Abstract
This review focuses on neuroimaging studies that examined stress processing and regulation and cognitive inhibitory control in patients with psycho-stimulant addiction. We provide an overview of these studies, summarizing converging evidence and discrepancies as they occur in the literature. We also adopt an analytic perspective and dissect these psychological processes into their sub-components, to identify the neural pathways specific to each component process and those that are more specifically involved in psycho-stimulant addiction. To this aim we refer frequently to studies conducted in healthy individuals. Despite the separate treatment of stress/affect regulation, stress-related craving or compulsive drug seeking, and inhibitory control, neural underpinnings of these processes overlap significantly. In particular, the ventromedial prefrontal regions including the anterior cingulate cortex, amygdala and the striatum are implicated in psychostimulant dependence. Our overarching thesis is that prefrontal activity ensures intact emotional stress regulation and inhibitory control. Altered prefrontal activity along with heightened striatal responses to addicted drug and drug-related salient stimuli perpetuates habitual drug seeking. Further studies that examine the functional relationships of these neural systems will likely provide the key to understanding the mechanisms underlying compulsive drug use behaviors in psycho-stimulant dependence.
Keywords: fMRI, cognitive control, reward, motivation, affect, impulsivity, decision making, cocaine, methamphetamine
The molecular and cellular neurobiology of drug addiction has recently been extensively reviewed in the literature (Di Chiara and Bassareo, 2007; Kalivas et al., 2005; Koob, 2006; Maldonado et al., 2006). Drawing heavily on rodent studies, previous reviews of the neuroscience of drug addiction have elaborated on the role of cortico-striatal pathways and extended amygdala circuitry in shaping habitual and compulsory drug use behaviors (Everitt et al., 2001; Everitt and Robbins, 2005; Everitt and Wolf, 2002; Koob, 2003; Koob, 2006; Robinson and Berridge, 2000, 2003). Others have reviewed genetic influences on stress responsivity and impulsivity that contribute to vulnerability to drug abuse (Kreek et al., 2005) and the pharmacology of cognitive processes of critical importance to substance dependence (Chamberlain et al., 2006; Cools and Robbins, 2004). Inspired by these earlier reviews, the current work takes a somewhat different perspective. This article reviews experimental findings on the alteration of frontal cortical functions in patients with psycho-stimulant dependence, within a framework of cognitive neuroscience. We do not distinguish between the frontal cortical deficits that are impaired as a result of drug use or those that occur prior to and contribute to drug use. However, making such distinction is of utmost importance in understanding the individual vulnerability to substance dependence and will require long-term longitudinal follow-up with detailed experimental and clinical profiling of a cohort of subjects.
The focus of this review is on human neuroimaging studies on inhibitory control and emotional stress regulation, with an attempt to integrate these findings with clinical observations where they are relevant. The unifying theme is the overlapping frontal cortical regions implicated in these cognitive and affective processes, although each of these processes appears to engage its own distinct neural circuitry and presumably contributes to the development of psycho-stimulant dependence. The anterior cingulate cortex (ACC), for instance, is widely and most consistently implicated in these processes, but each of these processes appears to engage the ACC at a different locale. Failure to activate the ventromedial prefrontal cortex including the perigenual ACC appears to be associated with impaired distress processing and decision making, which is characteristic of patients with substance misuse (section 2). Furthermore, it is hypothesized that self control under stress or an aroused state (e.g., drug craving) requires an intact braking mechanism to prevent such psychological experiences from escalating into habitual drug taking. We have observed that patients with cocaine dependence are impaired in activation of a sub-region in the rostral ACC during inhibitory control, independent of other cognitive and affective processes (section 1). This sub-region of ACC is situated at a locale where cognitive and affective functions overlap. Thus, altered anterior cingulate activity appears to be a critical neural marker of psycho-stimulant dependence. Our review of the literature suggests that different regions of the ACC process and regulate emotional stress and drug craving, control behavioral impulses and thus prevent such psychological experiences from translating into drug seeking and consumption. With an analytic approach, the current review delineates the specificity of cingulate activity while revealing a pathway common to various cognitive and affective deficits implicated in psycho-stimulant dependence.
Drug addiction has been conceptualized as a syndrome of impaired response inhibition and salience attribution (Goldstein and Volkow, 2002). This model proposes that in addicted individuals, the saliency of the abused drug and its associated cues is enhanced in the reward and motivation circuitry at the expense of natural reinforcers. It is hypothesized that drugs of abuse reset the threshold of the reward circuitry and concomitantly decrease its sensitivity to natural reinforcers. As a result, “wanting” of the abused drug becomes a primary motivational drive for the addicted individual (Robinson and Berridge, 2003). Without appropriate inhibitory control and an intact decision-making mechanism evaluating the delayed consequences, such an ill-motivated state easily perpetuates into a vicious cycle of withdrawal, craving, bingeing and intoxication, and relapse.
Emotional stress is also well known to contribute to drug use and relapse (Sinha, 2001; Sinha, 2005; Sinha, 2007). Drug dependent individuals frequently have difficulty managing stressful life events. Exposure to negative affect, stress and withdrawal-related distress has also been shown to aggravate drug craving (Childress et al., 1994;Cooney et al., 1997; Fox et al., 2007; Sinha et al., 1999; Sinha, 2000) and stress-related increases in drug craving and arousal are predictive of relapse outcomes (Sinha et al., 2006; Sinha 2007). Withdrawal related alterations in brain stress circuits are well documented (Breese et al., 2005; Koob et al., 2004) and these neuroadaptations in stress circuits may contribute to the increased salience of drug and drug-related stimuli in a variety of challenge or “stress” contexts (Robinson and Berridge, 2000; Sinha, 2001). Furthermore, we have also proposed that state-related alterations in cortico-striatal-limbic circuits may contribute to reduced access to adaptive coping such that response inhibition, behavioral flexibility and problem solving are affected during increasing levels of emotional stress or challenge (Sinha, 2001; Sinha et al., 2006).
We propose that disrupted inhibitory control and emotional stress processing along with compulsive drug seeking constitute the core of frontostriatal-limbic functional deficits in patients with psycho-stimulant dependence. These cognitive endophenotypes and their underlying neural processes may thus be expedient targets for psycho- and pharmaco- therapy for patients with a substance use disorder (SUD). Here we review the neuroimaging studies addressing inhibitory control, stress processing, and stress-related drug craving and compulsive drug seeking, with the goal of identifying the shared neurobiological substrates that could represent the core dysfunction in psycho-stimulant dependence. Although we focus primarily on patients with psycho-stimulant dependence, we do not exclude other substance use disorders. Thus, the term substance addiction (dependence) is also used where it is applicable and relevant.
1. Cognitive inhibitory control
Inhibitory control is an important executive function. The ability to suppress a prepotent response or a habit allows appropriate actions to meet complicated task demands and adaptation to changing environments. Impaired inhibitory control contributes to substance misuse. Individuals who bear an impulsive personality trait or who demonstrate impulsive behaviors are more likely to engage in substance misuse (Acton, 2003; Askenazy et al., 2003; Butler and Montgomery, 2004; Cloninger, 1988; Dawe, 2004; Masse and Tremblay, 1997; Moeller, 2001; Tarter et al., 2003; Tarter et al., 2004). For instance, in a sample of more than one thousand adolescents, indicators of disinhibition assessed at age 11 predicted age of first drink at age 14 (McGue et al., 2001). Elkins and colleagues showed that behavioral disinhibition is distinctly associated with early onset of nicotine, alcohol and illicit drug use (Elkins et al., 2006). On the other hand, the psychological and neural processes underlying the association of impulsivity and substance misuse are less clear. In particular, most behavioral and imaging studies have employed paradigms involving psychological processes other than inhibitory control, and have thus not ascertained the specificity in the association between deficits in inhibitory control and substance misuse.
1.1. Multiple psychiatric syndromes are characterized by impaired inhibitory control
Impairment in a number of different behavioral tasks has been taken to implicate a deficit in inhibitory control in patients with SUD. For instance, in a color word Stroop task, participants verbally name the color of “color” words printed in the same or different colors (e.g., “yellow” printed in yellow versus “yellow” printed in red). The increased interference effect, measured as the reaction time difference between incongruent (“yellow” in red) and congruent (“yellow” in yellow) trials, has been suggested to reflect impaired inhibitory control in substance abusers as compared to control participants. Such an increased interference effect has been found for patients with benign epilepsy (Chevalier et al., 2000), attention deficit hyperactivity disorder (Carter et al., 1995), Alzheimer’s disease (Amieva et al., 2004), Parkinson’s disease (Henik et al., 1993), bipolar disorder and schizophrenia (Zalla et al., 2004), and opiate dependence (Mintzer, 2002). Other studies have employed continuous performance tests (CPT) and used the number of commission errors as a behavioral proxy for failed inhibitory control. For instance, participants view a series of 5-digit numbers on a computer screen and respond to a number when it matches the previous number (Swann et al., 2002). Occasionally a number is displayed that differs by one digit from the number to be matched. A response to this number is scored as a commission error or “false alarm.” Increased commission errors in this or other similar CPTs have been associated with alcohol consumption, compared to a placebo drink (Dougherty et al., 1999), and with a greater number of suicide attempts (Dougherty et al., 2004), and are more common in individuals with anorexia nervosa (Butler and Montgomery, 2005), attention deficit hyperactivity disorder (Epstein et al., 2003), reading disorder (Beale et al., 1987), alcohol dependence (Bjork et al., 2004), cocaine dependence (Moeller et al., 2005), borderline personality (Paris et al., 1999) and schizophrenia (Nuechterlein and Dawson, 1984), as compared to healthy controls. A correlation between self-reported impulsivity measures and commission errors in the CPT has also been reported (Dougherty et al., 2003; Swann et al., 2002).
Successful performance in both the Stroop task and the CPT clearly requires some aspect of inhibitory control. One inhibits the pre-potent tendency to read the color word in the Stroop task and the motor routine prematurely activated prior to the processing of contextual information in the CPT. However, the Stroop task and CPT also involve linguistic processing/working memory and attentional control, processes that could be compromised in patients with a neuropsychiatric disorder. The fact that patients with a variety of different clinical conditions are found to be impaired in the Stroop task and CPT reflects the complexity of the cognitive processes involved in the execution of these behavioral tasks.
