Abstract
The presence of a small intestinal lactoferrin receptor (SI-LfR) has been suggested in the pig, but remains to be identified. LfR has been suggested to play a key role in the internalization of lactoferrin (Lf) and to facilitate absorption of iron bound to Lf. The aim of this study was to identify the pig SI-LfR cDNA, determine its mRNA and protein expression during different stages of intestinal development. The coding region of the pig LfR cDNA was cloned by PCR using conserved sequences among species. LfR mRNA expression and protein abundance were measured in proximal small intestine from piglets at 1 week (pre-weaning), 3 weeks (weaning) and 6 months (post-weaning) of age by quantitative (real-time) RT-PCR (Q-PCR) and Western blot, respectively. Intestinal brush border membrane vesicles (BBMV) were also isolated to examine LfR abundance on the apical membrane. We determined the pig SI-LfR open reading frame (ORF) consists of 972 bp, resulting in a protein with a molecular mass ~135 kD and ~35 kD under non-reducing and reducing conditions, respectively. Using Q-PCR, we determined LfR expression significantly increased with age in the duodenum and reciprocally decreased in the jejunum. Intestinal LfR protein expression was maintained at all timepoints in the jejunum; however, in the duodenum LfR abundance reached maximum levels at 6 months. In BBMV fractions, LfR abundance significantly increased with age. Taken together our findings demonstrate the presence of a human SI-LfR homologue in pig, with mRNA and protein expression concomitantly regulated in the duodenum and inversely regulated in the jejunum. These findings suggest a mechanism by which pig Lf can be internalized in the intestine.
Keywords: lactoferrin, lactoferrin receptor, pig
1. Introduction
Specific lactoferrin receptors (LfRs) have been shown to be present at the surface of different tissues and cell types, including small intestine, liver, neutrophils, bone and brain (Suzuki et al. 2005), and a receptor-mediated pathway has been proposed for the variety of lactoferrin (Lf) activities. The LfR cDNA has been cloned only for humans (Suzuki et al. 2001) and the mouse (Suzuki et al. 2004), and its tissue distribution has been studied in the mouse by immunohistochemistry, showing most prominent staining in the brush border of the small intestinal epithelium (Suzuki et al. 2004). Recently, we demonstrated that mouse LfR was abundantly expressed from birth until postnatal day 20 (Lopez et al. 2006), suggesting a mechanism by which Lf is internalized, and highlighting its importance during early infancy. However, it should be noted that virtually all studies on the identification of LfR in other species have focused on its binding to Lf, and there is no information with regard to whether it is the homologous LfR cDNA in other species. In addition, little is known about the distribution of LfR along the small intestine longitudinal axis.
Lf is an 80 kD glycoprotein that can reversibly bind 2 ferric ions. It is the major iron-binding protein in human milk, and is among the few proteins that can survive gastric and pancreatic enzyme proteolysis (Lönnerdal 1991; Lönnerdal et al. 1995) as it is found intact in significant quantities in the stool of breast-fed infants (Davidson et al. 1987). Furthermore, transepithelial transport of Lf was found to occur in human preterm infants (Hutchens et al. 1991). It has been suggested that Lf may play specific roles during infancy, one of which is to facilitate iron absorption. Independent of iron-binding properties, Lf has also been shown to have antimicrobial activities, stimulate cytokine production, enhance cell proliferation, and regulate mucosal immunity (Ward et al. 2003).
The piglet has long been recognized as an excellent model for human infants, due to the similarity of its gastrointestinal tract physiology to that of humans (Alpers 2000; Bertolo et al. 2003; Domeneghini et al. 2006; Miller et al. 1987; Wu et al. 2004). Notably, compared to other commonly used animal models, pigs have higher concentrations of milk Lf, ranging from 1.2 mg/ml in colostrum to 0.3 mg/ml in mature milk (Elliot et al. 1984; Gislason et al. 1993), making it a good model to investigate the role of milk Lf during infancy and to examine the response to milk feeding and weaning. We have previously shown by binding studies the existence of a receptor for Lf in both pig duodenum and jejunum (Gislason et al. 1993; Gislason et al. 1995). Isolated brush border membrane vesicles were used and a Kd of 0.3 μM was obtained. In addition, the binding of Lf to piglet enterocytes was highly specific, as bovine Lf, but not pig transferrin (Tf) significantly inhibited the binding. Furthermore, the number of binding sites for Lf in the piglet (Gislason et al. 1993) was similar to what was found in the non-human primate (Davidson and Lönnerdal, 1988). Altogether, these data suggest that the pig is a useful model to study interactions between Lf and its receptor and to evaluate the differential expression of LfR along the small intestine.
The objectives of this study were to utilize the piglet as a model to identify the pig homologue of the human SI-LfR cDNA, determine LfR mRNA and protein expression pattern in the small intestine, and abundance at the apical membrane of the enterocytes during various stages of intestinal development.
