et al., 1977) following the placement of VMH lesions. Disruptive effects of VMH lesions on females’ receptive responsiveness during estrus were also reported in sheep (Clegg et al., 1958) and to a lesser extent in the cat (Leedy and Hart, 1985). We therefore compared the effects of lesions of the VMH and MPOA/AH on females’ display of the limp acceptance behavior normally shown by sexually receptive female ferrets (Baum, 1976) in response to a male’s neck grip. We also monitored females’ display of aggressive bites and swats directed toward the male in response to his attempted neck grip. Finally, insofar as VMH lesions have long been known to induce hyperphagia and obesity in a variety of species (reviewed in King, 2006), we monitored ferrets’ body weights from the time of brain surgery to see whether there was any correlation between lesion-induced changes in body weight and subjects’ odor/partner preference or receptivity.
Methods
Animals
Retired breeder female (ovo-hysterectomized) and male (castrated) European Fitch ferrets were purchased from Marshall Farms (North Rose, NY, USA). Females were housed in pairs, and males were housed individually in modified rabbit cages under a long-day (16L/8D) photoperiod. Ferrets were fed Purina ferret chow once daily, and water was available ad libitum. Animal housing and all experiments were conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Boston University Animal Care and Use Committee.
Placement of brain lesions
Female ferrets received bilateral electrolytic lesions targeting either the VMH, POA/AH or sham lesions using sterile technique under combined xylazine (4 mg/kg)/ketamine (35 mg/kg) anesthesia. Following induction of anesthesia, subjects’ heads were shaved, cleaned with Betadine and fixed in a Kopf stereotaxic apparatus equipped with a squirrel monkey head adapter and ferret ear bars. Following retraction of the overlying skin and muscle layers, holes were drilled in the skull over each hemisphere, and a tungsten electrode (0.5 mm diameter) insulated with a bilayer epoxy (except for 1 mm at the tip) was lowered into the brain using the following coordinates which were determined empirically: VMH/sham=2.7 mm anterior to the intra-aural line, 0.8 mm lateral to the midline sinus and 12.8 mm below dura; POA/AH=4.9 mm anterior to the intra-aural line, 0.6 mm lateral to the midline sinus and 11.9 mm below dura. The anode of a Grass Lesion Maker was connected to the lesioning electrode, while the cathode was inserted into the animal’s anus. Lesions were made by passing 2.5 mA of direct current for 60 s. Sham lesions were performed using identical surgical procedure without passing any electrical current.
Experimental design
Beginning 3 days after brain surgery (or 2 days after arrival in the animal colony for stimulus animals), all ferrets began receiving daily subcutaneous injections of either testosterone propionate (TP; 5 mg/kg; males) or estradiol benzoate (EB; 15 μg/kg; females) dissolved in sesame oil. These steroid hormone treatments were continued for the duration of the study. Ferrets’ body weights were recorded once each week, and the data were used to adjust weekly the amount of TP and EB given to male and female ferrets, respectively. Beginning 7–14 days after the onset of EB injections, when females’ vulval diameters were at least 1 cm, subjects began receiving a series of post-operative behavioral tests of odor and partner preference which extended over a 12- to 16-day period. Subjects next received home cage tests of odor discrimination capacity over a 2- to 4-day period followed 4–7 days later by a single test of sexual receptivity.
Y-maze tests of odor and partner preference
Ferrets were tested in a Y-maze (Kelliher and Baum, 2001) that allowed for the presentation of different combinations of odor, auditory, visual and behavioral stimuli from stimulus male and female ferrets that received daily injections of TP and EB, respectively. The maze consisted of a “start” box, two “goal” boxes (all 18 × 12 × 12 in.) and a stainless steel box (48 × 36 × 12 in.) divided by a stainless steel triangle into a Y-shaped maze connecting the start box with the 2 goal boxes. The Plexiglas doors of the goal boxes were constructed so that they prevented physical contact with the stimulus behind the doors while allowing the passage of air from the goal box into the maze. One set of doors was constructed of opaque Plexiglas, while the other pair was made of clear Plexiglas (details below). The entire maze was covered with Plexiglas panels that created an airtight seal. Air was pulled into the maze through the goal boxes using an exhaust fan, which was attached to the start box with PVC tubing. This air was vented from the test room. The maze was wiped down with a diluted bleach solution followed by 95% ethanol before the onset of tests for each subject. During each subject’s repetitive trials, the maze was cleaned with 95% ethanol whenever the subject soiled the apparatus.
Female ferrets received three separate series of tests (4 days of testing for each series) for their preferences to approach Y-maze goal boxes as follows: (1) subjects could choose to approach volatile body odors emitted from anesthetized male vs female stimulus ferrets behind opaque Plexiglas goal box doors. After subjects approached a particular anesthetized stimulus ferret during each trial, the goal box door was lifted and the subject was allowed to make nasal contact with the stimulus animal for ~2 s, as in our previous study (Cloe et al., 2004). We reasoned that such direct physical contact would lead to an activation of VNO inputs, possibly enhancing the reward salience of the particular suite of odor stimuli emitted from male vs female stimulus ferrets (Woodley et al., 2004). (2) In the second series of Y-maze tests, subjects could choose to approach volatile body odors, visual and auditory cues emitted from awake male vs female ferrets kept behind clear Plexiglas goal box doors. Subjects were not allowed to have any physical contact with stimulus ferrets after each of these trials. (3) Finally, in a third series of tests, subjects could choose to approach volatile body odors, visual and auditory cues emitted from awake male vs female ferrets tethered in the respective goal boxes with no goal box door present. Subjects were given ~10 s for a brief physical interaction with the stimulus ferret chosen on each trial. During these latter tests, the stimulus male and female ferrets were slightly sedated with a combination of xylazine (.02 mg/kg) and ketamine (2 mg/kg) so that they did not struggle with their tether chains but remained awake and behaviorally active.
Each of the three stimulus conditions was presented over four consecutive test days (12 days in total). Each day’s testing began by placing a subject in the start box, whereupon the door was raised and the subject was allowed to approach stimuli emitted from either of the two goal boxes containing a male or a female ferret (free trial). A choice for male or female stimuli was recorded when the subject either made nasal contact with a goal box door (test series 1 and 2) or crossed the plane of the door (test series 3). The latency to approach the preferred goal box was also recorded during each trial. Subjects were given up to 120 s to make a choice. If this did not happen, the trial was counted as void whereupon the subject was returned to the start box and the trial was repeated (this happened very infrequently). After each free trial, a Plexiglas barrier was placed in the Y-maze to block access to the goal box chosen by a particular subject on the previous free trial. The start box door was then raised so that the subject could only approach the goal box not chosen on the previous trial (guided trial). In this way, subjects were frequently reminded of the location of the alternative social stimuli available in the two goal boxes. Daily test sessions were comprised of eight free trials and seven guided trials (a total of 32 free trials and 28 guided trials over the 4 days of testing for each stimulus condition). Male and female stimulus ferrets were placed in the same respective goal boxes during tests on any particular day; however, the location of each type of stimulus was alternated on successive days. The mean percentage of free trials (out of 32) during which each subject approached the male and female stimuli as well as latency to approach those stimuli was computed for each of the 3 stimulus conditions.
