Abstract
Background
Peak exercise capacity (VO2peak) is a measure of the severity of chronic heart failure (CHF), however few indices of resting cardiopulmonary function have been shown to predict VO2peak. A prolonged circulation time has been suggested as an index of increased severity of CHF. The aim of this study was to investigate the relationship between resting lung-to-lung circulation time (LLCT) and VO2peak in CHF.
Methods
30 CHF patients (59±13 yr, NYHA: 1.9±1.0) undertook the study. Each subject completed resting pulmonary and echocardiography measures and an incremental exercise test. LLCT was measured using the reappearance of end tidal acetylene (PET,C2H2) following a single inhalation. Univariate and multivariate stepwise linear regression was used to determine the predictors of VO2peak.
Results
Univariate correlates of VO2peak (group mean: 1.53 ±0.44.l.min−1) included LLCT (r =−0.75), inspiratory capacity (r = 0.41), ejection fraction (r=0.33), peak early flow velocity (r=−0.39) and the ratio of early to late flow velocity (r=−0.31). LLCT was the only independent predictor where VO2peak=3.923–0.045 (LLCT); R2 = 54%.
Conclusion
These results suggest that resting LLCT determined using the soluble inert gas technique represents a simple, non-invasive method that provides additional information regarding exercise capacity in CHF.
INTRODUCTION
Peak oxygen consumption (VO2peak) measured during maximal cardiopulmonary exercise testing has been shown to be a strong predictor of the severity of CHF1. However the measurement of VO2peak is not routine in the clinical setting. Currently there are few indices of resting cardiopulmonary function that correlate strongly with VO2peak in CHF. Indices of resting left ventricular (LV) systolic function such as ejection fraction (EF) have been shown to be poor correlates VO2peak in this patient group.2
Recent studies have reported that indices of resting pulmonary and LV diastolic function may be better predictors of VO2peak in CHF patients3, 4 when compared to other indices of resting LV systolic function. Nannas and colleagues3 reported that inspiratory capacity (IC) was an independent predictor of VO2peak. Likewise, Meyer and colleagues4 found that resting indices of LV diastolic function such as peak early flow velocity (E), peak late flow velocity (A), the ratio of the early transmitral flow velocity to atrial flow velocity (E/A) were univariate correlates of VO2peak in CHF patients.
While these resting cardiopulmonary measures may provide some insight into the severity of disease in CHF, changes in the resting circulation time may provide additional information. The lung-to-lung circulation time (LLCT) represents the transit time for a bolus of blood through the pulmonary and systemic circulations. Given this, alterations in LLCT at rest in CHF patients may reflect not only changes in CO but also changes in blood volume and blood flow distribution.
We have recently developed a non-invasive measurement of LLCT using the soluble gas acetylene (C2H2). Using this method, we are able to determine the time taken for a bolus of C2H2 to travel from the lungs, through the systemic circulation and then back to the lungs i.e. the lung-to-lung circulation time. This technique was developed from methods for determining cardiac output (CO) using soluble gases5, where recirculation is a well-described phenomenon. While other methods for estimating circulation time exist, these either require invasive techniques6, extensive monitoring using polysomnography7 or the inhalation of hypoxic gas mixtures.8 The method we propose has advantages over others in that it is non invasive and a relatively simple maneuver for the patient to perform that does not require the inhalation of a hypoxic gas mixture. Moreover the LLCT reflects the time taken to circumnavigate the entire pulmonary and systemic circulations, rather than the arterial circulation time alone.6–8 Given this, changes in LLCT at rest for CHF patients may reflect changes in CO and blood flow distribution.
Recent studies have suggested that a prolongation or lengthening of the resting circulation time may be a potential index of underlying cardiac dysfunction in CHF, particularly in patients with central sleep apnea.7, 9–11 In a separate study Wolff et al 8 found that resting lung to ear circulation time was approximately 25% longer in 8 patients with CHF when compared with 6 healthy age matched controls.
