Abstract
Biofortification of staple foods with iron (Fe) in the form of ferritin (Ft) is now possible, both by conventional plant breeding methods and transgenic approaches. Ferritin-iron (Ft-Fe) from plants and animals is absorbed well (25-30%) by human subjects, but little is known about dietary factors affecting its absorption. We used human intestinal Caco-2 cells and compared Fe absorption from animal Ft and FeSO4 to determine the effects of inhibitors and enhancers, such as phytic acid, ascorbic acid, tannic acid, calcium, and heme. When post-confluent cells were co-incubated with 59Fe-labeled (1 μM) FeSO4 and dietary factors, at different molar ratios of dietary factor to Fe (phytic acid:Fe,10:1, ascorbic acid:Fe, 50:1, tannic acid:Fe, 50:1, calcium:Fe,10:1, and hemin:Fe,10:1), all inhibited uptake from FeSO4, except ascorbate, confirming earlier studies. In contrast, these dietary factors had little or no effect on Fe uptake from undigested Ft or ferritin digested in vitro at pH 4, except tannins. However, results after in vitro digestion of Ft at pH 2 were similar to those obtained for FeSO4. These results suggest that Fe uptake occurs from both undigested as well as digested Ft, but possibly via different mechanisms. The Fe-Ft stability shown here could minimize Fe-induced oxidation of Fe-supplemented food products.
Introduction
Iron deficiency is the most common nutrient deficiency in the world, resulting primarily from insufficient intake of dietary iron (Fe) and/or poor Fe bioavailability due to intake of inhibitors, such as phytate and polyphenols (tannins) in plant-based diets [1]. According to the World Health Organization (WHO) Regional Office for the Western Pacific (WPRO) 2005 report, over 3.5 billion people are affected by Fe deficiency world-wide. To combat Fe deficiency, Fe supplementation programs have been implemented globally where Fe is routinely given as ferrous salts [2]; however, such treatment has been associated with adverse effects such as constipation, diarrhea and decreased growth [3, 4]. Iron fortification of staple food components such as flour, salt, fish sauce, condiments, etc. is also used, but lack of centralized manufacturing facilities, low bioavailability of the fortified Fe, and product instability have limited the success of this approach. An alternative approach that has been proposed is biofortification of staple foods, such as cereals or legumes, with a form of bioavailable Fe [5, 6].
Ferritin is an iron storage molecule consisting of a protein shell that encloses an Fe core with up to 4500 Fe atoms in the form of solid, hydrated ferric oxide mineral [7]. Helical bundles of 24 subunits assemble to form the native protein structure [8, 9]. Some earlier studies showed that ferritin-Fe (Ft-Fe) is poorly absorbed [10-12] while other studies suggested good bioavailability [13]. It is likely that the disparity among these studies was due to the labeling techniques used. For example, conventional extrinsic labeling is not likely to result in exchange of the isotope and Fe within the insoluble mineral core, while intrinsic labeling may not be valid as various methods have been used to induce ferritin (inflammation, antibody-coating of erythrocytes, etc) from animal sources and Fe in this type of ferritin may not be representative of that inside “native” ferritin. Although animal and plant ferritin (phytoferritin) differ with regard to subunit structure and mineral core composition, several studies suggest that Fe from either source may be equally available for absorption. Studies in anemic rats have shown that soybean meal, in which a major part of Fe is in ferritin, as well as purified animal ferritin are as efficient as ferrous sulfate in restoring hemoglobin levels [14]. Studies on recombinant forms of Ft in iron-deficient and anemic rats also show high bioavailability of Ft-Fe [15, 16]. A study in humans using intrinsically labeled soybeans showed that Fe from this source is well utilized, suggesting that Fe from plant ferritin is absorbable [17]. Using a modified extrinsic labeling technique, by which Fe was removed from ferritin by extensive dialysis and then slowly restored by adding Fe together with the Fe isotope, we have recently shown that Fe given as ferritin in a composite breakfast meal is as well absorbed as Fe from ferrous sulfate [18, 19], regardless if the ferritin is from an animal (horse spleen) or plant (soybeans) source. Thus, it is likely that phytoferritin may serve as a source of bioavailable Fe in biofortification programs.
Since several dietary factors may limit the absorption of dietary Fe, it is important to evaluate whether these factors also affect Ft-Fe uptake. Phytic acid is found in cereals and legumes and has been shown to inhibit Fe absorption in humans [20] and in cell culture models [21-23]. Polyphenols (e.g. tannic acid), which are present in tea, red wine, etc., are also potent inhibitors of ferrous Fe uptake [23-25]. Calcium has also been shown to decrease Fe uptake [26, 27] and though the mechanisms behind this inhibition are not clearly known, it has been speculated that calcium might hinder Fe uptake because of intraluminal interactions [27]. In contrast, ascorbic acid has been shown to enhance Fe uptake in humans and in cell culture models [1, 25, 28] Finally, studies using heme, a well-absorbed form of dietary Fe found in meat, which provides one-third of the dietary Fe in many Western societies, have shown that heme-Fe absorption and cellular Fe level are inversely related [29], suggesting that Fe absorbed from heme gets incorporated into the common cellular Fe pool, thus augmenting Fe status of the cell.
