Abstract
Extreme climatic events are predicted to increase in frequency and magnitude, but their ecological impacts are poorly understood. Such events are large, infrequent, stochastic perturbations that can change the outcome of entrained ecological processes. Here we show how an extreme flood event affected a desert rodent community that has been monitored for 30 years. The flood (i) caused catastrophic, species-specific mortality; (ii) eliminated the incumbency advantage of previously dominant species; (iii) reset long-term population and community trends; (iv) interacted with competitive and metapopulation dynamics; and (v) resulted in rapid, wholesale reorganization of the community. This and a previous extreme rainfall event were punctuational perturbations—they caused large, rapid population- and community-level changes that were superimposed on a background of more gradual trends driven by climate and vegetation change. Captured by chance through long-term monitoring, the impacts of such large, infrequent events provide unique insights into the processes that structure ecological communities.
Keywords: desert rodents, incumbency advantage, punctuational dynamics
As global climate change scenarios consistently forecast, recent years have witnessed increases in the intensity and frequency of extreme climatic events, including greater variability in precipitation in the southwestern United States (1–4). These events have raised questions about their ecological impacts on the structure and dynamics of populations and communities. Changing climate has caused short-term changes in primary productivity and abundances of individual species (5–8), as well as longer-term shifts in geographic ranges, phenology, and vegetation structure (7–11) and even evolutionary changes (12). Increasing variability of rainfall has been shown to alter the competitive dynamics and species composition of plant communities (13, 14). Still missing, however, is a general perspective on how such large perturbations affect assemblages of diverse kinds of organisms (10, 15–17).
Extreme climatic events, such as floods and droughts, are stochastic perturbations that have the potential to alter the outcomes of entrained population and community processes. The assembly of communities is a dynamic process in which species composition changes as some species, native or exotic, colonize or increase in abundance while other species decrease or go locally extinct (18–20). Although driven in part by deterministic intrinsic interactions, such as competition (21) and predation (22), community assembly is also influenced by stochastic extrinsic perturbations, such as physical disturbances (23), disease epidemics, and colonization of new species (24). Extreme climatic events represent massive, infrequent abiotic perturbations that can differentially affect certain species, thereby altering species composition, resource use, and interactions (10, 14–16). Effects of such perturbations on the long-term dynamics of communities are neither well documented nor well understood, most likely as a result of their rarity and stochasticity. By their very nature, infrequent unpredictable phenomena will never be subject to statistical inference that requires replication, control of variables, or large sample sizes. Nevertheless, impacts of these events can provide valuable insights into the processes—extrinsic and intrinsic, biotic and abiotic, deterministic and stochastic—that structure communities.
Long-term monitoring of community dynamics provides unique opportunities to observe extreme climatic events and to document their ecological impacts (8, 25–27). Since 1977, a community of seed-eating rodents near Portal, AZ, has been censused monthly, and two extreme precipitation events have been documented. The first occurred in 1983 when Tropical Storm Octave brought 129 mm of rain (≈50% of average annual rainfall) over 6 days (25). The second and most recent occurred when a summer thunderstorm dropped 30 mm of rain in <2 h on August 14, 1999, causing sheet flooding to an average depth of ≈35 cm (28). These two events had large, but strikingly different, impacts on the rodent community.
Here we document the immediate and long-term impacts of the 1999 flood and then briefly compare them to the impacts of the 1983 event. The long-term study includes standardized monthly censuses of rodents, which allow us to (i) measure species-specific responses; (ii) compare community composition and dynamics before and after; and (iii) evaluate the effects of both massive perturbations and background processes, both stochastic and deterministic phenomena.
The Setting: Pre-Flood Community Dynamics
Since 1977, the seed-eating rodent community at Portal has experienced gradual yet profound changes due to climate and vegetation change (29, 30). Higher-than-average winter precipitation increased shrub cover 3-fold (29). Total abundance of seed-eating rodents fluctuated in the short term and increased significantly over the three decades (Fig. 1) (31). Major changes in rodent species composition also occurred (30). Previously dominant, large-bodied kangaroo rats declined; Dipodomys spectabilis, formerly the second most abundant species and a keystone species of desert grasslands, went locally extinct in 1994 (25). These declines were compensated by large increases in smaller pocket mice characteristic of shrub habitat, especially Chaetodipus penicillatus and Chaetodipus baileyi (31, 32). The latter, a native species previously restricted to shrubbier habitats in the surrounding region, first appeared on the site in 1995 (Fig. 2E) (33). Colonization by C. baileyi followed a protracted drought when rodent abundance was well below the long-term average (Fig. 1) (28), and one species, the kangaroo rat Dipodomys merriami, comprised >80% of the total (Fig. 2A). After colonization in 1995, C. baileyi increased near-exponentially (33), but by the time of the 1999 flood it was still a distant third in relative abundance (8%) compared with the kangaroo rats D. merriami and Dipodomys ordii (56% and 15%, respectively) (Fig. 2).
