Abstract
Somatic cell gene targeting combined with nuclear transfer cloning presents tremendous potential for the creation of new, large-animal models of human diseases. Mouse disease models often fail to reproduce human phenotypes, underscoring the need for the generation and study of alternative disease models. Mice deficient for CFTR have been poor models for cystic fibrosis (CF), lacking many aspects of human CF lung disease. In this study, we describe the production of a CFTR gene–deficient model in the domestic ferret using recombinant adeno-associated virus–mediated gene targeting in fibroblasts, followed by nuclear transfer cloning. As part of this approach, we developed a somatic cell rejuvenation protocol using serial nuclear transfer to produce live CFTR-deficient clones from senescent gene-targeted fibroblasts. We transferred 472 reconstructed embryos into 11 recipient jills and obtained 8 healthy male ferret clones heterozygous for a disruption in exon 10 of the CFTR gene. To our knowledge, this study represents the first description of genetically engineered ferrets and describes an approach that may be of substantial utility in modeling not only CF, but also other genetic diseases.
Introduction
Cystic fibrosis (CF) is the most common autosomal recessive condition affecting white individuals. Although the CFTR gene — which encodes an epithelial chloride channel that is defective in CF — was cloned nearly 2 decades ago, progress toward treatments has been hindered by the lack of an animal model that reproduces the life-threatening lung infections observed in CF patients. While certain CF mouse models demonstrate phenotypic alterations in response to bacterial agarose bead challenge in the lung (1), they fail to develop the natural progression of spontaneous disease seen in humans. This is most likely due to species-specific expression of alternative chloride channels in their airways (2, 3). The domestic ferret (Mustela putorius furo) has been considered as an alternative species for modeling CF, given the high degree of similarity between ferret and human lung biology and known utility as a model for other types of lung infection, such as SARS (4) and influenza (5) virus.
The domestic ferret is an attractive alternative species to model CF for several reasons. First, ferrets and humans share a remarkably similar airway cytoarchitecture (6–8), a feature not shared by humans and mice. Second, the expression pattern of the CFTR gene is extremely similar in ferret and human airways (9, 10), with the highest levels in submucosal glands. Bioelectric and pharmacologic properties of the CFTR chloride channel in ferret airway epithelia are also similar to those seen in human airway epithelia (11). In contrast to those of mice, ferret and human tracheobronchial airways contain abundant submucosal glands that express high levels of CFTR (9, 10, 12); these glands have been shown to be critical for airway innate immunity in the ferret (13), as predicted for humans (14, 15). Gene transfer to ferret airway epithelia with several adeno-associated virus (AAV) serotypes has also been shown to be very closely conserved to that seen in human airway epithelia (16); again, a conservation not shared with mice (3). In addition, the ferret has a 42-day gestation time and reaches sexual maturity in 5–6 months (17), making it one of the more rapidly reproducing species for animal modeling by somatic cell nuclear transfer (SCNT). Together, these studies point to the potential of the ferret to be a good species on which to model CF lung disease in humans.
Since the birth of Dolly (18), the concept of combining SCNT with gene targeting in somatic cells has held tremendous potential for the development of new animal models of human disease such as CF. However, to date, this approach has failed to deliver on such potential due to technical challenges. A few laboratories have successfully applied SCNT with gene-targeting technology in livestock for agricultural and biomedical (organ transplantation and protein production) applications (19–22). However, to our knowledge, no report has yet to apply this application to generate better non-rodent disease models. The development of robust gene-targeting technologies that are compatible with SCNT have also been rate limiting, slowing progress in this area. Additionally, efficient nuclear transfer (NT) cloning procedures for potentially useful species such as ferret have lagged behind those for larger species such as sheep (8%–10%) (18, 23), cattle (10%–20%) (24, 25), and pigs (5.5%) (26). These cloning efficiencies of 5%–20% have been sufficient to generate gene-targeted sheep, cattle, and pigs (19–22). While ferret cloning from highly reprogrammable somatic cumulus cells has been reported (27), SCNT methods with fibroblasts (the best cell type for gene targeting) have yet to be developed. Thus, further optimization of fibroblast-based SCNT cloning procedures are needed to facilitate the development of genetic models in the ferret.