Similarly, although patients with SUDs are impaired in gambling tasks where they tend to select actions associated with short-term gains, even when they lead to long-term losses, this impairment does not appear to be specific to individuals with substance misuse. The same impairment was also found in patients with attention deficit hyperactivity disorder (Toplak et al., 2005), Korsakoff syndrome(Brand et al., 2005), catatonic schizophrenia (Bark et al., 2005), anorexia nervosa (Cavedini et al., 2004), traumatic brain injury (Levine et al., 2005), multiple sclerosis (Kleeberg et al., 2004), depression patients recovering from subcaudate tractotomy (Dalgleish et al., 2004), and in individuals with old ages (Denburg et al., 2005). A deficit in motivation or attentional control, which occurs more commonly across different clinical conditions, could possibly explain their impairment in the gambling task.
1.2. Stop-signal task as a tool to study response inhibition
The stop-signal task or the go/no-go task has also been used to study inhibitory control in patients with SUD (Fallgatter et al., 1998; Fillmore, 2002; Hester and Garavan, 2004; Kaufman et al., 2003). The stop-signal task is a motor response inhibition task and has been a valuable tool in studying the psychological and neural mechanisms underlying response inhibition (Logan, 1994; Logan and Cowan, 1984). In this behavioral task, the dominant or more frequent stimulus constitutes a go signal requiring the subjects to respond within a time window and therefore sets up a prepotent response tendency (as in compulsory drug seeking). In the less frequent stop trial, a stop signal appears after the go signal and instructs the subjects to refrain from making the response. The ease with which one can withhold a response depends on the time interval between the go and stop signals, or the stop-signal delay (SSD): the longer the SSD the more difficult it is for one to stop and vice versa.
Response inhibition in the stop-signal task can be characterized in several ways. First, by using a few different, well-spaced SSDs and computing the percentage of the stop trials with the response successfully withheld at each SSD (higher percentages at short SSDs), one can construct an inhibitory function for individual subjects. That is, the inhibitory function represents an integrated percentage of successful stop trials. The inhibitory function can then be compared between different subject groups. Another index useful to characterize response inhibition is the stop signal reaction time or SSRT, which describes the time for the stop signal to be processed so a response can be withheld. Based on a horse race model, behavioral performance in the stop-signal task can be interpreted in terms of statistically independent go and stop processes racing toward their finishing line or activation threshold. The horse race model allows estimation of the SSRT based on the subject’s probability of inhibiting responses to the stop signal and the distribution of RT in the go trials (Logan, 1994; Logan and Cowan, 1984). A longer SSRT indicates poor response inhibition. Finally, SSRT in the stop-signal task can also be obtained via a staircase procedure to update SSD trial by trial. The SSD decreases by a specified step (to make it easier to stop) if the subject fails at a previous stop trial and increases by the same step if the subject succeeds. With the staircase procedure, a “critical” SSD can be computed that represents the time delay required for the subject to succeed in withholding a response in half of the stop trials (Levitt, 1970). The SSRT is then estimated by subtracting the “critical” SSD from the average RT of the go trials.
The two behavioral indices, the inhibitory function and SSRT, have been widely used to describe impaired inhibitory control in people with neurological (Aron and Poldrack, 2005; Aron et al., 2003; Dimitrov et al., 2003; Reiman et al., 1997; Stewart and Tannock, 1999) or psychiatric conditions, including patients with SUDs (Armstrong and Munoz, 2003; Badcock et al., 2002; Dimoska et al., 2003; Fillmore, 2002; Li et al., 2006c; Monterosso et al., 2005; Oosterlaan and Sergeant, 1996; Overtoom et al., 2002; Rubia et al., 1998; Schachar et al., 1995). Fillmore and Rush (2002) used a choice reaction stop-signal task to study inhibitory control in chronic cocaine users (Fillmore, 2002). They found that, compared to control subjects, cocaine users displayed a lower probability of inhibiting their responses and required more time to inhibit responses to stop signals. A more recent study also observed prolonged SSRT in chronic methamphetamine users than controls (Monterosso et al., 2005). Other studies have documented the acute effects of cocaine (Fillmore et al., 2002) and alcohol (Fillmore and Rush, 2001; Mulvihill et al., 1997) on response inhibition. Taken together, these studies have highlighted altered behaviors and regional brain activations during response inhibition in patients with SUDs.
While well suited to study inhibitory function, the stop-signal task involves processes during sensorimotor transformation that are distinct from inhibitory control and yet can have a direct impact on the measure of response inhibition. One such process is response readiness. A previous study demonstrated an association between response readiness and stop signal processing and suggested that this effect needs to be accounted for in order to attribute prolonged SSRT to impaired response inhibition (Li et al., 2005b). Briefly, it is well known that in a reaction time (RT) task, RT decreases with an increased duration for response preparation (Bertelson and Tisseyre, 1968; Woodrow, 1914), a result that has been termed fore-period (FP) effect. We derived a FP effect for the go trial RT as an index of response readiness and found that the SSRT correlated positively with the FP effect. Another factor that needs to be accounted for is performance monitoring. In an RT task, the RT of a correct response is prolonged following an error, compared to other correct responses, and this RT difference (an error monitoring or EM effect) is thought to reflect cognitive processes involved in error correction (Rabbit, 1966). A recent study showed that subjects who demonstrated shorter SSRT in a stop signal task also appeared to have greater EM effect (Li et al., 2006a). Moreover, we showed that although patients with cocaine dependence demonstrated prolonged SSRT, compared to healthy controls, such difference vanished in a covariance analysis that accounted for the EM effect (Li et al., 2006c). These results suggest that task-related factors such as response preparation and performance monitoring need to be accounted for in the examination of stop signal performance. Furthermore, given the ubiquity of response preparation and error monitoring in cognitive performance, the inclusion of these task covariates should perhaps be considered in other behavioral tasks as well. On the other hand, the association between error monitoring and response inhibition in the stop signal task is correlational in nature. One thus cannot rule out the possibility that these two processes address two complementary aspects of impulsivity in patients with SUDs (Li et al., 2006c)
1.3. Neural correlates of stop-signal inhibition
Functional magnetic resonance imaging (fMRI) has also been used to explore the neural processes of inhibitory control in patients with SUDs. Employing a go/no-go task, Kaufman and colleagues found greater commission and omission errors in current cocaine users, compared to healthy controls (Kaufman et al., 2003). They also found cingulate and medial frontal hypoactivation during successful no-go inhibitions and errors of commission in cocaine patients, compared to healthy controls, and suggested that cocaine addiction is accompanied by disruption of neural circuitry critical for cognitive control. In another go/no-go fMRI study in which working memory demand is manipulated, current cocaine users demonstrated greater errors in response inhibition under increased working memory demand without showing difference in response prepotency, when their performance was compared to control subjects (Hester and Garavan, 2004). It was suggested that, since working memory demands were shown to increase during drug craving, this ‘dysexecutive’ pattern of brain activation may play a role in predisposing cocaine users toward relapse. Although these studies have highlighted altered regional brain activation in patients with cocaine dependence during performance of a go/no-go task, whether the findings speak directly to the neural correlates of inhibitory control remains unclear.
In fact, many previous fMRI studies have compared brain activation during blocks of go and mixed go/stop trials. Although such contrasts may reveal the activation associated with response inhibition, they may also reflect differences associated with other cognitive and affective processes that are unequally represented between these blocks. For instance, the go block contains more motor responses, while the mixed go/stop block contains higher task demand with greater signal and performance monitoring, greater oddball attention effect, and more emotional frustration because errors are more frequently made. With these potential confounds, earlier studies have localized a wide array of brain regions including prefrontal and cingulate cortices, the basal ganglia, and cerebellum, showing greater activation in the mixed than in the go blocks (Menon, 2001; Rubia et al., 2001). With go and stop trials intermixed, event-related fMRI studies circumvented most of these confounding variables (Durston et al., 2002; Garavan et al., 1999; Konishi et al., 1998; Liddle et al., 2001). In particular, by contrasting successful and unsuccessful inhibitions, Rubia and colleagues identified right inferior frontal cortex as specifically mediating response inhibition (Rubia et al., 2003) and (Rubia et al., 2005). However, while this contrast controlled for certain pre-response differences between successful and failed inhibition, it did not control for differences in stop-signal monitoring and post-response processing (Li et al., 2006a).
Our recent study attempted to address these confounds by taking advantage of the individual variability in stop signal performance (Li et al., 2006a). We recruited 24 healthy adults to participate in a tracking stop signal task, in which the stop signal delay was updated trial by trial depending on their performance, based on a staircase procedure (Levitt, 1970). We computed the SSRT for each individual subject, on the basis of a horse race model (Logan et al., 2005). Assuming that shorter SSRT would result from greater activation of brain regions that mediate response inhibition, we contrasted subjects with short and long SSRT, grouped by a median split. Importantly, by comparing these two groups of subjects who showed no differences in their inhibition failure rate, we could control for the confounding effect of signal monitoring. This contrast also allowed the evaluation of post-response processes as covariates. Subjects rated their emotional frustration when they completed three fourths of the task. Moreover, we computed for each individual subject an error monitoring (EM) effect, as described in the previous section, to index performance monitoring.
The results showed greater activation in the short SSRT group in the left anterior pre-supplementary motor area (pre-SMA, BA 8), pre-central gyrus (BA 9), and rostral anterior cingulate cortex (rACC, BA 32/24). Although the emotional frustration rating did not differ between the two groups, subjects with short SSRT showed a marginally greater EM effect than those with long SSRT. We thus performed a covariance analysis with the EM effect as covariate and obtained greater activation in the same brain regions and, in addition, in the left medial frontal gyrus (BA 10), in the short than the long SSRT group.
Another intriguing finding from our study is that the activation of the anterior pre- SMA but not the rACC was correlated with SSRT across individual subjects. This result suggests that while the anterior pre-SMA is positioned during the sensorimotor transformation to influence moment-to-moment variation in stop signal performance, the rACC plays a control-level and perhaps cross-modal role in mediating response inhibition. Further studies would be warranted to investigate this possibility. In another study we observed that, compared to healthy individuals, abstinent patients with cocaine dependence showed decreased activation in the rACC during stop signal inhibition, independent of behavioral performance including SSRT, frustration rating and the EM effect (Li et al., In press). Furthermore, in cocaine dependent patients, the activity of rACC was correlated with their subjective rating of difficulty in impulse control as assessed by the Difficulty in Emotion Regulation Scale (DERS: Gratz and Roemer, 2004). The impulse control subscale of the DERS assessed the ability of self-control with questions as “when I’m upset, I lose control over my behaviors ?” This finding thus extends previous work of Garavan and colleagues by ascertaining the functional specificity of ACC hypoactivation in patients with cocaine dependence (Hester and Garavan, 2004; Kaufman et al., 2003).