To date, there has been no examination of the relationship between LLCT and VO2peak in CHF. We propose that LLCT may be an index of the severity of CHF and that individuals with a longer resting LLCT will have a lower VO2peak. We hypothesized that resting LLCT determined using a soluble gas method would be a independent predictor of VO2peak in patients with CHF over and above other previously published resting indices of pulmonary3 and LV diastolic function.4 To assess this hypothesis we measured VO2peak, resting LLCT, pulmonary function and resting indices of LV systolic and diastolic function of 30 CHF patients referred to the Mayo laboratory.
METHODS
Subject Details
The characteristics of the subjects that volunteered for this study are outlined in Table 1. In total, 30 subjects with CHF (NYHA I–IV) aged 27–79 years who had a clinical diagnosis of heart failure for at least 3 months participated in the study. The percentages for the broad etiological classification for heart failure were as follows: ischemic dilated cardiomyopathy (38%); idiopathic dilated cardiomyopathy (48%); valvular heart disease (10%) and other (4% i.e. one patient, secondary to radiation therapy). Details of the medications prescribed to the patients are outlined in Table 1. The Mayo Clinic Institutional review board approved the study and all subjects signed a written consent form prior to commencing.
Table 1.
Subject Characteristics and Etiology of Chronic Heart Failure. Results are mean ± SD. BMI: Body mass index; NYHA: New York Heart Association Classification. ACE: Angiotensin-converting Enzyme Inhibitor; For cardiovascular medications, data reflect the ratio of the number of patients on the medication to the total number of patients.
| Age (yr) | 59 ± 13 |
| Male/Female | 20/10 |
| BMI (kg·m−2) | 27.7 ± 7.3 |
| NYHA Functional Classes | |
| I | 14 |
| II | 7 |
| III | 7 |
| IV | 2 |
| Etiology of Heart Disease | |
| Ischemic dilated cardiomyopathy | 12 |
| Idiopathic dilated cardiomyopathy | 14 |
| Valvular heart disease | 3 |
| Other (chemotherapy) | 1 |
| Cardiovascular Medications | |
| ACE | 23/30 |
| Digoxin | 23/30 |
| Beta Blocker | 26/30 |
| Diuretic | 22/30 |
Incremental Exercise Test
Each subject completed an incremental exercise test on a treadmill to determine VO2peak. Subjects commenced walking on the treadmill at 2 mph, 0% grade for a warm up period of 3–4 min. Following the warm up period the grade and/or speed of the treadmill was increased by approximately 2 mets every 2 minutes until the subject could no longer exercise because of either exhaustion or cardiac symptoms or significant ECG or blood pressure changes.4
During the incremental exercise test, oxygen uptake (VO2) was measured breath-by-breath using a metabolic measuring system (CPX-D, Medical Graphics Corporation, St. Paul, MN, USA). The gas exchange and heart rate data were averaged over 30-s intervals.
Ideally we would have determined maximal oxygen consumption (VO2max) for each subject. The technical criteria for achieving VO2max has typically been defined as achieving two the following during the incremental exercise test: 1) a plateau in oxygen uptake (VO2) despite an increasing exercise intensity 2) a respiratory exchange ratio of 1.1 or better.12 In our study few subjects achieved the technical criteria for achieving VO2max and hence the highest VO2 measured during the final minute of the incremental exercise was designated as VO2peak. Peak exercise values were calculated as the average of the two highest consecutive 30-s values obtained prior to termination of exercise. At the completion of the test, ventilation (VE) was plotted against carbon dioxide production (VCO2) to determine the VE/VCO2 slope for each subject.
Spirometry
Forced vital capacity (FVC); forced expiratory volume in 1 second (FEV1) and IC were measured with the subject in seated position with a closed-circuit pulmonary function testing system (Medical Graphics, USA) using American Thoracic Guidelines13.
Echocardiography
All measurements were made using standard two-dimensional echocardiography with the patient in a supine position according to the recommendations of the American Society of Echocardiography.14 Left ventricular stroke volume and CO were calculated using the methods of Lewis et al15, whereas LV EF was determined using the modified Simpson’s rule.16 Transmitral inflow velocity was obtained from a 2-dimensional apical window with pulsed wave Doppler function facilitating the calculation of the E, A and E/A ratio.