In this study, we examined the effects of various dietary factors on Fe uptake from both intact and digested Ft as compared to uptake of Fe from ferrous sulfate. In vitro digestion of Ft was conducted at pH 4 and pH 2, representing infant and adult digestive conditions, respectively, to determine the effects of pH on Ft protein digestion and Fe uptake. We conducted this study in Caco-2 cells (a human adenocarcinoma cell line that differentiates into a small intestine-like epithelium) which have been successfully used by us [22, 29] and others [21, 23] to examine factors affecting intestinal Fe absorption. Results from this cell model have been shown to correlate well with data from human studies [30, 31].
Materials and Methods
Reagents
Animal ferritin (horse spleen) was purchased from Calzyme Laboratories (San Luis Obispo, CA) and then dialyzed to remove the iron core following the protocol previously described [18]. Briefly, iron was removed by thioglycolic acid reduction and dialysis. All steps were performed at 4°C unless otherwise specified. Ferritin was mixed with 2% thioglycolic acid at a 1:1 ratio, gently purged with nitrogen, and stored at 4 °C for 1 h. This mixture was then placed in dialysis tubing [Spectra/Pore CE (MW cutoff: 10 000); Spectrum, Rancho Dominguez, CA] and dialyzed against 1% thioglycolic acid and 0.05 mmol HEPES/L. The dialysate was changed every 8 h for 3 d. On day 4, the dialysate consisted of 0.05 mmol HEPES/L only. The final dialysate change on day 4 consisted of 0.15 mmol HEPES/L and 0.1 mmol NaCl/L. The iron-free ferritin protein (<10 Fe atoms/protein molecule) was then stored at 4 °C until reconstitution of the mineral inside the ferritin protein. The apo-protein shell was then reconstituted using ferrous iron with a radioactive iron tracer (holo-Ft). Radioactive iron (59Fe as FeSO4; specific activity 27.7 mCi/mg) was purchased from Perkin Elmer (Boston, MA). All other reagents were purchased from Sigma (St. Louis, MO), unless otherwise stated. Prior to the administration of intact Ft to cells or to digestion, it was subjected to buffer exchange using Centricon filter tubes to ensure removal of loosely bound surface radioactivity. A 30kD molecular weight cut-off membrane filtered surface bound radioactivity (filtered fraction) into a collection tube, thus separating it from the reconstituted protein (filtrate). Radioactivity associated with the filtered fraction was then measured using a gamma counter. Filtrate fractions yielding filtered fractions with non-specific radioactivity close to background values were used for experimental purposes. Thus, the non-specific radioactivity removal was over 99.9% efficient.
Cell culture
Caco-2 cells were obtained from American Type Culture Collection (Manassas, VA) and were cultured in MEM (GIBCO, Gaithersburg, MD) containing 10% fetal bovine serum (Sigma) and antibiotics. They were maintained at 37°C with constant humidity in a 5% CO2-95% air atmosphere. All experiments were conducted between the 35th and 40th passage and at confluence (day 14) allowing for proper tight-junction formation.
In vitro digestion of ferritin
Radiolabeled ferritin was subjected to in vitro digestion to study the effects of digestion on ferritin-Fe uptake, and the subsequent influence of dietary factors. The pH of the solution of 1 μM Fe as holo-ferritin was lowered to either 4.0 or 2.0 with 1N HCl. Upon acidification, 2% pepsin (porcine, 4,200 U/mg, Sigma) was added and the mixture was incubated in the dark with shaking for 30 min to simulate gastric digestion. To simulate intestinal digestion, the pH was adjusted to 7.0 with 1 N NaHCO3, followed by addition of 0.4% pancreatin (porcine, 8 × USP, Sigma). Samples were incubated in the dark with shaking for 30 min, followed by addition of 0.4% alpha-antitrypsin (human, 10,000 U/mg, Sigma) to quench digestive activity.
Treatment of Caco-2 cells with digested and undigested ferritin
Post-confluent cells were washed with PBS and treated either with intact or with digested radiolabeled ferritin (containing 1 μM Fe) for 1 h at 37°C. Ferritin was digested under both infant (pH 4) and adult (pH 2) conditions. Medium was aspirated and cells were extensively washed with ice-cold PBS and then solubilized with 1N NaOH. Radioactivity associated with the cell fraction was measured in a gamma counter (Gamma 8500, Beckman, Irvine, CA). Cell protein was assessed using the Bradford assay [32]. Data are expressed as pmol Fe/μg cell protein relative to control cells treated with 1 μM radiolabeled FeSO4.
Treatment of Caco-2 cells with dietary factors and iron as ferritin or ferrous sulfate
Post-confluent cells were washed with PBS and treated either with radioactive (0.5 μCi 59Fe) FeSO4 (1 μM) or with radioactive ferritin (intact or digested) with 1 μM of Fe in the presence or absence of dietary factors at 37°C for 1 h. Concentrations of phytic acid, calcium and hemin (bovine, Sigma) were 10 μM each, resulting in a dietary factor to Fe molar ratio of 10:1. The instability of the heme molecule warranted the use of hemin, the more stable form of heme. Tannic acid and ascorbic acid did not impact Fe uptake in a statistically significant manner at concentrations of 10 μM, therefore 50 μM concentrations were used resulting in tannic acid and ascorbic acid to Fe molar ratios of 50:1 each. Medium was aspirated and cells were extensively washed with ice-cold PBS and then solubilized with 1 N NaOH. Radioactivity associated with the cell fraction was measured in the gamma counter. Data are expressed as percent of Fe uptake relative to control (non-treated) cells.
