Abstract
The proinflammatory cytokines interleukin (IL)-1 and tumor necrosis factor (TNF) promote HIV type 1 viral replication in vitro. In the present studies, HIV production was increased in the macrophagic U1 cell line expressing the HIV genome after exposure to IL-1β, osmotic stress, or surface adhesion, suggesting a confluence of signaling pathways for proinflammatory cytokines and cell stressors. The p38 mitogen-activated protein kinase (MAPK) mediates both cytokine and stress responses; thus the role of this kinase in HIV production was investigated. HIV production as measured by p24 antigen correlated with changes in the expression of a specific (non-alpha) isoform of p38 MAPK. In the presence of a specific p38 MAPK inhibitor (p38 inh), IL-1β-induced HIV production was suppressed by more than 90% and IL-1β-induced IL-8 production was suppressed completely, both with IC50 of 0.01 μM. p38 inhibition blocked cell-associated p24 antigen and secreted virus to a similar extent. The p38 inh also decreased constitutive HIV production in freshly infected peripheral blood mononuclear cells by up to 50% (P < 0.05). Interruption of p38 MAPK activity represents a viable target for inhibition of HIV.
Keywords: interleukin 1
Recent advances in the molecular pathogenesis of HIV type 1 infection has underscored the interaction of virus with host cytokines (1). In vitro experiments in the U1 cell line or in freshly obtained human peripheral blood mononuclear cells (PBMC) have documented increased HIV production after stimulation by proinflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF) (2). Several activities mediated by these cytokines result from activation of p38 mitogen-activated protein kinase (MAPK), for which at least five isoforms have been described (3). Synthesis of IL-1 or TNF also requires activation of the p38 MAPK in vitro or in vivo. Investigation of the functions of p38 MAPK has been aided by the development of a family of pyridinyl imidazole molecules that specifically block p38 MAPK and interrupt IL-1 and TNF signaling. In the present studies, we investigated the role of p38 MAPK and the effect of a specific inhibitor of p38 MAPK on HIV production in vitro. We used the U1 cell line, which contains two copies of incorporated HIV genome and responds to IL-1 or TNF with increased HIV expression. In addition, we studied the role of p38 MAPK in PBMC freshly infected with HIV.
MATERIALS AND METHODS
MAPK inhibitor (p38 inh) Materials.
The p38 SB 203580 (kindly supplied by John C. Lee and Peter R. Young, SmithKline Beecham) was dissolved in dimethyl sulfoxide. Ultrapure crystalline NaCl (Aldrich) was dissolved in sterile, pyrogen-free water at a stock concentration of 2 M. To inactivate pyrogens, the solution was autoclaved for 1 hr (4). Recombinant human IL-1β was kindly supplied by Sclavo, Siena, Italy. TNF-binding protein (TNFbp) was a construct of two recombinant TNF extracellular receptor p55 chains linked by polyethylene glycol (kindly supplied by Carl Edwards, Amgen). Cells were grown in RPMI 1640 tissue culture medium containing 2.5 mM l-glutamine, 25 mM Hepes (Mediatech, Herndon, VA), with 100 units/ml penicillin and 100 μg/ml streptomycin (GIBCO/BRL).
U1 Cells.
U1 cells were obtained from the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Cells were grown in T-75 polystyrene culture flasks (Corning) suspended in medium consisting of RPMI with 10% (vol/vol) heat-inactivated fetal bovine serum (Mediatech). When cell density achieved approximately 1 × 106 per ml, cells were centrifuged and resuspended in fresh medium at a density of 2 × 106 per ml. Cells were evaluated for viability by trypan blue exclusion (>95%). Five hundred microliters of cell suspension was added to either round-bottomed 12 × 75-mm polypropylene tubes (Falcon) or to 24-well flat-bottomed polystyrene plates (Falcon). The final concentration was 1 × 106 cells per ml in a volume of 1.0 ml. Stocks of IL-1β or NaCl were diluted in medium and added to cell cultures at final concentrations indicated. For NaCl, the stock solution was diluted in pyrogen-free water to 20 times the final concentration and 50 μl was added to 1.0 ml cultures. The final osmolarity of the cultures accounts for the 5% dilutional effect of the added water as described (4). The p38 inh or TNFbp was incubated with cells 1 hr before the addition of stimuli. Cultures were incubated for 24 or 48 hr at 37°C in 5% CO2, after which time they were frozen (−70°C) and thawed (37°C) for three cycles before assay for HIV p24 antigen, IL-8, or TNF. For experiments evaluating cell-associated versus secreted p24 antigen, supernatants were removed and the cell pellets were resuspended in 1.0 ml fresh medium. Resuspended cells and supernatants were frozen and thawed for three cycles and p24 was measured.