Here we report the production of cloned domestic ferrets heterozygous for a disrupted CFTR gene. Critical to this success was the development of recombinant AAV (rAAV) gene-targeting methods in fetal ferret fibroblasts and a serial NT cloning technique to rejuvenate senescent gene-targeted cells prior to cloning of full-term ferrets. Using this serial cloning technique, 8 healthy CFTR gene–disrupted male ferrets were obtained from 11 recipient jills. This study has demonstrated the feasibility of AAV-mediated genetic manipulation in the ferret using SCNT. Such an approach may also be of utility in genetic manipulation and disease modeling in other species.
Results
rAAV-mediated targeting of the CFTR gene in ferret fetal fibroblasts.
rAAV has been previously shown to facilitate homologous recombination between its viral DNA and cellular genomic DNA of infected fibroblasts (28). Therefore, we chose to use this virus to target the CFTR gene in ferret fibroblasts. A ferret BAC library (CHORI-237) was constructed by the BACPAC Resources Center, Children’s Hospital Oakland Research Institute, and used to isolate an approximately 150-kb genomic fragment containing exon 10 of the CFTR gene. Genomic sequences from this BAC clone were used to generate a rAAV targeting vector harboring a PGK promoter–driven neomycin resistance gene cassette flanked by sequences encompassing CFTR exon 10 and the adjacent introns (Figure 1). Male fetal fibroblasts derived from 28-day fetuses were used for CFTR gene targeting with rAAV serotype-2 (rAAV2). Fibroblasts were infected with rAAV2 virus at a multiplicity of infection of 100,000 particles per cell and subsequently serially diluted into 96-well plates and placed under G418 selection. During optimization of this procedure, we found that the number of cells seeded into each well and the timing of G418 section after plating were the most critical variables to the subcloning process, which targeted 15%–30% of wells with surviving clones by 15 days. Typically, seeding 200–500 cells per well and initiation of selection (300 μg/ml G418) at day 2 following passaging into 96-well plates was optimal.
Figure 1. CFTR gene targeting in ferret fibroblasts.
(A) Genomic fragment containing exon 10 of the ferret CFTR gene (top) and the rAAV CFTR targeting vector (bottom). Arrows mark nested primers used for PCR screening of targeting events. Restriction sites and probes used for Southern blot confirmation of targeting events are also shown. (B) Southern blot analysis of fibroblasts derived from the CL-B96 CFTR gene–targeted 21-day NT cloned ferret embryo using restriction sites and probes marked in A. The originating fibroblast line used for targeting is shown in lanes 1 and 4, while the CFTR gene targeted fibroblasts are shown in lanes 2 and 3. Arrowheads to the right of blots indicate the nontargeted (filled) and gene-targeted (open) CFTR alleles.
Following replica plating of each primary 96-well plate of selected fibroblast clones, a single plate was used for PCR screening of flanking sequences outside each targeting arm of the vector (Figure 1A). In total, approximately 500 clones were typically screened in a single experiment. Although the efficiency of gene targeting ranged from 0.5%–2% depending on the experiment, we found that the largest hurdle was the rapid senescence of PCR-positive targeted clones once they were expanded to 24-well plates. Initially, this was an obstacle that prevented direct confirmation of gene targeting by Southern blotting prior to SCNT.
Rejuvenation of PCR-positive CFTR-targeted senescent fibroblasts by SCNT.
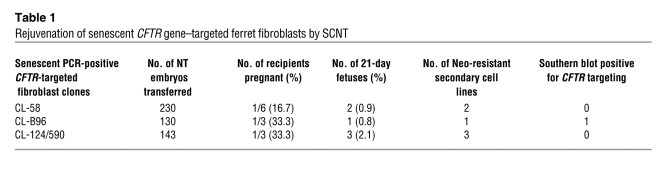
CFTR-targeted ferret fibroblast clones senesce rapidly during expansion following the initial round of PCR screening; therefore, it was necessary to develop methods for rejuvenating and expanding these candidate CFTR-targeted clones for Southern blot confirmation of the CFTR-targeting events. To this end, we performed several rounds of SCNT on 3 independent PCR-positive CFTR-targeted clones and successfully cloned six 21-day ferret fetuses by NT (Table 1). Each of these NT fetuses was dissociated with trypsin, and primary fetal fibroblasts were generated under selection with G418. DNA was then derived from each of these primary fibroblast lines and used for Southern blotting. From these cultures, one of the 3 original senescent fibroblast lines (CL-B96) gave rise to secondary NT-derived fetal fibroblasts with a “clean” CFTR gene-targeting event and no other integrations, as shown by Southern blotting (Figure 1B). The remaining 2 senescent fibroblast lines gave rise to NT-derived fetal fibroblasts that were neomycin resistant but had an insertion of the rAAV vector at a nonhomologous site in the genome. NT-derived fetal fibroblasts from the CL-B96 rejuvenated fibroblast clone were expanded to passage 5 for SCNT cloning of live-birth ferrets.