Habituation/dishabituation tests of odor discrimination
Subjects’ ability to discriminate volatile odorants emitted from either anal scent gland secretions or urine collected from male and female ferrets was assessed in the home cage using a habituation/dishabituation paradigm (Woodley and Baum, 2003). These tests were given over two consecutive days. Each day’s test consisted of a series of nine consecutive two-minute presentations of different odor stimuli with one-minute intervals between each presentation. The investigator pipetted 17 μl of odor stimulus (or vehicle) onto a 1 cm square piece of filter paper which was then secured to a plastic weigh boat. The weigh boats containing odor stimuli were inserted into a wire mesh holder attached to the front of home cage so that subjects could not make direct nasal contact with the stimuli being presented. Scent investigation was scored with a stopwatch whenever the animal’s nose came within 1 cm of the odorant stimulus. The order of stimulus presentation (urinary or anal scent gland odorants) was randomized across subjects. However, on each test day, the male stimuli were always presented first followed by the female stimuli.
Urine was collected from gonadectomized stimulus male and female ferrets previously treated with TP and EB, respectively. Individual stimulus ferrets were placed for 4 h alone in a clean cage alone over a stainless steel collecting tray. Undiluted urine that had not been contaminated with fecal matter was combined from 3 same-sex stimulus ferrets and frozen until use. On test days during which subjects’ ability to discriminate male vs female urinary odors was assessed, each subject was initially presented with 3 consecutive spots of deionized water followed by three consecutive spots of male followed by 3 consecutive spots of female urine. Frozen anal scent glands from two breeding males and two estrous females were purchased from Marshall Farms. Glands were thawed, whereupon the contents were removed and sonicated. The secretions from two individuals of the same sex were combined and diluted 1:100 in mineral oil, whereupon aliquots were stored at −20°C until use. Again, on test days when the ability to discriminate male vs female anal scents was assessed, each subject was initially presented with 3 consecutive spots of mineral oil followed by 3 consecutive spots of male followed by 3 consecutive spots of female anal scent gland odorants.
Receptivity tests
A sexually active stimulus male was habituated for 10 min in a clean, stainless steel, modified rabbit cage equipped with a clear, Plexiglas front. A subject was then placed in the cage along with the male, and the animals’ interaction was videotaped for 45 min. If the stimulus male stopped his attempts to neck grip the female subject for more than 3 min, the videotaping was paused and the disinterested male was replaced with a new stimulus male whereupon video taping was resumed. Later, the video tapes were played back and the behaviors were analyzed as follows: the duration (in seconds) of the stimulus male’s attempted as well as successful neck gripping of the female subject was recorded along with the time that the female subjects displayed a limp, unresisting (receptive) posture in response to either a successful or attempted neck grip. An index of each female’s sexual receptivity, the “acceptance quotient”, was computed by dividing the time the subject displayed a limp, unresisting receptive posture by the total time spent by the male either attempting or maintaining a neck grip on the subject (Baum, 1976). The number of bites and paw swats made by the female in response to the male’s attempted neck grips was also counted.
Histological procedures
After behavioral tests were completed, ferrets were given an i.p. injection of SleepAway (sodium pentobarbital; Butler Co., Columbus, OH; 130 mg/kg) whereupon they were transcardially perfused with 0.1 M PBS followed by 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde for 2 h followed by cryoprotection in 30% sucrose for 48 h at 4°C. Brains were sectioned coronally at 30 μm using a freezing sledge microtome. Every other section (60 μm intervals) was collected as a set (2 sets total). One set was immediately mounted onto gelatin-coated slides in exact sequential order and stained with cresyl violet to assess any lesion damage. The extent of each individual subject’s lesion was scored on a series of coronal templates of the ferret forebrain without knowledge of the subject’s behavioral performance. After group membership was assigned, individuals’ maps of lesion damage were compiled to create summary diagrams.
Data analysis
Statistical analyses were carried out using SigmaStat software for Windows (ver. 3.1; Systat, Inc.). Y-maze preference scores, sexual receptivity and body weight data (at each of 4 different times during the study) were analyzed across all groups using non-parametric Kruskal–Wallis 1-way ANOVAs. Provided a significant H value was obtained, 2-tailed Mann–Whitney U tests were used to make post hoc comparisons of pairs of means. One-tailed binomial tests were used to determine whether individual groups’ preference for male versus female stimuli in Y-maze tests differed significantly from chance. Habituation/dishabituation data were analyzed using 1-tailed Wilcoxon signed-rank tests to compare investigation times on the third presentation of one stimulus and the first presentation of a subsequent stimulus within each group of ferrets. A between-groups comparison of the time spent investigating male anal scent gland odorants was made using a Kruskal–Wallis ANOVA, followed by 2-tailed Mann–Whitney U tests for post hoc comparisons of pairs of means.
Results
Extent of lesion damage
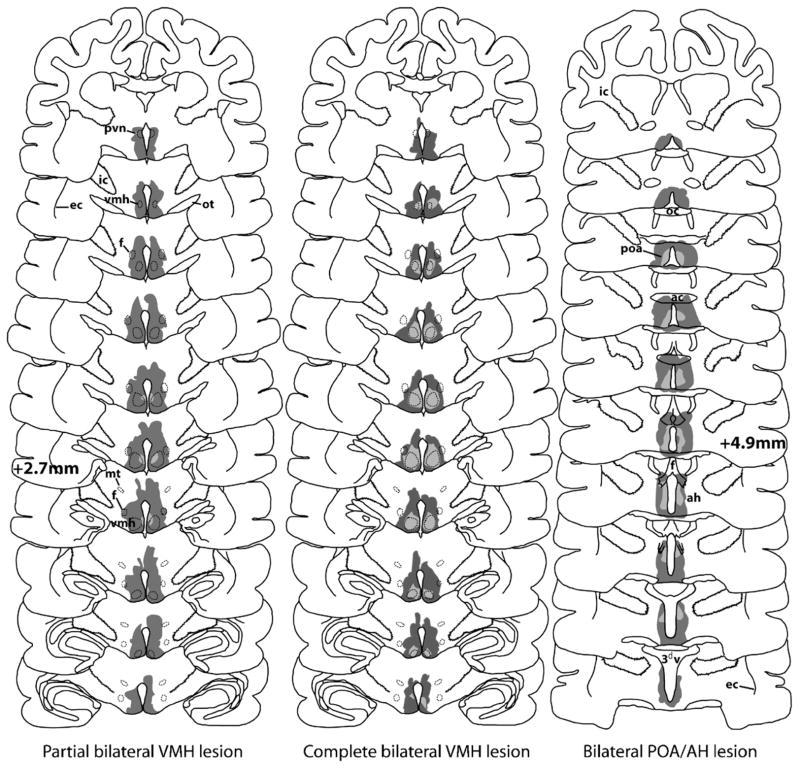
Based on an analysis of the extent of damage present in histological brain sections, female subjects that received brain lesions were divided into three groups according to lesion localization: partial bilateral VMH lesion (PBVMHx, n=11), complete bilateral VMH lesion (CBVMHx, n=6) and bilateral preoptic area/anterior hypothalamus lesion (BPOA/AHx, n=5). Schematic maps of the maximal extent as well as the common overlap of lesion damage in these three groups of females are shown in Fig. 1. Note that a variable amount of lesion damage was present just dorsal to as well as rostral and caudal to the areas of common lesion damage present in each of the 3 groups of experimental subjects. No evidence of neural damage was found in sham-operated females (n=8).
