Abstract
Long-term memories are influenced by the emotion experienced during learning as well as by the emotion experienced during memory retrieval. The present article reviews the literature addressing the effects of emotion on retrieval, focusing on the cognitive and neurological mechanisms that have been revealed. The reviewed research suggests that the amygdala, in combination with the hippocampus and prefrontal cortex, plays an important role in the retrieval of memories for emotional events. The neural regions necessary for online emotional processing also influence emotional memory retrieval, perhaps through the reexperience of emotion during the retrieval process.
Keywords: emotion, memory, retrieval, amygdala, prefrontal cortex
Retrieval of Emotional Memories
Memories of our experiences are likely characterized by representations in the form of neuronal activity. Activity among a network of neurons represents a code for the experience of, say, a birthday party. When this network is activated by some cue that triggers a reexperience of that event, we are said to have recollected the birthday party. Emotional events are often remembered with greater accuracy and vividness (though these two characteristics do not always go together) than events lacking an emotional component (Reisberg & Hertel, 2005). This enhanced memory for emotional events has been attributed to interactions between the amygdala and other neural areas such as the hippocampus and prefrontal cortex (PFC) (Cahill & McGaugh, 1996). The amygdala is active during emotional situations, and this activity influences the encoding and consolidation of the memory trace for the emotional event (see LaBar & Cabeza, 2006; Phelps, 2004, for a review). These effects on the “front end” of memory (attention, encoding, the early stages of consolidation) have been well documented, but the influence of emotion and amygdala activity on memory retrieval has been more difficult to demonstrate. This difficulty is partly due to the fact that, by definition, an emotional event exerts its influence during the initial experience of the event. Understanding the influence of emotion on retrieval mechanisms could have utility in treating disorders of emotion and memory such as depression and posttraumatic stress disorder (PTSD; Davis & Whalen, 2001; Elzinga & Bremner, 2002), in which emotion may influence all phases of memory. Given that it is at the retrieval stage that the pathological effects of negative emotion are most often observed (Elzinga & Bremner, 2002; Williams & Scott, 1988), perhaps this is also where treatment may work most effectively. Recent work on the neurobiology of retrieval, extinction learning, and potential treatments for psychopathology highlight the broad influence that emotion exerts on memory retrieval mechanisms. In this review, I examine this influence and discuss the applications of this work across many areas of psychology.
The focus here is on research findings from studies that address the relationship between emotion and memory retrieval, with the latter term defined broadly. These findings come from studies of cognitive psychology, animal learning, and cognitive neuroscience, and the goal is to integrate these findings into a coherent framework addressing the potential underlying commonalities that could inform basic scientists, as well as clinicians, on the associations between emotion and memory retrieval. The perspective taken at the outset is that there are many examples of the influence of emotion on memory retrieval across subdisciplines of psychology and that it may be possible to integrate these findings by seeking common ground in the behavioral and neural mechanisms that underlie this influence. I have chosen to review only a selection of influential work from the areas of cognitive psychology and animal learning, while comprehensively covering recent work in cognitive neuroscience that was explicitly designed to address the emotion and memory retrieval connection. Finally, I have chosen to organize the review by methodology, such that animal research is presented separately from human neuropsychology and functional imaging research. Given the differences in techniques and dependent measures across the various research methodologies, this organization was thought to be most straightforward.
Definitions
In reviewing the broad influence that emotion may have on memory retrieval, it is important to be clear about the definitions of emotion and the varieties of memory that may be influenced by emotion. Research on emotion and human cognition has often focused on mood–the background affective state of an individual, lasting on the order of minutes and often not having a specific known antecedent (Bower & Forgas, 2000). Studies examining mood and retrieval are discussed in the background section on cognitive psychology, as this work has been seminal in the theories describing affective influences on cognition (see Eich, Kihlstrom, Bower, Forgas, & Niedenthal, 2000). Emotions, by contrast, are short-lived cognitive and somatic reactions to specific environmental or cognitive antecedents (Scherer, 2000). The majority of the work reviewed concerns the retrieval of emotional information, especially of emotional autobiographical experiences and emotionally salient stimuli presented in a laboratory. Throughout this review, affect is used to describe both mood and emotion in a more general fashion, although the specific terms mood and emotion are used when each is appropriate.
Memory retrieval refers to “the access, selection, reactivation, or reconstruction of stored internal representations” (Dudai, 2002). This definition points to the processes that make up memory retrieval. In this review, I focus on those processes that lead up to successful retrieval (access and selection) and on successful retrieval itself. Event-related potential (ERP) and functional neuroimaging studies have demonstrated different patterns of neural activity associated with the processes of access and selection. Although studies of retrieval in neuropsychological patients are unable to differentiate these processes, we can nevertheless examine the phenomenological characteristics of memory retrieval in these patients to infer the roles of particular brain structures in retrieval processes. In the case of animal research addressing the confluence of affect and memory retrieval, most of the work has used conditioning paradigms. In these studies, the conditioned stimulus (CS) serves as a retrieval cue, perhaps leading to the reactivation of a memory trace, which is measured as the conditioned response (CR). These studies have provided a wealth of information, especially with regard to the neural basis of the interactions between affect and memory. Research on humans, by contrast, has focused primarily on the retrieval of episodic memories of emotional stimuli (from the laboratory) or of events (from one’s autobiography). Like the animal studies, these learning studies in humans rely on a retrieval cue that may or may not lead to the reactivation of a memory and are measured as a button press or verbal response. In this review, I include the results from animal conditioning paradigms as well as those from human studies on the retrieval of emotional episodic memories. As mentioned above, my goal is to examine the broader influence that affect has on retrieval, for example, the influence of a negative mood state on memory for an emotionally neutral word list, the retrieval of emotional episodic memories, or the extinction of a conditioned emotional response. Although the behavioral and neurobiological underpinnings of these examples are at least partially distinct, it is the common ground among them that is of interest here.
Background From Cognitive Psychology
Within the information-processing framework of cognitive psychology, information is first encoded and then consolidated and stored in long-term memory. Within this “modal model” of information processing, information that receives attention and elaboration is more likely to be subsequently available for retrieval (Brown & Craik, 2000). More important, memories are not believed to be stored in an all-or-none form, ala a storehouse model, but as a collection of attributes, which may include factors such as the time and place of the experience, the initial phoneme of a word, or the affective valence that a word carries (Underwood, 1969). Access to these attributes may determine whether a memory is retrieved, and research on phenomena such as tip-of-the-tongue has suggested that individual attributes may be sufficient to support successful memory retrieval (Koriat, Levy-Sadot, Edry, & de Marcas, 2003). Retrieval, then, is a reconstruction of a previous experience, and this reconstruction may be influenced by many variables present at the time of retrieval (Bartlett, 1932; Roediger & McDermott, 2000). Research on memory distortions has highlighted the malleable nature of memory and revealed various factors that contribute to these distortions, showing that many of them play a role during retrieval processing (Roediger & McDermott, 2000). For example, factors such as prior retrieval, cue manipulation, and imagination can impact or even change what is retrieved from memory.
Considerable work has addressed the variety of cues available at the time of memory testing and how these cues affect retrieval success. The principle of encoding specificity asserts that a cue to retrieval will be more effective if it recapitulates some aspect of the encoding of that memory (Tulving & Thomson, 1973). For example, if the word palm is learned in the context of a “palm tree,” the word hand will be less effective as a retrieval cue than a word that invokes the image of a tree. Just as contextual effects can influence encoding, they can also affect the performance of retrieval attempts. The concept of “transfer-appropriate processing” suggests that when an individual processes information in the same manner at encoding and retrieval, memory performance should be more successful (C. D. Morris, Bransford, & Franks, 1977). If word pairs are learned on the basis of semantic relationships, for example, then retrieval cues that stimulate thinking about word meaning will be more effective than retrieval cues that act at the level of word phonology. These task-specific effects at retrieval are referred to as retrieval mode or retrieval orientation (Rugg & Allan, 2000) and indicate that the cognitive processes undertaken during a retrieval attempt can influence the outcome of that attempt.
Affect could exert an influence over retrieval processes at the level of either the item (memory for an emotionally provocative stimulus) or the task (affective state that a participant happens to be in during retrieval attempt). If the encoding of an event is accompanied by an affective state that is (at least partly) recapitulated at the time of retrieval, then the affect may serve as a cue, leading to a greater likelihood of retrieval. Examples of this type of process are found in the phenomena of mood-dependent and mood-congruent memory (Bower, 1981; Bower & Forgas, 2000; Eich, 1995). Mood-dependent memory is the enhanced recollection of information previously encountered in a specific mood state when one reexperiences that mood state at retrieval. In mood-congruent memory, the mood state during learning is not a factor, but the match between the affective characteristics of the to-be-remembered stimuli and the mood state at retrieval is key. In a classic study on mood-dependent memory, participants learned a word list in either a happy or sad mood; they then learned a second word list in either the same or a different mood state (Bower, Monteiro, & Gilligan, 1978). Finally, recall memory for the initial word list was tested in either the same or a different mood state. In cases in which the moods during the encoding and retrieval sessions matched, memory performance was enhanced relative to that under conditions in which the moods between the two sessions were incongruent. The authors attributed this effect to a role of mood in disambiguating between words learned in one context over another. In other words, for the retrieval of a particular word list, the mood at the time of retrieval serves as a cue to distinguish what words were encountered in what list.
Mood-dependent memory has been difficult to demonstrate reliably1 (Bower & Mayer, 1989), but mood-congruent memory is a robust phenomenon that has been documented across many studies and in various populations (Blaney, 1986; Bower & Forgas, 2000; Lewis, Critchley, Smith, & Dolan, 2005; Weingartner, Miller, & Murphy, 1977). In one study, either negative or positive moods were induced prior to retrieval attempts for negative or positive real-life events (Teasdale & Fogarty, 1979). Negative events were retrieved more quickly when the participant was in a negative mood, and positive events were retrieved more quickly when the participant was in a positive mood. Similar results were found when participants studied positive, negative, and neutral words in a neutral mood state and were then tested for retrieval in either a positive or negative mood. Participants more accurately retrieved positive words when in a positive mood and negative words when in a negative mood, whereas their retrieval of neutral words was unaffected by mood manipulation (Teasdale & Russell, 1983).
Bower and Forgas have described mood-congruency effects, and more generally the influence of affect on cognition, in terms of the affect priming theory (Bower, 1981, 1987; Bower & Forgas, 2000). According to their model, affect—in the form of either a mood state or a more short-lived emotion—is stored in a network of associations. The activation of an affective state will lead to some level of activation among the other components of the network; memories associated with that affective state are more likely to be retrieved than other memories not associated with that state. Activation of, for example, the sad “node” by a sad mood during testing gives the retrieval of materials associated with sadness a greater likelihood of successful retrieval. A more recent version of the affect priming theory—the affect infusion model (AIM)—has been put forth by Forgas (1995) and further elaborated by Bower and Forgas (2000). AIM attempts to specify the conditions under which affect is prone to “infuse” into cognition. Mood-congruency effects arise most often in situations in which an individual must generate a response (such as free recall) than under more circumscribed experimental contingencies (such as recognition memory; see Eich, 1995). In addition to playing a role in memory, this infusion of affect may influence decision making, social processing, or other processes in which constructive cognitive function (or the active generation of new information) is necessary (Forgas, 1995). The influence of affect on cognition generally, and on retrieval specifically, is similar to the influence of other contextual cues. The more ambiguous the situation (i.e., the less the cueing environment captures the original to-be-remembered item), the more affect may be expected to influence cognitive function. This model integrates contextual information from both the individual and the situation to better explain the influence affect has on cognition.
