Abstract
Salicylic acid-induced protein kinase (SIPK) and wounding-induced protein kinase (WIPK), two distinct members of the mitogen-activated protein (MAP) kinase family, are activated in tobacco resisting infection by tobacco mosaic virus (TMV). WIPK activation by TMV depends on the disease-resistance gene N because infection of susceptible tobacco not carrying the N gene failed to activate WIPK. Activation of WIPK required not only posttranslational phosphorylation but also a preceding rise in its mRNA and de novo synthesis of WIPK protein. The induction by TMV of WIPK mRNA and protein also occurred systemically. Its activation at the mRNA, protein, and enzyme levels was independent of salicylic acid. The regulation of WIPK at multiple levels by an N gene-mediated signal(s) suggests that this MAP kinase may be an important component upstream of salicylic acid in the signal-transduction pathway(s) leading to local and systemic resistance to TMV.
Plant disease resistance frequently is associated with the formation of necrotic lesions, known as the hypersensitive response (HR), alterations in cell wall structure at the sites of infection, increases in endogenous salicylic acid (SA) levels, and activation of a complex array of defense-related genes, including the pathogenesis-related (PR) genes (1, 2). In addition to these local responses, the uninfected portions of the plant usually develop systemic acquired resistance (SAR), which is manifested as enhanced resistance to a subsequent challenge by the initial or even unrelated pathogens (3). Activation of these defense responses usually is governed by a “gene-for-gene” interaction between a plant resistance (R) gene and a pathogen avirulence (Avr) gene, or initiated by the plant recognition of non-race-specific elicitors such as elicitins (1, 4–6).
Plant recognition of pathogens occurs either at the surface of the plasma membrane or in the cytoplasm. Recent studies have revealed that various components in the plant defense signaling pathway(s) exhibit structural and functional conservation to those identified in animals. For example, several R gene products, including the N gene [which confers resistance to tobacco mosaic virus (TMV) in tobacco], share homology with the interleukin 1 receptor and Toll protein, both of which are involved in the induction of immune responses in mammals and Drosophila, respectively (4, 5). In addition, a variety of signaling events, such as Ca2+ flux, H2O2 burst generated by the activation of an NADPH oxidase, protein phosphorylation/dephosphorylation, and generation of oxylipin signaling molecules, have been associated with the induction of plant and animal defense responses (1, 2, 4, 7).
Protein kinases and phosphatases have been implicated, through the use of their inhibitors, in the induction of several defense responses including medium alkalization, reactive oxygen species generation, defense gene activation, and hypersensitive cell death (8–14). Kinase activities with characteristics of protein kinase C or mitogen-activated protein (MAP) kinase have been associated with these processes (13, 15, 16). The MAP kinase cascade is one of the major pathways by which extracellular stimuli are transduced into intracellular responses in yeast and mammalian cells (17–19). In mammals, two of the three subgroups of the MAP kinase family, the stress-activated protein kinase/Jun N-terminal kinase and the p38 kinase, are activated in response to various stress signals, including UV and ionizing radiation, hyperosmolarity, oxidative stress, and cytokines (19).
A variety of MAP kinase genes have been isolated by PCR-based homology cloning from several plant species (20, 21). In addition, several kinase activities believed to be MAP kinases, based on the fact that they preferentially phosphorylate myelin basic protein (MBP) and are themselves phosphorylated on tyrosine residues upon activation, have been shown to be activated by stress stimuli. These include the tobacco wounding (cutting)-activated 46-kDa kinase (22, 23), the fungal elicitor-activated 47-kDa kinase from tobacco (13), the harpin-activated 49-kDa kinase from tobacco (24), and the wounding-, systemin- and oligosaccharide-activated 48-kDa kinase from tomato (25). The wounding-activated 46-kDa protein kinase was believed to be encoded by WIPK, a member of tobacco MAP kinase family, because this gene is rapidly induced at the mRNA level by wounding (22). Studies using an antibody against the C-terminal peptide of the alfalfa MMK4 have linked the alfalfa MMK4 to cold, drought, and mechanical stresses (26, 27). The same antibody was also used to demonstrate that parsley ERMK may encode the 45-kDa MBP kinase activated by Pep25 elicitor derived from the Phytophthora sojae glycoprotein elicitor (15).
A 48-kDa SA-induced protein kinase, termed SIPK, was identified in tobacco, and its corresponding gene has been cloned by using peptide sequences obtained by microsequencing of the purified protein (28). This MAP kinase recently was shown to be activated by various fungal elicitors (29) and also by wounding (30). In this report, we demonstrated that TMV infection also activates the 48-kDa SIPK and, in addition, a 44-kDa kinase in tobacco plants carrying the resistance gene N. By using a WIPK-specific antibody, this 44-kDa kinase was shown to be encoded by WIPK. In contrast to SIPK and MAP kinases from yeast and mammals, activation of WIPK is preceded by a rise in mRNA levels and de novo synthesis of WIPK protein. Based on the discovery that WIPK gene activation is N gene-dependent, systemic, and SA-independent, we suspect that WIPK may be an important signaling component upstream of SA in both local and systemic defense responses of tobacco to TMV.
