Abstract
Conserved herpesviral protein kinases (CHPKs) are a group of enzymes conserved throughout all subfamilies of Herpesviridae. Members of this group are serine/threonine protein kinases that are likely to play a conserved role in viral infection by interacting with common host cellular and viral factors; however along with a conserved role, individual kinases may have unique functions in the context of viral infection in such a way that they are only partially replaceable even by close homologues. Recent studies demonstrated that CHPKs are crucial for viral infection and suggested their involvement in regulation of numerous processes at various infection steps (primary infection, nuclear egress, tegumentation), although the mechanisms of this regulation remain unknown. Notwithstanding, recent advances in discovery of new CHPK targets, and studies of CHPK knockout phenotypes have raised their attractiveness as targets for antiviral therapy. A number of compounds have been shown to inhibit the activity of human cytomegalovirus (HCMV)-encoded UL97 protein kinase and exhibit a pronounced antiviral effect, although the same compounds are inactive against Epstein-Barr Virus (EBV)-encoded protein kinase BGLF4, illustrating the fact that low homology between the members of this group complicates development of compounds targeting the whole group, and suggesting that individualized, structure-based inhibitor design will be more effective. Determination of CHPK structures will greatly facilitate this task.
Keywords: herpesviruses, protein kinases, serine/threonine kinases, protein kinase inhibitors, antivirals compounds
1. INTRODUCTION
Herpesviruses are among the most persistent of all pathogens owing to the fact that they co-evolved with their hosts for a long period of time and are relatively harmless in immunocompetent hosts. As opportunistic agents, herpesviruses [in particular Epstein-Barr Virus (EBV), Kaposi Sarcoma Herpesvirus (KSHV) and human cytomegalovirus (HCMV)] cause severe diseases with significant morbidity and mortality in immunocompromised patients. The population of such patients grows steadily due to AIDS, organ transplantation, cancer, and aging. Since the successful 1982 introduction of the anti-herpetic agent acyclovir, a number of anti-herpesvirus drugs have been and are currently approved [1]. However, most of these compounds, either directly or indirectly, target viral DNA polymerase (reviewed in [2]) and prolonged use of these antivirals is limited due to emergence of resistant (and cross-resistant) mutants as well as severe side effects. Hence, the combination of these factors emphasizes the need for improved therapies to treat herpesviruses-related complications, particularly by exploring new viral targets. Conserved herpesvirus-encoded protein kinases (CHPKs) are an attractive target for antiviral therapy due to (i) their role in viral infection, and (ii) their uniqueness, that is, very low sequence homology with cellular counterparts, and even among themselves, may allow development of highly selective inhibitors. In addition, development of inhibitors of protein kinases involved in the life cycle of a virus is not only of potential therapeutic interest, but can also furnish useful information about the pathways of viral gene expression and replication.
2. HERPESVIRUSES
Herpesviruses are enveloped viruses with virion size over 100 nm. The linear double-stranded DNA genomes (125 – 240 kb) encode up to 200 open reading frames, including a repertoire of enzymes for viral DNA replication as well as protein modifications (such as phosphorylation). DNA replication and capsid assembly occur in the nucleus. All members of Herpesviridae have a biphasic infection cycle consisting of latent and replicative (lytic) phases. Latency is characterized by limited gene expression, lack of virion production, and, in the case of gammaherpesviruses, is associated with immortalization and transformation of infected cells. In contrast, most of viral genes are expressed in a cascade manner during the lytic cycle, including viral reactivation, and large numbers of infectious virus particles are released [3]. Primary infection results in lifelong persistence of the virus in the host. Eight herpesviruses infect humans: alphaherpesviruses - herpes simplex virus 1 and 2 (HSV-1 and -2), and varicella-zoster virus (VZV); betaherpesviruses - human cytomegalovirus (HCMV) and human herpesviruses 6 and 7 (HHV-6 and -7); and gammaherpesviruses - Epstein-Barr virus (EBV) and Kaposi Sarcoma associated herpesvirus (KSHV). The differences between different subfamilies include host range, type of cells they are able to infect, and the length of the replication cycle.
3. CONSERVED HERPESVIRUS PROTEIN KINASES
All avian and mammalian herpesviruses encode protein kinases. A subset of these enzymes, exemplified by the HSV UL13 gene product, is conserved throughout all subfamilies of Herpesviridae [4, 5] and will be referred to as conserved herpesvirus protein kinases (CHPKs). HSV UL13 homologues encoded by other human herpesviruses include: VZV ORF47 [6], EBV BGLF4 [7, 8], HCMV UL97 [9-11], HHV-6 U69 [12], and KSHV ORF36 [13]. Putative substrates of CHPKs identified to date are summarized in Table 1, and characteristics of the individual CHPKs, as well as their common features, are discussed below.
Table 1.
Viral and cellular targets of herpesvirus-encoded protein kinases
| CHPK§ | Substrates | |
|---|---|---|
| Viral | Cellular | |
| HSV-1 UL13 |
gE/gI [118], ICP0 [119], ICP22/Us1.5 [120], VP22 [15], US3 [18] |
CKIIß [45], EF-1δ [88], p60 [121], RNA Pol II [122] |
| VZV ORF47 | gE [26], ORF32 [28], ORF62 [25], ORF63 [23], ORF9 [27] |
|
| HCMV UL97*/** |
UL44 [41, 42] | EF-1δ [87], p32 and lamin A/C [47] |
| HHV-6 U69* | U69 [12] | |
| EBV BGLF4* |
EA-D [7, 8], EBNA-LP [66], Z [65], EBNA2 [67], BZLF1 [65] |
CKIIß [45], EF-1δ [69], condensin [63], MCM4 [64] |
| KSHV ORF36* |
K-bZIP [79] | JNK [80] |
3.1. Herpes simplex virus UL13
Studies of HSV mutants, in which UL13 is deleted, yielded conflicting data. While earlier studies demonstrated dispensability of UL13 for viral replication (at least in cell culture) [14, 15], later studies claimed that such mutants exhibit impaired replication in a cell type-dependent manner [16], with reduced expression levels of immediate-early ICP0 protein and a subset of late proteins, including UL26, UL26.5, UL38, UL41 and US11 [17]. A number of viral and cellular UL13 putative targets have been identified (Table 1); however, the biological significance of UL13-mediated phosphorylation remains unclear. Kato et al. [18] recently suggested UL13 may be involved in regulation of nuclear egress, based on the fact that another HSV protein kinase, US3, which regulates nuclear egress of alphaherpesviruses [19, 20], is a physiological substrate for UL13, and that UL13 deletion resulted in aberrant localization of HSV egress factors UL31 and UL34 [18]. The UL13 protein has also been implicated in promoting tegument dissociation [21].
Daikoku et al. [22] purified HSV-2 UL13 from infected cells. The kinase activity of the purified protein was optimal at pH-9.0 and in the absence of NaCl. Casein, phosvitin, and, to some extent, histone, but not protamine, were efficiently phosphorylated. Phosphoamino acid analysis of phosphorylated casein revealed that UL13 phosphorylates serine and threonine residues, but not tyrosine. The UL13 kinase activity was resistant to treatment with heparin and CK I-7, inhibitors of protein kinases CK2 and CK1 respectively, but sensitive to the bioflavonoid quercetin.
3.2. Varicella-Zoster virus ORF47
VZV ORF47 protein is related to HSV UL13 [5]. The ORF47 protein autophosphorylates and phosphorylates the major immediate-early transactivator, IE62 [23-25], as well as IE63 [23], gE [26], ORF9 [27], and ORF32 proteins [28]. VZV gE is essential for replication [29] and requires ORF47 phosphorylation to mediate cell fusion and TGN trafficking for virion assembly [26]. The ORF47 protein is dispensable for VZV replication in vitro, as shown in studies of ROka47S, a recombinant virus described by Heineman and Cohen [30], in which ORF47 transcription was blocked by a stop codon mutation. In contrast, the ORF47 protein is essential for VZV infection of differentiated human T-cells, skin xenografts in the SCID-hu model of VZV infection in vivo [31], and immature dendritic cells [32]. Rahaus et al. [33] suggested that both VZV ORF47 and ORF66 protein kinases play key roles in activating the PI3/Akt/GSK-3α/β pathway, which plays an essential role in viral infection.
