Abstract
Four experiments were conducted to determine whether vomeronasal organ (VNO) inputs in male mice mediate the rewarding properties of estrous female urinary odors. Sexually naive male mice with either an intact (VNOi) or lesioned (VNOx) VNO preferred to investigate female urine over water in Y-maze tests. Subsequently, VNOi males ran significantly more quickly and remained in nasal contact longer with estrous female urine than with male urine, whereas VNOx males investigated these odors equally. In home-cage habituation–dishabituation tests, VNOi males also investigated female urine significantly longer than did VNOx males, although both groups investigated female urine longer than other non-body odors. Finally, female urinary odors induced Fos in the nucleus accumbens core of VNOi males but not of VNOx males. Our results suggest that female urinary odors retain some incentive value in VNOx males. However, once direct nasal contact is made with female urine, VNO inputs further activate forebrain mechanisms that amplify the reward salience of this stimulus for the male mouse.
Keywords: accessory olfactory bulb, nucleus accumbens, pheromones, sexual behavior
Social communication in rodents occurs via volatile as well as nonvolatile components of urine and other body odorants (Brown, 1979). Several studies (Lin, Zhang, Block, & Katz, 2005; Schaefer, Yamazaki, Osada, Restrepo, & Beauchamp, 2002; Schaefer, Young, & Restrepo, 2001) suggest that volatile components of urine are detected by receptor neurons in the main olfactory epithelium and processed in the main olfactory bulb, whereas other studies suggest that nonvolatile components of urine (Luo, Fee, & Katz, 2003) as well as extraorbital lacrimal gland secretions (Kimoto, Haga, Sato, & Touhara, 2005) are detected by receptor neurons in the vomeronasal organ (VNO) and processed in the accessory olfactory bulb (AOB). Socially relevant olfactory signals from both the main and accessory systems are integrated in the medial amygdala prior to being conveyed to different hypothalamic regions (Boehm, Zou, & Buck, 2005; Kevetter & Winans, 1981a, 1981b; Licht & Meredith, 1987). We (Pankevich, Baum, & Cherry, 2004) reported that surgical VNO removal (VNOx) eliminated the normal preference of male mice to remain in nasal contact with estrous female as opposed to male urine in the home cage. Likewise, VNO removal significantly reduced the preference of female mice to directly investigate male as opposed to female urine spots in Y-maze tests (Keller, Pierman, Douhard, Baum, & Bakker, 2006). Taken together, these results raise the possibility that inputs from the VNO-accessory olfactory system are rewarding and thereby enhance the motivation of mice to approach and maintain physical contact with nonvolatile urinary odors from opposite-sex conspecifics. Such a view was first proposed by Beauchamp and coworkers (Beauchamp, Martin, Wysocki, & Wellington, 1982), who observed that VNOx in male guinea pigs caused a progressive reduction over many days of testing in the investigation of female urine. This decline in urine investigation depended on repeated exposure to the stimulus as opposed to the simple passage of time after surgery (Beauchamp, Wysocki, & Wellington, 1985), suggesting that a learned approach response initially mediated by the main olfactory system had extinguished in the absence of rewarding inputs from the VNO-accessory olfactory system. In a similar vein, deficits in prey consumption were observed in snakes after VNOx (Kubie & Halpern, 1979), and it was hypothesized (Halpern, 1987) that VNO inputs augment the reinforcing value of prey items.
We further assessed this VNO-reward hypothesis by studying the effect of VNO removal on the preference of male mice to approach and directly investigate different urinary odorants (estrous female urine versus water in Experiment 1, estrous female versus male urine in Experiment 2) in Y-maze tests given over 10 consecutive days. We hypothesized that VNOx males, like VNO-intact (VNOi) males, would initially prefer to approach estrous female urine over the other stimulus (water in Experiment 1, male urine in Experiment 2); however, over subsequent tests VNOx males’ preference to investigate estrous urine would wane in the absence of rewarding inputs from the VNO-accessory olfactory system. We found in Experiment 1 that both groups of mice preferred to approach female urine over water, perhaps because of the novelty of the urinary odors. Therefore, in Experiment 3 we compared the time that VNOi and VNOx males investigated urinary odors over other non-body odors in home-cage habituation–dishabituation tests. Finally, in Experiment 4 we compared in VNOi and VNOx males the ability of direct nasal contact with estrous female urinary odorants to stimulate Fos immunoreactivity (Fos-IR) in different segments of the accessory olfactory projection pathway to the hypothalamus, in the mesolimbic dopamine system including the nucleus accumbens (core [AcbC] and shell [AcbSh] regions) and ventral tegmental area, and in the basolateral nuclei of the amygdala. Several of these latter forebrain regions have previously been implicated in mediating the rewarding characteristics of a variety of natural incentive stimuli, as well as abused drugs (Kelley & Berridge, 2002).
Method
Experimental Subjects
All procedures were approved by the Boston University Animal Care and Use Committee. Seventy-five male and 5 female Swiss Webster mice (Taconic, Germantown, PA) were purchased at 5–6 weeks of age and were initially housed in same-sex groups under a reversed 12-hr light–dark photoperiod with food and water provided ad libitum. Sexually naive males were used in this study because we wanted to monitor subjects’ hard-wired investigative responses to urinary odorants in the absence of previous mating experience. Several days after their arrival at Boston University, males were housed individually, and 1 day later they underwent bilateral castration via a single midline incision while they were under ketamine (120 mg/kg) and xylazine (12 mg/kg) anesthesia. During this surgery, all males also received a subcutaneous implant of a Silastic capsule (length = 1.5 cm, inner diameter = 0.10 cm, outer diameter = 0.22 cm) between the shoulder blades that contained crystalline testosterone (Pankevich, Deedy, Cherry, & Baum, 2003). As a result, any differences in behavioral responses of VNOi and VNOx males to urinary odors could not be attributed to group differences in odor-induced testosterone secretion and subsequent neural actions of this steroid. Two days later, mice underwent either bilateral removal of the VNO or sham surgery (Wysocki & Wysocki, 1995), again under ketamine and xylazine anesthesia. Briefly, a midline incision was made in the soft palette, and the underlying bone was exposed. In VNOi males, the incision was closed at this point with absorbable sutures. For VNOx males, the VNO was removed, the cavity was packed with gel foam, and the incision was closed. Mice were carefully monitored after surgery for bleeding and/or breathing difficulties and were allowed 1 week to recover before the onset of behavioral testing.
Urine was collected from 5 adult, gonadally intact Swiss Webster males and from 5 adult, ovariectomized Swiss Webster females that received estradiol benzoate (20 μg, 48 and 24 hr before urine collection) and progesterone (500 μg, 4 hr before urine collection). Mice were held by the scruff of the neck, and urine was collected into tubes using a funnel. Urine from at least 3 mice of the same sex was pooled, thoroughly mixed, and stored in 50-μl aliquots at −80 °C. Aliquots of urine were thawed for use during each day’s behavioral tests.
Experiment 1: Female Urine Versus Water Y-Maze Preference
The preference of male mice to approach and investigate volatile and nonvolatile urinary odors was assessed using a Y maze constructed of Plexiglas (Bakker, Honda, Harada, & Balthazart, 2002). The distance from the start box to each of the goal boxes was 108 cm, and the arms leading to the two goal boxes had different colors on the walls and different textures in the Plexiglas floors to facilitate subjects’ association of particular odor cues with their location in the Y maze. The goal box backsides were closed off with perforated Plexiglas doors, whereas the start box of the maze was closed off with a wire mesh that enclosed an electric fan. The fan drew air over the urinary or water stimuli placed in the goal boxes at the end of each arm. This air, in turn, carried any volatiles emitted from these stimuli through the length of the maze and over the subject that was placed in the start box. Subjects were tested on consecutive days during the dark phase of the light–dark cycle under low-level white lighting.