Infection of PBMC with HIV.
Studies were approved by the Combined Human Investigation Review Board of the University of Colorado Health Sciences Center. PBMC were isolated from the leukocyte-rich residual after plateletphoresis in healthy donors. Procedures for isolation and culture of PBMC were adapted from previous techniques (5). Thirty-milliliter aliquots of plateletphoresis by-product were added to 20 ml Histopaque 1077 (Sigma) in a 50-ml polypropylene tube (Falcon) and centrifuged at room temperature (RT) for 30 min at 400 × g. The PBMC layer was collected and washed twice in calcium- and magnesium-free PBS (GIBCO/BRL). Cells were resuspended at 1 × 106 per ml in R3 medium consisting of RPMI 1640 with 20% (vol/vol) fetal bovine serum (Mediatech), and 5% (vol/vol) IL-2 (Hemagen, Waltham, MA), supplemented with an additional 5% (vol/vol) IL-2 (Hemagen) and 3.3 μg/ml phytohemagglutinin-L (Sigma). After culture for 48 hr, PBMC were infected with HIV.
The lymphocytotropic azidothymadine-sensitive HIV strain AO18A (kindly provided by Daniel R. Kuritzkes) was titered as described previously (5, 6) and frozen (−70°C) until used. To infect PBMC, cells were incubated with 200 tissue culture-infective doses HIV per 1 × 106 PBMC for 3 hr at 37°C in a volume of 100 μl R3 medium in a 50-ml polypropylene tube (Falcon). Cells then were washed in 10 ml R3 medium, pelleted, and resuspended at 2 × 106 per ml. One hundred-microliter HIV-infected PBMC suspensions were aliquoted into 96-well flat-bottomed polystyrene tissue culture plates with an additional 100 μl of R3 medium or R3 medium containing p38 inh. In one well of each experiment, 1.0 μM p38 inh in a volume of 5.0 μl was added on each day of incubation. Cultures were incubated at 37°C in 5% CO2 for 4 days, after which time they were frozen (−70°C) and thawed (37°C) for three cycles before assay for HIV p24 antigen or IL-8.
p38 MAPK Western Blotting.
U1 cells were cultured at 1 × 106 per ml in polypropylene tubes for 24 hr as described above. After incubation, cells were pelleted and lysed in 100 μl culture media and 100 μl SDS sample buffer (0.0625 M Tris, pH 6.8/3% SDS/10% glycerol/0.001% bromophenol blue/0.05 M DTT). Equal aliquots of protein (approximately 100 μg) were subjected to SDS/PAGE as described by Laemmli (7), except that the separating gel consisted of 10% acrylamide and 0.13% bisacrylamide to allow better separation of the proteins (8). After electrophoresis, proteins were transferred to Immobilon-P membranes (Millipore). The membranes were blocked with Tris-buffered saline (TBS), pH 7.4, containing 0.1% Tween and 5% nonfat milk for 1 hr at RT. The membranes were then washed in TBS and incubated with 0.2 μg/ml rabbit anti-p38 MAPK antibody (C-20, Santa Cruz Biotechnology) for 2 hr at RT followed by secondary anti-rabbit antibodies coupled to horseradish peroxidase for an additional 1.0 hr. Bound horseradish peroxidase was visualized by enhanced chemiluminescence (Amersham).
Immunoassays.
Assay for HIV p24 antigen was performed by using an ELISA kit (Immunotech, Westbrook, ME). Before cytokine assay, 10% (vol/vol) Triton X-100 was added to freeze–thaw lysates (final concentration of 0.5% Triton X-100). IL-8 and TNFα were measured by using liquid-phase electrochemiluminescence assay (IGEN, Gaithersburg, MD) with a lower limit of detection of 40 pg/ml (9, 10). Triton X-100 added to the standard curves at 0.5% final concentration had no effect on the detection of IL-8 or TNFα.