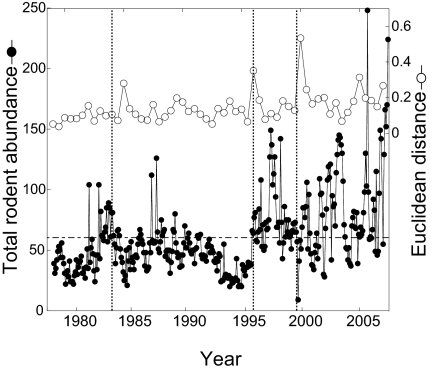
Fig. 1.
Total number of rodents captured during each monthly trapping session on the control plots (filled circles) and the change in average community composition, as measured by ED, between subsequent 6-month periods (open circles). Three notable events are marked by the vertical dashed lines: 1983 Tropical Storm Octave, 1995 colonization by C. baileyi, and the 1999 flood event. The long-term mean for rodent abundance is indicated by the horizontal dotted line, revealing the period of markedly low rodent abundance preceding the colonization of the site by C. baileyi. A record low number of rodents was captured after the flood in 1999, but total abundance returned to preflood levels within 3 months.
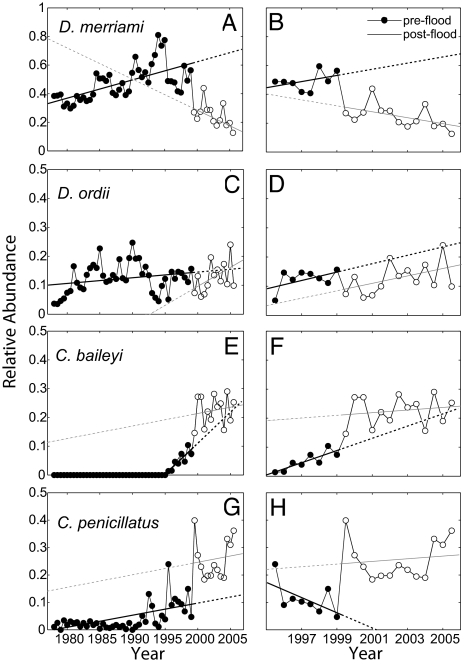
Fig. 2.
Temporal trends in relative abundances (6-month averages) of the four most common rodent species on the control plots. Trends since the beginning of the study (1978–2005) are shown in A, C, E, and G, and trends only since the arrival of C. baileyi in September 1995 are shown in B, D, F, and H. The solid trend lines represent the ordinary least squares regression lines (see Table 1 for relevant statistics) and are extrapolated by using dashed lines. The relative abundances of the two species of pocket mice (Chaetodipus spp.) are significantly higher than expected after the flood and have remained so through the present, whereas those of the historically most abundant species, Merriam's kangaroo rat (D. merriami), and its congener, D. ordii, are significantly lower than expected.
Results of the Flood
The impact of the downpour and resulting sheet flooding was immediately evident. An all-time record low number of rodents was captured the following month (Fig. 1). Species were affected differentially. Six of the eight seed-eating rodent species present before the flood suffered dramatically increased mortality. These included the kangaroo rats, Dipodomys spp., the historically dominant members of the community; <10% of the individuals present in the 6 months before the flood survived to be recaptured in the subsequent 6-month period, much lower than the preflood survival rate of 35–45% (Fig. 3). For example, only seven of the 103 marked D. merriami individuals were recaptured after the flood. Interestingly, however, the flood caused no detectable mortality in the two pocket mouse species C. baileyi and C. penicillatus. In the 6 months after the flood, C. baileyi survivorship remained near preflood levels (33.8% vs. 36.6%), whereas C. penicillatus survivorship actually increased (48.5% vs. 28.6%) (Fig. 3). Moreover, survivorship of both pocket mouse species was elevated above long-term average rates for two subsequent 6-month periods (Fig. 3).
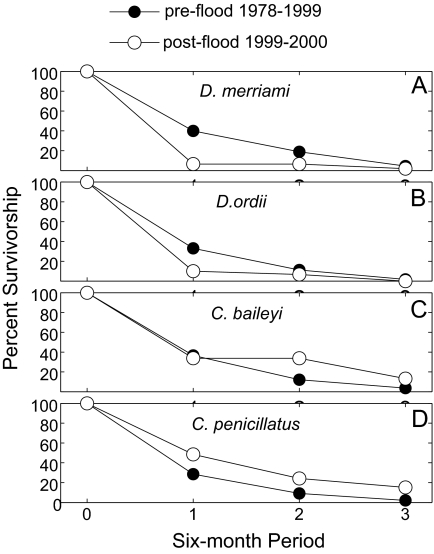
Fig. 3.