Eighteen male subjects were individually placed in the Y maze for 15 min on 2 consecutive days and allowed to investigate the entire apparatus with 50 μl of distilled water placed on a glass slide in each goal box (no data were recorded). On the 3rd day, each subject was placed in the start box, whereupon the door was raised and the subject was allowed to roam in the Y maze for 5 min, again with water present in both goal boxes (no-odor test). Next, subjects were tested for 10 consecutive days with 50 μl of estrous female urine in one arm and 50 μl of water in the other (test sessions with odor cues present). For each subject, the location of the stimuli in the two goal arms was kept constant during these trials, with the urine presented in the right-side arm for half of the subjects and in the left-side arm for the others. Odor stimuli were kept in the same location for each subject to maximize the likelihood that subjects’ choice of arms displayed during daily tests reflected their preference for a particular stimulus as opposed to their confusion about its location in the Y maze. Finally, subjects were tested for 1 additional day with 50 μl of water again placed in both goal boxes (no-odor test) to determine whether subjects’ investigation of the goal box site that previously contained female urine would return to the preodor test baseline. An observer who was unaware of subjects’ VNO lesion status recorded both the latency to first approach and directly investigate (nasally touch) each urine and water stimulus and the total time subjects spent in nasal contact with each stimulus in the two arms of the maze. The data were scored using an iPAQ Pocket PC (Hewlett-Packard, Palo Alto, CA) and Noldus software (Noldus Information Technology, Wageningen, The Netherlands). At the end of these tests, all subjects were given nine daily 5-min sessions in the Y maze without odor cues present in order to extinguish any association of visual and tactile cues in the goal arms with the odor cues previously presented. The Y maze was cleaned with 70% alcohol before the daily testing of each subject.
Experiment 2: Female Versus Male Urine Y-Maze Preference
One week after the completion of Experiment 1, the same male subjects were again habituated for 15 min on 2 consecutive days to the Y maze with water present in both goal boxes (no data were collected). The next day, each subject was placed in the start box, whereupon the door was raised and the subject was allowed to roam in the Y maze for 5 min, again with water present in both goal boxes (no-odor test). Next, subjects were tested for 10 consecutive days with 50 μl of estrous female urine in one arm and 50 μl of male urine in the other (test sessions with odor cues present). Again, the location of the two stimuli in the goal boxes was held constant for each subject, with the location of the male and female urinary stimuli counterbalanced in half the subjects of each group. Finally, subjects were tested for 1 additional day with 50 μl of water placed in both goal boxes (no-odor test) to determine whether subjects’ investigation of the goal box sites that previously contained female or male urine would return to preodor test baseline.
For both Experiments 1 and 2, group comparisons of goal-box-stimulus investigation latencies and durations were analyzed using two-way repeated measures analyses of variance (ANOVAs) followed by Student–Newman–Keuls post hoc tests. In addition, to determine whether VNOx males progressively spent less time than VNOi males investigating each of the urinary stimuli presented in Experiments 1 and 2, we computed slopes of regression lines based on investigation times across the 10 test sessions with odor cues present for each subject. Mean slopes were then computed for VNOi and VNOx males for each stimulus (except water, for which investigation durations were mostly 0 s) and compared between the two groups using two-tailed t tests.
Experiment 3: Investigation of Non-Body Odors, Constituents of Urine, and Urine Itself in Home-Cage Habituation–Dishabituation Tests
The motivation of VNOi and VNOx male mice to investigate two non-body odors (geraniol and isoamyl acetate), two constituents of urine (heptanone and dimethyl pyrazine; DMPZ), as well as male and estrous female urine itself was assessed using home-cage habituation–dishabituation tests (Baum & Keverne, 2002). Results of a previous study (Trinh & Storm, 2003) suggested that geraniol and isoamyl acetate can only be detected by receptors in the main olfactory epithelium and not by the VNO, whereas the rest of these stimuli can be detected by receptors in both systems. A new cohort of sexually naive males (VNOi = 10; VNOx = 8) was individually housed in plastic cages (29 cm × 18 cm × 13 cm) in which the bedding was not changed for a minimum of 48 hr before behavioral tests. These tests were conducted during the dark phase of the 12-hr light–dark cycle using low light illumination, and the experimenter was unaware of the surgical status of the subjects during all tests. Beginning 1 week after VNO surgery, subjects received a series of daily habituation–dishabituation tests. Prior to each test session, a subject’s cage top with food and water was removed and replaced with an empty cage top. Odorants were presented to the subjects using a plastic weigh boat with filter paper attached, which was lowered down the inside of the cage wall to the mouse’s eye level via a fine wire. In the course of each daily testing session, each subject received three 2-min presentations (at 1-min intervals) of mineral oil (tests in which odorants other than urine were presented) or of deionized water (tests in which urine was subsequently presented), followed by three 2-min presentations (at l-min intervals) of one odor of a pair of odors, and followed finally by three 2-min presentations of the second odor in an odor pair. Subjects were initially presented with mineral oil followed by geraniol or heptanone (100 μl of a 50-mM solution on filter paper)—the same volume and concentration used previously (Trinh & Storm, 2003). Half of the subjects in the VNOi and VNOx groups were presented first with geraniol followed by heptanone, and the other half of the subjects were presented with these odors in the reverse order. Subsequently, on a 2nd day of testing, all subjects were reexposed to the same odors but in the opposite sequence. Using a Psion (Psion Teklogix, London, United Kingdom) handheld computer and Noldus software (Noldus Information Technology), an observer recorded the number of seconds during each 2-min stimulus presentation in which a subject had direct nasal contact with the filter paper containing mineral oil or these different odorant stimuli. On subsequent test days, the above testing procedure was used to compare the motivation of male subjects to investigate isoamyl acetate versus DMPZ (again, 100 μl of a 50-mM solution) followed finally by estrous female versus male urine (10 μl of each type of undiluted urine).
Data from each day’s tests were analyzed using one-tailed Wilcoxon tests to compare (a) the difference between the number of seconds that subjects spent investigating the third presentation of the mineral oil or water stimuli versus the first odorant presented and (b) the difference between the number of seconds spent investigating the third presentation of the first odorant versus the first presentation of the second odorant. Non-parametric Wilcoxon tests were used because of the relatively large number of “0” values obtained during the third presentation of mineral oil, water, or various odorants. In addition, for each subject a mean was computed of the time spent investigating each odorant when it was first presented during tests given on 2 consecutive days. Within each group of males, the grand means of these investigation times for different odorants were compared using nonparametric within-groups Friedman ANOVAs. Post hoc comparisons of the mean time spent investigating estrous female urine versus each of the other odorants presented were subsequently made using two-tailed Wilcoxon tests.
Experiment 4: Fos-IR Induced in Different Forebrain Regions by Estrous Female Urine
Male subjects from Experiments 1 and 2 plus additional (VNOi = 20; VNOx = 6) male mice that had previously been exposed to estrous female urinary odors in another behavioral study, but which had never received mating experience, were used in Experiment 4. Beginning 1–2 weeks after the completion of these behavioral studies, 30 μl of either distilled water or estrous female urine was pipetted onto the oronasal groove of each male. Immediately thereafter, each subject was placed in a clean cage for 90 min with a glass slide containing 30 μl of the same stimulus that had previously been placed on the nose. Males were then deeply anesthetized using CO2, whereupon they were perfused transcardially with 0.1-M phosphate-buffered saline (PBS), pH 7.4, followed by 4% paraformaldehyde (wt/vol) in 0.1-M PBS. Brains were postfixed for 4 hr in 4% paraformaldehyde (wt/vol), followed by 3 days in 30% sucrose (wt/vol) in 0.1-M PBS at 4 °C. Olfactory bulbs were separated from the forebrains and individually frozen in optimal cutting temperature compound (Tissue-Tek; Miles, Elkhart, IN) and stored at −80 °C until sectioning. The two hemispheres of each olfactory bulb were separately cut sagittally at 30 μm with a freezing sledge microtome. Every section from one hemisphere and every other section from the opposite hemisphere were processed for soybean-agglutinin conjugated with horseradish peroxidase (SBA-HRP) staining to verify successful VNO removal (Wysocki & Wysocki, 1995). All sagittal sections from both hemispheres of mice used in Experiment 3 were also processed for SBA-HRP. VNOi males had robust SBA-HRP staining of the AOB glomerular layer (see Figure 1A), whereas VNOx males had no such staining (see Figure 1B). Only male subjects that showed no SBA-HRP staining throughout both olfactory bulbs were included in the VNOx group for each experiment. There were traces of SBA-HRP staining in a majority of olfactory bulb sections of 8 out of the 30 males that underwent VNO removal surgery in these experiments. Data from these mice were excluded from the present study.
