Abstract
The enhanced production of monocytes expressing pro-inflammatory markers such as the integrin CD11b in patients with hypercholesterolemia may promote vascular inflammation and exacerbate atherogenesis. The objective of the present study was to determine whether hypercholesterolemia stimulates the production of CD11b+ monocytes in bone marrow, and whether the renin-angiotensin system participates in this process and thus provides a target for therapeutic intervention. The dietary induction of hypercholesterolemia in adult male cynomolgus monkeys was accompanied by increased bone marrow cellularity and elevated peripheral blood and bone marrow monocyte CD11b expression. Isolated bone marrow CD34+ hematopoietic stem cells (HSCs) evaluated by in vitro functional assays exhibited enhanced myeloproliferative capacity and differentiation into CD11b+ monocytes. Treatment of hypercholesterolemic monkeys with the angiotensin II AT1 receptor blocker losartan for 15 weeks reduced bone marrow cellularity, suppressed peripheral blood and bone marrow monocyte CD11b expression, and normalized CD34+ cell function assays. All variables returned to pretreatment levels 6 weeks after discontinuation of losartan treatment. Hypercholesterolemia was associated with increased CD34+ cell AT1 receptor expression and an exaggerated in vitro myeloproliferative response to angiotensin II stimulation that positively correlated to plasma LDL concentrations. In vitro exposure to native low-density lipoproteins (LDL) also increased CD34+ cell AT1 receptor expression and the myeloproliferative response to angiotensin II stimulation in a dose-dependent and receptor-mediated manner. Our data provide support for a positive regulatory role of plasma LDL on AT1 receptor-mediated HSC differentiation and the production of pro-atherogenic monocytes. LDL-regulated HSC function may explain in part hypercholesterolemia-induced inflammation as well as the anti-inflammatory and anti-atherosclerotic effects of AT1 receptor blockers.
Keywords: Hypercholesterolemia, LDL, monocyte, CD34, angiotensin, hematopoietic stem cell, hematopoiesis
1. INTRODUCTION
The vascular infiltration of blood-borne monocytes induced by hypercholesterolemia is an obligatory early event in atherogenesis that is dependent on the adhesion of monocytes to endothelium. Sustained monocyte adhesion required for transendothelial migration of monocytes into the intima is a complex process requiring monocyte expression of integrins that include the β2 heterodimer variants CD11a/CD18, CD11b/CD18 and CD11c/CD18. Elevated monocyte expression of CD11b observed in patients with hypercholesterolemia is considered a indication of monocyte activation and heightened atherogenic potential given the positive correlation between CD11b expression and adhesion to endothelium 1. The renin-angiotensin system (RAS) may play a role in this activation process since hypercholesterolemia upregulates AT1 receptor expression in a variety of tissues2 and in vitro stimulation of AT1 subtype receptors by angiotensin (Ang) II directly increases monocyte CD11b.3 The suppression of monocyte CD11b by AT1 receptor blockade in patients with coronary artery disease4 and in monkeys with hypercholesterolemia-induced atherosclerosis5 provides additional evidence that the RAS regulates the phenotype of circulating monocytes and their atherogenicity. The anti-inflammatory action of Ang II receptor blockers (ARBs) reflected by diminished CD11b expression may be an important mechanism by which this drug class exerts its anti-atherosclerotic effects independent of blood pressure-lowering. Nevertheless, it remains unclear how hypercholesterolemia shifts monocyte development into activated and pro-atherogenic phenotypes, whether AT1 receptors mediate these events, and at what stages of development ARBs exert their anti-inflammatory influence.
A recent study in hypercholesterolemic mice6 demonstrated that the lack of AT1 receptors in donor bone marrow (BM) cells inhibited atherosclerotic lesion development despite intact recipient vascular AT1 receptors. These results signify the importance of BM cell AT1 receptors to the atherogenic process and are consistent with the earlier demonstration that BM AT1 receptors are necessary for normal monocyte development and tissue macrophage infiltration.7 Hematopoietic stem cells (HSCs) identified by the surface glycoprotein CD34 are the origin of the majority of tissue macrophages found in atherosclerotic lesions.7 Stimulation of CD34+ HSC AT1 receptors with Ang II preferentially enhances the production of myeloid progenitor cells in culture.8 The dietary induction of hypercholesterolemia enhances BM myelopoiesis in animal models of atherosclerosis9,10 which may then facilitate atherogenesis by increasing production of pre-activated myeloid lineage inflammatory cells predisposed to participate in atherogenesis. The potential for hypercholesterolemia to increase AT1 receptor expression is reportedly a function of the response to elevated low density lipoprotein (LDL) concentrations11 which may also be a mechanism whereby hypercholesterolemia aggravates hypertension. In clinical studies, the pressor response to Ang II infusion was related to LDL cholesterol concentration even in normocholesterolemic or mildly hypercholesterolemic patients.12 The same concentration-dependent upregulation of vascular smooth muscle cell AT1 receptors by exposure to LDL and the enhanced proliferative response to subsequent Ang II stimulation13 may also regulate Ang II-mediated effects on other cell types including HSCs. We proposed that a direct stimulatory effect of increased plasma LDL concentration on HSC AT1 receptor expression may explain the exaggerated myelopoiesis observed in experimental hypercholesterolemia. We evaluated this hypothesis by evaluating CD34+ HSC function in response to diet-induced hypercholesterolemia and systemic AT1 receptor blockade in a monkey model of atherosclerosis. To more directly determine the influence of LDL on the BM RAS, we also investigated the interaction between LDL receptor- and AT1 receptor-mediated HSC function.
2. METHODS
2.1 Animals and Experimental Protocol
Fourteen adult, male cynomolgus (Macaca fascicularis) monkeys (average weight 4.86 ± 0.89 kg) imported from Indonesia were fed a commercial diet (Monkey Chow #5038, Purina Mills-LabDiet, St. Louis, MO) for 35 weeks to establish a normocholesterolemia (NC) baseline. Monkeys were then fed a high-cholesterol-diet (0.067 mg cholesterol/kJ) for an additional 36 weeks. Fifteen weeks after the induction of hypercholesterolemia (HC), monkeys were assigned to groups receiving a single daily subcutaneous injection of either 100 mg losartan (n = 7) or vehicle (n = 7) for 15 weeks. All experimental procedures other than treatment were performed while monkeys were lightly anesthetized with ketamine hydrochloride (10 mg/kg IM, Fort Dodge Laboratories, Fort Dodge, IA). Housing, environmental enrichment and experimental procedures were approved by the Institutional Animal Care and Use Committee and performed in accordance with the standards established by the US Department of Health and Human Resources and US Drug Administration.