Survival of the four most abundant rodent species during the period immediately before and after the flood compared with survival before the flood. Survival is measured as the percentage of marked individuals that survived from one 6-month period to each of three subsequent 6-month periods, as indicated by recapture. Preflood survival is calculated over the course of the entire study (filled circles) and is compared with survival from the 6-month period before the August 1999 flood event to the following periods (open circles). The survivorship of the kangaroo rats (Dipodomys spp.) decreased markedly as a result of the flood, whereas that of the pocket mice (Chaetodipus spp.) remained at or above preflood levels.
Total rodent abundance returned to preflood levels within 3 months (Fig. 1). A lasting effect of the flood, however, was to alter species' population trends (Fig. 2). The data points and regression lines in Fig. 2 A, C, E, and G depict the long-term changes in relative abundance of the four most abundant species: D. merriami, D. ordii, C. penicillatus, and C. baileyi. The points and lines in Fig. 2 B, D, F, and H show the trends for these same species, but only since 1995, when C. baileyi colonized. Both depictions clearly show the striking differences between the pre- and postflood population trends for all four species: the kangaroo rats, which experienced high rates of mortality due to the flood, never regained their previous abundances, whereas the pocket mice, which showed no evidence of flood-caused mortality, immediately increased and then maintained permanently elevated relative abundances. On both time scales, D. merriami had accounted for ≈50% of the rodents and had been increasing before the flood (Fig. 2 A and B and Table 1). Immediately after the flood, however, D. merriami's relative abundance declined and has continued to decline, remaining 62% lower on average than predicted by extrapolating preflood trends (Table 1). Similarly, D. ordii, the less abundant kangaroo rat, also showed statistically significant but quantitatively less dramatic declines, with relative abundances 16% lower on average than expected by extrapolating preflood trends (Fig. 2 C and D and Table 1).
Table 1.
Temporal trends in relative abundance before and after the flood
| Species | Preflood RA (mean ± SD) | Preflood OLS regression | r2 | Postflood RA (mean ± SD) |
t | P value | |
|---|---|---|---|---|---|---|---|
| Observed | Expected | ||||||
| Preflood since 1978 | |||||||
| D. merriami | 0.48 ± 0.12 | y = 0.0062x + 0.340 | 0.39 | 0.25 ± 0.08 | 0.66 ± 0.02 | −14.2 | <0.001 |
| D. ordii | 0.12 ± 0.05 | y = 0.0012x + 0.089 | 0.10 | 0.11 ± 0.04 | 0.15 ± 0.00 | −2.56 | 0.034 |
| C. baileyi | 0.05 ± 0.03 | y = 0.0094x − 0.332 | 0.65 | 0.22 ± 0.05 | 0.13 ± 0.03 | 5.78 | <0.001 |
| C. penicillatus | 0.04 ± 0.05 | y = 0.0022x − 0.010 | 0.34 | 0.26 ± 0.07 | 0.10 ± 0.01 | 8.94 | <0.001 |
| Preflood since 1995 | |||||||
| D. merriami | 0.49 ± 0.06 | y = 0.0106x + 0.062 | 0.16 | 0.25 ± 0.08 | 0.62 ± 0.04 | −11.6 | <0.001 |
| D. ordii | 0.13 ± 0.03 | y = 0.0068x − 0.150 | 0.24 | 0.11 ± 0.04 | 0.20 ± 0.03 | −5.35 | <0.001 |
| C. baileyi | 0.05 ± 0.03 | y = 0.0094x − 0.332 | 0.65 | 0.22 ± 0.05 | 0.13 ± 0.03 | 5.78 | <0.001 |
| C. penicillatus | 0.11 ± 0.06 | y = 0.0143x + 0.692 | 0.35 | 0.26 ± 0.07 | 0.01 ± 0.02 | 15.36 | <0.001 |
Shown are data from the four most common rodent species from before the flood [1978–1999 or 1995–1999 (after C. baileyicolonization)] compared with observed and expected trends after the event (1999–2005). Trends were characterized by using OLS regression (all P values < 0.05), and differences between Observed and Expected were tested with paired ttests on arcsine-transformed data. RA, relative abundance.
The flood triggered immediate and sustained increases in the two pocket mouse species. C. baileyi, which had been increasing steadily since its colonization in 1995 (Fig. 2 E and F), increased at an even greater rate immediately after the flood, to 116% of the relative abundance predicted by extrapolating prestorm trends (Fig. 2 E and F and Table 1). Only 1 year after the flood, by 2000, C. baileyi had reached a relative abundance (27%) that it would not have attained until 2009 if preflood trends had continued unabated (Table 1). The greatest changes occurred in C. penicillatus, which generally had been increasing over the course of the entire study but had begun to decline after the colonization of C. baileyi (Fig. 2 G and H). Analyses at both time scales show that after the flood C. penicillatus attained its highest abundances, up to 350% greater than the long-term average (Fig. 2 G and H and Table 1).