1.4. Summary
Impairment in inhibitory control contributes to drug use and relapse. Among a myriad of cognitive tasks employed to examine inhibitory control, the stop-signal task appears to hold greater potential in isolating the neural correlates of response inhibition, because it offers a venue to control for signal monitoring (which requires sustained attention) and post-response processing. A recent imaging study highlights the importance of anterior pre-SMA and rACC cortices in mediating response inhibition. Compared to healthy individuals, patients with cocaine dependence demonstrated hypoactivation of the rACC during stop signal inhibition, despite no difference in performance. This latter finding specifies the functional significance of ACC hypoactivity in these patients. The ACC is also involved in the regulation of emotional stress and stress-induced drug craving (Sections 2). Thus, the finding that ACC is involved in inhibitory control is particularly intriguing, because impulsivity most often occurs in a specific behavioral context, as for instance, when stress or high arousal states such as during exposure to drug-related salient stimuli is part of the contingency. It would thus be of great relevance to examine response inhibition in patients with SUDs under a behavioral context that can potentially elicit drug use, such as when one is craving a drug or experiencing emotional distress.
2. Stress processing and regulation
Most models of drug addiction have proposed that stress increases the risk of drug abuse and relapse (Koob and Le Moal, 1997; Sinha, 2001). Both animal and human studies provide evidence linking drug abuse and stress during all stages of life, including early life stress (see Sinha, 2001; Goeders, 2003, for a review). For instance, monkeys that were peer-reared early in their lives consumed significantly more alcohol as adults, compared with mother-reared monkeys (Higley et al., 1991). More recently, Kosten and colleagues demonstrated that neonatal isolation enhanced acquisition of cocaine self-administration in rats (Kosten et al., 2000). In human studies, several studies show positive association between alcohol consumption and stress levels (reviewed in Sinha, 2001). Exposure to negative affect, stress, and withdrawal-related distress also increases drug craving (Childress et al., 1994; Cooney et al., 1997; Sinha et al., 1999, Sinha, 2000). Preclinical evidence indicates that chronic cocaine use alters the frontal-limbic-striatal pathways involved in stress processing and inhibitory control (Manstch et al., 2003; Beveridge et al., 2005). Similarly, human laboratory studies have shown an enhanced sensitivity to stress and alcohol/drug craving in cocaine abusers compared to social drinkers suggesting that both exposure to stress and drug related stimuli promote a compulsive drug seeking state in cocaine dependent individuals (Fox et al., 2007). On the other hand, social drinkers report mild increases in craving with exposure to alcohol cues and no increase in craving under stress conditions. The above findings suggest a bidirectional relationship between stress and psychostimulant use, where high levels of stress may facilitate the reinforcing effects of drug use thereby promoting enhanced acquisition, whereas altered stress and reward pathways lead to an increased drug wanting and seeking state in chronic drug users when they are exposed to stressors and drug related stimuli. This compulsive drug seeking state increases the risk of relapse and continued drug use, and contributes to maintenance of psycho-stimulant dependence.
2.1. Stress processing and the ventral striatum
An important marker of stress processing and distress regulation is the hypothalamic-pituitary-adrenal response to stress, most commonly assessed by plasma or salivary cortisol levels in humans. In Positive Emission Tomography (PET) studies using [11C]raclopride, several studies have now shown that acute stress exposure increases dopamine release in the ventral striatum (VS). For example, in a small sample study, Pruessner et al (2004) found that healthy students who had low early life maternal care showed greater dopamine release in the ventral striatum during an acute psychological stress task as compared to students with a history of high early life maternal care. Furthermore, cortisol response during the stress task was correlated significantly (r=.78) to VS dopamine release. Using PET, Oswald et al., (2005) also demonstrated that acute amphetamine challenge-related subjective “high” responses and concomitant increase in dopamine in the VS were each significantly associated with the amphetamine-induced cortisol responses. More recently, the same group has also shown a similar significant relationship between cortisol levels and dopamine release in the VS using a psychological stress task (Oswald et al., 2006).
Findings from these studies on the VS and the mesolimbic dopamine system suggest that the VS also plays a role in stress experience in addition to its involvement in reward processing. These data are consistent with a growing body of human imaging studies and preclinical data indicating ventral striatal activity during aversive conditioning, experience of aversive, pain stimuli and in anticipation of aversive stimuli (Sorg & Kalivas, 1991; McCullough & Salamone, 2001; Becerra et al., 2001; Jensen et al., 2003). Stress increases mesolimbic dopamine transmission in laboratory animals (Piazza et al., 1989; 1996), and these findings have often been cited as one possible mechanism for stress-induced increases in vulnerability to psycho-stimulant self-administration (Piazza & LeMoal 1998). The evidence points to a role for the VS beyond reward processing, and one that more broadly involves motivation and attention to behavioral response during salient (aversive or appetitive) events (Berridge & Robinson, 1998; Bindra, 1978; Ikemoto and Panksepp, 1999; Salomone et al., 1997). Others have assigned it a ‘motivational salience’ role functioning as a gateway from motivation to action, regardless of the valence of the motivation (Salomone, 1994; Horvitz, 2002). As the medial PFC and ACC also play a role both in stress and reward processing, this raises the issue of the possible inter-relationships between the VS and the ACC. There are cortical inputs from the PFC to the VS and also interactions between the VS and the amygdala which itself receives cortical afferents (McGeorge & Faull, 1989; Berendse et al., 1992; Voorn et al., 2004). Lesioning of the medial PFC in laboratory animals decreases reinstatement of responding for drug with exposure to stress, drug related cues and drug itself (Kalivas & McFarland, 2003; Shaham et al., 2003). These data suggest that the PFC may exercise some control over instrumental learning and responding for drug. However, the extent to which PFC may regulate VS activity particularly under conditions of stress are not well understood, and future studies need to directly assess the separate role of ACC-VS circuitries in stress and in the context of psycho-stimulant dependence to understand more fully how stress contributes to motivation to use and abuse psycho-stimulants.
2.2. Stress-related brain activation and drug craving
It is well known that emotional stress contributes to substance use and relapse (Sinha, 2001; Sinha, 2005). Stress has been consistently shown to increase drug craving and compulsive drug seeking in cocaine addicted individuals, and with greater sensitivity to stress-induced craving and arousal in cocaine patients compared to matched controls (Sinha et al., 2003; Fox et al., 2007). Furthermore, stress-induced craving and stress arousal is predictive of cocaine relapse outcomes (Sinha et al., 2006; Sinha, 2007). However, the neural processes underlying this association in humans remain unclear, see Capriles et al., 2003; Stewart, 2003, for a review of the rodent studies). In particular, there have been very few neuroimaging studies directly examining emotional stress processing in patients with SUDs.
Our recent fMRI study compared abstinent patients with cocaine dependence with demographically matched healthy controls during script-guided stress imagery (Sinha et al., 2005). Both stress and neutral scripts were developed prior to the scan and based on individual personal real life experiences. Imagery vividness, stress and cocaine craving ratings as well as heart rate were recorded during each imagery session. Regional brain activations were compared between cocaine dependent subjects and healthy controls, separately for stress and neutral trials. Despite similar increases in anxiety ratings and changes in heart rate during stress, cocaine dependent patients showed significantly less activation than controls in specific frontal and para-limbic regions, such as the anterior cingulate cortex (ACC), left hippocampal/parahippocampal region, right fusiform gyrus, and the right postcentral gyrus. Previous studies by our group and others have shown that the medial prefrontal cortex, including the ACC, is involved in emotion and anxiety regulation (Lane et al., 1998; Beauregard et al. 2001; Critchley et al. 2001; Sinha et al., 2004). Therefore, the finding that patients failed to activate ACC and related circuits during stress would be consistent with impairments in emotion and stress regulation. On the other hand, cocaine-dependent patients had increased activity in the caudate and dorsal striatum region during stress, activation that was significantly associated with stress-induced cocaine craving ratings. A recent report also showed that dorsal striatum activity was associated with drug cue-induced cocaine craving (Volkow et al., 2006). Greater craving-related activation in the dorsal striatum is consistent with the hypothesis that this region plays a key role in the transition from instrumental learning to habit learning and involved in driving compulsive drug seeking in addiction (Everitt & Robbins, 2005). Interestingly in a preliminary relapse analysis, only rostral ACC activity during stress was associated with time to cocaine relapse and number of days of cocaine use during follow-up (Sinha & Li, 2007). These data highlight the involvement of the ACC regulating stress and associated craving states that are known o increase the risk of cocaine relapse.
Another fMRI study highlighted the sex differences between cocaine dependent men and women in processing emotional information (Li et al., 2005a). Compared to men, cocaine dependent women demonstrated greater activation in left middle frontal, anterior cingulate, and inferior frontal cortices and insula, and right cingulate cortex during stress imagery. Region of interest analysis showed that the change of activity in left anterior cingulate and right posterior cingulate cortices both correlated inversely with increases in stress-induced drug craving ratings. Moreover, the activity of the right posterior cingulate correlated inversely with change in heart rate in women but not men cocaine users. The greater left frontolimbic activity in women suggests that women, at least cocaine-using women, might use more verbal coping strategies than do men while experiencing stress. The results also suggest a distinct role of the cingulate cortices in modulating stress-induced cocaine craving. Other studies on this same cohort of subjects indicate the important modulatory effect of personality traits and the synergistic effect of multiple substance use on stress-related brain activation (Li et al., 2005c; Li et al., 2006b; Li and Sinha, 2006).