In vitro activity of ORF47 has been studied with the protein immunoprecipitated from VZV-infected cells [6], or recombinant protein expressed in mammalian cells [23]. It preferentially phosphorylates serine residues, utilizing both ATP and GTP as phosphate donors, and its activity is optimal in the presence of Mn2+ (rather than Mg2+) and 50 – 250 mM KCl [6]. Kenyon et al. [23] reported up to an 18-fold increase in autophosphorylation of ORF47 in the presence of polyamines. Conflicting data are reported as to whether ORF47 contributes to nucleoside analogue metabolism: Koyano et al. [34] studied growth inhibition by nucleoside analogues of COS cells expressing VZV-TK or ORF47, and their results suggested that ACV and BV-araU were phosphorylated by TK only, whereas GCV, OXT-G and cOXT-G were phosphorylated by both TK and ORF47 (or by cellular factors activated by ORF47). In contrast, Suzutani et al. [35], studying sensitivity of viruses deficient in TK or ORF47 genes (or combination of both) to anti-herpesvirus nucleoside analogues, showed no changes in sensitivity in the absence of ORF47 and suggested that this kinase is not involved in phosphorylation of the tested compounds.
3.3. Human cytomegalovirus UL97
HCMV UL97 is probably the most widely studied CHPK, both in the biochemical sense and in the context of viral infection. UL97 is the only protein in the CHPK group for which the nuclear localization signal has been identified [36], and it is also a component of the tegument in mature virions [37, 38]. Studies of HCMV mutants lacking most of the UL97 gene demonstrated their severe replication deficiency in fibroblast infection, and thus confirmed the importance of the UL97 gene product for viral replication [39]. Replication of these mutants could be partially restored by expression of rat cytomegalovirus (RCMV) UL97 protein or EBV BGLF4, but not by HSV UL13 [40]. The involvement of UL97 was implicated in regulation of viral DNA synthesis [41-43], modification of cellular transcriptional and translational factors [44, 45], and viral nuclear egress [43, 46, 47]. The connection between UL97 and viral DNA synthesis is thought to occur through its interaction with, and phosphorylation of, the HCMV DNA polymerase processivity factor UL44 [41, 42], and the region responsible for this interaction has been mapped between aa 366 and aa 459 [42]. Both proteins co-localized in the viral replication centers, and treatment with inhibitors of viral DNA synthesis (cidofovir, CDV) or UL97 activity (NGIC-I and Gö6976) prevented this co-localization. However, as in the case of EBV BGLF4 and EA-D [8, 48], no direct evidence has been obtained yet to connect the phosphorylation status of UL44 with its functions in viral DNA replication. UL97 has also been reported to phosphorylate elongation factor 1 delta [45] and RNA polymerase II carboxyl-terminal domain [44], but again, the physiological relevance of these modifications remains unclear. The best-substantiated claim connects UL97 with viral nuclear egress. Three research groups have independently presented evidence for accumulation of viral capsids in the nucleus in the absence of UL97 protein [43, 46] and started to uncover the mechanism by which UL97, through interaction with cellular p32 protein, destabilizes nuclear lamina [47]. Recently, UL97 has been implicated in regulation of tegumentation, since its deletion resulted in aberrant aggregation of tegument proteins such as pp65 and ppUL25 [49], and changed subcellular distribution of the viral structural protein assembly sites [50].
Biochemical studies have been reported with UL97 protein expressed in heterologous systems, followed by affinity purification or immunoprecipitation. These studies demonstrated the ability of UL97 to autophosphorylate [11] and to phosphorylate certain exogenous protein substrates [51, 52] as well as two nucleoside homologues, GCV and ACV [53], although UL97 does not show homology with known nucleoside kinases [36]. Phosphorylation occurred in the presence of either Mg2+ or Mn2+, and both ATP and GTP could be used as phosphate donors in protein phosphorylation. Unusually, the optimal activity of pUL97 was observed at 1.5M NaCl and pH-9.5 [11]. Baek et al., mapped the autophosphorylation sites within the UL97 N-terminal region and demonstrated the importance of specific amino acids (arginine or lysine) in the P+5 position from the phosphorylated residue for the efficiency of histone 2B phosphorylation. The authors speculated that HCMV UL97 might regulate gene expression by histone phosphorylation and chromatin condensation [52]. The relation between UL97 autophosphorylation and efficient phosphorylation of exogenous substrates (both protein and GCV) remains unclear, with a number of conflicting reports that either support the necessity of autophosphorylation for allophosphorylation activity [42, 54] or describe autophosphorylation mutants that retain high levels of allophosphorylation activity [51].
3.4. Human herpesvirus 6 U69
HHV-6-encoded U69 is a homologue of HCMV UL97 [55]. The purified protein autophosphorylates and phosphorylates casein and histone (substrates that typically phosphorylated by serine/threonine protein kinases), but not endolase (a typical substrate for tyrosine protein kinases). Phosphorylation occurs predominantly on serine residues, with both ATP and GTP utilized as phosphate donors. The reaction requires both Mg2+ and Mn2+, and reaches optimal kinetics at physiological pH and low NaCl concentration. In the same study, U69-expressing baculoviruses exhibited higher susceptibility to GCV in plaque reduction experiments [12] suggesting that its product is involved in GCV phosphorylation. These data were further confirmed by GCV metabolic studies in a recombinant vaccinia virus system in which both U69 and UL97 were expressed. Analyses of metabolites showed increased GCV phosphorylation in the presence of both proteins, but the level of this phosphorylation was approximately 10-fold lower in cells expressing U69 [56]. Nevertheless, U69 seemed to play a role in GCV phosphorylation, since GCV-resistant HHV-6 strains carry an M318V substitution in U69 [57, 58], which corresponds to M460I and M460V substitutions in UL97, one of the most frequently observed mutations conferring GCV resistance in clinical HCMV isolates.
Similar to other CHPKs, HHV-6 U69 localized in nucleus [56], its importance for the viral infection as well as physiologically relevant targets have never been studied. Based on homology with UL97, one can predict that U69 will be crucial for viral infection, but this needs to be verified experimentally.
3.5. Epstein-Barr Virus BGLF4
EBV BGLF4 protein exhibits early expression kinetics with high levels throughout the EBV lytic phase [59]. It is detected mainly in the nuclei of EBV-infected cells [59, 60]. The NLS, although not clearly defined, is localized on the C-terminus of the protein [59]. We have recently reported [61] that the EBV mutant phenotype, in which BGLF4 protein level was knocked down by RNAi, exhibited properties similar to a HCMV UL97 knockout mutant [39], in particular by retention of nucleocapsids in the nucleus. Moreover, we have shown that the knockdown of BGLF4 abolished the expression of BFLF2 (a homologue of HSV UL31), a viral factor that is directly involved in nuclear egress [62] thus demonstrating that BGLF4 is involved in regulation of nuclear egress. Lee et al. [63] reported that transient expression of EBV BGLF4 protein induces unscheduled chromosome condensation, nuclear lamina disassembly, and stress fiber rearrangements, independently of cellular DNA replication and cyclin-dependent protein kinase 2 activity. BGLF4 interacts with condensin complexes, the major components in mitotic chromosome assembly, and induces condensin phosphorylation at CDK1 consensus motifs. BGLF4 also stimulates the decatenation activity of topoisomerase II, suggesting that it may induce chromosome condensation through condensin and topoisomerase II activation. The authors speculate that gammaherpesvirus kinases may induce multiple premature mitotic events to provide more extrachromosomal space for viral DNA replication and successful egress of nucleocapsid from the nucleus. Finally, BGLF4 has been reported to phosphorylate components of the cellular replication origin binding MCM4-MCM6-MCM7 complex [64], and, since such a phosphorylation inhibits helicase activity of MCM4, Kudoh et al. hypothesize that BGLF4 may play a role in blocking cellular DNA replication during EBV lytic infection. The EBV BGLF4 protein is a part of the tegument [60, 65] and has been shown to dissociate from it in a phosphorylation-dependent manner [65]. Although it phosphorylates a number of viral and cellular targets (Table 1), the biological relevance of EBV-BGLF4-mediated phosphorylation (except for its part in viral nuclear egress) remains hypothetical, and even when this phosphorylation has been linked to reduction of transcriptional activity for EBNA2 and EBNA-LP [66, 67], its consequences in the context of viral infection have not been explored.
Recombinant EBV BGLF4 has been expressed in insect cells [68, 69]. The purified protein autophosphorylates and phosphorylates histone and myelin basic protein [68, 69], utilizes both ATP and GTP as phosphate donors, and requires both Mn2+ and Mg2+, pH-7.4 and 300 mM KCl for optimal activity [7].