These changes in species' population trends resulted in an immediate and permanent change in the composition of the rodent community. Using Euclidean distance (ED) to quantify shifts in species composition between successive 6-month intervals reveals three episodes of dramatic change (Fig. 1). The largest (ED = 0.53) occurred at the time of the 1999 flood; the magnitude of this change was nearly four times greater than the long-term average (0.14 ± 0.08). Furthermore, after this large shift, the new community composition was maintained, as evidenced by postflood EDs near the long-term average (Fig. 1). Before the flood, D. merriami was consistently the dominant rodent species, comprising 32–81% (mean = 48.3 ± 12.1%) of the individuals and 20–82% (mean = 43.5 ± 17.9%) of the biomass. Subsequently, four species, D. merriami, D. ordii, C. penicillatus, and C. baileyi, have been approximately codominant, with none accounting for >27% of the individuals or biomass, on average (Table 1) (30). After the flood D. merriami reached record low relative abundances, whereas the two Chaetodipus species reached record highs (Fig. 2).
Discussion
The August 1999 flood and its repercussions at our Portal study site were idiosyncratic. However, the impact of this event on the desert rodent community offers a unique perspective that should be of general interest, especially to those concerned about ecological responses to extreme events and climatic changes.
An Extreme Event.
The 1999 flood was indeed an extreme event, the only one of its kind observed in our 30-year study. Its immediate impact was highly differential across species (Fig. 3). The two species of kangaroo rats suffered >90% mortality due to the flood, whereas the two species of pocket mice experienced no detectable mortality. The deaths were probably due to drowning, because bipedal kangaroo rats are poor swimmers and poor climbers. The decreases in relative abundance of kangaroo rats, and in the overall abundance of the rodents (Fig. 1), were the greatest recorded in the 30-year history of the study. If this event is representative, the susceptibility of other kinds of organisms to impacts of extreme weather and climate change may also be highly differential across species (16). Which species die or survive may depend on highly specific, seemingly idiosyncratic features of biology.
Resetting Long-Term Population Trends.
One consequence of the flood was that long-term trends in the relative abundances of the four most abundant species were reset or accelerated by the perturbation (Fig. 2). The kangaroo rat D. merriami, the most dominant species for the 22 years before the flood, still has not recovered 8 years after the perturbation. In contrast, the pocket mouse C. baileyi, which had colonized the study site in 1995 and had been increasing steadily, experienced accelerated population growth, likely facilitated by the greatly reduced abundance of its closest competitor, D. merriami. Moreover, the other pocket mouse, C. penicillatus, which had reached relative abundances of <10% before the flood, now comprises >35% of the community. These results show that large short-term perturbations can have effects on populations that last much longer than would be expected from simple recovery dynamics.
Changing Community Composition.
An immediate and long-lasting effect of the above population-level responses was a wholesale reorganization of the rodent community, a permanent shift of unprecedented magnitude to a new community composition dominated by pocket mice and not seen in the 22 years before the flood (Figs. 1 and 2). This community reorganization resulted in the invading species, C. baileyi, becoming the most abundant species in 2000, displacing D. merriami. C. penicillatus became the most abundant species in 2005 and has held that position through June 2007. Previously, another dramatic change in community composition marked the colonization of the site by the pocket mouse C. baileyi in 1995 (Fig. 1) after a prolonged period of below-average precipitation (28). The ongoing population increase of this species was accelerated by the flood, and it became the most abundant species <5 years after its arrival. So community structure and dynamics can be reset on a new, relatively stable trajectory by both biotic and abiotic perturbations. One kind of biotic perturbation, seen here, is the invasion of a new species, which can be facilitated by abiotic disturbance. An important process in these dynamics is immigration from the pool of individuals and species in surrounding habitats (the metacommunity). The rapidity of population increases and the low frequencies of reproductive individuals (our unpublished data) indicate that the increases of pocket mice immediately after the flood were due predominantly to immigration.
Comparison to 1983 Flood Event.
The third major shift in community composition during the 30-year history of the study occurred in response to another extreme climatic event, the excessive rainfall associated with Tropical Storm Octave in October 1983 (Fig. 1). The nature of this storm and its impact on the rodent community were markedly different from the August 1999 thunderstorm. In contrast to the 2-h thunderstorm, Octave lasted for 6 days. Rather than causing sheet flooding and immediate mortality, the prolonged heavy rainfall saturated the soil to great depth, likely causing spoilage of the seeds stored by rodents in deep granaries (25, 28). The greatest impact was on the largest and second most abundant rodent, the kangaroo rat D. spectabilis. Already decreasing gradually in abundance because of vegetation change before the storm, it suffered unprecedented mortality over the ensuing months, the winter and spring of 1983–1984 (25). The effect of Octave was to reset the existing decline, so that D. spectabilis never recovered its prestorm abundance and went locally extinct in 1994.