Figure 1.
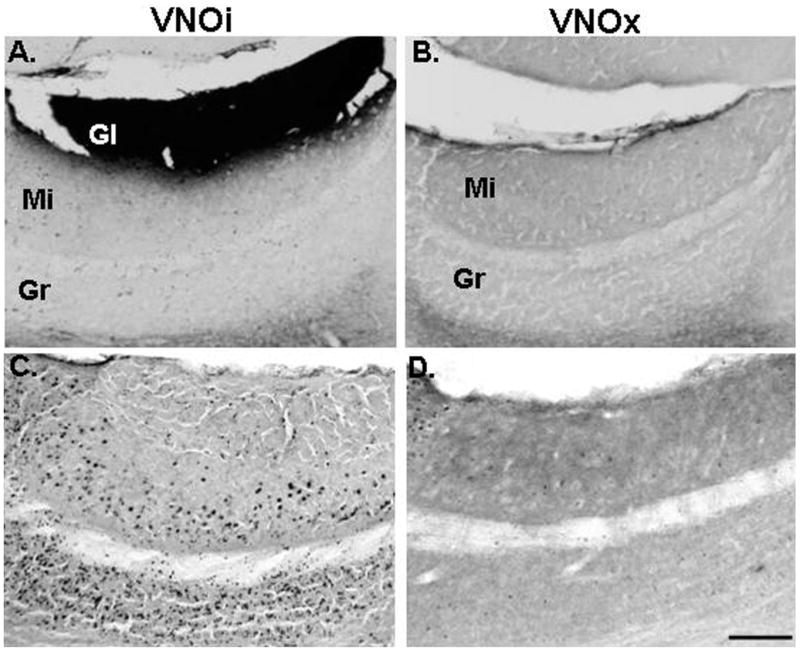
Representative photomicrographs show sagittal sections of the accessory olfactory bulb (AOB) that were stained with soybean agglutinin-horse radish peroxidase (Panels A and B) or with antibody to Fos protein (Panels C and D). Sections are shown from male mice that had previously received a sham removal of the vomeronasal organ (VNOi; Panels A and C) or bilateral VNO removal (VNOx; Panels B and D). AOB sections immunostained for Fos protein are shown from a VNOi male (Panel C) and a VNOx male (Panel D) following nasal application of estrous female urine. Scale bar = 100 μm. Gl = glomerular layer of the AOB; Mi = mitral cell layer of the AOB; Gr = granule cell layer of the AOB.
The right hemisphere of each forebrain was sectioned coronally at 30 μm, and every other section along with all sagittal sections of the olfactory bulb from the left hemisphere were processed immunohistochemically to visualize Fos protein, which was taken as an index of neuronal activation. A full description of the method has been published (Halem, Cherry, & Baum, 1999). Briefly, brain sections were pretreated with 7.5% normal goat serum (vol/vol) in 0.1% Triton X-100/PBS solution for 3 hr. Tissue was then incubated for 18 hr at 4 °C in primary Fos antibody (Santa Cruz Biotechnologies, Santa Cruz, CA) diluted 1:3000 in 2% normal goat serum (vol/vol) in 0.1% Triton X-100/PBS. Tissue was rinsed three times in 1.5% normal goat serum (vol/vol) in 0.1% Triton X-100/PBS (wt/vol), followed by a 1-hr incubation in biotinylated goat anti-rabbit immunoglobulin G (Vector Laboratories, Burlington, CA) diluted 1:200 in 2% normal goat serum (vol/vol) in 0.1% Triton X-100/PBS (wt/vol). Tissue was rinsed in PBS prior to incubation in avidin-biotin-peroxidase solution (ABC Kit; Vector Laboratories) for 45 min. Tissue was rinsed again in PBS followed by reaction with 3,3′-diaminobenzidine with nickel intensification (DAB Kit; Vector Laboratories) for 5 min. After a final rinse, sections were mounted on gelatin-coated slides and coverslipped.
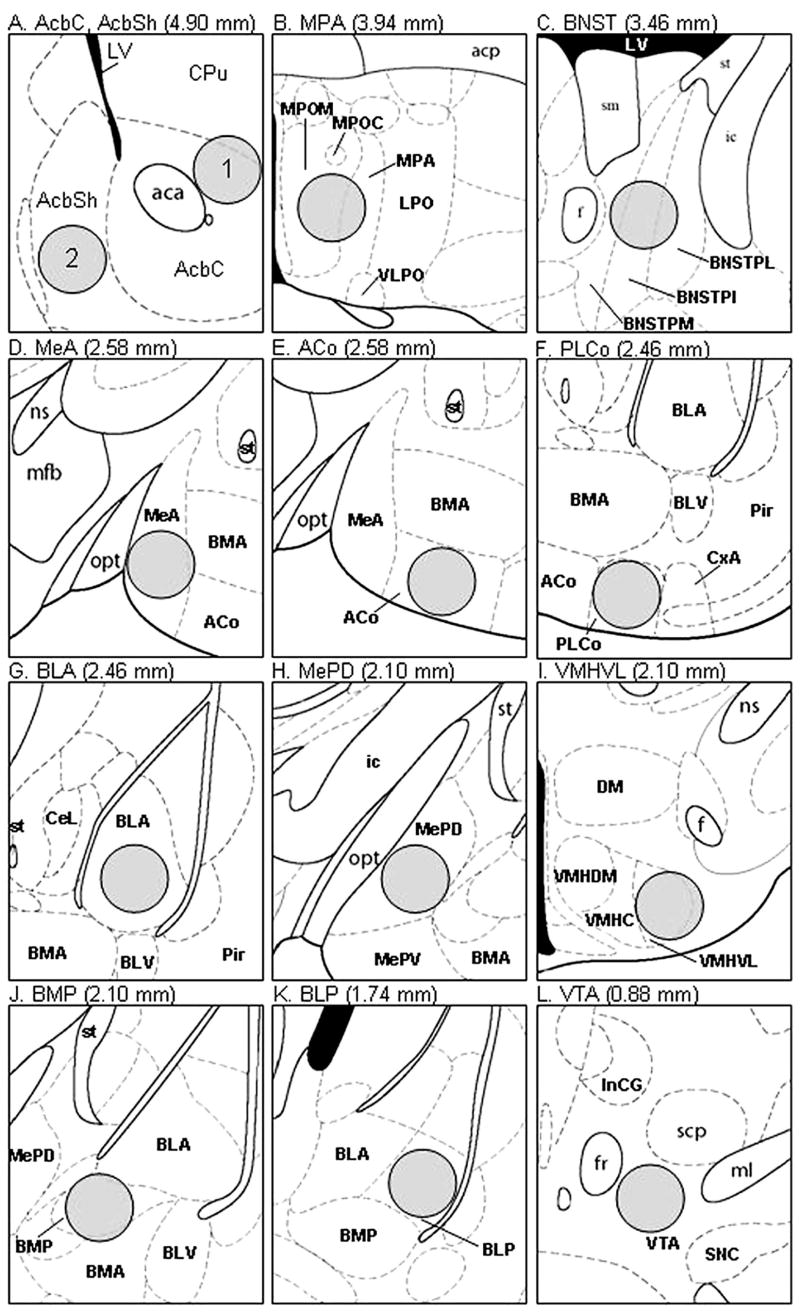
Two anatomically matched brain sections were selected for each subject, and the location of Fos-immunoreactive (IR) cells was recorded using a camera lucida microscope attachment. We examined regions receiving inputs from the VNO including the mitral and granule cell layers of the AOB, the anterior portion (MeA) and posterior dorsal portion (MePD) of the medial amygdala, the bed nucleus of the stria terminalis (BNST), the ventral lateral ventromedial hypothalamus (VMHVL), and the medial preoptic area (MPA; Halpern & Martinez-Marcos, 2003). We also examined corticoamygdaloid sites that receive input from the main olfactory bulb including the anterior (ACo) and posterolateral (PLCo) cortical amygdaloid nucleus, areas of the corticolimbic amygdala including the posterior part of the basomedial amygdaloid nucleus (BMP) as well as the anterior (BLA) and posterior (BLP) parts of the basolateral amygdaloid nucleus that have been shown to mediate conditioned sexual reward (Everitt, Cador, & Robbins, 1989) and more recently to be involved in vomeronasal-olfactory reward (Moncho-Bogani, Martinez-Garcia, Novejarque, & Lanuza, 2005), and areas of the mesolimbic dopamine pathway (Salamone, 2002) including the AcbC and AcbSh and the ventral tegmental area (VTA). See Figure 2 for drawings of the specific sites where Fos-IR cells were counted in each of these brain regions. The number of Fos-IR cells was counted in standard areas within each of the above-mentioned brain regions. These Fos results for each brain region were analyzed using a one-way ANOVA, and post-hoc comparisons of pairs of means were made using the Fisher’s least significant difference test.
