Abstract
Acetylcholine allows the elicitation of visually evoked behaviors mediated by the frog optic tectum, but the mechanisms behind its effects are unknown. Although nicotinic acetylcholine receptors (nAChRs) exist in the tectum, their subtype has not been assessed. By using quantitative autoradiography, we examined the binding of [3H]cytisine and [125I]α-bungarotoxin in the laminated tectum. In mammalian systems, these radioligands bind with high affinity to α4 nAChR subunits and α7 nAChR subunits, respectively. [3H]Cytisine demonstrated high specific binding in adult frogs in retinorecipient layer 9, intermediate densities in layer 8, and low binding in layers 1–7 of the tectum. [3H]Cytisine binding was significantly higher in the tecta of adults than in those of tadpoles. Lesioning the optic nerve for 6 weeks decreased [3H]cytisine binding in layers 8/9 by 70 ± 1%, whereas 6-month lesions decreased binding by 76 ± 3%. Specific binding of [125I]α-bungarotoxin in adults was present only at intermediate levels in tectal layers 8 and 9, and undetectable in the deeper tectal layers. However, the nucleus isthmi, a midbrain structure reciprocally connected to the tectum, exhibited high levels of binding. There were no significant differences in tectal [125I]α-bungarotoxin binding between tadpoles and adults. Six-week lesions of the optic nerve decreased tectal [125I]α-bungarotoxin binding by 33 ± 10%, but 6-month lesions had no effect. The pharmacokinetic characteristics of [3H]cytisine and [125I]α-bungarotoxin binding in the frog brain were similar to those demonstrated in several mammalian species. These results indicate that [3H]cytisine and [125I]α-bungarotoxin identify distinct nAChR subtypes in the tectum that likely contain non-α7 and α7 subunits, respectively. The majority of non-α7 receptors are likely associated with retinal ganglion cell terminals, whereas α7-containing receptors appear to have a different localization.
Indexing terms: cholinergic, visual system, autoradiography
The neurotransmitter acetylcholine (ACh) plays a role in both the processing and functional organization of visual information in mammals (Nobili and Sannita, 1997). The cerebral cortex is densely innervated by a cholinergic projection from the basal forebrain (Sato et al., 1987b). Acetylcholine supplied from this projection influences both neuronal excitability (Sato et al., 1987a; Roerig et al., 1997) and receptive field size (Greuel et al., 1988) in the striate cortex, the primary visual center. In addition, ACh contributes to the developmental plasticity of the visual cortex, affecting ocular dominance column plasticity (Imamura and Kasamatsu, 1989), the connectivity of inputs from the lateral geniculate (Liu et al., 1994), and the induction of long-term potentiation (Bröcher et al., 1992) and long-term depression (Kirkwood et al., 1999). It is also thought to serve as a permissive factor, along with norepinephrine and serotonin, in visual map formation (Bear and Singer, 1986; Gu and Singer, 1993, 1995). The mechanisms by which ACh exerts these effects are, however, still unclear.
The optic tectum, the main visual area of nonmammalian vertebrates, has served as a model system for the investigation of mechanisms subserving visual signal processing and the structural organization of topographic maps (Constantine-Paton et al., 1990; Cline, 1991, 1998; Ernst et al., 1998; Udin and Grant, 1999). In the frog, most of the ACh released in the tectum is synthesized by another midbrain structure, the nucleus isthmi (Desan et al., 1987; Wallace et al., 1990; Marín and González, 1999). Ablation of the nucleus isthmi produces a visual scotoma even though retinal input remains intact (Gruberg et al., 1991). Visual responses in the tectum can also be enhanced by direct stimulation of the nucleus isthmi (King and Schmidt, 1991) or application of ACh (Fite and Wang, 1986). Consistent with the idea that ACh is important to proper visual system functioning is the fact that toxins directed against the cholinergic system disrupt the topography of the activity-dependent retinotectal map (Schmidt, 1985, 1995).
One way in which ACh could be influencing the activity of the retinotectal system is through nicotinic ACh receptors (nAChR). Acetylcholine binding to nAChRs directly gates cation channels that can cause rapid depolarization of the neuron (Role and Berg, 1996). They are composed of a diverse set of protein subunits and consequently have a wide range of structural and pharmacological properties. Central nAChRs maintain the pentameric structure of neuromuscular nAChRs. Most central nAChRs consist of two α and three β subunits, but some are homopentamers comprised of α subunits only. Each subunit type has several isoforms: eight genes code for the α subunits (designated α2–9), and three genes express the β subunits (β2–4). Ectopic expression in Xenopus oocytes has shown that different functional combinations of α and β subunits confer differences in ion permeability, channel open time, and cellular regulation of the nAChR (Role and Berg, 1996; Wonnacott, 1997; Fenster et al., 1997).
Differential distribution of nAChR-subunit mRNA in the mammalian brain suggests that a range of nAChR subtypes exists (Boulter et al., 1986, 1990; Goldman et al., 1987; Wada et al., 1988; Deneris et al., 1988, 1989; Duvoisin et al., 1989; Schoepfer et al., 1990; Couturier et al., 1990; Seguela et al., 1993; Elgoyhen et al., 1994). The two predominant nAChR subtypes are differentiated by their sensitivity to inhibition by low concentrations of α-bungarotoxin (Lindstrom, 1995; Marks et al., 1998). Nicotinic AChRs that are insensitive to α-bungarotoxin have relatively high affinity for nicotinic agonists. Most of these receptors bind cytisine, a selective ligand for the α4 nAChR subunit (Whiting and Lindstrom, 1988; Flores et al., 1992). α-Bungarotoxin-sensitive nAChRs are thought to be homo-oligomers of the α7 gene product (Seguela et al., 1993). The α7 nAChR is of particular interest because its calcium permeability may be greater than that of the N-methyl-D-aspartate subtype of glutamate receptors (Rogers and Dani, 1995). It could therefore play a significant role in biochemical changes important for neuronal plasticity (Role and Berg, 1996).
Although some nicotinic receptor-like molecules have been identified in the tectum by using immunocytochemical techniques (Sargent et al., 1989; Titmus et al., 1999), their subtype and the extent to which they are present has not been investigated. In this study, we used [3H]cytisine and [125I]α-bungarotoxin to demonstrate the existence, laminar distribution, developmental expression, and pharmacology of two distinct nAChRs in the optic tectum of the frog, Rana pipiens.
Materials and Methods
Animals
Adult Rana pipiens were purchased from either Charles Sullivan (Nashville, TN) or J.M. Hazen (Alburg, VT). These adult animals were kept in 10-gallon glass tanks and fed mealworms. Tadpoles were raised in the laboratory from fertilized eggs and kept in 4-gallon plastic tubs. They were fed boiled nettle until they were large enough to eat boiled Romaine lettuce. The animals were staged according to the criteria put forth by Taylor and Kollros (1946). Animal care and all surgical procedures used were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Tissue collection and preparation
Binding site localization
Tissue was collected from adult frogs after they were deeply anesthetized by intraperitoneal injection (100 μl/15 g animal weight) of 2% ethyl m-aminobenzoate (MS-222). Their chest cavities were opened, and they were perfused through the conus arteriosus with ice-cold (4°C) buffer (Tris-HCl, 50 mM, NaCl, 100 mM, pH 7.4). The brains were extracted, the pia mater was removed, and the unfixed tissue was quick-frozen in −25°C isopentane. The frozen brains were then cut along the sagittal or frontal plane into 20-μm sections on a cryostat (Reichert-Jung, Cryocut 1800, West Germany). The sections were thaw-mounted on Superfrost Plus slides (VWR Scientific Products, St. Louis, MO) and dried overnight in a vacuum dessicator at 4°C. The mounted sections were then stored at −80°C until use or directly prepared for radioligand binding the next day.
Receptor autoradiography was performed through standard methods (Leslie et al., 1988). Briefly, the tissue was slowly brought to room temperature, preincubated in buffer for 15 minutes, then incubated in a buffer solution containing radioligand. Nonspecific binding was determined on adjacent tissue sections by the addition of excess nicotine (10 μM) to the radioligand solutions. Incubations with [3H]cytisine (5 nM; 32 Ci/mmol) were run for 90 minutes at 4°C and were terminated by a 5-minute wash in fresh buffer solution, a 5-minute wash in 10% buffer, and a 10-second dip in deionized water. Experiments using [125I]α-bungarotoxin (2.5 nM; 2,200 Ci/mmol) had bovine serum albumin (1 mg/ml) added to the buffers in order to protect the radioligand from proteases endogenous to the tissue. [125I]α-Bungarotoxin incubations lasted for 3 hours at room temperature and were ended with a 20-minute wash in fresh buffer, two 20-minute washes in 10% buffer, and a 10-second dip in deionized water (adapted from Aubert et al., 1996). After washing, the tissue was dried under a gentle stream of ambient air and placed in a vacuum dessicator overnight at room temperature. The following day quantitative standards (Amersham Corp., Arlington Heights, IL) and the treated tissue were set together against 3H-Hyperfilm (Amersham). The film was exposed for an appropriate length of time (generally 6 weeks for [3H]cytisine and 2 weeks for [125I]α-bungarotoxin) and the resulting autoradiograms were captured for digital analysis. Finally, the treated tissue was defatted with xylene and ethanol, and then Nissl-stained with thionin (Clarke et al., 1985). The brightfield images were then captured for morphological analysis (see Data Analysis). Both radioligands were purchased from New England Nuclear (Boston, MA). All other reagents were purchased from Sigma (St. Louis, MO).
Developmental studies
Tissue from adults and tadpoles (stages V, X, XV, XX, XXV; Taylor and Kollros, 1946) was prepared for autoradiography as described above. The preparation of tadpole tissue was similar to that of adults except that the animals were anesthetized by immersion in 0.1% MS-222, decapitated, and the whole heads were quick-frozen in −25°C isopentane. In order to avoid differences in radioligand concentration between experiments the binding runs were performed on all stages at the same time with the same incubation solutions. Furthermore, to avoid possible sensitivity differences in the 3H-Hyperfilm, the treated tissue from all stages was exposed to the same piece of film.