Comparison of these two extreme rainfall events shows both similarities and differences. Both storm events caused massive and highly species-specific mortality of dominant species, which resulted in long-lasting shifts in community composition. The causes of deaths, the time lag between the event and mortality, and the species affected, however, were totally different. The soaking of soil and spoilage of seed stores that caused the gradual die-off in D. spectabilis, a larder hoarding species, had no detectable impact on D. merriami, whose seed stores in shallow surface caches presumably dried rapidly and were undamaged. So, again, a general message from comparing the two storms is that extreme events can have species-specific impacts, which depend crucially on seemingly idiosyncratic details of biology but can nevertheless set in motion wholesale and long-lasting reorganization of the community.
Intersection of Extrinsic Perturbations and Intrinsic Interactions.
The dynamics of the rodent community at Portal can be characterized as continually changing, with relatively gradual long-term trends due to background climate and vegetation change punctuated by wholesale reorganizations initiated by infrequent extrinsic perturbations (i.e., short-term extreme rainstorms) with massive impacts (25, 29, 30). To understand the impacts of the extrinsic abiotic drivers, it is essential to take account of the intrinsic competitive interactions among the rodent species. Most of the time, the seed-eating rodents at Portal are food-limited, and competition is intense because they share a common resource (34–36). This is evidenced by (i) zero-sum dynamics, such that the collective rate of energy use by all rodent species remained relatively constant over the 30 years (31, 32); and (ii) consistent, positive responses of small species, especially pocket mice, to our kangaroo rat removal experiments (33, 37). From this evidence, we infer that (i) the long-term change in precipitation regime was a gradual perturbation that favored some species to the detriment of others, resulting in gradual compositional change and long-term compensatory dynamics driven by competition for limited resources (32); and (ii) the extreme storms were large perturbations that also differentially impacted species and resulted in community reorganization under the influence of strong competition. However, in the second case, reassembly, driven by either immigration after the localized thunderstorm or reproduction after the spatially extensive tropical storm, was very rapid, and a new relatively stable species composition was established in a matter of months. Although some responses to these different types of perturbations were similar, the resulting compositions of the reassembled communities were very different because of the interaction between the selective mortality and preexisting species composition and environmental conditions.
Overcoming Incumbency Advantage.
Reassembly after the flood did not change the identity of the four most abundant species, but it did change the interactions among these species. The long-term increase of D. merriami in response to the increase in shrubby vegetation and the decline of its larger competitor, D. spectabilis, was reversed (Fig. 2), and the ensuing decline of D. merriami allowed pocket mice to increase and ultimately dominate the community. These dynamics indicate that the differential mortality caused by the flood altered the preexisting dominance hierarchy, allowing formerly subordinate species that survived the event to dominate. The best explanation for these changes is that resident individuals and species had an incumbency advantage (38). Such a phenomenon has been documented for territorial individuals within species (39, 40). Here, however, loss of incumbency advantage altered interspecific competitive interactions, with profound, long-lasting effects on community structure. Such an advantage is not surprising in these desert rodents, which have established home ranges, seed stores, and burrow systems, and which rely on acquired knowledge of their territory for food finding and predator avoidance (41). Incumbency facilitates not only defense of the territory by the resident individual, but also inheritance of the home range by an offspring or conspecific upon the death of the resident. Under background conditions the effect of the incumbency advantage was to slow down and stabilize population and community dynamics.
The extreme storm event had a destabilizing effect. By causing wholesale mortality of the dominant resident rodents, the perturbation largely eliminated their incumbency advantage, made competitive interactions more nearly equal, and allowed new individuals of previously subordinate species to colonize and establish territories. The mortality of dominant residents made available the resources that they had stored and defended, and previously subordinate rodents were able to immigrate and increase. The effect of the flood in facilitating immigration of previously rare native species was similar to effects of other perturbations in facilitating colonization of exotic invasive species (24, 38, 42, 43). So, disrupting the incumbency advantage is one way that an extrinsic abiotic perturbation can interface with intrinsic competitive processes to alter assembly rules and cause wholesale community reorganization. Similar responses have been reported in plant communities in response to fire or grazing (38). Priority effects, a form of incumbency advantage for sessile organisms, are frequently invoked to account for such dynamics (44).
Furthermore, our long-term record of community dynamics on ecological time scales seems to be similar to the long-term fossil record of species dynamics on evolutionary time scales. At Portal, gradual long-term trends were punctuated by mass mortality triggered by two extreme precipitation events. In the fossil record, gradual long-term trends were punctuated by mass extinctions triggered by asteroid impacts or other perturbations (45). And in both cases massive mortality of previously dominant taxa removed their priority effects, allowed rapid increases in some surviving, previously subordinate taxa, and resulted in novel species compositions.
Concluding Remarks.
