Abstract
The medicinal herb, Panax notoginseng, has been used for thousands of years in traditional Chinese medicine and possesses anti-inflammatory properties. Dendritic cells (DCs) play a central role in the regulation of both inflammation and adaptive immunity. The aim of this study was to investigate the potential for notoginseng extracts to modulate Toll-like receptor (TLR) ligand-induced activation of cultured DC2.4 cells. Following stimulation with LPS, CpG or poly(I:C) and treatment with 0–50 µg/ml notoginseng extract for 24 hours, DCs were evaluated for various phenotypic and functional readouts. Notoginseng reduced the LPS-, CpG- and poly(I:C)-induced production of TNF-α by DC2.4 cells. Also, IL-6 production by notoginseng-treated cells stimulated with LPS and CpG but not poly(I;C) was reduced when compared to controls. TLR ligand-induced CD40 expression was attenuated by notoginseng. In contrast, notoginseng decreased CD86 levels on DCs activated with LPS and poly(I:C) but not CpG. Inhibition of TNF-α production was time-dependent in LPS-stimulated cells, occurring only with pretreatment or concurrent treatment of notoginseng but not after delayed addition of the herbal extract. Additionally, ginsenoside Rg1 more effectively inhibited LPS-stimulated cytokine production by DC2.4 cells than ginsenoside Rb1. Taken together, these results demonstrate that notoginseng inhibits the production of specific inflammatory molecules and innate immune responsiveness by DCs following TLR activation.
Keywords: Panax notoginseng, Ginsenosides, DC2.4, Dendritic cells, LPS, Inflammation, Immunity, Toll-like receptor
1. Introduction
Dendritic cells (DCs) are the primary professional antigen presenting cells of the immune system. They have the specialized ability to recognize, capture and process antigen (Ag) in both peripheral blood and tissues (Figdor et al., 2004). Like other immune cells, DCs are capable of recognizing microbial components using pattern-associated molecular patterns (PAMPs) that are common constituents of bacteria and viruses. Many PAMPS bind to specific pattern-recognition receptors on DCs, including Toll-like receptors (TLRs) resulting in immune activation. TLR stimulation leads to up-regulation of inflammatory mediators such as the pro-inflammatory cytokines TNF-α and IL-6. Subsequently, DCs mature and migrate to secondary lymphoid organs, where they interact with naïve T cells and induce antigen-specific immune responses (Geijtenbeek et al., 2004). This process of DC maturation includes the sequential loss of endocytotic/phagocytic receptors, upregulation of costimulatory molecules such as CD40 and CD86, and changes in morphology (Banchereau et al., 2000).
Toll-like receptors are a major family of cell-bound pattern-recognition receptors that sense infection via recognition of PAMPs and signal DCs for activation. For example, TLR3 recognizes viral double-stranded RNA and induces innate immunity, TLR4 is the primary LPS receptor and TLR9 is the receptor for bacterial DNA (Tsujimoto et al., 2006). Natural and synthetic ligands such as poly(I:C), LPS and CpG have been used extensively to model microbial activation of TLR3, TLR4 and TLR9, respectively. Binding of ligands to these TLRs induces nuclear factor kappa B (NFκB) activation and subsequent upregulation of numerous immune and inflammatory genes (Banchereau et al., 2000; Hoshino et al., 2002). Studies suggest modulation of these immune and inflammatory mediators can regulate the generation, development and progression of several inflammatory diseases including rheumatoid arthritis and atherosclerosis (Sharma and Li, 2006; Wang et al., 2006). Because DCs are key regulators of inflammation and immunity, one viable approach for the treatment of inflammatory and autoimmune diseases may be found via the intentional modulation of DC fate and function (Figdor et al., 2004; Pulendran et al., 1997).
In the last decade there has been a dramatic increase in the use of dietary supplements including herbal products. Information about the safety and efficacy of most natural products is vastly inadequate. This insufficiency stems from an unregulated industry, which is in stark contrast to the tightly regulated pharmaceutical industry. Under the Dietary Supplement Health and Education Act of 1994, supplements in the U.S. are not subjected to the same safety requirements that apply to prescription and over-the-counter medications (Berman and Straus, 2004; Institute of Medicine, 2005). This means that unlike conventional drugs, manufacturers of natural products are not required to conduct rigorous tests to demonstrate safety, efficacy or mechanisms of action (Goldma, 2001; Institute of Medicine, 2005). Recently, a survey by the U.S. National Academy of Sciences showed that a majority of consumers believe that herbs are just as safe, effective and cost-efficient as non-herbal medicines (Klepser and Klepser, 1999). In light of these beliefs and the increased usage of herbals, rigorous investigation is needed to demonstrate safety, mechanisms of action and efficacy of these products (Berman and Straus, 2004).
Ginseng is a widely consumed medicinal herb, both in the United States and worldwide (Barnes et al., 2004). Over thirteen different species of ginseng have been identified. Within the Panax genus, extracts of Panax notoginseng typically contain higher ginsenoside concentrations than formulations of the more widely used Panax ginseng (Harkey et al., 2001). Ginsenoside compounds are saponins which are unique to the Panax species and are purported to possess the primary pharmacological activity of ginseng (Harkey et al., 2001). These compounds are also used as markers for quality control and standardization of Panax species. Over twenty different saponins have been identified in notoginseng including ginsenosides, notoginsenosides and gypenoside (Dong et al., 2003). Of these, the ginsenosides Rg1, Rb1, Rd1 and notoginsenoside R1 are considered the major bioactive components (Dong et al., 2003).
To date, several studies exist describing the immunomodulatory effects of notoginseng and ginsenosides; however, specific mechanisms of action of these phytochemicals remain to be defined. Some of the reported immunologic effects of notoginseng and its constituents include the anti-allergic and anti-inflammatory activities of ginsenoside Rh1 (Park et al., 2004), a reduction in TNF-α levels by ginsenoside Rb1 (Smolinski and Pestka, 2003), an increase in both humoral and cell-mediated immune responses by ginsenoside Rg1 (Kwan, 1995), and a decrease in phospholipase 2 activity and neutrophil numbers by whole P. notoginseng extract (Li and Chu, 1999). Additionally, metabolites of notoginseng have been shown to promote human dendritic cell maturation and Th1 polarization in vitro (Fogel-Petrovic et al., 2004).