In contrast to betaherpesviruses, gammaherpesviruses encode functional thymidine kinase (TK) [70-72]. When expressed in EBV-negative cells, EBV TK conferred moderate sensitivity to GCV, BVDU, ACV and PCV; in contrast, BGLF4 conferred sensitivity to GCV only [73]. Neither has been shown to directly phosphorylate GCV in vitro ([74] and Gershburg and Pagano, unpublished data).
3.6. Kaposi Sarcoma-associated herpesvirus (KSHV) ORF36
KSHV ORF36 protein is a serine protein kinase that is localized in the nucleus [13]. In vitro protein kinase assays indicated that this viral protein is autophosphorylated and that the lysine residue in the catalytic kinase subdomain II was essential for enzymatic activity [13]. ORF36 is transcribed as two polycistronic transcripts that are initiated from promoters that are active in the early stage of the viral life cycle and inducible by hypoxic conditions [75]. Their kinetics have been reported both as late [76, 77] or early/early-late as verified by the treatment with CDV, an inhibitor of viral DNA synthesis [78]. ORF36 was found in replication/transcription complexes in infected cells and packaged in mature virions [79]. The ORF36 protein has been shown to phosphorylate components of the c-Jun N-terminal protein kinase signal transduction pathway, which in turn activated c-Jun in the activating protein 1 transcription complex [80]. Recently, Izumiya et al. [79] showed that ORF36 interacts with and phosphorylates the transcriptional regulator K-bZIP at Thr111, and both proteins are recruited to selected viral promoters as well as the Ori-Lyt region. This threonine residue of K-bZIP is also the target of the cyclin-dependent kinase CDK1. K-bZIP activity is also modified by sumoylation [81], and phosphorylation at Thr111 seem to change levels of sumoylation. The authors of the study propose a model whereby ORF36 switches K-bZIP from being a strong repressor of K-Rta, which targets immediate-early genes, to a transactivator that synergizes with K-Rta to activate early and late viral gene expression [79]. Coincidently, EBV BZLF1 gene product, the homologue of K-bZIP, is also modified by sumoylation [82] and phosphorylation [83-85]; however, the significance of sumoylation of Zta on EBV transcription or of an interplay between the sumoylation and phosphorylation remain to be studied.
In vitro kinase activity of the KSHV ORF36 has been studied on partially purified GST-fused protein expressed in mammalian cells. The kinase autophosphorylates on serine residues and prefers Mn2+ over Mg2+ at pH-7.5 and no salt [13].
Similar to EBV, KSHV encodes a thymidine kinase, ORF21 [74], and both ORF21 and ORF36 have been shown to confer sensitivity to GCV [86] with ORF36 being more efficient. In contrast to EBV [73], both proteins did not confer significant sensitivity to PCV and BVDU [86].
3.7. Common features and biological role(s) of CHPKs
CHPKs were identified based on the motifs diagnostic of conserved regions within the catalytic domains of protein kinases [4, 5]. Noteworthy are that the sequences of CHPKs encoded by beta- and gammaherpesviruses diverge from those of cellular counterparts sufficiently to raise the question of whether they are in fact protein kinases [4]. Nevertheless, despite low sequence homology within the group [40], CHPKs represent a group of protein kinases with some distinct common features and potentially conserved biological role(s) in viral infection. First, the CHPKs are commonly packaged into virions as component of the tegument [21, 37, 38, 65] implying their involvement in the formation, maintenance, and/or disassembly of virion structures through the phosphorylation of tegument components [21, 27, 65, 79]. Second, the CHPKs localize in the nuclei of infected cells [36, 56, 59, 60], which, along with shown interaction with the viral DNA Pol processivity factor [7, 8, 13, 36, 41, 42, 48, 79], indicates their involvement in viral DNA replication. Third, all CHPKs autophosphorylate and phosphorylate a common substrate - cellular translation factor EF-1δ [45, 69, 87, 88]. An interesting feature of the interaction between UL13 homologues and EF-1δ is that both cellular protein kinase cdc2 and UL13 homologues phosphorylate the same EF-1δ amino acid residue [45]. These observations suggest that UL13 homologues may share a function that mimics the cellular cdc2 protein kinase [89]. Fourth, in at least two studies CHPKs were able to complement each other functionally: HCMV UL97 partly substituted for HSV UL13 [90], and EBV BGLF4 complemented the replication of delta-UL97 HCMV [40]. Importantly, this complementation is not always reciprocal - HSV UL13 was unable to complement HCMV UL97 [40]. This phenomenon could be explained by the fact that alphaherpesviruses encode an additional protein kinase (US3 gene product homologues) and therefore the two protein kinases carry out different biological functions, whereas beta- and gammaherpesviruses encode only one multifunctional protein kinase. In fact, beta- and gammaherpesviruses-encoded protein kinases may represent dual-specificity kinases, which are able to phosphorylate both protein and certain nucleoside targets. The latter assertion has only been verified for purified UL97, which phosphorylated both GCV and ACV in vitro [53], although other CHPKs conferred sensitivity to GCV when expressed recombinantly [9, 10, 12, 56-58, 73, 86, 91, 92] or when their expression is induced in infected cells [93]. Otherwise, the ability of CHPKs to confer sensitivity to certain nucleotides could be explained by induction of cellular enzymes that metabolize nucleosides; however this hypothesis has never been tested. Fifth, CHPKs can utilize both ATP and GTP as a phosphate donor and prefer Mn2+ to Mg2+ for optimal activity; salt concentration and pH vary.
Romacker et al. [40] recently reported structural studies of several CHPKs using a molecular modeling approach with cellular Cdk2 as a template. The modeling was possible for HCMV UL97 and RCMV R97, but not for HSV UL13 and EBV BGLF4. Moreover, the authors acknowledged that the accuracy of the resulting models is limited by low sequence homology between target and template and indicate that the modeling was rather intended to confirm overall fold than to address structural details [40]. Hence, the conclusions of this report reiterate the need for experimental determination of CHPKs structure.
4. CHPK inhibitors and their antiviral activities
With recognizing CHPKs as potential targets for antiviral drug development [94], a number of compounds that exhibit both anti-CHPK and antiviral activity have been identified.
4.1. Maribavir
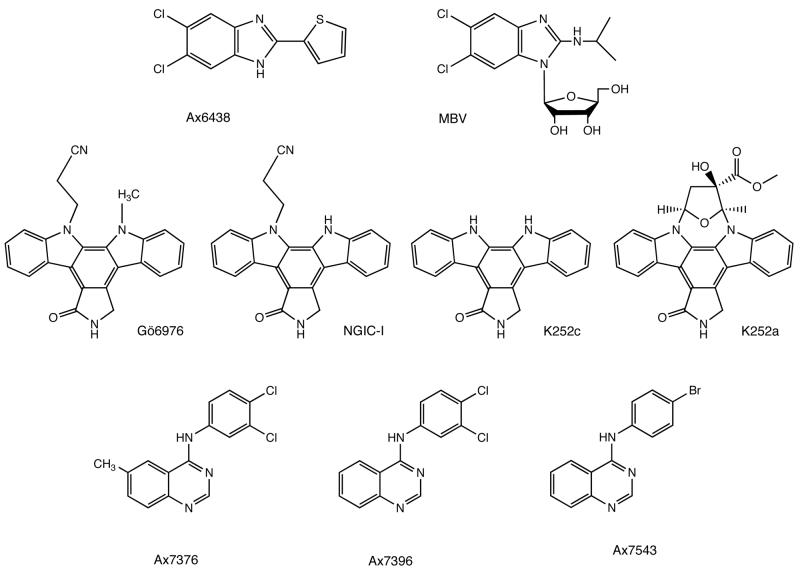
5,6-dichloro-2-(isopropylamino)-1,ß-l-ribofuranosyl-1-H-benzimidazole (1263W94 or maribavir, MBV) [95, 96] (Fig. 1) is a potent and selective inhibitor of HCMV and EBV replication [97-99], but is inactive against HSV-1 and -2, VZV, HHV-6 and -7, and KSHV [98]. MBV showed significant antiviral potency in vitro against different HCMV strains, including strains resistant to GCV (mutations at 460, 520, 594 in pUL97), foscarnet (Thr700Ala in the viral polymerase) and BDCRB (Asp344Glu and Ala355Val in pUL89) [97, 100, 101]. MBV inhibited viral DNA synthesis, however this effect was not mediated by inhibition of the viral DNA polymerase, but rather by a novel mechanism. Several lines of evidence imply that UL97 protein kinase is a target for MBV. First, MBV-resistance of HCMV has been mapped in UL97 at amino acid positions L397R [97], V353A and T409M [102]; all three mutations located upstream of UL97 mutations linked to GCV resistance, closer to kinase domains that are associated with ATP-binding and phosphotransfer. Second, MBV treatment exhibited a phenotype similar to the UL97-knockout [46]. Third, MBV inhibited UL97 kinase activity in vitro [52]. However, several MBV-resistant HCMV strains were isolated recently [103, 104] that carry mutations in pUL27, whereas the UL97 gene remained intact. Since EBV is also inhibited by MBV, we have tested the effects of the compound on EBV BGLF4 and found that the kinase was absolutely insensitive to MBV treatment both in vitro [68] and in cell culture [8]. Therefore, the mechanism of action of MBV remains elusive. Despite that, MBV is the only compound with potential connections to CHPKs that is currently in clinical development by ViroPharma for the prevention of cytomegalovirus (CMV) infections in transplant patients. It has been tested in several phase I/II studies by both GlaxoSmithKline and ViroPharma, in which the drug demonstrated antiviral activity, oral bioavailability and an acceptable safety and tolerability profile [105-109]. A phase III study with MBV as a prophylactic agent in CMV seropositive patients undergoing allogeneic stem cell transplants has been initiated, and another phase III trial in solid organ transplant patients is planned. MBV was granted fast-track status by the US FDA in February 2006 for the prevention of CMV infection in allogeneic bone marrow and solid organ transplant patients, and received orphan drug status in the US for the prevention of CMV viraemia and disease in at-risk populations. The patents covering MBV are held by GlaxoSmithKline and expire in 2015.