2.2 Physiological Studies
Triplicate blood pressure and heart rate measurements were performed at the same time of day and 3 hours after treatment with an automated air plethysmograph (VascuMap model AP 102V, Carolina Medical Inc., King, NC).5
2.3 Peripheral Blood and Bone Marrow Analysis
Plasma total cholesterol (TC), LDL-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), very low density lipoprotein-cholesterol (VLDL-C), Ang II and losartan concentrations, peripheral blood (PB) white blood cell (WBC) total and differential counts, and CD14+/CD45+ monocyte CD11b expression were determined as previously described in this species.5,14 The total area under the plasma concentration versus time curve (AUCtotal) for losartan was calculated by the trapezoidal rule15 using the maximum plasma concentration (Cmax) and the time at which Cmax occurred. The BM (BM) mononuclear cell (MNC) fraction was isolated from humerus BM aspirates by density gradient centrifugation.16 Numbers of BM MNCs were determined by manual counting using a hemocytometer. Immunophenotyping of PB and BM MNCs was performed by flow cytometry analysis of fluorescein isothiocyanate (FITC) and phycoerythrin (PE) fluorescence with mouse-derived monoclonal primary antibodies determined by the manufacturers to cross-react with cynomolgus monkey antigens: CD14 (FITC-conjugated, clone M5E2, BD Pharmingen, San Diego, CA), CD45 (PE-conjugated, clone TÜ116, BD Pharmingen; FITC-conjugated, clone MB4-6D6, Miltenyi Biotec Inc., Auburn, CA), CD11b (PE-conjugated, clone ICRF44, BD Pharmingen), and CD34 (PE-conjugated, clone 563, BD Pharmingen). LDLRs on CD34+ cells were detected using a mouse monoclonal antibody to human LDLR (clone 15C8, Oncogene Research Products, Boston, MA) together with a goat anti-mouse secondary FITC-conjugated antibody (IgG2b, BD Pharmingen)17 IgG isotype-identical irrelevant monoclonal antibodies (BD Pharmingen) served as controls to exclude non-specific staining from analysis.
2.4 Isolation of Bone Marrow CD34+ Cells
We used positive immunomagnetic selection was used to isolate CD34+ cells from 1 × 108 BM MNCs according to the manufacturer’s instructions (EasySep PE Selection Kit, StemCell Technologies, Vancouver, BC). Cell purity was assessed by flow cytometry analysis of CD34 expression using an FITC-conjugated mouse monoclonal antibody to a CD34 epitope (clone 561, Dynal, Oslo, Norway) different from the epitope used for quantification and isolation. Both clones 563 and 561 exhibit cross-reactivity with cynomolgus monkey BM cells, and cynomolgus monkey CD34+ cells isolated using clone 563 demonstrate significant growth potential in clonogenic assays.16 Cell surface expression of AT1 receptors by purified PE-labeled CD34+ cells was determined by two-color flow cytometry analysis as previously described18 using a primary rabbit polyclonal antibody to the N-terminal extracellular domain of AT1 receptors (Santa Cruz Biotechnology, Santa Cruz, CA), and a secondary FITC-conjugated F(ab’)2 fragment goat anti-rabbit IgG (H+L) antibody (Sigma, St. Louis, MO). Expression levels of AT1 receptors on CD34+ cells were quantified as mean fluorescent intensity (MFI) with FloJo analysis software (Tree Star, Inc., Ashland, OR).
2.5 In vitro evaluation of CD34+ HSC function
The colony-forming ability of CD34+ HSCs was assayed as previously described in this species19 according to the manufacturer’s directions in Methocult GF H4434 (StemCell Technologies). The methylcellulose-based culture medium consisted of optimal concentrations of growth factors to support the differentiation of nonhuman primate CD34+ cells into colony forming units of granulocytes and monocytes (CFU-GM), erythroid burst-forming units (BFU-E) and mixed colonies containing granulocyte-macrophage, megakaryocyte and erythroid elements (CFU-GEMM). After 14 days of incubation at 37°C in a humidified atmosphere of 5% CO2 in air, colonies containing at least 50 cells were enumerated by light microscopy and classified according to established criteria. After counting, cells were washed in PBS to remove culture medium, resuspended in serum-free Iscove’s modified Dulbecco’s medium (IMDM, Gibco, Grand Island, NY) and 25 mM HEPES buffer, and analyzed by flow cytometry for monocyte content as cells expressing both CD11b and CD14.20
Long-term BM cultures (LTBMCs) that simulate hematopoiesis by supporting differentiation of HSC into hematopoietic-lineage progeny were performed by seeding CD34+ cells over marrow stromal cell feeder layers of human HS-5 cells (CRL-11883, American Type Culture Collection, Rockville, MD).21 To achieve the formation of a stable feeder monolayer, 1 × 105 HS-5 cells were plated in 24 well plates 72 hours before the addition of CD34+ cells. HS-5 cells were maintained in RPMI medium 1640 (Gibco) supplemented with 5% FBS, 1% L-glutamine, 1% sodium pyruvate, and 1% penicillin-streptomycin. Suspended CD34+ cells were overlaid on HS-5 stromal layers at a concentration of 1 × 105 cells·well-1 in 24-well culture plates. LTBMCs were incubated at 37°C in a 5% CO2, fully humidified atmosphere, and were demi-populated and fed on a weekly basis. Non-adherent (NA) cells comprised of CD34+ cell-derived hematopoietic-lineage progeny were collected every week for 6 weeks after seeding, and immunophenotyped for differentiation into CD11b+/CD14+ monocytes by flow cytometry.22 HS-5 cells were grown without added CD34+ cells and analyzed as controls for cells expressing CD11b/CD14.
2.6 In vitro response of CD34+ HSCs to Ang II and nLDL
CD34+ cells were resuspended at 5 × 104 cells/ml in serum-free StemSpan (Stem Cell Technologies) supplemented with 5% lipoprotein-deficient serum (LPDS),23 100 ng/mL rhFlt3-ligand, 100 ng/mL rhSCF, 20 ng/mL rhIL-3. After 24 h, the cells were washed, manually counted, and resuspended at 5 × 105 cells/mL. One hundred μL aliquots were added to individual cells of a 96-well microtiter plate in medium with and without 1 μM Ang II. Losartan was added at a final 1 μM concentration to some wells 30 min before the addition of Ang II. The plates were incubated for 24 h at 37°C in 5% CO2 in air. Afterwards, the cells were washed and placed in methylcellulose growth medium. Colonies were counted 14 days after plating and then evaluated after resuspension for differentiation into CD11b+/CD14+ monocytes by flow cytometry.