2. Materials and Methods
2.1. Animals and sample collection
This study complied with the Guide for the Use and Care of Laboratory Animals and was approved by the Animal Research Services at the University of California, Davis, which is accredited by the Animal Association for the Accreditation of Laboratory Animal Care. Female Yorkshire market pigs (Sus scrofa) were obtained from the University of California, Davis Swine Center at various ages during postnatal development: 1 week, 3 weeks (day of weaning) and 6 months. Animals were exclusively suckled from birth to 3 weeks (21 days) and animals 3 weeks or older were fed standard commercial pig chow. The animals were fasted 12 h prior to euthanization. Animals aged 1 week and 3 weeks were euthanized by cardiac injection with Euthanol 6 (26% sodium pental barbital, Fort Dodge-Wyeth, Madison, NJ, USA), and 6 months old animals were killed by electric shock and exsanguination. Immediately following euthanization/killing, segments of small intestine were identified and either placed in RNALater (Applied Biosystems, Foster City, CA, USA) for RNA isolation or immediately frozen on dry ice for protein isolation. All tissue samples were maintained at −80°C until analysis.
2.2. Cloning of pig LfR cDNA
Double-stranded cDNA was generated from isolated pig intestinal tissue placed in 5–10 ml of RNALater. After removal of RNALater solution, total RNA was extracted using TRiZol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. RNA was diluted to 2 μg/μL in DEPC-treated water (Ambion, Austin, TX, USA) and integrity was evaluated by electrophoresis through 2% agarose gel containing 400 μg/ml ethidium bromide (Sigma Aldrich, St. Louis, MO, USA). cDNA was generated from 2 μg RNA using the RT-system according to the manufacturer’s instructions (Applied Biosystems). PCR for partial ORF was performed with the following degenerate primers designed based on conserved LfR mRNA sequences among various species (Fig. 1) using the ClustalW program at the European Bioinformatics Institute (http://www.ebi.ac.uk/clustawl/), LfR sense primer 5′-GTGGGYGATCGCTGGTCCA-3′, LfR antisense primer 5′-CCTCCTCCACCRATGCAGTG-3′. PCR amplification was performed in 50 μl reaction mixture with 3 μl cDNA in the presence of 0.3 mM dNTP, 0.2 μM sense and antisense primers, 10 μl 5X high fidelity buffer, and 0.2 μl (2 U/μl) iProof High-Fidelity DNA Polymerase (Bio-Rad, Hercules, CA, USA). The cycling protocol was: initial denaturation at 98 °C for 1 min, 45 cycles of denaturation at 98 °C for 10 sec, annealing at 62 °C for 30 sec, extension at 72 °C for 30 sec, and final extension at 72 °C for 10 min. The PCR product was cloned into the plasmid vector pBluescript SK(+) (Stratagene, La Jolla, CA), and the insert was sequenced (Division of Biological Sciences Automated DNA Sequencing Facility, UC Davis, Davis, CA, USA) using the T7 and T3 primers.
Figure 1.
Conserved region alignment of putative lactoferrin receptors from Rattus norvegicus (GenBank accession no. NM_001034946), Mus musculus (GenBank accession no. NM_010584), Homo sapiens (GenBank accession no. NM_017625), Pan troglodytes (GenBank accession no. XM_513928), and Xenopus tropicalis (GenBank accession no. NM_203598). Asterisks indicate identical nucleotide sequences identified and underline sections indicates sequences used to generate primers.
2.3 Real-time relative RT-PCR
To determine expression of pig LfR mRNA in isolated intestinal tissue (n=6), quantitative (real-time) RT-PCR (Q-PCR) reactions were carried out using the ABI 7900 HT real-time thermocycler (Applied Biosystems, Foster City, CA), using the SYBR Green detection system (Applied Biosystems). Gene-specific primers were chosen using Primer Express software (Applied Biosystems) to span exons in order to avoid co-amplification of genomic DNA: pig LfR, sense primer 5′-TGGAATGGTTCCCGGAGAA-3′, antisense primer 5′-GCAACTCCGAGGCAGGAAA-3′ and pig β-actin; sense primer 5′-CTCCTTCCTGGGCATGGA-3′, antisense primer: 5′-CGCACTTCATGATCGAGTTGA-3′. Q-PCR was performed in duplicate in 25 μl reaction mixtures using the following protocol: 50 °C for 2 min; 95 °C for 10 min; 40 cycles of 95 °C for 15 s, 60 °C for 1 min, followed by a final stage of 95 °C for 15 s, 60 °C for 15 s; and 95 °C for 15 s. The linearity of the dissociation curve was analyzed using the ABI 7900 HT software and the mean cycle time of the linear part of the curve was designated cycle time (Ct). Each sample was analyzed in duplicate and normalized to β-actin using the following equation: ΔCtGENE=CtGENE−Ctactin. The fold change was calculated using the following equation: 2(−ΔΔCtGENE) where ΔΔCtGENE=ΔCtGENE of the control − ΔCtGENE of each pig. Values are mean fold change ± SD.
