Abstract
A regular heart beat is dependent on a specialized network of pacemaking and conductive cells. There has been a longstanding controversy regarding the developmental origin of these cardiac tissues which also manifest neural-like properties. Recently, we have shown conclusively that during chicken embryogenesis, impulse-conducting Purkinje cells are recruited from myocytes in spatial association with developing coronary arteries. Here, we report that cultured embryonic myocytes convert to a Purkinje cell phenotype after exposure to the vascular cytokine, endothelin. This inductive response declined gradually during development. These results yield further evidence for a role of arteriogenesis in the induction of impulse-conducting Purkinje cells within the heart muscle lineage and also may provide a basis for tissue engineering of cardiac pacemaking and conductive cells.
In the mature avian heart, pacemaking action potentials generated at the sinoatrial node (Fig. 1a), spread through the myocytes of both atria initiating contraction of these cells. Impulse from the atrial myocardium does not propagate directly into the ventricular myocardium, but instead converges on the atrioventricular node, where, after a brief delay, it is propagated rapidly through the highly coupled cells of the atrioventricular bundle. Finally, activation is spread into ventricular muscle via a subendocardial and intramural network of Purkinje fibers (1), thereby synchronizing contraction of the ventricular chambers of the heart. Until recently, little was known about the mechanisms that regulate the differentiation and patterning of this essential tissue because it had been unclear whether impulse generation and conduction tissues arose from myocyte or neural crest lineages (2). This question has been recently settled, in large part, by retroviral cell lineage studies (3) in which we showed that Purkinje fibers differentiate from a subset of beating embryonic myocytes invariably along the developing coronary arterial bed.
Figure 1.
Periarterial differentiation of Purkinje fibers in vivo and ET-dependent induction of the impulse-conducting cells in vitro. (a) Arrangement of the avian cardiac conduction system consisting of the proximal (blue) and distal (green) subcomponents. Immunolabeling of Cx42 (b and c) and sMHC (e and f) (green signals) in conducting cells, cMyBP-C (b–e, red signals) in contractile myocytes, sarcomeric myosins (d, green signals) in both cell types, and smooth muscle α-actin (f, red signal) in coronary arterial bed of the ventricular myocardium. Conversion of isolated E3 myocytes from cMyBP-C positive (red signal) to sMHC-positive (green signal) after exposure to ET (10−7M) for 1 day (g), 3 days (h), and 5 days (i). SAN, the sinuatrial node; AVN, the atrioventricular node; B, the atrioventricular bundle and bundle branches; P, the Purkinje fiber network. Asterisks indicate the lumen of arteries.
The definite mapping of Purkinje fiber progenitor cells to a myocyte lineage (3), and not to neural crest, now allows us to study the mechanisms of Purkinje fiber differentiation by analyzing the process by which heart cells are converted from a contractile to a conductile lineage. The recruitment of cells to the conduction system at periarterial sites, within clonally derived populations of differentiated myocytes, has led us to hypothesize that embryonic myocytes may be induced to form Purkinje fibers by receiving paracrine signals originating from arterial vascular tissues (3, 4). Endothelin (ET) is one such paracrine factor abundant in the arterial system of the heart and is secreted by endothelial cells in a shear stress-dependent manner (5, 6). In the embryonic heart, ET receptors are expressed ubiquitously by all myocytes (7). In this study, we examined the response of cultured embryonic myocytes to various blood vessel-associated cytokines including ET, fibroblast growth factor, and platelet-derived growth factor. Evidence is presented that beating embryonic myocytes are competent to respond to ET and can be prompted by this factor to further differentiate into Purkinje fibers.
MATERIALS AND METHODS
Cell Culture.