Circulation Time Measurement
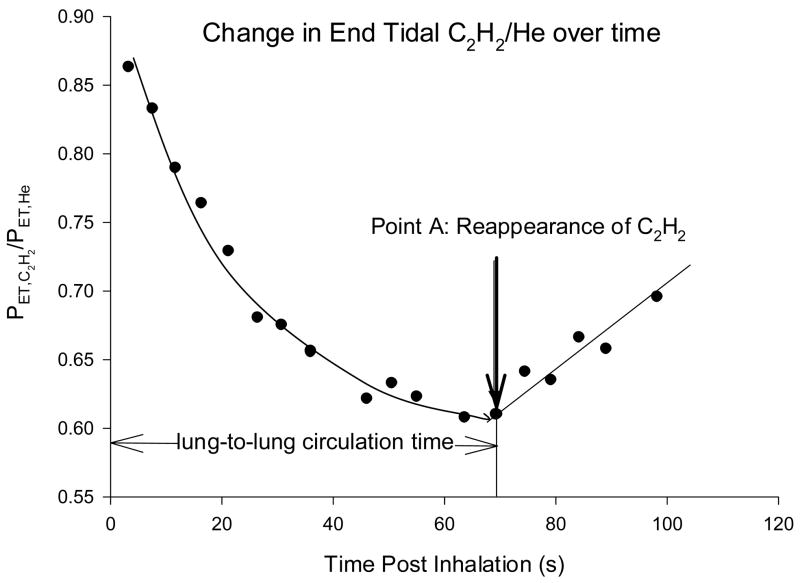
Lung-to-lung circulation time was measured using a acetylene bolus tracking method with the subject in a seated position. Each subject inhaled a single breath of a 0.65% C2H2, 9.0% Helium (He), normoxic gas mix. With end tidal gas monitoring in place, the subject then continued to breathe room air. Acetylene is an inert gas that is highly soluble in blood. Helium is an insoluble tracer gas that is used to account for any breath-by-breath variation in the distribution of C2H2 within the lung. Following inhalation, the PET,C2H2/PET,He will fall as C2H2 is carried away from the lungs via both perfusion and ventilation (Figure 1). The dissolved C2H2 is distributed through the systemic circulation and eventually appears in mixed venous blood, which causes a slight rise in alveolar (end-tidal) air, at which point the PET,C2H2/PET,He will start to rise (Figure 1). The time take for PET,C2H2/PET,He to rise following the initial inhalation is measured as the LLCT. To determine the point at which PET,C2H2/PET,He begins to rise we assumed an initial exponential decay in PET,C2H2/PET,He signal. The rise in PET,C2H2/PET,He following recirculation was assumed to be linear (Figure 1). The end tidal point representing the intersection between the exponential decay in PET,C2H2/PET,He and the linear rise in PET,C2H2/PET,He following recirculation was designated the final point prior to recirculation (Point A, Figure 1). LLCT was calculated from the difference in time at Point A to time zero (time at the peak C2H2 value during inhalation of the test gas, Figure 1). Using this particular method, the within tester reliability was r=0.92 for 28 resting LLCT assessments healthy subjects. The coefficient of variance for repeated measures of resting LLCT was 4.9 ± 2.8% (mean ± SD).
Figure 1.
Graphical representative of the change in breath-by-breath end tidal C2H2/He following a single inhalation of 0.9% C2H2, 9% He for a single subject. Following inhalation C2H2/He falls as C2H2 is distributed in the pulmonary blood volume. At the point when a significant volume of C2H2 is returned to the right side of the heart and re-enters the pulmonary circulation, C2H2/He will start to rise. The time taken from inhalation (time 0) until where C2H2/He shows a significant rise and consistent rise was calculated as being the circulation time.
Currently there is no gold standard estimate of LLCT. Previous invasive studies evaluating arterial circulation time used invasive techniques with a labeled dye (indocyanine green) introduced into the pulmonary artery and measuring the time taken for a detectable amount of dye to arrive at the pulmonary artery.6 While these techniques introduce dye directly into the pulmonary artery, they are still dependent on the distribution of the dye throughout the circulatory system, similar to the soluble gas method that we propose.