2.4 Western blot analysis
Isolated duodenal and jejunal tissues (0.5 g) were homogenized in Hepes-EDTA buffer (20 mM Hepes, pH 7.4, 1 mM EDTA, 250 mM sucrose/protease inhibitor mixture containing 4-(2-aminoethyl)benzenesulfonyl fluoride, trans-epoxysuccinyl-L-leucylamido(4-guanidino)butane, bestatin, leupeptin, aprotonin, and sodium EDTA), as previously described (McMahon et al. 1998). BBMVs were prepared as previously described (Lopez et al. 2006). Briefly, 200 μg of total homogenate was centrifuged at 5000 g for 10 min at 4°C. The pellet was collected and re-suspended in Tris buffer (2 mM Tris, 50 mM mannitol), incubated for 20 min with 10 mM CaCl2 on ice and centrifuged at 3,600 × g for 10 min. The supernatant was collected and centrifuged at 38,000 g for 20 min. The pellet was re-suspended in Tris buffer as described above and centrifuged at 38,000 g for 20 min. The final pellet was re-suspended in 250 μL Tris buffer containing 1 × complete protease inhibitor (Sigma). BBMV enrichment was determined by a >20-fold increase in alkaline phosphatase activity as previously described (Chandler et al. 1991). Protein concentrations were determined using the Bradford assay (Bio-Rad).
An equal amount of total protein (10–18 μg) was electrophoresed by SDS-PAGE under non-reducing or reducing conditions and transferred onto nitrocellulose membrane at 350 mA for 1 h. Membranes were blocked overnight in 5% nonfat milk in PBS/0.1% Tween-20 (PBST) overnight at 4°C. Blots were incubated at room temperature with LfR anti-serum (1:1000) in 5% nonfat milk in PBST for 1 h, and washed 3X with PBST. LfR anti-serum was previously characterized by our group (Suzuki et al. 2001). The LfR anti-serum was produced in rabbits against a chemically synthesized peptide (CTVGDRWSSQQGSKAD) which corresponds to part of the deduced human LfR amino acid sequence (Genemed Synthesis Inc., South San Francisco, CA, USA). Proteins were detected using HRP conjugated donkey anti-rabbit IgG (1:20,000; GE Healthcare-Amersham, Piscataway, NJ, USA), visualized with Super Signal Femto chemiluminescent reagent (Pierce, Rockford, IL, USA), and exposed to autoradiography film. Relative band density was quantified using the Chemi-doc Gel Quantification System (Bio-Rad).
2.5 Statistical analysis
All data were analyzed by Prism (GraphPad Software, San Diego, CA, USA). To analyze the change in LfR expression with increasing age in duodenum and jejunum individually, one-way ANOVA was used; if any significant differences were detected (P < 0.05), differences between ages were identified using Tukey’s test, and each pair of duodenum and jejunum data was compared by Student’s t-test. Values presented are means (of 3 experiments) ± SD. Differences were considered significant when P < 0.05.
3. Results
3.1 Cloning of pig LfR cDNA
Our bioinformatics approach was based on the sequences of previously identified LfRs from other species, and 2 short conserved regions were identified (Fig. 1). To obtain a cDNA fragment, PCR was performed using templates from pig duodenum and jejunum with combinations of 2 degenerate primers, leading to the cloning and sequencing of ~450 bp cDNA fragment. By Blast searching, a sequence showing 99% homology (accession no. AY609994) to the cloned pig ORF was identified in National Center for Biotechnology Information (NCBI) genome database (http://www.ncbi.nlm.nih.gov/genome/guide/pig/). Further analysis revealed the cDNA of the putative pig LfR was composed of 1215 bp. The ORF was 972 bp, with a predicted protein composed of 324 amino acid residues. Using a proteomic server SignalP V3.0 (http://www.cbs.dtu.dk/services/SignalP/), a signal sequence cleavage site has been predicted between amino acids 23 and 24.
3.2 Developmental expression of LfR mRNA
In duodenum, LfR mRNA expression significantly increased with age (P<0.05). When the piglets were 3 weeks old, mRNA was 3-fold higher than at 1 week of age, and by 6 months, LfR mRNA expression was 4.5-fold higher than the level at 1 week (Fig. 2). Conversely, in the jejunum LfR mRNA expression peaked at early infancy (1 week) and significantly decreased at 3 weeks and 6 months of age. We also noted that during early infancy (1 week) LfR mRNA in jejunum was significantly higher than in duodenum (5.05 ± 1.10 vs 1.00 ± 0.33; P<0.05); however, this difference was not significant at 3 weeks and 6 months (Fig. 2). β-actin mRNA levels did not change with age and served as an appropriate control gene (data not shown).
Figure 2.
