Abstract
Discovery of immunologically relevant antigens in prostate cancer forms the basis for developing more potent active immunotherapy. We report here a strategy using the transgenic adenocarcinoma of mouse prostate (TRAMP) model, which allows for the functional identification of immunogenic prostate tumor antigens with relevance for human immunotherapy. Using a combination of active tumor vaccination in the presence of CTL-associated antigen 4 (CTLA-4) in vivo blockade, we elicited tumor-specific T cells used to expression clone the first T cell-defined TRAMP tumor antigen, called Spas-1 (stimulator of prostatic adenocarcinoma specific T cells-1). Spas-1 expression was increased in advanced primary TRAMP tumors. We show that the immunodominant SPAS-1 epitope SNC9-H8 arose from a point mutation in one allele of the gene in TRAMP tumor cells, and that immunization with dendritic cells pulsed with SNC9-H8 peptide resulted in protection against TRAMP-C2 tumor challenge. In humans, the Spas-1 ortholog SH3GLB2 has been reported to be overexpressed in prostate cancer metastases. Additionally, we identified a nonmutated HLA-A2-binding epitope in the human ortholog SH3GLB2, which primed T cells from healthy HLA-A2+ individuals in vitro. Importantly, in vitro-primed T cells also recognized naturally processed and presented SH3GLB2. Our findings demonstrate that our in vivo CTLA-4 blockade-based T cell expression cloning can identify immunogenic cancer antigens with potential relevance for human immunotherapy.
Keywords: T cell antigen, immunotherapy, TRAMP mice, prostatic neoplasms
The goal of immunological approaches to tumor therapy is the induction of antitumor responses of sufficient strength to eradicate disseminated tumor and to prevent the reoccurrence of metastases. Until recently, the application of immunotherapy to prostate cancer has been limited compared with more immunogenic tumors such as melanoma or renal cell carcinoma. The inherent low immunogenicity of prostate cancer has been one reason that has long prevented the functional identification of tumor antigens using methods first pioneered by Boon and colleagues (1). Although irradiated prostate tumor cells can be and have been used as a crude source of tumor antigens in prostate cancer immunotherapy (2), knowledge of the targets of the T cell response to prostate cancer would allow the development of highly specific approaches to immunotherapy via specific immunization. Currently, a number of candidate tumor antigens have been targeted for prostate cancer solely based on their relatively restricted expression to the prostate or prostate cancer. These include prostate-specific antigen (PSA) (3), prostatic acid phosphatase (PAP) (4), prostate-specific membrane antigen (PSMA) (5), and a few other gene products (6). In these studies, potential relevant T cell epitopes were identified through a reverse immunology approach where T cell epitopes are predicted through computer algorithms according to known HLA-binding motifs. However, because these antigens are self-proteins, they remain poorly immunogenic. It is only through the recent development of more effective vaccination strategies that some of the preexisting immune tolerance to these self-antigens was overcome. As a result, promising biological outcomes are emerging in clinical trials (6, 7). We believe, however, that the efficacy of prostate cancer immunotherapies could be further improved if target antigens were identified through a functional approach rather than selected a priori based upon tissue expression.
Previously, we have shown that blockade of the inhibitory signals mediated by the T cell surface molecule CTLA-4 can greatly enhance T cell responses and, in many cases, result in tumor rejection in several mouse tumor systems (8, 9). In the case of B16 melanoma, tumor rejection through CTLA-4 blockade in combination with a GM-CSF-producing melanoma tumor cell vaccine was accompanied by autoimmune depigmentation of normal melanocytes, evidence that the antitumor T cell response was also, in part, directed to normal, bona fide self-antigens shared between the tumor, the vaccine, and melanocytes (10). One of these antigens was identified as the melanocyte differentiation antigen tyrosinase-related protein 2 (TRP-2) (11). Significantly, TRP-2 has also been shown to be a major target for T cells in melanoma patients and is currently in clinical trials as a melanoma vaccine.
