Abstract
A hallmark of the pathology of Alzheimer's disease is the accumulation of the microtubule-associated protein tau into fibrillar aggregates. Recent studies suggest that they accumulate because cytosolic chaperones fail to clear abnormally phosphorylated tau, preserving a pool of toxic tau intermediates within the neuron. We describe a mechanism for tau clearance involving a major cellular kinase, Akt. During stress, Akt is ubiquitinated and degraded by the tau ubiquitin ligase CHIP, and this largely depends on the Hsp90 complex. Akt also prevents CHIP-induced tau ubiquitination and its subsequent degradation, either by regulating the Hsp90/CHIP complex directly or by competing as a client protein with tau for binding. Akt levels tightly regulate the expression of CHIP, such that, as Akt levels are suppressed, CHIP levels also decrease, suggesting a potential stress response feedback mechanism between ligase and kinase activity. We also show that Akt and the microtubule affinity-regulating kinase 2 (PAR1/MARK2), a known tau kinase, interact directly. Akt enhances the activity of PAR1 to promote tau hyperphosphorylation at S262/S356, a tau species that is not recognized by the CHIP/Hsp90 complex. Moreover, Akt1 knockout mice have reduced levels of tau phosphorylated at PAR1/MARK2 consensus sites. Hence, Akt serves as a major regulator of tau biology by manipulating both tau kinases and protein quality control, providing a link to several common pathways that have demonstrated dysfunction in Alzheimer's disease.
Keywords: Alzheimer's disease, cell signaling, chaperone, MARK2/PAR1
The roles of the chaperones CHIP (carboxyl terminus of Hsc70-interacting protein) and Hsp90 (heat shock protein 90) are highly conserved throughout species, and both proteins are critical for quality control and stress recovery systems in most cell types. CHIP is a ubiquitin ligase that is essential for mitigating stress-related proteins after a cellular stress response (1, 2). CHIP binds with both Hsp70 and Hsp90 and thus has an opportunity to interact with and degrade a number of proteins through the Hsp70 or Hsp90 scaffold. It is now known that degradation of the microtubule-associated protein tau (tau), which is a significant component of Alzheimer's disease (AD) pathology, can be controlled by the Hsp90/CHIP complex (3, 4). This complex can facilitate the degradation of aberrant tau or promote its refolding back into native tau, depending on the presence of various Hsp90 binding partners (3). The impact of Hsp90 binding to a client is largely based on the repertoire of additional chaperones and/or cochaperones that are part of the complex at the time of the client/Hsp90 interaction. For example, tau degradation was prevented by deleting CHIP but enhanced by deleting prostaglandin E synthase 3 (P23). In addition, tau phosphorylated by microtubule affinity-regulating kinase 2 (PAR1/MARK2) was not recognized by the CHIP/Hsp90 complex and thus failed to get ubiquitinated or degraded, providing a link associating tau phosphorylation with degradation.
The master cellular kinase Akt has also been established as a client of the Hsp90 complex, and it can be degraded by inhibiting Hsp90 ATPase activity with small molecules aptly named Hsp90 inhibitors (5–7); however, the mechanism of degradation and the involvement of CHIP in this process have yet to be determined. Moreover, as many of the proteins comprising the Hsp90 complex are phosphorylated directly, we reasoned that, although Akt may indeed be a client of the Hsp90/CHIP complex, perhaps Akt was also regulating its activity during these periods of interaction and consequently may impact tau biology in a distinct way. Abnormal tau accumulation results from hyperphosphorylation of tau by a number of kinases, Akt included; however, regulation of tau degradation by Akt and the Hsp90/CHIP complex may play an even greater role in the abnormal accumulation that occurs with diseases of aging. Establishing such a relationship would also demonstrate a functional consequence of a kinase/chaperone signaling network that could be involved in the regulation of a number of other important proteins within the cell. Here we investigated whether CHIP, acting in concert with Hsp90, could promote Akt degradation. We also explored whether Akt could regulate the activity of the CHIP/Hsp90 complex, and we examined what impact this regulation might have on the ability of the chaperone complex to facilitate tau degradation, particularly with regard to degradation-resistant tau phosphorylated by PAR1/MARK2.
Results
Ablation of CHIP Leads to Increased Akt in Mouse Brain.