Monkey nLDL was isolated as previously described5 and added to suspensions of CD34+ cells isolated from normocholesterolemic monkeys at final protein concentrations of 25-100 μg/mL. The addition of 10 mmol/L BHT to LDL preparations prevented LDL oxidation. In some experiments, a monoclonal antibody (Clone IgG-C7, Research Diagnostics, Inc., 1:200 dilution) previously shown to inhibit nLDL-mediated receptor activation24 or a non-specific IgG (10 μg/mL) was added to CD34+ cell suspensions 30 min before the addition of nLDL.24 Stimulation of CD34+ HSCs with 1 μM Ang II after incubation with nLDL was carried out in the absence or presence of 1 μM losartan.
2.7 Statistics
Analysis of variance (ANOVA) with Bonferroni-Dunn comparison was used to determine within-group and between-group differences in values determined at NC and HC baselines, after 15 weeks of treatment, and at 6 weeks post-treatment. ANOVA and correlation statistics were performed using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA). Probability values < 0.05 were considered significant.
3. RESULTS
3.1 Effects of Hypercholesterolemia and Losartan on Plasma and Hemodynamic Variables
Plasma TC, LDL-C, HDL-C, VLDL-C and Ang II concentrations and hemodynamic data at the NC baseline, HC baseline 15 weeks after dietary induction of HC, and at the end of treatment (TX) and post-treatment (Post-TX) periods are presented in Table 1. The induction of HC in monkeys preassigned to either vehicle or losartan treatments increased plasma TC, LDL-C, and VLDL-C concentrations (P < 0.05), but had no effect on plasma Ang II concentrations. Blood pressures and heart rates were also unaffected by HC. Plasma Ang II concentrations measured in blood samples acquired 3 h after subcutaneous administration of 100 mg losartan were increased 10-fold compared to pre-treatment values, and were similar to values previously reported with chronic subcutaneous losartan administration in this model.5 Plasma losartan concentrations averaged 1.36±0.21 ug/mL at 3 h after injection and 0.237±0.06 ug/mL at 8 h after treatment. The losartan AUCtotal (524±26 nmol/h·L-1) was comparable to values observed in humans given 50 mg losartan p.o.,15 and was associated with prevention of the pressor response to infused Ang II as determined in preliminary studies. Six weeks after discontinuation of losartan, plasma Ang II concentrations returned to pre-treatment levels, and were not different from values in monkeys treated with vehicle.
Table 1.
Plasma and hemodynamic values
| NC | HC | Tx | Post-Tx | |||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | Losartan | Vehicle | Losartan | Vehicle | Losartan | Vehicle | Losartan | |
| TC, mmol L-1 | 2.9 ± 0.2 | 3.3 ± 0.3 | 8.5 ± 1.4* | 8.6 ± 1.5* | 8.5 ± 2.0* | 8.0 ± 1.1* | 8.0 ± 1.5* | 8.4 ± 1.3* |
| HDL, mmol L-1 | 0.80 ± 0.02 | 0.84 ± 0.02 | 1.5 ± 0.4* | 1.1 ± 0.4* | 1.5 ± 0.03* | 1.2 ± 0.04* | 1.2 ± 0.03* | 1.2 ± 0.04* |
| LDL-C, mmol L-1 | 2.1 ± 0.1 | 2.4 ± 0.1 | 5.9 ± 0.9* | 6.1 ± 0.8* | 6.1 ± 0.8* | 5.9 ± 0.7* | 5.9 ± 0.9* | 5.9 ± 0.8* |
| VLDL-C, mmol L-1 | 0.12 ± 0.02 | 0.13 ± 0.03 | 0.82 ± 0.06* | 0.84 ± 0.04* | 0.79 ± 0.03* | 0.72 ± 0.05* | 0.71 ± 0.05* | 0.82 ± 0.06* |
| Ang II, fmol mL-1 | 22 ± 2 | 20 ± 2 | 29 ± 6 | 26 ± 3 | 31 ± 3 | 353 ± 33*† | 27 ± 2 | 27 ± 3 |
| SBP, mm Hg | 114 ± 4 | 110 ± 8 | 116 ± 5 | 113 ± 7 | 118 ± 4 | 110 ± 4 | 119 ± 5 | 107 ± 7 |
| DBP, mm Hg | 51 ± 4 | 43 ± 5 | 44 ± 3 | 43 ± 5 | 46 ± 4 | 40 ± 2 | 49 ± 4 | 42 ± 4 |
| HR, beats min-1 | 166 ± 12 | 152 ± 6 | 158 ± 8 | 156 ± 9 | 164 ± 9 | 151 ± 9 | 157 ± 8 | 155 ± 5 |
P < 0.05, differences compared to NC baseline.
P < 0.05, compared to vehicle treatment.
Tx, 15 weeks of treatment; Post-Tx, 6 weeks post-treatment. TC, total plasma cholesterol concentration; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoproteincholesterol concentration; VLDL-C, very low density lipoprotein concentration; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
3.2 Effects of Hypercholesterolemia and Losartan on PB and BM MNC Parameters
Complete PB WBC and monocyte counts, PB monocyte CD11b expression, and the count of BM MNCs determined in monkeys before and after the induction of HC and during the treatment and post-treatment periods are shown in Table 2. The total PB WBC count, monocyte count, and percentage of CD14+/CD45+ cells within the monocyte gate in monkeys assigned to vehicle- and losartan-treatment groups were unchanged by HC, treatment, or recovery from treatment. In contrast, the number of BM MNCs recovered from BM aspirates was greater (P < 0.05) compared to NC baseline after 15 weeks of HC diet in monkeys preassigned to both treatment groups. After 15 weeks of treatment, the numbers of BM MNCs remained greater than at NC baseline in monkeys in both treatment groups (P < 0.05), but were lower (P < 0.05) in the losartan- compared to the vehicle-treated group. Six weeks after discontinuing treatment, the number of BM MNC in losartan-treated monkeys returned to the same level as in vehicle-treated monkeys (P > 0.05). The percentage of cells expressing CD11b within both PB and BM monocyte gates was increased (P < 0.05) 5-fold and 10-fold, respectively, by the induction of dietary HC. Losartan had no effect on CD11b expression by monocytes before the third week of treatment (data not shown). Compared to vehicle-treated monkeys, both PB and BM monocyte CD11b expression were normalized by losartan treatment, but returned to pretreatment levels 6 weeks after ending treatment.