Previously, we have demonstrated that P. notoginseng has immunomodulatory effects on murine macrophages in vitro (Rhule et al., 2006). In the present study we hypothesized that notoginseng will reduce the production of inflammatory mediators in TLR ligand-stimulated murine dendritic cells. To test our hypothesis, DC2.4 cells were stimulated with LPS, CpG or poly(I:C) and treated with notoginseng. The production of pro-inflammatory cytokines as well as the expression of accessory molecules important in DC activation and function were assessed following notoginseng treatment. Concentration- and time-dependent studies were carried out using LPS as the prototypical inflammatory stimulus. Finally, the effects of the purified ginsenosides Rb1 and Rg1 on LPS-induced TNF-α and IL-6 production were compared to our whole notoginseng extract. Our results demonstrate for the first time the immunomodulatory effects of notoginseng and purified ginsenosides on murine DCs in response to several TLR ligands. These results demonstrate that notoginseng selectively attenuates the production of pro-inflammatory mediators by DC2.4 cells following stimulation in vitro.
2. Materials and Methods
2.1 Chemicals
Notoginseng (NotoG™) extracts from the plant, Panax notoginseng (Burk) F. H. Chen ex C.Y. Wu & K.M. Feng were generously provided by Technical Sourcing International, Inc. (TSI, Missoula, MT). Notoginseng was extracted from the root of the plant using ethanol and contained high levels of the ginsenosides Rb1 and Rg1 (35% and 34% of the whole extract, respectively) as determined by HPLC analysis (unpublished data). Notoginseng extracts did not contain detectable levels of Escherichia coli or Salmonella enterica (unpublished data). Certification of analyses was approved by Xia Ronglong (QA manager, TSI). The extract was dissolved in complete media (see below) or culture-grade DMSO (Sigma-Aldrich, St. Louis, MO) and sterile-filtered through a 0.22 µM Millipore membrane. The purified ginsenosides Rb1 (CAS number: 41753-43-9) and Rg1 (CAS number: 22427-39-0) were purchased from Indofine Chemical Company, Inc. (Hillsborough, NJ). Lipopolysaccharide (LPS) from Escherichia coli (055:B5) was obtained from Sigma-Aldrich, and CpG oligonucleotide and poly (I:C) were purchased from InvivoGen (San Diego, CA).
2.2 Cell Culture
DC2.4 cells, a murine dendritic cell line (Zhenhai et al., 1997), were kindly provided by Dr. Kenneth Rock (University of Massachusetts Medical Center, Worcester, MA). Cells were grown in complete media comprised of DMEM (GibcoBRL, Grand Island, N.Y), supplemented with 10% FBS (Hyclone, Logan, UT), 10 mM HEPES, 2 mM L-glutamine and 50 µg/ml gentamicin (GibcoBRL, Grand Island, N.Y). DC2.4 cells were maintained at 37°C in a humidified incubator with 5% CO2. Cells were maintained via weekly passage and utilized for experimentation at 60–80% confluency.
2.3 Cell Activation and Treatment
DC2.4 cells (1 × 106) were cultured in 1 ml complete media for 24 hrs in 6-well plates. Cells were then stimulated with 1 µg/ml LPS, 0.5 µM CpG or 12.5 µg/ml poly(I:C) and treated with varying concentrations of notoginseng or purified ginsenosides for an additional 24 hours. In some experiments, DC2.4 cells were pre-treated with notoginseng for 24 hrs before LPS stimulation. Supernatants were collected after 24 hours and frozen at −20 °C for subsequent evaluation of cytokines. Additionally, cells were harvested for flow cytometric evaluation of costimulatory molecules.
2.4 Cytokine Assays
Levels of IL-6 and TNF-α in supernatants from cultured cells were analyzed by enzyme-linked immunosorbent assay (ELISA). Samples were evaluated per the manufacturer’s recommendations using mouse cytokine-specific BD OptEIA ELISA kits (BD PharMingen, San Diego, CA).
2.5 Flow cytometry
The expression of costimulatory molecules on DC2.4 cells was determined by flow cytometric analysis as previously described (Shepherd et al., 2001). Briefly, DC2.4 cells were harvested and washed with PAB (1% bovine serum albumin and 0.1% sodium azide in PBS). Non-specific staining of cells was blocked with 30 µg per sample of purified rat and/or hamster IgG (Jackson ImmunoResearch, West Grove, PA). Fluorochrome-conjugated antibodies to mouse CD86 and CD40, and their corresponding isotype controls were purchased from BDPharmingen. One hundred thousand events per sample were collected from viable cells (as determined by light scatter profiles and PI staining) using a BD FACSAria flow cytometer, analyzed by FACSDiva (version 4.0) software (BD Biosciences, San Jose, CA) and histograms generated using FCS Express (version 3) software (De Novo Software, Thornhill, Ontario).
2.6 NFκB Assay
DC2.4 cells (1 × 106 cells per well) were stimulated with 1 µg/ml LPS and treated with 50 µg/ml notoginseng for 0, 30 and 90 minutes in 6-well plates. At each time point, cells were harvested and nuclear protein extracts prepared using the Active Motif nuclear lysis kit (Active Motif, Carlsbad, CA). Protein content of nuclear extracts was measured using a BCA protein assay kit (Pierce, Rockford, IL). NFκB p65 binding activity was determined using the Active Motif TransAM NFκB p65 kit according to the manufacturer’s directions. Briefly, 2.5 µg of nuclear protein was incubated for 1 hour in a 96-well plate coated with the NFκB consensus oligonucleotide sequence (5’-GGGACTTTCC-3’). NFκB p65 specific binding was quantified by absorbance (450nm) using a VersaMax spectrophotometer (Molecular Devices, Sunnyvale, CA).
2.7 Statistics
All statistical analyses were performed using GraphPad Prism 4.0a for the Macintosh (GraphPad Software, San Diego, CA). Differences between two means were analyzed by Student’s t-test. Data sets with multiple comparisons were evaluated by one-way analysis of variance (ANOVA) with Dunnett's post test. Values of p < 0.05 were determined to be significant.