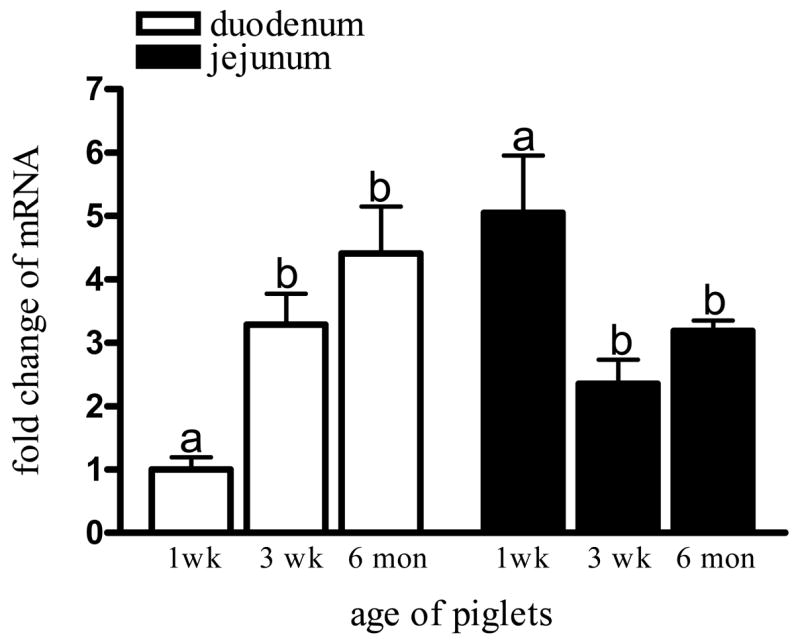
LfR mRNA expression in pig duodenum and jejunum. The extracted mRNA was analyzed by Q-PCR. PCR products were quantified for relative levels of mRNA using image analysis by comparing LfR with β-actin. At week 1, LfR expression was significantly lower in duodenum compared to jejunum (Student’s t-test, P<0.05); however, this difference disappeared with age. LfR expression significantly increased with age in duodenum and decreased in jejunum. Values are means ± SD, different letters indicate significant difference over time.
3.3 Development of LfR protein expression
We detected a major band of ~135 kD (Fig. 3; Lane 1) under non-reducing conditions and a band at ~35 kD under reducing conditions, suggesting that the native LfR exists as a four 35 kD subunit protein complex under non-reducing conditions (Fig 3: Lane 2). The specificity of human LfR anti-serum to the pig LfR was verified by Western blot of small intestine protein resolved by SDS-PAGE and incubated with excess peptide antigen. Under reducing conditions, we verified that pre-absorption with excess antigen blocked detection of pig LfR specific band corresponding to a protein at ~35 kD (Fig. 3; Lane 3). This anti-serum was therefore used for subsequent studies without antibody purification.
Figure 3.
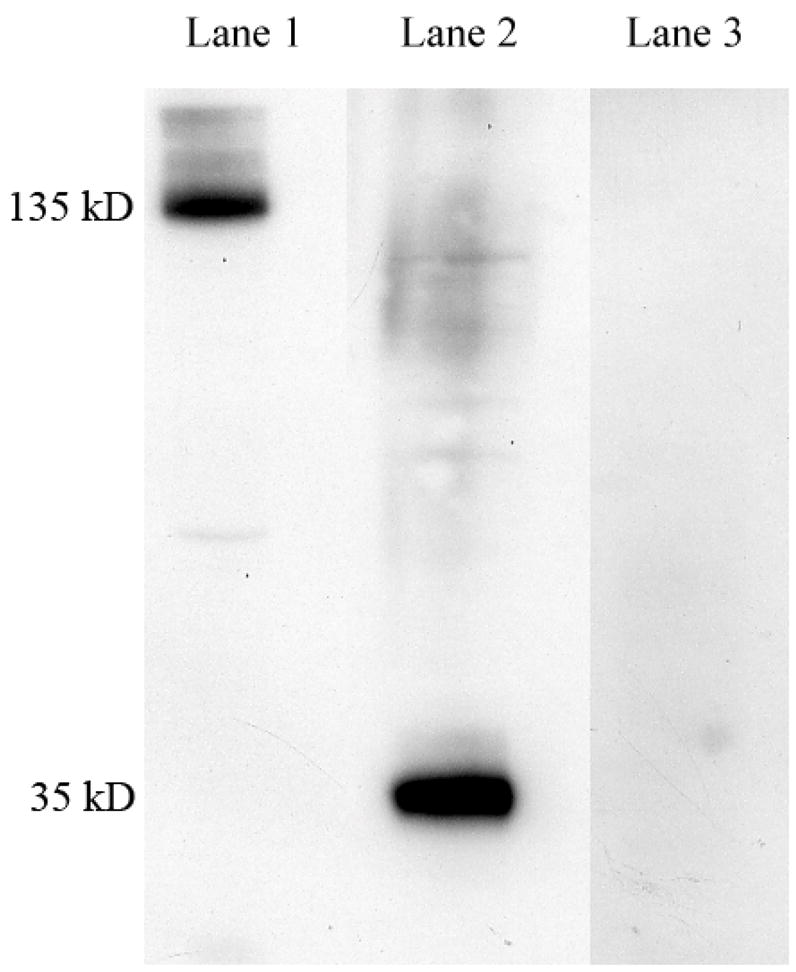
SDS-PAGE analysis of 3 weeks old pig duodenum and characterization of antibody specificity. Lane 1: protein (18 μg) resolved without beta-mercaptoethanol in the sample buffer; Lane 2: protein (18 μg) resolved with beta-mercaptoethanol in the sample buffer; Lane 3: protein (18 μg) resolved with beta-mercaptoethanol in the sample buffer, and anti-serum (1:1000) incubated with excess antigen (0.8 mg/ml peptide) for 2 h prior to blotting of nitrocellulose membranes with electrophoresed proteins.