Western blots of undigested vs. digested Ft
Ferritin was digested under mild (pH 4.0) or normal (pH 2.0) digestive conditions. Equal amounts of undigested and digested Ft were resolved by gel electrophoresis (5 % polyacrylamide) in SDS under non-reducing conditions. Proteins were transferred by electroblotting onto nitrocellulose membrane, which was blocked with 5% nonfat milk in PBST at 4°C overnight. The membrane was washed with PBST and incubated with purified human ferritin antibody (DAKO, Copenhagen, Denmark) 2 μg/mL) in PBST for 45 min at room temperature. The membrane was washed with several changes of PBST and proteins were detected with donkey anti-rabbit IgG, horseradish peroxidase-linked (Amersham, Buckinghamshire, England) for 45 min at room temperature in 5% nonfat milk/PBST. Proteins were visualized by chemiluminescence SuperSignal Femto (Pierce) and exposed to X-ray film for the optimum exposure time. We also compared the effect of these digestion conditions on lactoferrin, a protein known to be resistant against proteolysis [33], and serum albumin, which is easily digested. This 4-20 % gradient gel was run in SDS and stained with Coomassie Blue.
Data analysis
Data are presented as means ± standard error of mean (SEM). Statistical analysis was performed using Graph Pad Prism v 3.02 (San Diego, CA). Significant effect of treatment was determined using Student's t-test or one-way analysis of variance (ANOVA) where indicated, P<0.05.
Results
Effect of digestion on Fe uptake from ferritin
Mean Fe uptake from intact 59Fe-labeled Ft by Caco-2 cells after 1 h of incubation was 0.41 ± 0.01 pmol/μg cell protein which was significantly lower (P<0.05) than uptake from FeSO4 (0.58 ± 0.03 pmol/μg cell protein). In vitro digestion of radiolabeled Ft at pH 4 and pH 2 significantly increased Ft-Fe uptake 2-fold to 0.92 ± 0.02 pmol/μg cell protein and 10-fold to 5.44 ± 0.26 pmol/μg cell protein, respectively. These results suggest that the in vitro digestion results in partial or complete degradation of the Ft molecule in a pH-dependent manner, and that Fe becomes more available for absorption.
Effect of dietary factors on Fe uptake from ferrous sulfate and undigested ferritin
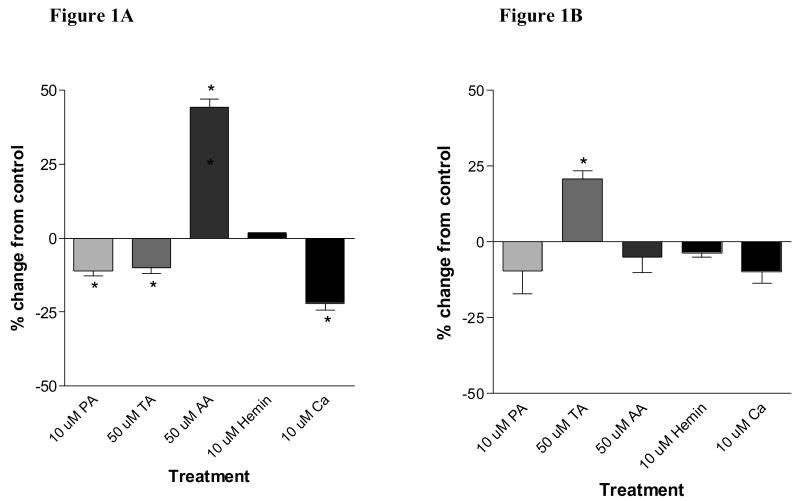
Three well-established inhibitors of non-heme Fe uptake, phytic acid (10 μM), tannic acid (50 μM) and calcium (10 μM) significantly inhibited Fe uptake from FeSO4 compared with untreated controls by 11.2 ± 0.8 %, 9.9 ± 1.1 % and 22.2 ± 1.1 %, respectively (Figure 1A). Moreover, ascorbic acid significantly increased ferrous Fe uptake by 44.1 ± 1.4% while hemin had no effect. In contrast, phytic acid, ascorbic acid, and calcium had no effect on Ft-Fe uptake (Figure 1B); however, tannic acid significantly enhanced Ft-Fe uptake to 120.8 ± 1.3 % of the untreated controls.
Figure 1.
Fe uptake by Caco-2 cells co-incubated with 1 μM FeSO4 (A) or 1 μM intact ferritin (B) and different dietary factors (PA: phytic acid; TA: tannic acid; AA: ascorbic acid) at 37°C for 1h. Data are presented as change in percentage of Fe uptake into the cells as compared to control (no treatment). Significant differences (P < 0.05) are indicated with an asterisk (*).