Myocytes were isolated from the ventricular segment of either E3, E5, or E10 chicken embryos by a conventional method and maintained as matrix-adherent cultures on four-chambered slide plates (≈2 × 104 cells/well) or as micromass suspensions by continuous rotation (65 rpm) according to the method of Clapham and coworkers (8). Either ET-1 (Sigma), Big-ET (Sigma), basic fibroblast growth factor (R & D Systems), or platelet-derived growth factor (R & D Systems) was added to cells precultured for 24 hr. Culture fluid containing these factors was then replaced every 12 hr thereafter. Control groups were treated in the same manner except that 1% BSA was used in place of the cytokines. In ET antagonist experiments, either BQ123 (Sigma) or BQ788 (American Peptide Company, Santa Clara, CA) was added with ET, bringing the final concentration of the added antagonists to 10−5 M in the culture fluid. The experiments were terminated by snap freezing the aggregates in liquid N2, storing them at −70°C until use.
Immunohistochemistry and Quantitative Analysis.
Frozen sections of hearts and cultured myocytes were fixed in methanol (−20°C) and single or double-immunolabeled, as described (3). Primary antibodies used include: an anti-Connexin42 (Cx42) polyclonal antibody (9); C315 (10), a mAb to cardiac myosin binding protein-C (cMyBP-C); ALD58, a mAb to slow myosin heavy chain (sMHC; refs. 11, 12); MF20, a mAb to sarcomeric myosin heavy chains; and an mAb to smooth muscle α-actin (Sigma). Two further polyclonal antibodies (V15KR and V15KG), with high specificities for both chicken Cx42 and its mammalian cognate Cx40, also were raised and used in this study. These probes were custom produced in rabbit and guinea pig against a peptide matching conserved residues 257–271 of rat Cx40 and 272–281 of chicken Cx42 by Research Genetics (Huntsville, AL). These new anti-Cx42 probes localized to conductive tissues in developing chicken heart in patterns identical to those we have characterized in previous studies of Cx42 (3, 9). Fluorescein- (green signal) or TexasRed- (red signal) conjugated secondary antibodies were used to visualize the primary antibodies. Myocyte aggregates were immunolabeled as whole mounts. In brief, aggregates were thawed from storage and then fixed in −20°C methanol for 1 hr. After fixation, aggregates were rehydrated in PBS containing 0.05% sodium azide (PBSA) for 5 min and then placed in blocking solution (0.1 M lysine, 0.1% of Triton X-100, and 0.05% of sodium azide in PBS) for 1 hr at room temperature on cavity slides. The aggregates were then incubated overnight in primary antibodies at room temperature. Except for extended 1-hr washing steps in PBS, bound primary antibody was subsequently detected by using standard second antibody-biotin (Amersham) and streptavidin-fluor (Amersham) localization protocols (3). Aggregates were additionally labeled with propidium iodide to delineate nuclei for cell counts. After labeling, aggregates were mounted on cavity slides with an antifade reagent (Molecular Probes, S-7461), coverslipped, and imaged with a Bio-Rad MRC-1000 laser-scanning confocal microscope. The percentages of sMHC+ myocytes were calculated from counts of sMHC+ and sMHC− myocytes in 10 aggregates from each treatment group. Within each aggregate, cells were counted in five sequential optical sections spaced at 10 μm intervals, the first optical section starting 5 μm below the top surface of each aggregate (average aggregate diameter ≈100 μm). Data was subjected to ANOVA statistical analysis, and the mean values were compared by using the students t test according to standard statistical practices.
RESULTS AND DISCUSSION
The conversion of contractile myocytes into conducting cells was monitored by probing for up-regulation of Purkinje fiber-specific gene products and for down-regulation of a myocyte-specific myofibrillar protein. Purkinje fiber-specific markers include Cx42 (9), a gap junctional isoform that functions in conduction of action potentials by Purkinje fibers (Fig. 1 b and c), and a slow twitch muscle myosin heavy chain, sMHC (3, 11, 12), uniquely present in Purkinje fibers (Fig. 1 e and f). In addition, we used a myosin-binding protein, cMyBP-C (10), as a marker for contractile myocytes. We found that cMyBP-C, a structural protein essential for normal muscle contractility (13, 14), was down-regulated when myocytes differentiated into Purkinje fibers (Fig. 1 b–e).