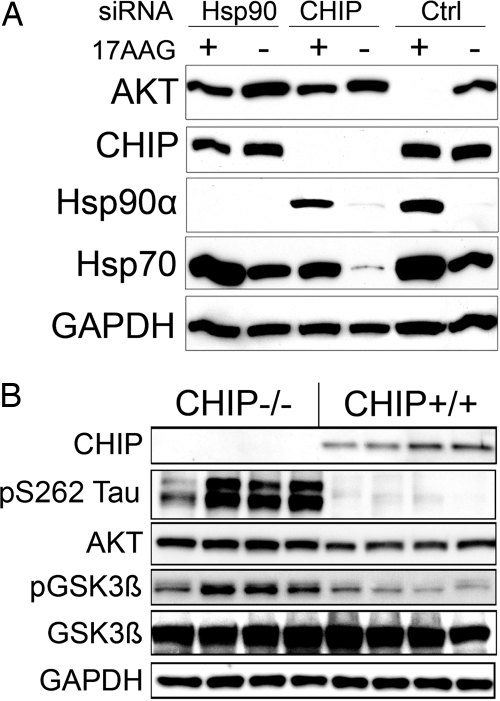
Basso et al. (5) found that, when Hsp90 was inhibited, Akt levels decreased, and we confirmed that result using the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG) in cells overexpressing Akt1 (Fig. 1A). Targeted knockdown of either CHIP or Hsp90α with siRNA [previously characterized (3)] attenuated 17-AAG-mediated degradation of Akt (Fig. 1A). As expected, 17-AAG increased the levels of both Hsp70 and Hsp90, whereas cells treated with CHIP siRNA had reduced basal Hsp70 levels (8). Knockdown of CHIP and Hsp90α also increased endogenous Akt levels relative to control [supporting information (SI) Fig. 7A].
Fig. 1.
The CHIP/Hsp90 complex is required for Hsp90 inhibitor-mediated Akt degradation. (A) HeLa cells transfected with siRNA pools for Hsp90α, CHIP, or a nonsilencing control were then transfected with WT Akt and treated with 0.5 μM 17-AAG for an additional 24 h. SiRNAs for both CHIP and Hsp90α prevented Akt degradation. (B) Brain homogenates from eight mice (four CHIP−/− and four CHIP+/+) were analyzed by Western blot using the indicated antibodies. Total Akt (P = 0.02) and pS9 GSK3β (P = 0.007) protein levels were significantly decreased (27% ± 7% and 39% ± 8%) in CHIP−/− mice compared with CHIP+/+ littermates after normalization to GAPDH and total GSK3β, respectively (SI Fig. 7B).
Given that CHIP ubiquitinates clients of the Hsp90 complex (9), such as Akt, we examined Akt levels in CHIP knockout mice. Compared with CHIP+/+ littermates, brain tissue homogenates from CHIP−/− mice showed significantly elevated levels of total Akt. Moreover, Akt's major cellular target, glycogen synthase kinase 3β (GSK3β), also exhibited significantly elevated levels in phospho-GSK3β Ser-9 compared with CHIP+/+ littermates, although total GSK3β was unchanged (Fig. 1B) (10). Phospho-tau was also elevated in CHIP−/− mice (Fig. 1B; quantitation in SI Fig. 7B).
To validate that this marked elevation in Akt levels and activity was indeed due to the absence of CHIP and not simply a result of aberrant kinase activation or stress-associated activities of the CHIP−/− mice, we examined levels of Akt in another diseased mouse model, twitcher, which has a similar phenotype caused by a naturally occurring nonsense mutation in the GALC (galactosylceramidase) gene (11). Unlike the CHIP knockout mice, the twitcher mice had no evidence of increased Akt levels or activity (SI Fig. 7C). These results indicate that the CHIP/Hsp90 complex is necessary for homeostatically regulating levels of Akt in the brain.
Interaction of Akt with CHIP in Brain Tissue.
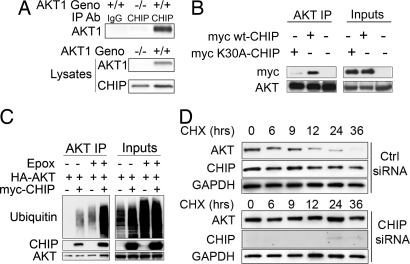
We then investigated the physiological interaction between Akt and CHIP in vivo. We coimmunoprecipitated CHIP from whole-brain homogenates of Akt1+/+ and Akt1−/− mice and determined that CHIP does bind to Akt1 (Fig. 2A). To check whether Hsp90 mediates the CHIP/Akt interaction, we used CHIP harboring the K30A mutation, which prevents CHIP from binding to Hsp70 and Hsp90 (SI Fig. 8A). The results confirmed that the CHIP/Akt interaction was largely dependent on Hsp90 or Hsp70, because mutated CHIP formed fewer complexes with Akt than WT CHIP (Fig. 2B).
Fig. 2.