Table 2.
Peripheral blood and bone marrow mononuclear cell values
| NC | HC | Tx | Post-Tx | |||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | Losartan | Vehicle | Losartan | Vehicle | Losartan | Vehicle | Losartan | |
| PB WBC × 106 mm-3 | 9.1 ± 1.0 | 10.3 ± 1.3 | 10.1 ± 0.9 | 10.9 ± 1.5 | 10.6 ± 1.5 | 10.6 ± 2.1 | 10.1 ± 2.9 | 10.4 ± 1.7 |
| PB Monocytes × 106 mm-3 | 2.6 ± 0.2 | 2.6 ± 0.2 | 2.5 ± 0.2 | 2.3 ± 0.3 | 2.9 ± 0.3 | 2.5 ± 0.3 | 2.4 ± 0.2 | 2.6 ± 0.4 |
| BMMNC × 106 mm-3 | 55 ± 6 | 50 ± 6 | 72 ± 6* | 72 ± 5* | 78 ± 6* | 62 ± 5*† | 81 ± 5* | 73 ± 6* |
| PB CD14+/CD45+ Gate,% | 76 ± 6 | 71 ± 7 | 81 ± 8 | 75 ± 4 | 72 ± 8 | 80 ± 8 | 76 ± 9 | 80 ± 9 |
| PB Monocyte CD11b+,% | 2.2 ± 0.1 | 2.3 ± 0.6 | 11.6 ± 2.3* | 12.4 ± 4.2* | 9.4 ± 2.2* | 3.6 ± 1.1† | 9.8 ± 2.8* | 12.0 ± 2.8* |
| BMMNC Monocyte CD11b+,% | 6.3 ± 0.6 | 8.3 ± 0.4 | 36.6 ± 6.3* | 42.4 ± 7.2* | 39.7 ± 8.2* | 13.6 ± 4.4† | 39.2 ± 7.8* | 32.0 ± 6.9* |
P < 0.05, differences from NC baseline.
P < 0.05, difference from vehicle-treated group.
Tx, 15 weeks of treatment; Post-Tx, 6 weeks post-treatment. PB WBC, peripheral blood white blood cell counts; BMMNC, bone marrow mononuclear cell counts.
3.3 Effects of Hypercholesterolemia and Losartan on CD34+ HSC Function
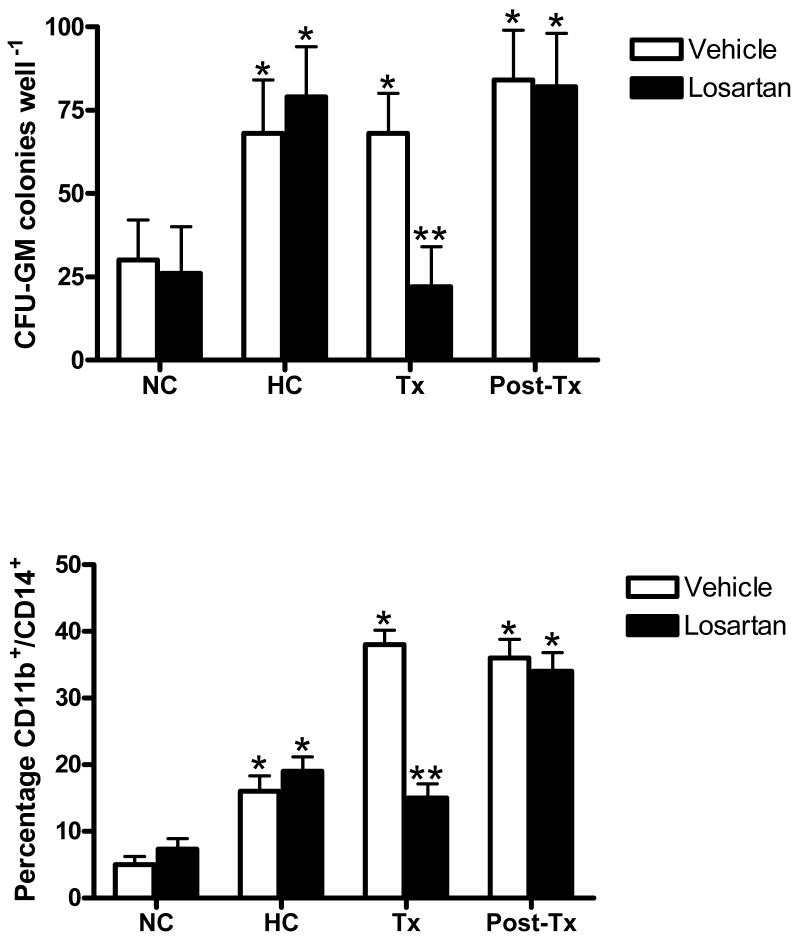
Following two rounds of isolation, the mean cell purity for BM MNCs expressing CD34 was 93.1 ± 2.2%, and the viability determined by trypan blue exclusion was > 95%. The myeloproliferative capacity of CD34+ HSCs, and CD11b+/CD14+ monocyte production by CD34+ HSCs from monkeys treated with vehicle or losartan are shown in Figure 1. HC enhanced the myeloproliferative capacity of CD34+ HSCs from monkeys preassigned to vehicle- and losartan-treatment groups as reflected by increased (P < 0.05) numbers of CFU-GM colonies compared to NC baseline values. The percentage of CD11b+/CD14+ monocytes within single-cell suspensions of clonogenic assay colonies were increased (P < 0.05) after 14 days of growth. CD34+ HSC differentiation was limited to the increased production of cells of the myeloid lineage since colony analysis of BFU-E (19 ± 6 and 21 ± 4 colonies·well-1, respectively) and CFU-GEMM (15 ± 3 and 16 ± 5 colonies·well-1, respectively) were not altered by the establishment of HC (P > 0.05). Monocyte CD11b expression, numbers of CFU-GM colonies, and the percentage of clonogenic assay-derived cells expressing CD11b/CD14 remained elevated above NC baseline values in vehicle-treated monkeys throughout the treatment period (P > 0.05). Six weeks after discontinuation of losartan treatment, circulating monocyte CD11b expression and CD34+ HSC colony formation and monocyte production returned to pre-treatment levels that were not different from those in vehicle-treated monkeys (P > 0.05).
Figure 1.