Beating myocytes from embryonic day 3–5 (E3–5) chicken ventricles were isolated and maintained as either substrate-adherent (Fig. 1 g and i) or suspended-aggregate micromass cultures (Fig. 2 a–f). Under these conditions, myocytes established spontaneous, rhythmic contractions within 18–20 hr. The cultures were then incubated with a recombinant ET peptide. On the first day of culture, beating myocytes were detected as cMyBP-C positive and sMHC-negative cells (Fig. 1g). Within 48–72 hr of incubation with ET, coexpression of sMHC and cMyBP-C in myocytes was observed (Fig. 1h). Over the next few days, a majority of cells became sMHC-positive (Fig. 1i). Coincident with the inductive up-regulation of Purkinje fiber-specific proteins, cMyBP-C was down-regulated significantly (Fig. 1 h and i). The ET-dependent induction of Cx42 was then examined in the micromass cultures. Because of the three-dimensional arrangement of cells in aggregates, micromass cultures were found to be a more appropriate system for studying the formation of the intermyocyte gap junctions (Fig. 2). Up-regulation of sMHC (Fig. 2 a and b) and down-regulation of cMyBP-C (Fig. 2 e and f) were both induced in myocytes exposed to ET. These changes in the aggregates occurred over a similar time course to that seen in substrate-adherent cultures. Cx42 was significantly up-regulated by ET (Fig. 2 c and d), resembling what is seen during Purkinje fiber differentiation in vivo (3).
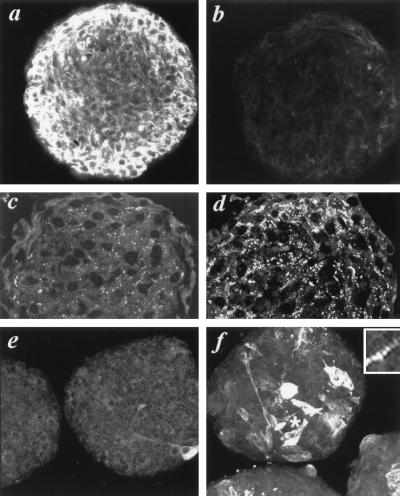
Figure 2.
Induction of Purkinje fiber phenotype in micromass cultures of embryonic myocytes. Confocal images of myocyte-micromass aggregates incubated for 48 hr in media containing no added ET (a, c, e) or 10−8 M ET (b, d, f) and immunolabeled for cMyBP-C (a, b), Cx42 (c, d), and sMHC (e, f). Inset shows the sarcomeric distribution of sMHC labeling adjacent the asterisk in a ET-treated aggregate.
sMHC-positive cells increased in response to ET in a dose-dependent manner over a range from 10−11 to 10−7 M ET (Fig. 3a). In contrast to the robust effects seen with ET, no detectable induction of Purkinje fiber phenotype was obtained in adherent cultures after exposure to its precursor big-ET (15) or by other blood vessel-associated factors, including fibroblast growth factor and platelet-derived growth factor (Fig. 3b). Hence, induction of Purkinje fiber phenotype appeared to be specific to ET. Indeed, the response to ET was neutralized by addition of either BQ123 or BQ788, selective antagonists of the ET-receptor subtypes (16), ET-A and ET-B respectively (Fig. 3c). On rare occasions, some cells in control cultures not exposed to ET were found to be sMHC-positive (e.g., Fig. 2e). The appearance of sMHC-positive cells in control cultures was not completely suppressed by addition of the receptor antagonists. These observations led us to speculate that the ET-dependent increase of cells exhibiting a Purkinje-like phenotype may have resulted from activated proliferation of cells already committed to a conductile lineage. However, as the effect of exogenous ET application was maintained in the presence of cytosine arabinoside (20 μg/ml), a potent inhibitor of cell proliferation, we concluded that this was unlikely. The exact source of these spontaneous sMHC-expressing cells remains unknown. One explanation may be a population of cells that had already been exposed to ET or precommitted by other mechanism(s) at or before isolation. Endogenous sources of ET in the embryonic tubular heart include the outflow tract where transient expression has been reported in endocardial endothelium (17).
Figure 3.