Fig. 1.
Schematic reconstruction of lesion damage in female ferrets with partial bilateral lesions of the ventromedial hypothalamic nuclei (VMH) (n=11), complete bilateral lesions of the VMH (n=6) or bilateral lesions centered at the border of the medial preoptic area and the anterior hypothalamus (POA/AH) (n=5). Regions of lesion damage common to all members of a group are shown in light gray, whereas the maximal extent of lesion damage sustained in any subject within each group is shown in dark gray. Coronal sections of the ferret brain are shown at 220 μm intervals, with the distance in front of the intra-aural line being indicated in the reconstructions of VMH as well as POA/AH lesions. Additional abbreviations: ic=internal capsule, ec=external capsule, ot=optic tract, f=fornix, mt=mammillothalamic tract, oc=optic chiasm, ac=anterior commissure, 3dv=third ventricle, poa=preoptic area, ah=anterior hypothalamus.
Odor and partner preference in Y-maze tests
Females in both the CBVMHx and PBVMHx groups approached odors emitted from the anesthetized male on significantly fewer trials than did females in either the sham-operated or BPOA/AHx groups (Fig 2; left top panel). Statistical analysis showed that there was an overall group effect (H=12.808, 3 df, p<0.005), and post hoc comparisons revealed that both the CBVMHx (p<0.008) and the PBVMHx (p<0.008) groups approached the male stimuli on significantly fewer trials than did sham-operated females. The difference between CBVMHx and POA/AHx females was also significant (p<0.05). Binomial tests showed that females in the sham-operated (x=0, p=0.004), PBVMHx (x=2, p=0.033) and BPOA/AHx (x=0, p=0.031) groups, but not in the CBVMHx group (x=1, p=0.109), showed a significant preference to approach the male as opposed to female odors. Further evidence that lesions of the VMH decreased females’ motivation to approach male odors in Y-maze tests derived from the observation (Fig. 2; left bottom panel) that the latency to approach an anesthetized male was significantly longer in CBVMHx females than in either sham-operated or BPOA/AHx females. Statistical analysis showed that there was an overall effect of group (H=15.290, 3 df, p<0.002), and post hoc comparisons showed that approach latency in CBVMHx females was significantly longer than in the sham-operated (p<0.05) or POA/AHx (p<0.009) groups. Furthermore, PBVMHx females approached the anesthetized male more slowly than females in the POA/AHx group (p<0.002).
Fig. 2.
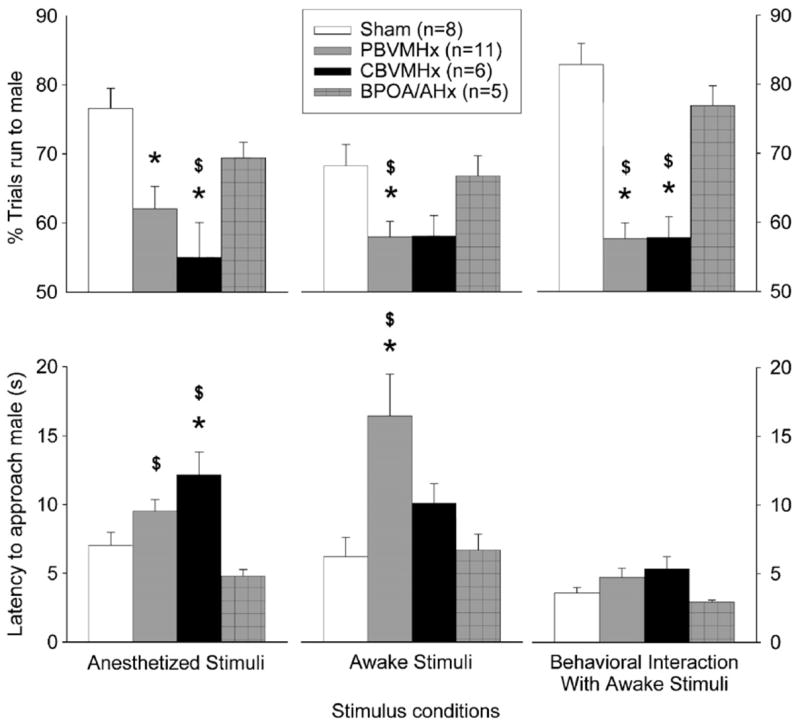
Effect of partial bilateral lesions of the ventromedial hypothalamic nuclei (PBVMHx), complete bilateral lesions of the ventromedial hypothalamic nuclei (CBVMHx), bilateral lesions centered at the border of the medial preoptic area and the anterior hypothalamus (BPOA/AHx) or a sham operation (Sham) on the approach (percentage of free trials; approach latency) of ovo-hysterectomized, estradiol-benzoate-treated female ferrets to male (as opposed to female) stimuli in Y-maze tests. See Methods text for more details about the three stimulus conditions used. Data are expressed as mean±SEM; number of subjects in each group is given in parentheses. *p<0.05 post hoc Mann–Whitney U test comparisons with the sham-operated group; $p<0.05 post hoc Mann–Whitney U test comparisons with the BPOA/AHx group.
Females with either partial or complete VMH lesions also approached an awake male behind a clear barrier on significantly fewer trials than did sham-operated or BPOA/AHx females (Fig. 2; middle top panel). There was an overall effect of group (H=10.641, 3 df, p<0.014), and post hoc comparisons showed that PBVMHx females approached the male stimuli on significantly fewer trials than either the sham-operated (p<0.003) or POA/AHx (p<0.02) females. Binomial tests revealed that the sham-operated (x=0, p=0.004), BPOA/AHx (x=0, p=0.031) and PBVMHx (x=1, p=0.006) females showed a significant preference to approach the awake male whereas no such preference was seen in the CBVMHx (x=1, p=0.109) females. Further evidence that lesions of the VMH decreased females’ motivation to approach awake males derived from the observation (Fig. 2; middle bottom panel) that latency to approach these animals was significantly longer in PBVMHx females than in either sham-operated or BPOA/AHx females. There was an overall effect of group (H=10.763, 3 df, p<0.013), with PBVMHx animals approaching males more slowly than either sham (p<0.05) or POA/AHx groups (p<0.05). There was also a non-significant trend for CBVMHx females to approach males more slowly than either of these latter 2 groups of control ferrets.