Affect could potentially exert item-specific effects on two forms of memory retrieval: familiarity and recollection. Moreover, it could be that emotional items are better remembered due to their influence on one or both of these forms of retrieval. In familiarity, an individual “knows” that an item has previously been encountered but cannot recall the context in which it was encountered. In recollection, the participant recalls the item in conjunction with contextual details of the original experience, and with this comes a sense of remembering, termed autonoetic consciousness (Tulving, 1985; M. A. Wheeler, Stuss, & Tulving, 1997). Familiarity and recollection may be distinguished through use of the remember/know procedure during recognition memory testing (Rajaram, 1993). This involves asking the participant to respond to items in terms of whether they have a specific recollection of the encoding event (“remember”) or whether the item is merely familiar (“know”). To assess the influence of emotion on these two aspects of memory, Ochsner (2000) measured remember/know responses during a recognition memory test for emotional and neutral scenes. He found that arousing emotional scenes (especially negative scenes, but to some extent positive scenes as well) led to greater recollection ratings than did the presentation of neutral stimuli, which were more likely to be rated as familiar. This effect was independent of the participants’ attention to affective characteristics of the stimuli at the time of encoding. Similar findings have been reported for the presentation of emotional words as opposed to scenes (Kensinger & Corkin, 2003). It is unclear, though, whether the retrieval boost provided by emotionally arousing stimuli is specific to enhanced recollective processes or is due to a combination of enhanced recollective experience and enhanced familiarity. The constructs of recollection and familiarity are thought to be orthogonal to one another, whereas in the remember/know testing technique, participants must choose one or the other (Yonelinas, Kroll, Dobbins, Lazzara, & Knight, 1998). This discrepancy between the underlying constructs and the methods of study suggests that the results must be interpreted with caution, leaving open the possibility that emotional arousal could influence both familiarity and recollection. Nevertheless, these studies allow researchers to conclude that the subjective experience of retrieving emotionally arousing memories is different from that of retrieving memories lacking in emotional arousal; the specific characteristics of this experience are the subject of great research interest and is revisited in the Neuroimaging Studies of Affect and Retrieval section.
Background From Animal Learning Studies
Studies of animal learning and memory almost always involve affect. Laboratory animals require incentive motivation, such as appetitive food or aversive shock, to demonstrate learning and memory capabilities. Additionally, these studies provide a basis from which to examine the interactions between affect and memory retrieval because memory can only be assessed through retrieval testing. A model system for examining the types of associations animals can store and the contextual cues that affect memory retrieval is classical conditioning (Bouton & Moody, 2004). When a CS no longer signals the appearance of an unconditioned stimulus (US), conditioned responding (CR) eventually stops, resulting in extinction. Although the performance of the CR is no longer detectable, the CS–US association is not erased, but merely changed—an example of latent learning wherein a learned response is expressed only under certain experimental contingencies. Thus, extinction is not unlearning, but rather the learning of new contextual contingencies. Many studies have documented that extinction may be reversed (that is, the CR is reinstated) through various contextual manipulations (Bouton, 1993). This contextual reinstatement of a learned response is a good way to assess memory retrieval: Changing the contextual cues makes it possible to reactivate a memory trace. Reinstatement may occur in response to changing the location from that in which extinction originally took place (renewal), or simply because of the passage of time (spontaneous recovery). Thus, contextual cues enable the animal to retrieve the memory trace of the CS–US association. This malleability serves as a flexible updating mechanism allowing an organism to keep track of the context in which a specific response is appropriate (Bouton & Moody, 2004).
Extinction is an example of new learning (Bouton, 1993) and depends on the processes involved in memory retrieval (Ouyang & Thomas, 2005). A great deal of research on the reinstatement of CR by contextual cues has shown that the hippocampus is necessary for both the acquisition and retrieval of contextual conditioning, including the conditioning to spatial context (Corcoran & Maren, 2001; Maren, Anagnostaras, & Fanselow, 1998), temporal context (Bouton, Westbrook, Corcoran, & Maren, 2006), and internal context (e.g., hunger or thirst; Kennedy & Shapiro, 2004). In one study, the hippocampus was pharmacologically inactivated after fear conditioning had been extinguished but before reexposure to the extinction context. Animals with inactivated hippocampi did not show the reduced freezing response indicative of extinction, whereas control animals (with no brain lesion) represented with the extinction context did show extinction (Corcoran & Maren, 2001). It is important to note that hippocampal inactivation did not alter unconditioned freezing behavior. This result, then, demonstrates that the hippocampus is necessary for the retrieval of fear memory context. Researchers have suggested that the role of the hippocampus in both the acquisition and retrieval of contextual information is to index and update the contingencies of environmental cues. When any of these cues changes, a new context is created. The reinstatement of context, either from the time of acquisition or from the time of extinction, is detected by the hippocampus, which is then able to retrieve the memory for the meaning of a stimulus in that context (Corcoran & Maren, 2001; Maren et al., 1998).
The neurobiology of extinction learning also relies on interactions between the medial PFC and the amygdala (Myers & Davis, 2002). Lesion and electrophysiological studies have shown that the medial PFC plays an integral role in the inhibition of CR observed in extinction (Milad & Quirk, 2002; Morgan & LeDoux, 1995). Pharmacological studies have demonstrated that the amygdala is also involved; the blockade of glutamate (the primary excitatory neurotransmitter) in this brain region impairs both the acquisition and extinction phases of conditioning (Falls, Miserendino, & Davis, 1992; Miserendino, Sananes, Melia, & Davis, 1990). The requirement for connections between the medial PFC and amygdala in extinction learning has been established through studies showing that stimulating medial PFC neurons reduces the firing of amygdala neurons (Quirk, Likhtik, Pelletier, & Pare, 2003; Rosenkranz, Moore, & Grace, 2003). It is interesting that this inhibition of amygdala activity is one mechanism whereby the medial PFC could affect extinction learning, as neurons in the amygdala project to brainstem regions controlling the behavioral and autonomic manifestations of conditioning. Specifically, reduced activity in the output neurons of the amygdala could alter affective learning and memory (Quirk et al., 2003). As PFC-mediated inhibition of the amygdala is reduced, a parallel reduction in extinction occurs.
Interactions between the amygdala and hippocampus are also necessary for the retrieval of emotional memories. The hippocampus and amygdala are heavily interconnected (Pitkänen, 2000), and their interaction in the encoding and consolidation of emotional memories has been well established (Packard & Cahill, 2001; Phelps, 2004). Seidenbecher, Laxmi, Stork, and Pape (2003) examined their functional connectivity in mice that had been fear conditioned by recording neuronal activity from the amygdala and the hippocampus during the retrieval of fear memory. They found significant synchronization of activity between the two structures during the initial presentation of the fear-inducing cue (CS+ originally paired with shock) and during reexposure to the training context. The amygdala and hippocampus also showed pronounced synchronization during the retrieval of both conditioned fear (through exposure to the original cue) and contextual fear (through exposure to the original testing environment), suggesting that these structures cooperate in the retrieval of fear memories (Seidenbecher et al., 2003). Paradigms such as extinction learning allow for cross-species comparison of behavior and neural activity. Similar classical conditioning paradigms have been conducted in humans, with measures of central and peripheral physiology as indices of conditioning (see the fMRI Studies section). The contextual cues used to reinstate previously extinguished CRs in conditioning paradigms may be compared with word cues that serve as reminders in verbal learning paradigms. It is possible, then, to compare factors, such as the affective value of retrieval cues across species, to better understand the cognitive and neural mechanisms underlying the retrieval of emotional information.
Neuropsychological Studies
The methods used in cognitive neuroscience (as in any discipline) often determine the inferences that can be drawn from the results. Thus, although neuropsychological studies of patients with brain damage have provided a great deal of information regarding brain–behavior relationships, there are caveats to inferring such relationships from brain lesion patients. For example, the potential for brain reorganization after damage and the fact that potential preexisting conditions could bias cognitive performance must be taken into account. Nevertheless, this work allows for claims of necessity: Brain damage that impairs the performance of a particular task demonstrates the necessity of that region in the performance of the task (Fellows et al., 2005; Rorden & Karnath, 2004). Such claims cannot be made for techniques such as functional neuroimaging, based on which no more than correlational inferences can be drawn to determine the connections between brain activity and cognitive function. Thus, the consideration of results from across methodologies allows for a more complete description of a phenomenon, especially when evidence from across these techniques converges upon a common conclusion.
Effects of Emotion on Encoding and Consolidation
The effects of brain damage on the encoding and consolidation of emotional material has been well documented (reviewed in Buchanan & Adolphs, 2004; Phelps, 2004), and it is well established that the amygdala is necessary for the enhancement of memory by emotion. What remains unclear from this work is what phases of memory require the amygdala. This is due, in part, to the fact that all of the lesion studies addressing the role of the amygdala in emotional memory have been conducted in patients with amygdala damage during the learning, consolidation, and retrieval phases of memory, making it impossible to determine the role of this structure specifically in memory retrieval.
Effects of Emotion on Autobiographical Retrieval
Our autobiographies often consist of very emotional experiences, which are remembered more vividly and forgotten more slowly than neutral events (Berntsen & Rubin, 2002). The study of the retrieval of autobiographical memory has a long history, dating back at least as far as Galton (1879). Galton’s techniques were revived by Crovitz and Schiffman (1974) in the form of a word-cued memory paradigm, in which individuals are presented with cue words, one at a time, and asked to report one memory in response to each word. People often recollect emotional events in a regular manner. For example, when asked to report and date their “most important memories,” people tend to report more events from between the ages of 10 and 30 (Rubin & Schulkind, 1997). Assessing the recollection of autobiographical memories is one method with which to examine the retrieval of emotional events in brain lesion participants.
Patients with medial temporal lobe (MTL) amnesia provide a unique opportunity to assess the role of emotion in memory retrieval. These individuals show impaired new learning and often a temporally limited retrograde amnesia but unimpaired memory for remote events (Bayley, Hopkins, & Squire, 2003; Reed & Squire, 1998), although this pattern has not been observed by all investigators (Moscovitch, Nadel, Winocur, Gilboa, & Rosenbaum, 2006; Nadel & Moscovitch, 1997).2 This pattern of deficits allows for the assessment of memory retrieval from the remote period before brain damage, when encoding and consolidation were presumably unaffected. This strategy was used to assess memory for emotional autobiographical events in MTL amnesics with and without damage to the amygdala (Buchanan, Tranel, & Adolphs, 2005). The layout of this study is presented in Figure 1. In the remote phase during which the event took place, the hippocampus and amygdala were intact, and thus participants presumably encoded and consolidated memories for their autobiographical experiences in a normal manner (all of the patients had damage due to anoxia or encephalitis as adults, thus abnormal brain processes such as seizure activity had no influence during this period). Damage to the MTL occurred before testing, allowing the assessment of memories for emotional events from the remote memory period.3 For the testing, patients were asked to describe the five most emotional events from their lives (although the time frame from which the memories were to come was not restricted by the study, nearly all of the memories reported by these patients came from the remote period, many years before brain damage). Additionally, they were given a word-cued memory task in which 30 words were presented, and each participant was asked to produce one memory in response (cf. Crovitz & Schiffman, 1974). For each phase of memory testing, participants were asked to rate their memories on pleasantness, intensity, significance, novelty, vividness, and frequency of rehearsal.
Figure 1.
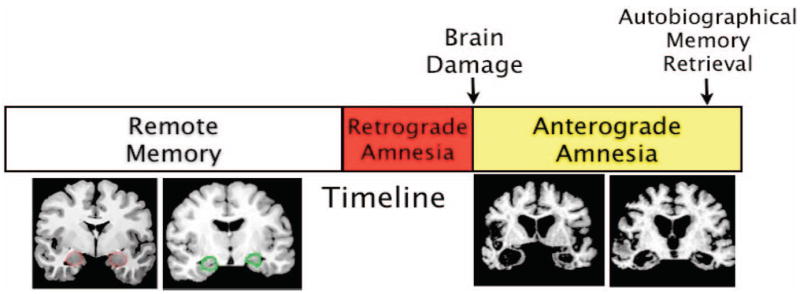
Schematic timetable of testing conducted in Buchanan et al. (2005). Autobiographical memory retrieval for events primarily from the remote memory epoch was tested in amnesic patients with damage to both the hippocampus and amygdala during the anterograde epoch. Intact hippocampal and amygdala during remote epoch allowed for assesment of the role of these structures in autobiographical retrieval.