Figure 2.
Drawings modified from the mouse brain atlas of Paxinos and Franklin (Paxinos & Franklin, 2001) showing the location of forebrain regions in which Fos-IR cells were counted in Experiment 4. The distance rostral to the interaural line is given in parentheses. The standard counting area of 0.1 mm2 is shown as a gray circle in each panel. A: (1) Accumbens nucleus, core (AcbC), (2) Accumbens nucleus, shell (AcbSh). B: Medial preoptic area (MPA). C: Bed nucleus of the stria terminalis (BNST). D: Medial amygdaloid nucleus, anterior part (MeA). E: Anterior cortical amygdaloid nucleus (ACo). F: Posterolateral cortical amygdaloid nucleus (PLCo). G: Basolateral amygdaloid nucleus, anterior part (BLA). H: Medial amygdaloid nucleus, posterodorsal part (MePD). I: Ventromedial hypothalamic nucleus, ventrolateral part (VMHVL). J: Basomedial amygdaloid nucleus, posterior part (BMP). K: Basolateral amygdaloid nucleus, posterior part (BLP). L: Ventral tegmental area (VTA). Additional abbreviations: LV = lateral ventricle; CPu = caudate putamen; aca = anterior commissure, anterior part; acp = anterior commisure, posterior part; MPOM = medial preoptic nucleus, medial part; MPOC = medial preoptic nucleus, central part; LPO = lateral preoptic area; VLPO = ventrolateral preoptic nucleus; sm = stria medullaris of the thalamus; st = stria terminalis; ic = internal capsule; f = fornix; BNSTPL = BNST, medial division, posterolateral part; BNSTPI = BNST, medial division, posterointermediate part; BNSTPM = BNST, medial division, posteromedial part; ns = nigrostriatal bundle; mfb = medial forebrain bundle; opt = optic tract; BMA = basomedial amygdaloid nucleus, anterior part; BLV = basolateral amydaloid nucleus, ventral part; Pir = piriform cortex; CxA = cortex–amygdala transition zone; CeL = central amygdaloid nucleus, lateral division; MePV = medial amygdaloid nucleus, posteroventral part; DM = dorsomedial hypothalamic nucleus; VMHDM = ventromedial hypothalamic nucleus, dorsomedial part; VMHC = ventromedial hypothalamic nucleus, central part; InCG = interstitial nucleus of Cajal, greater part; fr = fasciculus retroflexus; scp = superior cerebellar peduncle (brachium conjuntivum); ml = medial lemniscus; SNC = substantia nigra, compact part. Reprinted from The Mouse Brain in Stereotaxic Coordinates (2nd ed.), G. Paxinos and K. B. J. Franklin, pp. 1–8, Copyright (2001), with permission from Elsevier.
Results
Experiment 1: Female Urine Versus Water Y-Maze Preference
Both VNOi and VNOx males approached and made physical contact with estrous female urine significantly faster on the first odor test session than they approached water during the no-odor test given a day earlier, F(1, 16) = 66.7, p < .001 (see Figure 3A). VNOi male mice approached estrous female urine significantly faster than did VNOx mice during the first odor test (p < .05) as revealed by post hoc analysis. However, over the subsequent nine odor test sessions the latency to investigate estrous female urine was similar in these two groups. During the final no-odor test session, both groups more slowly approached the water that replaced the female urine spots in the relevant goal boxes, F(1, 16) = 29.9, p < .001.
Figure 3.
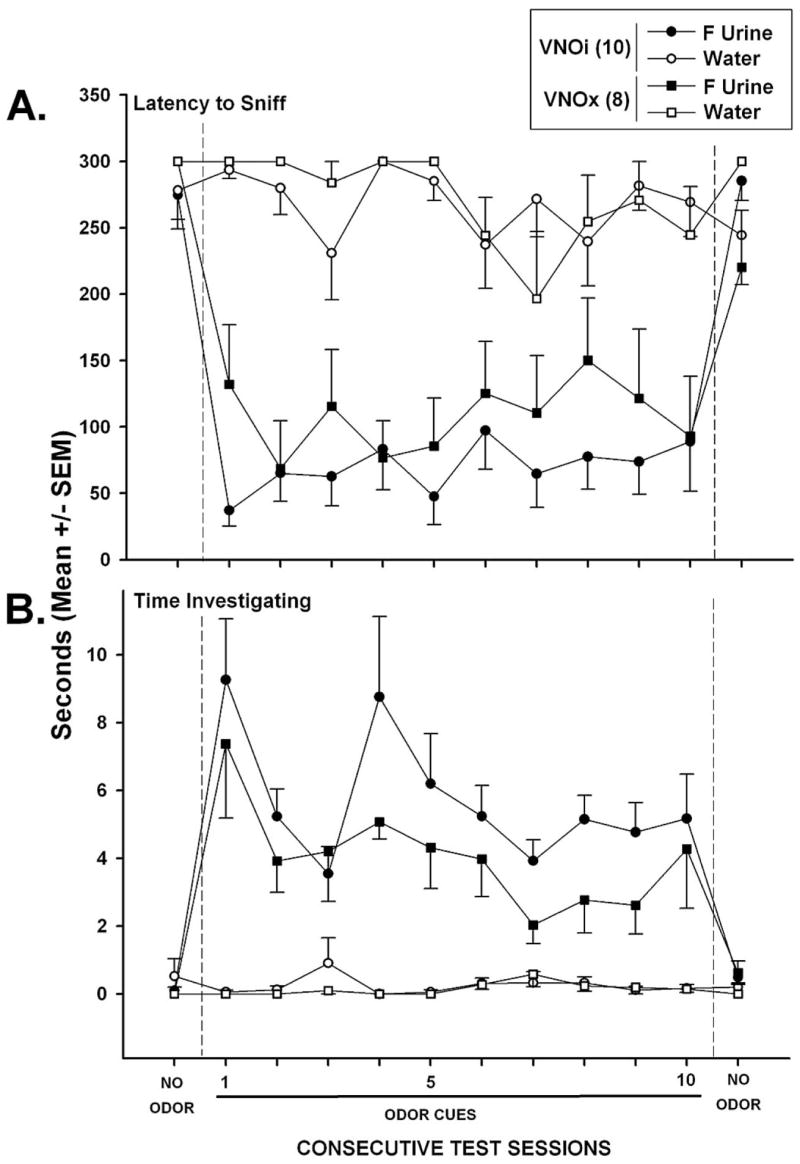
Effect of bilateral removal of the vomeronasal organ (VNOx) or sham operation (VNOi) of male mice on their latency to approach and sniff (A) as well as the time spent investigating with direct nasal contact either estrous female (F) urine or water in the two goal boxes of a Y maze (B). Initially, water was placed in each goal box during habituation tests given over 3 consecutive days; data are shown here only for the 3rd of these habituation days (no odor). Next, subjects were tested on 10 consecutive days with estrous F urine or water presented in the respective goal boxes (odor cues). Finally, subjects received one final no-odor test session when water was presented in each goal box. The number of subjects in each group is shown in parentheses.
Both groups also spent significantly more time investigating estrous female urine spots during the first odor test session than they had spent investigating water during the no-odor test given the previous day, F(1, 16) = 35.1, p < .001 (see Figure 3B). There were no significant group differences in the amount of time subjects spent investigating the female urine spots over the 10 tests with odor cues present. Over these tests, the slopes (M ± SEM) of the regression lines for times investigating the female urine in the VNOi and VNOx males were −0.205 ± 0.120 and −0.589 ± 0.193, respectively. These values did not differ significantly from each other, although the value for VNOx males differed significantly from zero, t(7) = 3.01, p < .02, suggesting that investigation of female urine by this group declined with repeated testing.