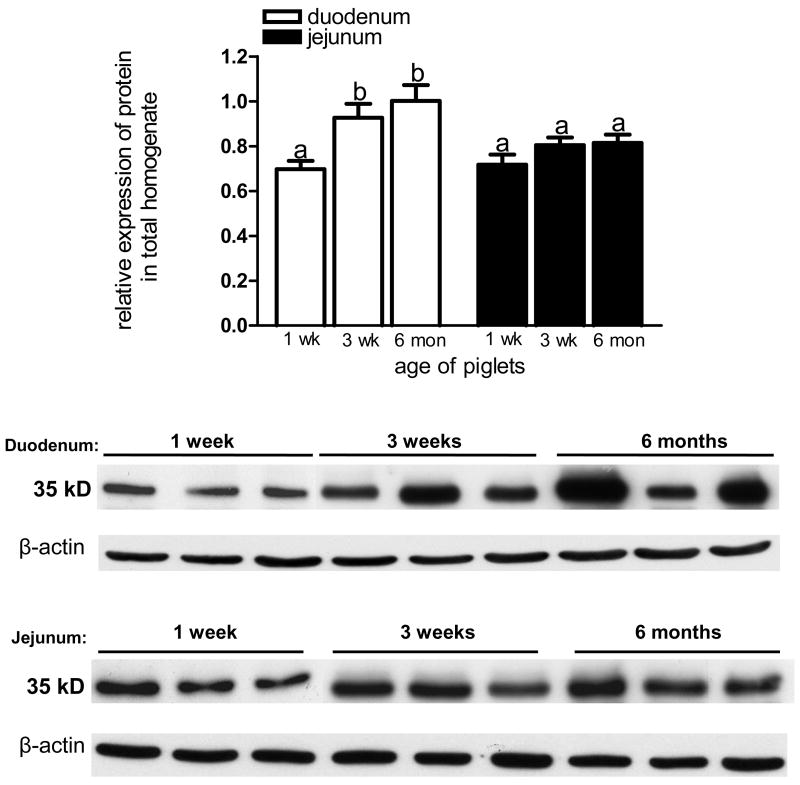
In total tissue homogenate, we observed a significant increase in LfR protein abundance in duodenum; LfR at 3 weeks and 6 months was significantly higher than at 1 week (Fig. 4). LfR expression in jejunum, however, remained unchanged throughout the 6 months postnatal period. Overall, the protein abundance in duodenum was higher when compared to jejunum at 3 weeks and 6 months, but these differences did not reach statistical significance (Fig. 4).
Figure 4.
LfR protein expression in total homogenates of pig duodenum and jejunum. Proteins (18 μg) were resolved by SDS-PAGE, transferred onto nitrocellulose membrane and immunoblotted with LfR anti-serum. LfR expression in duodenum increased after 1 week of age, but did not change in jejunum. Values are means ± SD, different letters indicate significant difference over time. All data were normalized to β-actin (n=3).
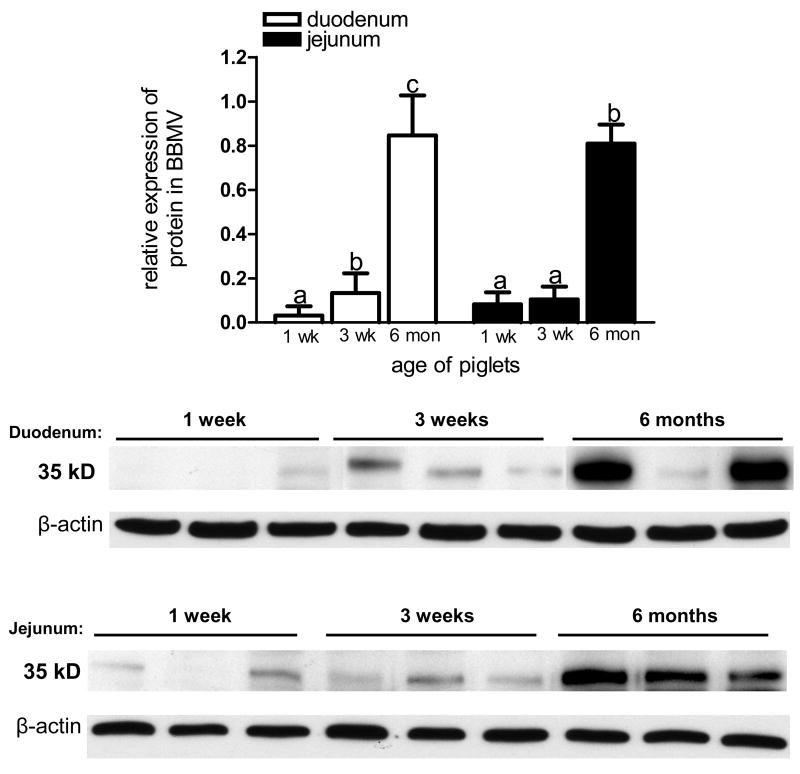
Our data showed a significant enhancement of LfR protein expression in isolated BBMV from duodenum and jejunum over the 6 months postnatal period (Fig 5). In the duodenum, LfR expression at 3 weeks and 6 months was significantly higher when compared to 1 week of age. Similarly, in BBMV from jejunum LfR expression was significantly increased at 6 months compared to 1 week and 3 weeks of age (Fig. 5). Our data also demonstrate that LfR was minimally expressed in BBMV from duodenum and significantly lower when compared to jejunum at 1 week of age (0.03 ± 0.05 vs 0.08 ± 0.03, respectively, P<0.05); however, LfR abundance was similar in duodenum and jejunum at 3 weeks and 6 months.