Finally, females with either partial or complete VMH lesions also approached a tethered male as opposed to a tethered female in the Y-maze goal boxes on significantly fewer trials than did sham-operated or BPOA/AHx females (Fig. 2; right top panel). There was an overall effect of group (H=21.211, 3 df, p<0.001), and post hoc comparisons revealed that both the CBVMHx (p<0.001) and the PBVMHx (p<0.001) groups approached the tethered male on significantly fewer trials than did sham-operated females. The differences between CBVMHx and POA/AHx females (p<0.009) and between PBVMHx and POA/AHx females (p<0.002) were also significant. Binomial tests showed that females in the sham-operated (x=0, p=0.004), PBVMHx (x=1, p=0.006), CBVMHx (x=0, p=0.016) and BPOA/AHx (x=0, p=0.031) groups showed a significant preference to approach the tethered male as opposed to the tethered female. There were no significant differences among the 4 groups of females in their latency to approach the tethered stimulus male (Fig. 2; right bottom panel).
Habituation/dishabituation tests of odor discrimination
All four groups of females showed significant (p<0.05) dishabituation responses upon the first presentation of both male and female volatile anal scent gland odors (Fig. 3). Females in the CBVMHx group spent significantly less time investigating male anal scents upon their first presentation than did females in either the sham-operated or BPOA/AHx groups (Fig. 3; inset bar graph). For the latter comparison, there was an overall effect of group (H=9.600, 3 df, p<0.022), and post hoc tests showed that CBVMHx females spent significantly less time investigating male anal scents than either sham-operated (p<0.001) or the POA/AHx (p<0.009) females. Sham-operated females spent significantly (p=0.039, 2-tailed Wilcoxon comparison) more time investigating the male as opposed to female anal scent gland odorants when they were first presented in these home cage tests whereas no such difference was seen in the other groups of female subjects. Finally, all four groups of females showed significant (p<0.05) dishabituation responses upon the first presentation of both male and female urinary odors (Fig. 4).
Fig. 3.
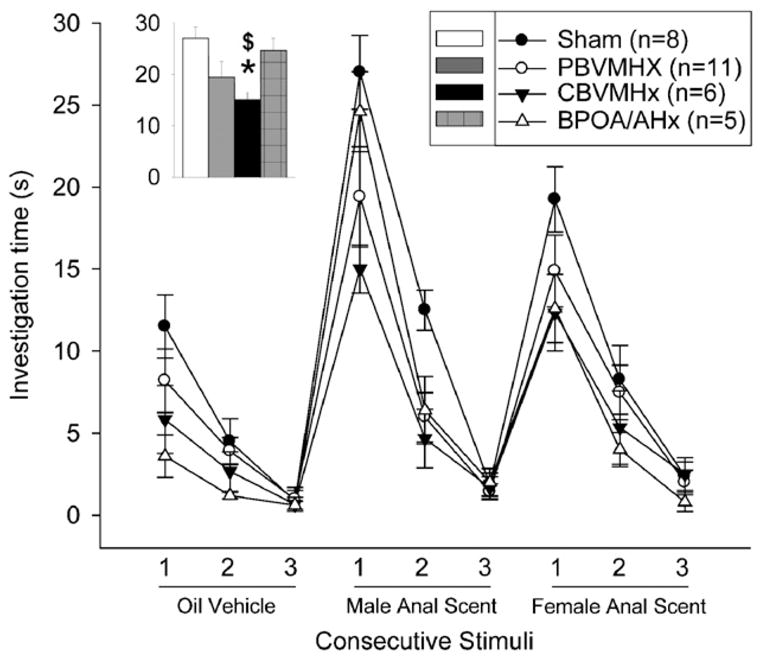
Effect of partial bilateral lesions of the ventromedial hypothalamic nuclei (PBVMHx), complete bilateral lesions of the ventromedial hypothalamic nuclei (CBVMHx), bilateral lesions centered at the border of the medial preoptic area and the anterior hypothalamus (BPOA/AHx) or a sham operation (Sham) on investigation by ovo-hysterectomized, estradiol-benzoate-treated female ferrets of a sequence of anal scent gland odorants (oil vehicle; male scent, female scent) presented in home cage habituation/dishabituation tests (more details in the Methods section). Inserted bar graph shows the investigation times of different groups of females for male anal scent when it was first presented (trial 1). Data are expressed as mean±SEM; number of subjects in each group is given in parentheses. *p<0.05 post hoc Mann–Whitney U test comparisons with the sham-operated group; $p<0.05 post hoc Mann–Whitney U test comparisons with the BPOA/AHx group.
Fig. 4.
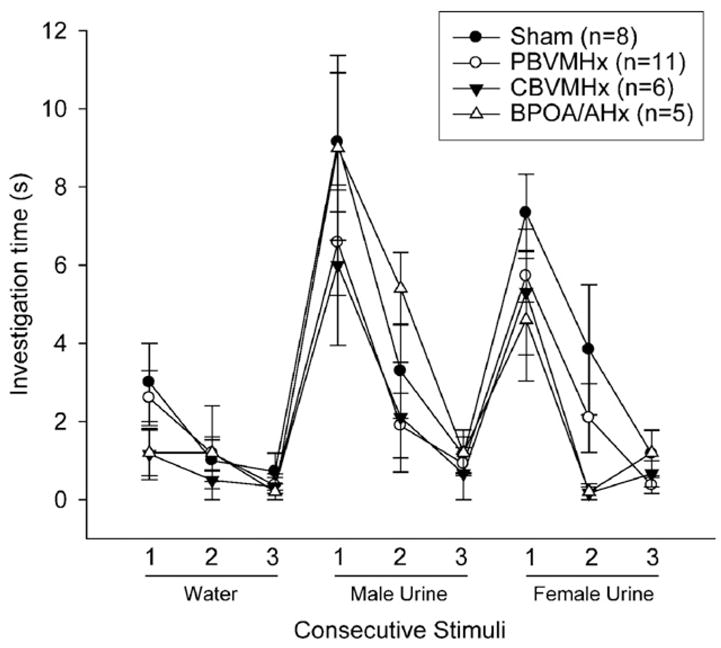
Effect of partial bilateral lesions of the ventromedial hypothalamic nuclei (PBVMHx), complete bilateral lesions of the ventromedial hypothalamic nuclei (CBVMHx), bilateral lesions centered at the border of the medial preoptic area and the anterior hypothalamus (BPOA/AHx) or a sham operation (Sham) on investigation by ovo-hysterectomized, estradiol-benzoate-treated female ferrets of a sequence of urinary odorants (water; male and female urine) presented in home cage habituation/dishabituation tests (more details in the Methods section). Data are expressed as mean±SEM; number of subjects in each group is given in parentheses.