Given that we are unable to establish a gold standard measure for LLCT validation, we chose to examine whether the measurement behaved in a manner we would predict in normal subjects during exercise. We measured LLCT and CO during exercise (25%, 50%, 75% and 100% VO2peak) in 8 healthy subjects (mean age 52 ± 5 yr). Sowton et al6 reported an inverse relationship between arterial circulation time and CO during exercise. That is, as CO rose, arterial circulation time fell. Extending this finding to LLCT, we therefore expected to find a fall in LLCT as CO rises during exercise.
Statistical Analysis
All data is presented as mean ± standard deviation (SD). Individual correlates of VO2peak and LLCT were assessed using univariate linear regression. Multivariate stepwise linear regression was used to evaluate independent predictors of VO2peak. Based on our hypothesis and the findings of previous studies we chose the following univariate correlates of VO2peak: resting hemodynamics (systolic and diastolic blood pressure, EF, HR, cardiac output and LLCT); resting pulmonary function (IC, FVC, FEV1, FEV1/FVC); resting echocardiographic measures (EF, E, A and E/A) and VE/VCO2. For the stepwise linear regression, predictive variables were chosen on the basis of high univariate regression and a low level of multicollinearity.17
RESULTS
The subjects that participated in this study had stable heart failure. The majority of patients were taking Angiotensin-converting Enzyme (ACE) inhibitors, beta blockers and digoxin (Table 1). Note that while 23 patients were taking ACE inhibitors, a further 4 patients were taking angiotensin receptor blockers. Of the subjects that participated in the study, the majority (45%) were NYHA classification I, 24% NHYA classification II, 24% were NYHA classification III and 7% were NYHA classification IV.
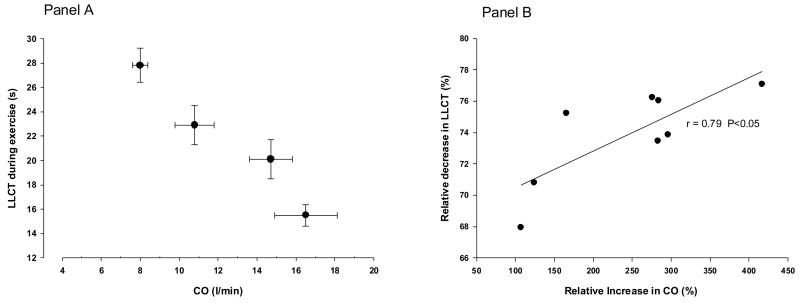
Figure 2 shows the relationship between LLCT and CO during exercise in 8 healthy subjects. Panel A clearly shows that, as CO increased there was a fall in our estimate of LLCT. Panel B shows the relative change in LLCT as a function of the relative change in CO during exercise. The relative change in LLCT was correlated (r =0.79) with the relative change in cardiac output.
Figure 2.
Panel A: Change in CO and lung-to-lung circulation time during exercise at 25%, 50%, 75% and 100% VO2peak in 8 healthy subjects; Panel B: Correlation of the relative change in CO with the relative change in lung-to-lung circulation time; Change in CO was positively correlated with the change in lung-to-lung circulation time (r=0.79, P<0.05). CO: Cardiac output; LLCT: Lung to lung circulation time.
For the 30 CHF patients, the mean VO2peak was 1.53 ±0.44.l.min−1. The mean peak oxygen uptake for the group relative to body mass was 19.1 ± 5.6 ml.kg−1.min−1. The mean peak heart rate achieved was 124 ± 22 beats.min−1 and the peak RER was 1.16 ±0.22.