Subjects in both groups spent very little time investigating the water stimuli during the 10 tests in which estrous female urine was present in the opposite arm of the Y maze. During the final no-odor test session, both groups spent significantly less time investigating the water that replaced the female urine spots in the relevant goal boxes, F(1, 16) = 14.4, p < .01.
Experiment 2: Female Versus Male Urine Y-Maze Preference
Both VNOi and VNOx males approached and directly investigated estrous female urine, F(1, 16) = 38.7, p < .001, as well as intact male urine, F(1, 16) = 163.8, p < .001, more quickly during the first session with urinary odor cues present than they did water during the no-odor test given a day earlier (see Figure 4A). Post hoc analysis revealed that during the first odor test session VNOx males approached intact male urine more quickly than did VNOi males (p < .05). During the subsequent nine test sessions with urinary odors present, VNOi male mice approached estrous female urine more quickly than intact male urine, F(1, 64) = 31.6, p < .001, whereas VNOx males approached these two stimuli with equal latencies. Both VNOi and VNOx males quickly stopped investigating the water spots that replaced the urine in the two goal boxes during the final no-odor test: female urine versus water, F(1, 16) = 136.3, p < .001, and male urine versus water, F(1, 16) = 5.1, p < .05.
Figure 4.
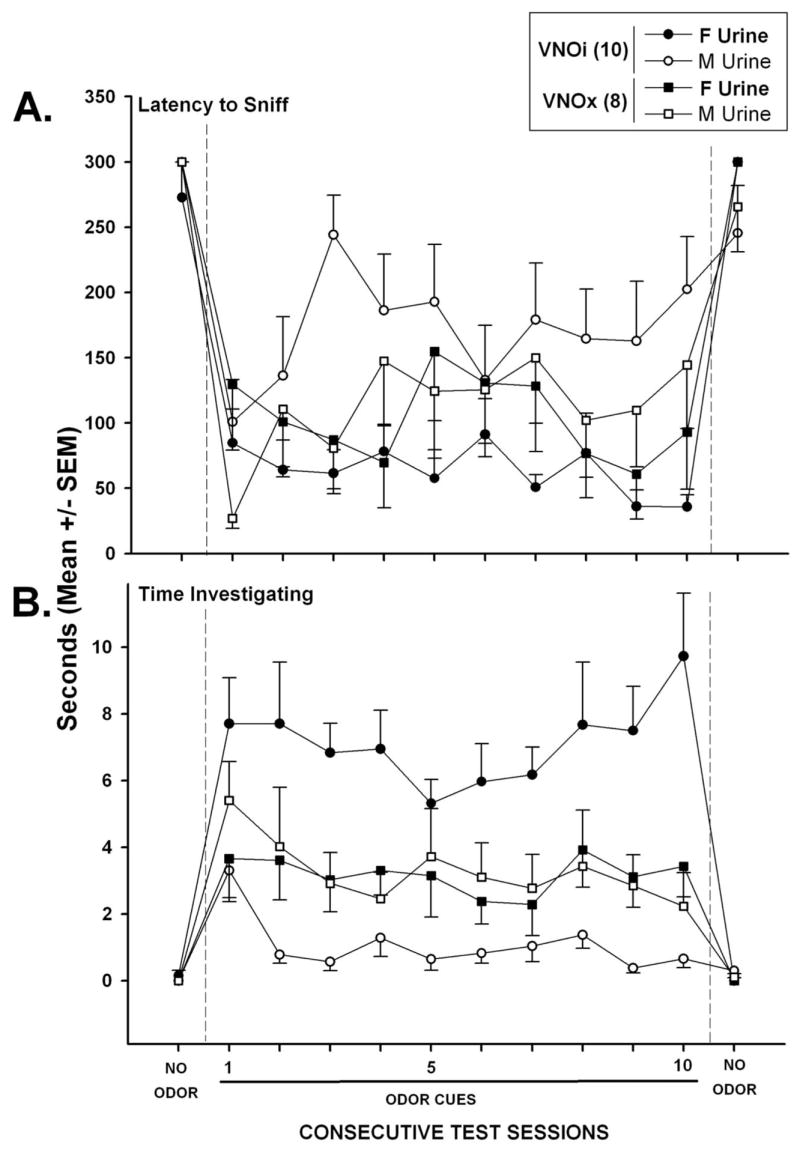
Effect of bilateral removal of the VNO (VNOx) or sham operation (VNOi) of male mice on their latency to approach and sniff (A) as well as the time spent investigating with direct nasal contact either estrous female (F) or intact male (M) urine placed in the goal boxes of a Y maze (B). See the Figure 3 caption for further details of testing. The number of subjects in each group is shown in parentheses.
Both VNOi and VNOx male mice investigated estrous female urine for a significantly longer time during the first odor test session than they spent investigating water during the no-odor test session given the previous day, F(1, 16) = 36.2, p < .001 (see Figure 4B). Post-hoc analysis showed that VNOi males spent significantly more time than VNOx males investigating female urine (p < .05). Across the remaining nine odor test sessions, VNOi male mice consistently investigated estrous female urine for longer times than they investigated intact male urine, F(1, 128) = 9.5, p < .05, whereas VNOx male mice investigated these two stimuli for equal times. Across the 10 tests sessions with odor cues present, the slopes (M ± SEM) of the regression lines for time investigating the female urine stimulus in the VNOi and VNOx groups were −0.108 ± 0.157 and −0.152 ± 0.172, respectively. These values did not differ significantly from each other or from zero. During these same tests, the slopes of the regression lines for time spent investigating the male urine stimulus in the VNOi and VNOx males were −1.088 ± 0.263 and −1.434 ± 1.961, respectively. These values did not differ significantly from each other; however, for VNOi males this slope was significantly different from zero, t(9) = 4.14, p < .01, reflecting a decrease in investigation of male urine over successive trials.
Both groups of males quickly stopped investigating the water that replaced the two urine stimuli during the final no-odor test session: female urine versus water, F(1, 16) = 33.4, p < .001, and male urine versus water, F(1, 16) = 7.4, p < .05.
Experiment 3: Investigation of Non-Body Odors, Constituents of Urine, and Urine Itself in Home-Cage Habituation–Dishabituation Tests
Both groups of male subjects showed an inconsistent ability to discriminate among mineral oil, geraniol, and heptanone. Thus, when these compounds were presented in this sequence (see Figure 5A), only VNOx males made a significant discrimination (mineral oil vs. geraniol). When tested on the reversed sequence of geraniol and heptanone the following day (data not shown), both groups successfully discriminated between these two stimuli but did not discriminate between mineral oil and heptanone. In a subsequent test, only VNOx males successfully discriminated between mineral oil and isoamyl acetate, whereas both groups discriminated between isoamyl acetate and DMPZ (see Figure 5B). When the order was reversed, both VNOi and VNOx male subjects successfully discriminated between mineral oil and DMPZ, whereas neither group discriminated between DMPZ and isoamyl acetate (data not shown). By contrast, both groups of male subjects reliably discriminated among water, female urine, and male urine (see Figure 5C) when they were presented in that sequence and when the sequence was reversed (data not shown). As also shown in Figure 5C, VNOi males investigated the first presentation of estrous female urine for a significantly longer time than did VNOx males, and the same significant difference was seen the next day when the sequence of female and male urine presentation was reversed (data not shown).
Figure 5.