Effect of dietary factors on Fe uptake from ferritin subjected to in vitro digestion
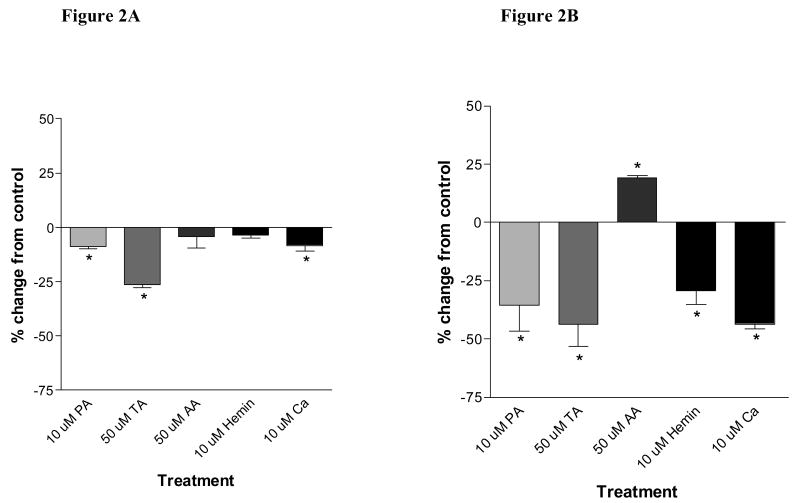
In contrast to the lack of effect observed on Fe uptake from undigested Ft, phytic acid significantly decreased Fe uptake from Ft digested at pH 4 (9.6 ± 0.9 %) and pH 2 (35.4 ± 5.6%), compared with undigested controls (Figure 2A). Contrary to its enhancing effect on Fe uptake from intact Ft, tannic acid significantly decreased Fe uptake from Ft digested at pH 4 (26.3 ± 0.6%) and pH 2 (43.8 ± 4.7%; Figure 2B). Calcium inhibited Fe uptake from Ft digested at pH 4 (8.4 ± 1.3%) and pH 2 (43.7 ± 1.0%) compared with Fe uptake from undigested Ft. There was no effect of ascorbic acid or hemin on Fe uptake from Ft digested at pH 4 but digestion at pH 2 increased Fe uptake in the presence of ascorbic acid by 19.1 ± 0.4% whereas hemin inhibited Fe uptake by 29.5 ± 2.8% as compared to undigested controls. This suggests that that with increasing severity of digestion conditions, Fe may be released from the mineralized core and may subsequently be made available for interactions with common dietary factors following its release.
Figure 2.
Fe uptake by Caco-2 cells co-incubated with 1 μM ferritin subject to digestion at pH 4 (A) and pH 2 (B) and different dietary factors (PA: phytic acid; TA: tannic acid; AA: ascorbic acid) at 37°C for 1h. Data are presented as change in percentage of Fe uptake into the cells as compared to control (no treatment). Significant differences (P < 0.05) are indicated with an asterisk (*).
Western blot of undigested Ft and digested Ft
Undigested and digested Ft were resolved by gel electrophoresis and Western blot analysis demonstrated significant degradation of Ft digested at pH 2 as compared to Ft digested at pH 4 (Figure 3A), further supporting the concept that digestion results in degradation of the native Ft protein and release of Fe. Intact ferritin protein (∼440 kD) occurs as a band at the top of the blot in the untreated sample and the sample digested at pH 4. Limited degradation of the Ft protein from “infant” or “compromised” digestion conditions (pH 4) resulted in occurrence of lower molecular weight bands at ∼160 kD, 60 kD and 21 kD. We speculate that this limited digestion of the protein possibly results in partial release of the inner Fe core. Harsher digestive conditions (pH 2), on the other hand, cause significant digestion of the protein coat, resulting in subunits with molecular weights too small to be detected as observed from the absence of any additional bands on the blot. Several types of Ft subunits have been observed in commercial ferritin preparations, both full length and fragments from a single cut. Sensitivity of a single site in the Ft subunit to proteolysis during isolation has been previously observed in commercial and fresh preparations of functional ferritin. Subunits generated during proteolysis have been shown to form cross-linked subunit dimers and larger aggregates were also observed [34]. As shown in Fig. 3B, digestion at pH 4 results in complete proteolysis of albumin, whereas both ferritin and lactoferrin are relatively resistant against proteolysis. Under these conditions (reducing gel), Ft is largely present in its 21 kD subunit, but some smaller fragments are also observed.
Figure 3.
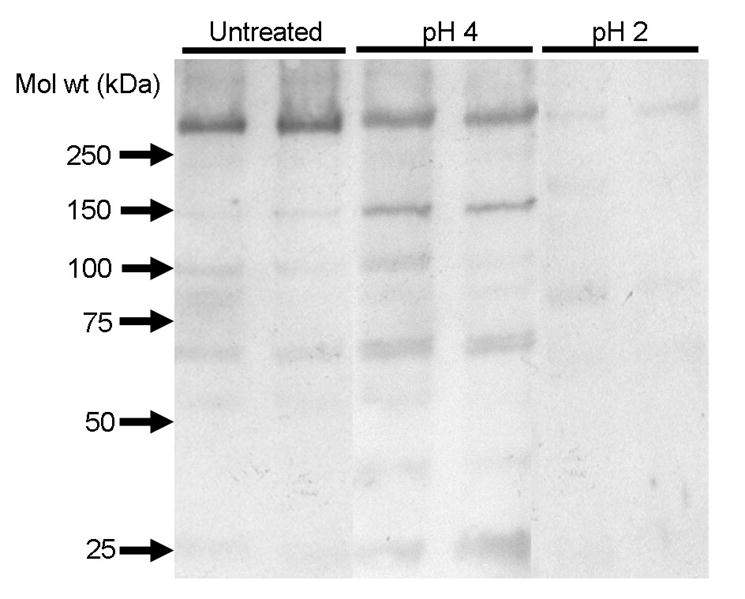
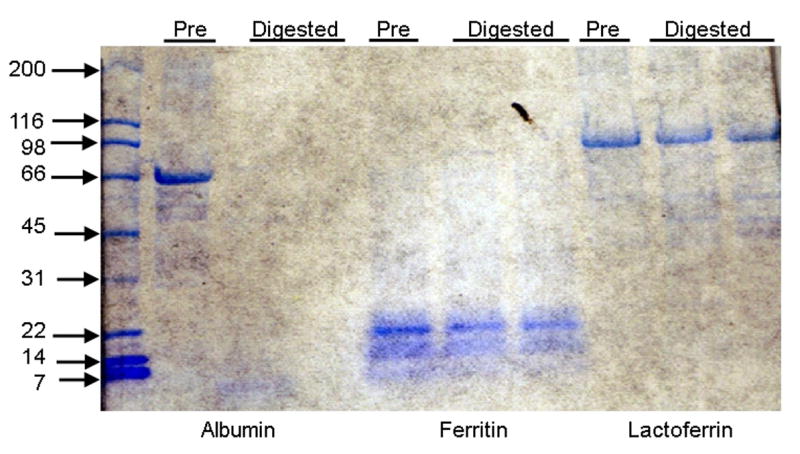
A. Intact and digested Ft were resolved by gel electrophoresis (5% gel) and Western blot analysis demonstrates significant degradation of Ft digested at pH 2 as compared to undigested Ft and Ft digested at pH 4. Limited degradation of the ferritin protein from “compromised” digestion (pH 4) results in occurrence of a several bands with lower molecular weights. Harsher digestive conditions (pH 2) caused significant digestion of the protein coat, resulting in subunits with molecular weights too small to be detected on a 5% gel, as observed from the absence of any additional bands for ferritin digested at pH 2 on the blot. B. Ferritin, serum albumin and lactoferrin were exposed to in vitro digestion at pH 4 and resolved by gel electrophoresis in a 4-20 % gradient in SDS under reducing conditions. Serum albumin was completely digested, whereas ferritin and lactoferrin were resistant against proteolysis.