Figure 1.
Anti-CHPK inhibitors: benzimidazoles – Ax6438 and MBV; indolocarbazoles - Gö6976, NGIC-I, K252C and K252A; quinozolines – Ax7376, Ax7396 and Ax7543. All listed compounds inhibited HCMV UL97 protein kinase both in vitro and in cell culture [91, 111-113, 115]; K252A also inhibited EBV BGLF4 protein kinase in vitro [68].
4.2. Indolocarbazoles
Slater et al. [110] showed the inhibitory effect of Arcyriaflavin A as well as its synthetic analogues (symmetrical indolocarbazoles) on HCMV infection in cultured cells. In follow-up studies Zimmermann et al. [111] and Marschall et al. [91, 112] attributed this inhibitory effect to direct targeting and inhibition of the pUL97 kinase activity. The compounds were effective against GCV-sensitive and -resistant HCMV strains, and selected compounds in nanomolar concentrations could reduce virus yield by three orders of magnitude [111]. Marschall et al. [112] also showed that the indolocarbazoles Gö6976 and NGIC-I (Fig. 1) do block the kinase activity in vitro. Reasoning that UL97 inhibitors may be efficient on EBV BGLF4, we have studied inhibitory effects of selected indolocarbazoles in vitro and in cell culture [68]. To our surprise, only one compound, K252a (Fig. 1), inhibited BGLF4 autophosphorylation in vitro; importantly both MBV and NGIC-I, which failed to inhibit BGLF4 activity, efficiently inhibited viral DNA replication at non-toxic concentrations [68]. These results illustrate a potential problem with development of inhibitors targeting CHPKs as a group. Low sequence homology, which likely translates into marked differences in structure, and makes the development of such inhibitors difficult if not impossible.
4.3. Quinazolines
In a recent report, Herget et al. [113] described a class of quinazolines as novel pUL97 inhibitors and provided evidence that selected quinazolines qualify for use in the development of anti-HCMV drugs: (i) the quinazolines selected for this study (Ax 7376, Ax 7396, Ax 7543) (Fig. 1) were highly potent and selective inhibitors of UL97 protein kinase activity in vitro (of 17 protein kinases tested, only the UL97 and EGFR protein kinases were efficiently inhibited); (ii) they significantly reduced sensitivity of UL97-expressing cells to GCV; (iii) the kinetics of HCMV inhibition and failure to inhibit replication of an HCMV mutant from which UL97 was deleted argue that pUL97 is the target responsible for the anti-HCMV activities of the quinazolines; (iv) clinical isolates of HCMV possess a quinazoline-sensitive phenotype even after they have acquired GCV and CDV resistance-conferring mutations in the UL97 gene; (v) the emergence of viral resistance to quinazolines was not observed at the frequency of resistance to GCV, as analyzed in long-term treatment experiments. It is noteworthy that the quinazolines selected for this study are structurally related to the drug gefitinib (Iressa; ZD1839), a well-characterized inhibitor of EGFR kinase approved for therapy of non-small cell lung cancer [114].
4.4. Anti-UL97/anti-HCMV high-throughput screening
Mett et al. [115] screened a library of 5000 compounds deduced from known protein kinase inhibitors and covering 60 different scaffolds. The compounds were tested for their ability to inhibit protein kinase activity of the purified GST-UL97 in vitro, whereas their cytotoxicity and capability to reduce UL97-mediated GCV toxicity were measured by the in-cell activity assays [112]. The study identified 93 compounds that were nontoxic, GCV-protective UL97 inhibitors, and antiviral effects of 26 compounds from this group were quantified in HCMV/GFP-based infection and plaque reduction assays. Seventeen compounds out of 26 tested demonstrated pronounced antiviral effects; some of them, such as benzimidazole Ax 6438 (Fig. 1) or quinazolines (described above), may serve as promising leads for further drug development.
5. CONCLUSIONS AND PERSPECTIVES
Conserved herpesvirus-encoded protein kinases are crucial for viral infection and represent attractive and novel targets for antiviral therapy. Although the biological significance of CHPKs is unquestioned, and a considerable number of putative substrates of CHPKs have been identified (Table 1), the biological relevance of their phosphorylation remains obscure. Studies of alphaherpesvirus protein kinases are complicated by the fact that these viruses encode at least one more protein kinase [116] and another protein with potential kinase activity [117]. Therefore early studies with mutant viruses lacking CHPK corresponding genes suggested their dispensability for viral infection [14, 15]. In contrast, studies of both beta- and gammaherpesviruses demonstrated the severe deficiency of such mutants [39, 61] and involvement of CHPKs in various steps of viral infection. The analyses are even more complicated due to the high efficiency of viral genomes, that is, their overlapping and bi-directional genes and transcriptional elements. Therefore, deletion of large fragments may unintendedly result in disruption of more than one viral function. One can envision two ways to address this issue: design of finer, point mutants that will affect kinase activity only - for instance, substitution of an invariable lysine in CHPKs subdomain II abolishes kinase activity and can be used to study the roles of CHPK-mediated phosphorylation in viral infection (such as in [16]); alternatively, development of CHPK-selective inhibitors, which, in addition to their therapeutic potential, will serve as tools in future studies of these protein kinases. The latter approach however requires extensive knowledge of enzyme structure and will greatly benefit from additional studies aimed to determine experimentally structures of CHPKs. Therefore future studies are warranted (i) to verify biologically relevant CHPK targets, (ii) to determine the structure of CHPKs, and (iii) to develop selective CHPK inhibitors, which may lead to effective antiviral drugs.
ACKNOWLEDGEMENTS
The authors thank Dr. David Shugar for helpful discussions. This work is supported by a research grant HL064851 from National Institutes of Health.