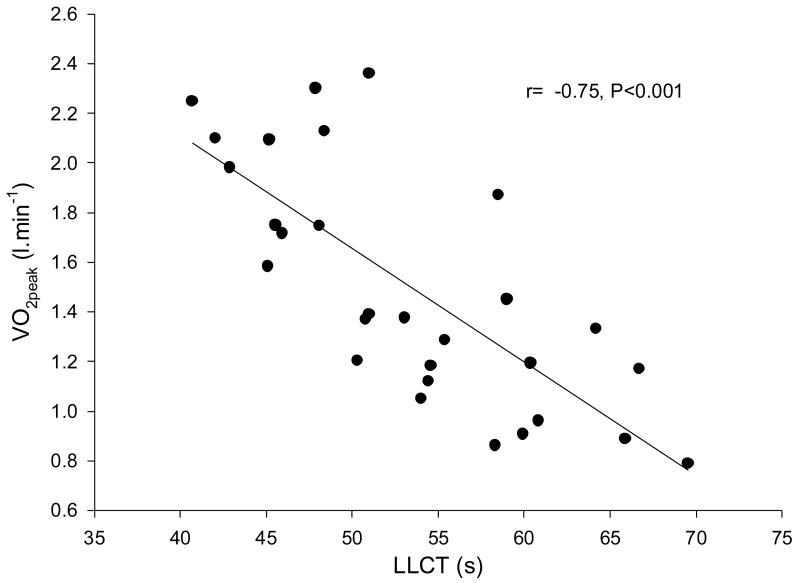
The relationship between VO2peak and LLCT is shown in Figure 3. Individuals with a longer resting LLCT generally had a poorer exercise capacity, whereas individuals with a shorter resting circulation time tended to have a greater exercise capacity. Hence we found that VO2peak was inversely related to LLCT (r=−0.75, P <0.001).
Figure 3.
Relationship between circulation time and peak exercise capacity in 30 patients with chronic hear failure. Circulation time was inversely related to peak exercise capacity, (r=−0.75, P<0.001)
Other univariate correlates of VO2peak are presented in Table 2. Apart from LLCT, significant univariate correlates of VO2peak were IC (P=0.01), EF (P=0.04), E wave (P=0.02), VE/VCO2 (P=0.04) and E/A ratio (P=0.05).
Table 2.
Univariate predictors of Peak Exercise Capacity and Lung-to-Lung Circulation Time for Chronic Heart Failure Patients. Results are mean ± SD. r: Univariate correlation coefficient; VO2peak: Peak oxygen uptake; LLCT: Lung-to-lung circulation time; NYHA: New York Heart Association Classification. SBP systolic blood pressure; DBP: Diastolic blood pressure; HR: Heart Rate; CO: Cardiac output; IC: Inspiratory capacity; FEV1: Forced expired volume in 1s; FVC: Forced vital capacity; EF: Ejection Fraction; E: Maximal early flow velocity; A: Maximal late flow velocity; E/A ratio: Maximal early to late flow velocity ratio.
| r (VO2peak) | r (LLCT) | ||
|---|---|---|---|
| Resting Hemodynamics | |||
| SBP (mmHg) | 109 ± 20 | 0.12 | −0.06 |
| DBP (mmHg) | 65 ± 11 | 0.17 | −0.29 |
| HR (beats·min−1) | 72 ± 17 | −0.29 | 0.05 |
| CO (l·min−1) | 4.6 ± 1.2 | −0.09 | −0.15 |
| LLCT | 53.7 ± 7.9 | −0.75‡ | |
| Peak Exercise Capacity | |||
| VO2peak (l·min−1) | 1.53 ± 0.44 | −0.75‡ | |
| VE/VCO2 | 36.5 ± 6.0 | −0.32* | 0.44‡ |
| Pulmonary Function | |||
| IC (l) | 2.5 ± 0.7 | 0.41* | −0.50‡ |
| FEV1 (l) | 2.8 ± 0.6 | 0.07 | −0.25 |
| FVC (l) | 3.7 ± 0.8 | 0.18 | −0.27 |
| Echocardiography | |||
| EF (%) | 27.1 ± 8.7 | 0.32* | −0.19 |
| E (m·s−1) | 0.82 ± 0.34 | −0.39* | 0.35* |
| A (m·s−1) | 0.65 ± 0.30 | 0.21 | −0.16 |
| E/A ratio | 1.8 ± 1.0 | −0.31* | 0.32* |
P<0.05;
P<0.01
Univariate correlates of LLCT are presented in Table 2. Significant univariate correlates of LLCT were VO2peak (P<0.001), IC (P=0.01), VE/VCO2 (P=0.01), E (P=0.02) and E/A (P=0.05).