Discussion
In this study we used a modified method for extrinsic labeling of Ft, by which the Fe mineral core is slowly dialyzed away with thioglycolic acid, and then slowly re-constituted by addition of Fe in the presence of the radioisotope. Detailed studies using Mössbauer spectroscopy and X-ray absorption fine structure (EXAFS) have shown that Fe in Ft reconstituted in this fashion is identical to native Fe in the core [35]. This method of extrinsic labeling was used in our previous studies in human subjects showing high bioavailability of Ft-Fe [18].
The Caco-2 cell model has been used extensively to study the intestinal uptake of Fe, as well as factors affecting this uptake. Although its validity to predict absorption of Fe in human subjects has not been universally demonstrated, it is believed that it is a good screening tool to evaluate various compounds prior to performing human studies [30, 31]. Iron, either in the form of radiolabeled ferrous sulfate (1μM Fe) or intact Ft (1μM Fe), administered to the apical surface of the Caco-2 monolayer was detected inside the cells, demonstrating that Caco-2 cells absorb ferrous Fe and Fe from intact Ft, albeit somewhat less from Ft during this time period (1 h). It is well-known that uptake of ferrous Fe uptake in intestinal cells is facilitated by divalent metal transporter-1 (DMT-1), which is located in the apical membrane of the enterocyte [36]. However, Fe sequestered within the mineral core of Ft is unavailable to this transporter; thus, another mechanism for Ft-Fe uptake must exist. While the mechanism responsible for Fe uptake from Ft is not yet known, the presence of putative Ft receptors has been documented for several cell types [37-42], and we have preliminary data indicating receptor-mediated uptake of Fe from Ft by Caco-2 cells (Kalgaonkar & Lönnerdal, to be submitted).
We observed significant effects of phytic acid, tannic acid, ascorbic acid and calcium on ferrous Fe uptake in Caco-2 cells, in agreement with results of other studies on dietary factors affecting absorption of non-heme Fe. Phytic acid, tannic acid and calcium significantly reduced ferrous Fe uptake whereas ascorbic acid resulted in a significant increase. These effects were not observed for Fe uptake from intact Ft, indicating that Fe within Ft is unable to interact with these dietary factors. Tannic acid increased Fe uptake from intact Ft, possibly by interfering with Ft or Ft mineral core assembly due to its amphoteric properties and releasing Fe for absorption. It is likely that ferritin is affected by normal digestive processes in the gastrointestinal tract. We therefore exposed ferritin to in vitro digestion, using a “gastric” pH of 2. However, legume ferritin is present in plastids [43] and it is uncertain whether pH within the plastids can be lowered to such an extent despite low surrounding pH. In addition, although most in vitro digestion studies have used a pH of 2 for the pepsin digestion because this has been assumed to be the pH of adult stomach [23, 25, 31], several recent studies on stomach aspirates from adult human subjects show that the postprandial pH is around 4, even up to 3 hrs after a meal [44, 45]. We therefore also used a “gastric” pH of 4 to evaluate this possibility. We have previously [46] used such a pH as an in vitro model to study protein digestion in infants, who have been shown to have a considerably higher stomach pH than adults [47]. Dietary factors (with the exception of tannic acid) demonstrated limited effects on Fe uptake from Ft digested at pH 4; however, following digestion at pH 2, pronounced effects of these factors on Fe uptake were observed. These observations again suggest that Ft provides a protective protein coat to its Fe core which is degraded in a pH dependent manner. It is thus possible that Ft in part escapes digestion and is present in the small intestine in intact form. Ft-Fe may then be taken up by another pathway, possibly explaining previous observations in humans in which dietary factors had limited or no effect on absorption of Ft-Fe. We have previously shown that lactoferrin, another large iron-binding protein known to be resistant against digestion [33], can be found in significant quantities in intact form in the stool of breast-fed infants [48]. In vitro studies, similar to those in our present study, show that lactoferrin can resist proteolytic digestion [46] and also be taken up and deliver its Fe into Caco-2 cells by a specific lactoferrin receptor [49]. Thus, some proteins are able to escape digestion and be taken up via endocytotic pathways.