3. Results
3.1 Notoginseng inhibits LPS-induced TNF-α and IL-6 production by DC2.4 cells
In this study, the immunomodulatory effects of notoginseng were characterized using DC2.4 cells, a murine dendritic cell line. DC2.4 cells were stimulated with 1 µg/ml LPS and concomitantly treated with 0, 5, 25 or 50 µg/ml notoginseng. In unstimulated DC2.4 cells, notoginseng did not evoke either TNF-α or IL-6 release above basal levels even at the highest concentration used (Fig. 1A and 1B, respectively). The addition of LPS resulted in a 2-fold and 23-fold increase in the production of TNF-α and IL-6, respectively. Notoginseng significantly inhibited the production of both TNF-α and IL-6 in a concentration-dependent manner. At the highest concentration of notoginseng tested (50 µg/ml), LPS-induced TNF-α production was reduced approximately 2-fold. In addition, nearly a 3-fold reduction in IL-6 production was observed at the 50 µg/ml concentration. Importantly, the reduction in LPS-induced cytokine production was not due to cytotoxicity, as viability of cells was similar to controls at all concentrations of notoginseng examined as assessed by trypan blue exclusion (data not shown).
Figure 1.
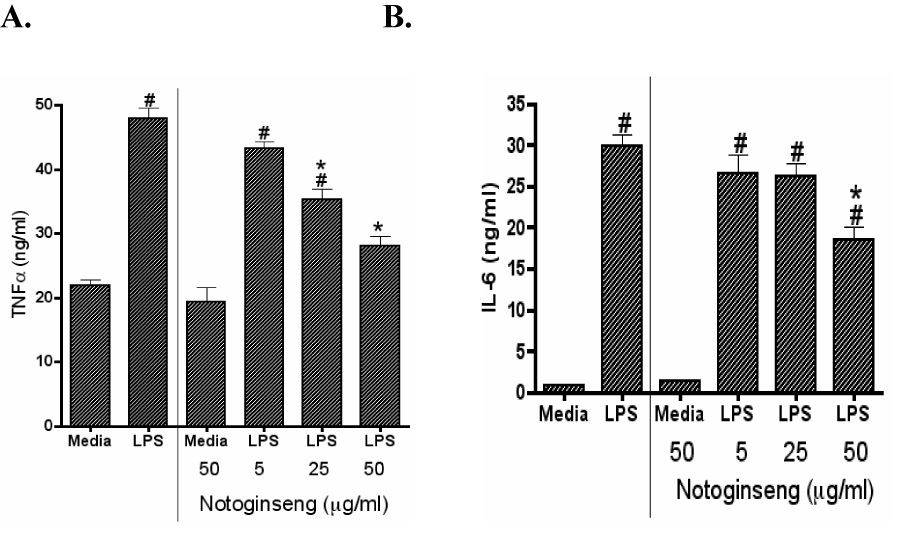
Notoginseng suppresses LPS-induced TNF-α and IL-6 production by DC2.4 cells. DCs were treated with 0, 5, 25 or 50 µg/ml of notoginseng and/or LPS (1µg/ml). Supernatants were collected after 24 hours and assayed for TNF-α (A) and IL-6 (B) production as described in the Materials and Methods. Data represents mean ± SEM of three samples. Hash (#) indicates significant differences between LPS-stimulated and unstimulated cells; asterisk (*) indicates significant differences between the LPS-stimulated control- and notoginseng-treated samples (p<0.05). Data are representative of three independent experiments.
3.2 The effects of notoginseng on TNF-α production are time-dependent
Notoginseng was evaluated for its potential to suppress TNF-α production by DC2.4 at varying time points relative to LPS stimulation. DC2.4 cells were either pretreated with 50 µg/ml of notoginseng for 24 hours prior to LPS addition (−24 hr timepoint), treated with notoginseng and LPS simultaneously (0 hr), or treated with notoginseng 8 hours after LPS addition (+8 hrs). All samples were collected 24 hours after LPS exposure. As expected, concomitant treatment of DC2.4 cell with LPS and notoginseng significantly suppressed the production of TNF-α after 24 hours of culture by more than 50 percent (Fig. 2). Pretreatment of DC2.4 cells with notoginseng for 24 hours resulted in a 20% decrease in TNF-α levels. In a separate experiment, removal of notoginseng from the medium after 24 hours of pre-treatment, but prior to LPS stimulation, also resulted in suppression of TNF-α following additional 24 hr incubation with LPS (data not shown). In contrast, addition of notoginseng 8 hours after LPS stimulation did not significantly inhibit TNF-α secretion, although a trend towards decreased levels was observed (Fig. 2).
Figure 2.
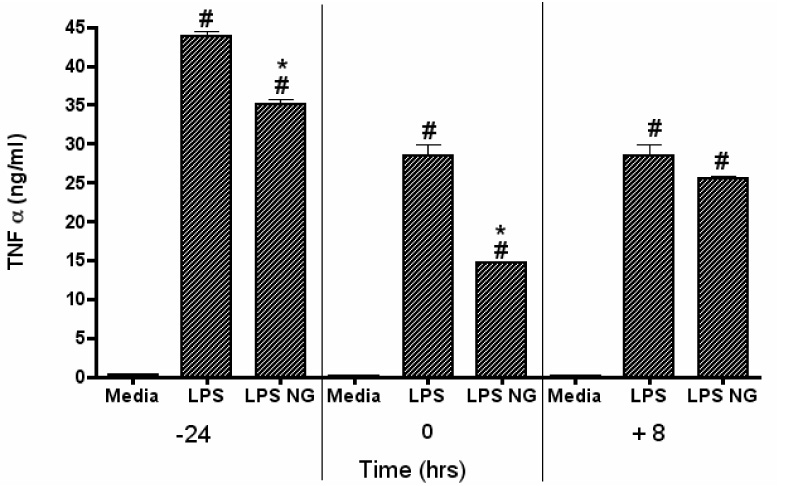
Variable notoginseng treatment affects LPS-induced production of TNF-α by DC2.4 cells. Cells were treated with 50 µg/ml notoginseng (NG) at three different time-points: −24, 0 and 8 hours, relative to LPS stimulation. Supernatants were collected 24 hours after LPS addition and TNF-α levels measured by ELISA. Data represents mean ± SEM of three samples. Hash (#) indicates significant differences between LPS-stimulated and unstimulated cells; asterisk (*) indicates significant differences between LPS-stimulated controls and LPS-stimulated, notoginseng-treated samples (p<0.05). Data are representative of three separate experiments.