CHIP binds Akt in a predominantly Hsp70/90-dependent manner, promoting its ubiquitination and degradation regardless of phosphorylation state. (A) CHIP coimmunoprecipitation from brain lysates of Akt1−/− and Akt1+/+ mice showed CHIP binding to endogenous Akt1 only in WT (+/+) mice. (B) HEK293 cells were transfected with HA-tagged WT Akt and myc-tagged WT CHIP, myc-CHIP harboring the K30A mutation, or empty vector. Akt coimmunoprecipitation showed that the K30A mutation in CHIP abrogated Akt/CHIP complex formation. (C) Cells were transfected with WT Akt and ubiquitin and either myc-CHIP or empty vector. After 24 h, 1 μM epoxomicin was added to media for 6 h. Akt coimmunoprecipitation showed enhanced CHIP/Akt complexes and ubiquitination of Akt in the presence of epoxomicin. (D) Cells transfected with CHIP or control siRNA for 72 h were then treated with 0.5 μM 17-AAG and 50 μM cycloheximide, and cells were harvested at indicated time points. CHIP siRNA prevented endogenous Akt degradation.
Based on that evidence, we determined whether CHIP promotes Akt ubiquitination in cell culture systems. Cells were cotransfected with WT Akt and myc-CHIP for 24 h and then treated with the protosomal inhibitor epoxomicin or vehicle for an additional 6 h. Coimmunoprecipitation of Akt revealed increased levels of polyubiquitinated Akt in cells cotransfected with CHIP (Fig. 2C); however, the amount of ubiquitin immunoreactivity markedly increased in the presence of the inhibitor, suggesting that Akt is processed in a proteasomal-dependent manner.
Next we determined whether CHIP is involved in the protein turnover of endogenous Akt. As shown in Fig. 2D, cycloheximide pulse–chase experiments showed that degradation of endogenous Akt decreased in Hs578t cells transfected with CHIP siRNA in the presence of 17-AAG compared with cells transfected with control siRNA and 17-AAG (Fig. 2D). Conversely, CHIP overexpression enhanced the 17-AAG-mediated degradation of overexpressed Akt (SI Fig. 8B). We also determined that CHIP interacted with Akt independent of its phosphorylation status, because both WT Akt and mutant Akt mock-phosphorylated at the T308 and S473 activation sites bound to CHIP (SI Fig. 8C). Together these results show that CHIP ubiquitinates and degrades Akt; however, this depends on the presence of Hsp90 and perhaps Hsp90 ATPase activity.
CHIP Levels in Akt1−/− Brain.
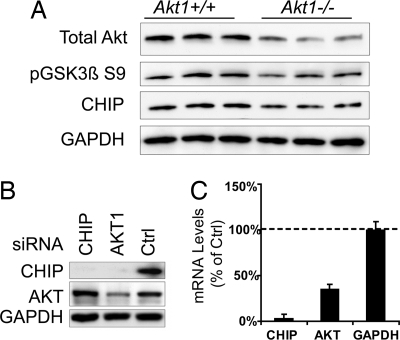
Previous work has suggested that the PI3K/Akt cascade is involved in the transcriptional regulation of the heat shock response (12, 13). Because CHIP maintains homeostasis by regulating the degradation of proteins during and after a stress response, we wanted to investigate the consequence of reducing Akt levels on CHIP, perhaps providing evidence to support Akt as a critical stress mediator. To determine whether Akt regulates CHIP expression, we explored the effect that Akt1 deletion had on CHIP levels in the brain. Akt1−/− brain homogenates had modestly, but statistically significantly, reduced CHIP protein levels (Fig. 3A; quantitation in SI Fig. 9A). Total Akt levels were reduced but not absent because of the presence of other Akt isoforms (14). Levels of phospho-GSK3β S9, a known consensus substrate of Akt, were also significantly reduced. This led us to speculate that Akt might regulate CHIP levels. SiRNA that targeted Akt1 (optimized in SI Fig. 10A) reduced both CHIP protein (Fig. 3B; second cell line in SI Fig. 9B) and mRNA levels (Fig. 3C), as determined by Western blot and real-time PCR, respectively. Surprisingly, overexpression of heat shock factor 1 (HSF1) or forkhead transcription factor 3A (FOXO3A), both of which are regulated upstream by the PI3K/Akt cascade, failed to reverse CHIP reductions induced by Akt siRNA (SI Fig. 9C). When Akt activity was inhibited with LY294002 or overexpressed with constitutive active Akt, neither CHIP protein (SI Fig. 9D) nor mRNA level (data not shown) was affected, indicating that CHIP expression is tied to Akt levels, but only to a threshold. These data suggest the presence of a feedback system based on Akt levels, not activity, that regulates the expression of CHIP through a previously uncharacterized signaling pathway.
Fig. 3.
Akt1−/− mice have reduced CHIP levels, and decreased Akt levels leads to lower CHIP expression. (A) Brain homogenates from six mice (three Akt1−/− and three Akt+/+) were analyzed by Western blot for total Akt levels, along with CHIP and pGSK3β S9 levels. For each of these proteins, expression was lower in Akt1−/− mice than in Akt1+/+ mice. Quantitation of CHIP reductions by densitometry showed a significant 17% reduction by Student's t test (P = 0.015) (SI Fig. 11A). (B) Hs578T cells transfected with Akt, CHIP, or control siRNAs for 72 h showed reductions in CHIP levels with either CHIP or Akt siRNA. (C) Cells were transfected in triplicate with the same siRNAs described in A. Real-time PCR for CHIP mRNA showed significantly reduced levels (>50%) in cells transfected with Akt siRNA. Primer specificity was confirmed with CHIP siRNA.
