Abstract
Mesothelin is a tumour differentiation antigen that is normally present on the mesothelial cells lining the pleura, peritoneum and pericardium. It is however highly expressed in several human cancers including malignant mesothelioma, pancreatic, ovarian and lung adenocarcinoma. The normal biologic function of mesothelin is unknown but recent studies have shown that it binds to CA-125 and may play a role in the peritoneal spread of ovarian cancer. The limited mesothelin expression in normal tissues and high expression in many cancers makes it an attractive candidate for cancer therapy. Three mesothelin targeted agents are in various stages of clinical evaluation in patients. These include SS1P (CAT-5001) a recombinant immunotoxin targeting mesothelin, MORAb-009 a chimeric anti-mesothelin monoclonal antibody and CRS-207 a live-attenuated Listeria monocytogenes vector encoding human mesothelin. These ongoing clinical trials will help define the utility of mesothelin as a target for cancer therapy.
Keywords: Mesothelioma, Ovarian cancer, Pancreatic cancer, lung cancer, tumor vaccine, monoclonal antibodies, clinical trial, CA-125, SS1P, MORAb-009
1. Introduction
Mesothelin is a differentiation antigen whose expression in normal human tissues is limited to mesothelial cells lining the pleura, pericardium and peritoneum.1,2 However, mesothelin is highly expressed in several human cancers, including virtually all mesotheliomas and pancreatic adenocarcinomas, and approximately 70% of ovarian cancers and 50% of lung adenocarcinomas.3–6 The mesothelin gene encodes a precursor protein of 71 kDa that is processed to a 31 kDa shed protein called megakaryocyte potentiating factor (MPF) and a 40 kDa fragment, mesothelin, that is attached to the cell membrane by a glycosyl-phosphatidylinositol (GPI) anchor1,7 (Figure 1). MPF was isolated from the culture supernatant of a pancreatic cancer cell line and was so named because it stimulated the megakaryocyte colony-forming activity of interleukin-3 in mouse bone marrow cultures.8 The biologic function of mesothelin is not known. However, results of recent studies suggest that the mesothelin may play a role in ovarian cancer metastasis by binding to MUC16/CA-125.9 A small amount of cell bound mesothelin is shed into the serum and has been shown to be elevated in patients with mesothelioma and ovarian cancer.10,11 These studies suggest that serum mesothelin could be useful for diagnosis and follow-up of these patients. This review focuses on mesothelin as a target for cancer therapy and summarizes the available pre-clinical data as well as on-going and planned clinical trials.
Figure 1.

Schematic of mesothelin. The human mesothelin (MSLN) gene encodes a precursor protein of 622 amino acids. On translocation into the endoplasmic reticulum (ER) the N-terminal signal peptide (red; residues 1–33) and the C-terminal glycosyl-phosphatidylinositol (GPI) anchor addition signal (blue; a predicted cleavage site: Ser598) are removed and the latter replaced with a GPI anchor. The MSLN precursor is cleaved into two products, mature megakaryocyte potentiating factor (MPF; residues Ser34 – Arg286) and the GPI-anchored membrane-bound mature MSLN (orange) starting from Glu296. The proteolytic cleavage region (green) contains a furin cleavage site at Arg295, and other protease cleavage sites including a trypsin cleavage site at Arg286. The four N-linked glycans (black lollipops; Asn57, Asn388, Asn488 and Asn515) are indicated.
2. Mesothelin expression in human cancers
Mesothelin gene expression in human cancers has been studied using serial analysis of gene expression (SAGE) tag analysis (http://www.ncbi.nlm.nih.gov/projects/SAGE/). High mRNA expression of mesothelin is found in mesothelioma, lung, ovarian and pancreatic adenocarcinomas. In addition, immunohistochemistry has helped delineate the frequency and pattern of mesothelin protein expression in these tumors (Table 2, Figure 2). These studies have been greatly facilitated by the commercial availability of a monoclonal antibody (mAb) 5B2 (Novocastra, Newcastle-on-Tyne, United Kingdom) that can detect mesothelin expression in paraffin embedded tissues.
Table 2.
Anti-mesothelin agents in clinic
| Agent | Class of drug | Status | Comments |
|---|---|---|---|
| SS1P (CAT-5001) | Recombinant immunotoxin against mesothelin | Two Phase I clinical trials completed | Phase I study in combination with chemotherapy for mesothelioma to open shortly |
| MORAb-009 | Chimeric anti-mesothelin monoclonal antibody | In Phase I clinical trial | Planned Phase II studies in pancreatic cancer and mesothelioma |
| CRS-207 | Listeria monocytogene-mesothelin vaccine | Pre-clinical development completed | Phase I clinical trial for mesothelin expressing malignancies to start 2007 |
Figure 2.