MATERIALS AND METHODS
Treatment of Tobacco.
Tobacco plants [Nicotiana tabacum cv. Xanthi nc (NN); N. tabacum cv. Xanthi (nn); and N. tabacum cv. Xanthi nc (NN)/NahG transgenic] were grown at 22°C in a growth room programmed for a 14-hr light cycle. Seven- to eight-week-old tobacco plants were either inoculated by rubbing fully expanded leaves with wet carborundum plus TMV (U1 strain, 1 μg/ml or as indicated in 50 mM phosphate buffer, pH 7.0) or buffer only (mock). After infection, plants were either maintained at 32°C for 48 hr before being transferred to 22°C for temperature shift experiments or maintained at 22°C throughout the infection in a growth chamber. Discs from the infected leaves were collected at various times, quickly frozen in liquid nitrogen, and stored at −80°C until analysis.
Preparation of Protein Extracts.
Leaf discs (4 discs, each ≈1 cm in diameter) were first ground to a fine powder in 1.5-ml microcentrifuge tubes using small, plastic pestles. After adding 0.25 ml extraction buffer (100 mM Hepes, pH 7.5/5 mM EDTA/5 mM EGTA/10 mM DTT/10 mM Na3VO4/10 mM NaF/50 mM β-glycerophosphate/1 mM phenylmethylsulfonyl fluoride/5 μg/ml antipain/5 μg/ml aprotinin/5 μg/ml leupeptin/10% glycerol/7.5% polyvinylpolypyrrolidone), the mixture was sonicated for 15 sec with a W-375 Sonicator (Heat System/Ultrasonics) fitted with a microprobe at setting 4 and 80% duty cycle. After centrifugation at 13,000 rpm for 30 min in a Microcentinfuge, supernatants were transferred into clean tubes, quickly frozen in liquid nitrogen, and stored at −80°C.
The concentration of protein extracts was determined by using the Bio-Rad protein assay kit with BSA as standard.
In-Gel Kinase Activity Assay.
The in-gel kinase activity assay was performed as described previously (28). The relative kinase activity was quantitated by using a PhosphorImager (Molecular Dynamics) and normalized to the level present at the zero time point for the 48-kDa kinase, which was given a value of 1.
Antibody Production and Immunoblot Analysis.
The peptide p44N (MADANMGAGGGQFPDFPS), which corresponds to the N terminus of the WIPK, was synthesized and conjugated to keyhole limpet hemocyanin carrier. Polyclonal antisera were raised in rabbits and purified by affinity column chromatography (Zymed). Immunoblot analysis was performed as described previously (29).
Immune-Complex Kinase Activity Assay.
For immune-complex kinase activity assay, protein extract (50 μg) was incubated with either Ab-p48N (2.5 μg) or Ab-p44N (2.5 μg) in immunoprecipitation buffer (20 mM Tris, pH 7.5/150 mM NaCl/1 mM EDTA/1 mM EGTA/1 mM Na3VO4/1 mM NaF/10 mM β-glycerophosphate/2 μg/ml antipain/2 μg/ml aprotinin/2 μg/ml leupeptin/0.1% Tween 20) at 4°C for 2 hr on a rocker. About 20 μl packed volume of protein A-agarose washed in immunoprecipitation buffer was added, and the incubation was continued for another 4 hr. Agarose bead–protein complexes were pelleted by brief centrifugation and washed three times with 1.5 ml immunoprecipitation buffer, once with immunoprecipitation buffer plus 1 M NaCl, and then three times with 1 ml kinase reaction buffer. Kinase activity in the complex (equivalent to 20 μg of starting protein) was assayed at room temperature for 20 min in a final volume of 25 μl containing 0.1 mg/ml MBP and 10 μM ATP with 1 μCi of [γ-32P]ATP. The reaction was stopped by the addition of SDS/PAGE sample loading buffer. After electrophoresis on an SDS/15% polyacrylamide gel, the phosphorylated MBP was visualized by autoradiography.
RNA Gel Blot Analysis.
RNA was extracted by using Trizol reagent (GIBCO/BRL) following the manufacturer’s instructions. Twenty micrograms of total RNA per lane was separated on 1.2% formaldehyde/agarose gels, transferred, and hybridized with random primer-labeled inserts consisting of either a full-length or a 3′ untranslated region of WIPK cDNA as described previously (29).
Treatment of Protein Extracts with Phosphatases.