Effects of dietary induction of hypercholesterolemia (HC) and treatment with losartan or vehicle during sustained HC on myeloid lineage colony formation (CFU-GM) (upper panel) and the percentage of CD11b+/CD14+ monocytic cells cells in clonogenic assays (lower panel). Each condition represents triplicate experiments from each monkey. *P < 0.05, differences from NC baseline. **P < 0.05, difference from vehicle-treated group.
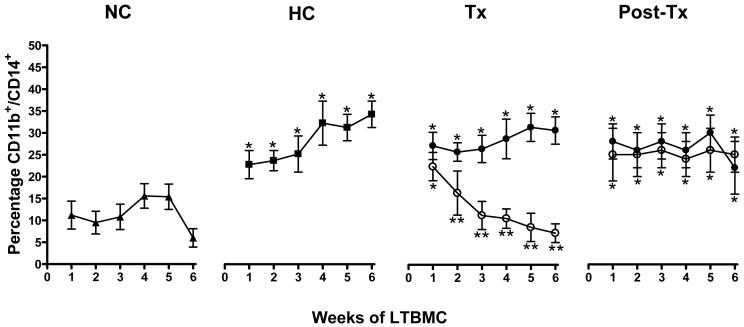
Results of weekly immunophenotyping of NA HSC progeny from LTBMCs established from CD34+ cells obtained at NC and HC baselines, after 15 weeks of treatment, and 6 weeks after discontinuing treatment are shown in Figure 2. The average NA cell recovery (1.27 ± 0.28 × 106 cells·well-1) was not affected by the dietary or treatment protocols, nor did it differ at any point during the 6-week LTBMC assay (P > 0.05). Induction of HC was associated with an increased percentage of NA cells co-expressing CD11b and CD14, a difference from NC baseline values which was maintained for the entire 6 weeks of LTBMC (P < 0.05). After 15 weeks of treatment, LTBMCs established from losartan-treated monkeys had reduced expression (P < 0.05) of CD11b/CD14 by NA cells for all but the first week of culture. Immunophenotyping results of LTBMC NA cells from vehicle-treated monkeys were never different from HC baseline values (P > 0.05). Six weeks after discontinuing treatment, no differences in NA cell CD11b/CD14 expression between treatment groups were observed, nor were values in either group different from pre-treatment values (P > 0.05). NA cells collected from control wells were few in number and expressed neither CD11b nor CD14.
Figure 2.
Analysis of long-term bone marrow culture (LTBMC) CD11b+/CD14+ monocytic cell production 1-6 weeks after seeding with CD34+ hematopoietic stem cells. The proportion of CD11b+/CD14+ nonadherent (NA) cells are represented as percentages of total NA cells recovered from LTBMCs. Each condition represents triplicate experiments from each monkey. Percentages are at normocholesterolemia (NC) baseline (▲), hypercholesterolemia (HC)baseline (■), end of tre atment (Tx), and end of the 6 week post-treatment (Post-Tx) periods in monkeys treated with vehicle (•) or losartan (○). *P < 0.05, differences from NC baseline. **P < 0.05, difference from vehicle-treated group.
3.4 Effects of Ang II and nLDL on CD34+ HSC function
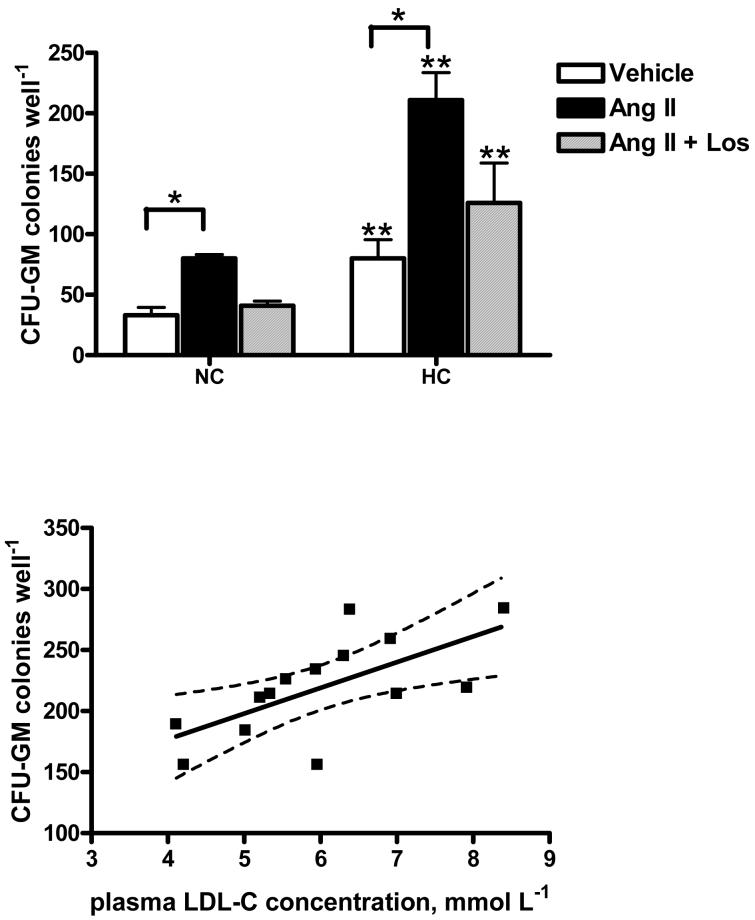
Compared to NC values, flow cytometry analysis of CD34+ cells indicated a doubling of AT1 receptor surface expression 15 weeks after induction of dietary HC (from 46.2±2.3 to 98± 6.1 MFI, P < 0.05). The size and granularity of CD34+ cells assessed by light scattering properties were unchanged throughout the experimental protocol. To determine whether the increase in CD34+ cell AT1 receptor expression was functionally relevant, CD34+ HSCs isolated from monkeys before and after the induction of HC were incubated with Ang II or vehicle and analyzed by clonogenic assays. Figure 3 shows that, compared to vehicle, Ang II stimulation increased (P < 0.05) CFU-GM formation by HSCs isolated from monkeys at both NC and HC baselines. A 2-fold increase in number of CFU-GM colonies formed in response to Ang II was observed in assays of HSCs from monkeys after HC induction compared to NC baseline. Losartan blocked the myeloproliferative effect of Ang II in both cases. Furthermore, plasma LDL concentrations and the in vitro myeloproliferative response of CD34+ HSCs to Ang II stimulation were positively correlated (P = 0.01, r2 = 0.43).
Figure 3.