Global warming is predicted to increase the frequency and magnitude of extreme climate and weather events (2, 4). Efforts to document these perturbations and to understand their environmental implications have been hampered by a lack of data, due in large part to a paucity of studies of sufficient duration and intensity (3, 13, 16). Consequently, many of the “extreme events” that have been studied are more frequent and predictable than the two storms documented here. For example, there is a substantial literature on the ecological impacts of the El Niño Southern Oscillation, and in particular on the effects of the high rainfall and severe drought during El Niño and La Niña years, respectively (8). Some of the impacts are similar to those reported here. In coastal Chile, for example, El Niño events resulted in substantial changes in desert rodent community composition, due in large part to immigration from surrounding habitats (46, 47). But other impacts are very different. For example, the main effect of El Niño was prolonged population increases fueled by a pulse of abundant new food resources, whereas the main effect of our severe storms was rapid population decreases due to catastrophic mortality. Moreover, El Niño did not have such a dramatic impact on the rodent community at Portal, where several El Niño events were not followed by increases in abundances (28).
Given the diverse phenomena that have been termed “extreme events,” the relatively small number of such events that have been studied, and the diverse ecological responses that have been documented at population, community, and ecosystem levels (see above), it is premature to make sweeping generalizations. Nevertheless, comparisons between the two extreme storms at Portal, and between them and other weather and climate events reported in the literature, suggest that there may be some emerging common themes. For example, massive differential mortality and resetting of community dynamics also occurred in plant communities in response to extreme drought conditions (16, 17). Mortality caused by a hurricane interacted with intrinsic predator–prey interactions to alter population trends in insular spider and lizard communities (48, 49). The effects of such perturbations would have been very difficult to predict, but, once documented, they provide invaluable insights into the processes of population dynamics and community assembly. Our study is unique, using intensive long-term sampling to document how extreme climatic events can decimate resident species populations, alter species composition and interspecific interactions, and influence invasion dynamics. These unexpected and important results emphasize the need for additional empirical and theoretical work on the ecological consequences of global climate change.
Methods
In 1977 J.H.B. and colleagues began a long-term study in the Chihuahuan Desert near Portal, AZ. Detailed information on the history of the study, monitoring protocols, experimental design, and previous results can be found in Brown (37). This study is restricted to the granivorous rodent community at the site, comprising eight species before the flood (D. merriami, D. ordii, C. baileyi, C. penicillatus, Perognathus flavus, Reithrodontomys megalotis, Peromyscus eremicus, and Peromyscus maniculatus). To assess trends over time, we calculated relative abundances of species, averaging monthly capture data over 6-month periods (January–June and July–December). Using relative rather than absolute abundances accounts for variation in total abundance of all species, which fluctuated with resource availability (Fig. 1). Trends in relative abundances before the flood are shown on two time scales: (i) to allow comparisons of long-term population dynamics, we analyzed all data since 1977; and (ii) to better characterize shorter-term trends before the flood and take into account the impact of the newly colonized species, we used only data after the first appearance of C. baileyi in 1995. Temporal trends were quantified by using ordinary least squares (OLS) regression. There were significant temporal autocorrelations in the population dynamics, but regressions were used only to describe trends, not to compare statistical significance. Expected abundances for 6-month periods after the flood were calculated by extrapolating the ordinary least squares regression equations; these postflood expected values were then compared with observed values for the corresponding 6-month period using paired t tests. Relative abundances were arcsine-transformed (). Percent survivorship of each rodent species was calculated as the percentage of individuals captured in one 6-month period (e.g., January–June 1999, immediately preceding the flood) that were recaptured in subsequent 6-month periods. The background rates of survivorship were calculated as the percentages of all individuals marked from 1978 through 1999 that were recaptured in subsequent 6-month periods. Because these values are simply percentages based on all data, there is only one value per species per 6-month period, and therefore no statistical comparison was performed. We used recapture rate as a surrogate for survivorship, because (i) it is a simple calculation, unburdened by assumptions about trappability, movement, and death; and (ii) the actual fate of each individual not recaptured is unknown and irrelevant to our conclusions, but the rapid, concerted, unprecedented disappearance of large numbers of individuals can only reasonably be attributed to mortality. To quantify changes in community composition through time, we followed Ernest and Brown (32) and used the ED to measure the difference in relative abundances of all species between successive 6-month periods.
ACKNOWLEDGMENTS.
We thank J. M. Chase, P. Chesson, N. M. Haddad, P. L. Meserve, S. Schwinning, and J. F. Weltzin for thoughtful reviews of the manuscript; S. K. M. Ernest, E. P. White, J. R. Goheen, and T. W. Perry for helpful discussion; and A. Ernest and the many others who assisted with the field work at Portal over the years. We thank the National Science Foundation Long-Term Research in Environmental Biology Program for funding (most recently Grant DEB-0702875). K.M.T. was supported by this grant and a National Science Foundation Graduate Research Fellowship.