Results from the study corroborate previous work demonstrating that people report more emotional than neutral experiences from their past. In addition, two important new findings emerged from the study. First, patients with MTL damage limited to the hippocampus retrieved autobiographical memories of emotional events that were strikingly similar to those from comparison participants, in terms of both the number of reported memories and the affective quality of those memories. These results indicate that the recollection of emotional autobiographical memories from the remote past is not dependent on the hippocampus proper. Second, and more interesting for the purposes of the present review, patients with damage to both the amygdala and the hippocampus retrieved fewer unpleasant autobiographical memories. Compared with the other groups, these patients also rated their unpleasant memories as less intense, significant, novel, and vivid compared with the other participants. Specifically, these amnesics’ ratings of unpleasant emotions were greater than 2 standard deviations below the mean of the ratings of the normal comparison group (see Figure 2). Also, these patients used fewer emotionally negative words in describing their memories than did the other participants (> 1.5 standard deviations below the mean of the other groups’ performance). This reduced use of negative emotional words was found in both the top five memories and in the word-cued memories and was specific to their recollections; they used the same number of negative emotional words as comparison participants when describing the contents of a visual scene in a control task. Results from these patients suggest that amygdala damage results in impoverished retrieval of unpleasant events in terms of both quantity and quality. More important, these effects are separate from the processes of encoding and consolidation, as the recounted events had occurred long before brain damage.
Figure 2.
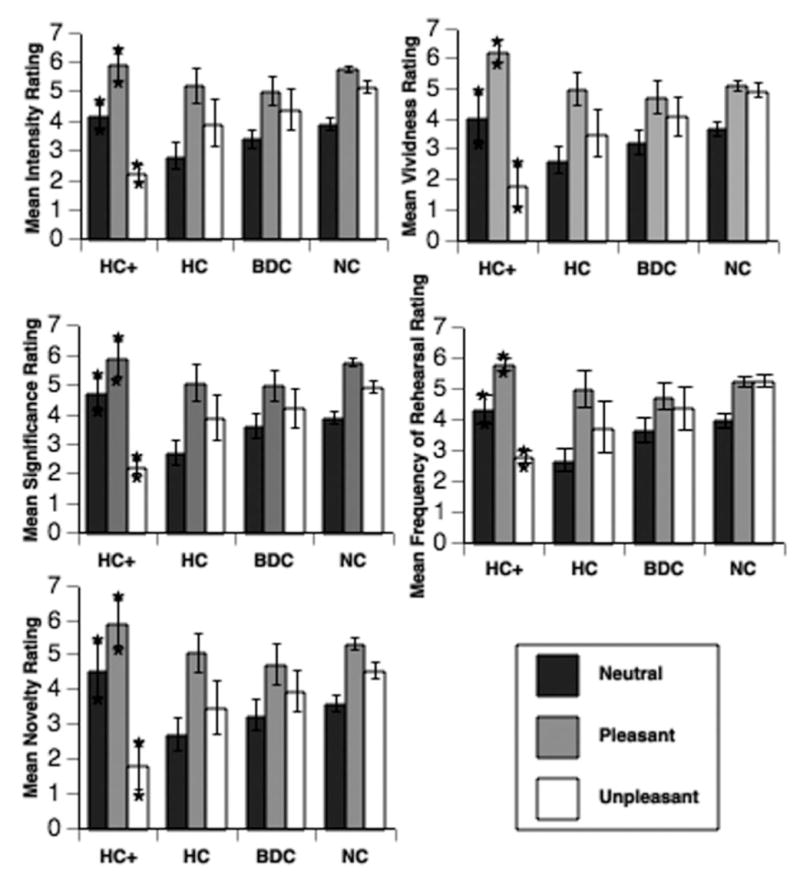
Ratings of emotional autobiographical memories from patients with damage to the hippocampus + amygdala (HC+), hippocampus only (HC), brain-damaged comparison (BDC), and normal comparison (NC) participants. HC+ participants rated their unpleasant memories as lower on intensity, vividness, significance, and novelty compared with the other participant groups. From “Emotional Autobiographical Memories in Amnesic Patients With Medial Temporal Lobe Damage,” by T. W. Buchanan, D. Tranel, and R. Adolphs, 2005, Journal of Neuroscience, 25, p. 3156. Copyright 2005 by the Society for Neuroscience. Reprinted with permission.
A question that remained from this work is whether these effects are specific to the right or left hemisphere. This is of interest because considerable research on the neuropsychology of emotion has suggested that the left and right cerebral hemispheres have different roles in the processing of emotional experience (Borod, 1993). These laterality effects have been described in terms of perception, recognition, experience, and recollective processes (Borod et al., 1998). It is unknown, however, what role the right versus left amygdala would play in the retrieval of emotional autobiographical experiences. This question was examined using the same experimental paradigm as previously described, this time in patients who had undergone temporal lobe resection for the treatment of epilepsy (Buchanan, Tranel, & Adolphs, 2006). These patients included 11 with right-sided damage and 12 with left-sided damage; in all cases, excisions included portions of the amygdala, anterior hippocampus, and surrounding cortex. Results showed a striking positivity effect in the patients with right-sided damage—they retrieved very few unpleasant memories when asked to report their top five emotional memories or word-cued memories. Additionally, these patients rated their unpleasant memories as less intense than those with left-sided damage and participants from the comparison group. These results are in line with those from previous research in right temporal lobectomy patients, which showed reduced recognition of negative emotions (Adolphs, Tranel, & Damasio, 2001; Anderson, Spencer, Fulbright, & Phelps, 2000). A possible explanation for this is that these patients are unable to recognize their own negative emotional recollections as being unpleasant. One line of research on the neural mechanisms of emotion emphasizes that an understanding of others’ emotional states depends on the ability to simulate their feeling state (Goldman, 1992; Iacoboni et al., 1999), and research on the neural mechanisms of emotion suggests that a network of structures in the right hemisphere may be necessary for this process (Adolphs, Damasio, Tranel, Cooper, & Damasio, 2000; Winston, O’Doherty, & Dolan, 2003). Perhaps damage to this right-hemisphere network in the right temporal lobectomy patients affected their recognition of negative emotional events from their own memories.
The primary commonality between the two studies described above is that patients who produced a positive bias in autobiographical memory have damage to the right anteromedial temporal lobe, including the amygdala. Together, results from these studies suggest that the right amygdala—regardless of the integrity of the left amygdala—may be a necessary component of the neural circuitry required for the retrieval of unpleasant, highly intense autobiographical experiences. These results are consistent with a model proposed by Markowitsch and colleagues (Markowitsch, 1998; Markowitsch et al., 2000) that describes a right-hemisphere network of regions, including the amygdala and its connection to PFC as necessary for the retrieval of emotional autobiographical memories. It remains unclear from these data what processes the amygdala may influence and why the deficits observed were specific to unpleasant memories. Research incorporating measures of the cognitive processes of memory retrieval along with measures of brain function may be able to address these issues.
Neuroimaging Studies of Affect and Retrieval
Research examining the neural correlates of memory retrieval has associated specific patterns of neural activity with the recognition of previously presented items. Several recent studies have addressed the retrieval of emotional memories that were encoded in laboratory studies, using either event-related brain potentials (ERPs) or functional magnetic resonance imaging (fMRI). These studies are summarized in Tables 1, 2, and 3.
Table 1.
Neuroimaging Studies of Human Extinction Learning
| Study | Description | Key finding |
|---|---|---|
| LaBar et al., 1998 | Acquisition and extinction of conditioning to shock US. | Amygdala activity during both phases of conditioning, habituated during extinction; associated with SCR measure of conditioning. |
| Phelps et al., 2004 | Acquisition and extinction (1-day later) of conditioning to shock US. | Amygdala activity during both phases of conditioning, habituated during extinction; associated with SCR measure of conditioning; medial PFC activity correlated with extinction success on Day 2. |
| Knight et al., 2004 | Between-subjects study of acquisition and extinction of conditioning to shock US. | Amygdala and hippocampus activity during acquisition; amygdala activity during initial extinction (changes in experimental contingencies). |
| Gottfried & Dolan, 2004 | Acquisition and extinction of conditioning to aversive odor US. | Amygdala and medial PFC activity during both phases of conditioning; activity in both regions habituated throughout both phases of conditioning. |
Note. US = unconditioned stimulus; SCR = skin conductance response; PFC = prefrontal cortex.
Table 2.
Neuroimaging Studies of Affect and Explicit Retrieval
| Study | Description | Key finding |
|---|---|---|
| Dolan, Lane, Chua, & Fletcher, 2000 | Recognition of previously presented IAPS pictures during PET scanning. | Left-amygdala activity in comparison of emotional versus neutral memory retrieval. |
| Taylor et al., 1998 | Encoding and recognition of IAPS pictures during PET scanning. | Left-amygdala activity during encoding but not retrieval of emotional stimuli. |
| Smith, Dolan, & Rugg, 2004 | ERP study of recognition of emotional context of pictures. | Increased magnitude old/new effects for pictures from an emotional compared with a neutral context. |
| Smith, Henson et al., 2004 | fMRI study of recognition of emotional context of pictures. | Left-amygdala and medial PFC activity during recognition of negative emotional context pictures. |
| Smith, Henson, Rugg, & Dolan, 2005 | fMRI study of source recognition of emotional context of pictures. | Left-amygdala activity during item recognition of negative emotional context pictures; right-amygdala activity during emotional source recognition. |
| Smith et al., 2006 | fMRI study with dynamic causal modeling of interactions during source recognition of emotion. | Increased connection strength between left amygdala and hippocampus during recognition of emotional context. |
| Maratos, Allan, & Rugg, 2000 | ERP study of recognition of emotional words. | Old/new effects were not different for negative and neutral words. |
| Maratos & Rugg, 2001 | ERP study of recognition of emotional context of words. | Increased magnitude old/new effects for words from an emotional compared with a neutral context. |
| Maratos et al., 2001 | fMRI study of recognition of emotional context of words. | Left-amygdala activity during recognition of negative emotional context words; bilateral medial PFC activity during recognition of positive emotional context words. |
| Dolcos et al., 2005 | fMRI study of recognition of emotional pictures conducted 1 year after encoding. | Right-amygdala and other medial temporal areas were more active during recognition success of emotional pictures. |
| Fenker et al., 2005 | fMRI study of recognition of neutral words paired with emotional facial expressions using R/K procedure. | Greater right-amygdala response to “know” responses for words paired with fearful expressions. |
| Sharot, Delgado, & Phelps, 2004 | fMRI study of recognition of emotional and neutral pictures using R/K procedure. | Greater right-amygdala response to “remember” responses for negative pictures. |
| Phelps et al., 2001 | fMRI study of instructed fear conditioning paradigm. | Left-amygdala activity during presentation of stimulus verbally linked to threat of shock. |
| Lewis et al., 2005 | fMRI study of mood-congruent memory. | Orbitofrontal cortex regions differentially index positive versus negative mood effects on memory. |
| Sterpenich et al., 2006 | fMRI study of recognition of neutral faces encoded in emotional context. | Right-amygdala and locus ceruleus index correct recognition of faces encoded in emotional context. |
| Sergerie, Lepage, & Armony, 2006 | fMRI study of encoding and recognition of fearful, happy, and neutral faces. | Right-amygdala activity at encoding was associated with remembered fearful faces; left-amygdala activity at retrieval was associated with remembered fearful faces. |
| Kensinger & Schacter, 2005 | fMRI study of accurate versus distorted memory for emotional objects. | Right-amygdala and left medial PFC activity during correct attributions of negative objects. |
Note. IAPS = International Affective Picture System; PET = position emission tomography; ERP = event-related potential; fMRI = functional magnetic resonance imaging; PFC = prefrontal cortex; R/K = Remember/Know.