Optic nerve lesions
Optic nerve lesions were performed in order to help determine whether the radioligand binding sites resided on retinal ganglion cells terminals or on other tectal elements. Animals were anesthetized by injection, and the optic nerve was accessed through the roof of the buccal cavity (Liu and Debski, 1995). The optic nerve was severed approximately 1 mm from the back of the right eye. The cut ends of the nerve were then cauterized with a Vetroson pocket cauterizer to prevent regeneration. The wound was sealed with Vetbond (3M Co., St. Paul, MN). Feeding resumed within 3 days after surgery, and the animals were allowed to survive for 6 weeks (short term) or 6 months (long term). Previous studies have shown that short-term lesions allow for the degeneration of most of the presynaptic retinal fibers in the tectum, whereas the long-term lesions allow for the degeneration of all retinal fibers (Matsumoto and Scalia, 1981; Kuljis and Karten, 1983; Sargent et al., 1989). Behavioral tests conducted during the survival period showed that animals responded to prey seen only through the left eye indicating that the cut optic nerve had not regenerated. At the end of the survival period the frogs were anesthetized, the optic nerve lesion was confirmed, and the brain was prepared for autoradiography as described above.
Binding characterization studies
Pharmacological analyses were used to determine the selectivity of the radioligands for nAChR-like sites. Saturation and competition analyses of nicotinic radioligand binding were performed through autoradiographic methods (Leslie et al., 1988). All experiments employed serial sections that were sorted so that differences in binding densities due to possible anatomical variations were evenly distributed. Nonspecific binding of the two radioligands was accounted for by competition with excess nicotine (10 μM) on adjacent sections. Saturation experiments with [3H]cytisine used eight concentrations of radioligand between 0.18 and 23.4 nM, whereas those with [125I]α-bungarotoxin used eight concentrations between 0.12 and 20.2 nM. Competition experiments with [3H]cytisine (5 nM) used eight concentrations of dihydro-β-erythroidine (DHBE; 100 pM to 1 mM), methyllycaconitine (MLA; 100 pM to 1 mM), and unlabeled α-bungarotoxin (100 pM to 300 μM). Competition experiments with [125I] α-bungarotoxin (2.5 nM) used eleven concentrations of MLA (100 fM to 100 μM), and eight concentrations of unlabeled cytisine and DHBE (1 nM to 10 mM).
Data analysis
Densitometry
Images were captured by using a digital camera (Dage CCD100, Michigan City, IN) connected to an Apple PowerPC running NIH Image 1.61 (NIH website). Binding densitometry was performed by comparing the optical densities of tissue autoradiograms to those made by the radioactive standards, which contained a known amount of radioactivity and were the same thickness as the tissue sections. The grayscale values of the standards were converted from nCi/mg wet tissue weight to fmol/mg wet tissue weight, based upon the specific activity of the radioligand being used. A standard curve, constructed from a third-degree polynomial equation, interpolated the grayscale value into the actual binding density of the radioligand in the tissue. In all experiments, specific binding was calculated as the difference between total and nonspecific binding. All graphical results were plotted and statistically analyzed with Graph Pad Prism 3.0 (Graph Pad Software, Inc., San Diego, CA).
Binding site localization
For localization studies, the stained tissue images were superimposed with their respective autoradiograms by using Adobe Photoshop 5.0. The staining procedure did not cause any appreciable shrinkage of the tissue because the tissue had already been dried during its preparation for autoradiography. Consequently, the alignment of the two types of images was precise. These experiments compared sections from a minimum of four animals per treatment group and were performed in duplicate or triplicate.
Developmental studies
For developmental studies, binding density measurements were constrained to a sampling area of 25 × 25 μm (625 μm2) in the superficial tectum. The purpose of this constraint was to ensure that we were sampling only from the retinorecipient, superficial tectal layers even in the youngest animals. The optic tectum matures following rostrocaudal and lateral-medial gradients: tectal layers and thickness are first established in rostrolateral regions and then extend progressively more caudomedially as the animal matures (Reh and Constantine-Paton, 1984). The size chosen for our sample area was based upon our own observations that the superficial layer of the posterolateral tectum in stage V animals was 95 ± 6-μm-thick (n = 4) when sliced in the sagittal plane. To further examine the anatomical differences in the tectum during development we sampled from three distinct areas of the retinorecipient layers in sagittal sections: midway at the highest point of the tectum, anterior at approximately 45° from the midpoint, and posterior at approximately 45° from the midpoint (Fig. 1). Significance across all of the stages and between areas of the same age was determined with one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. These studies compared sections from four animals per stage with at least three sections from each animal.
Fig. 1.

Schematic of a sagittal section through the adult optic tectum illustrating the location of the anterior, middle, and posterior tectal areas sampled in the developmental studies. Densitometry readings were taken from a 625-μm2 region in retinorecipient layer 9 for each of these areas. Scale bar = 500 μm.
Optic nerve lesions and pharmacological analyses
The entirety of layers 8 and 9 of the adult optic tectum were quantified in the optic nerve lesion studies and pharmacological experiments because the majority of tectal binding was present in these layers. In the lesion experiments using [3H]cytisine, layers 1–7 were also analyzed because specific binding was present in those layers in normal animals and a qualitative difference in those layers was readily apparent between afferented and deafferented lobes. Significant differences between afferented and deafferented tectal lobes in the lesion studies were determined by a Student's paired, two-tailed t-test. In the [125I]α-bungarotoxin competition experiments, the average level of nonspecific binding was reported as 0% total specific binding. All of these experiments used sections from at least four animals and were performed in duplicate or triplicate.
Results
Localization of binding sites in the optic tectum
The optic tectum contains alternating fibrous and cellular laminae (Székely and Lázár, 1976). The most superficial of these laminae is designated layer 9 and is a plexiform layer containing only scattered cell bodies. The majority of tectal afferents, including those from retinal ganglion cells and many from the nucleus isthmi, come into layer 9 where they synapse with tectal dendrites. Most of the cell bodies from which these dendrites originate are found deep within the tectum in layer 6, its main cellular layer. We first wanted to demonstrate whether [3H]cytisine and [125I]α-bungarotoxin would bind specifically to the optic tectum and to identify the laminar location of those binding sites. The location of [3H]cytisine and [125I]α-bungarotoxin binding sites was determined by digital imaging, which allowed the results of standard receptor autoradiography and tissue staining to be superimposed.
In adult frogs, [3H]cytisine (5 nM) bound at high density in a band restricted to tectal layer 9 (Fig. 2A,C). In lateral sections this band ran from the rostral to caudal tectum with little observable change in position or width. However, in more medial sections, the high-density binding in layer 9 was not as wide nor did it extend to the most posterior tectal regions (data not shown). These results were consistent with the thinning and shortening of the retinorecipient layer demonstrated in anatomical studies (Scalia, 1976). Intermediate binding densities were seen in layer 8, and their lateral to medial profile was identical to that of layer 9 binding. Layers 1–7 had low levels of [3H]cytisine binding sites, but changes in the expression of binding sites in these layers were not evident between lateral and medial sections. Nonspecific binding, as determined by the addition of excess nicotine (10 μM) was not detected when [3H]cytisine was used at 5 nM (Fig. 2D). However, increasing the radioligand concentration above 10 nM resulted in a nonspecific binding value that was 5–10% of the total binding. [125I]α-Bungarotoxin binding was also found in the adult optic tectum and was primarily localized to its superficial layers (Fig. 2E,G). Only intermediate densities of binding were seen in layers 8 and 9, and, unlike the [3H]cytisine autoradiograms, there was no clear demarcation between these two layers in terms of binding density. Rather, the binding sites formed a broad, diffuse band that encompassed both of these layers. This band was similar to that seen for [3H]cytisine in that it did not extend into the most posterior regions of medial tectal sections. However, unlike the [3H]cytisine binding pattern, there was also a decrease in binding density evident in the posterior regions of the lateral tectum. Very little, if any, specific binding was detected in layers 1 through 7, but very strong binding was found in a pretectal region. Nonspecific binding, as determined by competition with nicotine (10 μM), ranged between 15% and 30% of the total binding (Fig. 2H). Higher concentrations of nicotine did not reduce the nonspecific binding any further, nor did competition with methyllycaconitine (MLA; 10 μM), a known antagonist at [125I]α-bungarotoxin sites (Barrantes et al., 1995).
Fig. 2.
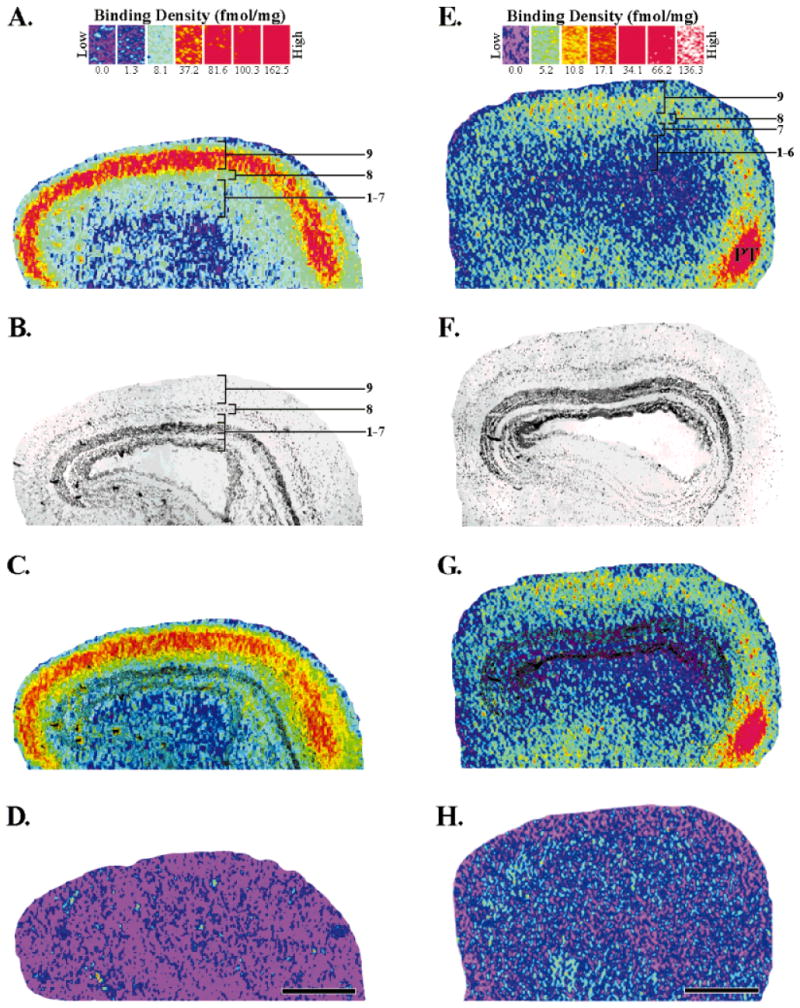
Localization of [3H]cytisine (A,C) and [125I]α-bungarotoxin (E,G) binding sites in the adult optic tectum. The binding scales above each column provide a respective index of binding density (fmol/mg wet tissue weight) for each radioligand (see Materials and Methods for details). A: Specific [3H]cytisine (5 nM) binding in the optic tectum. The binding sites were most dense (red) in layer 9. Layer 8 had intermediate densities (yellow/green), whereas layers 1–7 had low densities (light green/light blue). Tectal layers were assigned by using the superimposed image seen in C. B: Brightfield image of the same tissue section that produced the autoradiogram (A). The optic tectum is a laminated structure consisting of alternating cellular and plexiform layers. The darkly stained, main cellular layer is layer 6. C: Superimposition of images A and B which accurately localizes the binding to specific, tectal layers. D: Nonspecific binding, as determined by the addition of nicotine (10 μM) to the incubation solution, was not detected when using [3H]cytisine at 5 nM. E: Total [125I]α-bungarotoxin binding (2.5 nM) in the optic tectum. The densest binding was seen in a pretectal area (PT). Intermediate binding densities were present in tectal layers 8 and 9. F: Brightfield image of the section that produced the autoradiogram in E. G: Superimposition of E and F localizes the binding to the tectal layers. H: Nonspecific [125I]α-bungarotoxin binding as determined by the addition of excess nicotine (10 μM). Anterior is to the right. Scale bars = 500 μm.