Footnotes
The authors declare no conflict of interest.
References
- 1.Gordon HB, Whetton PH, Pittock AB, Fowler AM, Haylock MR. Simulated changes in daily rainfall intensity due to the enhanced greenhouse effect: implications for extreme rainfall events. Clim Dyn. 1992;8:83–102. [Google Scholar]
- 2.Easterling DR, et al. Climate extremes: Observations, modeling, and impacts. Science. 2000;289:2068–2074. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- 3.Weltzin JF, et al. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience. 2003;53:941–952. [Google Scholar]
- 4.Diffenbaugh NS, Pal JS, Trapp RJ, Giorgi F. Fine-scale processes regulate the response of extreme events to global climate change. Proc Natl Acad Sci USA. 2005;102:15774–15778. doi: 10.1073/pnas.0506042102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polis GA, Hurd SD, Jackson CT, Pinero FS. El Niño effects on the dynamics and control of an island ecosystem in the Gulf of California. Ecology. 1997;78:1884–1897. [Google Scholar]
- 6.Gannon MR, Willig MR. In: Forest Biodiversity in North, Central, and South America and the Caribbean: Research and Monitoring. Dallmeier F, Comisky J, editors. Washington, DC: Parthenon; 1998. pp. 271–291. [Google Scholar]
- 7.Holmgren M, Scheffer M, Ezcurra E, Gutierrez JR, Mohren GMJ. El Niño effects on the dynamics of terrestrial ecosystems. Trends Ecol Evol. 2001;16:89–94. doi: 10.1016/s0169-5347(00)02052-8. [DOI] [PubMed] [Google Scholar]
- 8.Holmgren M, et al. Extreme climatic events shape arid and semiarid ecosystems. Front Ecol Environ. 2006;4:87–95. [Google Scholar]
- 9.Allen CD, Breshears DD. Drought-induced shift of a forest-woodland ecotone: Rapid landscape response to climate variation. Proc Natl Acad Sci USA. 1998;95:14839–14842. doi: 10.1073/pnas.95.25.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmesan C, Root TL, Willig MR. Impacts of extreme weather and climate on terrestrial biota. Bull Am Meteorol Soc. 2000;81:443–450. [Google Scholar]
- 11.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 12.Grant BR, Grant PR. Evolution of Darwin's finches caused by a rare climatic event. Proc R Soc London Ser B. 1993;251:111–117. [Google Scholar]
- 13.Knapp AK, et al. Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science. 2002;298:2202–2205. doi: 10.1126/science.1076347. [DOI] [PubMed] [Google Scholar]
- 14.Chesson P, et al. Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia. 2004;141:236–253. doi: 10.1007/s00442-004-1551-1. [DOI] [PubMed] [Google Scholar]
- 15.Paine RT, Trimble AC. Abrupt community change on a rocky shore: Biological mechanisms contributing to the potential formation of an alternative state. Ecol Lett. 2004;7:441–445. [Google Scholar]
- 16.Miriti MN, Rodriguez-Buritica S, Wright SJ, Howe HF. Episodic death across species of desert shrubs. Ecology. 2007;88:32–36. doi: 10.1890/0012-9658(2007)88[32:edasod]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Haddad NM, Tilman D, Knops JMH. Long-term oscillations in grassland productivity induced by drought. Ecol Lett. 2002;5:110–120. [Google Scholar]
- 18.Fox BJ. Species assembly and the evolution of community structure. Evol Ecol. 1987;1:201–213. [Google Scholar]
- 19.Tilman D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA. 2004;101:10854–10861. doi: 10.1073/pnas.0403458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavaleta ES, Hulvey KB. Realistic species losses disproportionately reduce grassland resistance to biological invaders. Science. 2004;306:1175–1177. doi: 10.1126/science.1102643. [DOI] [PubMed] [Google Scholar]
- 21.Kelt DA, Taper ML, Meserve PL. Assessing the impact of competition on community assembly: A case-study using small mammals. Ecology. 1995;76:1283–1296. [Google Scholar]
- 22.Stoks R, McPeek MA. Predators and life histories shape Lestes damselfly assemblages along a freshwater habitat gradient. Ecology. 2003;84:1576–1587. [Google Scholar]
- 23.Burke MJW, Grime JP. An experimental study of plant community invasibility. Ecology. 1996;77:776–790. [Google Scholar]
- 24.Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends Ecol Evol. 2002;17:170–176. [Google Scholar]
- 25.Valone TJ, Brown JH, Jacobi CL. Catastrophic decline of a desert rodent, Dipodomys spectabilis: Insights from a long-term study. J Mamm. 1995;76:428–436. [Google Scholar]