The effect of Ang II AT1 receptor stimulation on myeloid lineage (CFU-GM) colony forming capacity of CD34+ hematopoietic stem cells (HSCs) isolated from monkeys at normocholesterolemia (NC) and hypercholesterolemia (HC) baselines (upper panel) and correlation between plasma LDL concentrations of monkeys and HSC myeloproliferative response to Ang II stimulation (lower panel). Each condition represents triplicate experiments from each monkey. *P < 0.05, difference from vehicle. **P < 0.05, NC vs. HC. Dotted lines represent 95% confidence intervals.
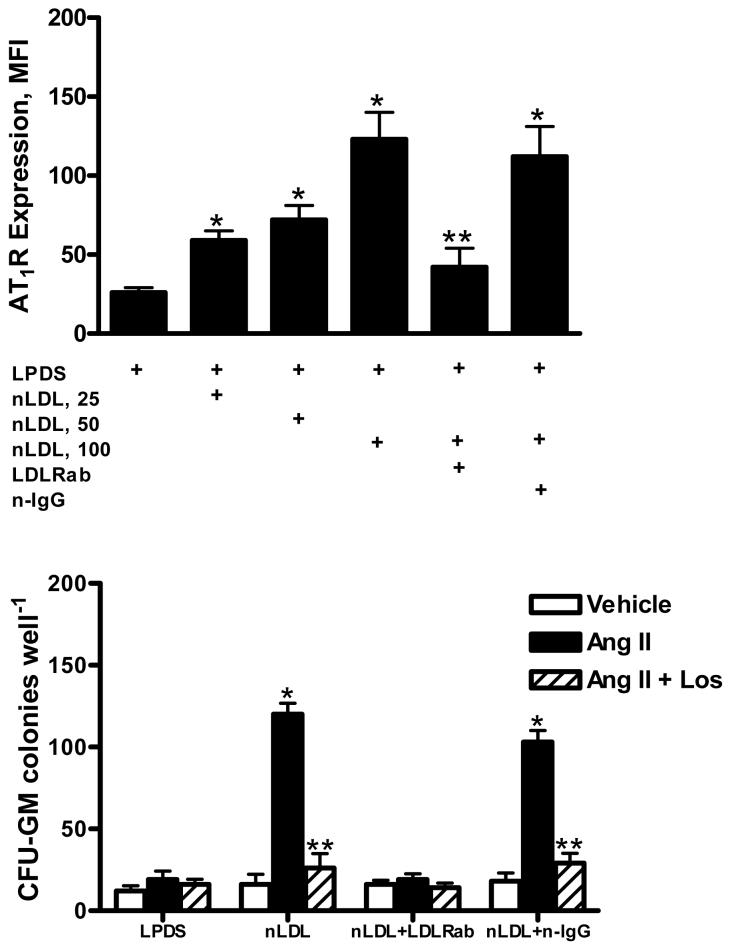
Analysis by flow cytometry demonstrated that 51 ± 8 % of CD34+ BM MNCs co-expressed immunodetectable LDLRs. To determine the regulatory action of nLDL on HSC AT1 receptor expression, CD34+ cells isolated from normocholesterolemic monkeys were incubated with increasing concentrations of nLDL isolated from normocholesterolemic monkeys, and the surface expression of AT1 receptors was estimated by flow cytometry. Figure 4 shows that exposure to nLDL increased (P < 0.05) the AT1 receptor expression by CD34+ cells in a dose-dependent fashion with a maximum response achieved at an nLDL concentration of 100 μg/mL Preincubation of HSCs with a neutralizing LDLR antibody prior to addition of nLDL prevented the stimulatory influence of Ang II. The colony forming capacity of CD34+ HSCs in response to Ang II AT1 receptor stimulation was evaluated in the absence of plasma lipoproteins as well as in the presence of 100 μg/mL nLDL. The addition of Ang II had no effect on HSC myeloproliferation when performed in cultures supplemented with LPDS. In contrast, the addition of 100 μg/mL nLDL to cultures was associated with a 5-fold increase in Ang II-stimulated CFU-GM colony formation. The effect on myeloproliferation exerted by nLDL was reduced (P < 0.05) by preincubation of HSCs with an LDLR neutralizing antibody.
Figure 4.
Effects of native LDL (nLDL) on CD34+ cell AT1 receptor (AT1R) expression (upper panel) and AT1R-mediated myeloproliferation (lower panel). A neutralizing LDL receptor antibody (LDLRab) or nonspecific IgG (n-IgG) was added in some experiments. Each condition represents triplicate experiments from each monkey. The data represent the mean ± SEM of 6 experiments. *P < 0.05, compared to LPDS. **P < 0.05, compared to vehicle.
DISCUSSION
4.1 Hypercholesterolemia simulates myelopoiesis and monocyte CD11b expression in monkeys
In the present study, monkeys provided a cholesterol-containing diet had increased numbers of BM MNCs and CD11b on both PB and BM monocytes. These results agree with results from other animal studies in which dietary hypercholesterolemia was associated with BM hypercellularity and increased monocytic precursor proliferative capacity.9,10 In the present study, the shift in CD34+ HSC function toward the production of CD11b+/CD14+ myeloid cells determined by in vitro assays paralleled the in vivo increase in PB and BM monocyte CD11b expression. The temporal relationship between CD34+ HSC activity and PB monocyte CD11b expression suggests that activated PB monocytic phenotypes derives from HC-driven HSC myeloproliferation.
4.2 Losartan normalizes HSC function in hypercholesterolemic monkeys
We previously showed that hypercholesterolemic monkeys treated with losartan had less atherosclerosis in coronary arteries and aorta than monkeys treated with vehicle, and that monocyte CD11b expression was simultaneously suppressed.5 Similar results from other studies using the ARB olmesartan in the same model confirmed the anti-atherosclerotic actions of systemic AT1 receptor blockade, as well as the anti-inflammatory characteristics of this drug class.25 In our original study, we observed that PB monocyte CD11b expression in hypercholesterolemic monkeys was reduced and remained suppressed for at least 2 weeks after discontinuation of losartan treatment.5 We hypothesized that residual effects related to blockade of AT1 receptor-mediated myelopoiesis within the BM was responsible for the delay in return to PB monocyte CD11b pretreatment values. Our results in the present study demonstrate that the reduction in CD11b expression was not a direct or immediate effect of losartan on monocytes since PB and BM monocyte CD11b expression was unaffected during the first 3 weeks of treatment with losartan (data not shown). Instead, the temporal relationship between normalization of HSC function and suppressed PB and BM monocyte CD11b expression suggest that the anti-inflammatory effects of losartan in the context of HC are related to blockade of CD34+ HSC AT1 receptors. Taken together, these results indicate that diet-induced HC initially invokes BM RAS activation and an AT1 receptor-mediated myelopoietic shift in HSC function concurrent with proinflammatory changes in both PB and BM monocytic phenotypes.