Akt siRNA Accelerates Tau Degradation by Inhibiting Hsp90.
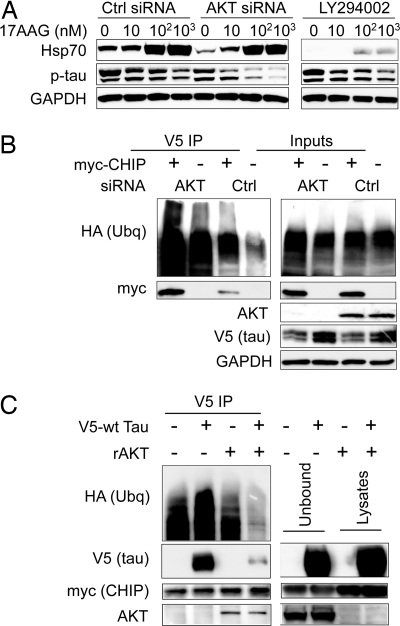
Given this novel interaction between Akt and CHIP in conjunction with the role of CHIP in contextual substrate-dependent stress recovery (1), we determined whether the presence of another CHIP substrate, in this case Akt, interferes with CHIP-mediated tau ubiquitination and degradation. First, we reduced Akt levels by siRNA and inhibited PI3K activity using LY294002 to determine how that would affect Hsp90 inhibitor-mediated tau degradation in cell culture. Consistent with our previous findings, we observed a dose-dependent decrease in phospho-tau levels after treatment with 17-AAG (4, 15). Both treatment with Akt siRNA (SI Fig. 10A) and exposure to LY294002 reduced 17-AAG-mediated induction of the heat shock proteins, although LY294002 had a much more pronounced effect (Fig. 4A and SI Fig. 10B). However, only Akt siRNA accelerated Hsp90 inhibitor-mediated tau degradation, suggesting that this process did not depend on HSF1 activation (Fig. 4A).
Fig. 4.
Reducing total Akt levels enhances phospho-tau degradation by Hsp90 inhibition and ubiquitination; altering PI3K activation reduces HSF1 activity but does not affect tau degradation. (A) Cells were transfected with control or Akt siRNA pools for 72 h and then transfected with V5-tau. After 24-h treatment with 0.5 μM 17-AAG or 100 μM LY294002 plus 17-AAG at the indicated concentrations, lysates were analyzed by Western blot. 17-AAG-mediated reductions in phospho-tau were enhanced by Akt siRNA but not LY294002. (B) Cells transfected with control or Akt siRNA for 48 h were then transfected with V5-tau, HA-ubiquitin, and myc-CHIP or empty vector. V5 coimmunoprecipitation revealed that Akt siRNA enhanced CHIP binding and ubiquitination of tau. (C) Cells were transfected with myc-CHIP and V5-tau or empty vector. CHIP/tau complexes were coimmunoprecipitated with an anti-myc antibody and incubated with 100 ng of recombinant Akt (rAkt) for 16 h. After washing, Western blot showed less tau bound to CHIP in those lysates incubated with rAkt. Inputs confirm the presence of rAkt in the unbound fraction.
Using coimmunoprecipitation, we found that, when we reduced Akt levels with siRNA, CHIP binding to and ubiquitination of tau was significantly enhanced (Fig. 4B). We also found that Akt and CHIP could independently and concomitantly bind tau, but the number of CHIP/tau complexes was reduced in the presence of Akt overexpression (SI Fig. 11). Coimmunoprecipitated CHIP/tau complexes from our cell culture model were then incubated with recombinant Akt. By adding recombinant Akt, we reduced the ability of CHIP to bind with tau (Fig. 4C), indicating that Akt may be a preferred substrate for CHIP, or that CHIP preferentially binds clients based on higher protein concentration, similar to the mechanism defined by Qian et al. (1). In summary, we found that reduced Akt levels enhanced CHIP-mediated tau ubiquitination and degradation and that Akt preferentially binds with CHIP compared with tau.
Akt Enhances PAR1-Mediated Phosphorylation of Tau.