ABBREVIATIONS
- CHPK
conserved herpesviral protein kinase
- HSV
herpes simplex virus
- VZV
varicellazoster virus
- HCMV
human cytomegalovirus
- EBV
Epstein-Barr virus
- KSHV
Kaposi sarcoma associated virus
- HHV
human herpesvirus
- AIDS
acquired immunodeficiency syndrom
- ICP0
infected-cell protein 0
- SCID
Severe Combined Immunodeficiency
- GCV
ganciclovir (2-amino-9-(1,3-dihydroxypropan-2-yloxymethyl)-3H-purin-6-one)
- OXT-G
oxetanocin G (2-amino-9-[(2R,3R,4R)-3,4-bis(hydroxymethyl)oxetan-2-yl]-3H-purin-6-one)
- ACV
acyclovir (2-amino-9-(2-hydroxyethoxymethyl)-3H-purin-6-one)
- BV-araU
bravavir (5-[(E)-2-bromoethenyl]-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl] pyrimidine-2,4-dione)
- CDV
cidofovir ([(2S)-1-(4-amino-2-oxo-pyrimidin-1-yl)-3-hydroxypropan-2-yl]oxymethylphosphonic acid)
- BVDU
brivudin (5-[(E)-2-bromoethenyl]-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione)
- PCV
penciclovir (2-amino-9-[4-hydroxy-3-(hydroxymethyl)butyl]-3H-purin-6-one)
- MBV
maribavir ((2S)-2-[5,6-dichloro-2-(propan-2-ylamino)benzoimidazol-1-yl]-5-(hydroxymethyl)oxolane-3,4-diol)
- NLS
nuclear localization signal
- RNAi
RNA interference
- EBNA2
Epstein-Barr nuclear antigen 2
- EBNA-LP
Epstein-Barr nuclear antigen leader peptide
- GST
Glutathione-S-transferase
- BDCRB
(2R,3R,4S,5R)-2-(2-bromo-5,6-dichlorobenzoimidazol-1-yl)-5-(hydroxymethyl)oxolane-3,4-diol
- CDK1
cyclin-dependent kinase 1
- JNK
c-Jun N-terminal kinase
- MCM
minichromosome maintenance proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.De Clercq E, Brancale A, Hodge AV, Field HJ. Antiviral Chemistry & Chemotherapy's current antiviral agents FactFile 2006 (1st edition) Antivir Chem Chemother. 2006;17:113–166. doi: 10.1177/095632020601700302. [DOI] [PubMed] [Google Scholar]
- 2.De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Pellett PE, Roizman B. In: Fields Virology. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, editors. Lippincott Williams & Wilkins; 2007. pp. 2479–2500. [Google Scholar]
- 4.Chee MS, Lawrence GL, Barrell BG. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989;70(Pt 5):1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- 5.Smith RF, Smith TF. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J Virol. 1989;63:450–455. doi: 10.1128/jvi.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng TI, Grose C. Serine protein kinase associated with varicella-zoster virus ORF 47. Virology. 1992;191:9–18. doi: 10.1016/0042-6822(92)90161-h. [DOI] [PubMed] [Google Scholar]
- 7.Chen MR, Chang SJ, Huang H, Chen JY. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EAD in vitro. J Virol. 2000;74:3093–3104. doi: 10.1128/jvi.74.7.3093-3104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershburg E, Pagano JS. Phosphorylation of the Epstein-Barr virus (EBV) DNA polymerase processivity factor EA-D by the EBV-encoded protein kinase and effects of the L-riboside benzimidazole 1263W94. J Virol. 2002;76:998–1003. doi: 10.1128/JVI.76.3.998-1003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Littler E, Stuart AD, Chee MS. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan V, Talarico CL, Stanat SC, Davis M, Coen DM, Biron KK. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;358:162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- 11.He Z, He YS, Kim Y, Chu L, Ohmstede C, Biron KK, Coen DM. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J Virol. 1997;71:405–411. doi: 10.1128/jvi.71.1.405-411.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansari A, Emery VC. The U69 gene of human herpesvirus 6 encodes a protein kinase which can confer ganciclovir sensitivity to baculoviruses. J Virol. 1999;73:3284–3291. doi: 10.1128/jvi.73.4.3284-3291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Lee D, Seo T, Chung J, Choe J. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) open reading frame 36 protein is a serine protein kinase. J Gen Virol. 2000;81:1067–1071. doi: 10.1099/0022-1317-81-4-1067. [DOI] [PubMed] [Google Scholar]
- 14.de Wind N, Domen J, Berns A. Herpesviruses encode an unusual protein-serine/threonine kinase which is nonessential for growth in cultured cells. J Virol. 1992;66:5200–5209. doi: 10.1128/jvi.66.9.5200-5209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulter LJ, Moss HW, Lang J, McGeoch DJ. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J Gen Virol. 1993;74:387–395. doi: 10.1099/0022-1317-74-3-387. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Nishiyama Y, Sata T, Kawaguchi Y. The role of protein kinase activity expressed by the UL13 gene of herpes simplex virus 1: the activity is not essential for optimal expression of UL41 and ICP0. Virology. 2005;341:301–312. doi: 10.1016/j.virol.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Purves FC, Ogle WO, Roizman B. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc Natl Acad Sci U S A. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato A, Yamamoto M, Ohno T, Tanaka M, Sata T, Nishiyama Y, Kawaguchi Y. Herpes simplex virus 1-encoded protein kinase UL13 phosphorylates viral Us3 protein kinase and regulates nuclear localization of viral envelopment factors UL34 and UL31. J Virol. 2006;80:1476–1486. doi: 10.1128/JVI.80.3.1476-1486.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagenaar F, Pol JM, Peeters B, Gielkens AL, de Wind N, Kimman TG. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J Gen Virol. 1995;76(Pt 7):1851–1859. doi: 10.1099/0022-1317-76-7-1851. [DOI] [PubMed] [Google Scholar]
- 20.Klupp BG, Granzow H, Mettenleiter TC. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J Gen Virol. 2001;82:2363–2371. doi: 10.1099/0022-1317-82-10-2363. [DOI] [PubMed] [Google Scholar]
- 21.Morrison EE, Wang YF, Meredith DM. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J Virol. 1998;72:7108–7114. doi: 10.1128/jvi.72.9.7108-7114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daikoku T, Shibata S, Goshima F, Oshima S, Tsurumi T, Yamada H, Yamashita Y, Nishiyama Y. Purification and characterization of the protein kinase encoded by the UL13 gene of herpes simplex virus type 2. Virology. 1997;235:82–93. doi: 10.1006/viro.1997.8653. [DOI] [PubMed] [Google Scholar]
- 23.Kenyon TK, Lynch J, Hay J, Ruyechan W, Grose C. Varicella-zoster virus ORF47 protein serine kinase: characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J Virol. 2001;75:8854–8858. doi: 10.1128/JVI.75.18.8854-8858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinchington PR, Fite K, Turse SE. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J Virol. 2000;74:2265–2277. doi: 10.1128/jvi.74.5.2265-2277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng TI, Keenan L, Kinchington PR, Grose C. Phosphorylation of varicellazoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J Virol. 1994;68:1350–1359. doi: 10.1128/jvi.68.3.1350-1359.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenyon TK, Cohen JI, Grose C. Phosphorylation by the Varicella-Zoster Virus ORF47 Protein Serine Kinase Determines whether Endocytosed Viral gE Traffics to the trans-Golgi Network or Recycles to the Cell Membrane. J Virol. 2002;76:10980–10993. doi: 10.1128/JVI.76.21.10980-10993.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spengler M, Niesen N, Grose C, Ruyechan WT, Hay J. Interactions among structural proteins of varicella zoster virus. Arch Virol Suppl. 2001:71–79. doi: 10.1007/978-3-7091-6259-0_8. [DOI] [PubMed] [Google Scholar]
- 28.Reddy SM, Cox E, Iofin I, Soong W, Cohen JI. Varicella-zoster virus (VZV) ORF32 encodes a phosphoprotein that is posttranslationally modified by the VZV ORF47 protein kinase. J Virol. 1998;72:8083–8088. doi: 10.1128/jvi.72.10.8083-8088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo C, Lee J, Sommer M, Grose C, Arvin AM. The requirement of varicella zoster virus glycoprotein E (gE) for viral replication and effects of glycoprotein I on gE in melanoma cells. Virology. 2002;304:176–186. doi: 10.1006/viro.2002.1556. [DOI] [PubMed] [Google Scholar]