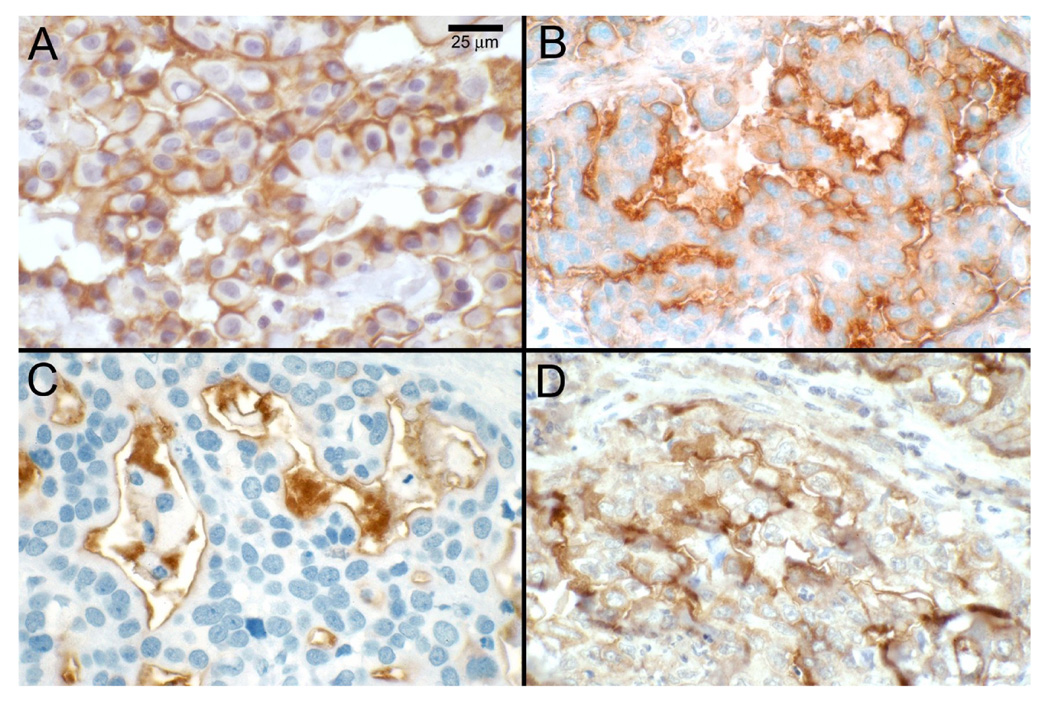
Mesothelin expression in human tumors. Mesothelin expression was detected by immunohistochemistry using monoclonal antibody (mAb) 5B2 in tissue specimens of patients with mesothelioma (A); ovarian cancer (B); pancreatic adenocarcinoma (C); and lung adenocarcinoma (D).
2.1 Mesothelioma
Mesothelin is highly expressed in epithelial malignant mesothelioma. In the original study by Chang and colleagues mesothelin expression was evaluated by mAb-K1 using frozen section tissues of patients with malignant mesothelioma.12 Out of the 23 pleural mesothelioma samples analysed all 15 epithelial mesothelioma had mesothelin expression, while 4 sarcomatous mesotheliomas were negative. In the four samples with biphasic mesothelioma only the epithelial component stained for mesothelin. Mesothelin expression in paraffin embedded mesothelioma tissue samples was studied by Ordonez using mAb 5B2.3 Out of the 55 mesothelioma specimens (44 epithelioid, 3 biphasic, and 8 sarcomatoid) studied mesothelin reactivity was noted in all epithelioid mesotheliomas and the epithelial component of biphasic mesotheliomas. However, none of the sarcomatous mesotheliomas expressed mesothelin. These results are in agreement with the results of Chang and colleagues that mesothelin is present in all epithelial mesotheliomas and is absent in the sarcomatous type. Although mesothelin is not a specific marker for mesothelioma a negative mesothelin immunostain strongly argues against the diagnosis of epithelioid mesothelioma.13
2.2 Pancreatic cancer
Argani and colleagues were the first to show mesothelin expression in pancreatic ductal adenocarcinoma.4 Using SAGE database they found the tag for mesothelin to be consistently present in pancreatic cancer libraries but not in normal pancreas. In addition, mesothelin mRNA expression was present in 4 of 4 resected primary pancreatic cancers and by immunohistochemistry all 60 resected primary adenocarcinomas were mesothelin positive. These results were confirmed by Hassan and colleagues who showed that mesothelin was expressed in all 18 cases of pancreatic adenocarcinomas examined but absent in normal pancreas and in chronic pancreatitis.14 Ordanez also showed mesothelin was expressed in majority of pancreatic adenocarcinomas, but was absent in islet cell tumors of the pancreas.6 In addition to pancreas mesothelin is also highly expressed in other adenocarcinomas of the biliary tree such as gallbladder cancer, and tumors of the common bile duct.14,15
2.3 Ovarian cancer
Using mAb-K1 Chang and colleagues demonstrated mesothelin expression in 10 out of 15 non-mucinous epithelial ovarian carcinomas while as it was absent in all 4 mucinous ovarian cancers examined.2 Using 5B2 anti-mesothelin antibody Ordanez noted mesothelin expression in 14 of 14 serous, 3 of 3 endometrioid, 6 of 8 clear cell and 3 of 6 mucinous ovarian carcinomas.6 Using tissue microarrays containing ovarian cancer specimens Frierson and colleagues showed that all 38 serous ovarian cancers expressed mesothelin while as only 1 of 8 mucinous ovarian tumor had mild mesothelin expression.16 In contrast to the studies of Ordanez and Frierson and colleagues who noted mesothelin expression in 100% of serous ovarian cancer, Hassan and colleagues noted mesothelin expression in 27 of 33 (82%) of serous ovarian cancers.5 Based on these results one can conclude that mesothelin expression is present in most serous ovarian cancers, which constitute the majority of epithelial ovarian cancers, but is also expressed to a lesser degree in other subtypes of ovarian cancer.