Developmental profile of [3H]cytisine and [125I]α-bungarotoxin binding site expression
We next wished to determine whether these binding sites, present in the adult system, were also found in the developing tectum. We examined the patterns and density of tectal binding in early, middle, and late tadpole stages and compared them to that found in the adult. For clarity in presenting these results, a schematic illustrating the anatomical relationship of the optic tectum to other areas of the frog brain is shown in Figure 3.
Fig. 3.

Schematic representation of a sagittal section through the frog brain that illustrates the general location and anatomical relationships of the brain areas depicted in Figure 4. Anterior is to the right.
The development of the optic tectum proceeds in an anterolateral to posteromedial fashion that mirrors the growth of retinal ganglion cell terminals into this structure (Reh and Constantine-Paton, 1984). During tectal development, new cells are added along the posteromedial border, behind the more mature, anterolateral areas (Currie and Cowan, 1974). Therefore, the tectum of a young animal is thick in anterolateral regions where afferents are synapsing with maturing tectal cells, but thin in posteromedial regions where cells are being formed and inputs have not yet invaded the superficial neuropil.
[3H]Cytisine binding was detected in every tadpole stage that was sampled (Fig. 4A). The tectal binding patterns in the tadpole were very similar to those in the adult, but a binding gradient matching the expected maturational gradient was evident. In all stages, the strongest [3H]cytisine binding was limited to the most superficial layer of the tectum, and in early stages (V, X) this binding was further constrained to the rostral half of the tectum. In tadpoles, a band of intermediate binding, which strongly resembled the binding in adult layer 8, was also found directly below the robust binding. These two bands decreased in binding density in more posterior regions, particularly in stages V–XX. Binding in the superficial posterior tectum was extremely low until stage XV, when the developing tectum began to assume the adult binding pattern. By stage XXV the binding pattern strongly resembled that of the adult, although the binding densities were lower than in the mature animal. All stages exhibited low binding densities in the deeper layers of the tectum, but stages V and X had slightly higher densities in those layers. Binding in the rostral and caudal thalamus was also observed at all stages.
Fig. 4.
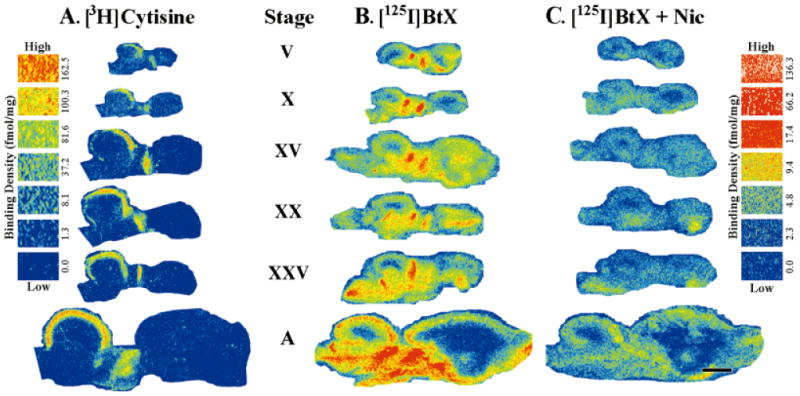
Development and distribution of [3H]cytisine and [125I]α-bungarotoxin binding sites in early (stages V, X), middle (stage XV), and late (stages XX, XXV) tadpole and adult (A) frog optic tectum. A: [3H]Cytisine binding was primarily constrained to the superficial, tectal layers in tadpoles and in adults. Binding was present in every stage examined, although at early stages it was restricted to the rostral half of the tectum. Invasion of binding of the caudal half of the tectum was gradual and progressive with high binding densities achieved only in adulthood. Binding was also evident in the thalamus at all stages examined. Nonspecific binding of [3H]cytisine (5 nM) was not detected (not shown). B: [125I]α-Bungarotoxin (BtX) binding sites in the frog brain were distinct from those seen with [3H]cytisine. Consistent [125I]α-bungarotoxin binding was seen in the superficial tectal layers throughout development. This binding also had a pattern of expression that paralleled the tectal maturation gradient by first presenting itself in the rostral tectum (stages V, X) and then, later in development, in the caudal tectum as well (stages XV and above). The levels of both total and nonspecific binding in the tectum noticeably increased between stage XXV and adult animals. Binding was also observed in the telencephalon and throughout the thalamus and midbrain tegmentum at all stages of development. C: Nonspecific binding, as determined by competition with nicotine (Nic; 10 μM), represented 10% to 30% of the total [125I]α-bungarotoxin binding depending on the brain area sampled. Scale bar = 1 mm.
Specific [125I]α-bungarotoxin binding was also present in the superficial layers of the tectum at every stage examined (compare Fig. 4B,C). As with [3H]cytisine, [125I]α-bungarotoxin binding was constrained by the pattern of tectal maturation, and binding sites were not present in immature areas. At younger stages (V, X) binding sites in the caudal half of the tectum were not detected. As in the adult, binding in the deeper layers of the tadpole tectum was not reliably above nonspecific values. Total binding in the adult tectum was qualitatively higher than in the tadpole stages. However, a concomitant increase occurred in nonspecific binding that made specific binding values equivalent to those in tadpoles (see Fig. 5). [125I]α-Bungarotoxin binding was also observed in the telencephalon and throughout the thalamus and midbrain tegmentum at all stages.
Fig. 5.
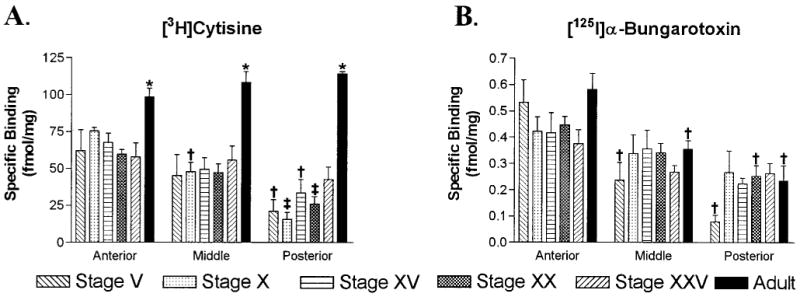
Quantitative analysis of [3H]cytisine and [125I]α-bungarotoxin binding in the retinorecipient layers of the tectum during development. A: Histograms displaying [3H]cytisine (5 nM) binding site densities for anterior, middle, and posterior regions of the optic tectum in tadpoles and adult frogs. Binding densities in every region of the adult tectum were equivalent and significantly higher than those found in any tadpole stage (*P < 0.02). In tadpoles, binding was present at fairly constant levels in the anterior tectum, at slightly lower levels in the middle tectum, and at still lower levels in the posterior tectum. Binding in the posterior tectum tended to increase with development. By stage XXV, binding densities in the posterior tectum were not significantly different from those in the anterior tectum. B: Histograms of [125I]α-bungarotoxin (2.5 nM) binding densities during development. Within the same age group, binding densities were greatest in the anterior tectum, intermediate in middle regions, and lowest in posterior ones. Within each of these regions, binding densities were equivalent across the different age groups. Error bars represent the standard error of the mean (S.E.M.; n = 4). † P < 0.05 compared to the anterior tectum of the same age, and ‡ P < 0.05 compared to both the anterior and middle tectum of the same age.
Quantitative analysis of the superficial layers in the developing tectum demonstrated the developmental pattern of expression for [3H]cytisine binding (Fig. 5A). Binding densities in the adult were similar in anterior, middle, and posterior tectum (P = 0.20, n = 4) and significantly higher than that found at any other developmental stage (P < 0.01, n = 4). There were no significant differences in binding densities between any tadpole stage for a given tectal region. However, at most tadpole stages, binding densities were greatest in anterior regions and smallest in posterior ones. In stages V–XX these decreasing anterior to posterior gradients were significant (P < 0.05, n = 4). By stage XXV, binding densities across the three regions appeared to plateau (P = 0.55, n = 4), reflecting the distribution in the adult. Absolute binding densities, however, remained low in relation to those in adults.
The quantitative analysis of developmental [125I]α-bungarotoxin binding also showed an expression gradient (Fig. 5B). In the adult, there was less binding in middle and posterior tectal regions than in the anterior tectum, and these differences were significant (P = 0.003, n = 4). Tectal binding densities were not significantly different between adults and other tadpole stages (P > 0.10, n = 4). Although qualitative analysis of total binding suggested a markedly higher density of [125I]α-bungarotoxin sites in the adult (see Fig. 4B), quantitation of specific binding demonstrated no such increase. In tadpoles, binding densities were generally highest in the anterior tectum and lowest in posterior regions across all stages. However, these regional differences were significant only at stages V and XX (P < 0.01, n = 4).
One very interesting and unexpected finding of these studies was the observation of [125I]α-bungarotoxin binding in the nucleus isthmi at several developmental stages. We pursued these observations with more experiments that were specifically targeted towards the demonstration of binding in the isthmi at all of the stages we had sampled previously. Figure 6 shows binding in the nucleus isthmi at stages V, XX, XXV, and adults. The nonspecific binding in the isthmi of stages X and XV is quite high, and could be due to regional differences that depend on the plane of section. However, quantification revealed that the total binding in the isthmi of these two stages was roughly twice that of the nonspecific binding in this region. Total binding in the nucleus isthmi of three stage X animals was 2.7 ± 0.7 fmol/mg with 1.5 ± 0.4 fmol/mg of nonspecific binding. Similarly, the isthmi of three stage XV animals had 3.2 ± 0.3 fmol/mg total binding and 1.5 ± 0.3 fmol/mg nonspecific binding. Thus, it appears that [125I]α-bungarotoxin binding sites are present in the nucleus isthmi from at least stage V onward.