Table 1 .
Rejuvenation of senescent CFTR gene–targeted ferret fibroblasts by SCNT
SCNT cloning of CFTR-targeted ferrets from fetal fibroblasts.
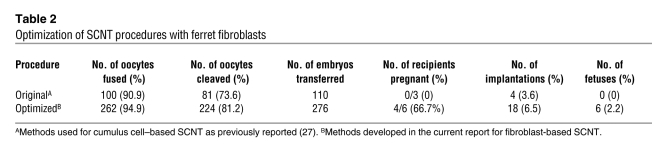
We have reported methods of SCNT cloning of ferrets using cumulus cells (27). However, in initial pilot studies, we observed that these previous methods were not compatible with efficient cloning from fetal fibroblasts. Thus, ferret SCNT protocols were optimized to resolve the major deficiencies preventing successful ferret cloning with fetal fibroblasts. Major variables included the age of recipient oocytes, alterations to media designed to reduce the stress during embryo manipulation, and optimization of the timing for oocyte activation to more efficiently promote nuclear remodeling. Using this optimized method for ferret fibroblasts, we observed a doubling in oocyte implantation as compared with the previous methods developed for cumulus cells (Table 2). This enhanced level of implantation also led to normal fetal development in 2.2% of implanted reconstructed embryos, whereas fetal development failed to occur when we used the previous methods employing cumulus cells (Table 2).
Table 2 .
Optimization of SCNT procedures with ferret fibroblasts
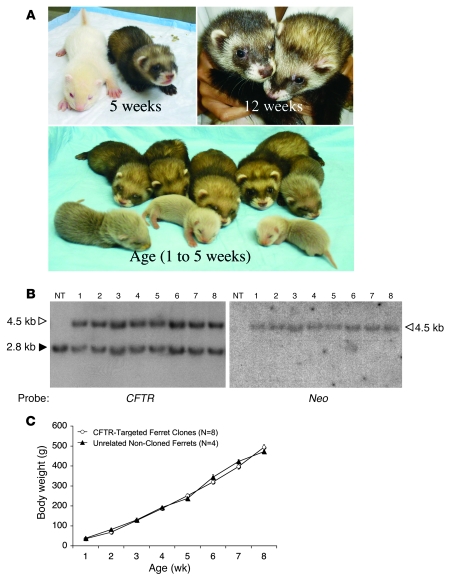
Using this improved method of SCNT with ferret fibroblasts, we proceeded to clone live-birth ferrets from the NT-rejuvenated, CFTR-targeted fibroblasts (CL-B96). In total, 11 recipient jills were each adoptively transferred with 35–60 reconstructed NT embryos derived from the CL-B96 line and allowed to develop to term (Table 3). Fourteen live pups were born by natural birth or C-section from these 11 jills. Twelve of these 14 pups appeared to be healthy at birth. In 3 cases however, the jills only nursed one pup in the litter or failed to nurse at all. Two abandoned pups were rescued by transfer to foster jills. However, it was not possible to do this in all cases of parental neglect, since surrogate jills were not always available. Two of the 14 pups born appeared weak and died; however, it was unclear whether the jills were lactating and surrogates were not available at the time. Southern blotting confirmed that all 8 of the surviving NT cloned ferrets (Figure 2A) were heterozygous for a single targeted allele of the CFTR gene (Figure 2B). Furthermore, these ferrets all remained healthy and had preweaning growth rates similar to those of noncloned pups (Figure 2C).
Table 3 .