Protein extracts were prepared in the absence of phosphatase inhibitors from TMV-infected leaves at 8 hr after shifting plants from 32°C to 22°C or 48 hr after infection at 22°C. Samples containing 20 μg of protein were treated with either the serine/threonine-specific phosphatase, PP-2A1 (0.25 unit in 30 μl; Upstate Biotechnology, Lake Placid, NY), or the tyrosine-specific protein phosphatase, YOP (2 units in 30 μl; NEB, Beverly, MA), for 20 min at 30°C in the presence or absence of a phosphatase inhibitor. The PP-2A1 inhibitor, okadaic acid, and YOP inhibitor, Na3VO4, were used at a concentration of 1 μM and 1 mM, respectively. After phosphatase treatment, kinase activity was detected by the in-gel kinase activity assay.
RESULTS
TMV Infection Activates the 48-kDa SIPK Along with a 44-kDa Kinase in Tobacco.
Previously, we reported the activation of SIPK by SA (28) and various fungal elicitors (29). Here, we describe our studies to determine whether infection by the viral pathogen TMV also activates SIPK and/or other kinases in the resistant tobacco cultivar Xanthi nc (NN). To more readily follow changes in kinase activity, we took advantage of the reversible, high-temperature inhibition of TMV-induced defense responses in these plants. At 32°C, TMV-infected tobacco fail to (i) produce elevated levels of SA, (ii) synthesize PR proteins, (iii) restrict virus multiplication and spread, and (iv) develop necrotic lesions. Upon shifting these plants to lower temperatures (22°C), all of the above defense responses are rapidly and strongly induced in a more synchronous manner (31).
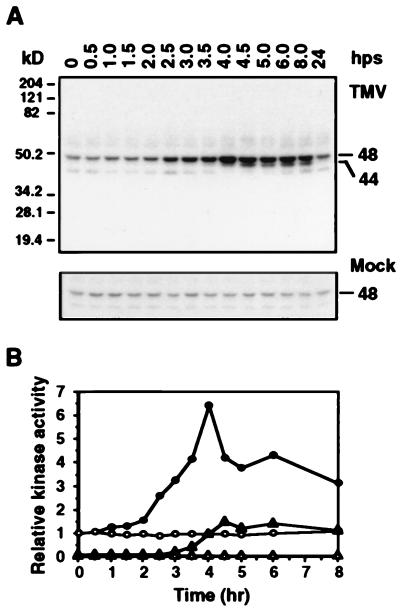
Using an in-gel kinase activity assay with MBP as the substrate (28), we observed that a 48-kDa kinase and a 44-kDa kinase were activated within 4 hr postshifting (hps) TMV- but not mock-infected plants to 22°C (Fig. 1). The size and substrate preference of the 48-kDa kinase were consistent with those of the previously identified SIPK. Moreover, this 48-kDa kinase was recognized by the SIPK-specific antibody Ab-p48N in an immune-complex kinase assay (Fig. 2A) (29), confirming its identity as SIPK.
Figure 1.
Activation of 48-kDa and 44-kDa kinases in TMV-infected tobacco. Tobacco plants carrying resistance gene N [N. tabacum cv. Xanthi nc (NN)] were inoculated with either TMV (U1 strain, 1 μg/ml in 50 mM phosphate buffer, pH 7.0) or buffer only (mock). After infection, plants were maintained at 32°C for 48 hr. Discs from the infected leaves were collected at various time after the plants were shifted back to 22°C (hps, hr postshift), and protein extracts were prepared. (A) In-gel kinase activity assay. Extracts containing 15 μg protein were electrophoresed in 10% SDS-polyacrylamide gels imbedded with 0.25 mg/ml of MBP in the separating gel. After protein renaturation, the kinase reaction was carried out as described in Materials and Methods. The sizes of activated kinases are given in kilodaltons. (B) The activities of 48-kDa kinase [in TMV-inoculated (•) and mock-inoculated (○) leaves] and 44-kDa kinase [in TMV-inoculated (▴) and mock-inoculated (▵) leaves] were quantitated by using a PhosphorImager, and the relative activities were plotted against time. Kinase activities were normalized to the level present at the zero time point for the 48-kDa kinase, which was given a value of 1.
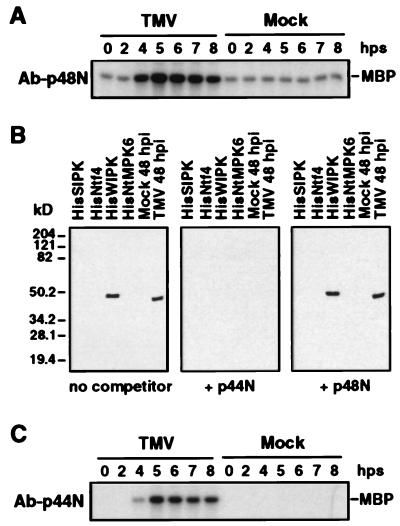
Figure 2.