Fig. 6.
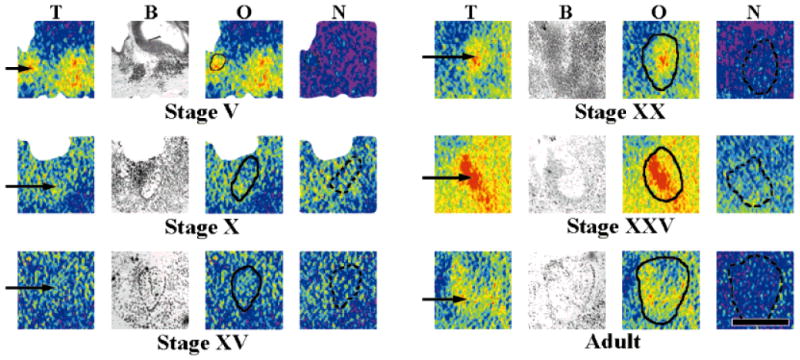
[125I]α-Bungarotoxin binding (2.5 nM) in the nucleus isthmi at different stages of development. Binding was clearly observable in the nucleus isthmi (arrows) of tadpole and adult tissue. In stages V, XX, XXV, and adults, binding densities were inhomogeneously distributed across this structure. A high level of nonspecific binding in stage X and stage XV tissue made it difficult to unambiguously identify binding sites with the nucleus isthmi (but see text). Comparisons of binding levels across the stages were not made because the results were from several individual experiments, and the treated tissue was not exposed to the same film. T, total [125I]α-bungarotoxin binding; B, brightfield image; O, overlay image for localization with the solid line outlining the nucleus isthmi; N, nonspecific binding with the dashed line outlining the nucleus isthmi. Anterior is to the right. Scale bar = 500 μm.
Effect of optic nerve lesions on [3H]cytisine and [125I]α-bungarotoxin binding in the tectum
In order to begin to understand whether [3H]cytisine and [125I]α-bungarotoxin sites within the tectum were present on afferent terminals or tectal cells, we performed lesions of the right optic nerve and allowed the animals to survive for short (6 weeks) or long (6 months) periods of time. Optic nerve lesions result in the degeneration of retinal ganglion cell terminals within the tectum and the disappearance of elements associated with them (Matsumoto and Scalia, 1981; Kuljis and Karten, 1983; Sargent et al., 1989).
Lesioning of the optic nerve resulted in a significant qualitative change in [3H]cytisine binding (Fig. 7A,B). Short-term deafferentation of the left tectal lobe reduced the binding in layers 8 and 9. A decrease in the normally low binding density present in layers 1–7 also occurred. Long-term lesions further decreased the binding in layers 8 and 9, but some binding remained in the deafferented lobe even after 6-month survival periods.
Fig. 7.

Effects of short and long term lesions of the optic nerve on [3H]cytisine (5 nM) and [125I]α-bungarotoxin (2.5 nM) binding site expression in the optic tectum. A: Representative autoradiogram of specific [3H]cytisine binding in a frontal section of the optic tectum after lesioning of the right optic nerve followed by a short (6-week) survival period. Binding in the deafferented (left) tectal lobe was dramatically reduced. B: [3H]Cytisine binding in the two tectal lobes after long term optic nerve lesion (6-month survival period). Residual binding in the superficial layers of the deafferented lobe was reduced even further by the extended survival time. C: Total [125I]α-bungarotoxin binding after lesioning and a 6-week survival time. Binding was marginally reduced in the deafferented (left) tectal lobe. D: Total [125I]α-bungarotoxin binding after a long-term lesion. No difference in binding was evident between the lesioned and unlesioned lobes. The four images should not be directly compared to one another because the experiments were performed at different times and exposed to different pieces of autoradiographic film. Quantitative analysis of these results is depicted in Figure 8. Scale bar = 1 mm.
The effects of optic nerve lesions on [125I]α-bungarotoxin binding in the tectum were quite different from those observed with [3H]cytisine. Six-week lesions reduced [125I]α-bungarotoxin binding in the superficial layers of the left tectal lobe (Fig. 7C), whereas there was no apparent effect of long-term lesions on this binding (Fig. 7D).
Quantitative analysis of radioligand binding in the tectum after lesions of the optic nerve confirmed our qualitative impressions. Short-term lesions reduced [3H]cytisine binding in layers 8 and 9 by 69–71% and the binding in layers 1–7 by 30–33% when compared to the afferented tectal lobe (Fig. 8A). Long-term lesions had similar effects on [3H]cytisine binding (Fig. 8B). Binding in layers 8 and 9 dropped 73–79%, whereas the binding in layers 1–7 was decreased by 20–36%.
Fig. 8.
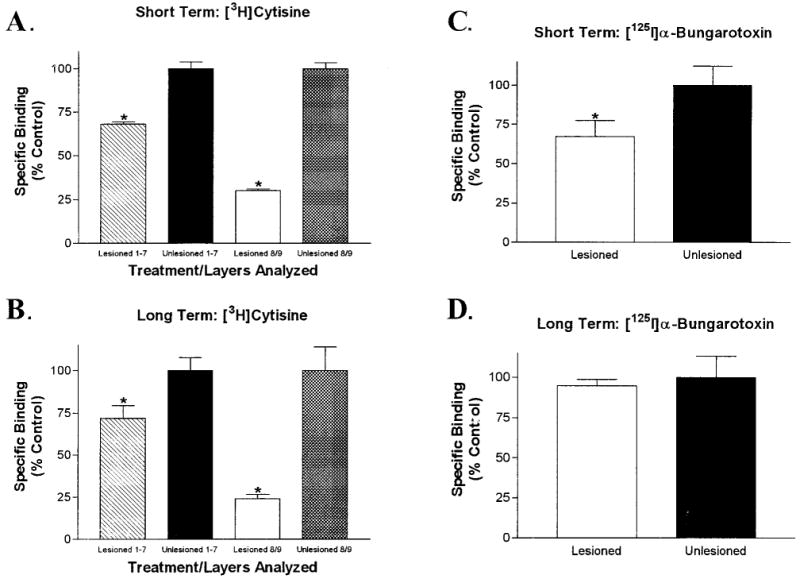
Analysis of [3H]cytisine (5 nM) and [125I]α-bungarotoxin (2.5 nM) binding in the retinorecipient layers of the tectum after lesions of the adult optic nerve. A: Quantitative analysis of [3H]cytisine binding after lesioning of the adult optic nerve with a short survival period. The binding in layers 1–7 of the deafferented lobe was reduced by 31.5 ± 1.5%, whereas the binding in layers 8 and 9 was decreased by 70 ± 1%. B: Long survival periods after optic nerve lesions had a similar effect on [3H]cytisine binding in the deafferented lobe. Binding in layers 1–7 was decreased by 28 ± 8% and that in layers 8 and 9 was reduced by 76 ± 3%. C: Short-term optic nerve lesions had a small, but significant, effect on specific [125I]α-bungarotoxin binding in the superficial layers of the deafferented lobe. Binding was reduced by 33 ± 10% in layers 8 and 9. D: Long-term optic nerve lesions did not affect specific [125I]α-bungarotoxin binding in tectal layers 8 and 9. There was no significant difference between afferented and deafferented lobes. Error bars represent the S.E.M. (*P < 0.05, paired, two-tailed t-test; n = 4 or 5).
As with the qualitative data, the effects of optic nerve lesions on quantitative [125I]α-bungarotoxin binding were different from those on [3H]cytisine binding. Short-term lesions induced a significant reduction (23–43%) of specific [125I]α-bungarotoxin binding in layers 8 and 9 of the deafferented lobe (Fig. 8C). Interestingly, there was no significant difference in the specific [125I]α-bungarotoxin binding between deafferented and afferented tectal lobes after long-term lesions (Fig. 8D). [125I]α-bungarotoxin binding in layers 1–7 was not analyzed because specific binding in those layers was not detectable. The densitometry data from all experiments with both [3H]cytisine and [125I]α-bungarotoxin was normalized to fmol binding/mg wet tissue weight, and the comparison of those data showed no significant difference in binding densities between short- and long-term survival periods (not shown).
Pharmacokinetics of [3H]cytisine and [125I]α-bungarotoxin binding
The nAChR subunit selectivity of the radioligands that we employed was determined in mammalian systems, not in frogs. Therefore, it was important to ensure that these radioligands had similar binding characteristics in the frog optic tectum. This was achieved by performing saturation and competition analyses of radioligand binding.
Figure 9A illustrates that [3H]cytisine bound with high affinity and saturable kinetics, both of which are indicators of specific binding to a receptor. Competition experiments of [3H]cytisine (5 nM) against increasing concentrations of unlabeled nicotinic compounds determined the inhibition constants (Ki) of those unlabeled compounds at the [3H]cytisine binding site (Fig. 9B). DHBE, a competitor at non-α7 nAChRs in mammalian systems (Whiting et al., 1991; Alkondon et al., 1999), had the highest affinity for [3H]cytisine sites and showed that [3H]cytisine binds at more than one receptor site (Ki1 = 40.3 pM; Ki2 = 24.5 nM). MLA, a compound selective for α7 nAChRs, had the next highest affinity but competed at only one receptor site (Ki = 3.29 μM). Unlabeled α-bungarotoxin had the lowest affinity for the [3H]cytisine binding site (Ki = 12.5 μM).
Fig. 9.

Pharmacological analysis of [3H]cytisine and [125I]α-bungarotoxin binding in layers 8 and 9 of the adult optic tectum. A: Saturation binding analysis of [3H]cytisine binding. The apparent dissociation constant (KD) was 0.47 ± 0.04 nM with a maximum binding capacity (Bmax) of 40.4 ± 0.71 fmol/mg. The data were best fit by an equation for binding to a single site. The inset is a Scatchard plot of the same data that yielded similar binding constants. B: Competition binding analysis of [3H]cytisine (5 nM) binding. Dihydro-β-erythroidine (DHBE), which is a competitive antagonist at non-α7 nicotinic acetylcholine receptors (nAChRs) in mammalian systems, competed with [3H]cytisine at two sites. The high affinity DHBE site demonstrated an inhibition constant (Ki) of 40.3 pM, whereas the low-affinity site had a Ki of 24.5 nM. Methyllycaconitine (MLA) and unlabeled α-bungarotoxin (BtX) are both high-affinity, competitive antagonists of the α7 nAChR in mammalian systems and did not compete with [3H]cytisine binding until micromolar concentrations of competitor were present. MLA had a Ki of 3.29 μM. Unlabeled BtX was unable to reduce [3H]cytisine binding to background levels and demonstrated a Ki of 12.5 μM. C: Saturation analysis of [125I]α-bungarotoxin binding demonstrated a KD = 0.81 ± 0.19 nM and a Bmax = 1.86 ± 0.10 fmol/mg. The data fit best to an equation for single-site binding, and Scatchard analysis (inset) again produced similar binding values. D: Competition of MLA, unlabeled cytisine, and DHBE against [125I]α-bungarotoxin (2.5 nM) binding. The competition curves fit to equations that produced the following inhibition constants: MLA = 2.3 pM and 2.6 nM, unlabeled cytisine = 17.6 nM, DHBE = 21.1 μ M. All points are the mean ± S.E.M. of specific binding (n = 4 or 5) for all graphs.