2.4 Lung cancer
Though earlier studies using mAb-K1 showed no expression of mesothelin in lung adnocarcinomas, several recent studies using the 5B2 anti-mesothelin mAb show high expression in these tumors.12 In the study by Ordonez looking at mesothelin expression in different human cancers mesothelin expression was present in 14 of 34 (41%) lung adenocarcinomas.6 This was confirmed in a large study by Miettinen and Sarlomo-Rikala who evaluated mesothelin expression in 596 lung carcinomas.17 In this study mesothelin expression was present in 78 of 148 (53%) adenocarcinomas, 15 of 118 (13%) large cell carcinomas, 29 of 124 (23%) squamous cell carcinomas and 0 of 41 (0%) small cell lung cancers. One unusual feature of the lung cancer is that there is more intracellular mesothelin reactivity than seen in mesotheliomas, ovarian or pancreatic cancer where the mesothelin staining is predominantly at the cell surface or normal mesothelial cells. Studies by Ho and colleagues have further delineated mesothelin expression in human lung cancer.18 Using NCI-60 cell line panel they detected mesothelin mRNA in 7 of 9 (78%) lung cancer cell lines. In 4 of the 7 cell lines in which mesothelin mRNA was detected, cell surface mesothelin expression was detected by flow cytometry. In addition, mesothelin mRNA was present in 10 of 12 (83%) lung adenocarcinomas samples obtained from patients. These results support earlier studies by Ordanez and Miettinen that show mesothelin expression by immunohistochemistry in about half the patients with lung adenocarcinoma.
2.5 Mesothelin expression in other human tumors
In addition to the above tumors mesothelin over-expression has been noted in some other human cancers. It is commonly expressed in squamous cell carcinomas of different sites such as cervix, lung and head and neck carcinomas as well as endometrial adenocarcinomas.6,19,20 Mesothelin expression by immunohistochemistry is infrequently present in colorectal, gastric and esophageal cancers.6 Mesothelin expression is absent in soft tissue sarcomas with the notable exception of biphasic synovial sarcomas.6 Cancer with absent mesothelin expression include melanomas, renal cell cancer, transitional cell carcinomas, thyroid cancer, breast cancer, prostate cancer and germ cell tumors.6
3. Mesothelin biology
The normal biologic function of mesothelin is not clear. Bera and colleagues generated mutant mice in which the mesothelin gene was inactivated, and neither mesothelin mRNA or protein was detected in the homozygous mutant mice.21 These mesothelin knockout mice did not have a detectable phenotype and both males and females produced offspring normally. These results suggest that in mice mesothelin function is not essential for growth or reproduction. Although the functions of mesothelin remain largely unknown, recent studies have shed light on the possible role of mesothelin in cancer biology.
3.1 Mesothelin binding to MUC16/CA-125 and ovarian cancer metastasis
MUC16/CA125 is a very large cell surface mucin, with an average molecular weight between 2.5–5 million Da., that is also heavily glycosylated with both O-linked and N-linked oligosaccharides.22 It is shed into the serum and is used for monitoring response to therapy in ovarian cancer.23 Rump and colleagues were the first to show that binding of CA-125 to membrane bound mesothelin mediates heterotypic cell adhesion since an anti-mesothelin antibody blocks this interaction.9 Their results suggest that mesothelin is a novel CA-125 binding protein and that CA-125 might lead to intra-peritoneal dissemination of ovarian carcinoma by binding to mesothelin present on normal mesothelial cells lining the peritoneal cavity. The biochemical basis of this interaction between mesothelin and CA-125 was further characterized by Gubbels and colleagues24 Using the MUC16 expressing ovarian cancer cell line OVCAR-3, they showed that it binds mesothelin while as OVCAR-3 derived sublines that do not express MUC16 do not bind mesothelin. They also showed that mesothelin has a very strong affinity for MUC 16 with an apparent Kd of approximately 5 nM and that mesothelin interacts with both soluble and cell surface associated forms of native MUC16. Take together these studies provide evidence that mesothelin and MUC16 binding may be important in the peritoneal spread of ovarian cancer. Inhibiting MUC16 binding to mesothelin could therefore be a potentially useful strategy to treat ovarian cancer.