Table 3.
Neuroimaging Studies of Emotional Autobiographical Memory
| Study | Description | Key finding |
|---|---|---|
| (Fink et al., 1996) | PET study of listening to affect-laden autobiographical memories. | Right mesial temporal activity, including the amygdala. |
| (Piefke et al., 2003) | fMRI study of reading emotional autobiographical memories. | Medial PFC activity during presentation of positive versus negative memories; right temporal activity during presentation of negative memories. |
| (Piefke, Weiss, Markowitsch, & Fink, 2005) | Same as above. | Greater right-insula activity in women during presentation of negative memories. |
| (Markowitsch et al., 2000) | PET study of processing real versus fictitious autobiographical memories. | Right-amygdala activity during real but not fictitious memories. |
| (Markowitsch, Vandekerckhove, Lanfermann, & Russ, 2003) | fMRI study of retrieval of strongly positive or strongly negative autobiographical events. | Bilateral medial PFC activity during retrieval of sad versus happy memories. |
| (Greenberg et al., 2005) | Event-related fMRI study of autobiographical memory retrieval. | Left amygdala and hippocampal; right-PFC activity during autobiographical retrieval compared with semantic retrieval. |
| (Addis, Moscovitch, Crawley, & McAndrews, 2004) | Event-related fMRI study of cue-induced retrieval of autobiographical events. | Emotion rating of memory modulated hippocampal activity during retrieval. |
Note. PET = positron emission tomography; fMRI = functional magnetic resonance imaging; PFC = prefrontal cortex.
ERP Studies
The so-called old/new effects measured from ERPs (a greater response to previously presented stimuli compared with that provoked by new items) serve as a reliable index of recognition memory (Rugg & Allan, 2000). Specifically, familiarity of stimulus items appears to be reflected by a bilateral potential that occurs over the frontal region at 300 ms after stimulus onset. Another “old/new” effect is often found in the left parietal region about 400 ms poststimulus onset, and this effect indexes the recollection of information, linking contextual information about when an event was encountered to the retrieval of the item itself (Rugg & Allan, 2000). A late-onset effect (around 500 ms–600 ms poststimulus) occurs in the right prefrontal region, and it indexes whether a retrieval attempt was successful (Rugg, Fletcher, Frith, Frackowiak, & Dolan, 1996). These regular patterns demonstrate the dissociable components of retrieval: detecting familiarity, recollecting the experience, and monitoring the success of the retrieval attempt. The millisecond time resolution of ERPs makes this methodology ideally suited to address the timing of emotional memory retrieval, and the neural signatures evoked by the retrieval of emotional versus neutral material makes it possible to assess the effects that emotion may have on retrieval processes.
In examining neural activity during the retrieval of emotional material, it is unclear whether observed differences reflect processes that occur specifically in the service of retrieval or more generally in the processing of emotional material. Recent studies have addressed this issue through the use of experimental paradigms involving an “emotional context.” In these paradigms, both neutral and emotionally provocative stimuli are presented simultaneously. Memory performance for the neutral stimuli is then tested outside of the emotional context. This setup allows for the manipulation of emotion at encoding while leaving the retrieval setting neutral. In one such study, target words were presented in sentences with either a negative or neutral meaning (Maratos & Rugg, 2001). For the target word gas, the neutral sentence was “She put the pan on the stove and turned on the gas” and the negative sentence was “She put her head in the oven and turned on the gas.” At test, participants were presented with the target word and asked to judge whether the word had appeared in a neutral or negative context (see Maratos & Rugg, 2001, Experiment 2). Emotional context was found not to influence the traditional old/new effects recorded over the frontal or parietal regions, indicating that the recognition of neutral and emotional material relies on the same neural processes. However, a qualitatively different effect of emotional source retrieval was found with regard to the late-positive potential that occurs at around 700 ms poststimulus, after the old/new left-parietal potential has occurred. This late-positive potential is similar to the response elicited by viewing emotional pictures (Schupp et al., 2000) and may reflect the affective evaluation of stimuli, whether they be from the external environment or the results of retrieval mechanisms.
Similar results were obtained with an emotional picture source retrieval task (Smith, Dolan, & Rugg, 2004). In this task, line drawings of neutral objects were presented along with emotional pictures. As in the previous study, the traditional old/new effects over the frontal and parietal cortices were the same for items from both emotional and neutral contexts. However, this study also showed that correct source retrieval of emotional stimuli elicited two separate effects that were not observed when neutral stimuli were retrieved: an early positivity (prior to the old/new left parietal potential) and (the previously reported) postretrieval, late-positive potential. This finding demonstrates that the retrieval of emotion influences neural activity via weak preretrieval potentiation and a more pronounced modulation of postretrieval processing. These studies show that the retrieval of emotional materials enlists the same neurophysiological signature responses as the retrieval of neutral stimuli but that emotion influences neural activity both before and after the traditional left-parietal recollection potential. These findings are consistent with the idea (discussed later in the Summary and Integration section) that neural mechanisms may act to recapitulate an emotional state early in the retrieval process and then produce a response similar to that elicited by the perception of an emotional stimulus upon successful recollection. Results from ERP studies give a good estimate of the time course of the effects of emotion on retrieval processing, but they cannot identify the specific neural regions involved in this processing. Studies addressing the anatomical specificity of these effects are discussed in the following sections.
fMRI Studies
Like ERP studies, research using fMRI has described neural processes that are reliably associated with memory retrieval (Buckner & Wheeler, 2001; Wagner, Shannon, Kahn, & Buckner, 2005). Unlike ERP research, fMRI techniques have the spatial resolution to better determine the neural regions producing these effects, albeit at a lower temporal resolution.4
Extinction learning in humans
The most direct way to address the common ground between the retrieval of emotional memories in nonhuman animals and humans is to use the same task. Extinction learning offers just this possibility because it is emotionally arousing, may readily be conducted in an fMRI environment, and allows for an objective measurement of conditioning from behavioral and/or autonomic responses. In these studies (reviewed in Table 1), CSs are either paired with shock (CS+) or not paired with shock (CS−). Measures of peripheral physiology, such as skin conductance, serve as the CR. Upon successful conditioning, the CS+ is presented without the US. Measures of neural activity during acquisition and extinction are collected to determine the neural signatures of these phases of emotional learning and retrieval. The results from neuroimaging studies assessing human extinction learning have been consistent with those obtained in nonhuman animal research: Acquisition is accompanied by an increase in the amygdala’s response to the CS+, and extinction is accompanied by a decrease in this response (LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; Phelps, Delgado, Nearing, & LeDoux, 2004). In these studies, amygdala activity was associated with the skin conductance response to the CS+ during both acquisition and extinction. However, the response of the medial PFC5 was associated with reduced CR during extinction, suggesting that the medial PFC inhibits amygdala activity during the extinction process (Phelps et al., 2004). This was consistent with previously reported findings from nonhuman animal research. In a study in which participants were presented with aversive odorants as UCS and visual stimuli as CS, followed by extinction, activity in the amygdala and medial PFC were greatest during the early stages of both acquisition and extinction (Gottfried & Dolan, 2004). These data, along with results from other studies (Knight, Smith, Cheng, Stein, & Helmstetter, 2004), suggest that these structures are most responsive to changes in experimental contingencies, regardless of whether the responses occur during initial learning or during the retrieval processes that take place in extinction learning. Studies of extinction learning demonstrate the common neural regions engaged during the retrieval of emotional experience across species.
Episodic retrieval
Studies of episodic memory retrieval in an fMRI environment have pointed to medial temporal, prefrontal, and parietal regions as the key structures that contribute to retrieval (Buckner & Wheeler, 2001; Henson, Rugg, Shallice, Josephs, & Dolan, 1999), with different processes of memory retrieval recruiting different neural regions. The retrieval attempt, in which the participant mounts a strategic effort to remember, elicits activity in the PFC (Buckner & Wheeler, 2001).6 Activity in the hippocampus (Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, 2000; Henson et al., 1999) and the posterior parietal cortex (Wagner et al., 2005) correlates with successful retrieval.7 Neural areas also track the retrieval of information across the different sensory domains differently, such that the recollection of a visual scene may result in the activity of visual cortices even in the absence of visual stimulation (M. E. Wheeler, Petersen, & Buckner, 2000). Similar results have been reported for the recollection of auditory (Nyberg, Habib, McIntosh, & Tulving, 2000) and olfactory (Gottfried, Smith, Rugg, & Dolan, 2004) information. Analysis of the effects of emotion on these regular patterns of retrieval-related neural activity make it possible to determine how emotion may affect the normal processes of retrieval. Also, comparing the neural activity that is recruited for the retrieval of neutral versus emotional items may reveal qualitatively different neural signatures.
One study examined the retrieval of emotional memories using an emotional context paradigm similar to that previously described, but it included positive emotional context stimuli in addition to neutral and negative contexts (Maratos, Dolan, Morris, Henson, & Rugg, 2001). More important, this study assessed the incidental retrieval of emotional context, as there were no specific instructions to retrieve the emotional context, only to judge whether the target words had or had not been previously presented. The comparison of responses to previously presented versus novel stimulus words activated the regions that had previously been reported in studies on memory retrieval. These old/new effects included activity in the left-lateral parietal, precuneus, cingulate, parahippocampal, and prefrontal cortices. A direct comparison between the responses to the recognition of “old” words from a negative context and “old” words from a neutral context showed greater activation in the regions that are not typically active in recognition tasks, including the amygdala. It is interesting to note that activity in the amygdala did not differentiate between the retrieval of negative versus positive words, indicating that activity in this structure may play a role in the retrieval of emotional material regardless of valence. Retrieval of old words from a positive context resulted in greater activation in the medial PFC compared with retrieval of old words from a neutral or negative context. This medial PFC activity, unlike that of the amygdala, was specific to the retrieval of positive words, perhaps reflecting a role in the processing of rewarding stimuli (O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001). These results suggest that the retrieval of emotional information recruits the regions that are also involved in the retrieval of neutral information, along with a subset of additional regions typically associated with emotional processing, including the amygdala and medial PFC.
Emotion and the cognitive operations of retrieval
It is not clear from the aforementioned studies, however, whether emotion influenced the process of retrieval or whether the incidental cue led to an emotional response to the products of retrieval. Results from ERP studies suggest that emotion may affect both the processing and the products of retrieval. To address this issue, Smith, Stephan, Rugg, and Dolan (2006) examined neural activity during the retrieval of emotional information using two different source retrieval tasks: emotion discrimination and nonemotion discrimination. In the emotion discrimination task, neutral objects were initially paired with negative emotional or neutral scenes, and at test, participants were asked with which type of scene (emotional or neutral) the object was originally paired. This task required the explicit recall of emotional context. In the nonemotion discrimination task, participants were asked whether the contextual scene contained people, regardless of emotional content. In both tasks, the perceptual stimuli and emotional context remained the same, but attention to emotion was either deliberate or incidental in the task requirements. These tasks take advantage of the “mode” of retrieval (or retrieval orientation), wherein the task demands affect neural processing during retrieval tasks (Duzel et al., 1999; Woodruff, Uncapher, & Rugg, 2006). If amygdala activity during retrieval reflects the processing that the participant is conducting—focusing on emotion context—then it should be more active during the emotion than the nonemotion discrimination task (an effect of retrieval orientation). By contrast, if amygdala activity merely reflects incidental retrieval of the context in which an object was originally encoded, then there should be no difference in amygdala activity between the discrimination tasks.