The binding of [125I]α-bungarotoxin was also of high affinity and was saturable (Fig. 9C). Competition analyses (Fig. 9D) showed that MLA was most selective for the [125I]α-bungarotoxin site and competed at two sites (Ki1 = 2.3 pM; Ki2 = 2.6 nM), followed by unlabeled cytisine (Ki = 17.6 nM) and DHBE (Ki = 21.1 μM). Interestingly, unlabeled cytisine exhibited a Ki in the nanomolar range instead of the micromolar range, but this result was not surprising because cytisine is known to compete with [125I]α-bungarotoxin and [3H]MLA sites in mammalian systems (Marks et al., 1986; Peng et al., 1999; Cortes-Burgos et al., 1999).
Discussion
We have shown that the nicotinic radioligands [3H]cytisine and [125I]α-bungarotoxin bind to distinct receptor sites in the brain of the frog, Rana pipiens. Although binding sites for both radioligands were present in the retinorecipient layers of the optic tectum, the distribution, density, and pharmacokinetics of these sites differed. Furthermore, their patterns of expression during development and their response to optic nerve lesions were also different from one another.
Presence of distinct nicotinic binding sites
The presence of nAChRs in brain areas responsible for visual processing is well-documented in many species. Previous work using monoclonal antibody (mAb) 22, which recognizes the main immunogenic region of the nAChR (Tzartos et al., 1981), indicates the presence of nicotinic receptors in layer 9 of Rana pipiens (Sargent et al., 1989), in layers 8 and 9 of Xenopus laevis (Titmus et al., 1999), and in retinorecipient layers of the goldfish (Henley et al., 1986) and the chick (Swanson et al., 1983). Furthermore, [3H]cytisine binding has been reported in the tectum of Xenopus (Titmus et al., 1996). Nicotinic receptors have also been shown in the superior colliculus, the structural homologue of the optic tectum in mammalian systems (Clarke et al., 1985; Swanson et al., 1987; Prusky and Cynader, 1988; Pauly et al., 1989; Dominguez del Toro et al., 1994; Happe et al., 1994; Nakayama et al., 1995; Aubert et al., 1996). Our work is particularly similar to the findings of Swanson et al. (1987) and Prusky and Cynader (1988) as the data indicate that the locations of receptors responsible for high-affinity, nAChR agonist binding are similar, yet distinct, from those which bind [125I]α-bungarotoxin.
Our findings with [3H]cytisine are consistent with an earlier, immunocytochemical study in the optic tectum of the leopard frog in that a high density of binding was observed in layer 9 (Sargent et al., 1989). The intermediate binding densities in layer 8 that we have found have not been reported in this animal but are consistent with binding patterns found in Xenopus (Titmus et al., 1999). However, unlike previous studies, we also observed nicotinic receptors in layers 1–7. A possible explanation for this result comes from our DHBE competition experiments, which indicated that more than one [3H]cytisine binding site is present in the tectum. In general it is thought that the high affinity binding of [3H]cytisine in mammalian systems corresponds to the α4β2 nAChR subtype (Whiting and Lindstrom, 1988; Flores et al., 1992). This may well account for the [3H]cytisine binding in layer 9 that appears to be also recognized by mAb 22 (see below). However, [3H]cytisine is also known to bind to nAChRs that lack the β2 subunit (Zoli et al., 1998) and may be doing so in our system to produce the low binding densities in the deeper tectal layers that have not been previously described using mAb 22.
Although an earlier study did not detect [125I]α-bungarotoxin sites in the optic tectum of Rana pipiens by using immunoprecipitation techniques (Sargent et al., 1989), the autoradiographic methods we employed clearly did so. The concentration of tectal [125I]α-bungarotoxin sites in our study was low (∼2 fmol/mg wet tissue weight), but binding densities in the adult nucleus isthmi and thalamus were much higher. In addition, other investigators have provided considerable evidence that α-bungarotoxin receptors exist in the optic tectum. [125I]α-Bungarotoxin binding sites are present in retinorecipient layers of the tectum in the goldfish (Oswald et al., 1980) and the chick (Polz-Tejera et al., 1975), which is consistent with our finding in Rana pipiens of binding sites in tectal layer 9. There is also indirect evidence for such sites from studies demonstrating that application of agents affecting α-bungarotoxin-sensitive receptors alter neuronal characteristics within the tectum. Application of α-bungarotoxin to the toad or goldfish tectum causes retinal ganglion cell terminals to sprout and move away from their appropriate positions (Freeman, 1977; Schmidt, 1985). In Xenopus, injection of α-bungarotoxin to the tectum blocks light-induced calcium flux into the retinal ganglion cells, whereas nicotine enhances it (Edwards and Cline, 1999). Furthermore, application of MLA to tectal slices decreases the frequency of glutamatergic, miniature excitatory postsynaptic currents induced by cholinergic agonists (Titmus et al., 1999).
Developmental expression of [3H]cytisine and [125I]α-bungarotoxin binding sites
Binding sites for both [3H]cytisine and [125I]α-bungarotoxin were present in developing tissue but absent from immature tectal areas. Both radioligands bound to the superficial tectal layers, but the binding did not reach the posterior extent of the tectum at younger stages (V, X). Radioligand binding was also less dense in those posterior regions during the majority of development, a pattern that is consistent with the normal, anatomical maturation of the tectum. However, the absolute density of binding throughout development and the rostrocaudal distribution of binding in the adult tectum were unmistakably different between [3H]cytisine and [125I]α-bungarotoxin.
Quantitative analysis of the density of [3H]cytisine sites throughout the life of the animals showed that these sites are expressed at every stage, and their density and distribution follows the gradients of both tectal maturation and tectal innervation by retinal afferents (Reh and Constantine-Paton, 1984). At every tadpole stage, binding was greatest in anterior regions and least in posterior ones. The posterior region demonstrated a tendency toward increased binding with increased age. However, a dramatic rise in binding density occurred in the postmetamorphic, juvenile period between stage XXV and full adulthood. During this period, the tectum and retina undergo extensive transformations due to growth, and the binocular visual map is finalized (Udin, 1989; Gruberg et al., 1989). An increase in nAChR density might enhance the total activity of the system and facilitate the final establishment and maintenance of the in-register retinotopic and binocular visual maps. Interestingly, peaks in the binding density of radiolabeled nicotinic agonists in the mammalian brain are closely correlated with peak periods of synaptogenesis. These peaks in expression are followed by a small decline that plateaus at adult levels, which are well above developmental values (Fiedler et al., 1987; Prusky et al., 1988a,b; Aubert et al., 1996). Therefore, the increased receptor density that we observe might be a product of the increasing complexity of afferent terminal arbors that occurs during tectal development (Udin, 1989; Cline and Constantine-Paton, 1990).
The peak binding density of [125I]α-bungarotoxin in the optic tectum was low when compared to that of [3H]cytisine, and no differences in binding existed between any of the stages. [125I]α-bungarotoxin sites were generally most dense in anterior tectal regions and low in the posterior regions. Unlike [3H]cytisine binding, this difference in rostrocaudal distribution was retained in the mature animal with adults having decreased densities in the middle and posterior tectum as compared to anterior regions. This suggests that either the source or the regulation of tectal [125I]α-bungarotoxin sites is different from that of [3H]cytisine sites. In mammals, the highest expression of [125I]α-bungarotoxin binding in the brain is also present during peak periods of synaptogenesis. However, after those peaks the density of binding decreases markedly (Fiedler et al., 1987; Fuchs, 1989), suggesting that the nAChRs that bind [125I]α-bungarotoxin subserve specific developmental processes during a critical period, and once that period has passed, the density of [125I]α-bungarotoxin receptors declines and their functions conceivably change. Such a decline was not seen in our system, suggesting a necessary, continued function for these receptors.
Location of binding sites within the tectum
In the mammalian brain, activation of presynaptic nAChRs has been shown to increase the release of many neurotransmitters (Grady et al., 1992; Gray et al., 1996; Li et al., 1998; Alkondon et al., 1999), and postsynaptic nAChRs that mediate current flow have also been demonstrated (Roerig et al., 1997; Zhang et al., 1996; Nong et al., 1999). Within the tectum, several lines of evidence suggest that at least some nAChRs are capable of modulating neurotransmitter release. First, nAChR-like receptors are present on the terminals of some retinal ganglion cells (Sargent et al., 1989). Second, injection of α-bungarotoxin into the tectal ventricle decreases the flow of calcium into retinal ganglion cell axons (Edwards and Cline, 1999), and third, application of nicotinic agonists to the tectum appears to increase the release of glutamate from retinal ganglion cells (Titmus et al., 1999).
Sargent et al. (1989) identified mAb 22 binding sites on retinal ganglion cell axons in Rana pipiens. The mAb 22 immunoreactivity is found in distinct sublaminae (a–c, e, and f) of layer 9. Our autoradiographic study lacks the anatomical resolution necessary to assess binding in the sublaminae of layer 9, but it is likely that [3H]cytisine recognizes the same sites as mAb 22 for two reasons. The strong band of [3H]cytisine binding in layer 9 is essentially identical to the pattern of mAb 22 immunoreactivity, and this immunoreactivity is lost after optic nerve lesioning (Sargent et al., 1989), a result identical to that which we obtained with [3H]cytisine. We found that lesioning of the optic nerve significantly reduced the binding of [3H]cytisine to the retinorecipient layers of the tectum after both short and long-term survival periods. Similar results have been obtained in Xenopus (Titmus et al., 1999) and in visual areas of the mammalian brain. In both the rat and cat, removal of an eye results in the loss of [3H]nicotine binding in the superior colliculus (Prusky and Cynader, 1988). Furthermore, electrolytic lesions of the feline lateral geniculate nucleus induce sharp decreases of [3H]nicotine binding in layer IV of the visual cortex, the primary layer for cortical afferents (Parkinson et al., 1988; Prusky et al., 1988b).