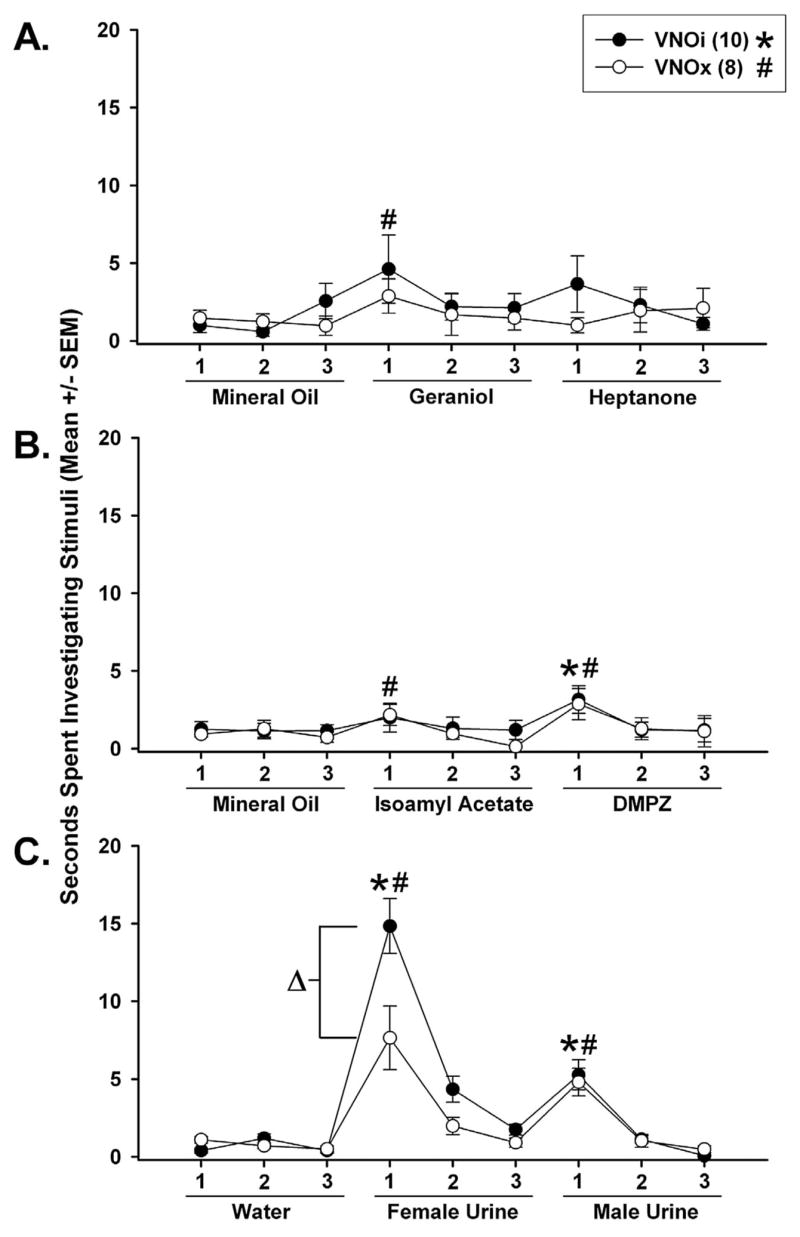
Effect of bilateral removal of the VNO (VNOx) or sham operation (VNOi) on the ability of male mice to discriminate geraniol from heptanone (A), isoamyl acetate from dimethyl pyrazine (DMPZ; B), and estrous female from intact male urine (C). On 3 consecutive days, subjects received a habituation–dishabituation test during which investigation times (direct nasal contact with each stimulus) were recorded in nine consecutive trails that consisted of three presentations of mineral oil or water, followed by three presentations of one of the paired odorants, followed by three presentations of the second odor of the pair (see Method section for further details). * and # p < .05, one-tailed Wilcoxon test comparisons with the third presentation of the previous stimulus for the respective groups. Δ p < .05, two-tailed Student’s t test between-groups comparisons of the time spent investigating the estrous female urine. The number of subjects in each group is shown in parentheses.
VNOi and VNOx male subjects spent significantly more time investigating estrous female urine than each of the other stimuli, including intact male urine, when they were first presented sequentially during habituation–dishabituation tests: Friedman ANOVA for VNOi males, χ2(5, N = 10) = 26.933, p < .01, and for VNOx males, χ2(5, N = 8) = 16.786, p < .01 (see Table 1).
Table 1.
Effect of Bilateral Vomeronasal Organ Removal on Time Spent by Male Mice Investigating Different Odors Presented Sequentially in Home-Cage Habituation–Dishabituation Tests
| Stimulus | VNOi (n = 10) | VNOx (n = 8) |
|---|---|---|
| Geraniol | 3.0 ± 1.0 | 2.0 ± 1.0 |
| Isoamyl acetate | 1.0 ± 0.2 | 3.0 ± 1.0 |
| Heptanone | 1.0 ± 0.3 | 4.0 ± 1.0 |
| Dimethyl pyrazine | 1.0 ± 0.2 | 3.0 ± 1.0 |
| Male urine | 6.0 ± 1.0 | 4.0 ± 1.0 |
| Female urine | 18.0 ± 2.0* | 7.0 ± 2.0* |
Note. Data are expressed as means (± SEM) of seconds spent investigating each odorant when it was first presented. VNOi = sham-operated males with an intact vomeronasal organ; VNOx = males from which the vomeronasal organ was removed bilaterally.
p < .05; two-tailed Wilcoxon post hoc comparisons with each of the other means in the same group of subjects.
Experiment 4: Fos-IR Induced in Different Forebrain Regions by Estrous Female Urine
Exposure to estrous female urine increased the number of Fos-IR AOB mitral and granule cells in VNOi males, but not in VNOx males (see Figure 1, Panels C and D). Exposure to estrous female urine caused significant increases in the number of Fos–IR AOB mitral, F(2, 42) = 45.2, p < .001, and granule, F(2, 42) = 27.4, p < .001, cells in VNOi males, whereas the number of Fos-IR cells in both the mitral and granule layers of the AOB were significantly lower in VNOx males exposed to estrous female urinary odors than in either of the other two groups of males (see Table 2). Likewise, in other segments of the vomeronasal projection pathway to the medial amygdala and hypothalamus, nasal application of estrous female urine caused significant increments in the number of Fos-IR neurons in VNOi, but not in VNOx, male subjects (see Table 2). Thus, there were significant overall group differences in the MeA, F(2, 41) = 3.6, p < .05, and MePD, F(2, 41) = 6.2, p < .05, subdivisions of the medial amygdala as well as in the BNST, F(2, 41) = 7.0, p < .05, and the VHMVL, F(2, 41) = 5.5, p < .05.
Table 2.
Effect of Bilateral Vomeronasal Organ Removal on the Ability of Estrous Female Urinary Odors to Induce Fos in Forebrain Regions of Male Mice
| Water
|
Estrous female urine
|
||
|---|---|---|---|
| Brain region | VNOi (n = 10) | VNOi (n = 20) | VNOx (n = 14) |
| Vomeronasal projection pathway | |||
| Mi | 34 ± 9 | 100 ± 8** | 10 ± 2* |
| Gr | 90 ± 29 | 225 ± 25** | 8 ± 2* |
| MeA | 7 ± 1 | 12 ± 1** | 8 ± 2 |
| MePD | 7 ± 2 | 13 ± 1** | 8 ± 1 |
| BNST | 4 ± 1 | 9 ± 1** | 5 ± 1 |
| VMHVL | 5 ± 1 | 11 ± 1** | 6 ± 1 |
| MPA | 6 ± 1 | 11 ± 1 | 9 ± 2 |
| Amygdaloid targets of main olfactory input | |||
| Aco | 15 ± 2 | 17 ± 2 | 16 ± 3 |
| PLCo | 12 ± 2 | 13 ± 2 | 16 ± 2 |
| Additional amygdaloid sites | |||
| BMP | 4 ± 1 | 9 ± 1* | 9 ± 2* |
| BLA | 7 ± 2 | 11 ± 1 | 11 ± 1 |
| BLP | 3 ± 1 | 3 ± 0.4 | 4 ± 1 |
| Mesolimbic dopamine System | |||
| AcbC | 5 ± 1 | 12 ± 3** | 5 ± 1 |
| AcbSh | 6 ± 1 | 10 ± 1 | 10 ± 2 |
| VTA | 6 ± 2 | 10 ± 1 | 8 ± 1 |
Note. Data are expressed as the number of Fos-immunoreactive cells per standard area in different brain regions. VNOi = sham-operated males with an intact vomeronasal organ; VNOx = males from which the VNO was removed bilaterally; Mi = mitral cell layer of the accessory olfactory bulb; Gr = granule cell layer of the accessory olfactory bulb; MeA = medial amygdaloid nucleus; MePD = medial amygdaloid nucleus, posterodorsal part; BNST = bed nucleus of the stria terminalis; VMHVL = ventromedial hypothalamic nucleus, ventrolateral part; MPA = medial preoptic area; Aco = anterior cortical amygdaloid nucleus; PLCo = posterolateral cortical amygdaloid nucleus; BMP = basomedial amygdaloid nucleus, posterior part; BLA = basolateral amygdaloid nucleus, anterior part; BLP = basolateral amygdaloid nucleus, posterior part; AcbC = accumbens nucleus, core; AcbSh = accumbens nucleus, shell; VTA = ventral tegmental area.
significantly different (p < .05) from VNOi water-exposed males.
significantly different (p < .05) from VNOi, water-exposed males and from VNOx urine-exposed males by Fisher’s least significant difference post-hoc tests.