Analysis of activity during the emotion discrimination task versus the nonemotion discrimination task showed increased bilateral activity in the amygdala, hippocampus, and medial PFC. As in previous work, brain activity during the retrieval of objects learned in an emotional versus neutral context (independent of retrieval discrimination task) included the left amygdala and left medial PFC. These findings indicate that both the correct emotional source retrieval as well as the emotional retrieval orientation activates the amygdala (see Kensinger & Schacter, 2006, for a different pattern of amygdala activity during the encoding of emotional source memory). The results further suggest that retrieval orientation toward emotional events recruited more emotion-related regions—bilateral amygdala and medial PFC—than did the incidental retrieval of emotional events. To further address the neural dynamics among these regions during retrieval tasks, Smith, Stephan, Rugg, and Dolan (2006) examined the functional connectivity among the amygdala, hippocampus, medial PFC, and fusiform gyrus using dynamic causal modeling, which allows for the assessment of the influence one brain area has on another at the neural level (Friston, Harrison, & Penny, 2003). They found that functional connectivity from the left hippocampus to the left amygdala (the analysis was only conducted in the left hemisphere) increased during both discrimination tasks. During the emotion discrimination task, functional connectivity from the amygdala to the hippocampus, as well as from the hippocampus back to the amygdala (bidirectional functional connectivity), increased. Implementing an affective retrieval orientation during the emotion discrimination task also engaged the medial PFC, which influenced the function of both the amygdala and hippocampus. Thus, functional connectivity between the hippocampus and amygdala is associated with the task of retrieving emotional information, and this mutual activation is further enhanced when attention to emotion is required for the performance of the task, perhaps through top-down effects from the medial PFC. Similar interactions between the amygdala and hippocampus have recently been described in relation to the encoding of emotional information (Dolcos, LaBar, & Cabeza, 2004), during which both the encoding and retrieval of emotional events may engender a more pronounced level of functional connectivity among the neural structures involved in the experience of emotion (amygdala, medial PFC) and in memory processes more generally (hippocampus).
Many neuroimaging studies of memory retrieval focus only on successful retrieval. By doing so, it is impossible to disentangle the effects of emotion on neural activity during the process of retrieval versus effects on neural activity in response to successful retrieval. By examining the neural activity during an unsuccessful retrieval attempt, it is possible to delineate those structures involved in the process, separate from the success, of a retrieval attempt. Smith, Henson, Rugg, and Dolan (2005) addressed this issue by examining the responses associated with successful versus unsuccessful emotional source retrieval. They reported right-amygdala activity during unsuccessful emotional retrieval (an index of the effect of emotion on the process of retrieval) and left-amygdala activity during successful emotional retrieval. Similar findings were reported by the same authors in a subsequent study (Smith et al., 2006; see the Internet-only supplementary materials of that article). These findings show that the emotional context of encoding influences retrieval processing regardless of the success of the retrieval attempt. Furthermore, the influence of emotion on retrieval processing does not always rise to the level of conscious awareness. These findings present an intriguing possibility for mood-congruency effects; initial exposure to a stimulus that is associated with emotion may bias subsequent response to, or retrieval of, that stimulus in the absence of awareness. The non-conscious processing of emotional stimuli activates the amygdala, often specifically in the right hemisphere (J. S. Morris, Öhman, & Dolan, 1999); the findings of Smith et al. (2005) demonstrated that these nonconscious effects extend beyond the initial exposure to affective stimuli and extend to the retrieval of the stimuli. From these studies, then, emotion leads to a qualitatively different pattern of brain activity during the deliberative search for emotional memories (e.g., retrieval orientation) as well as during the retrieval process when an individual is unaware of the emotional nature of the searched-for stimulus.
Associations among neural regions may influence not only the accuracy of memory for emotion but also the phenomenological characteristics. Memories for emotional experiences are rated as more vivid and are held with greater confidence than those for more neutral experiences (Talarico, LaBar, & Rubin, 2004; Talarico & Rubin, 2003). Another phenomenological characteristic of memory is whether an experience is recollected or merely seems familiar (Tulving, 1985). Research has demonstrated that the MTL memory system shows different activity profiles to familiarity versus recollection in memory retrieval paradigms (Yonelinas, Otten, Shaw, & Rugg, 2005). Also, the retrieval of emotional events is associated with a greater experience of recollection compared with neutral events (Kensinger & Corkin, 2003; Ochsner, 2000) and may rely on neural regions outside the areas traditionally associated with memory retrieval (parietal cortex, dorsolateral PFC). Sharot, Delgado, and Phelps (2004) examined the neural correlates of the phenomenology of retrieval for emotional stimuli. Participants were presented with negative emotional and neutral scenes immediately before an fMRI scanning session, during which a recognition memory test, including the remember/know procedure, was conducted. The participants reported remembering more of the emotional scenes while producing a greater number of know responses to the neutral scenes. Neuroimaging data showed greater amygdala activity in response to remembering emotional scenes, whereas the parahippocampal cortex exhibited greater activity in response to remember responses for neutral scenes. Similar results were reported by Dolcos, LaBar, and Cabeza (2005) for tests involving a retention interval of 1 year. Thus, amygdala activity may enhance the subjective feeling of remembering during the retrieval of emotional material. This increased amygdala activity may contribute to the heightened feeling of vividness of, and confidence in, emotional versus neutral memories.
Recapitulation of encoding activity at retrieval
Just as visual imagery relies on visual cortical areas (Kosslyn, Thompson, Kim, & Alpert, 1995) and sensory-specific retrieval activates cortical areas specific to the particular sensory stimulus retrieved (Wheeler et al., 2000), perhaps the retrieval of emotional experiences relies on the recapitulation of brain activity involved in the original experience of an emotional state. The research described above suggests that during the retrieval of emotional memories, neural structures typically associated with emotion are activated along with neural structures involved more generally in memory retrieval. The true test of this assertion would be to examine neural activity both during the encoding and retrieval of emotional stimuli. Smith, Henson, Dolan, and Rugg (2004) showed that the encoding and retrieval of emotional context activated several common areas, including the left amygdala and left angular gyrus. Another study showed that this recapitulated emotional context extends to other regions that are highly interconnected to the amygdala. Fenker, Schott, Richardson-Klavehn, Heinze, and Duzel (2005) presented nonemotional words along with fearful or neutral facial expressions in an encoding session and then measured neural activity during word retrieval, for the expressed purpose of examining the incidental retrieval of facial emotion. They found that the recollection of words (assessed using the remember/know paradigm) previously paired with emotional faces elicited greater activity in the fusiform face area (FFA)8 compared with recollection of words paired with neutral faces. Previous research has shown considerable interconnections between the amygdala and the FFA in the processing of emotional faces (Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004). These results indicate that these connections are active not only during the online processing of emotional faces but also during the incidental retrieval of emotional faces. This suggests that stimuli from the environment and from internal representations elicit similar patterns of neural activity during emotional processing.
Neural basis of mood-congruent retrieval
The neural basis of the affect priming model (Bower, 1981; Bower & Forgas, 2000) was tested in a study examining mood-congruency effects on neural activity during the retrieval of emotional words during positive and negative mood states (Lewis et al., 2005). In an encoding session, participants were presented with positively and negatively valenced words while neural activity was measured using fMRI. During a subsequent recognition task, neural activity associated with positive- and negative-mood inductions, as well as with a recognition memory task for the previously presented words, was measured. Analysis of the data allowed for the testing of two specific questions: (a) Does mood-induced matching of neural activity during the encoding and retrieval of affect-congruent stimuli occur? and (b) What process of memory is affected by mood congruency? With regard to the first question, congruent neural activity was measured in the medial PFC (subgenual region) during the retrieval of positive stimuli in a positive mood state, and congruent activity was measured in a more lateral region of the PFC (posterolateral orbitofrontal cortex) during the retrieval of negative stimuli in a negative mood state. These regions have previously been implicated in the processing of positive and negative affective states, respectively (O’Doherty et al., 2001; Phan, Wager, Taylor, & Liberzon, 2002). These findings provide some neural support for the affect-priming theory of mood-congruent memory in that some of the same neural regions involved in the encoding of emotional stimuli are also recruited during the retrieval of these stimuli (although not the amygdala, as would be predicted on the basis of previous work).
In order to address the second question, Lewis et al. (2005) examined the neural activity associated with the correct recognition (yes response to previously presented stimuli) of mood-congruent stimuli versus the correct rejection (no response to novel stimuli) of mood-congruent stimuli (regardless of valence). If mood-congruency effects modulate the activity of neural areas associated with correct retrieval, such as the anterior PFC (BA 9/10), then these areas should show greater activity when retrieval mood and stimulus affect match. By contrast, if mood-congruency effects modulate the activity of areas associated with the retrieval search process, such as dorsolateral PFC (BA 44/6), then these regions should show greater activity when retrieval mood and stimulus affect match (regardless of retrieval success). This analysis showed that mood congruency is associated with significantly greater activity in regions typically associated with retrieval search (dorsolateral PFC and precuneus) and not with those implicated in successful retrieval. These results suggest that mood congruency acts at the level of search processing rather than at the level of retrieval success processing, lending support to the idea that affect guides the search process for the retrieval of memory. Also, the results further suggest that areas traditionally associated with memory retrieval—posterior parietal cortex and dorsolateral PFC—are influenced by affect during retrieval processing.
Autobiographical retrieval
The study of autobiographical memory retrieval in the context of functional neuroimaging presents a number of challenges. Studies that have attempted this line of research have used auditory or visual cues of participants’ own memories, which were collected prior to the scanning session. Responses to these cues are then compared with responses elicited by various control tasks, including exposure to others’ autobiographical memories, presentation of recent versus remote memory cues, and comparison of emotional versus “less emotional” autobiographical cues (for a review, see Maguire, 2001). Additionally, the time course of the retrieval period, the success of the retrieval attempt, and the cognitive set (e.g., retrieval orientation) of the participants are all factors that could influence neural activity in these tasks (see Cabeza et al., 2004; Greenberg et al., 2005, for further discussion of these issues). In spite of these challenges and limitations, this line of research has provided some intriguing results. In this review, I focus only on those studies that have explicitly manipulated or measured emotion in reference to autobiographical memory (see Table 3 ).
One of the methods used in functional imaging to study autobiographical memories is the comparison of neural responses to participants’ own versus others’ recollections. In one of the first neuroimaging investigations of autobiographical memory, Fink et al. (1996) conducted a positron emission tomography (PET) study in which participants listened to sentences from their own and others’ autobiographical memories. These memories were described as “affect-laden,” although ratings of emotionality were not reported. When neural responses to personal autobiographical memories were compared with responses to others’ memories (impersonal condition), activity in the right temporal lobe, including in the amygdala, increased significantly. Similar findings were reported from a PET study by Markowitsch et al. (2000), in which greater right temporal activity, including in the amygdala, occurred during exposure to personal autobiographical experiences versus fictitious experiences. Other studies have reported increased medial PFC responses to positive versus negative stimuli (Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003). By contrast, subtraction of the activity associated with negative recollection from that associated with positive recollection resulted in greater right-middle temporal cortex activity. Although these findings are generally consistent in showing a pattern of right temporal and medial PFC activity in the recollection of emotional autobiographical events, it is difficult to determine from these studies what processes are engaged by the task. The use of a block design in these studies leaves open the possibility that the observed differences in activity across conditions is related to retrieval orientation (Rugg & Allan, 2000) or mood state (Bower, 1981). Additionally, it is unclear whether retrieval was successful and what phase of retrieval corresponded to the various neural activations. For example, the search process most likely relies on areas distinct from those indexing retrieval success (Buckner & Wheeler, 2001).