The concentration of Ft in plants is usually quite low, or about 50-70 mg/kg [50]. Thus, a 50 g meal of soybeans would contain at most 0.5 mg of Ft-Fe. Conventional plant breeding techniques may increase the Ft content 2-3 times, but the amount of Fe would still be low as compared to the Fe requirement of Fe-deficient children and women. Expression of recombinant Ft in transgenic plants offers an opportunity to increase the Ft content of legumes to a much higher extent [51]. However, it is not yet known whether a substantially increased Ft content of such plants will lead to a corresponding increase in the Fe content; the ability of the plant to acquire Fe from the soil and transport it to the bean/seed may be a limiting factor. Further studies are needed to explore the possibility of over-expressing recombinant Ft in plants and its effect on the Fe content.
In conclusion, the results from this study strongly suggest that Fe from undigested Ft is absorbed by intestinal cells and that while common dietary factors dramatically affect ferrous Fe uptake, they have a limited effect on Fe uptake from undigested Ft. We speculate that this limited effect may be due to the protective protein coat provided by Ft and facilitated through an uptake pathway for Ft-Fe distinct from that of ferrous Fe. However, once digested, Fe release from the mineralized core occurs allowing for interaction with dietary factors. Taken together, these results show that Fe is well absorbed from both undigested and digested animal Ft and it is therefore likely that biofortification of staple foods with Ft will yield Fe in a bioavailable form. Although dietary factors will affect absorption of Ft-Fe, to an extent depending on the degree of digestion, the impact of them will be similar to or less than those affecting Fe uptake from FeSO4, a bioavailable form of Fe but prone to cause changes in color and organoleptic properties of products fortified with this compound. In contrast, Ft-Fe is inside the mineral core and protected by a protein shell and when present in foods will minimize Fe-induced oxidative damage to the product.
Acknowledgments
We are grateful to Drs. Elizabeth Theil and Shannon L Kelleher for their significant input to this study and critical review of the manuscript.
Supported by NIH grant # R01 HL56169. Presented in part at the International BioIron meeting in Prague, Czech Republic, June 2005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Sandberg AS. Bioavailability of minerals in legumes. Br J Nutr. 2002 Dec;88 3:S281–5. doi: 10.1079/BJN/2002718. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Exposito AB, Villalpando S, Rivera JA, Griffin IJ, Abrams SA. Ferrous sulfate is more bioavailable among preschoolers than other forms of iron in a milk-based weaning food distributed by PROGRESA, a national program in Mexico. J Nutr. 2005 Jan;135:64–9. doi: 10.1093/jn/135.1.64. [DOI] [PubMed] [Google Scholar]
- 3.Hyder SM, Persson LA, Chowdhury AM, Ekstrom EC. Do side-effects reduce compliance to iron supplementation? A study of daily- and weekly-dose regimens in pregnancy. J Health Popul Nutr. 2002 Jun;20:175–9. [PubMed] [Google Scholar]
- 4.Dewey KG, Domellof M, Cohen RJ, Landa Rivera L, Hernell O, Lonnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr. 2002 Nov;132:3249–55. doi: 10.1093/jn/132.11.3249. [DOI] [PubMed] [Google Scholar]
- 5.Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F. Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol. 1999 Mar;17:282–6. doi: 10.1038/7029. [DOI] [PubMed] [Google Scholar]
- 6.Lucca P, Hurrell R, Potrykus I. Fighting iron deficiency anemia with iron-rich rice. J Am Coll Nutr. 2002 Jun;21:184S–90S. doi: 10.1080/07315724.2002.10719264. [DOI] [PubMed] [Google Scholar]
- 7.Theil EC. Iron, ferritin, and nutrition. Annu Rev Nutr. 2004;24:327–43. doi: 10.1146/annurev.nutr.24.012003.132212. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Theil EC. Ferritins: dynamic management of biological iron and oxygen chemistry. Acc Chem Res. 2005 Mar;38:167–75. doi: 10.1021/ar0302336. [DOI] [PubMed] [Google Scholar]
- 9.Masuda T, Mikami B, Goto F, Yoshihara T, Utsumi S. Crystallization and preliminary X-ray crystallographic analysis of plant ferritin from Glycine max. Biochim Biophys Acta. 2003 Jan 31;1645:113–5. doi: 10.1016/s1570-9639(02)00523-x. [DOI] [PubMed] [Google Scholar]
- 10.Layrisse M, Martinez-Torres C, Renzy M, Leets I. Ferritin iron absorption in man. Blood. 1975 May;45:689–98. [PubMed] [Google Scholar]
- 11.Lynch SR, Beard JL, Dassenko SA, Cook JD. Iron absorption from legumes in humans. Am J Clin Nutr. 1984 Jul;40:42–7. doi: 10.1093/ajcn/40.1.42. [DOI] [PubMed] [Google Scholar]