Statistical Analysis.
Data are presented as means ± SEM. For data presented as percent change, the baseline (medium alone) value was subtracted from the value of each experimental condition as described in each legend. Group means are compared by ANOVA using Fisher’s least significant difference.
RESULTS
HIV Is Induced by IL-1β, Surface Adhesion, and Osmotic Stress in U1 Cells.
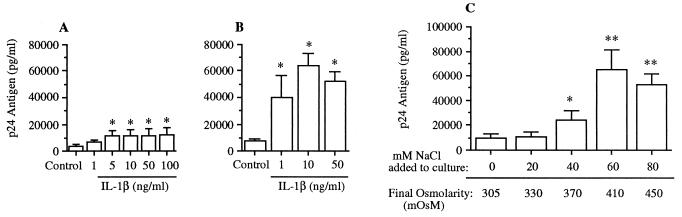
Fig. 1A shows results from U1 cells cultured in polypropylene tubes for 48 hr. Cells exposed to medium alone (control) produced 3,749 ± 1,351 pg/ml HIV p24 antigen. IL-1β added at concentrations of 1 or 5 ng/ml increased p24 levels to 6,702 ± 1,699 and 11,165 ± 3,635 pg/ml, respectively. At 10, 50, or 100 ng/ml IL-1β, there was no further significant increase in HIV production. The maximum increase in p24 production was 3.2-fold (compared with control cells), observed with IL-1β at 100 ng/ml. The data in Fig. 1B are from similar experiments performed with U1 cells cultured in polystyrene wells. Unstimulated cells produced 7,557 ± 1,760 pg/ml p24 antigen. The addition of IL-1β at concentrations of 1, 10, or 50 ng/ml resulted in p24 production of 39,673 ± 16,747, 63,989 ± 9,208, and 52,389 ± 18,411 pg/ml, respectively. There was an 8.5-fold increase in p24 production compared with control cultures by 10 ng/ml IL-1β. Thus, surface adhesion potentiates the stimulatory effect of IL-1β on HIV production.
Figure 1.
Production of HIV p24 antigen in U1 cells exposed to IL-1β or hyperosmolarity. In A and B, U1 cells were incubated for 48 hr in medium alone (control) or with medium containing IL-1β at the concentrations indicated. In A, experiments were performed in polypropylene tubes, whereas in B, experiments were performed in polystyrene wells. Total HIV p24 antigen (mean ± SEM) was measured by ELISA. Data are derived from three experiments in A or from five to seven experiments in B. ∗, P < 0.05 compared with unstimulated control. (C) U1 cells were cultured in medium alone (final osmolarity of 305 mosM) or with added NaCl at the final osmolarity shown under the horizontal axis. After 48 hr, total p24 was measured. n = 3 separate experiments. ∗, P < 0.05; ∗∗, P < 0.01 compared with no addition of NaCl.
Previous studies in our laboratory have shown that like IL-1 and TNF, osmotic stress activates p38 MAPK (4, 11). Therefore, we examined the effect of osmotic stimulation on HIV production. As shown in Fig. 1C, HIV was up-regulated by simply increasing the osmolarity of the culture using NaCl. Cells in medium alone (control) produced 9,410 ± 3,415 pg/ml HIV p24 antigen after 48 hr of incubation in polystyrene plates. NaCl induced a dose-dependent increase in p24 antigen production. Maximum p24 production was observed in the presence of additional 60 mM NaCl (final osmolarity of 410 mosM), which resulted in 65,081 ± 16,023 pg/ml p24 antigen, a 7-fold increase compared with control cultures.
Production of HIV in U1 Cells Is Dependent on p38 MAPK.
The stimulation of HIV antigen by signals that activate p38 MAPK suggested a direct involvement of this kinase in HIV production. To examine this further, U1 cells were incubated with the specific p38 inh, SB 203580, that has been shown previously to block the activity of p38 MAPK and production of proinflammatory cytokines when stimulated by endotoxin, hyperosmolarity, or IL-1 (4, 12, 13). In three separate experiments, the inhibitor (1.0 μM) had no significant effect on cell proliferation or viability (assessed by trypan blue exclusion) when added alone or in the presence of 10 ng/ml IL-1β (data not shown).