3.2 Mesothelin regulation by Wnt-1 and Wnt-5a
The Wnt ligands are secreted glycoproteins that play an important role in intracellular signaling and regulate a variety of biological processes including cell growth, cell differentiation and apoptosis. A mis-regulation of Wnt signaling has been shown to lead to the development of several human cancers.25 Using the mouse mammary epithelial cell line C57mg, Prieve and Moon showed that mesothelin was up-regulated by Wnt-1 both by the stable expression of Wnt-1 in C57mg cells and as well as by co-culturing C57mg cells with Wnt-1 secreting cells.26 They also demonstrated that mesothelin expression was induced by Li+, an inhibitor of GSK-3β that mimics Wnt-1. In contrast to Wnt-1, Wnt-5a down-regulated mesothelin expression, perhaps through antagonism of endogenous Wnt/β-catenin signaling. These results suggest that mesothelin expression can be altered by Wnt proteins. Interestingly, mesothelin is highly expressed in mesothelioma, lung, ovarian, and pancreatic carcinomas which have constitutive activation of Wnt signaling.6,27
3.3 Humoral anti-mesothelin immune response in cancer patients
To determine whether a spontaneous humoral B cell response to mesothelin is present in patients with mesothelin expressing cancers Ho et al used a sensitive ELISA to detect mesothelin-specific IgG antibodies in serum of patients with advanced mesothelioma and ovarian cancer28. Elevated levels of mesothelin-specific antibodies were detected in the sera of 27 of 69 (39%) patients with mesothelioma and 10 of 24 (42%) patients with epithelial ovarian cancer when compared with a normal control population. Mesothelin specific antibodies were present at a higher frequency in patients whose tumors had strong mesothelin expression by immunohistochemistry. These results suggest that the immunogenicity of mesothelin is associated with its high expression on the tumor cells and serologic recognition of mesothelin is cancer related. The presence of a mesothelin specific B-cell response in a significant proportion of patients with mesothelin expressing tumors supports on-going efforts to use mesothelin as a therapeutic cancer vaccine.
3.4 Prognostic significance of mesothelin expression in ovarian cancer
The significance of tumor mesothelin expression with clinical outcome in ovarian cancer was studied by Yen and colleagues29 In this study tumor mesothelin expression by immunohistochemistry was correlated with clinical outcome in 198 patients with ovarian serous carcinoma. Mesothelin immunoreactivity was present in 55% of serous carcinomas with similar expression in both high grade and low grade tumors. The results of this study showed that in patients with high-grade advanced-stage ovarian cancer treated with optimal debulking surgery and chemotherapy, diffuse tumor mesothelin immunostaining correlated significantly with prolonged survival. Mesothelin expression did not correlate significantly with patient age, tumor site, tumor grade, in vitro drug resistance and tumor cell differentiation. The authors speculate that a humoral or T cell immune response to mesothelin-expressing ovarian carcinoma cells could result in reduction of tumor load leading to the prolonged patient overall survival. However, the prognostic significance of mesothelin expression in ovarian cancer and other mesothelin expressing tumors needs to be validated in large prospective studies.
4. Mesothelin targeted therapies
The limited expression of mesothelin on normal human tissues and high expression in several human cancers makes mesothelin an attractive candidate for cancer therapy. These therapies include agents that target cell surface mesothelin or elicit an immune response against mesothelin. Agents that are in the clinic or about to enter clinical trials include CAT-5001, MORAb-009 and CRS-207 (Table 2).
4.1 SS1P (CAT-5001)
SS1P is a recombinant immunotoxin consisting of an anti-mesothelin Fv linked to a truncated Pseudomonas exotoxin that mediates cell killing.30,31 After binding to mesothelin, the immunotoxin is internalized via clathrin coated pits, undergoes processing in the endocytic compartment and the immunotoxin fragment containing the ADP-ribosylation domain is transported to the endoplasmic reticulum and then translocated to the cytosol where it inhibits elongation factor-2 leading to inhibition of protein synthesis and ultimately cell death.32 Pre-clinical studies have shown that SS1P is cytotoxic to cell lines expressing mesothelin and causes complete regression of mesothelin expressing tumor xenografts in nude mice.7 In addition SS1P is cytotoxic to tumor cells obtained directly from human patients. Tumor cells obtained from patients with ovarian cancer undergoing surgery were grown in three dimensional organotypic cultures and treated with SS1P.33 After treatment the organotypic gels were formalin fixed and evaluated for light microscopic examination and apoptosis. SS1P caused a dose dependent increase in tumor cell death and apoptosis. Similarly tumor cells established from ascites of patients with peritoneal mesothelioma are very sensitive to SS1P with an IC50 of 0.08 to 3.9 ng/ml.34 These studies show that mesothelin is highly expressed on tumor cells obtained directly from patients with mesothelioma and are very sensitive to treatment with SS1P.