- 30.Heineman TC, Cohen JI. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J Virol. 1995;69:7367–7370. doi: 10.1128/jvi.69.11.7367-7370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moffat JF, Zerboni L, Sommer MH, Heineman TC, Cohen JI, Kaneshima H, Arvin AM. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc Natl Acad Sci U S A. 1998;95:11969–11974. doi: 10.1073/pnas.95.20.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu H, Cohen JI. Varicella-zoster virus open reading frame 47 (ORF47) protein is critical for virus replication in dendritic cells and for spread to other cells. Virology. 2005;337:304–311. doi: 10.1016/j.virol.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Rahaus M, Desloges N, Wolff MH. Varicella-zoster virus requires a functional PI3K/Akt/GSK-3alpha/beta signaling cascade for efficient replication. Cell Signal. 2007;19:312–320. doi: 10.1016/j.cellsig.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Koyano S, Suzutani T, Yoshida I, Azuma M. Analysis of phosphorylation pathways of antiherpesvirus nucleosides by varicella-zoster virus-specific enzymes. Antimicrob Agents Chemother. 1996;40:920–923. doi: 10.1128/aac.40.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzutani T, Ogasawara M, Shibaki T, Azuma M. Susceptibility of protein kinase (ORF47)-deficient varicella-zoster virus strains to anti-herpesvirus nucleosides. Antiviral Res. 2000;45:79–82. doi: 10.1016/s0166-3542(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 36.Michel D, Pavic I, Zimmermann A, Haupt E, Wunderlich K, Heuschmid M, Mertens T. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J Virol. 1996;70:6340–6346. doi: 10.1128/jvi.70.9.6340-6346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Zeijl M, Fairhurst J, Baum EZ, Sun L, Jones TR. The human cytomegalovirus UL97 protein is phosphorylated and a component of virions. Virology. 1997;231:72–80. doi: 10.1006/viro.1997.8523. [DOI] [PubMed] [Google Scholar]
- 38.Wolf DG, Honigman A, Lazarovits J, Tavor E, Panet A. Characterization of the human cytomegalovirus UL97 gene product as a virion-associated protein kinase. Arch Virol. 1998;143:1223–1232. doi: 10.1007/s007050050370. [DOI] [PubMed] [Google Scholar]
- 39.Prichard MN, Gao N, Jairath S, Mulamba G, Krosky P, Coen DM, Parker BO, Pari GS. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J Virol. 1999;73:5663–5670. doi: 10.1128/jvi.73.7.5663-5670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romaker D, Schregel V, Maurer K, Auerochs S, Marzi A, Sticht H, Marschall M. Analysis of the structure-activity relationship of four herpesviral UL97 subfamily protein kinases reveals partial but not full functional conservation. J Med Chem. 2006;49:7044–7053. doi: 10.1021/jm060696s. [DOI] [PubMed] [Google Scholar]
- 41.Krosky PM, Baek MC, Jahng WJ, Barrera I, Harvey RJ, Biron KK, Coen DM, Sethna PB. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J Virol. 2003;77:7720–7727. doi: 10.1128/JVI.77.14.7720-7727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marschall M, Freitag M, Suchy P, Romaker D, Kupfer R, Hanke M, Stamminger T. The protein kinase pUL97 of human cytomegalovirus interacts with and phosphorylates the DNA polymerase processivity factor pUL44. Virology. 2003;311:60–71. doi: 10.1016/s0042-6822(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 43.Wolf DG, Courcelle CT, Prichard MN, Mocarski ES. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc Natl Acad Sci U S A. 2001;98:1895–1900. doi: 10.1073/pnas.98.4.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baek MC, Krosky PM, Pearson A, Coen DM. Phosphorylation of the RNA polymerase II carboxyl-terminal domain in human cytomegalovirus-infected cells and in vitro by the viral UL97 protein kinase. Virology. 2004;324:184–193. doi: 10.1016/j.virol.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Kawaguchi Y, Kato K, Tanaka M, Kanamori M, Nishiyama Y, Yamanashi Y. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1delta. J Virol. 2003;77:2359–2368. doi: 10.1128/JVI.77.4.2359-2368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krosky PM, Baek MC, Coen DM. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J Virol. 2003;77:905–914. doi: 10.1128/JVI.77.2.905-914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marschall M, Marzi A, aus dem Siepen P, Jochmann R, Kalmer M, Auerochs S, Lischka P, Leis M, Stamminger T. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J Biol Chem. 2005;280:33357–33367. doi: 10.1074/jbc.M502672200. [DOI] [PubMed] [Google Scholar]
- 48.Seaman WT, Holley-Guthrie E, Gershburg E, Dickerson SJ, Delecluse H-J, Pagano JS, Kenney SC. BMRF1 transcriptional function, but not phosphorylation by the BGLF4-encoded protein kinase, is important for Epstein-Barr virus replication. 2007 Submitted. [Google Scholar]
- 49.Prichard MN, Britt WJ, Daily SL, Hartline CB, Kern ER. Human cytomegalovirus UL97 Kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J Virol. 2005;79:15494–15502. doi: 10.1128/JVI.79.24.15494-15502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azzeh M, Honigman A, Taraboulos A, Rouvinski A, Wolf DG. Structural changes in human cytomegalovirus cytoplasmic assembly sites in the absence of UL97 kinase activity. Virology. 2006;354:69–79. doi: 10.1016/j.virol.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 51.Baek MC, Krosky PM, Coen DM. Relationship between autophosphorylation and phosphorylation of exogenous substrates by the human cytomegalovirus UL97 protein kinase. J Virol. 2002;76:11943–11952. doi: 10.1128/JVI.76.23.11943-11952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baek MC, Krosky PM, He Z, Coen DM. Specific Phosphorylation of Exogenous Protein and Peptide Substrates by the Human Cytomegalovirus UL97 Protein Kinase. Importance of the P+5 position. J Biol Chem. 2002;277:29593–29599. doi: 10.1074/jbc.M202312200. [DOI] [PubMed] [Google Scholar]
- 53.Talarico CL, Burnette TC, Miller WH, Smith SL, Davis MG, Stanat SC, Ng TI, He Z, Coen DM, Roizman B, Biron KK. Acyclovir is phosphorylated by the human cytomegalovirus UL97 protein. Antimicrob Agents Chemother. 1999;43:1941–1946. doi: 10.1128/aac.43.8.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michel D, Kramer S, Hohn S, Schaarschmidt P, Wunderlich K, Mertens T. Amino acids of conserved kinase motifs of cytomegalovirus protein UL97 are essential for autophosphorylation. J Virol. 1999;73:8898–8901. doi: 10.1128/jvi.73.10.8898-8901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gompels UA, Nicholas J, Lawrence G, Jones M, Thomson BJ, Martin ME, Efstathiou S, Craxton M, Macaulay HA. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology. 1995;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 56.De Bolle L, Michel D, Mertens T, Manichanh C, Agut H, De Clercq E, Naesens L. Role of the human herpesvirus 6 u69-encoded kinase in the phosphorylation of ganciclovir. Mol Pharmacol. 2002;62:714–721. doi: 10.1124/mol.62.3.714. [DOI] [PubMed] [Google Scholar]
- 57.Manichanh C, Olivier-Aubron C, Lagarde JP, Aubin JT, Bossi P, Gautheret-Dejean A, Huraux JM, Agut H. Selection of the same mutation in the U69 protein kinase gene of human herpesvirus-6 after prolonged exposure to ganciclovir in vitro and in vivo. J Gen Virol. 2001;82:2767–2776. doi: 10.1099/0022-1317-82-11-2767. [DOI] [PubMed] [Google Scholar]
- 58.Safronetz D, Petric M, Tellier R, Parvez B, Tipples GA. Mapping ganciclovir resistance in the human herpesvirus-6 U69 protein kinase. J Med Virol. 2003;71:434–439. doi: 10.1002/jmv.10510. [DOI] [PubMed] [Google Scholar]
- 59.Gershburg E, Marschall M, Hong K, Pagano JS. Expression and localization of the Epstein-Barr virus-encoded protein kinase. J Virol. 2004;78:12140–12146. doi: 10.1128/JVI.78.22.12140-12146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang JT, Yang PW, Lee CP, Han CH, Tsai CH, Chen MR. Detection of Epstein-Barr virus BGLF4 protein kinase in virus replication compartments and virus particles. J Gen Virol. 2005;86:3215–3225. doi: 10.1099/vir.0.81313-0. [DOI] [PubMed] [Google Scholar]