A previous study employed positron emission tomography (PET) to localize alterations in regional cerebral blood flow (rCBF) during mental imagery of a personal anger-associated scene and of an emotionally neutral scene in cocaine-dependent men (Drexler et al., 2000). Compared to the neutral imagery condition, the anger condition was associated with greater decreases in rCBF in multiple areas of the frontal cortex (particularly the right inferior frontal gyrus), left posterior insula, left fusiform gyrus, and midbrain. Conversely, this same inferior frontal area was activated by the anger imagery in nicotine-dependent men, who participated as a control group in the study. The anger imagery was also associated with increases in rCBF in the right fusiform gyrus, right and left middle occipital gyri, left post-central gyrus, left medial frontal gyrus, left cuneus, and in the left anterior cingulate gyrus. The study showed that cue-induced anger in cocaine-dependent men was associated with decreased activity in frontal cortical areas that are involved in response monitoring and inhibition. The lack of this association in nicotine-dependent men suggests a possible deficit in anger regulation associated with cocaine dependence and a possible link between cocaine dependence, violence, and relapse.
The imaging studies reviewed here identify specific areas of the limbic and corticostriatal circuitry involved in stress or other aspects of affective processing in patients with psychostimulant addiction. In particular, the medial PFC and ACC is a common region implicated in studies of stress and emotion regulation. The ACC as described in section 1 and in this section is implicated both in behavioral inhibitory control and affect regulation, and thus may play an important role in stress-related compulsive drug seeking. Furthermore, stress processing engages ventral striatum in healthy individuals and the dorsal striatum in cocaine patients, and both striatal regions have been implicated in drug craving (see also section 3). Thus, the evidence points to an overlapping neural circuitry in inhibitory control and stress processing and stress-induced drug craving.
2.3. Prefrontal-amygdala coupling and stress regulation
Although imaging studies on stress processing in patients with SUD are few, it has drawn much attention in research on patients with mood and anxiety disorders. Given the high comorbidity between post-traumatic stress disorder (PTSD) and SUD, studies of patients with PTSD would elucidate how altered frontal processing during stress regulation is associated with substance misuse. For instance, neuroimaging studies employing either PET, fMRI or Single Positron Emission Computed Tomography (SPECT) have consistently found in patients with PTSD, compared to control subjects, hypoactivation in rostral anterior cingulate or BA 32 during recall of personal traumatic events (Bremner et al., 1999; Britton et al., 2005; Lanius et al., 2001; Lanius et al., 2004; Liberzon et al., 2003; Lindauer et al., 2004; Shin et al., 1999; see also Liberzon and Phan, 2003 for a review). Retrieval of emotionally charged words related to childhood trauma, compared to neutral words, also decreased activation in BA 32 (Bremner et al., 2003). Another recent fMRI study showed that threat-related visual distractors activated the ventral anterior cingulate (Bishop et al., 2004). Moreover, participants with higher anxiety levels demonstrated less activity level in this brain region, again suggesting a role of this cingulate sub-region in regulating (in this case, irrelevant) emotional stimuli. Another frontal area that showed hypoactivation in PTSD patients during trauma imagery is the BA 10, located anterior and ventral to BA32 (Lanius et al., 2001; Liberzon et al., 2003; Shin et al., 2004). Several of these studies have also observed hyperactivation of the amygdala (Rauch et al., 2000; Shin et al., 2004) and the posterior cingulate cortex (Bremner et al., 1999; Lanius et al., 2004) associated with decreased activation in these medial frontal regions during trauma recall or viewing of fearful face stimuli. Affect dysregulation as a result of altered medial frontal activation may result in greater susceptibility of these patients to emotionally threatening stimuli, which elicited greater activation in the amygdala. A similar functional coupling between rostral ACC and amygdala may also be involved in pain regulation and the placebo effect (Petrovic et al., 2004; Petrovic et al., 2005).
Cingulate cortex has long been recognized as an important structure in cognitive and emotional control. In contrast to a more dorsal sub-region in the ACC that activates in response to maintenance of working memory, conflict monitoring, error processing and other cognitive manipulations, the subcallosal and/or perigenual cingulate has been associated with processing of emotional experiences ((Bush et al., 2000). Other studies have implicated the medial prefrontal cortex (MPFC) in the representation of sympathetic arousal (Damasio, 1994). A recent study provides evidence that activity in this brain region co-varies negatively with basal skin conductance (SCR) level (Nagai et al., 2004). In other words, higher MPFC activity is associated with lower physiological arousal. Consistent with its role in emotional and autonomic regulation, MPFC or BA 10 was more activated when subjects suppressed negative affect evoked by arousing and aversive pictures, along with attenuated activity in subcortical limbic regions including the nucleus accumbens and amygdala (Phan et al., 2005). Equally relevant are the studies of Lévesque and colleagues, who showed that other prefrontal structures including the dorsolateral and orbital prefrontal cortices are involved in reappraisal and active suppression of negative emotions (Lévesque et al., 2003; Lévesque et al., 2004; see also Ochsner and Gross, 2005, for a review).
The functional correlation between the medial frontal cortex and amygdala is also demonstrated for extinction learning in humans (Phelps et al., 2004). In this study, participants were exposed to conditioned visual stimuli, which were associated with mild wrist shock as an unconditioned stimulus, as well as to non-conditioned visual stimuli on the first day and went through extinction the next two days. Blood oxygenation level dependent (BOLD) signals in the amygdala (conditioned minus non-conditioned stimulus) correlated positively with the conditioned response (skin conductance changes, conditioned stimulus – unconditioned stimulus). While a dorsal anterior cingulate region (BA 32) demonstrated greater activation to the conditioned than to the non-conditioned stimulus, both the ventromedial prefrontal cortex (BA 10) and the subgenual anterior cingulate (BA32, 24 and 25) showed the opposite pattern of activity, with decreased BOLD signal to the conditioned stimulus. Moreover, the subgenual anterior cingulate demonstrated activity (conditioned minus non-conditioned stimulus) positively correlated with the extinction process. That is, the perigenual anterior cingulate showed less deactivation related to the conditioned stimulus as skin conductance changes also decreased to the conditioned stimulus throughout extinction. Note that although the more rostral BA 10 did not show a significant correlation with extinction, overall the area shows activity closely resembling the perigenual cingulate, both in striking contrast to the more dorsal sub-region of BA 32. Further studies are required to distinguish the roles of rostral anterior cingulate (BA 32) and ventral medial prefrontal cortex (BA 10) in affective processing and emotional regulation. Overall, these results suggest a frontal mechanism in the extinction of fear. Failure of this regulatory process can increase individual vulnerability to anxiety symptoms and the risk for the development of an anxiety disorder. Furthermore, failure of this regulatory process may also contribute to substance misuse as environmental cues can reinstate drug seeking behaviors (Goeders. 2002; Kelley et al., 2005; Quirk and Gehlert, 2003).
The finding of functional decoupling between medial frontal cortical and amygdala activity during experience of emotionally aversive conditioning is paralleled by recent work combing genotype mapping and neuroimaging. A recent fMRI study showed that a genetic variant associated with low expression of the monoamine oxidase-A (MAO-A) predicted hyper-responsivity of the amygdala during emotional arousal, with diminished reactivity of regulatory prefrontal regions, compared with the high expression allele (Meyer-Lindenberg et al., 2006). In a series of fMRI studies, individuals with one or two copies of the short allele of the serotonin transporter (5-HTT) promoter polymorphism, which has been associated with reduced 5-HTT expression and function and increased fear and anxiety-related behaviors, exhibit greater amygdala activity in response to fearful stimuli, compared with individuals homozygous for the long allele (Bertolino et al., 2005; Hariri et al., 2002; Hariri et al., 2005). Another study examined the effects of cognitive appraisal on the prefrontal-amygdala activity by comparing brain activation when subjects label (requiring cognitive evaluation) or simply match visually fearful and threatening stimuli (Hariri et al., 2003). This contrast showed greater activation of amygdala and parahippocampal gyri during matching and of prefrontal structures including the anterior cingulate cortex or BA 32 during labeling. Moreover, prefrontal activity was negatively correlated with that of the amygdala. The effect of 5-HTT polymorphism on the prefrontal regulation of amygdala activity was further established in a recent imaging study, which found that the functional coupling of the activity of these two brain regions explained about one third of variation in temperamental anxiety (Pezawas et al., 2005). These genotype-related alterations in the anatomy and function of an amygdala-cingulate feedback circuit critical for emotion regulation implicate a developmental, systems-level mechanism underlying normal emotional reactivity and genetic susceptibility for mood disorders (Pezawas et al., 2005). These and other aspects of the interaction between the prefrontal cortex and amygdala have been summarized in recent reviews (Brown and Hariri, 2006; Grace, 2006; Hariri et al., 2006; Shin et al., 2006).
The studies on the role of the prefrontal cortex and amygdala in affective processing have important implications for the association between emotional distress and substance use. The high co-morbidity between PTSD and SUD provides support for the contribution of stress and affect dysregulation to substance misuse (Brady and Sinha, 2005; Coffey et al., 2002, Jacobsen et al., 2001; Najavits et al., 1998; Back et al., 2000). In addition to the high co-occurrence of SUD and PTSD, there is evidence that PTSD may be uniquely deleterious to SUD treatment outcome. For example, comparisons between SUD-PTSD comorbid patients and patients with either a SUD alone or a SUD and a comorbid psychiatric condition other than PTSD reveal that SUD–PTSD patients have a higher addiction severity, are more likely to have comorbid psychiatric disorders, have poorer substance use treatment outcome, and have a higher number of inpatient admissions (Brady et al., 1994; Najavits et al., 1998; Ouimette et al., 2000). It would thus be very relevant to examine whether the afore-described genotypic diathesis in serotonergic neurotransmission would also predispose individuals to substance use and relapse, in association with increased emotional reactivity and impaired stress regulation.
2.4. Somatic markers and decision making
The involvement of the frontal cortex in substance misuse may go beyond stress processing. For instance, prefrontal dysfunction has been shown in individuals with psycho-stimulant dependence, along with impaired performance on a number of different decision making tasks (Bolla et al., 2003; Paulus et al., 2001). In particular, in the somatic marker hypothesis, the ventromedial prefrontal cortex (VMPFC) is postulated to play an important role in processing “marker” signals carried by bodily states, including bodily expression of emotions and feelings but extending to autonomic arousal that assist in reasoning and decision making (Damasio, 1996). Evidence supporting this hypothesis derives from studies of patients with lesions in the VMPFC using the Iowa Gambling Task (Bar-On et al., 2003; Bechara, 1996; Bechara et al., 1999). Note that the VMPFC described in these studies was not limited to BA 10. The extent of the lesions also included perigenual cingulate cortex and some lateral extents of the orbitofrontal cortex (Bechara et al., 2000; Bechara, 2004). It was found that, although both VMPFC patients and healthy controls exhibited changes in skin conductance responses (SCR) following reward or penalty, healthy controls but not the patients showed such autonomic changes prior to the selection from a risky deck. It is suggested that the absence of such anticipatory SCRs in patients with prefrontal damage reflects their insensitivity to future outcomes.