Recent studies have looked at the anti-tumor activity of SS1P in combination with radiation therapy or chemotherapy. Athymic nude mice bearing A431/K5 mesothelin expressing tumors were treated with radiation alone, SS1P alone or the two agents in combination.35 The results of this study showed that mice treated with low-dose radiation and SS1P or high-dose radiation and SS1P had a statistically significant prolongation in time to tumor doubling or tripling compared with control, SS1P or radiation alone treated mice. Since radiation treatment increased the cell surface expression of mesothelin, it is possible that the increased anti-tumor activity of SS1P in combination with radiation is partly due to enhanced mesothelin expression, making the cells more sensitive to SS1P treatment. Combination of SS1P with tumor-directed radiation might be useful in the treatment of locally advanced pancreatic cancer since mesothelin is highly expressed in these tumors and not normal pancreatic tissues, and because radiation is commonly used in this setting. To study the possible synergy between SS1P and chemotherapy immunodeficient mice bearing A431/K5 mesothelin expressing tumors were treated with SS1P alone, chemotherapy alone or the two in combination.36 This combination treatment was synergistic causing long-lasting remissions. Synergy was observed with paclitaxel (taxol), cisplatin and cyclophosphamide. Our initial hypothesis was that the increased activity of SS1P in combination with paclitaxel was due to paclitaxel-induced damage to endothelial cells resulting in increased SS1P uptake in the tumor. However, using radiolabeled SS1P we did not observe any increase in tumor SS1P levels in mice treated with paclitaxel.36 It appears that other factors such as shed mesothelin in the tumor microenvironment may contribute to this synergy and are being studied in our laboratory. Given the non-overlapping toxicities and different modes of action of SS1P and chemotherapeutic agents, combining them could potentially result in increased anti-tumor activity in patients.
Two Phase I studies of SS1P have just been completed. These studies which were designed to test the safety, maximum tolerated dose (MTD) and pharmacokinetics of SS1P used two different strategies for SS1P administration. In one study SS1P was administered as an intravenous bolus infusion over 30 minutes (SS1P bolus infusion study) while as in the other study SS1P was given as a continuous i.v. infusion over 10 days (SS1P continuous infusion study).
In the SS1P bolus infusion study, SS1P was given every other day (QOD) for 6 doses.37 A total of 17 patients were treated using this schedule and the MTD was 18 µg/kg/dose. The dose-limiting toxicity (DLT) included grade 3 urticaria (1 patient) and grade 3 vascular leak syndrome (2 patients). Since the dose-limiting toxicities were observed in patients who had received more than 4 doses of SS1P, the protocol was amended to treat patients QOD for 3 doses to allow further SS1P dose escalation. Using this schedule of administration 17 patients were treated and the MTD was established as 45 µg/kg/dose. SS1P was well tolerated with hypoalbuminemia, fatigue, edema as most common side effects. The DLT was reversible pleuritis and was observed in 2/2 patients treated with 60 µg/kg of SS1P and in 1/9 patients treated at the dose of SS1P 45 µg/kg. This DLT was due to SS1P targeting normal mesothelial cells expressing mesothelin, similar to the toxicity observed in toxicological studies in cynomolgus monkeys. Anti-tumor activity was noted in several heavily pre-treated patients treated with SS1P QOD × 6 or × 3 schedule. Out of the 34 patients treated (20 mesothelioma, 12 ovarian cancer and 2 pancreatic cancer) 4 had minor response and 19 had stable disease including complete resolution of ascites in two patients. A patient with peritoneal mesothelioma had complete resolution of abdominal ascites, required no further treatment and died of unrelated cause more than 5 years from initial treatment. Similarly in a patient with ovarian cancer there was resolution of abdominal and pelvic ascites that lasted 6 months. Pharmacokinetic analysis showed a dose dependent increase in area under the curve. At the MTD of 45 µg/kg the mean peak SS1P concentration was 483 ng/ml with a half-life of 466 minutes. The results of this study showed that an immunotoxin targeting mesothelin is safe and had anti-tumor activity in heavily pretreated patients.
In the SS1P continuous infusion study, patients received continuous administration of SS1P via portable pumps over 10 days. A total of 24 patients with mesothelin expressing mesothelioma, ovarian and pancreatic cancer were treated. Most common toxicities were similar to that observed in the bolus infusion study including hypoalbuminemia, weight gain, edema and fatigue. The MTD of SS1P given as a continuous iv infusion over 10 days was 18 µg/kg. Some anti-tumor activity was also observed in this study (Dr. R. Kreitman, Laboratory of Molecular Biology, National Cancer Institute).
Since SS1P given as a bolus infusion has a prolonged half-life and can be given at much higher dose and several patients showed anti-tumor response, this is the schedule that is being pursued for its further clinical development. Based on pre-clinical studies in animal models that show marked synergy when SS1P is combined with chemotherapy, clinical trials of SS1P in combination with chemotherapy are about to start for the treatment of mesothelin expressing malignancies.