Receptivity tests
Female ferrets in the CBVMHx group showed a significant decrement in receptive behavior in response to attempted neck grips by a stimulus male (Fig. 5). Statistical analyses showed that there were significant overall differences among the 4 groups in acceptance quotient (H=12.730, 3 df, p<0.005), bites per minute (H=15.385, 3 df, p<0.002) and swats per minute (H=13.135, 3 df, p<0.004). Subsequent post hoc comparisons showed that CBVMHx displayed significantly lower acceptance quotients than sham-operated (p<0.001) or POA/AHx (p<0.03) females coupled with significantly more bites per minute than sham-operated (p<0.002) or POA/AHx (p<0.004) females. CBVMHx females also displayed significantly more swats per minute than sham-operated (p<0.002) or POA/AHx (p<0.009) females.
Fig. 5.
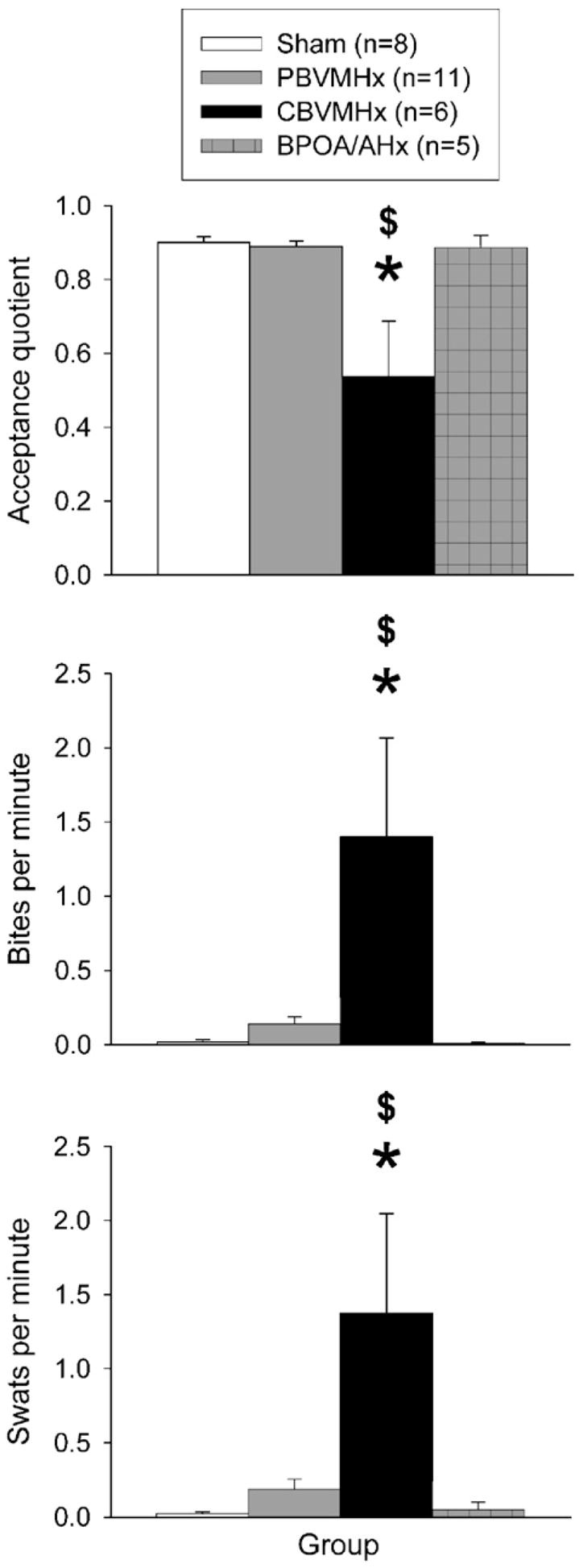
Effect of partial bilateral lesions of the ventromedial hypothalamic nuclei (PBVMHx), complete bilateral lesions of the ventromedial hypothalamic nuclei (CBVMHx), bilateral lesions centered at the border of the medial preoptic area and the anterior hypothalamus (BPOA/AHx) or a sham operation (Sham) in ovo-hysterectomized, estradiol-benzoate-treated female ferrets on 3 indices of sexual receptivity (acceptance quotient: ratio of the time that females displayed a limp acceptance posture to the time that a stimulus male either maintained or attempted to maintain a neck grip on the female; frequency/minute of bites and paw swats shown by female subjects in response to attempted neck grips by a stimulus male). Data are expressed as mean±SEM; number of subjects in each group is given in parentheses.
Post-operative body weight gain
At the time of brain surgery, there were no significant differences in body weight among the 4 groups of females. By contrast, at different post-operative time points, there were significant increases in body weights in all three groups of lesioned females while the weights of sham-operated females remained unchanged (Fig. 6). Significant overall group differences in body weight were seen at the start of EB treatment (H=9.919, 3 df, p<0.019) as well as at the start (H=8.577, 3 df, p<0.035) and end (H=15.210, 3 df, p<0.002) of behavioral testing. Subsequent post hoc comparisons at the start of EB treatment showed that CBVMHx (p<0.008) and PBVMHx (p< 0.006) females weighed more than sham-operated females. Likewise, comparisons made at the start of testing showed that CBVMHx (p<0.001) and PBVMHx (p<0.002) females weighed significantly more than sham-operated females. At both the start of EB and the start of testing, BPOA/AHx females’ body weights did not differ significantly from those of females with VMH lesions. At the end of behavioral testing, CBVMHx (p<0.001), PBVMHx (p<0.005) and POA/AHx (p<0.03) females all weighed significantly more than sham-operated females, and CBVMHx females weighed significantly (p<0.02) more than POA/AHx females. Further analysis showed that correlation coefficients between body weight at the end of behavioral testing and the percentage of free Y-maze trials run by all 17 females with some degree of VMH damage towards the anesthetized (r=−0.007), awake (r=−0.140) and tethered (r=0.146) stimulus male were not statistically significant. Likewise, the correlation between body weight and acceptance quotients (r=0.023) for these same females was also not significant.
Fig. 6.