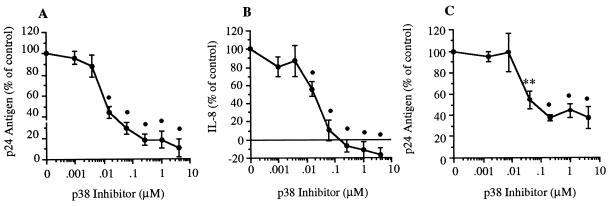
U1 cells were cultured in polypropylene tubes for 24 hr (Fig. 2 A and B) or in polystyrene plates for 48 hr (Fig. 2C). Cells were incubated in medium alone (control), with IL-1β at 10 ng/ml, or with IL-1β in the presence of the p38 inh. As shown in Fig. 2A, the p38 inh reduced IL-1β-induced p24 production in a dose-dependent manner. The maximal inhibition (90 ± 8%) was seen at an inhibitor concentration of 4.0 μM in polypropylene tubes. We also assessed IL-8 production in these cultures. As shown in Fig. 2B, p38 inh completely reduced IL-8 production compared with cells stimulated with IL-1β alone. For production of p24 antigen (Fig. 2A) and IL-8 (Fig. 2B), the 50% inhibitory concentration was approximately 0.01 μM. Control cultures (medium alone) produced 3,125 ± 391 pg/ml p24 antigen, and cultures stimulated with IL-1β produced 8,992 ± 1,509 pg/ml. For IL-8, the concentrations were 686 ± 76 pg/ml and 1,460 ± 134 pg/ml, respectively.
Figure 2.
Effect of p38 MAPK inhibition on IL-1β-induced p24 antigen and IL-8 in U1 cells. (A) U1 cells were cultured for 24 hr in the presence of IL-1β alone (10 ng/ml) or with IL-1β plus p38 inh (0.00098–4.0 μM). Total production of p24 antigen was measured after 24 hr, and results were expressed as percent change compared with stimulation with IL-1β (set at 100%). (B) Total IL-8 was measured in the same cultures as shown in A. The horizontal line above the x axis indicates 100% inhibition. Mean ± SEM p24 antigen or IL-8 production is shown (n = 7). •, P < 0.001 compared with control. (C) Cells were cultured in polystyrene wells for 48 hr in the presence of IL-1β alone (10 ng/ml) or IL-1β with serial 5-fold dilutions of p38 inh (0.0016–5.0 μM). Total production of p24 antigen was measured, and results were expressed as percent p24 change compared with cultures stimulated with IL-1β alone. Mean ± SEM p24 antigen is shown (n = 3). ∗∗, P < 0.01; •, P < 0.001 compared with control.
Fig. 2C shows similar experiments conducted in polystyrene plates. p38 inh significantly reduced HIV production in response to IL-1β stimulation. Maximal inhibition (compared with cultures with IL-1β alone) was 62.7%, in the presence of 0.2 μM p38 inh. In these experiments, p24 production was 14,143 ± 9,581 pg/ml in control (media alone), and 113,917 ± 6,384 pg/ml when stimulated with IL-1β.
Because a significant role for intermediate production of TNF in HIV synthesis in U1 cells has been demonstrated (2, 14), we measured TNF directly in IL-1β-stimulated cultures as depicted in Fig. 2 (A and B). TNF levels were below the detection limit (40 pg/ml). TNF bioactivity may be present in cultures despite the absence of detectable TNF protein. Induction of membrane-bound TNF has been observed in U1 cells, and cell surface-bound TNF may be functional by a juxtacrine mechanism despite the absence of measurable TNF (15). Therefore, we stimulated HIV synthesis in U1 cells with IL-1β in the presence or absence of the TNF inhibitor TNFbp. Addition of IL-1β to U1 cells stimulated p24 antigen production an average of 3.4-fold compared with control cultures. Coincubation of cells with TNFbp reduced p24 antigen production to 2.4-fold over stimulation with IL-1β alone, representing a 30% reduction in IL-1β-induced HIV (data not shown, n = 3). These data are consistent with a role for intermediate TNF production in IL-1β-induced HIV in U1 cells, as described by other investigators (16). However, IL-1β-induced HIV suppression by TNFbp was incomplete, suggesting a non-TNF-mediated component of IL-1β activation (see Fig. 2A).
p38 Inhibition Blocks both Cell-Associated and Secreted p24 Antigen.