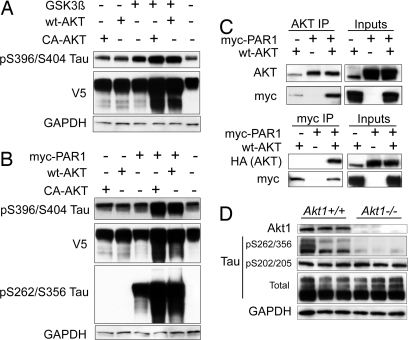
We previously showed that tau phosphorylated by PAR1/MARK2 could not be recognized by CHIP or degraded by Hsp90 inhibition (3, 4). Given the individual relationships between Akt and GSK3β, and between PAR1/MARK2 and GSK3β, we wanted to investigate the potential interaction among all three of these proteins, particularly in the context of how their interaction affects tau degradation. Cells were cotransfected with constitutively activated (CA) or WT Akt together with CA-GSK3β or PAR1/MARK2. Cells cotransfected with GSK3β and Akt had increased levels of tau phosphorylated at S396/S404, the preferred site for GSK3β activity, compared with cells transfected with GSK3β alone (Fig. 5A). Total tau levels (V5) also increased only in the presence of Akt, again implying Akt's role in preventing tau degradation. Tau phosphorylated at S262/S356 was not detected in any condition (data not shown).
Fig. 5.
PAR1 and Akt interact, enhancing the acceleration of phospho-tau accumulation; Akt1−/− mice have selectively reduced levels of tau phosphorylated at the PAR1 consensus sites on tau at S262/S356. (A) Cells were transfected with V5-tau and GSK3β alone or with either WT Akt or CA Akt harboring a myristoylation signal for 48 h and analyzed by Western blot. Tau phosphorylated at S396/S404 was elevated in cells cotransfected with each Akt plasmid and GSK3β. Total tau levels were also elevated (V5). Tau phosphorylated at S262/S356 was undetectable (data not shown). GAPDH was used for loading normalization. (B) Cells were transfected with V5-tau and PAR1 alone, or PAR1 together with either WT Akt or CA Akt, for 48 h and analyzed by Western blot. Tau phosphorylated at S396/S404 was elevated in cells cotransfected with each Akt plasmid and PAR1. Total tau levels were also elevated (V5). Tau phosphorylated at S262/S356 was detectable only in cells transfected with PAR1. GAPDH was used for loading normalization. (C Upper) Cells were transfected with WT Akt, myc-PAR1, or both for 48 h. Akt coimmunoprecipitation and myc immunoblotting showed that PAR1 bound to Akt. (C Lower) Reverse coimmunoprecipitation with myc demonstrated Akt binding with overexpressed Akt. Inputs refer to cell lysates before coimmunoprecipitation. (D) Brain homogenates from six mice (three Akt1−/− and three Akt+/+) were analyzed by Western blot using the indicated antibodies. Deletion of Akt1 was confirmed, and tau phosphorylated at S262/S356 was significantly lower in Akt1−/− mice. Total tau and GAPDH levels were unchanged.
Conversely, cells cotransfected with PAR1/MARK2 and either Akt vector had increased pS396/S404 tau immunoreactivity (Fig. 5B), which is consistent with previous findings that pS262/S356 can prime subsequent tau phosphorylation by other kinases (16). We found that tau was phosphorylated at S262/S356 when PAR1/MARK2 was overexpressed alone; however, cotransfection with either CA or WT Akt markedly enhanced immunoreactivity for this epitope (Fig. 5B). Again, total tau levels (V5) were higher when Akt was cotransfected with PAR1/MARK2. Given this potentially synergistic interaction between PAR1/MARK2 and Akt, we performed coimmunoprecipitation studies of cell lysates overexpressing WT HA-tagged Akt and myc-tagged PAR1. Pull-down studies with an Akt antibody showed Akt binding to PAR1 (Fig. 5C). The reverse immunoprecipitation between WT Akt and myc-PAR1 further validated the binding of these two kinases. We investigated how this interaction influenced PAR1/MARK2-mediated tau phosphorylation at S262/S356 in vivo by using brain tissue from Akt1−/− mice. Although total levels of tau were unchanged, we found significantly reduced levels of pS262/S356 tau (Fig. 5D) in Akt1−/− mice compared with WT littermates. As expected, Akt1 was not detected from the Akt-null homogenates. Therefore, Akt appears to either be activating MARK2/PAR1 through a direct interaction, or perhaps Akt primes tau, making it more accessible for MARK2/PAR1 activity. Together, these data reveal a new mechanism whereby Akt may prevent proteasomal degradation of tau on two fronts—by reducing the CHIP/tau interaction directly and by enhancing tau phosphorylation at S262/S356, which is not recognized by CHIP.