- 12.Skikne B, Fonzo D, Lynch SR, Cook JD. Bovine ferritin iron bioavailability in man. Eur J Clin Invest. 1997 Mar;27:228–33. doi: 10.1046/j.1365-2362.1997.970645.x. [DOI] [PubMed] [Google Scholar]
- 13.Sayers MH, Lynch SR, Jacobs P, Charlton RW, Bothwell TH, Walker RB, Mayet F. The effects of ascorbic acid supplementation on the absorption of iron in maize, wheat and soya. British journal of haematology. 1973 Feb;24:209–18. doi: 10.1111/j.1365-2141.1973.tb05741.x. [DOI] [PubMed] [Google Scholar]
- 14.Beard JL, Burton JW, Theil EC. Purified ferritin and soybean meal can be sources of iron for treating iron deficiency in rats. J Nutr. 1996 Jan;126:154–60. doi: 10.1093/jn/126.1.154. [DOI] [PubMed] [Google Scholar]
- 15.Murray-Kolb LE, Takaiwa F, Goto F, Yoshihara T, Theil EC, Beard JL. Transgenic rice is a source of iron for iron-depleted rats. J Nutr. 2002 May;132:957–60. doi: 10.1093/jn/132.5.957. [DOI] [PubMed] [Google Scholar]
- 16.Chang YJ, Jo MY, Hwang EH, Park CU, Kim KS. Recovery from iron deficiency in rats by the intake of recombinant yeast producing human H-ferritin. Nutrition. 2005 Apr;21:520–4. doi: 10.1016/j.nut.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Murray-Kolb LE, Welch R, Theil EC, Beard JL. Women with low iron stores absorb iron from soybeans. Am J Clin Nutr. 2003 Jan;77:180–4. doi: 10.1093/ajcn/77.1.180. [DOI] [PubMed] [Google Scholar]
- 18.Davila-Hicks P, Theil EC, Lonnerdal B. Iron in ferritin or in salts (ferrous sulfate) is equally bioavailable in nonanemic women. Am J Clin Nutr. 2004 Oct;80:936–40. doi: 10.1093/ajcn/80.4.936. [DOI] [PubMed] [Google Scholar]
- 19.Lonnerdal B, Bryant A, Liu X, Theil EC. Iron absorption from soybean ferritin in nonanemic women. Am J Clin Nutr. 2006 Jan;83:103–7. doi: 10.1093/ajcn/83.1.103. [DOI] [PubMed] [Google Scholar]
- 20.Hurrell RF. Influence of vegetable protein sources on trace element and mineral bioavailability. J Nutr. 2003 Sep;133:2973S–7S. doi: 10.1093/jn/133.9.2973S. [DOI] [PubMed] [Google Scholar]
- 21.Han O, Failla ML, Hill AD, Morris ER, Smith JC., Jr Inositol phosphates inhibit uptake and transport of iron and zinc by a human intestinal cell line. J Nutr. 1994 Apr;124:580–7. doi: 10.1093/jn/124.4.580. [DOI] [PubMed] [Google Scholar]
- 22.Skoglund E, Lonnerdal B, Sandberg AS. Inositol phosphates influence iron uptake in Caco-2 cells. J Agric Food Chem. 1999 Mar;47:1109–13. doi: 10.1021/jf980745c. [DOI] [PubMed] [Google Scholar]
- 23.Glahn RP, Wortley GM, South PK, Miller DD. Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: studies using an in vitro digestion/Caco-2 cell model. J Agric Food Chem. 2002 Jan 16;50:390–5. doi: 10.1021/jf011046u. [DOI] [PubMed] [Google Scholar]
- 24.Tuntawiroon M, Sritongkul N, Brune M, Rossander-Hulten L, Pleehachinda R, Suwanik R, Hallberg L. Dose-dependent inhibitory effect of phenolic compounds in foods on nonheme-iron absorption in men. Am J Clin Nutr. 1991 Feb;53:554–7. doi: 10.1093/ajcn/53.2.554. [DOI] [PubMed] [Google Scholar]
- 25.Engle-Stone R, Yeung A, Welch R, Glahn R. Meat and ascorbic acid can promote Fe availability from Fe-phytate but not from Fe-tannic acid complexes. J Agric Food Chem. 2005 Dec 28;53:10276–84. doi: 10.1021/jf0518453. [DOI] [PubMed] [Google Scholar]
- 26.Hallberg L. Does calcium interfere with iron absorption? Am J Clin Nutr. 1998 Jul;68:3–4. doi: 10.1093/ajcn/68.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Roughead ZK, Zito CA, Hunt JR. Inhibitory effects of dietary calcium on the initial uptake and subsequent retention of heme and nonheme iron in humans: comparisons using an intestinal lavage method. Am J Clin Nutr. 2005 Sep;82:589–97. doi: 10.1093/ajcn.82.3.589. [DOI] [PubMed] [Google Scholar]
- 28.Han O, Failla ML, Hill AD, Morris ER, Smith JC., Jr Ascorbate offsets the inhibitory effect of inositol phosphates on iron uptake and transport by Caco-2 cells. Proc Soc Exp Biol Med. 1995 Oct;210:50–6. doi: 10.3181/00379727-210-43924. [DOI] [PubMed] [Google Scholar]
- 29.Follett JR, Suzuki YA, Lonnerdal B. High specific activity heme-Fe and its application for studying heme-Fe metabolism in Caco-2 cell monolayers. Am J Physiol Gastrointest Liver Physiol. 2002 Nov;283:G1125–31. doi: 10.1152/ajpgi.00443.2001. [DOI] [PubMed] [Google Scholar]
- 30.Au AP, Reddy MB. Caco-2 cells can be used to assess human iron bioavailability from a semipurified meal. J Nutr. 2000 May;130:1329–34. doi: 10.1093/jn/130.5.1329. [DOI] [PubMed] [Google Scholar]