Stepwise multivariate analysis indicated that the only independent predictor of VO2peak was LLCT accounting for 54% of the variance. The equation for VO2peak using LLCT as an independent predictor was:
DISCUSSION
There are two important findings from this particular study. Firstly, we demonstrated that a non-invasive soluble gas technique provides a potentially valid and reliable estimate of LLCT in CHF and that as CO increased, LLCT fell. Secondly we showed that resting LLCT is an independent predictor of VO2peak in CHF.
The advantages of the soluble gas technique is that it is non invasive and relatively simple to perform. Recent studies have measured circulation times in CHF patients using polysomnography7, 18. This method however, requires extensive monitoring and the detection of apneic episodes in order to estimate circulation time. If no apneic episodes are detected then circulation time cannot be calculated. Other methods require the inhalation of 100% N2 mixture8, which may be unsafe in severe CHF patients.
Our results suggest that resting LLCT is an independent predictor of VO2peak in CHF over and above other recently published indices of resting cardiopulmonary function.3, 4 Nanas and colleagues3 found that IC was correlated (r=0.71, P<0.001) and an independent predictor of VO2peak in 51 CHF patients (Mean NYHA classification: 2.8) who had a normal FEV1/FVC ratio. The results of the current study support those of Nannas et al3, albeit with a slightly lower univariate correlation coefficient. The finding that individuals with a lower IC tended to have a lower exercise capacity is not surprising. Typically CHF has been associated with a restrictive lung pattern19 resulting in a decrease in TLC and hence IC. The mechanism for the loss of TLC in CHF remains controversial with factors such as changes in lung fluid balance, cardiomegaly, increased central blood volume, respiratory muscle weakness and airway remodeling being cited as potential causes.20–23
However unlike Nannas et al3, we did not find that IC was an independent predictor of VO2peak in CHF. After accounting for the variance due to LLCT, we found that adding IC did not significantly improve the model for determining VO2peak. This would suggest that any variance in VO2peak due to IC is accounted for the variance in the LLCT measurements. Indeed, Table 2 shows that there was a significant correlation between LLCT and IC. The strong relationship between LLCT and VO2peak would therefore explain the fact that IC is not an independent predictor in the current study.
The results of this study are similar to those of Meyer et al4 who reported that indices of resting LV diastolic function correlated with VO2peak in CHF patients. In the current study E, E/A and EF were significantly univariate correlates of VO2peak. Indeed, Meyer et al4, showed such indices (LV chamber stiffness parameter –k, E, A. E/A) were all univariate correlates of VO2peak in CHF patients with defined LV systolic dysfunction. Of these, only the LV chamber stiffness parameter was an independent predictor of VO2peak which accounted for which for 56% of the variance. Unfortunately, we were unable to examine LV chamber stiffness and thereby make any conclusions on the relationship between this index and LLCT. Notably, there was a weak but significant relationship between LLCT and E and E/A and as such, when the variance in VO2peak due to LLCT was taken into account, the addition of either E or E/A did not alter the model for VO2peak.
Overall, our results suggest that resting LLCT is a ‘better’ predictor of exercise capacity than the other measures of pulmonary and LV diastolic function obtained in the current study. As such we may have expected to find a significant relationship between resting CO and LLCT in this patient group. However the finding that there is a relationship between resting CO and circulation time appears controversial. On one hand, Hall and colleagues7 reported an inverse relationship between lung to ear circulation time and resting CO (r=−0.72, P<0.006), in 13 individuals with central sleep apnea (8 of whom were CHF patients). Alternatively a recent study by Wolff et al8 failed to find a significant relationship between resting lung to ear circulation time and resting CO in CHF patients.