The somatic marker hypothesis has been extended to involve the amygdala (Bechara et al., 1999). Patients with bilateral lesions of the amygdala failed to demonstrate anticipatory changes in SCR, analogous to patients with VMFPC lesions. Moreover, amygdala patients failed to show SCR changes following reward and penalty, consistent with their failure in learning conditioned associations. Interestingly, those with SUDs demonstrated similar deficits as observed in VMPFC patients, suggesting that failure to integrate somatic markers as a result of a functional deficit of this prefrontal region may help to account for substance misuse (Bechara, 2002). Moreover, individuals with SUDs demonstrated normal SCR to punishment in the gambling task and normal acquisition of conditioned SCR to an aversive loud sound (Bechara, 2002). This latter finding suggests the integrity of amygdala function and supports the hypothesis that the poor decision-making in some patients with SUD is associated with defective somatic state activation that is linked to a dysfunctional VMPFC. Thus, the dysfunctional VMPFC may contribute to the transition from casual substance taking to compulsive and uncontrollable behavior observed in some patients with SUD (Bechara, 2002).
Criticisms of the somatic marker hypothesis include the finding that participants may have more knowledge than previously thought to guide their choices, which raises the question to what extent the somatic marker is influencing their decisions (Maia and McClelland, 2004; see also Bechara et al., 2005, for their reply). Also, there has not been strong empirical evidence linking peripheral sensory feedback to Iowa Gambling Task performance, which is crucial to the tenability of the hypothesis (see Dunn et al., In Press, for a review). In its current form, the somatic marker hypothesis also has to reconcile with the afore-described prefrontal-amygdala regulation of emotional state. Under the latter conceptual framework, patients with VMFPC lesions will have a hyperactive amygdala, which contribute to their excessive reactivity to emotional stress and susceptibility to drug use. On the other hand, the somatic marker hypothesis proposes that amygdala lesion would lead to an impairment in learning the association between emotional/autonomic arousal and actions, and in turn, to poor decision making (hence susceptibility of drug use). It thus appears that the VMPFC serves to maintain amygdala reactivity within a range where it can reliably signal emotional/autonomic arousal. Amygdala hyperactivity and hypoactivity may both contribute to substance abuse but through different neural pathways.
2.5. Summary
Stress responses engage the ventral and dorsal striatum, a subcortical structure implicated in motivation to seek drugs, thus providing direct evidence linking emotional stress processing to a critical psychological process mediating substance misuse. Studies of patients with PTSD and patients with ventromedial prefrontal lesions provide parallel evidence for a role of the prefrontal-amygdala circuitry in regulating emotional reactivity. A deficit in this regulatory process could either result in a heightened response to emotional stress or in impairment in association learning that can lead to disadvantageous decision making, , which in turn contribute to drug use. Further work is required to characterize the exact extent of prefrontal cortex that is involved in regulating amygdala and striatal function. Studies employing a paradigm other than the gambling task, particularly ones that allow temporal separation of task events, are warranted to provide auxiliary evidence for the somatic marker hypothesis. Perhaps two neural pathways to drug abuse can be delineated, with one linking to stress regulation and the other linking to impaired somatic marker processing.
3. Stress Regulation, Craving and Inhibitory Control – An Integrative Model
Inhibitory control and stress regulation are critical processes involved in effectively adapting to the environment. In the previous section 1 and section 2 we’ve reviewed neuroimaging evidence indicating impairments in both of these areas in patients with psychostimulant dependence. In particular, in section 2 we summarized that stress and drug-related stimuli similarly increase drug craving along with stress-related arousal in cocaine patients as compared to controls (Sinha et al., 2003; Fox et al., 2007). These findings suggest that greater levels of stress and arousal activate the reward and motivation circuits, and more so in patients than in healthy volunteers as documented by our findings on neural correlates of stress-induced cocaine craving (Sinha et al., 2005). These data are extend previous research indicating activation of amygdala, ventral and dorsal striatum and the ACC during cue induced cocaine craving states (Childress et al., 1999; Kilts et al., 2001; 2004; Volkow et al., 2006).
On the other hand, as highlighted in section 1 and 2, psychostimulant dependence is associated with profound alterations in prefrontal and anterior cingulate circuits involved in inhibitory control, decision making and in regulating distress states. In each case, the findings indicate hypoactivity of prefrontal and ACC regions in cocaine patients as compared to controls (Hester and Garavan, 2004; Kaufman et al., 2003; Li et al., In Press). Animal studies have consistently shown that lesions of the dorsomedial PFC, including the ACC region, prevents the reinstatement of responding for drug elicited by stress, drug cues and drug itself (Kalivas & McFarland, 2003; Shaham et al., 2003). This PFC mediation of reinstatement appears to be dependent on specific striatal-limbic and thalamocortical circuits, the functional integrity of which is critical in processing of sensorimotor, cognitive and emotional information required for adaptation and goal-directed behaviors (Alexander et al., 1986; Graybiel, 2005; Haber, 2003; Goto & Grace, 2005; Kalivas & McFarland, 2003). Lesions of these neostriatal-prefrontal circuits in animals and in patients with various striatal system disorders impair prefrontally dependent cognitive and behavioral regulation functions such as working memory, set shifting and executive control (Dunnet et al., 2005). Furthermore, distinct alterations in neuronal plasticity in these circuits are associated with chronic psychostimulant abuse (Gould, 2006; Hyman, 2005; Hyman et al., 2006; Kalivas, 2007; Kalivas and Hu, 2006; Lu et al., 2006; Self and Choi, 2004). Such findings have led researchers such as Everitt & Robbins (2005) to postulate that in SUDs and particularly in psychostimulant dependence, there is a transition from voluntary actions under the control of the medial PFC and ACC circuits, to more habitual modes of responding as seen in compulsive drug seeking or craving states driven by the dorsal striatum-based habit learning circuitry. From the perspective of stress regulation and adaptation, Arnsten and colleagues have elegantly shown dysfunction in prefrontal catecholamine projections during high levels of stress, which may render the PFC ‘off-line’ thereby allowing the habitual and more automatic responses to regulate behavior (Arnsten & Goldman-Rakic, 1998; Arnsten, 1998; Arnsten & Li, 2005). Here we integrate these similar perspectives to present a simplified schematic (see Figure 1) of our central thesis that dysfunction in the prefrontal-ACC circuitry in patients with psychostimulant dependence renders them susceptible to compulsive drug seeking especially during conditions of high stress and arousal (i.e., during exposure to stress, drug-related stimuli or drug taking) that is marked by increased activity in the limbic-striatal circuitries (amygdala-hippocampus, thalamus and dorsal striatum). Thus as shown in figure 1, when prefrontal-ACC circuitries are ‘online,’ there is good inhibitory control and low levels of craving or compulsive drug seeking. However, in states with hypoactivity of the PFC-ACC circuits, there is increased activity of the stress and reward salience circuitry and increased compulsive drug seeking resulting in high susceptibility to drug use and relapse.
FIGURE 1.
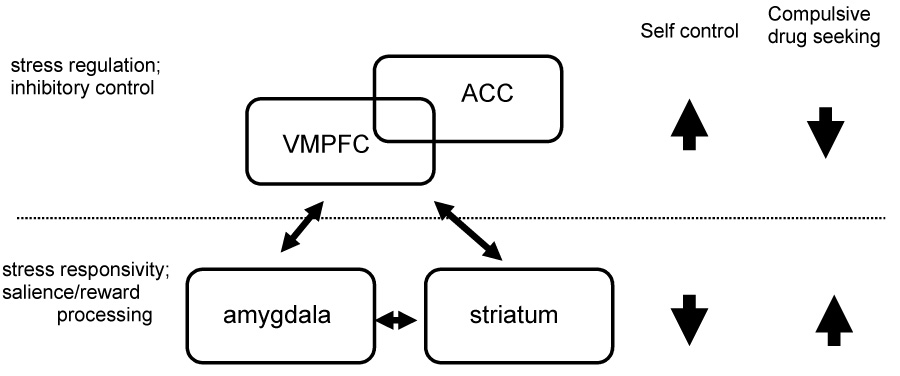
A schematic model to account for prefrontal-limbic dysfunctions associated with compulsory drug seeking. Prefrontal including anterior cingulate cortex regulates emotional stress and inhibitory control. Prefrontal hypoactivity along with heightened responses in the striatal-limbic circuitries to stress and drug-related salient stimuli contribute to compulsive drug seeking.
4. General Summary
The literature of human imaging studies reviewed here suggests that multiple brain regions are affected in psycho-stimulant dependence. Distinct brain areas are involved in impulse control and stress regulation. Importantly, these cognitive and affective processes also share common neural substrates. This review illustrates that one common neurobiological substrate of particular importance is the anterior cingulate cortex which is involved in controlling response inhibition and regulating emotional stress and stress and drug cue-related craving. Further studies are warranted to delineate both the role of each cingulate sub-region and the interaction of these sub-regions in mediating these processes. Furthermore, the specific cingulate sub-regions may involve unique molecular constituents that are keys to the development of novel therapeutics targeting different pathogenetic mechanisms underlying psycho-stimulant dependence.
The studies reviewed are not exhaustive. Other psychological processes such as altered delayed discounting of value, which have been associated with substance misuse, have been reviewed elsewhere (De Wit and Richards, 2004; Hester et al., 2006; see Bickel and Marsch, 2001; Bickel et al., In Press; Monterosso and Ainslie, In press, for a review). Measures of reflection impulsivity – the tendency to gather and evaluate information before making a decision – also appears to address distinct psychological functions of importance of substance misuse (Clark et al., 2006; Morgan, 1998; Morgan et al., 2002; 2006). Furthermore, studies have demonstrated the association of cognitive bias to drug cues and negative affect to drug use behaviors in patients with SUD using modified Stroop tasks (Bolla et al., 2004; Goldstein et al., 2007; see also Cox et al., 2006; McCusker, 2001, for a conceptual overview). As imaging data are accumulated with these paradigms, a synthesis of these results would be warranted in the future.