In this study, we describe the use of anti-CTLA-4 therapy in the TRAMP model (12–14) for the identification of prostate tumor antigens. In previous studies, we demonstrated that CTLA-4 blockade by itself or in combination with a GM-CSF-expressing TRAMP tumor cell vaccine retarded the growth of transplantable TRAMP prostate tumors (unpublished results) and primary tumors spontaneously arising in the transgenic TRAMP mice (13). The same vaccination caused autoimmune prostatitis in syngeneic C57BL/6 mice, suggesting that TRAMP tumors may express prostate tissue-specific antigens that could become potential targets for immunotherapy (13). We hypothesized that, as demonstrated in the melanoma model, some of the target antigens of the immune prostatitis generated in the mouse will have human orthologs relevant to human prostate cancer. Importantly, because these targets are identified by their capacity to stimulate T cells, by definition they would be validated immunologically, making them ideal targets for immunotherapy. We applied the vaccination strategy of CTLA-4 in vivo blockade in combination with a GM-CSF-expressing TRAMP-C2 (TRAMPC2-GM) cell vaccine to generate a potent anti-TRAMP tumor response in nontransgenic, syngeneic C57BL/6 mice. The tumor-specific T cells were used to identify the first immunologically validated prostate cancer rejection antigen. We refer to this antigenic target in prostate cancer as SPAS antigen because of its ability to “stimulate prostatic adenocarcinoma-specific T cells.” We demonstrate that SPAS-1 has a human ortholog known as SH3GLB2, which is immunogenic in humans in vitro, making it a potentially attractive candidate target antigen for the development of antigen-targeted immunotherapies in humans.
Results
Generation of TRAMP-Reactive T Cell Hybridomas and Identification of Spas-1.
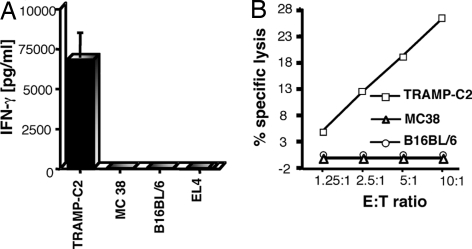
TRAMP-specific CD8+ T cell lines were established from spleens and lymph nodes of mice vaccinated with TRAMPC2-GM in combination with anti-CTLA-4 antibody treatment. We have shown that this vaccination protocol was sufficient to lead to complete rejection of transplantable TRAMP tumors (unpublished results). The function and specificity of the T cell line were assessed by using standard assays for IFN-γ production (Fig. 1A) and cytotoxicity (Fig. 1B) in response to incubation with a panel of syngeneic C57BL/6-derived tumors of different cellular origins. In both assays, the T cell line recognized TRAMP-C2 cells but not other tumors, including a melanoma (B16BL6), a colon adenocarcinoma (MC38), and a lymphoma (EL4) (Fig. 1A). To facilitate expression cloning of antigens responsible for stimulating the CD8+ T cell lines, we established T cell hybridomas by fusing the CD8+ T cells with the LacZ-inducible fusion partner BWZ.36 (15). Eight BTZ (C57BL/6-derived, TRAMP-reactive, LacZ inducible) hybridoma clones were generated and tested for retention of reactivity by measuring induction of LacZ activity after incubation with tumor cells [supporting information (SI) Fig. 7A]. Incubation with anti-Db but not anti-Kb blocking monoclonal antibodies resulted in partial inhibition of T cell activation, suggesting that eight of eight BTZs tested were specific for a TRAMP-C2 antigen presented by H-2Db MHC molecules (SI Fig. 7B). Next, we screened a cDNA library generated from TRAMP-C2 tumor cells with one of the T cell hybridoma subclones, BTZ5.65. A single cDNA clone was isolated from the library that was capable of stimulating BTZ5.65 and the remaining panel of T cell hybridomas (data not shown). The 1.0-kb cDNA insert contained 462 nt of an open-reading frame followed by an untranslated 3′ region (3′UTR) of 509 nt including a polyA tail. A full-length transcript was isolated that encoded a predicted protein of 395 aa (SI Fig. 8). We named this gene Spas-1, for stimulator of prostatic adenocarcinoma-specific T cells (GenBank accession no. EF676083). Search of the GenBank database revealed the existence of a human ortholog of mouse SPAS-1 of unknown function designated SH3-domain, GRB2-like, endophilin B2 (SH3GLB2, GenBank accession no. AF257319). Sequence alignment showed that the human and mouse genes share 90% identity at the nucleotide level and 96% identity at the amino acid level.