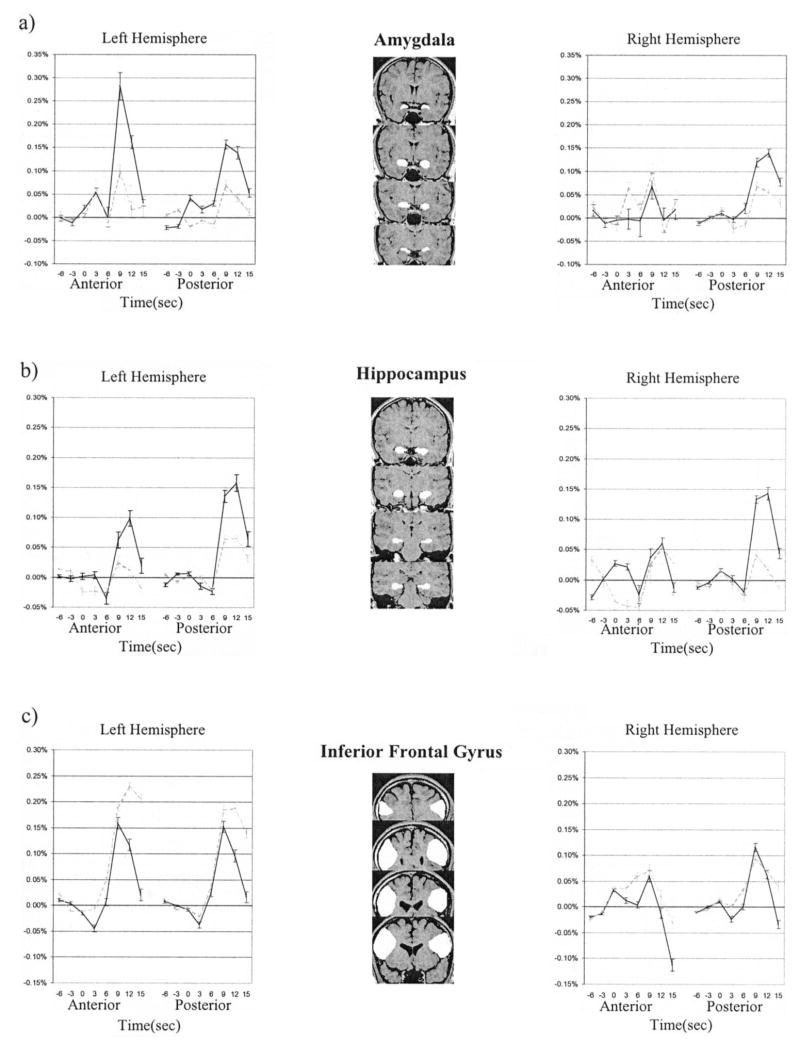
In an attempt to address some of these issues, Greenberg et al. (2005) used an event-related fMRI paradigm to examine emotional autobiographical memory. In this study, participants were presented with self-produced autobiographical memory cues derived from a prescanning session. A total of 50 autobiographical cues were presented for 24 s each, with instructions to “. . . try to re-experience and maintain the memory in mind until the end of the trial. . . .” These memories had been rated an average of 5.0 on a 1–7 scale of valence (with 1 indicating that the memory was very negative and 7 indicating that it was very positive). More important, to control for the effects of retrieval success, only those trials in which participants reported successful retrieval were included in the analysis. A region of interest (ROI) analysis focusing on the amygdala, hippocampus, and inferior PFC, was conducted, with specific attention paid to the time course of activity during the retrieval trial. Responses to these autobiographical cues were compared with responses during semantic trials during which participants were presented with a category word and asked to produce as many exemplars of that category as they could recall. Retrieval of autobiographical memories produced increased activity in the amygdala and hippocampus compared with that stimulated by semantic retrieval. However, the semantic retrieval task resulted in greater activity in the left inferior frontal gyrus. These differential patterns of neural activity developed over time, reaching peaks at 9–12 s poststimulus onset (see Figure 3). A correlation analysis showed that retrieval of autobiographical memories resulted in greater functional connectivity among the amygdala, hippocampus, and medial PFC than retrieval of semantic memories. This finding is consistent with those from other human imaging studies and nonhuman animal research, which suggest an increased functional connectivity between the amygdala and hippocampus during the retrieval of emotional stimuli cued by neutral contexts (Seidenbecher et al., 2003; Smith et al., 2006). These findings demonstrate that, after controlling for retrieval success, the recollection of emotional autobiographical memories activates the amygdala and hippocampus bilaterally, and these regions show greater functional connectivity under these conditions than during retrieval of semantic information.
Figure 3.
Hemodynamic responses of voxels in the amygdala, hippocampus, and inferior frontal gyrus during the retrieval of autobiographical (AM; solid lines) or semantic (SM; stippled lines) memories. y-axes represent average signal change. From “Co-Activation of the Amygdala, Hippocampus and Inferior Frontal Gyrus During Autobiographical Memory Retrieval,” by D. L. Greenberg et al., 2005, Neuropsychologia, 43, p. 665. Copyright 2005 by Elsevier. Reprinted with permission.
It is difficult to determine the role of amygdala and medial PFC activation in these studies of autobiographical retrieval. The activity could potentially be due to the reexperience of the emotional event, as suggested by the late-positive potential recorded during the retrieval of emotional memories (Smith, Dolan, & Rugg, 2004). Findings from Greenberg and colleagues (2005), and those from Smith, Henson, et al. (2004; Smith et al., 2005, 2006), suggest that this activity of traditionally “emotional” brain regions may function in retrieval processes such as the registration of item familiarity, the experience of recollection, or success monitoring. The cues used in these experiments were intrinsically neutral, yet evoking the memory of emotional stimuli or events led to increased amygdala and medial PFC activity. These findings are consistent with the idea that amygdala and medial PFC activity aids in the retrieval of previously experienced emotional situations, perhaps through the evocation of an internal emotional state that serves as a retrieval cue.
Summary and Integration
The reviewed literature has pointed to the influence that affect may have on the processes of memory retrieval, at both psychological and neurobiological levels. It suggests that exposure to a reminder of an emotional event elicits brain activity similar to that taking place during the original event. This activity—in both the amygdala and medial PFC—in turn elicits an affective state similar to that experienced during the original event. It is important to note that recognition of affective stimuli in the environment elicits the activity of the amygdala and medial PFC without necessarily eliciting a subjective experience of emotion such as a change in mood state (Phan et al., 2002). The internal representation of previously experienced emotional stimuli may elicit a transient emotional state, though one that is sufficient to cue the retrieval of an emotional event. The notion that affective experience serves as a cue for memory retrieval is not a novel concept; it forms the basis of the affect priming theory described earlier (Bower & Forgas, 2000). The goal here is to further specify the cognitive and neural mechanisms that make the effects of emotion on retrieval possible.
The retrieval of an emotional event may be cued by direct exposure to a specific reminder of an event or by a partial reminder that initiates the processes required to retrieve the memory for that event. This reminder of an emotional event, such as a picture of the event, may lead to the activation of the amygdala and medial PFC in much the same way as the original exposure to an emotional event. The emotional state is triggered through connections from the amygdala and medial PFC to hypothalamic and brainstem centers whose activation leads to autonomic and somatic responses. This emotional state may occur simultaneously with the retrieval attempt for the event or through the recruitment of areas involved in memory retrieval more generally, such as the dorsolateral PFC and parietal cortex. The cognitive-emotional state thus initiated by successful retrieval may then further bias retrieval processes on the basis of the reexperience of the emotional state.
Specific reminders of an emotional event are less common than reminders that only partially cue an emotional event. The reexperience of an affective state may serve either as a selective reminder of the original encoding of a particular event or as a reminder of similar affective experiences from the past. Even the attempt to retrieve an emotional memory may establish the affective state necessary to influence the cognitive and neurobiological processes of retrieval (see Smith et al., 2005, 2006). A partial reminder of an emotional event, such as a fragment of a conversation, may trigger a search process for an emotional event associated with the context of the conversation. In this case, the search process itself leads to activation of the amygdala and medial PFC. Connections between these and other retrieval-related brain structures contribute to the recollection of the original emotional event. Upon recollection of the event, brain regions associated with retrieval success may become active, along with further activity of the emotion-related structures in response to the internally generated emotional stimulus. The instantiation of an affective state then proceeds in the same way as in the direct response to an emotional stimulus. The reexperience of an affective state serves as a selective reminder for the original encoding of a particular event, or as a reminder of similar affective experiences from the past. In this sense, one may think of an affective experience serving as a cue to reexperience an affective state in much the same way as a stimulus that is similar to an established CS may lead to a generalized CR in classical conditioning. When one is reminded of a negative affective experience, this may serve as a cue of the original experience as well as a cue of other negatively affective events. This kind of generalization may form the basis of mood-congruent memory, wherein the prevailing mood state enhances the retrieval of previously encountered stimuli with a matching emotional valence, although the stimulus may be unrelated to establishing the mood state.
The emotional brain, then, is not a passive responder to emotional information retrieved through the actions of other “cognitive” brain structures. Rather, the amygdala and medial PFC may play active roles in the retrieval of this information by producing an affective state. Recent work on emotion recognition has suggested this active role of the amygdala in seeking out emotional information in the environment (Adolphs et al., 2005). The amygdala may play this active role in the search for emotional information not only from the environment but also from past experience as well. Ultimately, an emotional event may be retrieved through search mechanisms instantiated via the amygdala–hippocampal connections. The establishment of a retrieval orientation toward emotional stimuli may depend on medial PFC activity, which further enhances the processing of amygdala and hippocampus. It may be necessary to recapitulate at least some aspects of the original event to have a true recollection of that event, and the affective state, triggered by the amygdala during the search process, serves as a potent contextual cue in this process. Just as external cues that are similar to the to-be-remembered item are the best cues for the retrieval of nonemotional information (ala encoding specificity and transfer-appropriate processing), emotional states similar to the original experience serve as potent cues to the retrieval of those experiences.
The retrieval of auditory, visual, and olfactory stimuli tends to activate regions of cortex involved in the perception of these senses, even in the absence of sensory stimulation (Buckner & Wheeler, 2001; Gottfried et al., 2004). Similarly, the specific sensory cortices that are active in mental imagery are the same as those active during the actual experience of a stimulus (Kosslyn, Ganis, & Thompson, 2001). The same pattern has been found for the recollection of emotional experience. For instance, both the initial exposure to and the retrieval of emotional stimuli often leads to amygdala and medial PFC activation (Smith, Henson, et al., 2004). This activation during retrieval may not be as extensive or as long-lasting as those observed during the actual experience of the stimulus, although the retrieved experiences are often rated as being highly vivid and intense (Buchanan et al., 2005; Talarico et al., 2004). Upon successful retrieval, the amygdala and medial PFC may respond in a manner similar to that observed during the perception of the original emotional stimulus. The coordinated activities of these structures is necessary for the optimal retrieval of salient events from the past.
The findings from neuropsychology and neuroimaging are consistent with the notion that the amygdala is involved in the reexperience of an emotional memory. Neuropsychology indicates that patients with amygdala damage have impaired retrieval of unpleasant emotional experiences (Buchanan et al., 2005, 2006), and the results from neuroimaging studies suggest that this impairment could be due to an inability either to mount a proper search process for emotional events or to recognize retrieved events as being emotional. I do not propose that unpleasant memories are stored in the amygdala, but rather that the amygdala may play a role in the recapitulation of these experiences through its influences on other neural structures, which then leads to the retrieval and reexperience of the memories through the orchestrated activities of the structures necessary for emotion and those necessary for memory retrieval. It is unclear from the studies that have been performed why amygdala damage would specifically affect the retrieval of negative emotional memories and not positive memories as well, especially given that neuroimaging studies demonstrate amygdala activity during the retrieval of both positive and negative emotional memories. Perhaps other neural regions, such as the medial PFC, are able to represent the pleasant emotional experiences from the past, making them more likely to be retrieved even in the absence of a functional amygdala. The involvement of the medial PFC in the retrieval of pleasant memories, as suggested by several neuroimaging studies, provides some support for this contention (Lewis et al., 2005; Maratos et al., 2001). The independent roles that various brain structures play in the responses to the process and products of retrieval deserve further research attention.