Although it is likely that the majority of [3H]cytisine sites reside on retinal afferents in Rana pipiens, our results also indicate that [3H]cytisine receptors are present on postsynaptic tectal cells. This is demonstrated by the fact that low binding is present in the nonretinorecipient layers 1–7. This interpretation of [3H]cytisine receptors on the dendrites and/or soma of tectal cells is consistent with the recent reporting of physiological responses in tectal cells after exposure to nicotinic agonists (Titmus et al., 1999). It is noteworthy that these cells also reduce significantly their expression of nAChR molecules following optic nerve lesions. Furthermore, [3H]cytisine binding exists in layers 8 and 9 even after long-term lesions that should result in the complete degeneration of any retinal ganglion cell terminals present in the tectum (Matsumoto and Scalia, 1981).
Changes in the density of [125I]α-bungarotoxin sites after optic nerve lesions were entirely different from the results we obtained with [3H]cytisine. Short-term lesions slightly decreased [125I]α-bungarotoxin binding in the retinorecipient layers, but long-term lesions had no effect. Our results are similar to those in the rat superior colliculus, which show that eye enucleation only slightly decreases [125I]α-bungarotoxin binding, and indicate that the majority of these sites are not located on retinal ganglion cell terminals (Swanson et al., 1987).
A possible source of the [125I]α-bungarotoxin binding in the tectum could be the nucleus isthmi. The nucleus isthmi is homologous to the mammalian parabigeminal nucleus, a cholinergic center in the brainstem that is connected to the superior colliculus (Mufson et al., 1986; Gruberg et al., 1989; Wallace et al., 1990). The strong [125I]α-bungarotoxin binding in the isthmi suggests a possible location of these sites within the tectum on the terminals of nucleus isthmi afferents. These terminals are found throughout tectal layers 8 and 9. The nucleus isthmi sends both a contralateral and ipsilateral projection to the tectal lobes. The ipsilateral projection innervates the majority of layer 9 (sublaminae a–f) of the tectal lobe, whereas the contralateral projection innervates only layers 8 and 9a (Gruberg and Udin, 1978; Gruberg and Lettvin, 1980). In Rana pipiens, fibers projecting from the contralateral isthmi are thought to be less dense in the caudal tectum than in more rostral tectal areas (Gruberg et al., 1989). The apparent decrease in the density of the contralateral isthmi projections in the posterior tectum would help explain the decreased [125I]α-bungarotoxin binding that we find in that region.
Recent observations have shown that staining for mAb 306, an antibody selective for α7-containing nAChRs, co-localizes with staining for an antibody against choline acetyltransferase but not mAb 22 (Debski and Sargent, 1999). These results suggest that α7 nAChR-like subunits, recognized by [125I]α-bungarotoxin, reside on presynaptic isthmotectal terminals.
Nevertheless, the decrease we observed in [125I]α-bungarotoxin binding after short-term lesions could mean that some of these sites are present on a select population of retinal ganglion cell axons even though this loss was seemingly restored after long-term lesions. One possible explanation for this restoration is that compaction of the superficial tectal layers induced by the degradation of retinal terminals caused a sampling artifact that resulted in an inflation of binding densities. As observed by others and ourselves, the retinorecipient layers are thinner in deafferented tectal lobes than in afferented lobes (Matsumoto and Scalia, 1981; Desan et al., 1987). Accordingly, deafferented lobes are also smaller upon gross examination when compared to afferented lobes. Another possible scenario is that [125I]α-bungarotoxin sites associated with tectal elements other than retinal ganglion cell terminals were upregulated following the lesion in such a way as to precisely account for the loss of retinal ganglion cell sites.
Selectivity of [3H]cytisine and [125I]α-bungarotoxin for nAChR subunits
The primary purpose of our pharmacokinetic studies was to determine whether [3H]cytisine and [125I]α-bungarotoxin bound to the frog optic tectum with similar affinity and selectivity for nAChR-like subunits as they did in mammalian systems. Table 1 shows that the saturation binding studies yielded affinities of [3H]cytisine and [125I]α-bungarotoxin binding to the tectum that were within an order of magnitude of those found in mammalian systems (Marks et al., 1986; Fiedler et al, 1987; Khan et al., 1994; Barrantes et al., 1995; Gopalakrishnan et al., 1995, 1997). Therefore, differences in the KD values can most likely be attributed to differences in tissue preparation and experimental protocols.
TABLE 1.
Comparison of the Apparent Affinity Constants of Nicotinic Ligands in the Adult Frog Optic Tectum With Those Found in Adult Mammalian Nervous Tissue1
| Ligand | Frog | Rat | Mouse | Human |
|---|---|---|---|---|
| (nM) | ||||
| [3H]Cytisine | 0.47 ± 0.04 | 0.44 ± 0.14 | 1.5 ± 0.72 | 0.21 ± 0.087 |
| [125I]α-Bungarotoxin | 0.81 ± 0.19 | 0.62 ± 0.015 | ∼0.323 | ∼0.76 |
All values are the mean ± S.E.M. (if available).
Purified recombinant human α7 nAChR, Gopalakrishnan et al., 1995.
Purified recombinant human α4β2 nAChR, Gopalakrishnan et al., 1997.
A more important line of evidence that [3H]cytisine and[125I]α-bungarotoxin were respectively binding to presumed non-α7 and α7 nAChR-like subunits comes from the competition studies. The rank order of inhibitor potencies at the [3H]cytisine and [125I]α-bungarotoxin sites paralleled that expected from studies in mammals (Happe et al., 1994; Barrantes et al., 1995; Rangwala et al., 1997; Alkondon et al., 1999). Compounds exhibiting selectivity for non-α7 nAChRs competed at [3H]cytisine sites with picomolar to nanomolar potency, whereas those with selectivity for α7 nAChRs competed with low affinity, micromolar potencies. The converse was generally consistent for competition studies at [125I]α-bungarotoxin sites. The Ki of ∼18 nM for unlabeled cytisine at the [125I]α-bungarotoxin site was considerably lower than the expected micromolar value (Marks et al., 1986; Rangwala et al., 1997). This result probably corresponds to a species difference in the agonist binding site of the α7-like receptor. This agonist site binds cytisine strongly enough to compete with [125I]α-bungarotoxin binding, but the affinity is not high enough for [3H]cytisine to remain bound after the treated tissue has been washed. Hence, there is no [3H]cytisine binding in the nucleus isthmi.
One final issue that should be addressed is the specificity of the radioligands for the individual nAChR subunits that are known to exist. Although [3H]cytisine is selective for nAChRs that contain α4 and β2 subunits (Whiting and Lindstrom, 1988; Flores et al., 1992), this compound is known to bind to brain tissue that lacks the β2 subunit and is an efficacious agonist at presumed α3β4 nAChRs (Zoli et al., 1998). Indeed, our own results with DHBE competition indicate more than one [3H]cytisine binding site. Furthermore, [3H]epibatidine (1 nM) binds to the tectum in the exact same pattern as [3H]cytisine, but it is competed by unlabeled cytisine at two sites (Butt, Pauly, and Debski, unpublished observations). Epibatidine has selectivity for mammalian nAChRs containing α3 and α4 subunits but possibly binds to α5 and α6 subunits as well (Houghtling et al., 1995; Zoli et al., 1998). Therefore, without genetic studies or more selective, functional antibodies to frog nAChRs, the identity of [3H]cytisine binding in the tectum can at best be classified as non-α7. A similar situation exists for [125I] α-bungarotoxin and its affinity for α7-like nAChRs. [125I]α-Bungarotoxin is not capable of differentiating between α subunits 7, 8, or 9 (Anand et al., 1993; Gerzanich et al., 1994, 1995; Cuevas and Berg, 1998). Therefore, the signal we have reported could originate from all three subunits or any combination of the three. The possibility that the [125I] α-bungarotoxin binding we see represents a combined signal is strengthened by the demonstration of the two-site pharmacokinetics of MLA competition at these sites. The results of these experiments are consistent with earlier reports that α8-containing nAChRs have a lower affinity for α-bungarotoxin than those with α7 subunits (Gerzanich et al., 1994, 1995). The α8 subunit has been reported in the chick optic tectum (Sargent, 1993). Its presence has not been investigated in the frog. Nevertheless, it is more likely that the low-affinity [125I]α-bungarotoxin site indicates α8 subunits rather than α9 subunits because α9 transcripts have only been found in non-neuronal, cochlear hair cells (Elgoyhen et al., 1994).
Conclusions
The results of the binding studies reported here support the conclusion that multiple subtypes of nAChR-like molecules exist in the retinorecipient layers of the optic tectum in Rana pipiens. [3H]Cytisine binding sites are primarily located on the axons of retinal ganglion cells, but some are also present on cells deep with the tectum. The majority of [125I]α-bungarotoxin sites are unlikely to be present on retinal afferents. The pharmacology of the [3H]cytisine and [125I]α-bungarotoxin sites indicates that they are binding predominantly to non-α7 and α7 nAChR subunits, respectively. The receptors identified by the radioligands are expressed early during retinotectal development and are maintained in the mature animal. They likely contribute to the known facilitatory effects of ACh on retinotectal activity and connectivity.
Acknowledgments
We thank Drs. Susan Udin, Robin Cooper, and Phil Bonner for their comments on various versions of the manuscript.
Grant sponsor: National Institute of Health; Grant number: EY-11913; Grant sponsor: National Institute of Mental Health; Grant number: 5T32MH19917.