Immune-complex kinase assays using sequence-specific antibodies against SIPK and WIPK. (A) Immune-complex kinase assay of TMV-activated kinase using SIPK-specific antibody, Ab-p48N. Protein extracts (50 μg) from TMV- or mock-inoculated leaf tissue were reacted with Ab-p48N (2.5 μg). The resultant antigen–antibody complex was precipitated with protein A-agarose beads and washed extensively before addition to a kinase assay mixture with [γ-32P]ATP and MBP as substrates. The reaction mixture, including the phosphorylated MBP, was then fractionated by SDS/PAGE. (B) An antibody raised against a peptide (p44N) corresponding to the unique N terminus of WIPK, Ab-p44N, specifically recognized the WIPK protein. Two nanograms each of recombinant HisSIPK, HisNtf4, HisWIPK, and HisNtMPK6 or 20 μg of protein extracts from 48-hr mock- or TMV-inoculated tobacco leaves (maintained throughout infection at 22°C) were subjected to immunoblot analysis with Ab-p44N in the absence or presence of 0.2 μg/ml competitor peptides p44N or p48N. (C) Immune-complex kinase assay of TMV-activated kinase using WIPK-specific antibody, Ab-p44N. Protein extracts (50 μg) from TMV- or mock-inoculated leaves were immunoprecipitated with Ab-p44N (2.5 μg), and the kinase activity of the immune-complex was determined as above. Times in A and C are given in hps from 32°C to 22°C.
The 44-kDa Kinase Activated by TMV Is Encoded by WIPK.
The size and substrate preference of the 44-kDa kinase suggested that it also might be a MAP kinase, possibly that encoded by WIPK. To confirm or refute this possibility, antibody was prepared in rabbits against a peptide corresponding to the unique N terminus (p44N) of WIPK and affinity purified. The specificity of the Ab-p44N was assessed by immunoblot analysis against a panel of different MAP kinases encoded by the tobacco SIPK, WIPK, Ntf4, and NtMPK6 genes, which were expressed as His-tagged fusion proteins in Escherichia coli. Ab-p44N recognized only the His-tagged WIPK protein (Fig. 2B). Addition to the immunoreaction of the competitor peptide p44N, but not the p48N (corresponding to the unique N terminus of SIPK), blocked binding of Ab-p44N to the His-tagged WIPK protein (Fig. 2B), further demonstrating the specificity of this antibody.
The WIPK-specific Ab-p44N then was employed to determine whether the TMV-induced 44-kDa kinase activity corresponded to the WIPK-encoded protein. At various times after shifting TMV- or mock-infected plants from 32°C to 22°C, protein extracts were prepared and subjected to an immune-complex kinase assay (Fig. 2C). Ab-p44N immunoprecipitated a kinase whose activity correlated with the activation kinetics of the 44-kDa kinase in TMV-infected plants (Fig. 1), thereby demonstrating that this kinase is encoded by WIPK. Interestingly, there was little, if any, WIPK activity in TMV-infected plants before shifting to 22°C, or in mock-infected plants before or after shifting (Figs. 1 and 2C).
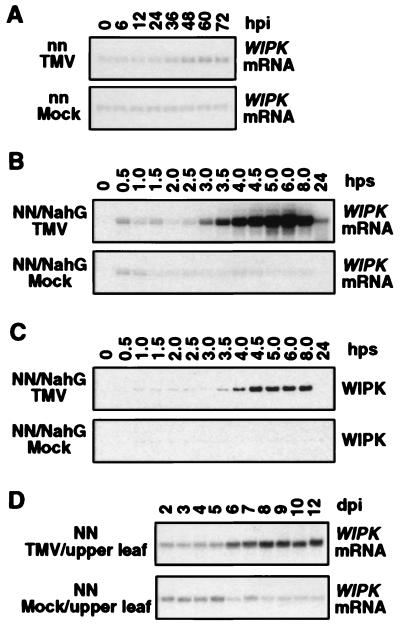
Activation of 44-kDa WIPK Is Preceded by an Increase in Its mRNA and de novo Synthesis of WIPK Protein.