Quantitation of ET-mediated conversion of embryonic ventricular myocytes into Purkinje fibers. (a) Dose dependency of response to ET in micromass cultures of E5 myocytes. (b) Induction of Purkinje fiber cells after 5 days exposure to ET, big-ET, basic fibroblast growth factor, and platelet-derived growth factor 10−7 M in monolayer cultures of E3 myocytes. (c) Neutralization of ET-mediated (at 10−8 M) conversion of E3 myocytes in micromass culture by BQ123 (at 10−5 M) and BQ788 (at 10−5M). (d) Age-dependency of conversion after 48-hr exposure to media containing ET (10−8 M) in micromass cultures of embryonic myocytes. Purkinje fiber (Pf)/M and bars indicate means and standard errors of sMHC+ cells within MF20-positive cells, respectively.
The results outlined above show that ventricular myocytes obtained from E3–5 hearts can be induced to convert from a contractile to a conductile phenotype after exposure to ET. Because the response to ET involved a marked down-regulation of cMyBP-C (a marker for contractile myocytes), it was concluded that the observed induction was distinct from the hypertrophic growth seen in myocytes of both neonatal and adult hearts after exposure to ET (18). We suggest that responsiveness of myocytes to ET may be a distinct, developmentally regulated process. Consistent with this hypothesis, it was found that the frequency of ET-dependent conversion of myocytes from contractile to conductile Purkinje phenotype declined with progression of embryonic development (Fig. 3d). It remains to be established which downstream component in the ET-signal pathway specifies the induction of a Purkinje fiber phenotype in embryonic myocytes, distinguishing this response from other ET-dependent behaviors seen in adult myocytes.
The present study demonstrates that embryonic myocytes are competent to respond to ET, a paracrine factor prominently secreted from cells in and around the coronary arterial bed, and can be induced to exhibit a Purkinje fiber-phenotype. The results are consistent with a model in which only that subpopulation of myocytes receiving this coronary-derived paracrine signal differentiates into Purkinje fibers, whereas those free from the inductive signal remain as contractile myocytes (3, 4). Coronary stem cells, the presumed main source of later ET expression, start migration into the heart at E3 (19, 20) and initiate formation of overt coronary vessels between E7 and E10. During this latter period, coronary hemodynamics begins to alter profoundly—developing arteries form connections to the aorta, and between E10 and E14, the vascular bed closes (21). Consistent with the development of a functional coronary bed, preferential expression of Cx42 in differentiating Purkinje fibers first becomes detectable after E10 (3). Based on this time course, it is suggested that the organization of a Purkinje fiber network may be regulated by a local, shear stress-induced expression of ET from coronary arteries.
How this highly soluble cytokine confines its activity so precisely to just a few cell layers of myocytes is unknown. There may be mechanisms that neutralize the inductive activity of ET in myocyte populations distal to the artery. Alternatively, the present data suggest that this restricted patterning may result from more subtle, developmentally regulated processes. Of particular relevance is the decreased responsiveness of myocytes to ET with increasing embryonic age between E3 and E10 (Fig. 3d). Significantly, the ability of ET to mediate its effect declines most precipitously at stages (i.e., between E7 and 10) when myocytes actually start to come into contact with developing arteries. Resolution of this issue will probably have to await a more complete understanding of the interaction between the varying temporal sensitivity of myocytes to ET and the spatial distribution of active ligand in the developing embryonic heart.
Because the conduction system is vital for generating and synchronizing the heart beat, dysfunction of this essential tissue is a direct cause of arrhythmias and conduction disturbances causing sudden cardiac death. Corrected function of this specialized cardiac tissue frequently involves the implantation of artificial pacemaking devices. Although recent work suggests the feasibility of transplantational repair of contracting myocardium (22), regeneration and/or repair of cardiac conduction tissue after heart injury or congenital disease has not been considered feasible. Our data clearly indicate that embryonic myocytes have the potential to convert their phenotype into Purkinje fiber conduction cells in response to a paracrine signal. In addition to furthering the understanding of the development of specialized myocardial tissues, this study may provide an approach for replacing and/or repairing dysfunctional tissue of the cardiac conduction system.