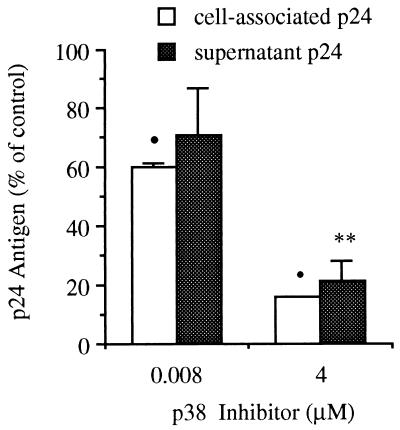
We explored the possibility of compartment-specific activity of p38 MAPK inhibition. We examined cell-associated and secreted p24 antigen production in the same cultures. These compartments reflect gene expression and virion release, respectively. After 24 hr of incubation in polypropylene tubes, supernatants were collected and the cell pellets were resuspended in fresh medium as described in Materials and Methods. Under these conditions, IL-1β-stimulated cell-associated and secreted p24 antigen concentrations were 4,044 ± 1,145 pg/ml and 2,613 ± 1,295 pg/ml, respectively. As shown in Fig. 3, compared with production after exposure to IL-1β alone (control), 4 μM p38 inh decreased production by 84.3% ± 0.3% and 78.8% ± 7.1% for cell-associated and secreted p24 concentration, respectively. p38 inh at 0.008 μM reduced stimulated p24 production by 40% ± 1.3% and 29% ± 15.4% for cell-associated and secreted p24 concentration, respectively (n = 3 separate experiments). These data demonstrate no significant differential effect of p38 inhibition on stimulated extracellular (virion) vs. intracellular (protein) production.
Figure 3.
Effect of p38 MAPK inhibition on cell-associated and secreted p24 antigen. U1 cells were cultured for 24 hr in polypropylene tubes followed by assay for cell-associated (open bars) and secreted (stippled bars) HIV p24 antigen as described in Materials and Methods. Cells were cultured in the presence of IL-1β alone (10 ng/ml, control) or with IL-1β plus p38 inh at 4 μM or 0.008 μM. Results are expressed as percent change compared with stimulation with IL-1β alone (set at 100%). n = 3 separate experiments. ∗∗, P < 0.01; •, P < 0.001 compared with IL-1β alone.
Inhibition of HIV by p38 inh Is Associated with a Decrease in a Specific p38 Isoform.
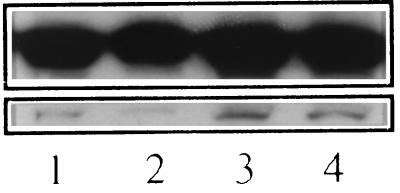
To better understand the relationship of p38 MAPK and HIV production, we examined the levels of p38 MAPK in the presence of the p38 inh. Five isoforms of p38 MAPK have been identified, of which p38α, p38β, and p38β2 are known to be inhibited by the pyridinyl imidazole class of inhibitors (3). We assessed the relative amounts of p38 protein associated with IL-1β stimulation of U1 cells by Western blotting by using an antibody raised against the C-terminal domain of the alpha form of p38 MAPK. As depicted in Fig. 4, at least 2 bands of p38 MAPK immunoreactivity were detected. The predominant upper bands (presumed to be the alpha p38 isoform) showed no notable change in association with exposure to the inhibitor. The amount of the lower bands was reduced with the addition of inhibitor, and the levels correlated with the reduced expression of HIV p24 antigen under these conditions. The addition of IL-1β increased the amount of this p38 isoform compared with media alone (control), and p38 inh blocked the IL-1β-stimulated increase in p38. Under these conditions, baseline (control)-associated and IL-1β-induced p24 antigen production were also inhibited by the presence of the p38 inh (data not shown and data presented in Fig. 2, respectively). Thus, after 24 hr of incubation in U1 cells, exposure to the p38 inh resulted in decreased levels of a p38 MAPK isoform that migrated faster than the alpha isoform on SDS gels. This p38 MAPK isoform was up-regulated by IL-1β, and its levels correlated with HIV production.