Discussion
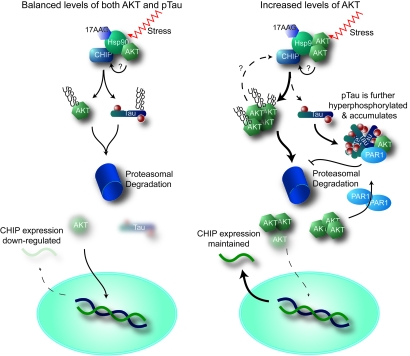
Because hyperphosphorylated tau pathology is present in the brains of AD patients, the role of kinases in the pathogenesis of AD has been studied extensively during the past two decades (17). Here we propose a role for Akt in AD pathogenesis, distinct from its kinase activity (Fig. 6). Our results suggest that Akt regulates the CHIP/Hsp90 complex; this role appears to have a greater impact on tau accumulation than phosphorylation. CHIP ubiquitinates and degrades Akt, particularly when Hsp90 ATPase activity is inhibited. Akt reduced CHIP-mediated tau ubiquitination and slowed its degradation. We also found a link between Akt and the critical tau kinase PAR1/MARK2, whereby Akt enhances phosphorylation of tau at S262/S356, a site that is not recognized by the CHIP/Hsp90 degradation complex. In particular, we show reduced phosphorylation of tau by PAR1/MARK2 in brain homogenates from Akt1−/− mice, providing additional evidence for cross-talk between Akt and PAR1/MARK2. Akt also appears to regulate the transcription of CHIP, but this regulation is tied to Akt level rather than activity. All of these findings point to a role for Akt as a mediator of chaperone biology, providing a distinct perspective about the role of kinases in protein degradation (5, 6).
Fig. 6.
Proposed mechanism by which unconventional Akt activity affects tau degradation and CHIP regulation. (Left) Under normal conditions, stress or Hsp90 inhibition (17-AAG) stimulates the CHIP/Hsp90 complex to recognize both Akt and phospho-tau, targeting them for proteasome-mediated degradation by ubiquitination. A consequence of reduced Akt levels would lead to decreased CHIP levels, either through direct transcriptional regulation or via a feedback mechanism. Akt may also regulate the CHIP/Hsp90 complex directly. (Right) As Akt levels increase with age or disease, the CHIP/Hsp90 complex either targets Akt over phospho-tau or alters CHIP activity. As tau accumulates, Akt and PAR1 synergize to enhance tau phosphorylation. Increased levels of Akt maintain CHIP expression.
We implicate the ubiquitin ligase CHIP as a critical component of this process (Fig. 1B). In fact, CHIP−/− mouse brain homogenates had increased levels of Akt and pGSK3β Ser-9, further suggesting the critical interaction between these two proteins (Fig. 1A). Although we have identified the mechanism of their interaction, we can only speculate on its purpose. For example, CHIP−/− mice have increased apoptotic activity throughout their bodies despite having elevated prosurvival Akt gene expression (2, 8). Therefore, Akt and CHIP might cooperate to balance apoptotic signaling. As Akt levels decrease, CHIP expression levels also decrease, promoting apoptosis (Fig. 3); however, as Akt levels increase, CHIP regulates Akt prosurvival activity by facilitating its degradation. Qian et al. (1) recently demonstrated a similar scenario for Hsp70, whereby CHIP maintained Hsp70 levels based on the cellular context. Thus, CHIP balances proteins that have a major impact on the cellular environment; however, if these protein levels fall below an acceptable limit, CHIP must be down-regulated to prevent further decreases. There also appears to be a threshold for CHIP levels, such that, despite Akt overexpression, CHIP remains constant, perhaps providing additional information about the mechanism of Akt oncogenesis: Akt levels within the cell might exceed the capacity of CHIP. Conversely, Akt siRNA dramatically reduced CHIP but only modestly affected Hsp induction. Because neither HSF1 nor FOXO3A rescued CHIP expression from Akt knockdown, we propose that a distinct pathway directly mediated by Akt is acting on the STUB1 (CHIP) promoter. Moreover, the disparity between LY294002-mediated and Akt siRNA-mediated Hsp induction suggests that a component of PI3K signaling that is distinct from the classical Akt cascade dramatically alters HSF1 activity.
Based on our previous work, which defined phospho-tau as a client of the Hsp90/CHIP complex (3, 4), we hypothesized that Akt has regulatory effects on the phospho-tau/CHIP interaction. Indeed, we found that tau degradation by Hsp90 inhibition was enhanced only when the endogenous Akt level, not Akt activity, was reduced (Fig. 4A). CHIP ubiquitination of Akt was also enhanced when Akt levels were reduced (Fig. 4B). Hence, Akt might regulate the Hsp90/CHIP complex by impairing or slowing the degradation of its anti-tau activity, and this regulation might occur by directly modifying the client-bound complex itself, competing with other substrates, or modifying the tau protein. The evidence we presented suggests that competition for CHIP binding plays a role. For example, when we overexpressed Akt, the number of CHIP/tau complexes decreased (SI Fig. 11A), and recombinant Akt abrogated the CHIP–tau interaction (Fig. 4C). This idea of substrate competition would be supported by work showing that CHIP-mediated stress recovery occurred by sequential ubiquitination of substrates and Hsp70 (1). However, given the role of Akt as a kinase and modulator of Hsp90 itself, along with our findings that Akt directly binds to tau (SI Fig. 11), multiple Akt-mediated mechanisms could be involved in the process of tau degradation. Our findings suggest that Akt be considered in a novel context with regard to AD; rather than strictly serving as a modifier of tau phosphorylation, Akt likely serves a much more important role as a regulator of tau degradation.