4.2 MORAb-009
MORAb-009 is a high-affinity chimeric (mouse/human) monoclonal IgG1/κ with high affinity and specificity for mesothelin. The heavy and light chain variable regions of mouse anti-mesothelin scFv (obtained by panning on mesothelin-positive cells a phage display library made from splenic mRNA of a mouse immunized with mesothelin cDNA) were grafted in frame with human IgG1 and kappa constant regions. Since MORAb-009 is a chimeric antibody containing only the mouse sequences that recognize human mesothelin, it should be less immunogenic and allow repeated administration to patients. Laboratory studies show that MORAb-009 kills mesothelin expressing cell lines via antibody dependent cellular cytoxicity (ADCC) and in addition it inhibits the binding of mesothelin to CA-125. Based on these preclinical studies a Phase I clinical trial of MORAb-009 has been initiated (www.clinicaltrials.gov/ct/show/NCT00325494). The primary objectives of this study are to evaluate the safety and tolerability of MORAb-009 in patients with mesothelioma, pancreatic cancer, and mesothelin expressing non-small cell lung cancer and ovarian cancer. Secondary objectives of this clinical trial are to study the pharmacokinetics, human anti-chimeric antibody formation and tumor responses in the treated patients. This study is ongoing and so far 11 patients have been treated on 4 different dose levels.38 These include 6 patients with mesothelioma, 3 with pancreatic cancer and 2 with ovarian cancer. No major adverse events have been observed and dose escalation is ongoing.
4.3 CRS-207
The rationale for mesothelin as a tumor vaccine is based on studies showing that mesothelin can elicit a strong CD8+ T cell response in patients.39 One of the mesothelin cancer vaccines in advanced stages of clinical development is CRS-207 (LmΔactA/ΔinlB/hMeso). This vaccine utilizes a live attenuated strain of the bacterium Listeria monocytogenes (Lm), a facultative intracellular bacterium, as the vector.40 The engineered vector CRS-100, has deletions of the two genes that encode the virulence determinants actA and internalin B (inlB), which results in a greater than 1000-fold decrease in virulence compared to the wild type Lm. CRS-100 is currently undergoing Phase 1 testing in patients with carcinoma and liver metastasis.41 CRS-207 is a live-attenuated Lm vaccine strain based on CRS-100 that encodes human mesothelin. Preclinical studies show that CRS-207 elicits human mesothelin-specific CD4+/CD8+ immunity in mice and in cynomolgus monkeys and exhibits therapeutic efficacy in tumor bearing mice.42 A Phase I clinical trial of CRS-207 for the treatment of patients with mesothelin expressing cancers is about to commence.
4.4 Mesothelin cancer vaccines
The utility of mesothelin as a tumor vaccine came from a clinical trial conducted by Jaffe and colleagues that involved vaccination of pancreatic cancer patients with GM-CSF transduced pancreatic cancer cell lines.43 Out of the 14 patients treated on this study 3 developed a postvaccination delayed-type hypersensitivity (DTH) response to the autologous tumor, that was associated with prolonged survival. 43 Subsequent immunologic studies showed that a strong and consistent induction of CD8+ T cell response to multiple HLA-A2, A3, and A24-restricted mesothelin epitopes occurred exclusively in the three patients who had developed a vaccine induced DTH response.39 In another vaccine study, T-cell lines derived from the native or the agonist mesothelin epitope were shown to lyse mesothelin expressing and HLA-2 positive pancreatic cancer, ovarian cancer and mesothelioma cell lines.44 These studies support the potential utility of mesothelin in peptide and / or vector-mediated immunotherapy protocols for the treatment of cancers that highly express mesothelin.
4.5 Mesothelin targeted ovarian cancer gene therapy
Pre-clinical studies have evaluated mesothelin as a target for adenoviral-mediated gene therapy. Adenoviruses containing the mesothelin promoter driving reporter gene expression were evaluated using established ovarian cancer cell lines and purified tumor cells obtained from patients.45 These studies showed that the mesothelin promoter is transcriptionally active in ovarian cancer cells but has significantly reduced activity in normal control cells. Also in the liver, which does not express mesothelin, mesothelin promoter activity was low making it a useful promoter for gene therapy. The utility of mesothelin for transductional gene therapy i.e. to direct gene therapy agents to targets highly expressed in specific tumors was also evaluated in this study. An adenovirus vector containing Fc-binding domain was conjugated to the mouse antihuman mesothelin mAb. This transductional targeting to mesothelin led to increased transduction rates in ovarian cancer cell lines as well as tumor cells obtained from patients. In contrast there was no increase in gene transfer rate using this construct in mesothelin negative human fibroblast cells or the teratocarcinoma cell line PA-1. These results show that mesothelin could be a potentially useful candidate for combined transductional and transcriptional adenovirus-based gene therapy.