Cloning of CFTR-targeted ferrets by SCNT
Figure 2. Cloning of CFTR-targeted ferrets.
(A) Cloned CFTR-targeted ferrets. The top left panel shows the first clone (sable coat color) at 5 weeks of age with its albino noncloned foster sibling. The other panels show sable clones with ages indicated. (B) Southern blot analysis of ear fibroblast DNA from the 8 CFTR-targeted cloned ferrets (nos. 1–8) using AflII-digested genomic DNA and the indicated CFTR and neomycin probes. NT, nontargeted unrelated ferret DNA. Arrowheads indicate the nontargeted (filled) and gene-targeted (open) CFTR alleles. (C) Preweaning growth rate of CFTR-targeted ferret clones as compared with unrelated noncloned ferrets. Results are shown as the mean ± SEM for the indicated n in each group.
Discussion
Combining rAAV-mediated gene targeting with SCNT cloning lays the foundation for numerous other applications in disease modeling with ferrets and other species (see the companion article in this issue; ref. 29). In previous reports of the generation of gene-targeted animals in pig (20, 21), cow (22), and sheep (19), linear fragments have been used to facilitate gene targeting with nonviral gene transfer methods. Important differences between these previous approaches and our current strategy are worth noting. First, gene-targeting experiments in pig and sheep fibroblasts have utilized expression from the endogenous promoter of the target gene to facilitate positive selection by insertion of an internal ribosome entry site upstream to the resistance marker gene (19–21). This was not feasible for targeting the CFTR locus, since this gene is not expressed in fibroblasts. A second strategy used to target the bovine gene encoding immunoglobulin-μ required an alternative approach, since this gene is not expressed in fibroblasts (22). In this context, a diphtheria toxin A gene was used as a negative marker to select against random integration events and led to a targeting efficiency of approximately 0.5%. This efficiency of targeting appears to be similar to that obtained in our experiments to target the ferret CFTR allele using rAAV.
Selection of gene-targeted fibroblasts can lead to rapid senescence. This was indeed the case in the ferret, where CFTR-targeted fibroblast clones rarely expanded beyond 1 × 106 cells. Factors affecting senescence appear to be linked to neomycin selection and stress induced by this process, as serial dilution cloning of nonselected fibroblast could easily be expanded to greater than 5 × 107 cells. This phenomenon may be one reason why there are few reports of gene-targeted animals produced by SCNT. Reversal of this phenotype in fibroblasts by NT, as described in this report, indicates that the causes of selection-based senescence are not permanent. Cell rejuvenation provides an alternative strategy to more efficiently produce gene-targeted animal models. Interestingly, senescence of CFTR-targeted fibroblasts was not a major obstacle in the cloning of CF pig models (see the companion article in this issue; ref. 29), suggesting that species-specific factors likely influence the biology of selection-induced senescence.
The creation of both ferret and pig CF models provides new opportunities for dissecting the pathophysiology of CF and testing of new therapies not previously approachable in mouse models of this disease. Furthermore, the fact that AAV-mediated gene targeting was successfully used to genetically engineer both ferret and pig models suggests that this technology may be generally applicable to modeling in any species. It remains unknown whether homozygous CFTR gene–disrupted ferrets will contract lung disease identical to that of humans. However, regardless of their phenotype, it is likely the field will learn much about CFTR biology from this model, as has been the case for CF mice. Under the proper light cycle, ferrets can reach sexual maturity in approximately 5–6 months. Hence, with a gestation time of 42 days, CFTR-deficient ferrets could be available in approximately 1–1.5 years. Since ferrets are a preferred model for other devastating infectious human lung diseases, such as H5N1 influenza (5) and SARS virus (4), the ability to generate genetically engineered ferrets may also be of significant utility to pandemic viral disease research.
Methods
Animals.
Ferrets were purchased from Marshall Farms. Female sable jills (virgin, 6–7 months of age) and albino jills (primipara, 9–12 months of age) were in estrus when delivered. Vasectomized male ferrets (albino, 12 months of age) were used for mating to induce follicular oocyte maturation in oocyte donor jills and to induce pseudopregnancy in NT embryo surrogate jill recipients. All ferrets were housed in separate cages under controlled temperature (20–22°C) and long daylight cycle (16 hours light/8 hours dark). Ferret chow was obtained from Marshall Farms. The use of animals in this study was carried out according to a protocol approved by the University of Iowa Institutional Animal Care and Use Committee and conformed to or exceeded NIH standards.