- 26.Marques SC, Azeiteiro UM, Martinho F, Pardal MA. Climate variability and planktonic communities: The effect of an extreme event (severe drought) in a southern European estuary. Estuar Coast Shelf Sci. 2007;73:725–734. [Google Scholar]
- 27.Miriti MN. Twenty years of changes in spatial association and community structure among desert perennials. Ecology. 2007;88:1177–1190. doi: 10.1890/06-1006. [DOI] [PubMed] [Google Scholar]
- 28.Brown JH, Ernest SKM. Rain and rodents: Complex dynamics of desert consumers. Bioscience. 2002;52:979–987. [Google Scholar]
- 29.Brown JH, Valone TJ, Curtin CG. Reorganization of an arid ecosystem in response to recent climate change. Proc Natl Acad Sci USA. 1997;94:9729–9733. doi: 10.1073/pnas.94.18.9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thibault KM, White EP, Ernest SKM. Temporal dynamics in the structure and composition of a desert rodent community. Ecology. 2004;85:2649–2655. [Google Scholar]
- 31.White EP, Ernest SKM, Thibault KM. Trade-offs in community properties through time in a desert rodent community. Am Nat. 2004;164:670–676. doi: 10.1086/424766. [DOI] [PubMed] [Google Scholar]
- 32.Ernest SKM, Brown JH. Homeostasis and compensation: The role of species and resources in ecosystem stability. Ecology. 2001;82:2118–2132. [Google Scholar]
- 33.Ernest SKM, Brown JH. Delayed compensation for missing keystone species by colonization. Science. 2001;292:101–104. doi: 10.1126/science.292.5514.101. [DOI] [PubMed] [Google Scholar]
- 34.Brown JH, Munger JC. Experimental manipulation of a desert rodent community: Food addition and species removal. Ecology. 1985;66:1545–1563. [Google Scholar]
- 35.Heske EJ, Brown JH, Mistry S. Long-term experimental study of a Chihuahuan Desert rodent community: 13 years of competition. Ecology. 1994;75:438–445. [Google Scholar]
- 36.Valone TJ, Brown JH. Effects of competition, colonization, and extinction on rodent species diversity. Science. 1995;267:880–883. doi: 10.1126/science.7846530. [DOI] [PubMed] [Google Scholar]
- 37.Brown JH. In: Issues and Perspectives in Experimental Ecology. Resetarits WL Jr, Bernardo J, editors. Oxford: Oxford Univ Press; 1998. pp. 71–95. [Google Scholar]
- 38.Corbin JD, D'Antonio CM. Competition between native perennial and exotic annual grasses: Implications for an historical invasion. Ecology. 2004;85:1273–1283. [Google Scholar]
- 39.Davies NB. Territorial defence in speckled wood butterfly (Pararge aegeria): Resident always wins. Anim Behav. 1978;26:138–147. [Google Scholar]
- 40.Takeuchi T. Matter of size or matter of residency experience? Territorial contest in a green hairstreak, Chrysozephyrus smaragdinus (Lepidoptera: Lycaenidae). Ethology. 2006;112:293–299. [Google Scholar]
- 41.Genoways HH, Brown JH, editors. Biology of the Heteromyidae. Lawrence, KS: Am Soc Mammalogists; 1993. Special Publication No. 10. [Google Scholar]
- 42.Moulton MP, Pimm SL. In: Community Ecology. Diamond J, Case TJ, editors. New York: Harper and Row; 1986. pp. 80–97. [Google Scholar]
- 43.Sher AA, Hyatt LA. The disturbed resource-flux invasion matrix: A new framework for patterns of plant invasion. Biol Inv. 1999;1:107–114. [Google Scholar]
- 44.Paine RT. In: The Changing Scenes in Natural Sciences. Goulden CE, editor. Special Publication 12, Academy of Natural Sciences; 1977. pp. 245–270. [Google Scholar]
- 45.Jablonski D, Sepkoski JJ. Paleobiology, community ecology, and scales of ecological pattern. Ecology. 1996;77:1367–1378. [PubMed] [Google Scholar]
- 46.Meserve PL, Milstead WB, Gutierrez JR. Results of a food addition experiment in a north-central Chile small mammal assemblage: Evidence for the role of “bottom-up” factors. Oikos. 2001;94:548–556. [Google Scholar]
- 47.Meserve PL, Kelt DA, Milstead WB, Gutierrez JR. Thirteen years of shifting top-down and bottom-up control. Bioscience. 2003;53:633–646. [Google Scholar]
- 48.Schoener TW, Spiller DA, Losos JB. Predators increase the risk of catastrophic extinction of prey populations. Nature. 2001;412:183–186. doi: 10.1038/35084071. [DOI] [PubMed] [Google Scholar]
- 49.Schoener TW, Spiller DA. Nonsynchronous recovery of community characteristics in island spiders after a catastrophic hurricane. Proc Natl Acad Sci USA. 2006;103:2220–2225. doi: 10.1073/pnas.0510355103. [DOI] [PMC free article] [PubMed] [Google Scholar]