- 61.Gershburg E, Raffa S, Torrisi MR, Pagano JS. Epstein-Barr Virus-Encoded Protein Kinase (Bglf4) Is Involved In Production Of Infectious Virus. J Virol. 2007 doi: 10.1128/JVI.02398-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonnella R, Farina A, Santarelli R, Raffa S, Feederle R, Bei R, Granato M, Modesti A, Frati L, Delecluse HJ, Torrisi MR, Angeloni A, Faggioni A. Characterization and intracellular localization of the Epstein-Barr virus protein BFLF2: interactions with BFRF1 and with the nuclear lamina. J Virol. 2005;79:3713–3727. doi: 10.1128/JVI.79.6.3713-3727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee CP, Chen JY, Wang JT, Kimura K, Takemoto A, Lu CC, Chen MR. EBV BGLF4 Kinase Induces Premature Chromosome Condensation through Activation of Condensin and Topoisomerase II. J Virol. 2007 doi: 10.1128/JVI.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kudoh A, Daikoku T, Ishimi Y, Kawaguchi Y, Shirata N, Iwahori S, Isomura H, Tsurumi T. Phosphorylation of MCM4 at sites inactivating DNA helicase activity of the MCM4-MCM6-MCM7 complex during Epstein-Barr virus productive replication. J Virol. 2006;80:10064–10072. doi: 10.1128/JVI.00678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asai R, Kato A, Kato K, Kanamori-Koyama M, Sugimoto K, Sairenji T, Nishiyama Y, Kawaguchi Y. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J Virol. 2006;80:5125–5134. doi: 10.1128/JVI.02674-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kato K, Yokoyama A, Tohya Y, Akashi H, Nishiyama Y, Kawaguchi Y. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J Gen Virol. 2003;84:3381–3392. doi: 10.1099/vir.0.19454-0. [DOI] [PubMed] [Google Scholar]
- 67.Yue W, Gershburg E, Pagano JS. Hyperphosphorylation of EBNA2 by Epstein-Barr virus protein kinase suppresses transactivation of the LMP1 promoter. J Virol. 2005;79:5880–5885. doi: 10.1128/JVI.79.9.5880-5885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gershburg E, Hong K, Pagano JS. Effects of maribavir and selected indolocarbazoles on Epstein-Barr virus protein kinase BGLF4 and on viral lytic replication. Antimicrob Agents Chemother. 2004;48:1900–1903. doi: 10.1128/AAC.48.5.1900-1903.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kato K, Kawaguchi Y, Tanaka M, Igarashi M, Yokoyama A, Matsuda G, Kanamori M, Nakajima K, Nishimura Y, Shimojima M, Phung HT, Takahashi E, Hirai K. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1delta (EF-1delta): EF-1delta is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J Gen Virol. 2001;82:1457–1463. doi: 10.1099/0022-1317-82-6-1457. [DOI] [PubMed] [Google Scholar]
- 70.de Turenne-Tessier M, Ooka T, de The G, Daillie J. Characterization of an Epstein-Barr virus-induced thymidine kinase. J Virol. 1986;57:1105–1112. doi: 10.1128/jvi.57.3.1105-1112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Littler E, Zeuthen J, McBride AA, Sorensen E. Trost, Powell KL, Walsh-Arrand JE, Arrand JR. Identification of an Epstein-Barr virus-coded thymidine kinase. Embo J. 1986;5:1959–1966. doi: 10.1002/j.1460-2075.1986.tb04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roubal J, Klein G. Synthesis of thymidine kinase (TK) in Epstein-Barr virus-superinfected Raji TK-negative cells. Intervirology. 1981;15:43–48. doi: 10.1159/000149213. [DOI] [PubMed] [Google Scholar]
- 73.Moore SM, Cannon JS, Tanhehco YC, Hamzeh FM, Ambinder RF. Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues. Antimicrob Agents Chemother. 2001;45:2082–2091. doi: 10.1128/AAC.45.7.2082-2091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gustafson EA, Chillemi AC, Sage DR, Fingeroth JD. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob Agents Chemother. 1998;42:2923–2931. doi: 10.1128/aac.42.11.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haque M, Wang V, Davis DA, Zheng ZM, Yarchoan R. Genetic organization and hypoxic activation of the Kaposi's sarcoma-associated herpesvirus ORF34-37 gene cluster. J Virol. 2006;80:7037–7051. doi: 10.1128/JVI.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jenner RG, Alba MM, Boshoff C, Kellam P. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J Virol. 2001;75:891–902. doi: 10.1128/JVI.75.2.891-902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paulose-Murphy M, Ha NK, Xiang C, Chen Y, Gillim L, Yarchoan R, Meltzer P, Bittner M, Trent J, Zeichner S. Transcription program of human herpesvirus 8 (kaposi's sarcoma-associated herpesvirus) J Virol. 2001;75:4843–4853. doi: 10.1128/JVI.75.10.4843-4853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu M, Suen J, Frias C, Pfeiffer R, Tsai MH, Chuang E, Zeichner SL. Dissection of the Kaposi's sarcoma-associated herpesvirus gene expression program by using the viral DNA replication inhibitor cidofovir. J Virol. 2004;78:13637–13652. doi: 10.1128/JVI.78.24.13637-13652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Izumiya Y, Izumiya C, Van Geelen A, Wang DH, Lam KS, Luciw PA, Kung HJ. Kaposi's sarcoma-associated herpesvirus-encoded protein kinase and its interaction with K-bZIP. J Virol. 2007;81:1072–1082. doi: 10.1128/JVI.01473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamza MS, Reyes RA, Izumiya Y, Wisdom R, Kung HJ, Luciw PA. ORF36 protein kinase of Kaposi's sarcoma herpesvirus activates the c-Jun N-terminal kinase signaling pathway. J Biol Chem. 2004;279:38325–38330. doi: 10.1074/jbc.M400964200. [DOI] [PubMed] [Google Scholar]
- 81.Izumiya Y, Ellison TJ, Yeh ET, Jung JU, Luciw PA, Kung HJ. Kaposi's sarcoma-associated herpesvirus K-bZIP represses gene transcription via SUMO modification. J Virol. 2005;79:9912–9925. doi: 10.1128/JVI.79.15.9912-9925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adamson AL, Kenney S. Epstein-barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies. J Virol. 2001;75:2388–2399. doi: 10.1128/JVI.75.5.2388-2399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daibata M, Humphreys RE, Sairenji T. Phosphorylation of the Epstein-Barr virus BZLF1 immediate-early gene product ZEBRA. Virology. 1992;188:916–920. doi: 10.1016/0042-6822(92)90553-2. [DOI] [PubMed] [Google Scholar]
- 84.El-Guindy AS, Paek SY, Countryman J, Miller G. Identification of constitutive phosphorylation sites on the Epstein-Barr virus ZEBRA protein. J Biol Chem. 2006;281:3085–3095. doi: 10.1074/jbc.M506076200. [DOI] [PubMed] [Google Scholar]
- 85.Kolman JL, Taylor N, Marshak DR, Miller G. Serine-173 of the Epstein-Barr virus ZEBRA protein is required for DNA binding and is a target for casein kinase II phosphorylation. Proc Natl Acad Sci U S A. 1993;90:10115–10119. doi: 10.1073/pnas.90.21.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cannon JS, Hamzeh F, Moore S, Nicholas J, Ambinder RF. Human herpesvirus 8-encoded thymidine kinase and phosphotransferase homologues confer sensitivity to ganciclovir. J Virol. 1999;73:4786–4793. doi: 10.1128/jvi.73.6.4786-4793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawaguchi Y, Matsumura T, Roizman B, Hirai K. Cellular elongation factor 1delta is modified in cells infected with representative alpha-, beta-, or gammaherpesviruses. J Virol. 1999;73:4456–4460. doi: 10.1128/jvi.73.5.4456-4460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawaguchi Y, Van Sant C, Roizman B. Eukaryotic elongation factor 1delta is hyperphosphorylated by the protein kinase encoded by the U(L)13 gene of herpes simplex virus 1. J Virol. 1998;72:1731–1736. doi: 10.1128/jvi.72.3.1731-1736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawaguchi Y, Kato K. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev Med Virol. 2003;13:331–340. doi: 10.1002/rmv.402. [DOI] [PubMed] [Google Scholar]
- 90.Ng TI, Talarico C, Burnette TC, Biron K, Roizman B. Partial substitution of the functions of the herpes simplex virus 1 U(L)13 gene by the human cytomegalovirus U(L)97 gene. Virology. 1996;225:347–358. doi: 10.1006/viro.1996.0609. [DOI] [PubMed] [Google Scholar]
- 91.Marschall M, Stein-Gerlach M, Freitag M, Kupfer R, van den Bogaard M, Stamminger T. Direct targeting of human cytomegalovirus protein kinase pUL97 by kinase inhibitors is a novel principle for antiviral therapy. J Gen Virol. 2002;83:1013–1023. doi: 10.1099/0022-1317-83-5-1013. [DOI] [PubMed] [Google Scholar]