Collection of fetal ferret fibroblast.
Fetal ferret fibroblasts were obtained from 28-dpc fetuses derived from a sable (female) × sable (male) mating (Marshall Farms) as previously described (30). Each fetus was treated individually. Karyotype analysis was performed on each embryo line, and only male fibroblasts with a normal chromosomal profile were used for SCNT and CFTR gene targeting with rAAV.
Cloning ferret CFTR genomic DNA.
Ferret genomic DNA was extracted from internal organs collected from an E28 female fetus and used to construct a ferret genomic BAC library through the BACPAC Resources Center (http://bacpac.chori.org) at Children’s Hospital Oakland Research Institute. The average length of the ferret genomic DNA inserts in this library is approximately 150 kbp. BAC clones encompassing ferret CFTR exon 10 were isolated from this library after screening with the CFTR exon 10 probe, synthesized according to the partial ferret CFTR cDNA sequence (gb:S82688) (9). BAC DNA from the CFTR-positive clone was prepared with the ΨCLONE BAC DNA isolation kit (Princeton Separations). Sequencing of the CFTR exon 10 and adjacent introns was initiated with 2 primers located inside the CFTR exon 10 region (primers: e10F: 5′-TGATGATTATGGGAGAGTTGGAGCC-3′ and e10R: 5′-GCATGCTTTGATGACACTCCTG-3′). Primer walking was used to sequence approximately 2 kb on each side of exon 10 to generate a contig for cloning of the targeting vector. Based on the obtained CFTR genomic sequence, primers for subcloning were designed, and two 2.0-kb CFTR fragments containing exon 10 and flanking intronic sequence were retrieved from BAC DNA by PCR with AccuPrime Pfx SuperMix (Invitrogen). The PCR products were cloned into the pBlunt4PCR vector with Topo cloning kit (Invitrogen) and confirmed by sequencing. The resultant plasmids were designated as pTopo-Left and pTopo-Right, encompassing exon 10 and left-arm or right-arm introns, respectively.
Generation of CFTR-targeting proviral vector.
To construct the AAV targeting vector centered on CFTR exon 10, a 0.97-kb left homologous arm and a 1.23-kb right homologous arm were retrieved by PCR from pTopo-Left and pTopo-Right, respectively. The primer set for the left arm was: 5′-ccatcgatGGCACCCCTGTGTTATCTTTCT-3′ (forward) and 5′-ccggtacctatcaGATCCAGGAAAACTGAGAGCAG-3′ (reverse). The right-arm set was: 5′-ccatcgatgcggccgcgagctcGCCTGGCACCATCAAAGAAAAC-3′ (forward) and 5′-ggactagtggatccGATGGCCTTTCCTTTGGATGGA-3′ (reverse) (lowercase letters indicate restriction enzyme sequences introduced for cloning). The reverse primer of the left arm and forward primer of the right arm are located at the center of exon 10. The 2 PCR products together with a 1.7-kb PGK promoter–driven neomycin resistance expression cassette were assembled and finally cloned into an AAV2 proviral plasmid, giving rise to vector harboring 2.3-kb ferret CFTR genomic DNA with a neomycin cassette inserted at the center of exon 10. The rAAV2 targeting virus (AV.CFtarg) was produced as previously described using a triple plasmid transfection procedure in 293 cells and purified over an iodixanol cushion followed by ion exchange HPLC (31).
Screening for CFTR-targeted fibroblast clones.