Figure 5.
LfR protein expression in BBMV from pig duodenum and jejunum. Proteins (10 μg) were resolved by SDS-PAGE, transferred onto nitrocellulose membrane and immunoblotted with LfR anti-serum. LfR expression in BBMV demonstrated an increase with age at both locations. Values are means ± SD, different letters indicate significant difference over time. All data were normalized to β-actin (n=3).
4. Discussion
Small intestinal LfRs have been suggested to serve as a specific receptor for Lf, an iron binding protein in milk, and mediate multiple functions of Lf. We used a pig model to study the expression and localization of LfR during development. Molecular cloning allowed the identification of a gene encoding the pig LfR. The resulting 1215 bp sequence shares 82% homology with the human LfR (GenBank accession no. NM_017625), and 79% homology with mouse LfR (GenBank accession no. NM_010584) sequences deposited in Genbank. The molecular mass calculated by direct translation of the cDNA was in agreement with the molecular mass (~35 kD) shown by Western blot. Under non-reducing conditions, SDS-PAGE revealed a single band of ~135 kD, suggesting that pig small intestine LfR exists as a 4-subunit protein complex. We have previously reported the presence of a similar tetramer of mouse small intestine LfR (Suzuki et al. 2004).
We found that LfR protein abundance increased significantly from 1 week to 3 weeks of age in the duodenum, whereas no change with age was observed for the jejunum. At 1 week of age, LfR mRNA expression was significantly higher than in duodenum; however, the protein abundance was similar at these two locations, which may be due to shorter half-life of mRNA in jejunum or a lag in protein synthesis in jejunum. More detailed studies are needed to investigate these possibilities. As can be noted, there was considerable variation among individual piglets in LfR expression, both for total homogenate and BBMVs. This may reflect different milk Lf concentrations or different iron status of the individual piglets. Especially, after they were weaned, they are confronting more exogenous pathogens, for which LfR could be a protective factor. The in-group variation may also be a result of different immune status of the individual piglets. In isolated BBMVs, LfR protein abundance increased with age in both duodenum and jejunum, reaching the highest values at 6 months of age. It therefore appears that a relatively higher proportion f LfR becomes localized to the apical membrane with increasing age. The newly synthesized LfR from the Paneth cell at the bottom of the crypt needs to migrate upwards along the villi in order to be present at the apical membrane to serve as a ready-to-be-used receptor for its ligand. The LfR that localizes within the enterocytes might be in a process of trafficking to the apical membrane, post-translational modification or recycling back from the apical membrane.
We have previously attempted to quantify LfRs in BBMVs isolated from piglet small intestine by using kinetic binding studies (Gislason et al. 1993), as no LfR antibody was available at that time. We found saturable and specific binding of pig Lf supporting the presence of a LfR, but found no difference in the number of binding sites or binding affinity between duodenum, jejunum and ileum (Gislason et al. 1993). In addition, we found no difference in the number of binding sites between the time points studied, i.e. at birth, 10 and 21 days postnatally (Gislason et al. 1993). The apparent discrepancies between these two studies could be explained by the different approaches chosen; in the binding study it is possible that Lf also bound to “pseudo-receptors” (other binding sites) as porcine Lf (GenBank accession no. ABJ98718) has a high pI of 8.73 (ScanSite PI/MW) and consequently is positively charged at the pH studied (similar to that of intestinal contents).
In neonatal piglets (1 week of age), we found relatively small amounts of LfR in the BBMV, but considerably more in the total homogenate, suggesting that the receptor is predominantly localized intracellularly. Although we do not yet know the physiological significance of the LfR, one proposed function of Lf is to provide iron to the newborn. This hypothesis is based on the fact that Lf is the major iron binding protein in human milk (Fransson et al. 1980; Fransson et al. 1983), can survive proteolytic degradation in the gastrointestinal tract and is found intact in the stool of breast-fed infants in significant quantities (Davidson et al. 1987). It was supported by the observation that transfected human intestinal carcinoma cells (Caco-2) over-expressing human LfR cDNA showed increased uptake of Lf and iron (Suzuki et al. 2001). In the mouse, we have shown that LfR is expressed soon after birth and is found at the enterocyte membrane, possibly facilitating uptake of iron via the mouse LfR (Lopez et al. 2006). This is also supported by our finding that DMT1 (divalent metal transporter-1) which is known to facilitate uptake of ferrous iron in the small intestine, is not properly located on the apical membrane in the neonatal period, but instead is found intracellularly in mice (Lopez et al. 2006), making it unlikely to be involved in iron absorption at this age. Studies in rat pups suggest that the increase in postnatal iron absorption results from multifactorial regulation and includes increased expression of DMT1 and changes in its localization within the enterocyte (Leong and Lönnerdal, 2003; Kelleher, 2006), also making DMT1 an unlikely pathway involved in iron absorption at young age. In the newborn piglet, non-specific macromolecular absorption is known to occur for some time after birth, possibly lessening the need for a membrane-exposed LfR during this time period. However, later during the nursing period, pinocytosis ceases and there would be a need for LfR on the apical membrane, which is what we found in this study. Thus, it is possible that LfR in piglet small intestine facilitates iron uptake from Lf. In a radioisotope study in piglets, using intrinsic labeling, we have shown high iron absorption from sow’s milk, suggesting that iron bound to pig Lf is highly bioavailable (Gislason et al. 1993). We have also shown in weanling mice that iron absorption from milk formula supplemented with iron in the form of Lf is higher than from formula with iron added as ferrous sulfate (Fransson et al. 1983), suggesting that LfR has functional significance for iron absorption in this species.