4.3 LDL receptor-mediated HSC AT1 receptor expression
Our study demonstrates that the induction of dietary HC increases CD34+ cell AT1 receptors and the in vitro myeloproliferative response to Ang II stimulation. We investigated the mechanism for the HC-induced shift in myelopoiesis by evaluating the effect of LDL on CD34+ HSC AT1 receptor expression and response to Ang II stimulation. The rationale for this study lay in earlier reports showing that the CD34+ HSC myeloproliferative response to Ang II was a function of serum concentration, with the proliferative response to Ang II suppressed and even reversed when serum concentrations were reduced or eliminated.26 Earlier investigations reported that the LDL subfraction in serum was responsible for in vitro growth stimulation of HSCs.27 These results taken together with those of the present study suggest that an interaction between Ang II and LDL exerts a substantial influence on myeloproliferation. The increased AT1 receptor expression by CD34+ cells incubated with nLDL, the subsequent exaggerated response to Ang II stimulation, and the ability to block these responses to nLDL exposure with an LDLR neutralizing antibody provide a plausible mechanism for enhanced HSC inflammatory capacity relative to increased plasma LDL concentrations.
4.4 Role of the bone marrow RAS in production of activated monocytes
The identification of a complete RAS in several tissues suggested that Ang II produced by hematopoietic, stromal, fat and other cells within the BM microenvironment could regulate hematopoiesis in an autocrine/paracrine manner. With the objective of identifying a local BM RAS that might function in a hematoregulatory capacity, we previously determined that mRNA and protein for all major components of the RAS were present in BM and that marrow stromal cells have a complete RAS that is capable of generating Ang II.28 The emerging model of the BM as an ancillary component of atherogenesis is supported by the present study and previous studies in animal models of HC-induced atherosclerosis which exhibited changes in BM consistent with amplified myelopoiesis. Collectively, these data suggest that HC contributes significantly to the functional disruption of BM HSCs. Moreover, activation of the BM RAS by HC may be an underlying mechanism linking HSC function, inflammation and the atherogenic process. The link between inflammation, atherosclerosis, hypercholesterolemia, and tissue RAS activation suggests that cholesterol-lowering with HMG-CoA reductase inhibitors (“statins”) may augment the anti-inflammatory and anti-atherosclerotic actions of AT1 receptor blockade. Studies combining ARB and statin therapy in patients with dyslipidemias hold promise for enhancement of the anti-inflammatory actions of ARB monotherapy since clinical trials indicate the benefit of a combination regimen in cardiovascular disease management and inflammation.29 An immunomodulatory effect of combined treatment was shown in hypercholesterolemic patients where treatment with statins reduced the number of AT1 receptors on thrombocytes.30 Further studies are warranted to investigate the benefit of the potentially synergistic actions of ARBs and statins on HSC function in the context of HC, and whether this combination therapy provides additional suppression of HC-induced inflammation.
ACKNOWLEDGEMENTS
In addition to National Heart, Lung, and Blood Institute Grants HL-068258 and HL-051952 (to C. M. Ferrario) and American Heart Association Grant 0130405N (to W. B. Strawn), the authors gratefully acknowledge grant support in part provided by Unifi (Greensboro, NC), the Farley-Hudson Foundation (Jacksonville, NC), and Merck & Co., Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Weber C, Erl W, Weber KS, Weber PC. HMG-CoA reductase inhibitors decrease CD11b expression and CD11b-dependent adhesion of monocytes to endothelium and reduce increased adhesiveness of monocytes isolated from patients with hypercholesterolemia. J Am Coll Cardiol. 1997;30:1212–1217. doi: 10.1016/s0735-1097(97)00324-0. [DOI] [PubMed] [Google Scholar]
- 2.Strehlow K, Wassmann S, Bohm M, Nickenig G. Angiotensin AT1 receptor over-expression in hypercholesterolaemia. Ann Med. 2000;32:386–389. doi: 10.3109/07853890008995944. [DOI] [PubMed] [Google Scholar]
- 3.Mateo T, bu Nabah YN, Abu TM, Mata M, Cerda-Nicolas M, Proudfoot AE, Stahl RA, Issekutz AC, Cortijo J, Morcillo EJ, Jose PJ, Sanz MJ. Angiotensin II-induced mononuclear leukocyte interactions with arteriolar and venular endothelium are mediated by the release of different CC chemokines. J Immunol. 2006;176:5577–5586. doi: 10.4049/jimmunol.176.9.5577. [DOI] [PubMed] [Google Scholar]
- 4.Khan BV, Navalkar S, Khan QA, Rahman ST, Parthasarathy S. Irbesartan, an angiotensin type 1 receptor inhibitor, regulates the vascular oxidative state in patients with coronary artery disease. J Am Coll Cardiol. 2001;38:1662–1667. doi: 10.1016/s0735-1097(01)01615-1. [DOI] [PubMed] [Google Scholar]
- 5.Strawn WB, Chappell MC, Dean RH, Kivlighn S, Ferrario CM. Inhibition of early atherogenesis by losartan in monkeys with diet-induced hypercholesterolemia. Circulation. 2000;101:1586–1593. doi: 10.1161/01.cir.101.13.1586. [DOI] [PubMed] [Google Scholar]
- 6.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 7.Nishida M, Fujinaka H, Matsusaka T, Price J, Kon V, Fogo AB, Davidson JM, Linton MF, Fazio S, Homma T, Yoshida H, Ichikawa I. Absence of angiotensin II type 1 receptor in bone marrow-derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest. 2002;110:1859–1868. doi: 10.1172/JCI200215045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers KE, Xiong S, Steer R, diZerega GS. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells. 2000;18:287–294. doi: 10.1634/stemcells.18-4-287. [DOI] [PubMed] [Google Scholar]