3.3 Notoginseng modulates the production of TNF-α and IL-6 by DCs stimulated with additional TLR ligands
Dendritic cells can be activated to secrete pro-inflammatory cytokines by numerous pathogens expressing different TLR ligands. In this regard, the responsiveness of DCs to varied TLR ligands following exposure to notoginseng was examined. DC2.4 cells were stimulated with 1 µg/ml LPS, 0.5 µM CpG or 12.5 µg/ml poly(I:C) and concomitantly treated with 50 µg/ml notoginseng. The addition of LPS, CpG and poly(I:C) significantly increased the production of TNF-α by DC2.4 cells (Fig. 3A) (Sparwasser et al., 1998; Tsujimoto et al., 2006). Notoginseng inhibited the production of TNF-α by 46%, 36% and 50% for LPS, CpG and poly(I:C), respectively (Fig. 3A). There was a significant increase in the production of IL-6 in LPS and CpG stimulated samples while, barely detectable levels of IL-6 were present with poly(I:C) stimulation (Fig 3B). Notoginseng reduced the levels of IL-6 in the LPS- and CpG- stimulated samples (Fig 3B). In contrast, notoginseng did not inhibit the limited production of IL-6 by the poly(I:C)-stimulated DC2.4 cells (Fig 3B). As previously observed, the reduction in TLR-induced cytokine production was not due to cytotoxicity, as viability of notoginseng-treated cells was similar to controls as determined by trypan blue exclusion (data not shown).
Figure 3.
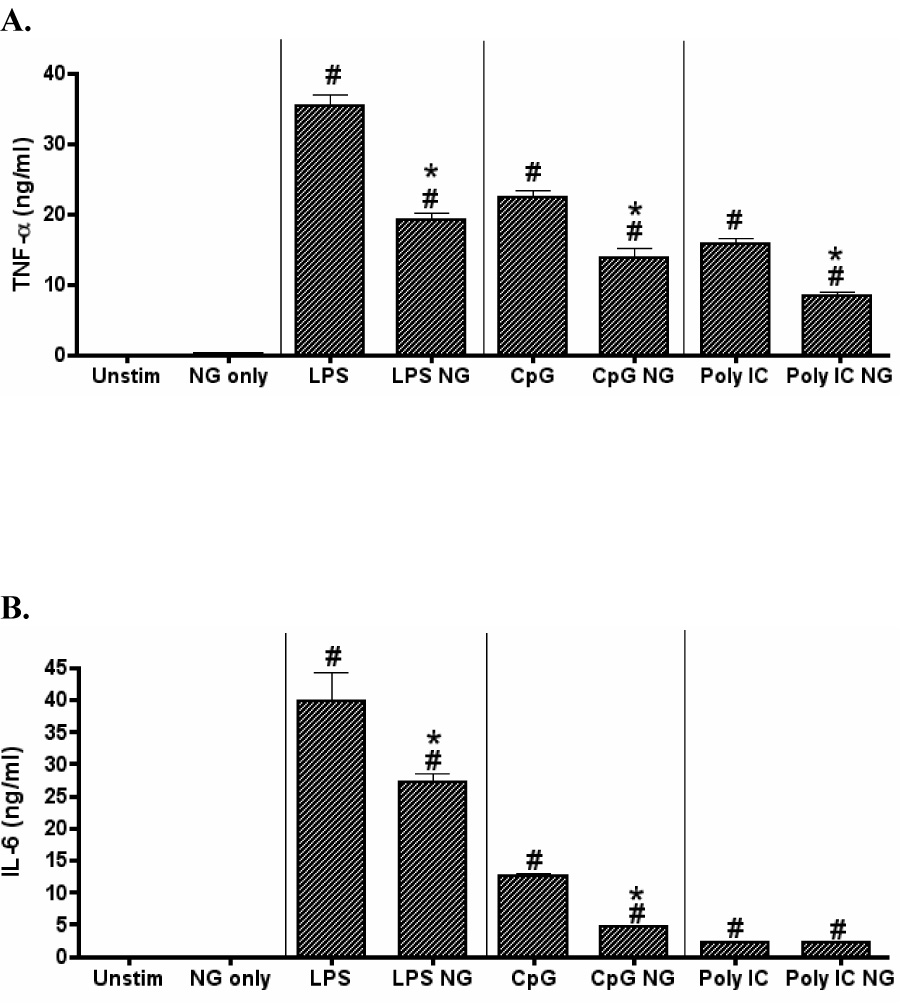
Notoginseng selectively reduces TLR ligand-induced production of TNF-α and IL-6 by DC2.4 cells. DCs were unstimulated or stimulated with LPS, CpG or poly(I:C) and concurrently treated with 50 µg/ml notoginseng (NG) for 24 hours. Supernatants were collected and assayed for TNF-α and IL-6 production by ELISA. Data represents mean ± SEM of three samples. Hash (#) indicates significant differences between stimulated and unstimulated cells; asterisk (*) indicates significant differences between the TLR ligand-stimulated control- and notoginseng-treated samples (p<0.05). Data are representative of three independent experiments.
3.4 Expression of TLR-induced costimulatory molecules by DCs is selectively affected by notoginseng
Because of the observed immunomodulatory effects of notoginseng on TLR-induced cytokine production, the potential for notoginseng to modulate costimulatory molecule expression on activated DCs was examined by flow cytometry. DC2.4 cells were treated concomitantly with each of the 3 TLR ligands and 50 µg/ml of notoginseng. The fluorescence intensity of two critical costimulatory molecules, CD40 and CD86, was assessed. Stimulation of DC2.4 cells with LPS, CpG and poly (I:C) significantly increased the expression of CD40 (Table 1 and Fig. 4) (Sparwasser et al., 1998). CD86 expression was significantly increased following LPS and poly(I:C) stimulation, but not with CpG stimulation (Fig. 4). Treatment of unstimulated DC2.4 cells with notoginseng did not affect CD40 expression; however, CD86 levels were decreased following exposure to the herbal extract alone (data not shown). Notoginseng treatment decreased CD40 expression on DCs stimulated with all examined TLR ligands (Fig. 4). Likewise, CD86 expression was reduced on notoginseng-treated DC2.4 cells that were stimulated by LPS or poly(I:C). In contrast, there was an increase in CD86 expression in CpG-stimulated, notoginseng-treated samples.
Table 1.