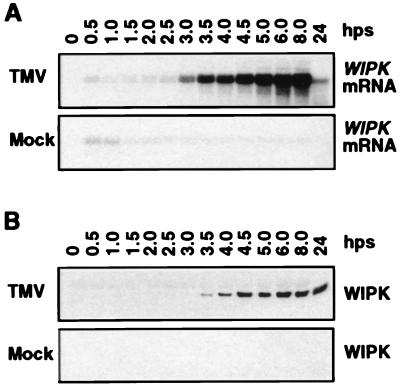
To assess the regulation of WIPK by TMV infection, its mRNA and protein levels were monitored in the inoculated leaves of temperature-shifted tobacco plants. WIPK mRNA levels increased substantially over background by 3 hps to 22°C and remained high at least until 8 hps before declining (Fig. 3A). After the increases in mRNA levels, WIPK protein began to accumulate around 3.5 hps, and the levels remained elevated throughout the time course (Fig. 3B). Increases in kinase activity also correlated with the kinetics of WIPK mRNA and protein accumulation (Figs. 1 and 3). A similarly coordinated increase in WIPK mRNA, protein, and kinase activity was detected in tobacco plants infected with TMV at 22°C and maintained at this temperature, conditions under which activation of defense responses are not blocked (Figs. 4). Thus, under two different infection conditions, WIPK activation is correlated with resistance to TMV and is regulated, at least in part, at the mRNA level. SIPK mRNA and protein levels were not altered by TMV infection (data not shown), consistent with the previous observation that SIPK is activated by other stimuli at the posttranslational level (29, 30).
Figure 3.
Activation of WIPK gene expression by TMV in tobacco plants [cv. Xanthi nc (NN)] after temperature shift. (A) Increase in steady-state levels of WIPK mRNA in TMV-infected plants. Duplicates of leaf discs used in Fig. 1 were extracted for total RNA, thus facilitating direct comparison of the induction kinetics of mRNA and enzymatic activity. Twenty micrograms of total RNA per lane was separated on 1.2% formaldehyde/agarose gels and transferred to Zeta-probe membranes. Blots were hybridized with random primer-labeled inserts consisting of either a full-length cDNA of WIPK (data shown) or its 3′ untranslated region (data not shown). Equal loading of RNA was confirmed by ethidium bromide staining of the rRNA (data not shown). (B) Increase of WIPK protein in TMV-infected tobacco after temperature shift. Samples containing 20 μg of protein from the leaf extracts used for Fig. 1A were separated on 10% SDS-polyacrylamide gels. After blotting to nitrocellulose, the WIPK protein was detected with Ab-p44N.
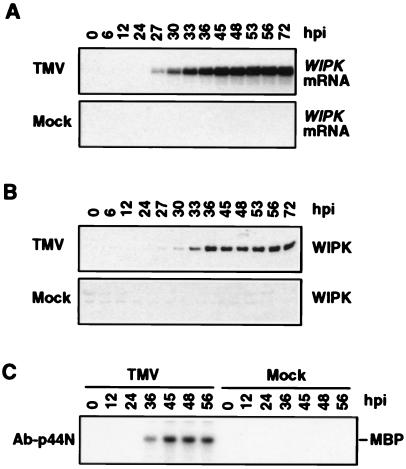
Figure 4.
Activation of WIPK by TMV in tobacco plants [cv. Xanthi nc (NN)] maintained at 22°C throughout infection. (A) Increase in steady-state levels of WIPK mRNA in TMV-infected tobacco plants. Tobacco plants were inoculated with TMV or buffer (mock) as in Fig. 1 except a higher concentration of TMV was used (5 μg/ml). Leaf discs were taken at the indicated times in hr postinoculation (hpi). Total RNA was prepared and analyzed for WIPK mRNA as described in Fig. 3. (B) Increase of WIPK protein in TMV-infected tobacco maintained at 22°C. Protein extracts were prepared from duplicate leaf discs to those used in A. Twenty micrograms of protein was analyzed by immunoblotting by using Ab-p44N as described in Fig. 3. (C) Induction of WIPK enzymatic activity in TMV-infected tobacco maintained at 22°C. Selected protein extracts from B were analyzed by immune-complex kinase assay by using WIPK-specific Ab-p44N as described in Fig. 2.
Phosphorylation of Tyrosine and Serine/Threonine Is Required for WIPK Activity.
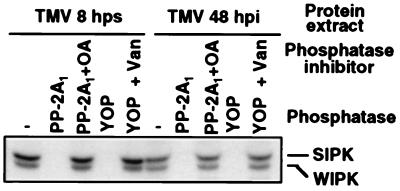
All MAP kinases characterized thus far are activated by dual phosphorylation of a TXY motif between subdomains VII and VIII of the catalytic kinase domain (18, 20, 21). This fact, plus the observations that the WIPK-encoded 44-kDa kinase activity was reduced dramatically by 24 hps compared with 8 hps (Fig. 1A) despite little, if any, change in protein level during this time period (Fig. 3B), led us to suspect that the TMV-mediated activation of WIPK also is regulated by posttranslational phosphorylation. To test this possibility, protein extracts prepared from TMV-infected plants at 8 hr after the temperature shift or at 48 hr after infection at 22°C were treated with either the serine/threonine-specific protein phosphatase PP-2A1 or the tyrosine-specific protein phosphatase YOP for 20 min before analysis by the in-gel kinase activity assay (Fig. 5). Both phosphatases inactivated the WIPK-encoded 44-kDa kinase, as well as 48-kDa SIPK, which previously was demonstrated to be regulated by posttranslational phosphorylation (28). Furthermore, inactivation of WIPK (and SIPK) by PP-2A1 and YOP could be prevented by the addition of okadaic acid, a PP-2A1 inhibitor, or vanadate, a tyrosine phosphatase inhibitor.