One major goal of addiction neuroscience research is to identify the cognitive and neural pathways by which occasional, voluntary drug use become habitual and ultimately compulsive (Everitt and Robbins, 2005). To this aim, a spate of pre-clinical and clinical research has delineated the importance of the reward, stress and cognitive inhibition circuitries in mediating this behavioral transition. Most of the studies, however, have approached these different neural systems in isolation, although drug use behaviors clearly do not result from a single, isolated cognitive or affective deficit. For instance, a deficit in inhibitory control may present as impulsivity, aggression or poor decision making but may not be associated with substance dependence without substance dependent patients concurrently experiencing drug craving. Likewise, impaired distress regulation can manifest as mood disturbances but may not lead to substance abuse if the reward and motivation system is otherwise intact. Thus, it would be important in future studies to examine the relationship between these neural systems, such as, for instance, how emotional distress and drug craving affect inhibitory control. Equally important are efforts to distinguish between functional deficits that result from prolonged drug use and those that cause drug use. This challenging task can be taken up by examining biological children of patients with SUD in longitudinal studies. Finally, with some caveats, lesion data or experiments can provide more convincing evidence linking brain regions with a specific psychological process (Naqvi et al., 2007; Vorel et al., 2007). Studies are thus warranted to systematically investigate substance use behaviors in patients with lesions to the frontolimbic structures that have been implicated in drug craving, emotional stress regulation, and cognitive inhibitory control in neuroimaging studies.
Acknowledgements
This work was supported by Yale Interdisciplinary Women's Health Research Scholar Program on Women and Drug Abuse (BIRCWH; K12-DA14038), funded by the NIH Office of Research on Women's Health and the National Institute on Drug Abuse. Support was also provided by NIH grants R01-DA11077 (RS), P50-DA16556 (RS), K02- DA17232 (RS), R03-DA022395 (Li), a research grant from the Alcoholic Beverage Medical Research Foundation (Li), and research grant (Li) from the Clinical Translational Science Award (NIH-UL1 RR024139) awarded to Yale University. We thank Dr. Tara Chaplin for her most helpful comments and edits of the manuscript. We are also grateful to three anonymous reviewers for their critical comments and discussions of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acton G. Measurement of impulsivity in a hierachical model of personality: implications for substance use. Substance Use Misuse. 2003;38:67–83. doi: 10.1081/ja-120016566. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amieva H, Lafont S, Rouch-Leroyer I, Rainville C, Dartigues JF, Orgogozo JM, Fabrigoule C. Evidencing inhibitory deficits in Alzheimer's disease through interference effects and shifting disabilities in the Stroop test. Arch Clin Neuropsychol. 2004;19:791–803. doi: 10.1016/j.acn.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Armstrong IT, Munoz DP. Inhibitory control of eye movements during oculomotor countermanding in adults with attention-deficit hyperactivity disorder. Exp Brain Res. 2003:444–452. doi: 10.1007/s00221-003-1569-3. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. The biology of being frazzled. Science. 1998;280(5370):1711–1722. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Askenazy F, Sorci K, Benoit M, Lestideau K, Myquel M, Lecrubier Y. Anxiety and impulsivity levels identify relevant subtyps in adolescents with at-risk behavior. Journal of Affective Disorders. 2003;74:219–227. doi: 10.1016/s0165-0327(02)00455-x. [DOI] [PubMed] [Google Scholar]
- Back S, Dansky BS, Coffer SF, Saladin ME, Sonne S, Brady KT. Cocaine dependence with and without posttraumatic stress disorder: A comparison of substance use, trauma history and psychiatric comorbidity. The American Journal on Addictions. 2000;9:51–62. doi: 10.1080/10550490050172227. [DOI] [PubMed] [Google Scholar]
- Badcock JC, Michie PT, Johnson L, Combrinck J. Acts of control in schizophrenia: dissociating the components of inhibition. Psychol Med. 2002:32. doi: 10.1017/s0033291701005128. [DOI] [PubMed] [Google Scholar]
- Ball T, Schreiber A, Feige B, Wagner M, Lucking CH, Kristeva-Feige R. The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. Neuroimage. 1999;10:682–694. doi: 10.1006/nimg.1999.0507. [DOI] [PubMed] [Google Scholar]
- Bar-On R, Tranel D, Denburg N, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126:1790–1800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Bark R, Dieckman S, Bogerts B, Northoff G. Deficit in decision making in catatonic schizophrenia: An exploratory study. Psychiatry Research. 2005;134:131–141. doi: 10.1016/j.psychres.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Beale IL, Matthew PJ, Oliver S, Corballis MC. Performance of disabled and normal readers on the Continuous Performance Test. J Abnorm Child Psychol. 1987;15:229–238. doi: 10.1007/BF00916351. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (Part I): Impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making, and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee G. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: Some questions and answers. Trends in Cognitive Sciences. 2005;9:159–162. doi: 10.1016/j.tics.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future consequences following damage to prefrontal cortex. Cereb Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Berendse HW, et al. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning or incentive salience? Brain Res Brain Res Review. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bertelson P, Tisseyre F. The time-course of preparation with regular and irregular foreperiods. Quat J Exp Psychol. 1968;20:297–300. doi: 10.1080/14640746808400165. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, Blasi G, Caforio G, Hariri AR, Kolachana B, Nardini M, Weinberger D, Scarabino T. Variation of human amygdala response during threatening stimuli as a finction of 5′HTTLPR genotype and personality style. Biological Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2006.09.016. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra D. How adaptive behavior is produced: a perceptual motivational alternative to response reinforcement. Behavioral and Brain Sciences. 1978;1:41–91. [Google Scholar]
- Bishop S, Duncan JS, Brett M, Lawrence A. Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature of Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol & Alcoholism. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. Journal of Neuropsychiatry Clin Neuroscience. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson K, Grant S, Contoreggi C, Links J, Metcalfe J, Weyl H, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brady KT, Killeen T, Saladin ME, Dansky BS, Necker S. Comorbid substance abuse and post-traumatic stress disorder: Characteristics of women in teatment. American Journals on Addictions. 1994;3:160–164. [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Brand M, Fujiwara E, Borsutsky S, Kalbe E, Kessler J, Markowitsch H. Decision-making deficits of Korsakoff patients in a new gambling task with explicit rules: Associations with executive functions. Neuropsychology. 2005;19:267–277. doi: 10.1037/0894-4105.19.3.267. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Lê AD, O'Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety and the risk of relapse. Alcoholism: Clinical and Experimental Research. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J, Narayan M, Staib L, Southwick S, McGlashan T, Charney D. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J, Vythilingam M, Vermetten E, Southwick S, McGlashan T, Staib L, Soufer R, Charney D. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological Psychiatry. 2003;53:879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Britton J, Phan K, Taylor S, Fig L, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern M, Lee G, Smith E, Sadeghi M, Saxena S, Jarvik M, London ED. Attentuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: A preliminary study. Psychiatry Research. 2004;130:269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern M, London ED, Childress AR, Lee G, Bota R, Ho M, Saxena S, Baxter LRJ, Madsen D, Jarvik M. Brain metabolic changes during cigarette craving. Archives General Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brown SM, Hariri AR. Neuroimaging studies of serotonin gene polymorphisms: exploring the interplay of genes, brain, and behavior. Cogn Affect Behav Neurosci. 2006;6:44–52. doi: 10.3758/cabn.6.1.44. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Butler G, Montgomery A. Impulsivity, risk taking, and recreational 'ecstasy' (MDMA) use. Drug and Alcohol Dependence. 2004;76:55–62. doi: 10.1016/j.drugalcdep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Butler G, Montgomery A. Subjective self-control and behavioral impulsivity coexist in anorexia nervosa. Eating Behaviors. 2005;6:221–227. doi: 10.1016/j.eatbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, J S. A role for the prefrontal cortex in stress-and-cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carter C, Krener P, Chaderjian M, Northcutt C, Wolfe V. Abnormal processing of irrelevant information in attention deficit hyperactivity disorder. Psychiatry Research. 1995;56:59–70. doi: 10.1016/0165-1781(94)02509-h. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Bassi T, Ubbiali A, Casolari A, Giordani S, Zorzi C, Bellodi L. Neuropsychological investigation of decision-making in anorexia nervosa. Psychiatry Research. 2004;127:259–266. doi: 10.1016/j.psychres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Robbins TW, Sahakian BJ. Neuropharmacological modulation of cognition. Curr Opin Neurol. 2006;19:607–612. doi: 10.1097/01.wco.0000247613.28859.77. [DOI] [PubMed] [Google Scholar]
- Chevalier H, Metz-Lutz MN, Segalowitz SJ. Impulsivity and control of inhibition in Benign Focal Childhood Epilepsy (BFCE) Brain Cogn. 2000;43:86–90. [PubMed] [Google Scholar]
- Childress A, Mozely PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, McLellan AT, MacRae J, Natale M, O'Brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? Journal of Substance Abuse Treatment. 1994;11:17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60:515–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Cloninger C, Sigvardsson S, Bowman M. Childhood personality predicts alcohol abuse in young adults. Alcohol Clin Exp Res. 1988;12:494–505. doi: 10.1111/j.1530-0277.1988.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Coffey S, Saladin M, Drobes D, Brady K, BDansky B, Kilpatrick D. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug and Alcohol Dependence. 2002;65 doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Cools R, Robbins TW. Chemistry of the adaptive mind. Philos Transact A Math Phys Eng Sci. 2004;362:2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative mood reactivity, and relapse in treated alcoholic men. Journal of Abnormal Psychology. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction-stroop test: Theoretical considerations and procedural recommendations. Psychol Bull. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Crockford D, Goodyear B, Edwards J, Quickfall J, El-Guebaly N. Cue-induced brain activity in pathological gamblers. Biological Psychiatry. 2005;58:787–795. doi: 10.1016/j.biopsych.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Daglish M, Weinstein A, Malizia A, Wilson S, Melichar J, Britten S, Brewer C, Lingford-Hughes A, Myles J, Grasby P, Nutt D. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. American Journal of Psychiatry. 2001;158:1680–1686. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- Daglish M, Weinstein A, Malizia AL, Wilson S, Melichar JK, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ. Functional connectivity analysis of the neural circuits of opiate craving: "more" rather than "different". Neuroimage. 2003;20 doi: 10.1016/j.neuroimage.2003.07.025. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Yiend J, Bramham J, Teasdale J, Ogilvie A, Malhi G, Howard R. Neuropsychological processing associated with recovery from depression after stereotactic subaudate tractotomy. American Journal of Psychiatry. 2004;161:1913–1916. doi: 10.1176/ajp.161.10.1913. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error: Emotion, Reason and the Human Brain. New York, NY: Crosset/Putnam; 1994. [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- David S, Munafo M, Johansen-Berg H, Smith S, Rogers RD, Matthews P, Walton R. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Behav Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebraska Symposium of Motivation. 2004;50:19–55. [PubMed] [Google Scholar]