Notoginseng selectively alters TLR-induced costimulatory molecule expression on DC2.4 cells.
| CD40a | CD86 | |
|---|---|---|
| Unstimulated | 192 ± 2 | 1224 ± 28 |
| LPS | 1484 ± 61# | 1630 ± 52# |
| LPS + Notoginseng | 932 ± 35#* | 1295 ± 115#* |
| CpG | 611 ± 34# | 1141 ± 33 |
| CpG + Notoginseng | 512 ± 13#* | 1408 ± 28#* |
| Poly(I:C) | 1627 ± 83# | 1366 ± 49# |
| Poly(I:C) + Notoginseng | 1098 ± 40#* | 929 ± 2#* |
DC2.4 cells were not stimulated or stimulated with LPS (1µg/ml), CpG (0.5µM) or poly(I:C) (12.5 µg/ml) and concomitantly treated with notoginseng (50 µg/ml) for 24 hours.
Mean Channel Fluorescence (MCF) was determined by flow cytometry. Data shown are representative of three independent experiments. Error bars indicate mean ± SEM of five samples
p<0.05 for the comparison of unstimulated and TLR-activated cells
p<0.05 for the comparison of TLR-stimulated cells treated with notoginseng or untreated
Figure 4.
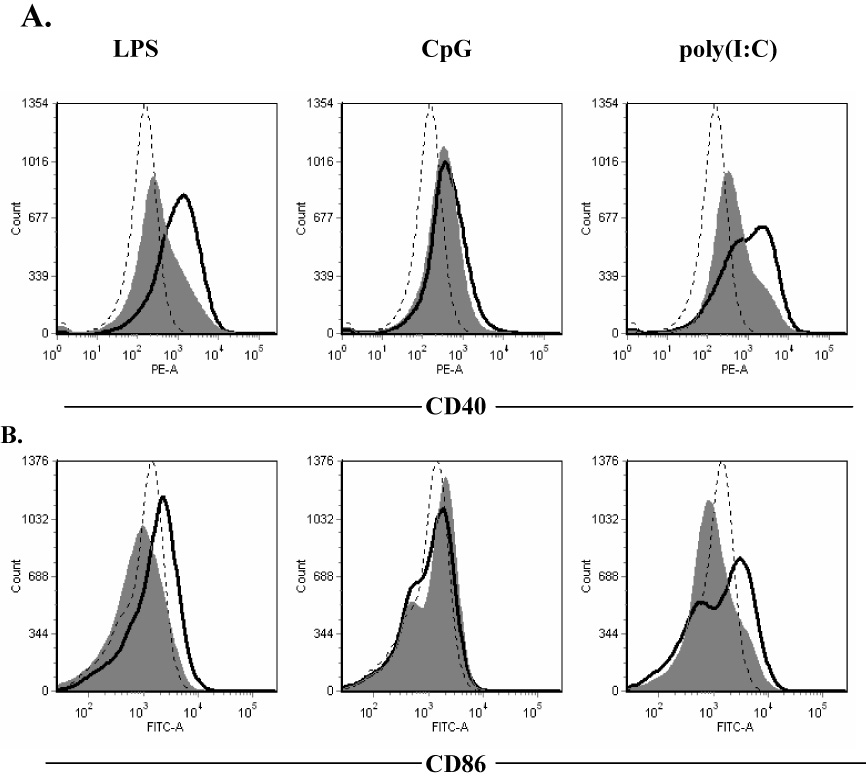
Notoginseng inhibits the expression of costimulatory molecules on DC2.4 cells following stimulation with TLR ligands. Cells were unstimulated (dashed line) or stimulated (black line) with LPS, CpG or poly(I:C) and concomitantly treated with 50 µg/ml notoginseng (gray histogram) for 24 hours. Representative histograms demonstrating the cell surface expression of CD40 (A) and CD86 (B) on activated DC2.4 cells were determined by FACS analysis. Data are representative of three independent experiments, each consisting of a minimum of three samples per treatment group.
3.5 The effects of purified ginsenosides Rb1 and Rg1 on TNF-α and IL-6 production in LPS-stimulated DC2.4 cells
Ginsenosides are believed to be responsible for the biological activity of notoginseng. The notoginseng extract used in our studies is comprised of several ginsenosides, primarily Rb1 (35%) and Rg1 (34%). Therefore, the potential inhibitory effects of the purified ginsenosides Rb1 and Rg1 on LPS-induced TNF-α and IL-6 production were evaluated in DC2.4 cells. To more closely mimic the approximate concentrations of ginsenosides present in the whole extract, Rb1 and Rg1 were standardized to concentrations found in 50 µg/ml of the whole notoginseng extract. Following LPS activation of DC2.4 cells, Rg1 significantly inhibited both TNF-α and IL-6 production (Fig. 5A and 5B). In contrast, Rb1 only inhibited the production of IL-6 and was less potent when compared to Rg1. Neither ginsenoside reduced the production of either TNF-α or IL-6 as effectively as the whole notoginseng extract. Interestingly, combination of the purified Rb1 and Rg1 compounds did not significantly decrease the production of either cytokine.
Figure 5.