Another way in which Akt indirectly modifies tau biology is through the impact that it has on tau phosphorylation by other kinases, particularly given this new function for Akt as a negative regulator of tau degradation. We demonstrated that tau phosphorylated by PAR1/MARK2 at S262/S356 was unrecognizable to CHIP (3, 4). Furthermore, Nishimura et al. (16) showed that PAR1/MARK2 might nucleate tau hyperphosphorylation by priming it for other kinases. Indeed, Akt significantly enhanced the accumulation of phospho-tau in combination with either GSK3β or PAR1/MARK2, suggesting that Akt abrogates tau degradation and provides a higher concentration of exposed substrate for other tau kinases.
Perhaps the most intriguing result was the combined effect of Akt and PAR1/MARK2 on tau, namely, increased phosphorylation of tau at epitopes other than those typically phosphorylated by PAR1/MARK2 (16). This suggested that Akt either increased tau levels by preventing degradation, allowing PAR1/MARK2 to prime tau for other endogenous kinases, or modified PAR1/MARK2 activity directly. Indeed we found that Akt and PAR1/MARK2 interact directly, suggesting that one might regulate the other's activity. Using Akt1−/− mice, we also found that pS262/S356 tau was selectively reduced compared with total tau levels (Fig. 5D). This further implied a direct and functional interaction between Akt and PAR1/MARK2 in the brain, one in which Akt regulated PAR1/MARK2 activity. However, this could also indicate the significance of Akt in regulating the CHIP/Hsp90 complex with regard to tau biology. As Akt levels were depleted, not only did PAR1/MARK2 activity decrease, but tau degradation was also enhanced. Indeed, Akt might bridge PAR1/MARK2 with the CHIP/Hsp90 complex to prevent tau degradation on multiple fronts. Moreover, although the impact of this Akt/PAR1/MARK2 complex on the CHIP/Hsp90 complex itself is not known, the implications are far-reaching for AD research given the nature of the signaling cascades in which these kinases are involved and their link with AD pathogenesis.
Materials and Methods
Materials.
Akt1−/− mouse tissues were obtained from M.J.B. CHIP−/− mice were originally obtained from C.P.
Plasmids.
PAR1 was provided by Bing-Wei Lu (Stanford University, Stanford, CA). Constitutively active GSK3β was provided by Ben Wolozin (Boston University, Boston). Myc-CHIP, V5-tau, and HA-ubiquitin were generated by L.P.'s laboratory. All Akt constructs were provided by J.Q.C. Myc-CHIP K30A mutant was provided by C.P. HSF1 plasmid was provided by Richard Voellemy (University of Miami, Miami). FOXO3A plasmid was provided by Wenlong Bai (University of South Florida).
Antibodies, siRNAs, and Chemicals.
12E8 (anti-pS262/S356 tau) was provided by Peter Seubert (Elan Pharmaceutials, South San Francisco, CA). PHF1 (anti-pS396/S404) and CP13 (pS202/T205 tau) were provided by Peter Davies (Albert Einstein College of Medicine, Bronx, NY). Tau 5 (anti-total tau) was provided by Lester Binder (Northwestern University, Chicago). Anti-V5 was obtained from Invitrogen. Anti-myc and anti-HA were obtained from Invitrogen. Anti-Hsp70 and HSP90α were obtained from Stressgen. Anti-GAPDH was obtained from Biodesign. Anti-Akt, Akt1, GSK3β, and GSK3β Ser-9 were obtained from Cell Signaling Technology. Anti-CHIP and E1 were generated by our group. Secondary antibodies were obtained from Southern Biotechnology Associates and Jackson Immunochemicals. All antibodies were used at a 1:1,000 dilution, except for PHF1 and CP13, which were used at 1:100, and V5, which was used at 1:5,000. All siRNAs were obtained from Qiagen and were previously described and characterized at 20 nM final concentration (3). Akt siRNAs were validated by Qiagen (Akt1-5HP and Akt1-10HP). 17-AAG (500 nM) was obtained from A.G. Scientific. LY294002 (100 μM) and epoxomicin (100 nM) were obtained from Calbiochem. Cycloheximide (50 μM) was obtained from Sigma–Aldrich.
Mouse Tissue.
Akt1−/− brain tissue was provided by M.J.B. CHIP−/− mice were generated by C.P. and bred at the Mayo Clinic.
Cell Culture and Transfections.