Figure 4.
Effect of p38 MAPK inhibitor on levels of specific isoforms of p38 MAPK. U1 cells (1 × 106 cells) were cultured in polypropylene tubes for 24 hr either in media alone (lane 1, control), with p38 inh (1 μM, lane 2), with IL-1β (10 ng/ml, lane 3), or with both p38 inh and IL-1β (lane 4). After incubation, cells were lysed and Western blotting was performed using an Anderson gel with anti-p38α antibody. (Upper) Bands representing the alpha isoform of p38 MAPK. (Lower) Bands resulting from a non-alpha form of p38 on the same gel.
Effect of p38 MAPK Inhibition on Constitutive HIV and IL-8 Production in PBMC.
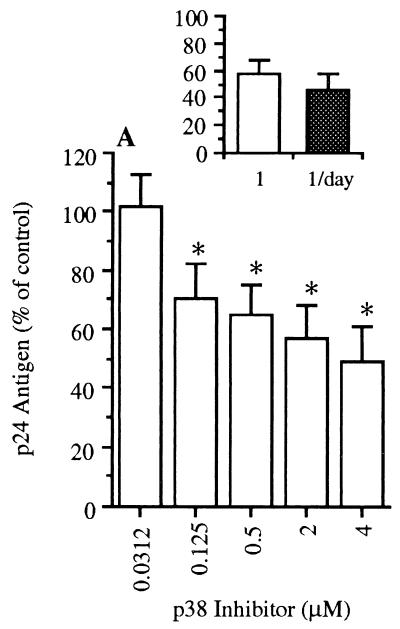
We next investigated the effect of p38 inh on HIV production in freshly infected PBMC. There was a dose-dependent inhibition of constitutive p24 production in the presence of p38 inh when added on the first day of culture. Maximum reduction was about 50% of control (medium alone), with significant inhibition at 0.125 μM. Also shown (Fig. 5 Inset) is a comparison of the effect of a single addition of the p38 inh (1.0 μM) at the time of the infection to that of adding inhibitor (1.0 μM) on each of the 4 days of culture. There was no further reduction in cultures receiving p38 inh on each day of incubation.
Figure 5.
Effect of p38 inh on production of p24 antigen in HIV-infected PBMC. After infection, PBMC were cultured in R3 medium alone (control) or with added p38 inh at the concentrations shown. Total p24 antigen was measured after 4 days, and values were expressed as percent p24 compared with control cultures (data not shown, set at 100%). Inset compares p24 antigen production in cultures exposed to p38 inh on the first day of incubation (1.0 μM) with cultures receiving inhibitor on each day of incubation (1.0 μM each day). The vertical axis of Inset represents percent production of p24 antigen compared with control (100%). Mean ± SEM p24 antigen is shown (n = 7). ∗, P < 0.05 compared with control.
IL-8 production in the same PBMC cultures was measured. In contrast to p24 production, there was no significant effect of the p38 inh on IL-8 production when added on the first day of culture. However, there was significant inhibitory effect on IL-8 production when inhibitor was added on each day compared with addition on day 1 only (61% reduction, P < 0.05, n = 3). This suggests that inhibition of p38 MAPK during the initial viral infection accounts for the decreased p24 antigen observed on day 4.
DISCUSSION
Experiments in U1 cells or PBMC have shown that stimulation of HIV production takes place after exposure to IL-1, TNF, or IL-6 (2). In this report, in addition to IL-1β induction of HIV in U1 cells, surface adhesion to polystyrene or osmotic stress also increased HIV expression. Both the magnitude and relative fold-increase in IL-1β-induced HIV compared with control were significantly greater for cells cultured in polystyrene compared with polypropylene. Although the mechanism for this difference is not established, other investigators have reported a role for the engagement of cell surface adhesion molecules in stimulating HIV production in vitro (17).