The weaning date of the pig varies in different studies. In the present study, piglets were weaned at 3 weeks, and the time points chosen, 1 week, 3 weeks, and 6 months, represent the actively nursing, weaning, and post-weaning stages of the piglet development. The high abundance of LfR in the small intestine at 6 months of age was intriguing as piglets normally are weaned at 3–4 weeks of age and therefore not exposed to milk Lf any longer. Although endocrine secretions, such as gastric fluid and pancreatic juice contain Lf, concentrations are low under normal circumstances and not likely to be of quantitative significance for iron absorption (Kruzel et al. 1998; Legrand et al. 2005). However, when we cloned the human LfR (Suzuki et al. 2001), we found that it was 100% homologous to a protein called intelectin, which was characterized as a lectin in mouse small intestine (Komiya et al. 1998). Intelectin plays a role in the defense against infection by recognizing galactofuranose in the carbohydrate chains of bacterial cell walls. In microarray analyses, intelectin was recently identified as the major antimicrobial gene differentially regulated post-infection in resistant BALB/c mice in response to parasite infection (Datta et al. 2005). Recently, intelectin was found in Paneth and goblet cells of the small intestine, and, in particular, on the brush border membrane of enterocytes (Wrackmeyer et al. 2006), which is similar to our observations for LfR in mouse intestine (Lopez et al. 2006). Wrackmeyer et al. suggested that intelectin serves as an organizer and stabilizer of the brush border membrane, preventing loss of digestive enzymes to the gut lumen and protecting the glycolipid domains from pathogens (Wrackmeyer et al. 2006). Thus, it is possible that LfR/intelectin is involved in the development of the gut local host-defense mechanism.
In conclusion, the cloning and the gene/protein expression analysis of pig LfR along the small intestinal axis over the 6-month postnatal period demonstrated the presence of a human SI-LfR homologue in pig, with mRNA and protein expression concomitantly regulated in the duodenum and inversely regulated in the jejunum. The data also strongly suggest that in the pig Lf can be transported through a LfR-mediated process. LfR may therefore be involved in iron absorption during early infancy and possibly acquires a unique function in host defense during later development.
Acknowledgments
This work was supported by NIH grant # HD-043240.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpers DH. Is glutamine a unique fuel for small intestinal cells? Curr Opin Gastroenterol. 2000;16:155. doi: 10.1097/00001574-200003000-00010. [DOI] [PubMed] [Google Scholar]
- Bertolo RF, Brunton JA, Pencharz PB, Ball RO. Arginine, ornithine, and proline interconversion is dependent on small intestinal metabolism in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E915–922. doi: 10.1152/ajpendo.00269.2002. [DOI] [PubMed] [Google Scholar]
- Chandler CJ, Harrison DA, Buffington CA, Santiago NA, Halsted CH. Functional specificity of jejunal brush-border pteroylpolyglutamate hydrolase in pig. Am J Physiol. 1991;260:G865–872. doi: 10.1152/ajpgi.1991.260.6.G865. [DOI] [PubMed] [Google Scholar]
- Datta R, deSchoolmeester ML, Hedeler C, Paton NW, Brass AM, Else KJ. Identification of novel genes in intestinal tissue that are regulated after infection with an intestinal nematode parasite. Infect Immun. 2005;73:4025–4033. doi: 10.1128/IAI.73.7.4025-4033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Lönnerdal B. Persistence of human milk proteins in the breast-fed infant. Acta Paediatr Scand. 1987;76:733–740. doi: 10.1111/j.1651-2227.1987.tb10557.x. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Lönnerdal B. Specific binding of lactoferrin to brush-border membrane: ontogeny and effect of glycan chain. 1988 Apr. 1988;254(4 Pt 1):G580–5. doi: 10.1152/ajpgi.1988.254.4.G580. [DOI] [PubMed] [Google Scholar]
- Domeneghini C, Di Giancamillo A, Arrighi S, Bosi G. Gut-trophic feed additives and their effects upon the gut structure and intestinal metabolism. State of the art in the pig, and perspectives towards humans. Histol Histopathol. 2006;21:273–283. doi: 10.14670/HH-21.273. [DOI] [PubMed] [Google Scholar]
- Elliot JI, Senft B, Erhardt G, Fraser D. Isolation of lactoferrin and its concentration in sows’ colostrum and milk during a 21-day lactation. J Anim Sci. 1984;59:1080–1084. doi: 10.2527/jas1984.5941080x. [DOI] [PubMed] [Google Scholar]