- DeFrank RS, Jenkins C, Rose RM. A longitudinal investigation of the relationships among alcohol consumption, psychosocial factors, and blood pressure. Psychosomatic Medicine. 1987;49:236–249. doi: 10.1097/00006842-198705000-00003. [DOI] [PubMed] [Google Scholar]
- Denburg N, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43:1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov M, Nakic M, Elpern-Waxman J, Granetz J, O'Grady J, Phipps M, Milne E, Logan G, Hasher L, Grafman J. Inhibitory attentional control in patients with frontal lobe damage. Brain Cognition. 2003;52:258–270. doi: 10.1016/s0278-2626(03)00080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Moeller FG, Harper RA, Marsh DM, Mathias CW, Swann AC. Familial transmission of Continuous Performance Test behavior: attentional and impulsive response characteristics. J Gen Psychol. 2003;130:5–21. doi: 10.1080/00221300309601271. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Moeller FG, Swann AC. Suicidal behaviors and drug abuse: impulsivity and its assessment. Alcohol Depend Drug. 2004;(76 Suppl):S93–S105. doi: 10.1016/j.drugalcdep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Moeller FG, Steinberg JL, Marsh DM, Hines SE, Bjork JM. Alcohol increases commission error rates for a continuous performance test. Alcohol Clin Exp Res. 1999;23:1342–1351. [PubMed] [Google Scholar]
- Drexler K, Schweitzer JB, Quinn CK, Gross R, Ely TD, Muhammad F, Kilts CD. Neural activity related to anger in cocaine-dependent men: A possible link to violence and relapse. American Journal of Addiction. 2000;9:331–339. doi: 10.1080/105504900750047382. [DOI] [PubMed] [Google Scholar]
- Due D, Huettel S, Hall W, Rubin D. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Dunn B, Dalgleish T, Lawrence A. The somatic marker hypothesis: A critical evaluation. Neuroscience Biobehavioral Review. doi: 10.1016/j.neubiorev.2005.07.001. In press. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Meldrum A, Muir JL. Frontal-striatal disconnection disrupts cognitive performance of the frontal-type in the rat. Neuroscience. 2005;135:1055–1065. doi: 10.1016/j.neuroscience.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Elliott R, Deakin B. Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: Evidence from functional magnetic resonance imaging studies in healthy human subjects. International Review of Neurobiology. 2005;65:89–116. doi: 10.1016/S0074-7742(04)65004-5. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between Continuous Performance Test performance measures and ADHD behaviors. J Abnorm Child Psychol. 2003;31:543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt B, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallgatter AJ, Wiesbeck GA, Weijers HG, Boening J, Strik WK. Event-related correlates of response suppression as indicators of novelty seeking in alcoholics. Alcohol Alcohol. 1998;33:475–481. doi: 10.1093/alcalc/33.5.475. [DOI] [PubMed] [Google Scholar]
- Fillmore M, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug & Alcohol Dependence. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Fillmore MI, Rush CR. Alcohol effects on inhibitory and activational response strategies in the acquisition of alcohol and other reinforcers: priming the motivation to drink. J Stud Alcohol. 2001;62:646–656. doi: 10.15288/jsa.2001.62.646. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Hays L. Acute effects of oral cocaine on inhibitory control of behavior in humans. Drug Alcohol Depend. 2002;67 doi: 10.1016/s0376-8716(02)00062-5. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced Sensitivity to Stress and Drug/Alcohol Craving in Abstinent Cocaine-Dependent Individuals Compared to Social Drinkers. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301470. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho J, Sperry L, Ross T, Salmeron B, Risinger R, Kelley D, Stein E. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross T, Stein E. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proc National Academy of Science. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton R, Bloomer C, Teneback C, Drobes D, Lorberbaum J, Nahas Z, DJ V. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives General Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The impact of stress on addiction. European Neuropsychopharmacology. 2003;13:435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. Journal of Pharmacology and Experimental Therapeutics. 2003;2301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Gottfried J, O'Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried J, O'Doherty J, Dolan RJ. Encoding predictive reward calue in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Disruption of cortical-limbic interaction as a substrate for comorbidity. Neurotox Res. 2006;10:93–101. doi: 10.1007/BF03033238. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dsyregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. 2004;26:41–54. [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr. Opin. Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- Grusser S, Wrase J, Klein S, Hermann D, Smolka M, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus D, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and intergrative networks. J Chem. Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant E, Munoz K, Kolachana B, Mattay VS, Egan MF, Weinberger D. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59:888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger D. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitoro A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser S, Flor H, Braus D, Buchholz H, Grunder G, Schrechenberger M, Smolka M, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. American Journal of Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Henik A, Singh J, Beckley D, Rafal R. Disinhibition of automatic word reading in Parkinson's disease. Cortex. 1993;29:589–599. doi: 10.1016/s0010-9452(13)80283-3. [DOI] [PubMed] [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81:251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: Effects of early experience, personality, and stress on alcohol consumption. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav. Brain Res. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Hyman S. Addiction to cocaine and amphetamine. Neuron. 1996;16:901–904. doi: 10.1016/s0896-6273(00)80111-7. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Ohara S, Matsumoto R, Kunieda T, Nagamine T, Miyamoto S, Kohara N, Taki W, Hashimoto N, Shibasaki H. Role of primary sensorimotor cortices in generating inhibitory motor responses in humans. Brain. 2000;123:1710–1721. doi: 10.1093/brain/123.8.1710. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behaviors: a unifying interpretation with special reference to reward-seeking. Brain res Brain Res Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: A review of the literature. American Journal of Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am J Addict. 2007;16:71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Hu XT. Exciting inhibition in psychostimulant addiction. Trends Neurosci. 2006;29:610–616. doi: 10.1016/j.tins.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas P, Volkow ND, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Ross T, Stein E, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiology and Behavior. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KPG. The neural correlates of cue-induced craving in cocaine-dependent women. American Journal of Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KPG. Neural activity related to drug craving in cocaine addiction. Archives of General Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kleeberg J, Bruggimann L, Annoni J, van Melle G, Bogousslavsky J, Schluep M. Altered decision-making in mulitple sclerosis: A sign of impaired emotional reactivity? Annals of Neurology. 2004;56:787–795. doi: 10.1002/ana.20277. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong G, Adams C, Varner J, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101 (Suppl. 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Koob G, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Research. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lanius R, Williamson P, Densmore M, Boksman K, Neufeld RW, Gati J, Menon RS. The nature of traumatic memories: A 4-T fMRI functional connectivity analysis. American Journal of Psychiatry. 2004;161 doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. American Journal of Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lévesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Lévesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural basis of emotional self-regulation in childhood. Neuroscience. 129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Levine B, Black S, Cheung G, Campbell A, O'Toole C, Schwartz M. Gambling task performance in traumatic brain injury: Relationships to injury severity, atrophy, lesion location, and cognitive and psychosocial outcome. Cognitive Behavioral Neurology. 2005;18:45–54. [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoaccoustics. Journal of Acoustic Society of America. 1970;49:467–477. [PubMed] [Google Scholar]
- Li C-S, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: A functional magnetic resonance imaging study. Biological Psychiatry. 2005a;57:487–494. doi: 10.1016/j.biopsych.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Yan P, Paliwal P, Constable R, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine dependent men. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301568. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Constable R, Sinha R. Imaging response inhibition in a stop-signal task: Neural correlates independent of signal monitoring and post-response processing. Journal of Neuroscience. 2006a;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Kosten TR, Sinha R. Antisocial personality and stress-induced brain activation in cocaine-dependent patients. Neuroreport. 2006b;17:243–247. doi: 10.1097/01.wnr.0000199471.06487.a2. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Mathalon DH, Krystal JH. Fore-period effect and stop signal processing time. Experimental Brain Research. 2005b;167:305–309. doi: 10.1007/s00221-005-0110-2. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Milivojevic V, Kemp KA, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug and Alcohol Dependence. 2006c;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Li CS, Milivojevic V, Constable RT, Sinha R. Recent cannabis abuse decreased stress-induced BOLD signals in the frontal and cingulate cortices of cocaine dependent individuals. Psychiatry Res. 2005c;140:271–280. doi: 10.1016/j.pscychresns.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R. Alexithymia and stress-induced brain activation in cocaine-dependent men and women. J Psychiatry Neurosci. 2006;31:115–121. [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Britton J, Phan K. Neural correlates of traumatic recall in posttraumatic stress disorder. Stress. 2003;6:151–156. doi: 10.1080/1025389031000136242. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan K. Brain-imaging studies of posttraumatic stress disorder. CNS Spectrums. 2003;8:641–650. doi: 10.1017/s109285290000883x. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer RJ, Booij J, Habraken JB, Uylings HB, Olff M, Carlier IV, den Heeten GJ, van Eck-Smit BL, Gersons BP. Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:853–861. doi: 10.1016/j.biopsych.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Logan GD. Dagenbach DC, TH, editors. Inhibitory Processes in Attention,Memory and Language. Academic Press, San Diego. 1994:189–239. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan J, Ding Y, Lin K, Pareto D, Fowler J, Biegon A. Modeling and analysis of PET studies with noreponephrine transporter ligands: the search for a reference region. Nucl Med Biol. 2005;32:531–542. doi: 10.1016/j.nucmedbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. American Journal of Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Maia T, McClelland J. A reexamination of the evidence for the somatic marker hypotehsis: What partcicpants really know in the Iowa gambling task. Proceedings of the National Academy of Science. 2004;101:16075–16080. doi: 10.1073/pnas.0406666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Leenders KL, Chevalley AF, Missimer J, Kunig G, Magyar S, Mino A, Schultz W. Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Res Rev. 2001;36:139–149. doi: 10.1016/s0165-0173(01)00089-3. [DOI] [PubMed] [Google Scholar]