Activation of p38 MAPK occurs after exposure of mammalian cells to IL-1 or TNF (18, 19). p38 also is activated by osmotic stress, ultraviolet irradiation, chemical stress, and mechanical forces (4, 11, 18, 20, 21). Studies of p38 MAPK signaling have been aided by the availability of pyridinyl imidazole compounds that are highly specific inhibitors of p38 MAPK (22). These inhibitors have been shown previously to block production of proinflammatory cytokines in vitro and in vivo (4, 12, 13, 23, 24). Young et al. (13) reported that pyridinyl imidazole inhibitors reduced endotoxin-induced cytokine production in isolated human PBMC. The ability of the p38 inh to block activation of an HIV long terminal repeat reporter construct has been evaluated in HeLa cells in vitro (25). The inhibitor reduced reporter activation in response to IL-1, TNF, ultraviolet irradiation, or osmotic stress (25). However, unlike the present studies, the effects of p38 MAPK inhibition on expression of competent HIV in a macrophagic cell line or in freshly infected PBMC remained unknown. Other investigators reported a role for the p38 MAPK in HIV production in T cells (26). The constitutive production of HIV in primary human T cells and in a T cell line (Jurkat) was inhibited by an antisense p38 mRNA by about 30% after 10 days of culture in vitro. In the present studies, the addition of p38 inh to U1 cells resulted in nearly complete (90%) reduction of HIV production after 24 hr of culture (Fig. 2A). In contrast, p38 inh resulted in a 62.7% suppression of p24 antigen production in U1 cells cultured in polystyrene plates for 48 hr (Fig. 2C). Under these conditions of increased stimulation, it is possible that signaling occurred by a p38-independent mechanism or that p38 inhibition was incomplete.
A comparison of the ability of SB203580 to inhibit cell-associated versus secreted p24 antigen after IL-1β stimulation showed no significant difference (Fig. 3). Therefore, there was no selective role for p38 in viral protein production and assembly (cell-associated p24) as opposed to viral secretion (secreted p24).
To identify p38 MAPK isoforms present in U1 cells, we subjected cell lysates to electrophoresis into Anderson gels. This method allows for fine resolution of proteins by size (8). Although the antibody used in these studies is directed against the C terminus of the alpha form of p38 MAPK, at least two p38 isoforms were detected in U1 cells (Fig. 4). There was no change in the amount of the alpha form of p38 induced by IL-1β and no regulation by p38 inh. In contrast, a p38 isoform was up-regulated by IL-1β and suppressed by p38 inh in a manner consistent with changes in HIV p24 antigen (Fig. 2). Although circumstantial, we speculate that regulation of HIV production by p38 inh in U1 cells is a result of the blockade of one (or more) specific p38 isoforms. One report to date has identified specific p38 MAPK isoforms with cell function in vitro. Myocyte apoptosis or hypertrophy was associated with activity of the alpha or beta p38 isoform, respectively (27).
We also examined the effect of p38 inh on HIV production in freshly infected PBMC. Recent studies have shown that macrophages are highly productive sources of HIV infection in infected individuals, underscoring a role for these cells in disease pathogenesis (28). When added at the time of infection, p38 inh reduced p24 production dose-dependently, with a maximal effect of about 50% inhibition at 4.0 μM inhibitor. The addition of p38 inh on each of the 4 days postinfection did not increase this inhibitory effect, indicating that most of the inhibition takes place at the time of infection. There was no similar reduction by a single addition of p38 inh on IL-8 production in the same cultures. However, there was significant reduction of IL-8 production when the inhibitor was added on each of the 4 days of culture.
These studies suggest that inhibition of p38 MAPK represents a possible strategy to reduce HIV expression. Inhibition of HIV production after p38 inhibition likely occurs by both direct (viral production and assembly) and indirect (suppression of IL-1 and TNF production and activity) mechanisms. Possible therapeutic regimens could include MAPK inhibition combined with more standard HIV antiretroviral agents. The strategy of MAPK inhibition has the attractive property that HIV should not develop resistance to such inhibitory agents, because the target is a cellular signal transduction molecule and is not virally encoded.