Figure 5.
Active 44-kDa WIPK required both threonine and tyrosine phosphorylation. Protein extracts were prepared in the absence of phosphatase inhibitors from TMV-infected leaves at 8 hr after shifting plants from 32°C to 22°C or 48 hr after infection at 22°C. Samples containing 20 μg of protein were treated with either the serine/threonine-specific phosphatase, PP-2A1 (0.25 unit in 30 μl), or the tyrosine-specific protein phosphatase, YOP (2 units in 30 μl), for 20 min at 30°C in the presence or absence of a phosphatase inhibitor. The PP-2A1 inhibitor, okadaic acid (OA), and YOP inhibitor, Na3VO4 (Van), were used at a concentration of 1 μM and 1 mM, respectively. After phosphatase treatment, kinase activity was detected by the in-gel kinase activity assay.
Activation of WIPK Is Resistance Gene N-Dependent, SA-Independent, and Systemic.
The activation of WIPK at the mRNA and posttranslational levels only under conditions in which defense responses are induced (e.g., after transfer to 22°C) suggests that this phenomenon is associated with resistance and may depend on the resistance gene N. In confirmation of this hypothesis, no increases in WIPK mRNA (Fig. 6A) or kinase activity (data not shown) were detected in TMV-inoculated leaves from the nearly isogenic Xanthi (nn) cultivar, which lacks the N gene. Interestingly, WIPK activation at the mRNA (Fig. 6B), protein (Fig. 6C), and enzymatic activity (data not shown) levels was essentially identical in wild-type Xanthi nc (NN) and transgenic Xanthi nc (NN) plants expressing the NahG gene, which encodes the SA-metabolizing enzyme salicylate hydroxylase (32). Thus, although many N gene-mediated defense responses are SA-dependent, activation of WIPK appears to be SA-independent.
Figure 6.
TMV activation of WIPK transcription in tobacco is N gene-dependent, SA-independent, and systemic. (A) WIPK mRNA induction in tobacco by TMV infection is N gene-dependent. TMV-susceptible tobacco plants [N. tabacum cv. Xanthi (nn), which lacks N resistance gene] were infected and WIPK mRNA was detected in the inoculated leaves by RNA blot analysis. (B) Induction of WIPK mRNA by TMV infection is SA-independent. Transgenic tobacco [cv. Xanthi nc (NN)] plants expressing the NahG gene were infected and WIPK mRNA was determined in the inoculated leaves by RNA gel blot analysis. (C) Increase of WIPK protein in TMV-infected NahG transgenic tobacco after temperature shift. Protein extracts were prepared from duplicate leaf discs to those used in B. Immunoblot analysis was performed as in Fig. 3. (D) Systemic induction of WIPK mRNA after TMV infection. Three leaves from each tobacco plant [cv. Xanthi nc (NN)] were either inoculated with TMV or buffer only (mock) and maintained at 22°C. At indicated days postinoculation (dpi), leaf discs were taken from the upper uninoculated leaves. Total RNA was isolated and WIPK mRNA levels were determined.
To determine whether WIPK activation is associated with SAR, another N gene-mediated phenomenon, both mRNA and protein levels were monitored in the upper uninoculated leaves of TMV-infected plants. WIPK mRNA (Fig. 6D) as well as protein (data not shown) increased over background by 6 days postinoculation (dpi). Previous analyses have demonstrated that SAR develops approximately 6 dpi (33); thus, the kinetics of WIPK induction in these uninoculated leaves is consistent with the possibility that it is involved in development of SAR.
DISCUSSION
In this study, we demonstrate that two MAP kinases, WIPK and SIPK, are activated by TMV infection of tobacco (Fig. 1). Activation of WIPK appears to be mediated by the resistance gene N because it was induced by TMV only in the tobacco cultivar carrying the N gene (Figs. 4A and 6A). Further evidence suggesting its involvement in N-mediated resistance is provided by temperature shift experiments (Figs. 1–3). N-mediated resistance to TMV is temperature-sensitive. At high temperatures (≥28°C), the TMV-infected, N gene-containing plants fail to (i) increase their SA and PR protein levels, (ii) restrict viral spread, (iii) develop necrotic lesions (31), or (iv) activate WIPK (Figs. 1–3). When these plants are shifted to lower temperatures, all of the above defense responses, including WIPK activation, are induced. Defining the precise role of WIPK in disease resistance will require alteration of WIPK expression and/or activation in transgenic plants and the identification of its substrate(s).