Targeting was initiated by infecting ferret primary fibroblasts derived from a male E28 fetus with AV.CFtarg at a multiplicity of infection of 100,000 particles per cell. On day 1 following infection, fibroblasts were subsequently serially diluted into twenty 96-well plates at 200–500 cells per well. These seeding densities allowed approximately 15%–30% of wells to give rise to G418-resistent clones. Selection was initiated on day 2 following replating by the addition of 300 μg/ml G418 to the media, and cells were cultured for an additional 15 days. Typically, this screening gave rise to approximately 500 G148-resistant clones, which were subsequently expanded into 3 replica 96-well plates. Once these replica plates reached confluence, a single plate was used for PCR screening of flanking genomic sequences outside each targeting arm of the vector and anchored sequences within the vector. Nested PCR screening was then performed for the predicted left-side homologous recombination event. Cells in the 96-well plates were directly lysed with 10 μl per well of lysis buffer (50 mM KCl, 1.5 mM MgCl2, 10 mM Tris, pH 8.5, 0.5% NP40, 0.5% Tween-20), and 10% of the cell lysate (1 μl) was used for PCR with the first-round PCR primer set: F1 (5′-TGGTTTCAAGGGAATGGGGTC-3′) and intR1 (5′-AAGCGAAGGAGCAAAGCTGCTA-3′). Two percent of the first-round PCR products was then used as template for the second-round PCR with primers: F2 (5′-GGTGCAGGAGGTGTTTTGTCATAGA-3′) and intR2 (5′-GCTAAAGCGCATGCTCCAGACT-3′). The positive clones were further confirmed by another nested PCR reaction against the right arm of the integration site using the first-round primer set: intF1 (5′-CGGACCGCTATCAGGACATAG-3′) and R1 (5′-TACGAAATGCAGCAAGCGCC-3′); and the second-round nested primer set: intF2 (5′-AGGTGTCATTCTATTCTGGGG-3′) and R2 (5′-CCCAGGCATCCCTGAAACT-3′). The clones that were PCR positive for both the left and right arms of the predicted integration event were expanded to 24-well plates prior to NT rederival of 21-day embryo fibroblasts. Fibroblasts derived from cloned 21-day embryos were used for isolation of genomic DNA and Southern blot confirmation of the integration event. Genomic DNAs were digested with either AflII (which has a unique site in the targeting vector) or BamHI (which does not cut within the targeting vector). Southern blotting was visualized with a 32P-labeled CFTR probe against the 5ι upstream intronic CFTR sequence of the left homologous arm or a Neo probe against the neomycin cDNA.
Rejuvenating fibroblast lines using SCNT.
Rederival of highly proliferative fetal fibroblasts from PCR-positive CFTR gene–targeted senescent cells was accomplished using SCNT. Immature oocytes were obtained from sable jills mated with vasectomized male ferrets 24 hours prior to collection. To retrieve the oocytes, jills were euthanized by administration of pentobarbital sodium injection (50–100 mg/kg, i.p). Preovulated follicles were punctured with fine forceps in mPBS (Dulbecco PBS supplemented with 0.1% [wt/vol] d-glucose, 36 mg/l pyruvate, and 0.4% [wt/vol] BSA) to release the cumulus-oocyte complexes (COCs). COCs were cultured in TCM-199 plus 10% FBS plus 10 IU/ml equine chorionic gonadotrophin (eCG; G4527; Sigma-Aldrich) plus 5 IU/ml of human chorionic gonadotrophin (hCG; C8554; Sigma-Aldrich). For enucleation, oocytes were transferred to mPBS medium containing 7.5 μg/ml cytochalasin B (CB; C6762; Sigma-Aldrich) in the micromanipulation chamber. Using Nomarski optics, the first polar body and chromosome spindle were aspirated with a minimal volume of oocyte cytoplasm with a 20-μm (inside diameter [ID]) PeizoDrill glass pipette (Humagen). A senescent fibroblast (diameter ≥40 μm) was inserted into the perivitelline space (PVS) of enucleated oocytes using another pipette (35 μm ID). This larger-diameter pipette was specifically needed to accommodate the larger size of senescent fibroblasts. The NT-reconstructed embryos were transferred into fusion medium (0.3–0.26 M mannitol, 0.1 mM MgCl2, 0.1 mM CaCl2, 0.5 mM HEPES, 0.01% [wt/vol] BSA), placed between 2 parallel electrodes and subjected to an electrical pulse of 1 DC of 180 V/mm for 30 μs from an ECM 2001 (BTX). The fused embryos were then activated with 5 mM ionomycin for 4 minutes, then 2 mM 6-dimethylaminopurine for 3 hours. Activated embryos were cultured for 24 hours and then were transferred into pseudopregnant albino recipient jills. A pseudopregnant state was achieved in surrogate albino virgin jills through mating with a vasectomized albino male 24 hours prior to embryo transfer. At E21 the fetuses were collected and fetal fibroblasts were established as described above, except 300 μg/ml G418 was added to the media. Once expanded, these cells were then used for Southern blot screening of the CFTR gene targeting event.