Literature Cited
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R, Peng X, Ballesta JJ, Lindstrom J. Pharmacological characterization of alpha-bungarotoxin-sensitive acetylcholine receptors immunoisolated from chick retina: contrasting properties of alpha 7 and alpha 8 subunit-containing subtypes. Mol Pharmacol. 1993;44:1046–1050. [PubMed] [Google Scholar]
- Aubert I, Cecyre D, Gauthier S, Quirion R. Comparative ontogenic profile of cholinergic markers, including nicotinic and muscarinic receptors, in the rat brain. J Comp Neurol. 1996;369:31–55. doi: 10.1002/(SICI)1096-9861(19960520)369:1<31::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Barrantes GE, Rogers AT, Lindstrom J, Wonnacott S. alpha-bungarotoxin binding sites in rat hippocampal and cortical cultures: initial characterisation, colocalisation with alpha 7 subunits and up-regulation by chronic nicotine treatment. Brain Res. 1995;672:228–236. doi: 10.1016/0006-8993(94)01386-v. [DOI] [PubMed] [Google Scholar]
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Boulter J, Evans K, Goldman D, Martin G, Treco D, Heinemann S, Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. Nature. 1986;319:368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Boulter J, O'Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, Gardner PD, Ballivet M, Deneris ES, McKinnon D, Heinemann S, Patrick J. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem. 1990;265:4472–4482. [PubMed] [Google Scholar]
- Bröcher S, Artola A, Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 1992;573:27–36. doi: 10.1016/0006-8993(92)90110-u. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT. Activity-dependent plasticity in the visual systems of frogs and fish. Trends Neurosci. 1991;14:104–111. doi: 10.1016/0166-2236(91)90071-2. [DOI] [PubMed] [Google Scholar]
- Cline HT. Topographic maps: developing roles of synaptic plasticity. Curr Biol. 1998;8:R836–839. doi: 10.1016/s0960-9822(07)00525-8. [DOI] [PubMed] [Google Scholar]
- Cline HT, Constantine-Paton M. NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection. J Neurosci. 1990;10:1197–1216. doi: 10.1523/JNEUROSCI.10-04-01197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Cortes-Burgos L, Stone M, Tinholt P, Wong EHF. Novel interactions at the alpha-7 nicotinic receptor as measured by [3H]-methyllycaconitine (MLA) Soc Neurosci Abstr. 1999;25:979. [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez MC, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Cuevas J, Berg DK. Mammalian nicotinic receptors with alpha7 subunits that slowly desensitize and rapidly recover from alpha-bungarotoxin blockade. J Neurosci. 1998;18:10335–10344. doi: 10.1523/JNEUROSCI.18-24-10335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie J, Cowan WM. Some observations on the early development of the optic tectum in the frog (Rana pipiens), with special reference to the effects of early eye removal on mitotic activity in the larval tectum. J Comp Neurol. 1974;156:123–141. doi: 10.1002/cne.901560202. [DOI] [PubMed] [Google Scholar]
- Debski EA, Sargent PB. Visualization of cholinergic input, presynaptic nicotinic AChR-like molecules and synaptic sites in the optic tectum. Soc Neurosci Abstr. 1999;25:980. [Google Scholar]
- Deneris ES, Connolly J, Boulter J, Wada E, Wada K, Swanson LW, Patrick J, Heinemann S. Primary structure and expression of beta 2: a novel subunit of neuronal nicotinic acetylcholine receptors. Neuron. 1988;1:45–54. doi: 10.1016/0896-6273(88)90208-5. [DOI] [PubMed] [Google Scholar]
- Deneris ES, Boulter J, Swanson LW, Patrick J, Heinemann S. Beta 3: a new member of nicotinic acetylcholine receptor gene family is expressed in brain. J Biol Chem. 1989;264:6268–6272. [PubMed] [Google Scholar]
- Desan PH, Gruberg ER, Grewell KM, Eckenstein F. Cholinergic innervation of the optic tectum in the frog Rana pipiens. Brain Res. 1987;413:344–349. doi: 10.1016/0006-8993(87)91026-2. [DOI] [PubMed] [Google Scholar]
- Dominguez del Toro E, Juiz JM, Peng X, Lindstrom J, Criado M. Immunocytochemical localization of the alpha 7 subunit of the nicotinic acetylcholine receptor in the rat central nervous system. J Comp Neurol. 1994;349:325–342. doi: 10.1002/cne.903490302. [DOI] [PubMed] [Google Scholar]
- Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: beta 4. Neuron. 1989;3:487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Edwards JA, Cline HT. Light-induced calcium influx into retinal axons is regulated by presynaptic nicotinic acetylcholine receptor activity in vivo. J Neurophysiol. 1999;81:895–907. doi: 10.1152/jn.1999.81.2.895. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Ernst AF, Jurney WM, McLoon SC. Mechanisms involved in development of retinotectal connections: roles of Eph receptor tyrosine kinases, NMDA receptors and nitric oxide. Prog Brain Res. 1998;118:115–131. doi: 10.1016/s0079-6123(08)63204-5. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler EP, Marks MJ, Collins AC. Postnatal development of cholinergic enzymes and receptors in mouse brain. J Neurochem. 1987;49:983–990. doi: 10.1111/j.1471-4159.1987.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Fite KV, Wang SR. Microiontophoresis and single-unit analysis of cholinergic drugs in the optic tectum of frog. Brain Behav Evol. 1986;28:198–206. doi: 10.1159/000118703. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Freeman JA. Possible regulatory function of acetylcholine receptor in maintenance of retinotectal synapses. Nature. 1977;269:218–222. doi: 10.1038/269218a0. [DOI] [PubMed] [Google Scholar]
- Fuchs JL. [125I]Alpha-bungarotoxin binding marks primary sensory area developing rat neocortex. Brain Res. 1989;5:223–234. doi: 10.1016/0006-8993(89)90640-9. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Anand R, Lindstrom J. Homomers of alpha 8 and alpha 7 subunits of nicotinic receptors exhibit similar channel but contrasting binding site properties. Mol Pharmacol. 1994;45:212–220. [PubMed] [Google Scholar]
- Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, Lindstrom J. Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol Pharmacol. 1995;48:774–782. [PubMed] [Google Scholar]
- Goldman D, Deneris E, Luyten W, Kochhar A, Patrick J, Heinemann S. Members of a nicotinic acetylcholine receptor gene family are expressed in different regions of the mammalian central nervous system. Cell. 1987;48:965–973. doi: 10.1016/0092-8674(87)90705-7. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Buisson B, Touma E, Giordano T, Campbell JE, Hu IC, Donnelly-Roberts D, Arneric SP, Bertrand D, Sullivan JP. Stable expression and pharmacological properties of the human alpha 7 nicotinic acetylcholine receptor. Eur J Pharmacol. 1995;290:237–246. doi: 10.1016/0922-4106(95)00083-6. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Molinari EJ, Sullivan JP. Regulation of human alpha4beta2 neuronal nicotinic acetylcholine receptors by cholinergic channel ligands and second messenger pathways. Mol Pharmacol. 1997;52:524–534. [PubMed] [Google Scholar]
- Grady S, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992;59:848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Greuel JM, Luhmann HJ, Singer W. Pharmacological induction of use-dependent receptive field modifications in the visual cortex. Science. 1988;242:74–77. doi: 10.1126/science.2902687. [DOI] [PubMed] [Google Scholar]
- Gruberg ER, Lettvin JY. Anatomy and physiology of a binocular system in the frog Rana pipiens. Brain Res. 1980;192:313–325. doi: 10.1016/0006-8993(80)90886-0. [DOI] [PubMed] [Google Scholar]
- Gruberg ER, Udin SB. Topographic projections between the nucleus isthmi and the tectum of the frog Rana pipiens. J Comp Neurol. 1978;179:487–500. doi: 10.1002/cne.901790303. [DOI] [PubMed] [Google Scholar]
- Gruberg ER, Wallace MT, Waldeck RF. Relationship between isthmotectal fibers and other tectopetal systems in the leopard frog. J Comp Neurol. 1989;288:39–50. doi: 10.1002/cne.902880104. [DOI] [PubMed] [Google Scholar]
- Gruberg ER, Wallace MT, Caine HS, Mote MI. Behavioral and physiological consequences of unilateral ablation of the nucleus isthmi in the leopard frog. Brain Behav Evol. 1991;37:92–103. doi: 10.1159/000114350. [DOI] [PubMed] [Google Scholar]
- Gu Q, Singer W. Effects of intracortical infusion of anticholinergic drugs on neuronal plasticity in kitten striate cortex. Eur J Neurosci. 1993;5:475–485. doi: 10.1111/j.1460-9568.1993.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995;7:1146–1153. doi: 10.1111/j.1460-9568.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Happe HK, Peters JL, Bergman DA, Murrin LC. Localization of nicotinic cholinergic receptors in rat brain: autoradiographic studies with [3H]cytisine. Neuroscience. 1994;62:929–944. doi: 10.1016/0306-4522(94)90484-7. [DOI] [PubMed] [Google Scholar]
- Henley JM, Mynlieff M, Lindstrom JM, Oswald RE. Interaction of monoclonal antibodies to electroplaque acetylcholine receptors with the alpha-bungarotoxin binding site of goldfish brain. Brain Res. 1986;364:405–408. doi: 10.1016/0006-8993(86)90857-7. [DOI] [PubMed] [Google Scholar]
- Houghtling RA, Davila-Garcia MI, Kellar KJ. Characterization of (+/-)(-)[3H]epibatidine binding to nicotinic cholinergic receptors in rat and human brain. Mol Pharmacol. 1995;48:280–287. [PubMed] [Google Scholar]
- Imamura K, Kasamatsu T. Interaction of noradrenergic and cholinergic systems in regulation of ocular dominance plasticity. Neurosci Res. 1989;6:519–536. doi: 10.1016/0168-0102(89)90042-4. [DOI] [PubMed] [Google Scholar]
- Khan IM, Yaksh TL, Taylor P. Ligand specificity of nicotinic acetylcholine receptors in rat spinal cord: studies with nicotine and cytisine. J Pharmacol Exp Ther. 1994;270:159–166. [PubMed] [Google Scholar]
- King WM, Schmidt JT. The long latency component of retinotectal transmission: enhancement by stimulation of nucleus isthmi or tectobulbar tract and block by nicotinic cholinergic antagonists. Neuroscience. 1991;40:701–712. doi: 10.1016/0306-4522(91)90006-a. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis RO, Karten HJ. Modifications in the laminar organization of peptide-like immunoreactivity in the anuran optic tectum following retinal deafferentation. J Comp Neurol. 1983;217:239–251. doi: 10.1002/cne.902170302. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Kornblum HI, Boyajian CL. Pharmacological characterization of ligand binding sites. In: Leslie FM, Altar CA, editors. Receptor localization: ligand autoradiography. 1988. [Google Scholar]; Venter JC, Harrison LC, editors. Receptor biochemistry and methodology. Vol. 13. New York: Alan R. Liss, Inc.; pp. 49–66. [Google Scholar]