WIPK activation in tobacco resisting TMV infection is a multistep process. In contrast to SIPK and the MAP kinases characterized in yeast and mammals, activation of WIPK required not only posttranslational phosphorylation (Fig. 5), but also preceding increases in its mRNA and protein levels (Figs. 3 and 4). Increases in mRNA levels of several plant MAP kinases previously have been associated with their activation (15, 22, 26, 27). However, the relatively slow elevation of mRNA levels observed suggests that altered mRNA levels were not directly responsible for the very rapid increases in kinase activity detected by in-gel kinase assay. In general, changes in protein levels were not monitored and, when tested, were found not to correlate with altered mRNA levels (27). In sum, this report demonstrates that the activation of a MAP kinase by the same extracellular stimulus or stress requires multiple steps including de novo synthesis of the MAP kinase protein.
WIPK originally was isolated based on an increase in its mRNA level after wounding; it was presumed to encode a wounding-activated 46-kDa MAP kinase (22). We have confirmed that wounding transiently induces WIPK at the mRNA level. However, there is little or no increase in WIPK protein after this very transient induction of WIPK mRNA. Furthermore, using the WIPK- and SIPK-specific antibodies Ab-p44N and Ab-p48N, respectively, we have discovered that the wounding-activated kinase is the 48-kDa SIPK, not the 44-kDa WIPK (30). The absence of wounding-induced elevations in WIPK enzymatic activity suggests that WIPK is not involved in the wounding responses, despite transient mRNA induction. By contrast, during the N gene-mediated resistance response of tobacco to TMV, there is sustained induction of WIPK mRNA, followed by de novo synthesis of WIPK protein and posttranslational activation of WIPK protein by phosphorylation.
Activation of WIPK by TMV infection was similar in wild-type and NahG transgenic tobacco (Fig. 6B), suggesting that its activation is SA-independent. Whether WIPK is a component upstream of SA in an N-mediated defense signaling pathway or is involved in a SA-independent pathway currently is unclear. However, the results of studies on transgenic tobacco constitutively expressing WIPK at the mRNA level by Seo and coworker (22) bear on this question. After wounding, these transgenic plants had elevated levels of SA and PR proteins; these two responses are not induced by wounding in wild-type plants. These results were thought to be a result of altered crosstalk between wounding responses and disease defense responses in the transgenic plants. This interpretation is called into question by the discovery that the wounding-activated protein kinase is encoded by SIPK rather than WIPK (30). Nonetheless, the transgenic results suggest a connection between SA biosynthesis/PR expression and WIPK. Assuming the transgenic plants constitutively expressed WIPK protein, as well as mRNA, then perhaps the upstream MAP kinase kinase (SIPK kinase) that turned on SIPK in response to wounding may also have been able to activate WIPK. If WIPK is upstream of SA in a defense signaling pathway, its activation would result in SA biosynthesis and PR gene expression. If this scenario, which is based on the assumption that the SIPK kinase is capable of activating WIPK, is correct, then what prevents inadvertent WIPK activation by wounding? In uninfected wild-type plants, there is little or no WIPK protein to serve as a secondary substrate of the SIPK kinase, thereby preventing crosstalk between these two MAP kinase cascades. In other words, the multiple steps required for the production of active WIPK may prevent its accidental activation.
Two other stimuli, in addition to TMV, activate WIPK. They are purified proteinaceous elicitins from the fungal pathogen Phytophthora spp. and infections by the harpin-producing bacterial pathogen Pseudomonas syringae pv. syringae (unpublished data). Interestingly, both elicitin and harpin, like TMV infection, cause HR-like host cell death. In mammals, the stress-activated protein kinase/Jun N-terminal kinase subfamily of MAP kinases participates in stress-induced apoptosis (34–37). Perhaps WIPK is similarly involved in pathogen-induced plant cell death.
The similarities between defense responses activated by different R gene products, as well as the conservation of structural motifs observed in these proteins, suggest that diverse plant–pathogen interactions activate a common signal transduction pathway(s) leading to disease resistance. Given that WIPK is activated in tobacco resisting TMV infection, it seems likely that its orthologs may participate in the defense response pathway(s) initiated by R gene products found in other plant species. In addition, defense signal(s) from different R genes within a plant species, as well as non-race-specific elicitors including elicitins and harpins, may converge on the same MAP kinase cascade(s). Determination of the role(s) of these MAP kinases in R gene-mediated defense signaling may, therefore, greatly enhance our understanding of the pathway(s) leading to resistance and may provide new opportunities for enhancing disease resistance in plants.