HeLa and HEK293 cells were grown in Opti-Mem plus 10% FBS and passaged every 3–5 days based on 90% confluence. Hs578T cells were grown in Leibovitz medium with 10% FBS. SiRNA and coimmunoprecipitation experiments were carried out as previously described (2, 3).
Coimmunoprecipitations and Western Blotting.
Akt and tau ubiquitination levels in HeLa and HEK293 cells was performed as previously described (18, 19). Briefly, cell supernatants were incubated with capture antibodies and Fc binding protein overnight at 4°C. Supernatants were washed and subjected to Western blot analyses after SDS/PAGE electrophoresis. Blots were probed with indicated antibodies.
Brain Tissue Preparation.
Hemi-sected mouse brain was frozen on dry ice for subsequent biochemical analyses. Mice lacking CHIP or Akt1 expression (CHIP−/−, Akt1−/−) were generated as previously described (8, 20). CHIP−/− and CHIP+/+ mice were humanely killed at postnatal day 30, and brains were quickly removed for dissection and frozen on dry ice for subsequent biochemical analyses. Similarly, Akt1−/− and Akt1+/+ mice were humanely killed at 3 months of age, and tissues were harvested.
Quantitative Real-Time PCR.
Real-time PCR was performed as previously described (21). Briefly, total RNA was prepared by using the RNeasy kit (Qiagen) and reverse-transcribed by using SuperScript II (Invitrogen). cDNA was amplified by using 2× SYBR green master mix (Applied Biosystems) and analyzed on the Bio-Rad Opticon.
Statistical Analyses.
For cell culture and animal tissue experiments, Student's t test was used to assess significance, where reductions or increases in protein levels were demonstrated by Western blot. GAPDH immunoreactivity was used to normalize for lane-to-lane variation. All replicates within each experiment and the values from subsequent replicate experiments were included in the analyses.
Supplementary Material
ACKNOWLEDGMENTS.
This research was supported by the Alzheimer's Association, CurePSP, the American Federation for Aging Research, and National Institutes of Health Grants R01-AG17216-08, K99-AG31291, and R01-DK56886.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709180105/DC1.
References
- 1.Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C. Nature. 2006;440:551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickey CA, Yue M, Lin WL, Dickson DW, Dunmore JH, Lee WC, Zehr C, West G, Cao S, Clark AM, et al. J Neurosci. 2006;26:6985–6996. doi: 10.1523/JNEUROSCI.0746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, et al. J Clin Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickey CA, Dunmore J, Lu B, Wang JW, Lee WC, Kamal A, Burrows F, Eckman C, Hutton M, Petrucelli L. FASEB J. 2006;20:753–755. doi: 10.1096/fj.05-5343fje. [DOI] [PubMed] [Google Scholar]
- 5.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. J Biol Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 6.Neckers L. Trends Mol Med. 2002;8:S55–S61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 7.Morano KA, Thiele DJ. EMBO J. 1999;18:5953–5962. doi: 10.1093/emboj/18.21.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai Q, Zhang C, Wu Y, McDonough H, Whaley RA, Godfrey V, Li HH, Madamanchi N, Xu W, Neckers L, et al. EMBO J. 2003;22:5446–5458. doi: 10.1093/emboj/cdg529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 11.Duchen LW, Eicher EM, Jacobs JM, Scaravilli F, Teixeira F. Brain. 1980;103:695–710. doi: 10.1093/brain/103.3.695. [DOI] [PubMed] [Google Scholar]
- 12.Morley JF, Morimoto RI. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillin A, Crawford DK, Kenyon C. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- 14.Owada Y, Utsunomiya A, Yoshimoto T, Kondo H. J Mol Neurosci. 1997;9:27–33. doi: 10.1007/BF02789392. [DOI] [PubMed] [Google Scholar]
- 15.Dickey CA, Eriksen J, Kamal A, Burrows F, Kasibhatla S, Eckman CB, Hutton M, Petrucelli L. Curr Alzheimer Res. 2005;2:231–238. doi: 10.2174/1567205053585927. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura I, Yang Y, Lu B. Cell. 2004;116:671–682. doi: 10.1016/s0092-8674(04)00170-9. [DOI] [PubMed] [Google Scholar]
- 17.Dickey CA, Petrucelli L. Expert Opin Ther Targets. 2006;10:665–676. doi: 10.1517/14728222.10.5.665. [DOI] [PubMed] [Google Scholar]
- 18.Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, et al. Hum Mol Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 19.Petrucelli L, Dawson TM. Ann Med. 2004;36:315–320. doi: 10.1080/07853890410031948. [DOI] [PubMed] [Google Scholar]
- 20.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 21.Dickey CA, Loring JF, Eastman PS, Montgomery JR, Gordon M, Morgan DG. J Neurosci. 2003;23:5219–5226. doi: 10.1523/JNEUROSCI.23-12-05219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.