- Li X, Rainnie DG, McCarley RW, Greene RW. Presynaptic nicotinic receptors facilitate monoaminergic transmission. J Neurosci. 1998;18:1904–1912. doi: 10.1523/JNEUROSCI.18-05-01904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J, Anand R, Peng X, Gerzanich V, Wang F, Li Y. Neuronal nicotinic receptor subtypes. Ann N Y Acad Sci. 1995;757:100–116. doi: 10.1111/j.1749-6632.1995.tb17467.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Debski EA. Origins of serotonin-like immunoreactivity in the optic tectum of Rana pipiens. J Comp Neurol. 1995;352:280–296. doi: 10.1002/cne.903520210. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jia W, Gu Q, Cynader M. Involvement of muscarinic acetylcholine receptors in regulation of kitten visual cortex plasticity. Brain Res Dev Brain Res. 1994;79:63–71. doi: 10.1016/0165-3806(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Romm E, Wehner JM, Collins AC. Nicotinic binding sites in rat and mouse brain: comparison of acetylcholine, nicotine, and alpha-bungarotoxin. Mol Pharmacol. 1986;30:427–436. [PubMed] [Google Scholar]
- Marks MJ, Smith KW, Collins AC. Differential agonist inhibition identifies multiple epibatidine binding sites in mouse brain. J Pharmacol Exp Ther. 1998;285:377–386. [PubMed] [Google Scholar]
- Marín O, González A. Origin of tectal cholinergic projections in amphibians: a combined study of choline acetyltransferase immunohistochemistry and retrograde transport of dextran amines. Vis Neurosci. 1999;16:271–283. doi: 10.1017/s0952523899162084. [DOI] [PubMed] [Google Scholar]
- Matsumoto DE, Scalia F. Long-term survival of centrally projecting axons in the optic nerve of the frog following destruction of the retina. J Comp Neurol. 1981;202:135–155. doi: 10.1002/cne.902020112. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Martin TL, Mash DC, Wainer BH, Mesulam MM. Cholinergic projections from the parabigeminal nucleus (Ch8) to the superior colliculus in the mouse: a combined analysis of horseradish peroxidase transport and choline acetyltransferase immunohistochemistry. Brain Res. 1986;370:144–148. doi: 10.1016/0006-8993(86)91114-5. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Shioda S, Okuda H, Nakashima T, Nakai Y. Immunocytochemical localization of nicotinic acetylcholine receptor in rat cerebral cortex. Brain Res Mol Brain Res. 1995;32:321–328. doi: 10.1016/0169-328x(95)00092-7. [DOI] [PubMed] [Google Scholar]
- Nobili L, Sannita WG. Cholinergic modulation, visual function and Alzheimer's dementia. Vision Res. 1997;37:3559–3571. doi: 10.1016/S0042-6989(97)00076-X. [DOI] [PubMed] [Google Scholar]
- Nong Y, Sorenson EM, Chiappinelli VA. Fast excitatory nicotinic transmission in the chick lateral spiriform nucleus. J Neurosci. 1999;19:7804–7811. doi: 10.1523/JNEUROSCI.19-18-07804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald RE, Schmidt JT, Norden JJ, Freeman JA. Localization of alpha-bungarotoxin binding sites to the goldfish retinotectal projection. Brain Res. 1980;187:113–127. doi: 10.1016/0006-8993(80)90498-9. [DOI] [PubMed] [Google Scholar]
- Parkinson D, Kratz KE, Daw NW. Evidence for a nicotinic component to the actions of acetylcholine in cat visual cortex. Exp Brain Res. 1988;73:553–568. doi: 10.1007/BF00406614. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Stitzel JA, Marks MJ, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain. Brain Res Bull. 1989;22:453–459. doi: 10.1016/0361-9230(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Peng JH, Lucero L, Fryer J, Herl J, Leonard SS, Lukas RJ. Inducible, heterologous expression of human alpha7-nicotinic acetylcholine receptors in a native nicotinic receptor-null human clonal line. Brain Res. 1999;825:172–179. doi: 10.1016/s0006-8993(99)01066-5. [DOI] [PubMed] [Google Scholar]
- Polz-Tejera G, Schmidt J, Karten HJ. Autoradiographic localisation of alpha-bungarotoxin-binding sites in the central nervous system. Nature. 1975;258:349–351. doi: 10.1038/258349a0. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Cynader MS. [3H]nicotine binding sites are associated with mammalian optic nerve terminals. Vis Neurosci. 1988;1:245–248. doi: 10.1017/s0952523800001504. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Arbuckle JM, Cynader MS. Transient concordant distributions of nicotinic receptors and acetylcholinesterase activity in infant rat visual cortex. Brain Res. 1988a;467:154–159. doi: 10.1016/0165-3806(88)90078-8. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Shaw C, Cynader MS. The distribution and ontogenesis of [3H]nicotine binding sites in cat visual cortex. Brain Res. 1988b;467:161–176. doi: 10.1016/0165-3806(88)90020-x. [DOI] [PubMed] [Google Scholar]
- Rangwala F, Drisdel RC, Rakhilin S, Ko E, Atluri P, Harkins AB, Fox AP, Salman SS, Green WN. Neuronal alpha-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. J Neurosci. 1997;17:8201–8212. doi: 10.1523/JNEUROSCI.17-21-08201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh TA, Constantine-Paton M. Retinal ganglion cell terminals change their projection sites during larval development of Rana pipiens. J Neurosci. 1984;4:442–457. doi: 10.1523/JNEUROSCI.04-02-00442.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerig B, Nelson DA, Katz LC. Fast synaptic signaling by nicotinic acetylcholine and serotonin 5-HT3 receptors in developing visual cortex. J Neurosci. 1997;17:8353–8362. doi: 10.1523/JNEUROSCI.17-21-08353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M, Dani JA. Comparison of quantitative calcium flux through NMDA, ATP, and ACh receptor channels. Biophys J. 1995;68:501–506. doi: 10.1016/S0006-3495(95)80211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Sargent PB, Pike SH, Nadel DB, Lindstrom JM. Nicotinic acetylcholine receptor-like molecules in the retina, retinotectal pathway, and optic tectum of the frog. J Neurosci. 1989;9:565–573. doi: 10.1523/JNEUROSCI.09-02-00565.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Hata Y, Hagihara K, Tsumoto T. Effects of cholinergic depletion on neuron activities in the cat visual cortex. J Neurophysiol. 1987a;58:781–794. doi: 10.1152/jn.1987.58.4.781. [DOI] [PubMed] [Google Scholar]
- Sato H, Hata Y, Masui H, Tsumoto T. A functional role of cholinergic innervation to neurons in the cat visual cortex. J Neurophysiol. 1987b;58:765–780. doi: 10.1152/jn.1987.58.4.765. [DOI] [PubMed] [Google Scholar]
- Scalia F. The optic pathway of the frog: Nuclear organization and connections. In: Llinás R, Precht W, editors. Frog neurobiology: a handbook. Berlin: Springer-Verlag; 1976. pp. 386–486. [Google Scholar]
- Schmidt JT. Apparent movement of optic terminals out of a local postsynaptically blocked region in goldfish optic tectum. J Neurophysiol. 1985;53:237–251. doi: 10.1152/jn.1985.53.1.237. [DOI] [PubMed] [Google Scholar]
- Schmidt JT. The modulatory cholinergic system in goldfish tectum may be necessary for retinotopic sharpening. Vis Neurosci. 1995;12:1093–1103. doi: 10.1017/s095252380000674x. [DOI] [PubMed] [Google Scholar]
- Schoepfer R, Conroy WG, Whiting P, Gore M, Lindstrom J. Brain alpha-bungarotoxin binding protein cDNAs and MAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990;5:35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Lindstrom J, Tzartos S, Schmued LC, O'Leary DD, Cowan WM. Immunohistochemical localization of monoclonal antibodies to the nicotinic acetylcholine receptor in chick midbrain. Proc Natl Acad Sci USA. 1983;80:4532–4536. doi: 10.1073/pnas.80.14.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM, Whiting PJ, Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci. 1987;7:3334–3342. doi: 10.1523/JNEUROSCI.07-10-03334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Székely G, Lázár G. Cellular and synaptic architecture of the optic tectum. In: Llinás R, Precht W, editors. Frog neurobiology: a handbook. Berlin: Springer-Verlag; 1976. pp. 407–434. [Google Scholar]
- Taylor AC, Kollros JJ. Stages in the normal development of Rana pipiens larvae. Anat Rec. 1946;94:7–28. doi: 10.1002/ar.1090940103. [DOI] [PubMed] [Google Scholar]
- Titmus MJ, Pleban L, Lima R, Udin SB. Cholinergic processes in Xenopus tectum. Soc Neurosci Abstr. 1996;22:761. [Google Scholar]
- Titmus MJ, Tsai HJ, Lima R, Udin SB. Effects of choline and other nicotinic agonists on the tectum of juvenile and adult Xenopus frogs: a patch-clamp study. Neuroscience. 1999;91:753–769. doi: 10.1016/s0306-4522(98)00625-3. [DOI] [PubMed] [Google Scholar]
- Tzartos SJ, Rand DE, Einarson BL, Lindstrom JM. Mapping of surface structures of electrophorus acetylcholine receptor using monoclonal antibodies. J Biol Chem. 1981;256:8635–8645. [PubMed] [Google Scholar]
- Udin SB. Development of the nucleus isthmi in Xenopus, II: branching patterns of contralaterally projecting isthmotectal axons during maturation of binocular maps. Vis Neurosci. 1989;2:153–163. doi: 10.1017/s0952523800012013. [DOI] [PubMed] [Google Scholar]
- Udin SB, Grant S. Plasticity in the tectum of Xenopus laevis: binocular maps. Prog Neurobiol. 1999;59:81–106. doi: 10.1016/s0301-0082(98)00096-3. [DOI] [PubMed] [Google Scholar]
- Wada K, Ballivet M, Boulter J, Connolly J, Wada E, Deneris ES, Swanson LW, Heinemann S, Patrick J. Functional expression of a new pharmacological subtype of brain nicotinic acetylcholine receptor. Science. 1988;240:330–334. doi: 10.1126/science.2832952. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Ricciuti AJ, Gruberg ER. Nucleus isthmi: its contribution to tectal acetylcholinesterase and choline acetyltransferase in the frog Rana pipiens. Neuroscience. 1990;35:627–636. doi: 10.1016/0306-4522(90)90334-z. [DOI] [PubMed] [Google Scholar]
- Whiting P, Schoepfer R, Lindstrom J, Priestley T. Structural and pharmacological characterization of the major brain nicotinic acetylcholine receptor subtype stably expressed in mouse fibroblasts. Mol Pharmacol. 1991;40:463–472. [PubMed] [Google Scholar]
- Whiting PJ, Lindstrom JM. Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J Neurosci. 1988;8:3395–3404. doi: 10.1523/JNEUROSCI.08-09-03395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;2:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Coggan JS, Berg DK. Synaptic currents generated by neuronal acetylcholine receptors sensitive to alpha-bungarotoxin. Neuron. 1996;17:1231–1240. doi: 10.1016/s0896-6273(00)80253-6. [DOI] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