Cloning of CFTR-targeted ferrets by SCNT.
The procedure described for rejuvenating fibroblast lines by SCNT was also used for cloning live-birth ferrets from Southern blot–confirmed E21 CFTR gene–targeted fibroblasts (CL-B96 clone) with minimal modification. Briefly, the same pipette was used for both enucleating the oocyte and insertion of fibroblasts into the PVS of enucleated oocytes. All other NT procedures were identical to those described above. The reconstructed embryos were transferred into albino primipara pseudopregnant jills. If natural birth did not occur at 42 days gestation, the recipients were treated with prostaglandin (Lutalyse; Pfizer; 0.5 mg to 1 mg i.m.) to induce labor. If no kits were delivered within 3 hours, 0.3 ml of oxytocin was subsequently administered to the jill. If these treatments were unsuccessful to induce labor within 8 hours, a C-section was performed. If the recipients failed to produce enough milk to feed kits, the young were fostered onto another jill when available.
Acknowledgments
The Cystic Fibrosis Foundation (J.F. Engelhardt and G.H. Leno); NIH grants HL61234 (to M.J. Welsh) and DK047967 (to J.F. Engelhardt); and the Center for Gene Therapy (DK054759) funded this research. The authors want to thank R. Scipioni Ball and her staff at Marshall Farms for their kind assistance with ferret care and reproduction. The authors also thank the personnel in the University of Iowa Animal Facility for their efforts in maintenance of the ferret colony and Shiva Patil and Heather Major for fibroblast karyotyping through the University of Iowa Children’s Hospital Cytogenetics Laboratory. We also gratefully acknowledge the assistance of Pieter J. de Jong (director) and Jeff Garnes at the BACPAC Resources Center at Children’s Hospital Oakland Research Institute for generating the ferret BAC library funded through an NHGRI-NIH White Paper Proposal (G.H. Leno and J.F. Engelhardt).
Footnotes
Nonstandard abbreviations used: AAV, adeno-associated virus; CF, cystic fibrosis; NT, nuclear transfer; rAAV, recombinant AAV; SCNT, somatic cell nuclear transfer.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:1578–1583 (2008). doi:10.1172/JCI34599.
Xingshen Sun and Ziying Yan contributed equally to this work.
References
- 1.Heeckeren A., et al. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. . J. Clin. Invest. 1997;100:2810–2815. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grubb B.R., Paradiso A.M., Boucher R.C. Anomalies in ion transport in CF mouse tracheal epithelium. Am. J. Physiol. 1994;267:C293–C300. doi: 10.1152/ajpcell.1994.267.1.C293. [DOI] [PubMed] [Google Scholar]
- 3.Liu X., Yan Z., Luo M., Engelhardt J.F. Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction. Am. J. Respir. Cell Mol. Biol. 2006;34:56–64. doi: 10.1165/rcmb.2005-0189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martina B.E., et al. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbarao K., Luke C. H5N1 viruses and vaccines. PLoS Pathog. 2007;3:e40. doi: 10.1371/journal.ppat.0030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercer R.R., Russell M.L., Roggli V.L., Crapo J.D. Cell number and distribution in human and rat airways. Am. J. Respir. Cell Mol. Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 7.Leigh M.W., et al. Postnatal development of tracheal surface epithelium and submucosal glands in the ferret. Exp. Lung Res. 1986;10:153–169. doi: 10.3109/01902148609061490. [DOI] [PubMed] [Google Scholar]
- 8.Wang X., Zhang Y., Amberson A., Engelhardt J.F. New models of the tracheal airway define the glandular contribution to airway surface fluid and electrolyte composition. Am. J. Respir. Cell Mol. Biol. 2001;24:195–202. doi: 10.1165/ajrcmb.24.2.3918. [DOI] [PubMed] [Google Scholar]
- 9.Sehgal A., Presente A., Engelhardt J.F. Developmental expression patterns of CFTR in ferret tracheal surface airway and submucosal gland epithelia. Am. J. Respir. Cell Mol. Biol. 1996;15:122–131. doi: 10.1165/ajrcmb.15.1.8679216. [DOI] [PubMed] [Google Scholar]