- Fransson GB, Keen CL, Lönnerdal B. Supplementation of milk with iron bound to lactoferrin using weanling mice: I. Effects on hematology and tissue iron. J Pediatr Gastroenterol Nutr. 1983;2:693–700. doi: 10.1097/00005176-198311000-00021. [DOI] [PubMed] [Google Scholar]
- Fransson GB, Lönnerdal B. Iron in human milk. J Pediatr. 1980;96:380–384. doi: 10.1016/s0022-3476(80)80676-7. [DOI] [PubMed] [Google Scholar]
- Gislason J, Douglas GC, Hutchens TW, Lönnerdal B. Receptor-mediated binding of milk lactoferrin to nursing piglet enterocytes: a model for studies on absorption of lactoferrin-bound iron. J Pediatr Gastroenterol Nutr. 1995;21:37–43. doi: 10.1097/00005176-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Gislason J, Iyer S, Hutchens TW, Lönnerdal B. Lactoferrin receptors in piglet small intestine: binding properties, ontogeny and regional distribution in the gastrointestinal tract. J Nutr Biochem. 1993;4:528–533. [Google Scholar]
- Gislason J, Jones B, Lönnerdal B, Hambraeus L. Iron absorption differs in piglets fed extrinsically and intrinsically 59Fe-labeled sow’s milk. J Nutr. 1992;122:1287–1292. doi: 10.1093/jn/122.6.1287. [DOI] [PubMed] [Google Scholar]
- Hutchens TW, Henry JF, Yip TT, Hachey DL, Schanler RJ, Motil KJ, Garza C. Origin of intact lactoferrin and its DNA-binding fragments found in the urine of human milk-fed preterm infants. Evaluation by stable isotopic enrichment. Pediatr Res. 1991;29:243–250. doi: 10.1203/00006450-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Kelleher SL. Effects of age and mineral intake on the regulation of iron absorption in infants. J Pediatr. 2006;149(5 Suppl):S69–73. [Google Scholar]
- Komiya T, Tanigawa Y, Hirohashi S. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochem Biophys Res Commun. 1998;251:759–762. doi: 10.1006/bbrc.1998.9513. [DOI] [PubMed] [Google Scholar]
- Kruzel ML, Harari Y, Chen CY, Castro GA. The gut. A key metabolic organ protected by lactoferrin during experimental systemic inflammation in mice. Adv Exp Med Biol. 1998;443:167–173. [PubMed] [Google Scholar]
- Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci. 2005;62:2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong WI, Bowlus CL, Tallkvist J, Lönnerdal B. Iron supplementation during infancy--effects on expression of iron transporters, iron absorption, and iron utilization in rat pups. Am J Clin Nutr. 2003 Dec;78(6):1203–1211. doi: 10.1093/ajcn/78.6.1203. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B. Lactoferrin binding to its intestinal receptor. Adv Exp Med Biol. 1991;310:145–150. doi: 10.1007/978-1-4615-3838-7_17. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- Lopez V, Suzuki YA, Lönnerdal B. Ontogenic changes in lactoferrin receptor and DMT1 in mouse small intestine: implications for iron absorption during early life. Biochem Cell Biol. 2006;84:337–344. doi: 10.1139/o06-059. [DOI] [PubMed] [Google Scholar]
- McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci U S A. 1998;95:4841–4846. doi: 10.1073/pnas.95.9.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ER, Ullrey DE. The pig as a model for human nutrition. Annu Rev Nutr. 1987;7:361–382. doi: 10.1146/annurev.nu.07.070187.002045. [DOI] [PubMed] [Google Scholar]
- Suzuki YA, Lönnerdal B. Baculovirus expression of mouse lactoferrin receptor and tissue distribution in the mouse. Biometals. 2004;17:301–309. doi: 10.1023/b:biom.0000027709.42733.e4. [DOI] [PubMed] [Google Scholar]
- Suzuki YA, Lopez V, Lönnerdal B. Mammalian lactoferrin receptors: structure and function. Cell Mol Life Sci. 2005;62:2560–2575. doi: 10.1007/s00018-005-5371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki YA, Shin K, Lönnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001;40:15771–15779. doi: 10.1021/bi0155899. [DOI] [PubMed] [Google Scholar]
- Ward PP, Mendoza-Meneses M, Cunningham GA, Conneely OM. Iron status in mice carrying a targeted disruption of lactoferrin. Mol Cell Biol. 2003;23:178–185. doi: 10.1128/MCB.23.1.178-185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrackmeyer U, Hansen GH, Seya T, Danielsen EM. Intelectin: a novel lipid raft-associated protein in the enterocyte brush border. Biochemistry. 2006;45:9188–9197. doi: 10.1021/bi060570x. [DOI] [PubMed] [Google Scholar]
- Wu G, Knabe DA, Kim SW. Arginine nutrition in neonatal pigs. J Nutr. 2004;134:2783S–2790S. doi: 10.1093/jn/134.10.2783S. [DOI] [PubMed] [Google Scholar]