The lack of a statistical relationship between LLCT and resting CO reported in the current study indicates that factors other than resting CO may affect LLCT in CHF patients. These may include factors which may affect blood flow distribution and a variation in blood volume7, 24, 25. Recent findings that an increased blood volume is associated with a poorer hemodynamic status in CHF suggest resting blood volume may be an important prognostic indicator in CHF24, 25. Moreover blood volume has been hypothesized to contribute changes in circulation time in CHF9. One would hypothesize that for a given CO, patients with a larger blood volume would have a longer LLCT. Hence some of the variation in resting LLCT we have observed may be due to variations in blood volume in the CHF group.
While we found no statistically significant relationship between resting LLCT and CO we did find that resting indices of LV diastolic function (E and E/A) had a weak but significant relationship with LLCT. This may suggest that LLCT is more sensitive to changes in resting indices of cardiac function rather than global changes in CO per se.
Finally we note that there may have been limitations in the method used to measure CO in the particular study. While echocardiograph provides a well-defined clinical measure of CO, other more accurate methods exist such as thermo dilution. A better correlation between LLCT and CO may have been found if the CO had been measured by a more accurate method like thermo dilution.
Limitations and Future Study
We acknowledge that we have not comprehensively validated the soluble inert gas method for determining LLCT with a gold standard measure of circulation time. While other invasive measures of circulation time exist (dye dilution), these rely on similar principles as the soluble inert gas we have proposed. As such any attempt to validate the soluble inert gas with a similar method would be a comparison of two similar techniques. Another potential limitation in our study was a lack of comprehensive measurements of diastolic function such as LV stiffness. The inclusion of LV stiffness may have resulted in the development of a more comprehensive model for predicting VO2peak using resting indices of cardiopulmonary function. While we have shown a significant relationship between resting LLCT and VO2peak in CHF, future studies should explore and compare the relationship between exercising LLCT in CHF and healthy subject populations. Moreover further studies may be necessary to confirm their results in other study groups, including, for instance, higher percentages of patients with more advanced heart failure. Further insight into specific factors that affect LLCT during exercise may be elucidated with this investigation.
In conclusion, this study found that resting LLCT could be reliably measured using an soluble gas technique and that LLCT was an independent predictor of exercise capacity in CHF. Our results support the hypothesis that CHF patients LLCTs may be an index of the severity of disease and that the assessment of resting LLCT may provide additional clinical information.
Acknowledgments
The authors of this study would like to thank Kathy O’Malley and Angela Heydmann for their assistance in the data collection and management of this project. This work was supported in part by National Institute of Health grant HL71478, the National Heart Foundation and the Heart Foundation Research Centre, Griffith University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 2.Carell ES, Murali S, Schulman DS, Estrada-Quintero T, Uretsky BF. Maximal exercise tolerance in chronic congestive heart failure. Relationship to resting left ventricular function. Chest. 1994;106(6):1746–52. doi: 10.1378/chest.106.6.1746. [DOI] [PubMed] [Google Scholar]
- 3.Nanas S, Nanas J, Papazachou O, Kassiotis C, Papamichalopoulos A, Milic-Emili J, et al. Resting lung function and hemodynamic parameters as predictors of exercise capacity in patients with chronic heart failure. Chest. 2003;123(5):1386–93. doi: 10.1378/chest.123.5.1386. [DOI] [PubMed] [Google Scholar]