- 10.Engelhardt J.F., et al. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat. Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 11.Liu X., Luo M., Zhang L., Ding W., Yan Z., Engelhardt J.F. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am. J. Respir. Cell Mol. Biol. 2007;36:313–323. doi: 10.1165/rcmb.2006-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi H.K., Finkbeiner W.E., Widdicombe J.H. A comparative study of mammalian tracheal mucous glands. J. Anat. 2000;197:361–372. doi: 10.1046/j.1469-7580.2000.19730361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dajani R., et al. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am. J. Respir. Cell Mol. Biol. 2005;32:548–552. doi: 10.1165/rcmb.2005-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verkman A.S., Song Y., Thiagarajah J.R. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am. J. Physiol. Cell Physiol. 2003;284:C2–C15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- 15.Wine J.J., Joo N.S. Submucosal glands and airway defense. Proc. Am. Thorac. Soc. 2004;1:47–53. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- 16.Liu X., et al. Comparative biology of rAAV transduction in ferret, pig and human airway epithelia. Gene Ther. 2007;14:1543–1548. doi: 10.1038/sj.gt.3303014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fox, J.G., and Bell, J.A. 1998. Growth, reproduction, and breeding. InBiology and diseases of the ferret. J.G. Fox, editor. Williams & Wilkins. Baltimore, Maryland, USA. 211–227. [Google Scholar]
- 18.Wilmut I., Schnieke A.E., McWhir J., Kind A.J., Campbell K.H. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 19.McCreath K.J., et al. Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. Nature. 2000;405:1066–1069. doi: 10.1038/35016604. [DOI] [PubMed] [Google Scholar]
- 20.Lai L., et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 21.Dai Y., et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat. Biotechnol. 2002;20:251–255. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 22.Kuroiwa Y., et al. Sequential targeting of the genes encoding immunoglobulin-mu and prion protein in cattle. Nat. Genet. 2004;36:775–780. doi: 10.1038/ng1373. [DOI] [PubMed] [Google Scholar]
- 23.Schnieke A.E., et al. Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science. 1997;278:2130–2133. doi: 10.1126/science.278.5346.2130. [DOI] [PubMed] [Google Scholar]
- 24.Wells D.N., et al. Coordination between donor cell type and cell cycle stage improves nuclear cloning efficiency in cattle. Theriogenology. 2003;59:45–59. doi: 10.1016/S0093-691X(02)01273-6. [DOI] [PubMed] [Google Scholar]
- 25.Yang X., et al. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat. Genet. 2007;39:295–302. doi: 10.1038/ng1973. [DOI] [PubMed] [Google Scholar]
- 26.Walker S.C., et al. A highly efficient method for porcine cloning by nuclear transfer using in vitro-matured oocytes. Cloning Stem Cells. 2002;4:105–112. doi: 10.1089/153623002320253283. [DOI] [PubMed] [Google Scholar]
- 27.Li Z., et al. Cloned ferrets produced by somatic cell nuclear transfer. Dev. Biol. 2006;293:439–448. doi: 10.1016/j.ydbio.2006.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell D.W., Hirata R.K. Human gene targeting by viral vectors. Nat. Genet. 1998;18:325–330. doi: 10.1038/ng0498-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers C.S., et al. Production of CFTR-null and CFTR-ΔF508 heterozygous pigs by adeno-associated virus–mediated gene targeting and somatic cell nuclear transfer. . J. Clin. Invest. 2008;118:1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z., et al. Nuclear transfer of M-phase ferret fibroblasts synchronized with the microtubule inhibitor demecolcine. J. Exp. Zoolog. A Comp. Exp. Biol. 2005;303:1126–1134. doi: 10.1002/jez.a.234. [DOI] [PubMed] [Google Scholar]
- 31.Yan Z., et al. Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia. J. Biol. Chem. 2006;281:29684–29692. doi: 10.1074/jbc.M604099200. [DOI] [PMC free article] [PubMed] [Google Scholar]