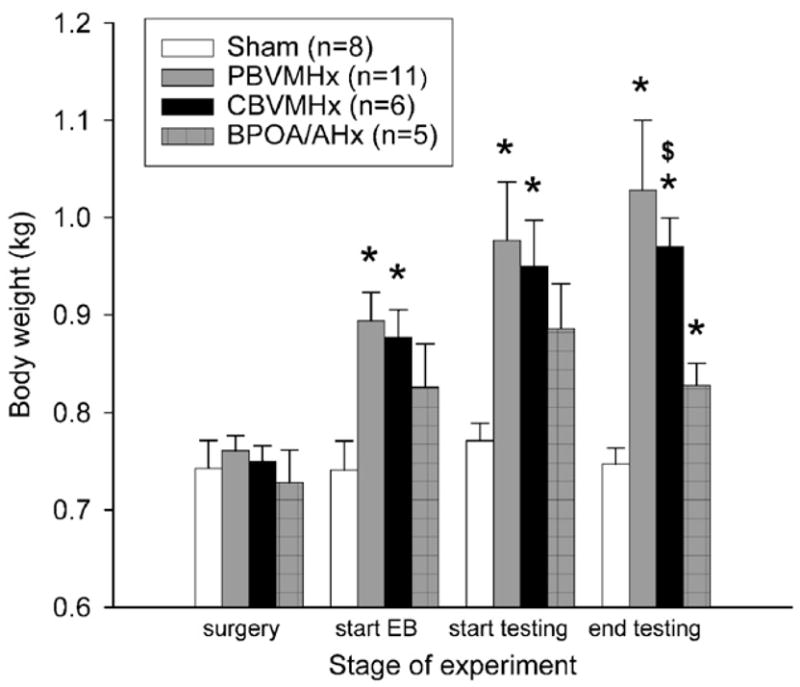
Effect of partial bilateral lesions of the ventromedial hypothalamic nuclei (PBVMHx), complete bilateral lesions of the ventromedial hypothalamic nuclei (CBVMHx), bilateral lesions centered at the border of the medial preoptic area and the anterior hypothalamus (BPOA/AHx) or a sham operation (Sham) in ovo-hysterectomized, estradiol-benzoate (EB)-treated female ferrets on body weights (expressed in kilograms; kg) at critical experimental time points. Data are expressed as mean±SEM; number of subjects in each group is given in parentheses. *p<0.05 post hoc Mann–Whitney U test comparisons with the sham-operated group; $p<0.05 post hoc Mann–Whitney U test comparisons with the BPOA/AHx group.
Discussion
Our main finding was that bilateral damage to the VMH significantly reduced the female-typical preference of ferrets to approach volatile body odors emitted from male as opposed to female stimulus ferrets. These deficits in olfactory investigation of opposite-sex body odors were best revealed in Y-maze tests using anesthetized stimulus subjects. Further evidence of a disruptive effect of VMH lesions on females’ interest in male body odorants was reflected in the significant reduction in the time that CBVMHx females spent investigating male (but not female) anal scent gland odorants when they were first presented during home cage assessments of subjects’ ability to discriminate between these odorants. Providing visual and auditory cues or the opportunity for a physical interaction with stimulus ferrets in Y-maze tests failed to reverse the reduced preference of females with VMH damage for male-derived olfactory stimuli. This outcome corroborates our previous suggestion (Kelliher and Baum, 2001) that olfactory inputs take precedence over other sensory modalities in determining ferrets’ sex partner preference. The present results are also consistent with the suggestion (Choi et al., 2005) that there is a neural circuit that includes the medial amygdala and the VMH which is selectively responsive to reproductively salient olfactory inputs. Indeed, body odors from male as opposed to female ferrets selectively augmented Fos expression in the VMH of female ferrets (Kelliher et al., 1998), and in the present study, lesioning of the VMH selectively reduced female ferrets’ motivation to approach volatile odors emitted from male conspecifics.
In the present study, as in our previous work (Paredes and Baum, 1995), bilateral lesions of the POA/AH failed to affect the preference of ovo-hysterectomized, estradiol-primed female ferrets to approach and interact physically with male vs female stimulus ferrets. In the present study, POA/AH lesions also failed to disrupt females’ preference to approach odors emitted from either anesthetized or awake male stimulus ferrets in Y-maze tests. These latter findings apparently differ from a recent report (Guarraci and Clark, 2006) that medial preoptic area (mPOA) lesions reduced the preference of estrous female rats to approach a male vs estrous female stimulus animal, although in that study the effects of mPOA lesions were most evident when actual physical contact (with mating) was allowed between subjects and stimulus rats. The absence of any effect of POA/AH lesions in female ferrets contrasts with our previous studies using male ferrets in which either excitotoxic (Paredes and Baum, 1995) or electrolytic (Kindon et al., 1996) lesions of the POA/AH caused a reversal of partner preference. These lesions, which bilaterally damaged the sexually dimorphic male nucleus (MN) of the ferret’s POA/AH (Tobet et al., 1986), thereby duplicated the preference profile seen in sham-operated control females. It is noteworthy that in the present experiment placement of VMH lesions simply reduced or in some cases eliminated females’ preference to approach male stimuli instead of causing them to switch their preference to other females. This combination of results suggests that VMH neurons play a central role in the processing of odor inputs leading to a female-typical profile of preference for male odors. In male ferrets, differentiation of a sexually dimorphic MN-POA/AH, which occurs during the last quarter of gestation in response to the action of estradiol formed by local aromatization of testosterone (Tobet et al., 1986), leads to the expression later in adulthood of a male-typical preference to seek out female body odors. Indeed, bilateral destruction of the MN-POA/AH was recently found (Alekseyenko et al., submitted for publication) to cause male ferrets to shift their preference from approaching opposite-sex (female) to same-sex (male) odors from anesthetized stimulus animals in Y-maze tests and to show a female-typical profile of Fos expression in the medial preoptic area following exposure to odors in soiled male bedding.
We observed that females with either partial or complete VMH lesions, like females given BPOA/AH or sham lesions, reliably discriminated male from female anal scent gland as well as urinary odors when they were presented in home cage habituation/dishabituation tests. This strongly suggests that the disruptive effects of either type of VMH lesion on females’ motivation to approach male stimuli in Y-maze tests was not a reflection of subjects’ inability to discriminate these odors but instead reflected a change in the reward salience of these odors to these animals. Even though they reliably detected male anal scent gland odorants in these tests, females with complete VMH lesions spent significantly less time than either sham-operated or BPOA/AHx females investigating anal scent gland odorants from males when they were first presented in a sequence of odors as part of home cage habituation/dishabituation testing. No such effect of VMH lesions was seen in habituation/dishabituation tests during which urinary odors were presented. All groups of females spent less than half as much time investigating male urinary odorants compared with male anal scents (compare the Y axes in Figs. 3 and 4), suggesting that anal scent gland odorants are considerably more salient than urinary odors to ferrets. Apparently VMH lesions only reduced females’ interest in these more salient olfactory stimuli.