There were no group differences in Fos responses to estrous female urinary odors in amygdaloid targets of main olfactory input. Of the three corticoamygdala areas counted, the posterior part of the BMP showed increases in Fos-IR in both VNOi and VNOx males after exposure to estrous female urinary odors, F(2, 41) = 3.9, p < .05. Finally, exposure to estrous female urine caused a significant increase in Fos-IR cells in one terminal segment of the mesolimbic dopamine pathway, the AcbC of VNOi, F(2, 41) = 3.6, p < .05, but not of VNOx male subjects.
General Discussion
The ability of VNO removal to affect the motivation of male mice to approach and make nasal contact with urinary odors from estrous females depended on the context in which this behavior was assessed. Thus, in Experiment 1, both VNOi and VNOx males ran more rapidly to estrous female urine than to water and preferred to investigate estrous female urine as opposed to water for equivalent times over 10 consecutive test days. The clear-cut preference of VNOx males to approach and investigate estrous female urine over water suggests that main olfactory inputs alone were sufficient to motivate them to approach this urinary stimulus. We cannot rule out the possibility that stimulus features, such as novelty, contributed to the preference of both VNOi and VNOx males to investigate female urine over water in these Y-maze tests. However, during home cage habituation–dishabituation tests (Experiment 3), both VNOi and VNOx male mice spent significantly more time investigating estrous female urine versus either of two novel non-body odorants (geraniol and isoamyl acetate) or two constituents of urine (heptanone and DMPZ) or male urine itself. This suggests that female urinary odors are especially salient stimuli in male mice, even in the absence of a functional VNO. In Experiment 1, there was a suggestion that VNOx males progressively spent less time over 10 consecutive test days investigating female urinary odors in so far as the negative slope of the regression line for these males (but not for VNOi control males) differed significantly from zero. However, the fact that the negative slopes of the regression lines for the VNOi and VNOx groups did not differ significantly from each other suggests that this is not a robust effect. Thus, the results of Experiment 1 do not provide strong corroboration of the previous observation (Beauchamp et al., 1985) that VNO removal led to the extinction of a preference for opposite-sex urinary odors. Indeed, our results suggest that female urinary odors retain significant incentive value even in VNOx males.
More support for the hypothesis (Beauchamp et al., 1985) that VNO removal attenuates the reward needed to maintain males’ interest in opposite-sex urinary odors was obtained in Experiment 2, in which male urine was substituted for water as an alternative incentive stimulus to estrous female urine in Y-maze preference tests. Here, a clear-cut effect of VNO removal on latency to approach as well as investigation times for the two stimuli emerged in male mice. Again, VNOx males, like VNOi males, approached both estrous female and male urine more quickly during the first test with odors present than they approached water spots during the final habituation test, suggesting that main olfactory inputs alone motivated subjects’ initial interest in urinary odors from any source. However, in nine subsequent tests VNOi males consistently ran more quickly and spent significantly more time investigating estrous female as opposed to male urine spots whereas VNOx males showed an equivalent preference to approach and investigate these two stimuli. These differences between VNOi and VNOx males were present at both the beginning and the end of a series of 10 consecutive tests, suggesting that, as in Experiment 1, there was no experience-dependent extinction of a preference for estrous urinary odors among VNOx males. In Experiment 3, as in Experiment 2, VNOi males spent significantly more time than VNOx males in direct nasal contact with estrous female urine when it was first presented after prior exposure to either water or male urine in home-cage habituation–dishabituation tests. Our results, by themselves, cannot definitively rule out the possibility that VNO removal disrupted a purely sensory function. However, they are also compatible with the hypothesis that main olfactory inputs motivate males to approach and directly contact female urine, whereupon VNO inputs further activate forebrain mechanisms that amplify the reward salience of this stimulus.
We (Pankevich et al., 2004) previously found that both sexually naive and experienced male mice with intact VNOs spent significantly more time investigating estrous female as opposed to male urine provided they had direct physical access to the stimuli when they were presented simultaneously in the home cage. No such preference was seen in VNOx males, even after they had received mating experience prior to testing for odor preference. This profile of results was confirmed and extended in the Y-maze tests given in Experiment 2. VNOi males (in this instance, sexually naive) spent significantly more time investigating estrous female as opposed to male urine over 9 consecutive days, whereas VNOx males investigated these two urinary stimuli for similar times. In two previous studies (Leypold et al., 2002; Stowers, Holy, Meister, Dulac, & Koentges, 2002), VNO signaling was disrupted in male mice by a null mutation of the TRP2 cation channel, and these males mounted male and female conspecifics indiscriminately. On the basis of these findings, both of these groups of authors suggested that VNO inputs are required for sex discrimination in mice. It seems unlikely, however, that the similar approach latencies and investigation durations for estrous female and male urinary odors seen in Experiment 2 reflected an inability of VNOx males to distinguish between these two stimuli. In our previous study (Pankevich et al., 2004), VNOx male mice resembled VNOi males in their ability to show significant dishabituation responses to male versus female urinary odors. A similar result was also obtained in Experiment 3. These results suggest that sex discrimination persists in male mice after VNO removal. A similar conclusion was reached from more recent studies using female mice (Keller et al., 2006) and from previous studies using male guinea pigs (Beauchamp et al., 1982), female hamsters (Petrulis, Peng, & Johnston, 1999), and female ferrets (Woodley, Cloe, Waters, & Baum, 2004).
In Experiment 4, the ability of estrous female urine to stimulate Fos-IR in the mitral and granule cell layers of the AOB was significantly lower in VNOx than in VNOi males. This result corroborates previous reports in male mice exposed to soiled female bedding (Pankevich et al., 2004) or allowed direct contact with a conspecific (Kumar, Dudley, & Moss, 1999), in female mice exposed to male urine (Keller et al., 2006), and in male hamsters exposed to female vaginal secretions (Meredith & Fernandez-Fewell, 1994; Swann, Rahaman, Bijak, & Fiber, 2001). We also observed significant increases in the number of Fos-IR neurons in four segments (MeA, MePD, BNST, and VMHVL) of the VNO projection pathway after nasal application of estrous urine to VNOi, but not to VNOx, males. This finding resembles previous reports that VNO removal attenuated mating-induced Fos expression in the medial amygdala of male rats (Kondo, Sudo, Tomihara, & Sakuma, 2003) and reduced the ability of female hamster vaginal secretions or mating stimulation to augment Fos expression in the medial amygdala and BNST of male hamsters (Fewell & Meredith, 2002), although in another study (Swann et al., 2001), VNO removal failed to attenuate the expression of Fos in the medial amygdala and BNST induced in male hamsters by female vaginal secretions. The latter failure of VNO removal to reduce odor-induced Fos responses may have reflected the use of male hamsters with extensive mating experience prior to the experiment. The fact that VNO removal blocked odor-stimulated Fos expression in all of the segments of the VNO projection pathway of male mice suggests that main olfactory inputs to the vomeronasal amygdala (Licht & Meredith, 1987) were not able to compensate for the deprivation of VNO inputs to this circuit.