4.6 Mesothelin as a target for radioimmunotherapy
Mesothelin as target for radioimmunotherapy was evaluated in vivo using nude mice bearing mesothelin expressing A431/K5 xenografts.46 This study used a pretargeting strategy that involves the administration of streptavidin (SA)-conjugated antibody to target the tumor, administration of a clearing agent to remove any circulating antibody-SA conjugate from the blood and then the injection of radiolabeled biotin that localizes to tumor tissue bearing SA-conjugated antibody. Mice bearing A431/K5 tumors were first injected with the anti-mesothelin tetravalent single-chain Fv-streptavidin fusion protein (SS1scFvSA) followed 20 hours later by a synthetic clearing agent to remove any unbound SS1scFvSA. This was followed 4 hours later by the administration of radiolabeled 90Y-1,4,7,10-tetraazacyclododecane-N,N’,N”,N’”-tetraacetic acid (DOTA)-biotin. Pretargeted therapy of A431/K5 tumor with 90Y doses of 11.2 – 32.4 MBq resulted in a dose-dependent tumor response. In mice that were treated with 32.4 MBq of 90Y, 86% survived tumor free for 110 days compared to a median survival of only 16 days in the untreated mice. Such a pre-targeted approach could potentially be useful in the clinic and reduce non-specific bone marrow toxicity. However, the high immunogenicity of SA, could limit repeated administration of this agent.
5.0 Conclusions
Mesothelin is a differentiation antigen present on normal mesothelial cells that is highly expressed in several cancers especially mesotheliomas, pancreatic, ovarian and lung cancer. This differential expression makes mesothelin an attractive candidate for targeted therapies. In Phase I studies of the recombinant anti-mesothelin immunotoxin SS1P objective anti-tumor responses were noted in several heavily pretreated patients. Based on these results and preclinical studies showing synergy with chemotherapy, Phase II studies of SS1P in combination with chemotherapy are about to commence for treatment of mesothelioma and ovarian cancer. MORAb-009, a chimeric anti-mesothelin mAb is currently in advanced stages of Phase I testing with Phase II studies in mesothelioma and pancreatic cancer planned. CRS-207, a Lm-mesothelin vaccine is about to commence Phase I testing in mesothelin expressing cancers. In addition, studies targeting mesothelin for gene therapy and radioimmunotherapy are in various stages of pre-clinical development.
Table 1.
Mesothelin Expression in Human Cancers*
| Tumor | Mesothelin expression | Comments | Ref. |
|---|---|---|---|
| Mesothelioma | 100% | Present in all epithelial mesotheliomas but absent in sarcomatous type | 3, 12 |
| Pancreatic cancer | 100% | Absent in normal pancreas and chronic pancreatitis | 4, 6, 14 |
| Ovarian cancer | 67–100% | Mostly in serous ovarian adenocarcinomas although it is also expressed to a lesser degree in other sub-types | 2, 5, 6, 16 |
| Lung adenocarcinoma | 41–53% | Some expression in squamous & large cell lung cancer but absent in small cell lung cancer | 6, 17 |
Detected by immunohistochemistry
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement The authors declare no conflict of interest
REFERENCES
- 1.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50:373–381. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- 3.Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–197. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 4.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 5.Hassan R, Kreitman RJ, Pastan I, et al. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13:243–247. doi: 10.1097/01.pai.00000141545.36485.d6. [DOI] [PubMed] [Google Scholar]
- 6.Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi N, Hattori K, Oh-eda M, et al. A novel cytokine exhibiting megakaryocyte potentiating activity from a human pancreatic tumor cell line HPC-Y5. J Biol Chem. 1994;269:805–808. [PubMed] [Google Scholar]
- 9.Rump A, Morikawa Y, Tanaka M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 10.Hassan R, Remaley A, Sampson ML, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 11.Robinson BWS, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 12.Chang K, Pai LH, Pass H, et al. Monoclonal antibody K1 reacts with epithelial mesothelioma but not lung adenocarcinoma. Am J Surg Pathol. 1992;16:259–268. doi: 10.1097/00000478-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Ordanez NG. What are the current best immunohistochemical markers for the diagnosis of epithelioid mesothelioma: A review and update. Hum Pathol. 2007;38:1–6. doi: 10.1016/j.humpath.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Hassan R, Laszik ZG, Lerner M, et al. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–845. [PubMed] [Google Scholar]