The present results showing that even partial bilateral destruction of the VMH eliminated female ferrets’ motivation to approach body odors from males extend results from several previous studies using different species showing that VMH lesions significantly reduced females’ proceptive sexual behaviors displayed towards a stimulus male. In an early study (Clark et al., 1981), bilateral lesions or surgical isolation of the VMH reduced approach behaviors otherwise displayed by estrous female rats towards a tethered stimulus male. A similar disruptive effect of VMH lesions was obtained in another study (Emery and Moss, 1984) in which estrous female rats were allowed to pace their contacts with a sexually active male. Likewise, placement of VMH lesions in female cats significantly reduced their approach and proceptive solicitation of neck grips and mounts from a stimulus male (Leedy and Hart, 1985). Finally, VMH lesions in female hamsters reduced their approach towards a stimulus male (Floody, 2002). The present results raise the possibility that all of the deficits in proceptive sexual behavior previously reported in female mammals following VMH lesions reflected a lesion-induced reduction in subjects’ motivation to approach opposite-sex (male) odors.
Our observation that complete bilateral lesions of the VMH caused a significant reduction in female ferrets’ acceptance of male neck grips coupled with increased display of aggressive behavior towards the stimulus male corroborates previous reports of reduced receptivity in a variety of vertebrate species including female whiptail lizard (Kendrick et al., 1995), rat (Clark et al., 1981; Mathews and Edwards, 1977; Pfaff and Sakuma, 1979), hamster (Malsbury et al., 1977), guinea pig (Goy and Phoenix, 1963), ewe (Clegg et al., 1958) and cat (Leedy and Hart, 1985) after the bilateral placement of VMH lesions. Partial lesions of the female ferret’s VMH failed to disrupt the display of receptive behavior. Our observation that the ability of VMH lesions to reduce receptivity in ferrets depended on the extent of the lesion damage corroborates previous reports in the female rat (Pfaff and Sakuma, 1979) and hamster (Malsbury et al., 1977) of a direct correlation between the extent of VMH lesion damage and resultant deficits in females’ receptivity.
In rats, estradiol receptors are localized in the ventro-lateral portion of the VMH (Pfaff and Keiner, 1973), and implantation of estradiol into this part of the VMH induced lordosis behavior and proceptive responses in ovariectomized female rats, provided subjects were subsequently treated with progesterone (Rubin and Barfield, 1983). In another study (Meisel et al., 1987), implantation of the estradiol receptor antagonist tamoxifen selectively into the VMH attenuated lordosis behavior in ovariectomized female rats given ovarian sex hormones. An autoradiographic study carried out using ovariectomized female ferrets (Baum et al., 1986) showed that estradiol binding cells are distributed throughout the VMH. This finding together with the present observation that only complete VMH lesions disrupted females’ display of receptive sexual behavior suggests that there is considerable redundancy in the ability of estradiol-responsive VMH neurons to promote the full expression of feminine acceptance behavior in response to a male’s neck grip. Our observation that even partial lesions of the VMH disrupted olfactory mate recognition in female ferrets raises the possibility that there is less redundancy in the capacity of estrogen-sensitive VMH neurons to control this function. Bilateral VMH lesions (either partial or complete) may have reduced females’ motivation to approach male odors by destroying estrogen-sensitive neurons or by interrupting fibers conveying olfactory information to the VMH. The present study cannot discriminate between these alternatives.
In the female ferret, as in many other vertebrate species (reviewed in King, 2006), bilateral lesion damage to the VMH caused a significant increase in body weight, which was present in both PBVMHx and CBVMHx females between the start and end of behavioral testing. It seems unlikely that lesion-induced gains in body weight (or in any hyperphagia leading to this weight gain), by itself, can explain the observed lesion-induced disruption of females’ sexual receptivity. Equivalent increments in body weight occurred in the 2 groups of female ferrets given VMH lesions, yet a significant reduction in acceptance behavior was seen only in females with complete VMH lesions (also, the correlation in these 2 groups of females between body weight at the end of testing and acceptance quotients was not significant). In Y-maze tests given with all three types of stimulus animals, both PBVMHx and CBVMHx females showed similar deficits in their approach to male stimuli. It seems unlikely, however, that these behavioral changes resulted simply from a loss of mobility associated with the increased weight gain that occurred in these two groups of females. First, there was again no correlation in these 2 groups of females between body weight and the percentage of trials on which they approached any of the 3 types of stimulus male in Y-maze tests. Second, both groups of females with VMH lesions were capable of running from the start box to the male goal box as quickly as the other 2 groups of females in tests given when subjects had a behavioral interaction with tethered stimulus ferrets in the goal boxes after each trial. Finally, females in the BPOA/AH group also experienced significant post-lesion increases in body weight (albeit somewhat lower ones than in VMH lesioned subjects). However, their performance in all Y-maze tests was indistinguishable from that of sham-operated females.
Results of the present study suggest that both female-typical, odor-based mate recognition and the capacity to display receptive sexual behavior in response to a male’s neck grip depend critically on the integrity of the female’s VMH. In male ferrets, perinatal exposure to testosterone of testicular origin and/or to estradiol synthesized by aromatization of this testosterone in the developing hypothalamus promotes a male-typical differentiation of a preference to seek out body odors emitted from females (Baum et al., 1990). Our prior experiments (Alekseyenko et al., submitted for publication; Kindon et al., 1996; Paredes and Baum, 1995) suggest that this male-typical profile of female-directed preference depends on the differentiation of sexually dimorphic MH-POA/AH which may override any VMH-dependent processing of body odors leading to a female-typical preference to approach male odors. In male rodents, the capacity to display sexually receptive (lordosis) behavior in response to adult ovarian hormone treatment is dramatically reduced due to the defeminizing action of estradiol during perinatal brain sexual differentiation (reviewed in Baum, 1979). By contrast, in male ferrets, no such defeminization of receptive capacity occurs in the course of brain sexual differentiation (Baum, 1976; Baum et al., 1990). New studies are needed to assess the possible role of the VMH in the capacity of male ferrets to show female-typical receptive sexual behavior.
Acknowledgments
This research was supported by NIH grant HD21094. We thank Dr. Olga Alekseyenko and Patricia Waters for technical assistance and the staff of the Boston University Animal Care Facility for caring for our ferrets.