The profile of significant Fos responses seen in VNOi males after exposure to estrous female urine (Experiment 4) provides evidence of neural activation in one segment of the mesolimbic dopamine system, the AcbC. This result corroborated findings of several previous studies in which significant increments in Fos expression were observed in the AcbC and/or the AcbSh of male rats (Bakker, Baum, & Slob, 1996; Lopez & Ettenberg, 2002; Paredes, Lopez, & Baum, 1998) and of ovariectomized, testosterone-primed female rats (Bressler & Baum, 1996) following exposure to soiled estrous female bedding. Likewise, exposure to soiled male bedding stimulated Fos-IR cells in the AcbSh of female mice (Moncho-Bogani et al., 2005). Studies using male rats (Hosokawa & Chiba, 2005; Kippin, Cain, & Pfaus, 2003; Lopez & Ettenberg, 2002) showed that the ability of estrous female bedding to augment Fos expression in either the AcbC or the AcbSh was greater in mice that had previously mated with a female. The use of sexually naive male mice in the present study may have therefore minimized the Fos responses observed in the AcbC and the AcbSh after exposure to estrous female odors. It is not known whether VNO or accessory olfactory and/or main olfactory inputs were responsible for the odor-induced stimulation of nucleus accumbens Fos expression reported in these previous studies. In Experiment 4, however, exposure to estrous female urinary odors failed to augment Fos-IR neurons in the AcbC of VNOx male mice. This outcome implies that the odor-induced Fos induction in the terminal segment of the mesolimbic dopamine system of VNOi males depended on the detection of urinary odorants by VNO receptors and the further transmission of this information via the accessory olfactory pathway to this reward circuit. A related phenomenon has been documented in the female prairie vole (Microtus ochrogaster), in which the release of dopamine in the nucleus accumbens has been implicated in mating-induced pair bonding (Gingrich, Liu, Cascio, Wang, & Insel, 2000). Such pair bonding was blocked in female prairie voles after VNO removal (Curtis, Liu, & Wang, 2001), perhaps, again, because VNO inputs are normally required to activate the mesolimbic dopamine reward system.
Acknowledgments
This work was supported by National Institutes of Health Grant MH59200. We thank Esther Kim for technical assistance.
References
- Bakker J, Baum MJ, Slob AK. Neonatal inhibition of brain estrogen synthesis alters adult neural Fos responses to mating and pheromonal stimulation in the male rat. Neuroscience. 1996;74:251–260. doi: 10.1016/0306-4522(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. Sexual partner preference requires a functional aromatase (cyp19) gene in male mice. Hormones and Behavior. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Hormones and Behavior. 2002;41:213–219. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Martin IG, Wysocki CJ, Wellington JL. Chemoinvestigatory and sexual behavior of male guinea pigs following vomeronasal organ removal. Physiology & Behavior. 1982;29:329–336. doi: 10.1016/0031-9384(82)90022-1. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Wysocki CJ, Wellington JL. Extinction of response to urine odor as a consequence of vomeronasal organ removal in male guinea pigs. Behavioral Neuroscience. 1985;99:950–955. doi: 10.1037//0735-7044.99.5.950. [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ. Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience. 1996;71:1063–1072. doi: 10.1016/0306-4522(95)00493-9. [DOI] [PubMed] [Google Scholar]
- Brown RE. Mammalian social odors. Advances in the Study of Behavior. 1979;10:107–161. [Google Scholar]
- Curtis JT, Liu Y, Wang Z. Lesions of the vomeronasal organ disrupt mating-induced pair bonding in female prairie voles (Microtus ochrogaster) Brain Research. 2001;901:167–174. doi: 10.1016/s0006-8993(01)02343-5. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cador M, Robbins TW. Interactions between the amygdala and ventral striatum in stimulus-reward associations: Studies using a second-order schedule of sexual reinforcement. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- Fewell GD, Meredith M. Experience facilitates vomeronasal and olfactory influence on Fos expression in medial preoptic area during pheromone exposure or mating in male hamsters. Brain Research. 2002;941:91–106. doi: 10.1016/s0006-8993(02)02613-6. [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. Journal of Neurobiology. 1999;39:249–263. [PubMed] [Google Scholar]
- Halpern M. The organization and function of the vomeronasal system. Annual Review of Neuroscience. 1987;10:325–362. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: An update. Progress in Neurobiology. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Chiba A. Effects of sexual experience on conspecific odor preference and estrous odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in male rats. Brain Research. 2005;1066:101–108. doi: 10.1016/j.brainres.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. European Journal of Neuroscience. 2006;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. Journal of Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster: I. Efferents of the “vomeronasal amygdala. Journal of Comparative Neurology. 1981a;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster: II. Efferents of the “olfactory amygdala. Journal of Comparative Neurology. 1981b;197:99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005 October 6;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Cain SW, Pfaus JG. Estrous odors and sexually conditioned neutral odors activate separate neural pathways in the male rat. Neuroscience. 2003;117:971–979. doi: 10.1016/s0306-4522(02)00972-7. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sudo T, Tomihara K, Sakuma Y. Activation of accessory olfactory bulb neurons during copulatory behavior after deprivation of vomeronasal inputs in male rats. Brain Research. 2003;962:232–236. doi: 10.1016/s0006-8993(02)03970-7. [DOI] [PubMed] [Google Scholar]
- Kubie J, Halpern M. Chemical senses involved in garter snakes prey trailing. Journal of Comparative and Physiological Psychology. 1979;93:648–667. doi: 10.1037/h0077061. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dudley CA, Moss RL. Functional dichotomy within the vomeronasal system: Distinct zones of neuronal activity in the accessory olfactory bulb correlate with sex-specific behaviors. Journal of Neuroscience. 1999;19:RC32. doi: 10.1523/JNEUROSCI.19-20-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proceedings of the National Academy of Sciences, USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht G, Meredith M. Convergence of main and accessory olfactory pathways onto single neurons in the hamster amygdala. Experimental Brain Research. 1987;69:7–18. doi: 10.1007/BF00247024. [DOI] [PubMed] [Google Scholar]
- Lin Y, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005 March 24;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- Lopez HH, Ettenberg A. Exposure to female rats produces differences in c-fos induction between sexually-naive and experienced male rats. Brain Research. 2002;947:57–66. doi: 10.1016/s0006-8993(02)02907-4. [DOI] [PubMed] [Google Scholar]
- Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003 February 21;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Meredith M, Fernandez-Fewell G. Vomeronasal system, LHRH, and sex behaviour. Psychoneuroendocrinology. 1994;19:657–672. doi: 10.1016/0306-4530(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Moncho-Bogani J, Martinez-Garcia F, Novejarque A, Lanuza E. Attraction to sexual pheromones and associated odorants in female mice involves activation of the reward system and basolateral amygdala. European Journal of Neuroscience. 2005;21:2186–2198. doi: 10.1111/j.1460-9568.2005.04036.x. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. Journal of Neuroscience. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Deedy EM, Cherry JA, Baum MJ. Interactive effects of testosterone and superior cervical ganglionectomy on attraction thresholds to volatile urinary odors in gonadectomized mice. Behavioral Brain Research. 2003;144:157–165. doi: 10.1016/s0166-4328(03)00073-1. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Lopez ME, Baum MJ. Testosterone augments neuronal Fos responses to estrous odors throughout the vomeronasal projection pathway of gonadectomized male and female rats. Hormones and Behavior. 1998;33:48–57. doi: 10.1006/hbeh.1998.1435. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Petrulis A, Peng M, Johnston RE. Effects of vomeronasal organ removal on individual odor discrimination, sex-odor preference, and scent marking by female hamsters. Physiology & Behavior. 1999;66:73–83. doi: 10.1016/s0031-9384(98)00259-5. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Functional significance of nucleus accumbens dopamine: Behavior, pharmacology, and neurochemistry. Behavioral Brain Research. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00281-4. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex-determined body odors: II. Relationship among odor maps, genetics, odor composition, and behavior. Journal of Neuroscience. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. Journal of Neuroscience. 2001;21:2481–2487. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male–male aggression in mice deficient for TRP2. Science. 2002 February 22;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Swann J, Rahaman F, Bijak T, Fiber J. The main olfactory system mediates pheromone-induced fos expression in the extended amygdala and preoptic area of the male Syrian hamster. Neuroscience. 2001;105:695–706. doi: 10.1016/s0306-4522(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nature Neuroscience. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Cloe AL, Waters P, Baum MJ. Effects of vomeronasal organ removal on olfactory sex discrimination and odor preferences of female ferrets. Chemical Senses. 2004;29:659–669. doi: 10.1093/chemse/bjh069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CJ, Wysocki LM. Surgical removal of the mammalian vomeronasal organ and its verification. In: Speilman AI, Brand JG, editors. Experimental cell biology of taste and olfaction. New York: CRC; 1995. pp. 49–57. [Google Scholar]