Acknowledgments
This work was supported by grants from the National Institutes of Health, the American Heart Association, the March of Dimes Birth Defects Foundation, the Mathers Foundation, and a fellowship program of the Charles H. Revson Foundation (to Y.W.). R.G.G is a Basil O’Connor Starter Scholar of the March of Dimes Birth Defects Foundation. T.M. is an Irma T. Hirschl Scholar.
ABBREVIATIONS
- ET
endothelin
- Cx
Connexin
- sMHC
slow myosin heavy chain
References
- 1.Davies F. J Anat. 1930;64:129–146. [PMC free article] [PubMed] [Google Scholar]
- 2.Gorza L, Vettore S, Vitadello M. Trends Cardiovasc Med. 1994;4:153–159. doi: 10.1016/1050-1738(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 3.Gourdie R G, Mima T, Thompson R P, Mikawa T. Development. 1995;121:1423–1431. doi: 10.1242/dev.121.5.1423. [DOI] [PubMed] [Google Scholar]
- 4.Mikawa T, Fischman D A. Annu Rev Physiol. 1996;58:509–521. doi: 10.1146/annurev.ph.58.030196.002453. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. Nature (London) 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 6.Masaki T, Kimura S, Yanagisawa M, Goto K. Circulation. 1991;84:1457–1468. doi: 10.1161/01.cir.84.4.1457. [DOI] [PubMed] [Google Scholar]
- 7.Clouthier D E, Hosoda K. Development (Cambridge, UK) 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- 8.Clapham D E, Shrier A, DeHaan R L. J Gen Physiol. 1980;75:633–654. doi: 10.1085/jgp.75.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gourdie R G, Green C R, Severs N J, Anderson R H, Thompson R P. Circ Res. 1993;72:278–289. doi: 10.1161/01.res.72.2.278. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda M, Koshida S, Sato N, Obinata T. J Mol Cell Cardiol. 1995;27:2275–2286. doi: 10.1016/s0022-2828(95)91731-4. [DOI] [PubMed] [Google Scholar]
- 11.Sartore S, Pierobon-Bormioli S, Schiaffino S. Nature (London) 1988;274:82–83. doi: 10.1038/274082a0. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Sanchez A, Bader D. J Cell Biol. 1985;100:270–275. doi: 10.1083/jcb.100.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins H, Conner D, Thierfelder L, Jarcho J A, MacRae C, McKenna W J, Maron B J, Seidman J G, Seidman C E. Nat Genet. 1995;11:434–437. doi: 10.1038/ng1295-434. [DOI] [PubMed] [Google Scholar]
- 14.Bonne G, Carrier L, Bercovici J, Cruaud C, Richard P, Hainque B, Gautel M, Labeit S, James M, Beckmann J, et al. Nat Genet. 1995;11:438–440. doi: 10.1038/ng1295-438. [DOI] [PubMed] [Google Scholar]
- 15.Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 16.Ihara M, Noguchi K, Saeki T, Fukuroda T, Tsuchida S, Kimura S, Fukami T, Ishikawa K, Nishikibe M, Yano M. Life Sci. 1992;50:247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- 17.Kurihara Y, Kurihara H, Oda H, Maemura K, Nagai R, Ishikawa T, Yazaki Y. J Clin Invest. 1995;96:293–300. doi: 10.1172/JCI118033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito H, Hirata Y, Hiroe M, Tsujino M, Adachi S, Takamoto T, Nitta M, Taniguchi K, Marumo F. Circ Res. 1991;69:209–215. doi: 10.1161/01.res.69.1.209. [DOI] [PubMed] [Google Scholar]
- 19.Mikawa T, Fischman D A. Proc Nat Acad Sci USA. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikawa T, Gourdie R G. Dev Biol. 1996;173:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 21.Rychter Z, R. Ostradal R. Folia Morphol (Prague) 1971;16:113–124. [PubMed] [Google Scholar]
- 22.Soonpaa M H, Koh G Y, Klug M G, Field L J. Science. 1994;264:98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]