- 4.Meyer TE, Karamanoglu M, Ehsani AA, Kovacs SJ. Left ventricular chamber stiffness at rest as a determinant of exercise capacity in heart failure subjects with decreased ejection fraction. J Appl Physiol. 2004;97(5):1667–72. doi: 10.1152/japplphysiol.00078.2004. [DOI] [PubMed] [Google Scholar]
- 5.Triebwasser JH, Johnson RLJ, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48(3):203–209. [PubMed] [Google Scholar]
- 6.Sowton E, Bloomfield D, Jones NL, Higgs BE, Campbell EJ. Recirculation time during exercise. Cardiovasc Res. 1968;2(4):341–5. doi: 10.1093/cvr/2.4.341. [DOI] [PubMed] [Google Scholar]
- 7.Hall MJ, Xie A, Rutherford R, Ando S, Floras JS, Bradley TD. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996;154(2 Pt 1):376–81. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]
- 8.Wolff CB, Checkley SK, Bhageerutty G, Bhatt H, Johnston A, Collier DJ, et al. Circulation time in man from lung to periphery as an indirect index of cardiac output. Adv Exp Med Biol. 2005;566:311–6. doi: 10.1007/0-387-26206-7_41. [DOI] [PubMed] [Google Scholar]
- 9.Andreas S. Central sleep apnea and chronic heart failure. Sleep. 2000;23(Suppl 4):S220–3. [PubMed] [Google Scholar]
- 10.Caples SM, Wolk R, Somers VK. Influence of cardiac function and failure on sleep-disordered breathing: evidence for a causative role. J Appl Physiol. 2005;99(6):2433–9. doi: 10.1152/japplphysiol.00676.2005. [DOI] [PubMed] [Google Scholar]
- 11.Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–40. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 12.Hagberg JM. Effect of training on the decline of VO2max with aging. Federation Proc. 1987;46:1830–1833. [PubMed] [Google Scholar]
- 13.American Thoracic Society. Standardization of spirometry, 1995 update. Am Rev Respir Dis. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 14.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, feigenbaum H, et al. Recommendations for quantification of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 15.Lewis J, Kuo L, Nelson J, Limacher M, Quinones M. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation. 1984;70(3):425–431. doi: 10.1161/01.cir.70.3.425. [DOI] [PubMed] [Google Scholar]
- 16.Parisi AF, Moynihan PF, Feldman CL, Folland ED. Approaches to determination of left ventricular volume and ejection fraction by real-time two-dimensional echocardiography. Clin Cardiol. 1979;2(4):257–263. doi: 10.1002/clc.4960020404. [DOI] [PubMed] [Google Scholar]
- 17.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. McGraw Hill; Boston, MA: 1996. pp. 217–530. [Google Scholar]
- 18.Ryan CM, Bradley TD. Periodicity of obstructive sleep apnea in patients with and without heart failure. Chest. 2005;127(2):536–42. doi: 10.1378/chest.127.2.536. [DOI] [PubMed] [Google Scholar]
- 19.Wasserman K, Zhang YY, Gitt A, Belardinelli R, Koike A, Lubarsky L, et al. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96(7):2221–7. doi: 10.1161/01.cir.96.7.2221. [see comment] [DOI] [PubMed] [Google Scholar]
- 20.Olson TP, Beck KC, Johnson JB, Johnson BD. Competition for intrathoracic space reduces lung capacity in patients with chronic heart failure. Chest. 2006;129:164–71. doi: 10.1378/chest.130.1.164. [DOI] [PubMed] [Google Scholar]
- 21.Chua TP, Coats A. The lungs in chronic heart failure. Eur Heart J. 1995;16:882–67. doi: 10.1093/oxfordjournals.eurheartj.a061019. [DOI] [PubMed] [Google Scholar]
- 22.Daganou M, Dimopoulou I, Alivizatos PA, Tzelepis GE. Pulmonary function and respiratory muscle strength in chronic heart failure: comparison between ischaemic and idiopathic dilated cardiomyopathy. Heart. 1999;81(6):618–20. doi: 10.1136/hrt.81.6.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puri S, Baker BL, Oakley CM, Hughes JM, Cleland JG. Increased alveolar/capillary membrane resistance to gas transfer in patients with chronic heart failure. Br Heart J. 1994;72(2):140–4. doi: 10.1136/hrt.72.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James KB, Troughton RW, Feldschuh J, Soltis D, Thomas D, Fouad-Tarazi F. Blood volume and brain natriuretic peptide in congestive heart failure: a pilot study. Am Heart J. 2005;150(5):984. doi: 10.1016/j.ahj.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J, Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. American Journal of Cardiology. 2004;93(10):1254–9. doi: 10.1016/j.amjcard.2004.01.070. [DOI] [PubMed] [Google Scholar]