Acknowledgments
The authors thank Michael W. Allen for technical assistance and Mary E. Rosandich, David L. Shugarts, and Russ K. Young for assistance with PBMC preparation and protocols. We also thank Dr. Carl Edwards for supplying the TNFbp and Dr. Daniel R. Kuritzkes for advice. These studies were supported by National Institutes of Health Grant AI 15614 (C.A.D.) and Department of Veterans Affairs Merit Award (K.A.H.).
ABBREVIATIONS
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- p38 inh
p38 MAPK inhibitor
- PBMC
peripheral blood mononuclear cells
- TNF
tumor necrosis factor
- TNFbp
TNF-binding protein
References
- 1.Fauci A S. Nature (London) 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 2.Poli G, Kinter A L, Vicenzi E, Fauci A S. Res Immunol. 1994;145:578–582. doi: 10.1016/s0923-2494(05)80036-7. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, McDonnell P C, Gum R J, Hand A T, Lee J C, Young P R. Biochem Biophys Res Commun. 1997;235:533–538. doi: 10.1006/bbrc.1997.6849. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro L, Dinarello C A. Proc Natl Acad Sci USA. 1995;92:12230–12234. doi: 10.1073/pnas.92.26.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson B, Coombs R W, Sannerud K, Rhame F S, Balfour H H. J Clin Micro. 1988;26:1416–1418. doi: 10.1128/jcm.26.7.1416-1418.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larder B A, Darby G, Richman D D. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 7.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 8.Anderson C, Brown P R, Gesteland R F. J Virol. 1973;12:241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deaver D R. Nature (London) 1995;377:758–760. doi: 10.1038/377758a0. [DOI] [PubMed] [Google Scholar]
- 10.Puren A J, Fantuzzi G, Gu Y, Su M S-S, Dinarello C A. J Clin Invest. 1997;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilly B C, Gaestel M, Engel K, Edixhoven M J, de Jong H R. FEBS Lett. 1996;395:133–136. doi: 10.1016/0014-5793(96)01028-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M-J, Heys J R, Landvatter S W, et al. Nature (London) 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 13.Young P, McDonnell P, Dunnington D, Hand A, Laydon J, Lee J. Agents Actions. 1993;39:C67–C69. doi: 10.1007/BF01972723. [DOI] [PubMed] [Google Scholar]
- 14.Folks T M, Clouse K A, Justement J, Rabson A, Duh E, Kehrl J H, Fauci A S. Proc Natl Acad Sci USA. 1989;86:2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barcellini W, Rizzardi G P, Marriott J B, Fain C, Shattock R J, Meroni P L, Poli G, Dalgleish A G. AIDS. 1996;10:835–842. doi: 10.1097/00002030-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Granowitz E V, Saget B M, Wang M Z, Dinarello C A, Skolnik P R. Mol Med. 1995;1:667–677. [PMC free article] [PubMed] [Google Scholar]
- 17.Shattock R J, Rizzardi G P, Hayes P, Griffin G E. J Infect Dis. 1996;174:54–62. doi: 10.1093/infdis/174.1.54. [DOI] [PubMed] [Google Scholar]
- 18.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 19.Freshney F W, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 20.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Lee J-D, Bibbs L, Ulevitch R J. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 22.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 23.Lee J C, Laydon J T, White J R. Agents Actions. 1994;41:C191–C192. [Google Scholar]
- 24.Badger A M, Bradbeer J N, Votta B, Lee J C, Adams J L, Griswold D E. J Pharmacol Exp Ther. 1996;279:1453–1461. [PubMed] [Google Scholar]
- 25.Kumar S, Orsin M J, Lee J C, McDonnell P C, Debouck C, Young P R. J Biol Chem. 1996;271:30864–30869. doi: 10.1074/jbc.271.48.30864. [DOI] [PubMed] [Google Scholar]
- 26.Cohen P S, Schmidtmayerova H, Dennis J, Dubrovsky L, Sherry B, Wang H, Bukrinsky M, Tracey K J. Mol Med. 1997;5:339–346. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Huang S, Sah V P, Ross J J, Brown J H, Han J, Chien K R. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 28.Orenstein J M, Fox C, Wahl S M. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]