Outstanding Issues
In recent years, many reports have shown that when a memory trace is activated by retrieval, the trace becomes malleable and subject to so-called reconsolidation (Dudai, 2006; Nader, 2003; Sara, 2000). This has led to controversy about how general the reconsolidation phenomenon is (see Cahill, McGaugh, & Weinberger, 2001; Dudai, 2004, 2006, for a full account of this lively debate). Reconsolidation occurs when an organism is trained on a task, and at some time later, the memory trace for this training is reactivated by reexposure either to a stimulus or to the context of the original learning. This reactivated trace is altered such that memory performance is either impaired (Nader, Schafe, & Le-Doux, 2000) or enhanced (Tronson, Wiseman, Olausson, & Taylor, 2006). Reconsolidation has been observed in appetitive (Torras-Garcia, Lelong, Tronel, & Sara, 2005)9 as well as aversive learning situations (Nader et al., 2000).
The potential for altering emotional memories during or immediately after retrieval is an intriguing possibility that has been explored extensively in animal models. The alteration of postretrieval emotional memory traces in humans, however, has not been conclusively demonstrated. The “first wave” of reconsolidation research led to conflicting reports as to whether electroconvulsive therapy produced amnesia for reactivated memory traces in humans (Misanin, Miller, & Lewis, 1968; Squire, Slater, & Chace, 1976). Although more recent human research has suggested that the memory trace for a motor sequence task can be disrupted through a reconsolidation-type mechanism (Walker, Brakefield, Hobson, & Stickgold, 2003), the learning of a motor sequence is quite distinct from emotional declarative memories. Although animal work has provided several demonstrations of reconsolidation of hippocampally mediated memories, it is not clear to what extent the same reconsolidation mechanisms affect human declarative memory traces, especially for emotional events. The similarities in brain mechanisms that underlie rodent and human emotional learning situations, such as fear conditioning, extinction, and reward learning, suggest that the labile state that is thought to be affected in animal reconsolidation paradigms should also be manipulable during memory retrieval in humans. However, evidence for reconsolidation of human emotional memory has thus far remained elusive.
The conventional stance with regard to cognitive therapy is that emotion can alter memory and other cognitive functions during, or after, the retrieval process (Beck, Rush, Shaw, & Emery, 1979; Brewin, 2006). Similarly, behavior therapy is based on the assumption that behavior is guided by associations between fear-inducing situations and benign environmental cues. Reexposure to (and therefore, retrieval of) these cues in the absence of fear or anxiety, then, should ameliorate the negative responses associated with contextual stimuli (Brewin, 2006; Wolpe & Lang, 1964). These two approaches to therapy, which form the basis of the most popular present form of psychotherapy, cognitive-behavior therapy (CBT), act on the premise that emotional memories are modified upon retrieval (Brewin, 2006). CBT has been applied to many varieties of psychopathology, including depression, generalized anxiety disorder, PTSD, obsessive-compulsive disorder, and social phobia (Brewin, 2006). Theoretical views of how CBT may act on memory representations include the idea that CBT may directly alter the representations of negative memories. Another possibility is that the relative weights of negative versus positive memories are altered through CBT, with successful treatment being the result of positive memories winning the retrieval competition (Brewin, 2006). In spite of the often reported bias toward negative memories in depression (Hertel, 2004), depressed individuals do have access to both positive and negative memories (Kendall, Howard, & Hays, 1989), and improved clinical outcomes in these individuals have been linked to the ability to activate positive memory traces (Scher, Ingram, & Segal, 2005). These findings, along with the fact that mood-dependent and mood-congruent memory effects may influence the kind of memories (more or less positive) nondepressed individuals recollect (Kendall et al., 1989), are consistent with the idea that the product of retrieval is dependent upon competition among internal memory states.
The phenomenon of reconsolidation is a potential mechanism whereby maladaptive memory traces may be altered through cognitive, behavioral, or pharmacological intervention (Dudai, 2006). Most of the studies that have demonstrated postretrieval reconsolidation effects have used traditional animal learning paradigms such as fear conditioning (Dudai, 2006; Nader et al., 2000). Techniques such as exposure therapy in human patient groups show that reexposure to previously noxious stimuli and contexts in the absence of negative consequences can alter emotional responses to the original fear-provoking stimuli (Foa & Kozak, 1986). Recent work with PTSD and phobias has been successful in modulating the reexperience of the traumatic event (or phobic object) by administering stress hormones during reexposure (Schelling et al., 2004; Soravia et al., 2006). All of these studies indicate that altering the retrieved representation of a fear-inducing stimulus through conditioning or pharmacological means can be effective in the treatment of these disorders. The malleability of the memory trace following retrieval, whether induced by CBT or pharmacological means, may thus allow for the amelioration of traumatic memories in individuals afflicted with psychopathology. Although the precise mechanisms whereby this modulation work are presently under investigation, the ideas derived from basic research on the retrieval of emotional memories hold considerable promise for the understanding and treatment of these disorders.
Conclusion
Emotion has long been known to influence what we remember. On the basis of the reviewed literature, it is clear that emotion can exert effects at the time of encoding, during retrieval search processes as well as during the experience of recollection. Models of affect and memory have described the situations in which emotion can influence cognition and, more specifically, memory retrieval (Bower & Forgas, 2000). By studying the function of the brain using ERPs and fMRI, it is possible to better understand the neural underpinnings of affect-cued memory retrieval. The neural processes involved in the recognition and experience of emotion are also involved in the retrieval of memories for emotional experiences. The amygdala and medial PFC work in combination with the hippocampus, lateral PFC, and the parietal cortex to accomplish the retrieval of emotional experience. These findings can be applied not only toward extending researchers’ basic understanding of the processes of memory and affect but also to the development of potential treatment options in the field of psychopathology. Whether this will make it possible to alleviate the pain patients with depression and PTSD experience in retrieving emotional memories remains a question for the scientific community to answer. However, it is hoped that by summarizing and integrating what is presently known about the mechanisms of affect and retrieval, the present review will help to stimulate further interest in this important topic.
Acknowledgments
The preparation of the article was supported by National Institute of Mental Health Grants R03 MH076815 and R01 MH067681.
Footnotes
Eich (1995) provided a comprehensive review of the factors necessary to demonstrate a true mood dependency effect and concluded that “. . . robust and reliable MDM [mood-dependent memory] effects can be revealed—but only within a restricted range of circumstances or conditions” (p. 74). The reader is refered to Eich (1995) and Bower and Forgas (2000) for a full discussion of the factors influencing mood-dependent memory.
Nadel and Moscovitch’s (1997) multiple trace theory posits that the hippocampal formation is always involved in the retrieval of long-term memory. By contrast, traditional consolidation theory proposes that long-term memories become independent of the hippocampal formation over time. The reader is referred to the citations for a full discussion of these issues.
The time course of retrograde amnesia was not addressed in this study, although previous studies with some of the same patients have investigated this topic (Tranel, Damasio, & Damasio, 2000).
The temporal resolution of fMRI studies is on the level of seconds, compared with the millisecond resolution of ERP and other neurophysiological techniques.
The sectors of the medial PFC that are involved in the retrieval of emotional material have been the focus of much research in nonhuman animals and humans. Especially in human research, the naming of the specific sector involved in a given task has sometimes been unclear. For this reason, I refer to regions, including the orbitofrontal cortex, ventromedial cortex, and subgenual cingulate cortex, collectively as medial prefrontal cortex. The reader is referred to Öngür and Price (2000) for a discussion of the anatomical organization of this area of the brain.
Considerable research has reported lateralized prefrontal activity in the encoding versus retrieval of long-term memory, and this has led to the hemispheric encoding/retrieval asymmetry (HERA) model (Tulving, Kapur, Craik, Moscovitch, & Houle, 1994). HERA proposes that the left PFC is differentially involved in the encoding of episodic memories and the retrieval of semantic memories, whereas the right PFC is more involved in the retrieval of episodic memories.
The correspondence between the left parietal potential from event-related potential studies, discussed above, and the parietal activity observed in fMRI studies provides a particularly strong argument for the role of parietal regions in retrieval processing.
The FFA is an area in the posterior MTL specifically involved in the processing of faces (Kanwisher, McDermott, & Chun, 1997).
More important, in this study, the pharmacological agent (DL-APV, an N-methyl-D-aspartate receptor antagonist) was administered via intracere-broventricular injection. Therefore, this effect cannot be ascribed to the amygdala alone but may involve other areas (such as the medial PFC) that are more often associated with positive emotion and reward.