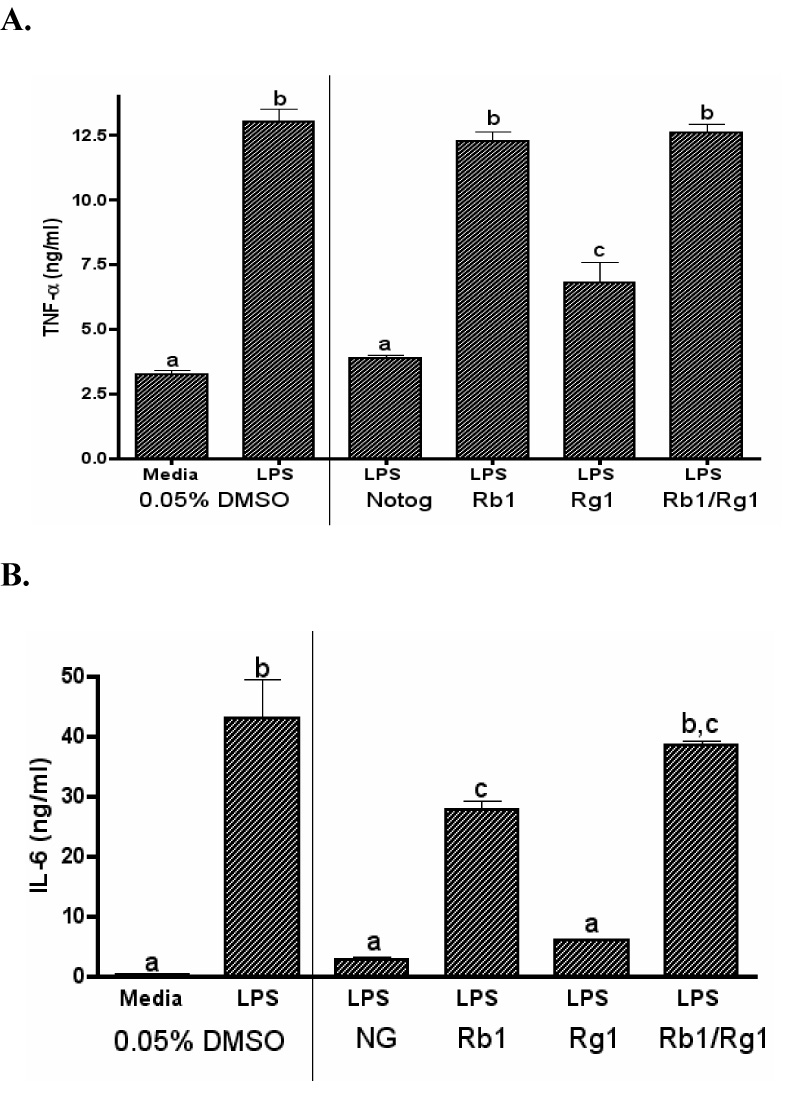
The production of TNF-α and IL-6 by DC2.4 cells is selectively suppressed by the whole notoginseng extract and purified ginsenosides. LPS-stimulated cells were treated with 50 µg/ml whole notoginseng extract (NG) or purified Rb1 and/or Rg1 ginsenosides. Supernatants were collected after 24 hours and assayed for TNF-α (A) and IL-6 (B) production by ELISA. Data represents mean ± SEM of 3 samples. Statistically significant differences (p<0.05) between treatment groups are indicated by different letters while groups sharing letters are not significantly different. Data are representative of two separate experiments.
3.6 Notoginseng does not alter LPS-induced NFκB p65 activity in DC2.4 cells
Translocation of NFκB to the nucleus is important for TLR ligand-induced inflammatory mediator production by dendritic cells. Given that notoginseng decreased the activation of DCs in our previous experiments and ginseng extracts have been shown to modulate NFκB activation in other immune cells, the effects of notoginseng on NFκB p65 nuclear levels were examined. DC 2.4 cells were either unstimulated (0 min), or stimulated with LPS for 30 or 90 mins and concurrently treated with 50 µg/ml notoginseng. Samples were then harvested and the nuclear fraction evaluated for NFκB p65 binding activity. LPS stimulation of DC2.4 cells resulted in increased NFκB p65 levels at both timepoints (data not shown). However, notoginseng did not significantly affect NFκB p65 activity in the cultured DCs.
4. Discussion and conclusions
Dendritic cells play an important role in the innate immune response to microbial pathogens (Granucci et al., 2005). Several studies have reported the effects of ginseng on various immune cell types; however, no information exists describing its effects on murine DCs. Previous studies on the effects of notoginseng on innate immunity have primarily focused on LPS-activated leukocytes. For this reason, we chose to examine the potential immunomodulatory effects of notoginseng on murine dendritic cells following stimulation with several TLR ligands, including LPS. Specifically, the effects of notoginseng on DC2.4 cells were examined following activation of TLR3, TLR4 and TLR9 by poly (I:C), LPS and CpG, respectively. The production of two critical inflammatory cytokines TNF-α and IL-6 were evaluated, as they are produced early during DC activation. Moreover, TNF-α is involved in the maturation of DCs. Along with IL-6, TNF-α can rapidly induce expression of costimulatory molecules such as CD86 on DCs thereby enhancing their interactions with T cells (Fujii et al., 2004). As LPS is a prototypical inflammatory stimulus, the effects of notoginseng were initially characterized following TLR4 activation. Notoginseng decreased the production of TNF-α and IL-6 by LPS-activated DC2.4 cells. Furthermore, these effects were demonstrated to be time-and concentration-dependent. Both pretreatment and concomitant notoginseng treatment significantly reduced TNF-α production. The effects were evident even when notoginseng was removed from the media prior to LPS stimulation of the dendritic cells (data not shown). These results suggest that notoginseng may bind to an external receptor on the DC2.4 cells, may be taken up by the DCs, or potentially both. Additionally, the actions of notoginseng most likely occur via intracellular changes, and not by binding LPS and preventing it from activating its receptor, as its presence is not required for the inhibition of TNF-α. When DCs were exposed to notoginseng 8 hours after LPS stimulation, no significant inhibitory effects were identified on TNF-α production. These results suggest that notoginseng may affect early signaling events in DCs following LPS-induced activation.
As previously mentioned, activation of DC can occur by a variety of pathogens through different TLRs (Napolitani et al., 2005). In these experiments, LPS, CpG and Poly (I:C) were used to model stimulation of DCs by both bacterial and viral pathogens (Sioud, 2006). Notoginseng reduced the production of TNF-α by DC2.4 cells in response to each of the TLR ligands tested while IL-6 production was reduced following LPS and CpG, but not poly(I:C) stimulation. Because the TLR-induced production of both of these pro-inflammatory cytokines was not completely abolished by notoginseng, DC function may not be significantly diminished. Thus, notoginseng may inhibit the inflammatory responsiveness of DCs without significantly affecting their ability to initiate adaptive immunity. If so, this medicinal herb might be expected to benefit individuals infected with microbial pathogens by limiting the production of inflammatory mediators such as TNF-α and IL-6 without affecting the generation of pathogen-specific adaptive immunity. This possibility is consistent with a previous report demonstrating that notoginseng-treated mice were less susceptible to the ill effects of experimental sepsis, effects which the authors attributed to a decreased inflammatory response to infection (Ahn et al., 2006).
The expression of TLRs can vary within different DC subpopulations (Krug et al., 2001). Currently, no information exists on the levels of expression for different TLRs in DC2.4 cells. Further experiments are therefore needed to characterize the level of expression of TLR3, TLR4 and TLR9 in DC2.4 cells to permit better understanding of how notoginseng may affect their expression and function. Also, it is becoming widely accepted that pathogens will trigger multiple TLRs during the course of infection. Thus, additional experiments designed to evaluate the effects of notoginseng on DCs stimulated with multiple TLR ligands would be expected to greatly enhance our understanding of the innate immune responsiveness of DCs.