Acknowledgments
We thank D’Maris Dempsey for assistance in preparing this manuscript. This work was supported by Grants MCB 9310371/9723952 from the National Science Foundation.
ABBREVIATIONS
- MAP
mitogen-activated protein
- MBP
myelin basic protein
- SA
salicylic acid
- SIPK
salicylic acid-induced protein kinase
- TMV
tobacco mosaic virus
- WIPK
wounding-induced protein kinase
- hps
hours postshifting
References
- 1.Hammond-Kosack K E, Jones J D G. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Shah J, Klessig D F. Genes Dev. 1997;11:1621–1639. doi: 10.1101/gad.11.13.1621. [DOI] [PubMed] [Google Scholar]
- 3.Ryals J A, Neuenschwander U H, Willits M G, Molina A, Steiner H-Y, Hunt M D. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bent A F. Plant Cell. 1996;8:1757–1771. doi: 10.1105/tpc.8.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker B, Zambryski P, Staskawicz B, Dinesh-Kumar S P. Science. 1997;276:726–733. doi: 10.1126/science.276.5313.726. [DOI] [PubMed] [Google Scholar]
- 6.Yu L M. Proc Natl Acad Sci USA. 1995;92:4088–4094. doi: 10.1073/pnas.92.10.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergey D R, Howe G, Ryan C A. Proc Natl Acad Sci USA. 1996;93:12053–12058. doi: 10.1073/pnas.93.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosskopf D G, Felix G, Boller T. FEBS Lett. 1990;275:177–180. doi: 10.1016/0014-5793(90)81466-2. [DOI] [PubMed] [Google Scholar]
- 9.Felix G, Grosskopf D G, Regenass M, Boller T. Proc Natl Acad Sci USA. 1991;88:8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felix G, Regenass M, Spanu P, Boller T. Proc Natl Acad Sci USA. 1994;91:952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viard M-P, Martin F, Pugin A, Ricci P, Blein J-P. Plant Physiol. 1994;104:1245–1249. doi: 10.1104/pp.104.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunigan D D, Madlener J C. Virology. 1995;207:460–466. doi: 10.1006/viro.1995.1105. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K, Shinshi H. Plant Cell. 1995;7:639–647. doi: 10.1105/tpc.7.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conrath U, Silva H, Klessig D F. Plant J. 1997;11:747–757. [Google Scholar]
- 15.Ligterink W, Kroj T, zur Nieden U, Hert H, Scheel D. Science. 1997;276:2054–2057. doi: 10.1126/science.276.5321.2054. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniam R, Després C, Brisson N. Plant Cell. 1997;9:653–664. doi: 10.1105/tpc.9.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herskowitz I. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 18.Seger R, Krebs E G. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 19.Kyriakis J M, Avruch J. BioEssays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 20.Hirt H. Trends Plant Sci. 1997;2:11–15. [Google Scholar]
- 21.Mizoguchi T, Ichimura K, Shinozaki K. Trends Biotechnol. 1997;15:15–19. doi: 10.1016/S0167-7799(96)10074-3. [DOI] [PubMed] [Google Scholar]
- 22.Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- 23.Usami S, Banno H, Ito Y, Nishihama R, Machida Y. Proc Natl Acad Sci USA. 1995;92:8660–8664. doi: 10.1073/pnas.92.19.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ádám A L, Pike S, Hoyos M E, Stone J M, Walker J C, Novacky A. Plant Physiol. 1997;115:853–861. doi: 10.1104/pp.115.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stratmann J W, Ryan C A. Proc Natl Acad Sci USA. 1997;94:11085–11089. doi: 10.1073/pnas.94.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonak C, Kiegeri S, Ligterink W, Barker P J, Huskisson N S, Hirt H. Proc Natl Acad Sci USA. 1996;93:11274–11279. doi: 10.1073/pnas.93.20.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bögre L, Ligterink W, Meskiene I, Barker P J, Heberle-Bors E, Huskisson N S, Hirt H. Plant Cell. 1997;9:75–83. doi: 10.1105/tpc.9.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Klessig D F. Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Du H, Klessig D F. Plant Cell. 1998;10:435–444. doi: 10.1105/tpc.10.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, S. & Klessig, D. F. (1998) Proc. Natl. Acad. Sci. USA, in press.
- 31.Malamy J, Hennig J, Klessig D F. Plant Cell. 1992;4:359–365. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- 33.Ward E R, Uknes S J, Williams S C, Dincher S S, Wiederhold D L, Aleander D C, Al-Goy P, Métraux J P, Ryals J A. Plant Cell. 1991;3:1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y-R, Meyer C F, Tan T-H. J Biol Chem. 1996;271:631–634. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- 35.Cuvillier O, Pirianov G, Kleuser B, Vanek P G, Coso O A, Gutkind S, Spiegel S. Nature (London) 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 36.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 37.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, et al. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]