References
- Alekseyenko OV, Waters P, Zhou H, Baum MJ. Bilateral damage to the sexually dimorphic medial preoptic area/anterior hypothalamus of male ferrets causes a female-typical preference for and a hypothalamic Fos response to male body odors. doi: 10.1016/j.physbeh.2006.10.005. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ. Effects of testosterone propionate administered perinatally on sexual behavior of female ferrets. J Comp Physiol Psychol. 1976;90:399–410. doi: 10.1037/h0077220. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Differentiation of coital behavior in mammals: a comparative analysis. Neurosci Biobehav Rev. 1979;3:265–284. doi: 10.1016/0149-7634(79)90013-7. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Gerlach JL, Krey LC, McEwen BS. Biochemical and radioautographic analysis of estrogen-inducible progestin receptors in female ferret brain and pituitary: correlations with effects of progesterone on sexual behavior and gonadotropin-releasing hormone-stimulated secretion of luteinizing hormone. Brain Res. 1986;368:296–309. doi: 10.1016/0006-8993(86)90574-3. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Erskine MS, Kornberg E, Weaver CE. Prenatal and neonatal testosterone exposure interact to affect differentiation of sexual behavior and partner preference in female ferrets. Behav Neurosci. 1990;104:183–198. doi: 10.1037//0735-7044.104.1.183. [DOI] [PubMed] [Google Scholar]
- Chang YM, Kelliher KR, Baum MJ. Steroidal modulation of scent investigation and marking behaviors in male and female ferrets (Mustela putorius furo) J Comp Psychol. 2000;114:401–407. doi: 10.1037/0735-7036.114.4.401. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Clark AS, Pfeifle JK, Edwards DA. Ventromedial hypothalamic damage and sexual proceptivity in female rats. Physiol Behav. 1981;27:597–602. doi: 10.1016/0031-9384(81)90228-6. [DOI] [PubMed] [Google Scholar]
- Clegg MT, Santolucito JA, Smith JD, Ganong WF. The effect of hypothalamic lesions on sexual behavior and estrous cycles in the ewe. Endocrinology. 1958;62:790–797. doi: 10.1210/endo-62-6-790. [DOI] [PubMed] [Google Scholar]
- Cloe AL, Woodley SK, Waters P, Zhou H, Baum MJ. Contribution of anal scent gland and urinary odorants to mate recognition in the ferret. Physiol Behav. 2004;82:871–875. doi: 10.1016/j.physbeh.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Emery DE, Moss RL. Lesions confined to the ventromedial hypothalamus decrease the frequency of coital contacts in female rats. Horm Behav. 1984;18:313–329. doi: 10.1016/0018-506x(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Floody OR. Time course of VMN lesion effects on lordosis and proceptive behavior in female hamsters. Horm Behav. 2002;41:366–376. doi: 10.1006/hbeh.2002.1776. [DOI] [PubMed] [Google Scholar]
- Goy RW, Phoenix CH. Hypothalamic regulation of female sexual behaviour; establishment of behavioural oestrus in spayed guinea-pigs following hypothalamic lesions. J Reprod Fertil. 1963;5:23–40. doi: 10.1530/jrf.0.0050023. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Clark AS. Ibotenic acid lesions of the medial preoptic area disrupt the expression of partner preference in sexually receptive female rats. Brain Res. 2006;1076:163–170. doi: 10.1016/j.brainres.2005.12.120. [DOI] [PubMed] [Google Scholar]
- Kelliher KR, Baum MJ. Nares occlusion eliminates heterosexual partner selection without disrupting coitus in ferrets of both sexes. J Neurosci. 2001;21:5832–5840. doi: 10.1523/JNEUROSCI.21-15-05832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher K, Baum M. Effect of sex steroids and coital experience on ferrets’ preference for the smell, sight and sound of conspecifics. Physiol Behav. 2002;76:1–7. doi: 10.1016/s0031-9384(02)00691-1. [DOI] [PubMed] [Google Scholar]
- Kelliher KR, Chang YM, Wersinger SR, Baum MJ. Sex difference and testosterone modulation of pheromone-induced neuronal Fos in the ferret’s main olfactory bulb and hypothalamus. Biol Reprod. 1998;59:1454–1463. doi: 10.1095/biolreprod59.6.1454. [DOI] [PubMed] [Google Scholar]
- Kendrick AM, Rand MS, Crews D. Electrolytic lesions to the ventromedial hypothalamus abolish receptivity in female whiptail lizards, Cnemidophorus uniparens. Brain Res. 1995;680:226–228. doi: 10.1016/0006-8993(95)00191-r. [DOI] [PubMed] [Google Scholar]
- Kindon HA, Baum MJ, Paredes RJ. Medial preoptic/anterior hypothalamic lesions induce a female-typical profile of sexual partner preference in male ferrets. Horm Behav. 1996;30:514–527. doi: 10.1006/hbeh.1996.0055. [DOI] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Leedy MG, Hart BL. Female and male sexual responses in female cats with ventromedial hypothalamic lesions. Behav Neurosci. 1985;99:936–941. doi: 10.1037//0735-7044.99.5.936. [DOI] [PubMed] [Google Scholar]
- Malsbury CW, Kow LM, Pfaff DW. Effects of medial hypothalamic lesions on the lordosis response and other behaviors in female golden hamsters. Physiol Behav. 1977;19:223–237. doi: 10.1016/0031-9384(77)90331-6. [DOI] [PubMed] [Google Scholar]
- Mathews D, Edwards DA. The ventromedial nucleus of the hypothalamus and the hormonal arousal of sexual behaviors in the female rat. Horm Behav. 1977;8:40–51. doi: 10.1016/0018-506x(77)90019-8. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Dohanich GP, McEwen BS, Pfaff DW. Antagonism of sexual behavior in female rats by ventromedial hypothalamic implants of antiestrogen. Neuroendocrinology. 1987;45:201–207. doi: 10.1159/000124726. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Baum MJ. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area/anterior hypothalamus. J Neurosci. 1995;15:6619–6630. doi: 10.1523/JNEUROSCI.15-10-06619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983;37:218–224. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Zahniser DJ, Baum MJ. Differentiation in male ferrets of a sexually dimorphic nucleus of the preoptic/anterior hypothalamic area requires prenatal estrogen. Neuroendocrinology. 1986;44:299–308. doi: 10.1159/000124660. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Baum MJ. Sexually dimorphic processing of somatosensory and chemosensory inputs to forebrain luteinizing hormone-releasing hormone neurons in mated ferrets. Endocrinology. 1997;138:1121–1129. doi: 10.1210/endo.138.3.4969. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Baum MJ. Effects of sex hormones and gender on attraction thresholds for volatile anal scent gland odors in ferrets. Horm Behav. 2003;44:110–118. doi: 10.1016/s0018-506x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Baum MJ. Differential activation of glomeruli in the ferret’s main olfactory bulb by anal scent gland odours from males and females: an early step in mate identification. Eur J Neurosci. 2004;20:1025–1032. doi: 10.1111/j.1460-9568.2004.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley SK, Cloe AL, Waters P, Baum MJ. Effects of vomeronasal organ removal on olfactory sex discrimination and odor preferences of female ferrets. Chem Senses. 2004;29:659–669. doi: 10.1093/chemse/bjh069. [DOI] [PMC free article] [PubMed] [Google Scholar]