- 15.Swierczynski SL, Maitra A, Abraham SC, et al. Analysis of novel tumor markers in pancreatic and biliary carcinomas using tissue microarrays. Hum Pathol. 2004;35:357–366. doi: 10.1016/j.humpath.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Frierson HF, Moskaluk CA, Powell SM, et al. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum Pathol. 200;34:605–609. doi: 10.1016/s0046-8177(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen M, Sarlomo-Rikala M. Expression of calretinin, thrombomodulin, keratin 5, and meosthelin in lung carcinomas of different types: an immunohistochemical analysis of 596 tumors in comparison with epithelioid mesotheliomas of the pleura. Am J Surg Pathol. 2003;27:150–158. doi: 10.1097/00000478-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Ho M, Bera TK, Willingham MC, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–1575. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 19.Chang K, Pastan I, Willingham M. Frequent expression of the tumor antigen CAK1 in squamous-cell carcinomas. Int J Cancer. 1992;51:548–554. doi: 10.1002/ijc.2910510408. [DOI] [PubMed] [Google Scholar]
- 20.Dainty LA, Risinger JI, Morrison C, et al. Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecol Oncol. 2007;105:563–570. doi: 10.1016/j.ygyno.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 21.Bera T, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000;20:2902–2906. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin BWT, Lloyd KO. Molecular cloning of the CA 125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 23.Bast RC, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 24.Gubbels JAA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50–65. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- 26.Prieve MG, Moon RT. Stromelysin-1 and mesothelin are differentially regulated by Wnt-5a and Wnt-1 in C57MG mouse mammary epithelial cells. BMC Dev Biol. 2003;3:2. doi: 10.1186/1471-213X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uematsu K, Kanazawa S, You L, et al. Evidence of dishevelled overexpression and transcriptional activity of β-catenin. Cancer Res. 2003;63:4547–4551. [PubMed] [Google Scholar]
- 28.Ho M, Hassan R, Zhang J, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 29.Yen JM, Hsu CY, Mao TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006;12:827–831. doi: 10.1158/1078-0432.CCR-05-1397. [DOI] [PubMed] [Google Scholar]
- 30.Chowdhury PS, Viner JL, Beers R, Pastan I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci USA. 1998;95:669–674. doi: 10.1073/pnas.95.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chowdhury PS, Pastan I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat Biotechnol. 1999;17:568–572. doi: 10.1038/9872. [DOI] [PubMed] [Google Scholar]
- 32.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 33.Hassan R, Lerner MR, Benbrook D, et al. Antitumor activity of SS(dsFv)PE38 and SS1(dsFv)PE38, recombinant antimesothelin immunotoxins against human gynecologic cancers grown in organotypic culture in vitro. Clin Cancer Res. 2002;8:3520–3526. [PubMed] [Google Scholar]
- 34.Li Q, Verschraegen CF, Mendoza J, Hassan R. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1(dsFv)PE38 towards tumor cell lines established from ascites of patient with peritoneal mesotheliomas. Anticancer Res. 2004;24:1327–1335. [PubMed] [Google Scholar]
- 35.Hassan R, Williams-Gould J, Steinberg SM, et al. Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts. Clin Cancer Res. 2006;12:4983–4988. doi: 10.1158/1078-0432.CCR-06-0441. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Xiang L, Hassan R, et al. Synergistic antitumor activity of taxol and immunotoxin SS1P in tumor-bearing mice. Clin Cancer Res. 2006;12:4695–4701. doi: 10.1158/1078-0432.CCR-06-0346. [DOI] [PubMed] [Google Scholar]
- 37.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus i.v. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007 doi: 10.1158/1078-0432.CCR-07-0869. (In press) [DOI] [PubMed] [Google Scholar]
- 38.Armstrong DK, Laheru D, Ma WW, et al. A phase 1 study of MORAb-009, a monoclonal antibody against mesothelin in pancreatic cancer, mesothelioma and ovarian adenocarcinoma. J Clin Oncol. 2007;25 (Suppl):14041. [Google Scholar]
- 39.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell response provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brockstedt DG, Giedlin MA, Leong ML, et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci USA. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giedlin MA, Bahjat KS, Prell RA, et al. Activation and expansion of hepatic NK cells promotes innate antitumor immunity and long-lived CD8+ T cell memory following treatment with attenuated Listeria monocytogenes; AACR Proceedings (Abstract #1877); 2007. Apr 14, [Google Scholar]
- 42.Brockstedt DG, Leong ML, Bahjat KS, et al. Live-attenuated L. monocytogenes encoding mesothelin for immunotherapy of patients with pancreas and ovarian cancers; AACR Proceedings (Abstract #1874); 2007. Apr 14, [Google Scholar]
- 43.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 44.Yokokawa J, Palena C, Arlen P, et al. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res. 2005;11:6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breidenbach M, Rein DT, Everts M, et al. Mesothelin-mediated targeting of adenoviral vectors for ovarian cancer gene therapy. Gene Ther. 2005;12:187–193. doi: 10.1038/sj.gt.3302404. [DOI] [PubMed] [Google Scholar]
- 46.Sato N, Hassan R, Axworthy DB, et al. Pretargeted radioimmunotherapy of mesothelin-expressing cancer using a tetravalent single-chain Fv-streptavidin fusion protein. J Nucl Med. 2005;46:1201–1209. [PubMed] [Google Scholar]