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. Journal of Neuroscience. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005 January 6;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. 2001;15:396–404. doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Spencer DD, Fulbright RK, Phelps EA. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;14:526–536. doi: 10.1037//0894-4105.14.4.526. [DOI] [PubMed] [Google Scholar]
- Bartlett FC. Remembering: A study in experimental and social psychology. Cambridge, England: Cambridge University Press; 1932. [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lesions. Neuron. 2003;38:135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Wiley; 1979. [Google Scholar]
- Berntsen D, Rubin DC. Emotionally charged autobiographical memories across the life span: The recall of happy, sad, traumatic, and involuntary memories. Psychology and Aging. 2002;17:636–652. doi: 10.1037//0882-7974.17.4.636. [DOI] [PubMed] [Google Scholar]
- Blaney PH. Affect and memory: A review. Psychological Bulletin. 1986;99:229–246. [PubMed] [Google Scholar]
- Borod JC. Emotion and the brain–anatomy and theory: An introduction to the special section. Neuropsychology. 1993;7:427–432. [Google Scholar]
- Borod JC, Obler LK, Erhan HM, Grunwald IS, Cicero BA, Welkowitz J, et al. Right hemisphere emotional perception: Evidence across multiple channels. Neuropsychology. 1998;12:446–458. doi: 10.1037//0894-4105.12.3.446. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Moody EW. Memory processes in classical conditioning. Neuroscience and Biobehavioral Reviews. 2004;28:663–674. doi: 10.1016/j.neubiorev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biological Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bower GH. Mood and memory. American Psychologist. 1981;36:129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- Bower GH. Commentary on mood and memory. Behavior Research & Therapy. 1987;25:443–455. doi: 10.1016/0005-7967(87)90052-0. [DOI] [PubMed] [Google Scholar]
- Bower GH, Forgas JP. Affect, memory, and social cognition. In: Eich E, Kihlstrom JF, Bower GH, Forgas JP, Niedenthal PM, editors. Cognition and emotion. New York: Oxford University Press; 2000. pp. 87–168. [Google Scholar]
- Bower GH, Mayer JD. In search of mood-dependent retrieval. Journal of Social Behavior and Personality. 1989;4:133–168. [Google Scholar]
- Bower GH, Monteiro KP, Gilligan SG. Emotional mood as a context for learning and recall. Journal of Verbal Learning and Verbal Behavior. 1978;17:573–578. [Google Scholar]
- Brewin CR. Understanding cognitive behaviour therapy: A retrieval competition account. Behavior Research & Therapy. 2006;44:765–784. doi: 10.1016/j.brat.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Brown SC, Craik FIM. Encoding and retrieval of information. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. Oxford, England: Oxford University Press; 2000. pp. 93–107. [Google Scholar]
- Buchanan TW, Adolphs R. The neuroanatomy of emotional memory in humans. In: Reisberg D, Hertel P, editors. Memory and emotion. New York: Oxford University Press; 2004. pp. 42–75. [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Emotional autobiographical memories in amnesic patients with medial temporal lobe damage. Journal of Neuroscience. 2005;25:3151–3160. doi: 10.1523/JNEUROSCI.4735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Memories for emotional autobiographical events following unilateral damage to medial temporal lobe. Brain. 2006;129(Pt 1):115–127. doi: 10.1093/brain/awh672. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nature Reviews Neuroscience. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, et al. Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. Journal of Cognitive Neuroscience. 2004;16:1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Modulation of memory storage. Current Opinion in Neurobiology. 1996;6:237–242. doi: 10.1016/s0959-4388(96)80078-x. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL, Weinberger NM. The neurobiology of learning and memory: Some reminders to remember. Trends in Neurosciences. 2001;24:578–581. doi: 10.1016/s0166-2236(00)01885-3. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. Journal of Neuroscience. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crovitz H, Schiffman H. Frequency of episodic memories as a function of their age. Bulletin of the Psychonomic Society. 1974;4:517–518. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Lane R, Chua P, Fletcher PC. Dissociable temporal lobe activations during emotional episodic memory retrieval. NeuroImage. 2000;11:203–209. doi: 10.1006/nimg.2000.0538. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences, USA. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. Memory from A to Z: Keywords, concepts and beyond. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annual Review of Psychology. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: The advantage of being refocused. Current Opinion in Neurobiology. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Duzel E, Cabeza R, Picton TW, Yonelinas AP, Scheich H, Heinze HJ, et al. Task-related and item-related brain processes of memory retrieval. Proceedings of the National Academy of Sciences, USA. 1999;96:1794–1799. doi: 10.1073/pnas.96.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich E. Searching for mood dependent memory. Psychological Science. 1995;6:67–75. doi: 10.1111/1467-9280.00249. [DOI] [PubMed] [Google Scholar]
- Eich E, Kihlstrom JF, Bower GH, Forgas JP, Niedenthal PM. Cognition and emotion. New York: Oxford University Press; 2000. [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? Journal of Affective Disorders. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. Journal of Neuroscience. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH. Method matters: An empirical study of impact in cognitive neuroscience. Journal of Cognitive Neuroscience. 2005;17:850–858. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- Fenker DB, Schott BH, Richardson-Klavehn A, Heinze HJ, Duzel E. Recapitulating emotional context: Activity of amygdala, hippocampus and fusiform cortex during recollection and familiarity. European Journal of Neuroscience. 2005;21:1993–1999. doi: 10.1111/j.1460-9568.2005.04033.x. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one’s own past: Neural networks involved in autobiographical memory. Journal of Neuroscience. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Forgas JP. Mood and judgment: The affect infusion model (AIM) Psychological Bulletin. 1995;117:39–66. doi: 10.1037/0033-2909.117.1.39. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Galton F. Psychometric experiments. Brain. 1879;2:148–162. [Google Scholar]
- Goldman A. In defense of the simulation theory. Mind and Language. 1992;7:104–119. [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nature Neuroscience. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Smith AP, Rugg MD, Dolan RJ. Remembrance of odors past: Human olfactory cortex in cross-modal recognition memory. Neuron. 2004;42:687–695. doi: 10.1016/s0896-6273(04)00270-3. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, LaBar K. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel P. Memory for emotional and nonemotional events in depression: A question of habit? In: Reisberg D, Hertel P, editors. Memory and emotion. Oxford, England: Oxford University Press; 2004. pp. 186–216. [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999 December 24;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall PC, Howard BL, Hays RC. Self-referent speech and psychopathology: The balance of positive and negative thinking. Cognitive Therapy and Research. 1989;13:583–598. [Google Scholar]
- Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. Journal of Neuroscience. 2004;24:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Memory enhancement for emotional words: Are emotional words more vividly remembered than neutral words? Memory & Cognition. 2003;31:1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Retrieving accurate and distorted memories: Neuroimaging evidence for effects of emotion. NeuroImage. 2005;27:167–177. doi: 10.1016/j.neuroimage.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. Journal of Neuroscience. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Koriat A, Levy-Sadot R, Edry E, de Marcas S. What do we know about what we cannot remember? Accessing the semantic attributes of words that cannot be recalled. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:1095–1105. doi: 10.1037/0278-7393.29.6.1095. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nature Reviews Neuroscience. 2001;2:635–642. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Kim IJ, Alpert NM. Topographical representations of mental images in primary visual cortex. Nature. 1995 November 30;378:496–498. doi: 10.1038/378496a0. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Smith AP, Dolan RJ. Brain mechanisms for mood congruent memory facilitation. NeuroImage. 2005;25:1214–1223. doi: 10.1016/j.neuroimage.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philosophical Transactions of the Royal Society B: Biological Science. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratos EJ, Allan K, Rugg MD. Recognition memory for emotionally negative and neutral words: An ERP study. Neuropsychologia. 2000;38:1452–1465. doi: 10.1016/s0028-3932(00)00061-0. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Dolan RJ, Morris JS, Henson RNA, Rugg MD. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39:910–920. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Rugg MD. Electrophysiological correlates of the retrieval of emotional and non-emotional context. Journal of Cognitive Neuroscience. 2001;13:877–891. doi: 10.1162/089892901753165809. [DOI] [PubMed] [Google Scholar]
- Maren S, Anagnostaras SG, Fanselow MS. The startled seahorse: Is the hippocampus necessary for contextual fear conditioning? Trends in Cognitive Sciences. 1998;2:39–42. doi: 10.1016/s1364-6613(98)01123-1. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ. Differential contribution of right and left amygdala to affective information processing. Behavioural Neurology. 1998;11:233–244. doi: 10.1155/1999/180434. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Thiel A, Reinkemeier M, Kessler J, Koyuncu A, Heiss WD. Right amygdalar and temporofrontal activation during autobiographic, but not during fictitious memory retrieval. Behavioural Neurology. 2000;12:181–190. doi: 10.1155/2000/303651. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Vandekerckhove MM, Lanfermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex. 2003;39:643–665. doi: 10.1016/s0010-9452(08)70858-x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002 November 7;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968 May 3;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990 June 21;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral Neuroscience. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morris CD, Bransford JD, Franks JJ. Levels of processing versus transfer appropriate processing. Journal of Verbal Learning and Verbal Behavior. 1977;16:519–533. [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences, USA. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinion in Neurobiology. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends in Neurosciences. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000 August 17;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Habib R, McIntosh AR, Tulving E. Reactivation of encoding-related brain activity during memory retrieval. Proceedings of the National Academy of Sciences, USA. 2000;97:11120–11124. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. Journal of Experimental Psychology: General. 2000;129:242–261. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Thomas SA. A requirement for memory retrieval during and after long-term extinction learning. Proceedings of the National Academy of Sciences, USA. 2005;102:9347–9352. doi: 10.1073/pnas.0502315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Cahill L. Affective modulation of multiple memory systems. Current Opinion in Neurobiology. 2001;11:752–756. doi: 10.1016/s0959-4388(01)00280-x. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Markowitsch HJ, Fink GR. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Human Brain Mapping. 2005;24:313–324. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The amygdala: A functional analysis. Oxford, England: Oxford University Press; 2000. pp. 31–116. [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram S. Remembering and knowing: Two means of access to the personal past. Memory & Cognition. 1993;21:89–102. doi: 10.3758/bf03211168. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Retrograde amnesia for facts and events: Findings from four new cases. Journal of Neuroscience. 1998;18:3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg D, Hertel P. Memory and emotion. New York: Oxford University Press; 2005. [Google Scholar]
- Roediger HL, McDermott KB. Distortions of memory. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. Oxford, England: Oxford University Press; 2000. pp. 149–162. [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. Journal of Neuroscience. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D, Schulkind M. Distribution of important and word-cued autobiographical memories in 20-, 35-, and 70-year-old adults. Psychology and Aging. 1997;12:524–535. doi: 10.1037//0882-7974.12.3.524. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Allan K. Event-related potential studies of memory. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. Oxford, England: Oxford University Press; 2000. pp. 521–538. [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Frackowiak RSJ, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learning & Memory. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schelling G, Kilger E, Roozendaal B, deq Uervain DJ, Briegel J, Dagge A, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: A randomized study. Biological Psychiatry. 2004;55:627–633. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Psychological models of emotion. In: Borod JC, editor. The neuropsychology of emotion. New York: Oxford University Press; 2000. pp. 137–162. [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003 August 8;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Lepage M, Armony JL. A process-specific functional dissociation of the amygdala in emotional memory. Journal of Cognitive Neuroscience. 2006;18:1359–1367. doi: 10.1162/jocn.2006.18.8.1359. [DOI] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nature Neuroscience. 2004;7:1376–1380. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- Smith AP, Dolan RJ, Rugg MD. Event-related potential correlates of the retrieval of emotional and nonemotional context. Journal of Cognitive Neuroscience. 2004;16:760–775. doi: 10.1162/089892904970816. [DOI] [PubMed] [Google Scholar]
- Smith AP, Henson RN, Dolan RJ, Rugg MD. fMRI correlates of the episodic retrieval of emotional contexts. NeuroImage. 2004;22:868–878. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- Smith AP, Henson RN, Rugg MD, Dolan RJ. Modulation of retrieval processing reflects accuracy of emotional source memory. Learning & Memory. 2005;12:472–479. doi: 10.1101/lm.84305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, et al. Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences, USA. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Slater PC, Chace PM. Reactivation of recent or remote memory before electroconvulsive therapy does not produce retrograde amnesia. Behavioral Biology. 1976;18:335–343. doi: 10.1016/s0091-6773(76)92295-1. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, D’Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewalle G, et al. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. Journal of Neuroscience. 2006;26:7416–7423. doi: 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico JM, LaBar KS, Rubin DC. Emotional intensity predicts autobiographical memory experience. Memory & Cognition. 2004;32:1118–1132. doi: 10.3758/bf03196886. [DOI] [PubMed] [Google Scholar]
- Talarico JM, Rubin DC. Confidence, not consistency, characterizes flashbulb memories. Psychological Science. 2003;14:455–461. doi: 10.1111/1467-9280.02453. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Fig LM, Decker LR, Minoshima S, Koeppe RA. The effect of emotional content on visual recognition memory: A PET activation study. NeuroImage. 1998;8:188–197. doi: 10.1006/nimg.1998.0356. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Fogarty SJ. Differential effects of induced mood on retrieval of pleasant and unpleasant events from episodic memory. Journal of Abnormal Psychology. 1979;88:248–257. doi: 10.1037//0021-843x.88.3.248. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Russell ML. Differential effects of induced mood on the recall of positive, negative and neutral words. British Journal of Clinical Psychology. 1983;22(Pt 3):163–171. doi: 10.1111/j.2044-8260.1983.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Torras-Garcia M, Lelong J, Tronel S, Sara SJ. Reconsolidation after remembering an odor-reward association requires NMDA receptors. Learning & Memory. 2005;12:18–22. doi: 10.1101/lm.80905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio A. Amnesia caused by herpes simplex encephalitis, infarctions in basal forebrain, and anoxia/ischemia. In: Boller F, Grafman J, editors. Handbook of neuropsychology. 2. Vol. 2. Amsterdam: Elsevier Science; 2000. pp. 85–110. [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nature Neuroscience. 2006;9:167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychologist. 1985;26:1–12. [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proceedings of the National Academy of Sciences, USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80:352–373. [Google Scholar]
- Underwood BJ. Attributes of memory. Psychological Review. 1969;76:559–573. [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003 October 9;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Weingartner H, Miller H, Murphy DL. Mood-state-dependent retrieval of verbal associations. Journal of Abnormal Psychology. 1977;86:276–284. doi: 10.1037//0021-843x.86.3.276. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: The frontal lobes and autonoetic consciousness. Psychological Bulletin. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory’s echo: Vivid remembering reactivates sensory-specific cortex. Proceedings of the National Academy of Sciences, USA. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Scott J. Autobiographical memory in depression. Psychological Medicine. 1988;18:689–695. doi: 10.1017/s0033291700008370. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. NeuroImage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Wolpe J, Lang PJ. A fear survey schedule for use in behavior therapy. Behavioral Research and Therapy. 1964;2:27–30. doi: 10.1016/0005-7967(64)90051-8. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Uncapher MR, Rugg MD. Neural correlates of differential retrieval orientation: Sustained and item-related components. Neuropsychologia. 2006;44:3000–3010. doi: 10.1016/j.neuropsychologia.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: Convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12:323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]