Interactions between accessory/costimulatory molecules on DCs and their ligands expressed on T cells are critical for the full activation of T cells (Banchereau et al., 2000). Ligation of CD40 on DCs acts as a maturation signal, enhancing antigen presentation and the expression of other co-stimulatory molecules; while CD86 is believed to be the most critical molecule for the amplification of T cell responses (Banchereau et al., 2000; Fujii et al., 2004). Previous studies from our laboratory demonstrated that notoginseng reduced the LPS-induced expression of both CD40 and CD86 on RAW 264.7 cells (Rhule et al., 2006). Similarly, CD86 and CD40 expression on LPS-activated DC2.4 cells was reduced using similar concentrations of notoginseng. Both molecules on DCs were also reduced by notoginseng treatment following poly(I:C) stimulation. In contrast, CD40 but not CD86 expression was reduced on DC 2.4 cells stimulated with CpG. Current studies in our laboratory are evaluating the functional significance of the notoginseng-induced changes in costimulatory molecule expression on DCs.
Ginsenosides are unique to the ginseng species and are believed to be the biologically active components of notoginseng. Because of the wide variability in the types and concentrations of ginsenosides present in ginseng extracts, it is crucial to define the immunomodulatory potential of different ginsenosides. In this study, the ginsenoside Rg1 inhibited the production of both TNF-α and IL-6, while Rb1 only affected the production of IL-6. Unexpectedly, when Rg1 was combined with Rb1, the inhibitory effect of Rg1 was lost. This result could be due to a number of factors including differential effects of Rb1 and Rg1 on cell membrane permeability, on the activation of disparate receptors and/or signal transduction pathways, or perhaps via the partial antagonism of Rg1 by Rb1 (Attele et al., 1999). These possibilities are consistent with a number of studies reporting that complex ginseng extracts can differentially affect immune cell function based on their specific ginsenoside profiles (Cho et al., 2002; Cho et al., 2001; Guermonprez et al., 2002; Joo et al., 2005). Future studies will therefore be required to elucidate the complex nature of ginsenoside interactions in dendritic cells.
The transcription factor NFκB plays an important role in the regulation of multiple signaling pathways that control the activation of many immune cells (Celec, 2004; Glass and Ogawa, 2006). TLR ligands activate NFκB proteins in DCs and subsequently affect their fate and function (Wang et al., 2007). Several studies using components of notoginseng suggest that these products may inhibit NFκB activation (Ahn et al., 2006; Chung et al., 1998; Keum et al., 2003). In our studies, notoginseng did not affect NFκB p65 activation. Thus, it is likely that this herbal exerts its anti-inflammatory effects through other pathways such as MAPK and AP1, or through other NFκB family members such as RelB or cRel. Alternatively, because DC2.4 cells are an immortalized cell line that are not terminally differentiated, they may respond differently than primary DCs following exposure to notoginseng. This observation is consistent with recent studies in our laboratory in which notoginseng reduced NFκB p65 nuclear levels and activity in LPS-stimulated bone marrow-derived dendritic cells (manuscript in preparation).
In summary, this study demonstrates that notoginseng inhibits the production of TNF-α and selectively decreases IL-6 production by DCs following TLR activation. Similarly, notoginseng differentially affected the expression of the costimulatory molecules CD40 and CD86 on DCs following activation by different TLR ligands. Collectively, our results demonstrate that notoginseng can decrease the inflammatory responsiveness of DCs to bacterial or viral stimuli. Further studies are needed to examine directly if the notoginseng-induced decreases in cytokines and accessory molecules by DCs alters their ability to initiate T cell-dependent adaptive immunity.
Supplementary Material
Acknowledgments
This research was supported by grants from NSF-EPSCoR (EPS00091995) and NCRR (P20 RR 017670). The authors thank Pamela Shaw and the CEHS Fluorescence Imagery core at UM for their expert assistance. We also thank Drs. Celine Beamer and Scott Wetzel for their helpful discussions and critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS, Jeong G, Song JY. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. European Journal of Immunology. 2006;36:37–45. doi: 10.1002/eji.200535138. [DOI] [PubMed] [Google Scholar]
- Attele AS, Wu JA, Yuan C-S. Ginseng Pharmacology, Multiple Constituents and multiple actions. Biochemical Pharmacology. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y-J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annual Review of Immunology. 2000:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Barnes P, Powell-Griner E, McFann K, Nahin R. Complementary and alternative medicine use among adults: United States, 2002. Advanced Data From Vital Health Statistics, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. 2004:343. [PubMed] [Google Scholar]
- Berman JD, Straus SE. Implementing a research agenda for complementary and alternative medicine. Annual Review of Medicine. 2004;55:239–254. doi: 10.1146/annurev.med.55.091902.103657. [DOI] [PubMed] [Google Scholar]
- Celec P. Nuclear factor kappa B--molecular biomedicine: the next generation. Biomedical Pharmacotherapy. 2004;58:365–371. doi: 10.1016/j.biopha.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Cho JY, Kim AR, Yoo ES, Baik KU, Park MH. Ginsenosides from Panax ginseng differentially regulate lymphocyte proliferation. Planta Medica. 2002;68:497–500. doi: 10.1055/s-2002-32556. [DOI] [PubMed] [Google Scholar]
- Cho JY, Yoo ES, Baik KU, Park MH, Han BH. In vitro inhibitory effect of protopanaxadiol ginsenosides on tumor necrosis factor (TNF)-alpha production and its modulation by known TNF-alpha antagonists. Planta Medica. 2001;67:213–218. doi: 10.1055/s-2001-12005. [DOI] [PubMed] [Google Scholar]
- Chung E, Lee KY, Lee YJ, Lee YH, Lee SK. Ginsenoside Rg1 down-regulates glucocorticoid receptor and displays synergistic effects with cAMP. Steroids. 1998;63:421–424. doi: 10.1016/s0039-128x(98)00043-9. [DOI] [PubMed] [Google Scholar]