- Masse L, Tremblay R. Behavior of boys in kindergarten and the onset of substance use during adolescence. Archives of General Psychiatry. 1997;54:62–68. doi: 10.1001/archpsyc.1997.01830130068014. [DOI] [PubMed] [Google Scholar]
- McClernon F, Hiott F, Huettel S, Rose J. Abstinence-induced changes in self-report craving correlate with event-realted fMRI responses to smoking cues. Neurpsychopharmacology. 2005;30 doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Salamone JD. Anxiogenic drugs beta-CCE and FG 7142 increase extracellular dopamine levels in nucleus accumbens. Psychopharmacology (Berl.) 1992;109:379–382. doi: 10.1007/BF02245888. [DOI] [PubMed] [Google Scholar]
- McCusker CG. Cognitive biases and addiction: an evolution in theory and method. Addiction. 2001;96:47–56. doi: 10.1046/j.1360-0443.2001.961474.x. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RM. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, R Hariri A, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer M, Stitzer ML. Cognitive impairment in methadone maintained patients. Drug Alcohol Dep. 2002;67:41–51. doi: 10.1016/s0376-8716(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Moeller F, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. The behavioral economics of will in recovery from addiction. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2006.09.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Morgan MJ. Recreational use of "ecstasy" (MDMA) is associated with elevated impulsivity. Neuropsychopharmacology. 1998;19:252–264. doi: 10.1016/S0893-133X(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, McFie L, Fleetwood H, Robinson JA. Ecstasy (MDMA): are the psychological problems associated with its use reversed by prolonged abstinence? Psychopharmacology (Berl) 2002;159:294–303. doi: 10.1007/s002130100907. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Impallomeni LC, Pirona A, Rogers RD. Elevated impulsivity and impaired decision-making in abstinent Ecstasy (MDMA) users compared to polydrug and drug-naïve controls. Neuropsychopharmacology. 2006;31:1562–1573. doi: 10.1038/sj.npp.1300953. [DOI] [PubMed] [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58:600–605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton R, Li X, Henderson S, Drobes D, Voronin K, George M. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Current Biology. 2005;15:122–128. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Critchley H, Featherstone E, Trimble M, Dolan R. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: A physiological account of a "default mode" of brain function. Neuroimage. 2004;22:243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Gastfriend DR, Barber JP, Reif S, Muenz LR, Blaine J, Frank A, Crits-Christoph P, Thase M, Weiss RD. Cocaine dependence with and without PTSD among subjects in the National Institute on Drug Abuse Collaborative Cocaine Treatment Study. American Journal of Psychiatry. 1998;155:214–219. doi: 10.1176/ajp.155.2.214. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorder. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Deichmann R, Crithcley H, Dolan R. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Sergeant JA. Inhibition in ADHD, aggressive, and anxious children: a biologically based model of child psychopathology. J Abnorm Child Psychol. 1996;24:19–36. doi: 10.1007/BF01448371. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul ME, Zhou Y, Kuwabara H, Wand GS. Glucocorticoids, stress, impulsivity and ventral striatal dopamine release. Alcoholism: Clinical and Experimental Research. 2006;30(6):281A. doi: 10.1111/j.1530-0277.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- Ouimette PC, Moos RH, Finney JW. Two-year mental health service use and course of remission in patients with substance use and posttraumatic stress disorders. J Stud Alcohol. 2000;61:247–253. doi: 10.15288/jsa.2000.61.247. [DOI] [PubMed] [Google Scholar]
- Overtoom CC, Kenemans JL, Verbaten MN, Kemner C, van der Molen MW, van Engeland H, Buitelaar JK, Koelega HS. Inhibition in children with attention-deficit/hyperactivity disorder: a psychophysiological study of the stop task. Biol Psychiatry. 2002;51:668–676. doi: 10.1016/s0006-3223(01)01290-2. [DOI] [PubMed] [Google Scholar]
- Paris J, Zelkowitz P, Guzder J, Joseph S, Feldman R. Neuropsychological factors associated with borderline pathology in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:770–774. [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Carlsson K, Petersson K, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. Jounral of Cognitive Neuroscience. 2004;16:1289–1301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing-induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant E, Verchinski B, Munoz K, LKolachana B, Egan M, Mataay V, Hariri A, Weinberger D. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan P, Moore G, Uhde T, Tancer M. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps E, Delgado M, Nearing K, LeDoux J. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Pathophysiological basis of vulnerability to drug abuse: Role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annual Review of Pharmacology and Toxicology. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends in Pharmacological Sciences. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Annals of the New York Academy of Science. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Rabbit PMA. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin M, Lasko N, Orr S, Pitman R. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Society of Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun LS, Chen K. Neuroanatomical correlates of externally and internally generated human emotion. American Journal of Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 Suppl 2:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rubia K, Oosterlaan J, Sergeant JA, Brandeis D, von Leeuwen T. Inhibitory dysfunction in hyperactive boys. Behav Brain Res. 1998;94:25–32. doi: 10.1016/s0166-4328(97)00166-6. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activation across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex isresponsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naïve adolescents. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav. Brain Res. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neuroscience Biobehavioral Reviews. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Schachar R, Mota VL, Logan GD, Tannock R, Klim P. Deficient inhibitory control in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 1995;23:411–437. doi: 10.1007/BF01447206. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum J, Shah N, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. American Journal of Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Sell L, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Activation of reward circuitry in human opiate addicts. European Journal of Neuroscience. 1999;11:1042–1048. doi: 10.1046/j.1460-9568.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- Sell L, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug & Alcohol Dependence. 2000;60:207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Self DW, Choi KH. Extinction-induced neuroplasticity attenuates stress-induced cocaine seeking: a state-dependent learning hypothesis. Stress. 2004;7:145–155. doi: 10.1080/10253890400012677. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology(Berl.) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shin L, Porr S, Carson M, Rauch S, Macklin M, Lasko N, Peters P, Metzger L, Dougherty D, Cannisatraro P, Alpert N, Fischman A, Pitman R. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumtic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lsko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuserelated PTSD: A PET investigation. American Journal of Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Stress and drug abuse. In: Steckler T, N.H.K., Reul JMHM, editors. Handbook of Stress and the Brain. Part 2 Stress: Integrative and Clinical Aspects. vol. 15. Elsevier, Amsterdam; 2005. pp. 333–356. [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Current Psychiatry Reports. Vol 9(5) doi: 10.1007/s11920-007-0050-6. In Press. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Renee-Aubin l, O'Malley SS. Psychological stress, drug cues and cocaine craving. Psychopharmacol. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: A functional magnetic imaging study. Psychopharmacol. 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Annals of the New York Academy of Sciences. 2004;1032:254–257. doi: 10.1196/annals.1314.032. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li C-SR. Imaging stress and cue-induced drug and alcohol craving: Association to relapse and clinical implications. Drug and Alcohol Review. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Anderson GA, Cooney N, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology. 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Smolka M, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus D. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: factors controlling the reinitiation of drug seeking after abstinence. Lincoln, NE: University of Nebraska Press; 2003. [PubMed] [Google Scholar]
- Stewart JA, Tannock R. Inhibitory control differences following mild head injury. Brain Cogn. 1999;41:411–416. doi: 10.1006/brcg.1999.1141. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51:988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Tapert S, Brown G, Baratta M, Brown S. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addictive Behaviors. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert S, Cheung E, Brown G, Frank L, Paulus M, Schweinburg A, Meloy M, Brown S. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug & Alcohol Depend. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. American Journal of Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Toma K, Honda M, Hanakawa T, Okada T, Fukuyama H, Ikeda A, Nishizawa S, Konishi J, Shibasaki H. Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation: an event-related fMRI study. J Neurosci. 1999;19:3527–3534. doi: 10.1523/JNEUROSCI.19-09-03527.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak M, Jain U, Tannock R. Executive and motivational processes in adolescents with Attention-Deficit-Hyperactivity Disorder (ADHD) Behavior and Brain Function. 2005;1:8. doi: 10.1186/1744-9081-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Li T. Drug addiction: the neurobiology of behaviour gone awry. Nature Reviews Neuroscience. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, M J, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral divide of the striatum. Trends in Neurosciences. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Bisaga A, McKhann G, Kleber HD. Insula damage and quitting smoking. Science. 2007;317:318–319. doi: 10.1126/science.317.5836.318c. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow N, Fowler J, Cervany P, Hitzemann R, Pappas N, Wong C, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Science. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. American Journal of Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wilson S, Sayette M, Delgado C, Fiez J. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine and Tobacco Research. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow H. The measurement of attention. Psychology Monographs. 1914;17:1–158. [Google Scholar]
- Wrase J, Grusser S, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus D, Heinz A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Journal of the Association of European Psychiatrists. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Yazawa S, Ikeda A, Kunieda T, Ohara S, Mima T, Nagamine T, Taki W, Kimura J, Hori T, Shibasaki H. Human presupplementary motor area is active before voluntary movement: subdural recording of Bereitschaftspotential from medial frontal cortex. Exp Brain Res. 2000;131:165–177. doi: 10.1007/s002219900311. [DOI] [PubMed] [Google Scholar]
- Zalla T, Joyce C, Szoke A, Schurhoff F, Pillon B, Komano O, Perez-Diaz F, Bellivier F, Alter C, Dubois B, Rouillon F, Houde O, Leboyer M. Executive dysfunction as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry Research. 2004;121:207–217. doi: 10.1016/s0165-1781(03)00252-x. [DOI] [PubMed] [Google Scholar]
- Zink C, Pagnoni G, Chappelow J, Martin-Skurski M, Berns G. Human striatal activiation reflects degree of stimulus saliency. NeuroImage. doi: 10.1016/j.neuroimage.2005.08.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink C, Pagnoni G, Martin-Skurski M, Chappelow J, Berns G. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
- Zink C, Pagnoni G, Martin M, Dhamala M, Berns G. Human striatal response to salient nonrewarding stimuli. Journal of Neuroscience. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