- 9.Averill LE, Meagher RC, Gerrity RG. Enhanced monocyte progenitor cell proliferation in bone marrow of hyperlipemic swine. Am J Pathol. 1989;135:369–377. [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman DL, Mogelesky TC, Liptak BF, Gerrity RG. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler Thromb. 1991;11:985–994. doi: 10.1161/01.atv.11.4.985. [DOI] [PubMed] [Google Scholar]
- 11.Nickenig G, Wassmann S, Bohm M. Regulation of the angiotensin AT1 receptor by hypercholesterolaemia. Diabetes Obes Metab. 2000;2:223–228. doi: 10.1046/j.1463-1326.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 12.John S, Delles C, Klingbeil AU, Jacobi J, Schlaich MP, Schmieder RE. Low-density lipoprotein-cholesterol determines vascular responsiveness to angiotensin II in normocholesterolaemic humans. J Hypertens. 1999;17:1933–1939. doi: 10.1097/00004872-199917121-00024. [DOI] [PubMed] [Google Scholar]
- 13.Nickenig G, Sachinidis A, Michaelsen F, Bohm M, Seewald S, Vetter H. Upregulation of vascular angiotensin II receptor gene expression by low-density lipoprotein in vascular smooth muscle cells. Circulation. 1997;95:473–478. doi: 10.1161/01.cir.95.2.473. [DOI] [PubMed] [Google Scholar]
- 14.Ettinger WH, Dysko RC, Clarkson TB. Prednisone increases low density lipoprotein in cynomolgus monkeys fed saturated fat and cholesterol. Arteriosclerosis. 1989;9:848–855. doi: 10.1161/01.atv.9.6.848. [DOI] [PubMed] [Google Scholar]
- 15.Yasar U, Forslund-Bergengren C, Tybring G, Dorado P, Llerena A, Sjoqvist F, Eliasson E, Dahl ML. Pharmacokinetics of losartan and its metabolite E-3174 in relation to the CYP2C9 genotype. Clin Pharmacol Ther. 2002;71:89–98. doi: 10.1067/mcp.2002.121216. [DOI] [PubMed] [Google Scholar]
- 16.Shibata H, Hanazono Y, Ageyama N, Nagashima T, Ueda Y, Hasegawa M, Ozawa K, Yoshikawa Y, Terao K. Collection and analysis of hematopoietic progenitor cells from cynomolgus macaques (Macaca fascicularis): Assessment of cross-reacting monoclonal antibodies. Am J Primatol. 2003;61:3–12. doi: 10.1002/ajp.10104. [DOI] [PubMed] [Google Scholar]
- 17.Duvillard L, Florentin E, Lizard G, Petit JM, Galland F, Monier S, Gambert P, Verges B. Cell surface expression of LDL receptor is decreased in type 2 diabetic patients and is normalized by insulin therapy. Diabetes Care. 2003;26:1540–1544. doi: 10.2337/diacare.26.5.1540. [DOI] [PubMed] [Google Scholar]
- 18.Rasini E, Cosentino M, Marino F, Legnaro M, Ferrari M, Guasti L, Venco A, Lecchini S. Angiotensin II type 1 receptor expression on human leukocyte subsets: A flow cytometric and RT-PCR study. Regul Pept. 2006 doi: 10.1016/j.regpep.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Drouet M, Mourcin F, Grenier N, Leroux V, Denis J, Mayol JF, Thullier P, Lataillade JJ, Herodin F. Single administration of stem cell factor, FLT-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: long-term follow-up of hematopoiesis. Blood. 2004;103:878–885. doi: 10.1182/blood-2003-05-1400. [DOI] [PubMed] [Google Scholar]
- 20.Kansas GS, Muirhead MJ, Dailey MO. Expression of the CD11/CD18, leukocyte adhesion molecule 1, and CD44 adhesion molecules during normal myeloid and erythroid differentiation in humans. Blood. 1990;76:2483–2492. [PubMed] [Google Scholar]
- 21.Winter SS, Sweatman JJ, Larson RS. Improved quantification of cell survival on stromal monolayers by flow cytometric analyses. Cytometry. 2000;40:26–31. [PubMed] [Google Scholar]
- 22.Krizanac-Bengez L, Moore PF, Barsoukov A, Sandmaier BM. The expression and differentiation pattern of cell antigens and adhesion molecules on the nonadherent cell population in canine long-term marrow culture: a biphasic development of myeloid and lymphoid cells. Tissue Antigens. 1998;51:141–155. doi: 10.1111/j.1399-0039.1998.tb02959.x. [DOI] [PubMed] [Google Scholar]
- 23.Poumay Y, Ronveaux-Dupal MF. Rapid preparative isolation of concentrated low density lipoproteins and of lipoprotein-deficient serum using vertical rotor gradient ultracentrifugation. J Lipid Res. 1985;26:1476–1480. [PubMed] [Google Scholar]
- 24.Han KH, Chen Y, Chang MK, Han YC, Park JH, Green SR, Boullier A, Quehenberger O. LDL activates signaling pathways leading to an increase in cytosolic free calcium and stimulation of CD11b expression in monocytes. J Lipid Res. 2003;44:1332–1340. doi: 10.1194/jlr.M200427-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki M, Takai S. Anti-atherosclerotic efficacy of olmesartan. J Hum Hypertens. 2002;16(Suppl 2):S7–12. doi: 10.1038/sj.jhh.1001393. [DOI] [PubMed] [Google Scholar]
- 26.Brunet dlG, Ivanovic Z, Leprivey-Lorgeot V, Praloran V. Angiotensin II that reduces the colony-forming ability of hematopoietic progenitors in serum free medium has an inverse effect in serum-supplemented medium. Stem Cells. 2002;20:269–271. doi: 10.1634/stemcells.20-3-269. [DOI] [PubMed] [Google Scholar]
- 27.Aye MT, Seguin JA, McBurney JP. Erythroid and granulocytic colony growth in cultures supplemented with human serum lipoproteins. J Cell Physiol. 1979;99:233–238. doi: 10.1002/jcp.1040990210. [DOI] [PubMed] [Google Scholar]
- 28.Strawn WB, Richmond RS, Ann TE, Gallagher PE, Ferrario CM. Renin-angiotensin system expression in rat bone marrow haematopoietic and stromal cells. Br J Haematol. 2004;126:120–126. doi: 10.1111/j.1365-2141.2004.04998.x. [DOI] [PubMed] [Google Scholar]
- 29.Sinha AK, Mehta JL. Modulation of atherosclerosis, blood pressure and arterial elasticity by statins. Adv Cardiol. 2007;44:315–330. doi: 10.1159/000096750. [DOI] [PubMed] [Google Scholar]
- 30.Nickenig G, Baumer AT, Temur Y, Kebben D, Jockenhovel F, Bohm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100:2131–2134. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]