- Dong TT, Cui XM, Song ZH, Zhao KJ, Ji ZN, Lo CK, Tsim KW. Chemical assessment of roots of Panax notoginseng in China: Regional and seasonal variations in its active constituents. Journal of Agricultural and Food Chemistry. 2003;51:4617–4623. doi: 10.1021/jf034229k. [DOI] [PubMed] [Google Scholar]
- Figdor CG, de Vries JM, Lesterhuis JW, Melief CJM. Dendritic cell immunotherapy: mapping the way. Nature Medicine. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- Fogel-Petrovic M, Long JA, Knight DA, Thompson PJ, Upham JW. Activated human dendritic cells express inducible cyclo-oxygenase and synthesize prostaglandin E2 but not prostaglandin D2. Immunology and Cell Biology. 2004;82:47–54. doi: 10.1111/j.1440-1711.2004.01213.x. [DOI] [PubMed] [Google Scholar]
- Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. Journal of Experimental Medicine. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TBH, van Vliet SJ, Engering A, Hart BA, Kooyk Yv. Self- and Nonself-Recognition by C-Type Lectins on Dendritic Cells. Annual Review of Immunology. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nature Reviews Immunology. 2006;6:44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- Goldma P. Herbal medicine today and the roots of modern pharmacology. Annals of Internal Medicine. 2001;135:594–600. doi: 10.7326/0003-4819-135-8_part_1-200110160-00010. [DOI] [PubMed] [Google Scholar]
- Granucci F, Foti M, Ricciardi-Castagnoli P. Dendritic cell biology. Advances in Immunology. 2005;88:193–233. doi: 10.1016/S0065-2776(05)88006-X. [DOI] [PubMed] [Google Scholar]
- Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annual Review of Immunology. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- Harkey MR, Henderson GL, Gershwin EM, Stern JS, Hackman RM. Variability in commercial ginseng products: an analysis of 25 preparations. American Journal of Clinical Nutrition. 2001;73:1101–1106. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. International Immunology. 2002;14:1225–1231. doi: 10.1093/intimm/dxf089. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Complementary and Alternative Medicine in the United States. The National Academies Press; 2005. pp. 1–33. [PubMed] [Google Scholar]
- Joo SS, Won TJ, Lee do I. Reciprocal activity of ginsenosides in the production of proinflammatory repertoire, and their potential roles in neuroprotection in vivo. Planta Medica. 2005;71:476–481. doi: 10.1055/s-2005-864145. [DOI] [PubMed] [Google Scholar]
- Keum YS, Han SS, Chun KS, Park KK, Park JH, Lee SK, Surh YJ. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutation Research. 2003:523–524. 75–85. doi: 10.1016/s0027-5107(02)00323-8. [DOI] [PubMed] [Google Scholar]
- Klepser T, Klepser EM. Unsafe and potentially safe herbal therapies. American Journal of Health-System Pharmacy. 1999;56:125–138. doi: 10.1093/ajhp/56.2.125. [DOI] [PubMed] [Google Scholar]
- Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. European Journal of Immunology. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kwan C. Vascular Effects of selected Antihypertensive drugs derived from traditional medicinal herbs. Clinical Experimental Physiology. 1995;22:S297–S299. doi: 10.1111/j.1440-1681.1995.tb02925.x. [DOI] [PubMed] [Google Scholar]
- Li SH, Chu Y. Anti-inflammatory effects of total saponins of Panax notoginseng. Zhongguo Yao Li Xue Bao. 1999;20:551–554. [PubMed] [Google Scholar]
- Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature Immunology. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E-K, Choo M-K, Joo Han M, Kim D-H. Ginsenoside Rh 1 possesses antiallergic and anti-Inflammatory activities. International Archives of Allergic Immunology. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraakovsky E. Developmental Pathways of Dendritic Cells in Vivo Distinct Function, Phenotype and Localization of Dendrtic cell subsets in FLT3 Ligand Treated Mice. The Journal of Immunology. 1997;159:2222–2231. [PubMed] [Google Scholar]
- Rhule A, Navarro S, Smith JR, Shepherd DM. Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells. Journal of Ethnopharmacology. 2006;106:121–128. doi: 10.1016/j.jep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Sharma R, Li DZ. Role of dendritic cells in atherosclerosis. Asian Cardiovascular & Thoracic Annals. 2006;14:166–169. doi: 10.1177/021849230601400220. [DOI] [PubMed] [Google Scholar]
- Shepherd DM, Steppan LB, Hedstrom OR, Kerkvliet NI. Anti-CD40 Treatment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-exposed C57Bl/6 mice induces activation of antigen presenting cells yet fails to overcome TCDD-induced suppression of allograft immunity. Toxicology and Applied Pharmacology. 2001;170:10–22. doi: 10.1006/taap.2000.9080. [DOI] [PubMed] [Google Scholar]
- Sioud M. Innate sensing of self and non-self RNAs by Toll-like receptors. Trends in Molecular Medicine. 2006;12:167–176. doi: 10.1016/j.molmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Smolinski AT, Pestka JJ. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb(1) (ginseng) and parthenolide (feverfew) Food and Chemical Toxicology. 2003;41:1381–1390. doi: 10.1016/s0278-6915(03)00146-7. [DOI] [PubMed] [Google Scholar]
- Sparwasser T, Koch ES, Vabulas RM, Heeg K, Lipford GB, Ellwart JW, Wagner H. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. European Journal of Immunology. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tsujimoto H, Efron PA, Matsumoto T, Ungaro RF, Abouhamze A, Ono S, Mochizuki H, Moldawer LL. Maturation of murine bone marrow-derived dendritic cells with poly(I:C) produces altered TLR-9 expression and response to CpG DNA. Immunology Letters. 2006;107:155–162. doi: 10.1016/j.imlet.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Fathman JW, Lugo-Villarino G, Scimone L, von Andrian U, Dorfman DM, Glimcher LH. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. The Journal of Clinical Investigation. 2006;116:414–421. doi: 10.1172/JCI26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang X, Hussain S, Zheng Y, Sanjabi S, Ouaaz F, Beg AA. Distinct Roles of Different NF-{kappa}B Subunits in Regulating Inflammatory and T Cell Stimulatory Gene Expression in Dendritic Cells. Journal of Immunology. 2007;178:6777–6788. doi: 10.4049/jimmunol.178.11.6777. [DOI] [PubMed] [Google Scholar]
- Zhenhai S, Reznikoff G, Dranoff G, Rock K. Cloned Dendritic Cells Can Present Exogenous Antigens on Both MHC Class I and Class II Molecules. Journal of Immunology. 1997;158:2723–2730. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