- 92.Metzger C, Michel D, Schneider K, Luske A, Schlicht HJ, Mertens T. Human cytomegalovirus UL97 kinase confers ganciclovir susceptibility to recombinant vaccinia virus. J Virol. 1994;68:8423–8427. doi: 10.1128/jvi.68.12.8423-8427.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng WH, Hong G, Delecluse HJ, Kenney SC. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J Virol. 2004;78:1893–1902. doi: 10.1128/JVI.78.4.1893-1902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shugar D. Viral and host-cell protein kinases: enticing antiviral targets and relevance of nucleoside, and viral thymidine, kinases. Pharmacol Ther. 1999;82:315–335. doi: 10.1016/s0163-7258(99)00004-2. [DOI] [PubMed] [Google Scholar]
- 95.Koszalka GW, Chamberlain SD, Harvey RJ, Frick LW, Good SS, Davis ML, Smith A, Drach JC, Townsend LB, Biron KK. Benzimidazoles for the treatment of human cytomegalovirus. Antivir Res. 1996;30:A43. [Google Scholar]
- 96.Townsend LB, Gudmundsson KS, Daluge SM, Chen JJ, Zhu Z, Koszalka GW, Boyd L, Chamberlain SD, Freeman GA, Biron KK, Drach JC. Studies designed to increase the stability and antiviral activity (HCMV) of the active benzimidazole nucleoside, TCRB. Nucleosides Nucleotides. 1999;18:509–519. doi: 10.1080/15257779908041486. [DOI] [PubMed] [Google Scholar]
- 97.Biron KK, Harvey RJ, Chamberlain SC, Good SS, Smith AA, 3rd, Davis MG, Talarico CL, Miller WH, Ferris R, Dornsife RE, Stanat SC, Drach JC, Townsend LB, Koszalka GW. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002;46:2365–2372. doi: 10.1128/AAC.46.8.2365-2372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams SL, Hartline CB, Kushner NL, Harden EA, Bidanset DJ, Drach JC, Townsend LB, Underwood MR, Biron KK, Kern ER. In vitro activities of benzimidazole D- and L-ribonucleosides against herpesviruses. Antimicrob Agents Chemother. 2003;47:2186–2192. doi: 10.1128/AAC.47.7.2186-2192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zacny VL, Gershburg E, Davis MG, Biron KK, Pagano JS. Inhibition of Epstein-Barr virus replication by a benzimidazole L-riboside: novel antiviral mechanism of 5, 6-dichloro-2-(isopropylamino)-1-beta-L-ribofuranosyl-1H-benzimidazole. J Virol. 1999;73:7271–7277. doi: 10.1128/jvi.73.9.7271-7277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McSharry JJ, McDonough A, Olson B, Talarico C, Davis M, Biron KK. Inhibition of ganciclovir-susceptible and -resistant human cytomegalovirus clinical isolates by the benzimidazole L-riboside 1263W94. Clin Diagn Lab Immunol. 2001;8:1279–1281. doi: 10.1128/CDLI.8.6.1279-1281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Drew WL, Miner RC, Marousek GI, Chou S. Maribavir sensitivity of cytomegalovirus isolates resistant to ganciclovir, cidofovir or foscarnet. J Clin Virol. 2006;37:124–127. doi: 10.1016/j.jcv.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 102.Chou S, Wechel LC, Marousek GI. Cytomegalovirus UL97 Kinase Mutations That Confer Maribavir Resistance. J Infect Dis. 2007;196:91–94. doi: 10.1086/518514. [DOI] [PubMed] [Google Scholar]
- 103.Chou S, Marousek GI, Senters AE, Davis MG, Biron KK. Mutations in the human cytomegalovirus UL27 gene that confer resistance to maribavir. J Virol. 2004;78:7124–7130. doi: 10.1128/JVI.78.13.7124-7130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Komazin G, Ptak RG, Emmer BT, Townsend LB, Drach JC. Resistance of human cytomegalovirus to the benzimidazole L-ribonucleoside maribavir maps to UL27. J Virol. 2003;77:11499–11506. doi: 10.1128/JVI.77.21.11499-11506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lalezari JP, Aberg JA, Wang LH, Wire MB, Miner R, Snowden W, Talarico CL, Shaw S, Jacobson MA, Drew WL. Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob Agents Chemother. 2002;46:2969–2976. doi: 10.1128/AAC.46.9.2969-2976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lorenzi PL, Landowski CP, Brancale A, Song X, Townsend LB, Drach JC, Amidon GL. N-methylpurine DNA glycosylase and 8-oxoguanine dna glycosylase metabolize the antiviral nucleoside 2-bromo-5,6-dichloro-1-(beta-D-ribofuranosyl)benzimidazole. Drug Metab Dispos. 2006;34:1070–1077. doi: 10.1124/dmd.105.009209. [DOI] [PubMed] [Google Scholar]
- 107.Ma JD, Nafziger AN, Villano SA, Gaedigk A, Bertino JS., Jr. Maribavir pharmacokinetics and the effects of multiple-dose maribavir on cytochrome P450 (CYP) 1A2, CYP 2C9, CYP 2C19, CYP 2D6, CYP 3A, N-acetyltransferase-2, and xanthine oxidase activities in healthy adults. Antimicrob Agents Chemother. 2006;50:1130–1135. doi: 10.1128/AAC.50.4.1130-1135.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swan SK, Smith WB, Marbury TC, Schumacher M, Dougherty C, Mico BA, Villano SA. Pharmacokinetics of maribavir, a novel oral anticytomegalovirus agent, in subjects with varying degrees of renal impairment. J Clin Pharmacol. 2007;47:209–217. doi: 10.1177/0091270006296765. [DOI] [PubMed] [Google Scholar]
- 109.Wang LH, Peck RW, Yin Y, Allanson J, Wiggs R, Wire MB. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2003;47:1334–1342. doi: 10.1128/AAC.47.4.1334-1342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Slater MJ, Cockerill S, Baxter R, Bonser RW, Gohil K, Gowrie C, Robinson JE, Littler E, Parry N, Randall R, Snowden W. Indolocarbazoles: potent, selective inhibitors of human cytomegalovirus replication. Bioorg Med Chem. 1999;7:1067–1074. doi: 10.1016/s0968-0896(99)00032-2. [DOI] [PubMed] [Google Scholar]
- 111.Zimmermann A, Wilts H, Lenhardt M, Hahn M, Mertens T. Indolocarbazoles exhibit strong antiviral activity against human cytomegalovirus and are potent inhibitors of the pUL97 protein kinase. Antiviral Res. 2000;48:49–60. doi: 10.1016/s0166-3542(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 112.Marschall M, Stein-Gerlach M, Freitag M, Kupfer R, van Den Bogaard M, Stamminger T. Inhibitors of human cytomegalovirus replication drastically reduce the activity of the viral protein kinase pUL97. J Gen Virol. 2001;82:1439–1450. doi: 10.1099/0022-1317-82-6-1439. [DOI] [PubMed] [Google Scholar]
- 113.Herget T, Freitag M, Morbitzer M, Kupfer R, Stamminger T, Marschall M. Novel chemical class of pUL97 protein kinase-specific inhibitors with strong anticytomegaloviral activity. Antimicrob Agents Chemother. 2004;48:4154–4162. doi: 10.1128/AAC.48.11.4154-4162.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8:303–306. doi: 10.1634/theoncologist.8-4-303. [DOI] [PubMed] [Google Scholar]
- 115.Mett H, Holscher K, Degen H, Esdar C, De Neumann BF, Flicke B, Freudenreich T, Holzer G, Schinzel S, Stamminger T, Stein-Gerlach M, Marschall M, Herget T. Identification of inhibitors for a virally encoded protein kinase by 2 different screening systems: in vitro kinase assay and in-cell activity assay. J Biomol Screen. 2005;10:36–45. doi: 10.1177/1087057104270269. [DOI] [PubMed] [Google Scholar]
- 116.Frame MC, Purves FC, McGeoch DJ, Marsden HS, Leader DP. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J Gen Virol. 1987;68(Pt 10):2699–2704. doi: 10.1099/0022-1317-68-10-2699. [DOI] [PubMed] [Google Scholar]
- 117.Chung TD, Wymer JP, Smith CC, Kulka M, Aurelian L. Protein kinase activity associated with the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) J Virol. 1989;63:3389–3398. doi: 10.1128/jvi.63.8.3389-3398.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ng TI, Ogle WO, Roizman B. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology. 1998;241:37–48. doi: 10.1006/viro.1997.8963. [DOI] [PubMed] [Google Scholar]
- 119.Ogle WO, Ng TI, Carter KL, Roizman B. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology. 1997;235:406–413. doi: 10.1006/viro.1997.8710. [DOI] [PubMed] [Google Scholar]
- 120.Ogle WO, Roizman B. Functional anatomy of herpes simplex virus 1 overlapping genes encoding infected-cell protein 22 and US1.5 protein. J Virol. 1999;73:4305–4315. doi: 10.1128/jvi.73.5.4305-4315.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bruni R, Fineschi B, Ogle WO, Roizman B. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0 is modified in a cell-type- specific manner and Is recruited to the nucleus after infection. J Virol. 1999;73:3810–3817. doi: 10.1128/jvi.73.5.3810-3817.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Long MC, Leong V, Schaffer PA, Spencer CA, Rice SA. ICP22 and the UL13 protein kinase are both required for herpes simplex virus-induced modification of the large subunit of RNA polymerase II. J Virol. 1999;73:5593–5604. doi: 10.1128/jvi.73.7.5593-5604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]