- 31.Yun S, Habicht JP, Miller DD, Glahn RP. An in vitro digestion/Caco-2 cell culture system accurately predicts the effects of ascorbic acid and polyphenolic compounds on iron bioavailability in humans. J Nutr. 2004 Oct;134:2717–21. doi: 10.1093/jn/134.10.2717. [DOI] [PubMed] [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Brines RD, Brock JH. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum. Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim Biophys Acta. 1983 Sep 13;759:229–35. doi: 10.1016/0304-4165(83)90317-3. [DOI] [PubMed] [Google Scholar]
- 34.Mertz JR, Theil EC. Subunit dimers in sheep spleen apoferritin. The effect on iron storage. The Journal of biological chemistry. 1983 Oct 10;258:11719–26. [PubMed] [Google Scholar]
- 35.Rohrer JS, Islam QT, Watt GD, Sayers DE, Theil EC. Iron environment in ferritin with large amounts of phosphate, from Azotobacter vinelandii and horse spleen, analyzed using extended X-ray absorption fine structure (EXAFS) Biochemistry. 1990 Jan 9;29:259–64. doi: 10.1021/bi00453a035. [DOI] [PubMed] [Google Scholar]
- 36.Sharp P, Tandy S, Yamaji S, Tennant J, Williams M, Singh Srai SK. Rapid regulation of divalent metal transporter (DMT1) protein but not mRNA expression by non-haem iron in human intestinal Caco-2 cells. FEBS letters. 2002 Jan 2;510:71–6. doi: 10.1016/s0014-5793(01)03225-2. [DOI] [PubMed] [Google Scholar]
- 37.Mack U, Storey EL, Powell LW, Halliday JW. Characterization of the binding of ferritin to the rat liver ferritin receptor. Biochim Biophys Acta. 1985 Dec 13;843:164–70. doi: 10.1016/0304-4165(85)90135-7. [DOI] [PubMed] [Google Scholar]
- 38.Takami M, Mizumoto K, Kasuya I, Kino K, Sussman HH, Tsunoo H. Human placental ferritin receptor. Biochim Biophys Acta. 1986 Oct 29;884:31–8. doi: 10.1016/0304-4165(86)90223-0. [DOI] [PubMed] [Google Scholar]
- 39.Adams PC, Powell LW, Halliday JW. Isolation of a human hepatic ferritin receptor. Hepatology. 1998 Jul-Aug;8:719–21. doi: 10.1002/hep.1840080402. [DOI] [PubMed] [Google Scholar]
- 40.Moss D, Fargion S, Fracanzani AL, Levi S, Cappellini MD, Arosio P, Powell LW, Halliday JW. Functional roles of the ferritin receptors of human liver, hepatoma, lymphoid and erythroid cells. J Inorg Biochem. 1992 Aug 15;Sep 15;47:219–27. doi: 10.1016/0162-0134(92)84067-w. [DOI] [PubMed] [Google Scholar]
- 41.Ramm GA, Britton RS, O'Neill R, Bacon BR. Identification and characterization of a receptor for tissue ferritin on activated rat lipocytes. J Clin Invest. 1994 Jul;94:9–15. doi: 10.1172/JCI117353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao QK, Kong PA, Gao J, Li FY, Qian ZM. Expression of ferritin receptor in placental microvilli membrane in pregnant women with different iron status at mid-term gestation. Eur J Clin Nutr. 2001 Aug;55:651–6. doi: 10.1038/sj.ejcn.1601195. [DOI] [PubMed] [Google Scholar]
- 43.Waldo GS, Wright E, Whang ZH, Briat JF, Theil EC, Sayers DE. Formation of the ferritin iron mineral occurs in plastids. Plant Physiol. 1995 Nov;109:797–802. doi: 10.1104/pp.109.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonian HP, Vo L, Doma S, Fisher RS, Parkman HP. Regional postprandial differences in pH within the stomach and gastroesophageal junction. Digestive diseases and sciences. 2005 Dec;50:2276–85. doi: 10.1007/s10620-005-3048-0. [DOI] [PubMed] [Google Scholar]
- 45.Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharmaceutical research. 2006 Jan;23:165–76. doi: 10.1007/s11095-005-8476-1. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki YA, Kelleher SL, Yalda D, Wu L, Huang J, Huang N, Lonnerdal B. Expression, characterization, and biologic activity of recombinant human lactoferrin in rice. J Pediatr Gastroenterol Nutr. 2003 Feb;36:190–9. doi: 10.1097/00005176-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Agunod M, Yamaguchi N, Lopez R, Luhby AL, Glass GB. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Am J Dig Dis. 1969 Jun;14:400–14. doi: 10.1007/BF02239360. [DOI] [PubMed] [Google Scholar]
- 48.Davidson LA, Lonnerdal B. Persistence of human milk proteins in the breast-fed infant. Acta paediatrica Scandinavica. 1987 Sep;76:733–40. doi: 10.1111/j.1651-2227.1987.tb10557.x. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki YA, Shin K, Lonnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001 Dec 25;40:15771–9. doi: 10.1021/bi0155899. [DOI] [PubMed] [Google Scholar]
- 50.Sczekan SR, Joshi JG. Isolation and characterization of ferritin from soyabeans (Glycine max) The Journal of biological chemistry. 1987 Oct 5;262:13780–8. [PubMed] [Google Scholar]
- 51.Theil EC, Burton JW, Beard JL. A sustainable solution for dietary iron deficiency through plant biotechnology and breeding to increase seed ferritin control. Eur J Clin Nutr. 1997 Nov;51 4:S28–31. [PubMed] [Google Scholar]