Fig. 1.
Generation of TRAMP-reactive CD8+ T cell lines and hybridomas. (A) The tumor specificity of the T cell line was determined by specific IFN-γ production in response to TRAMP-C2, MC38, B16BL6, or EL4 target cells. Standard deviations of triplicate cultures are shown. (B) The tumor specificity of the T cell line was determined by cytotoxicity using a standard JAM assay. T cells were cultured at varied effector to target cell ratios (E:T) with TRAMP-C2 or MC38 EL4 target cells.
Tissue Distribution of Murine Spas-1.
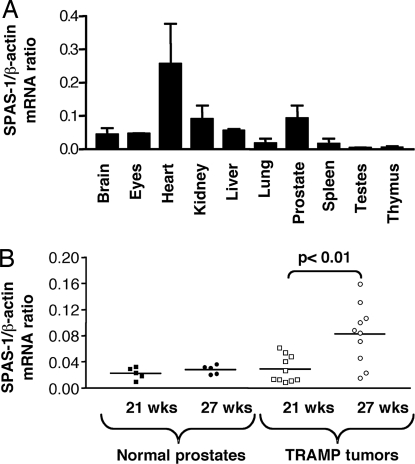
Real-time quantitative RT-PCR was performed to assess the expression of Spas-1 in normal mouse tissues. Spas-1 expression was not limited to the prostate but was found in other tissues, with the highest level of expression in the heart (Fig. 2A). To determine whether Spas-1 expression levels changed during tumorigenesis, real-time RT-PCR was performed on prostate tissues from age-matched C57BL/6 wild-type and TRAMP mice. Based on previous studies, TRAMP mice start developing severe intraepithelial hyperplasia of the prostate ≈20 weeks of age, progressing to full neoplasia by the time they are 24–30 weeks old (16). We therefore chose to determine levels of Spas-1 expression in prostate tumors from both 21- and 27-week-old TRAMP mice to determine expression changes during tumor progression. The expression levels of Spas-1 were significantly increased in prostate tumor tissues from 27-week-old TRAMP mice compared with normal prostate tissues from nontransgenic mice or prostate tissues from 21-week-old TRAMP mice (Fig. 2B).
Fig. 2.
Spas-1 expression is not restricted to the prostate but is increased in older TRAMP tumors. (A) Mouse Spas-1 expression in normal tissues: quantitative real-time PCR was performed on two individual panels of cDNA prepared from normal mouse tissues obtained from two adult C57BL/6 mice. Representative data from three experiments are shown. The error bars represent the standard deviation between two different mouse cDNA samples analyzed in the same experiment. (B) Mouse Spas-1 expression in normal versus tumor prostate: the level of Spas-1 expression was determined in a panel of normal prostates and primary TRAMP tumors from 21- and 27-week-old mice. Representative data from two experiments are shown. The P value (unpaired Student's t test) was <0.01 in both experiments.
Identification of the SPAS-1 T Cell Epitope.
To identify the antigenic peptide recognized by the BTZ1.4 T cell hybridoma, we limited our search to the last 152 C-terminal amino acids of the protein, which are encoded by the original SPAS-1 cDNA clone. We used the BIMAS computer algorithm to predict possible H-2Db-binding peptide motifs in this region. Eight synthetic peptides with the highest predicted binding scores (H-2Db-1 to H-2Db-8) were tested in vitro for their ability to stimulate BTZ1.4. Although the H-2Db-3 peptide (THVNHLHCL) led to activation of BTZ1.4, this activation appeared to be very weak, because even at a 100 nM peptide concentration, the activation plateau had not been reached (SI Fig. 9A). Through testing of deletion mutants, the antigenic region of SPAS-1 was narrowed down to the first 78 nt of the cDNA insert, the same region that also encoded the H-2Db-3 peptide (SI Fig. 9B). To ascertain this was indeed the correct antigenic peptide, we tested a set of overlapping minigenes spanning the predicted region for expression and presentation of the correct peptide to BTZ1.4 (SI Fig. 9C). Interestingly, the minigene coding for the predicted H-2Db-3 peptide did not lead to any T cell activation. The actual minimal peptide that led to the highest T cell activation was not THVNHLHCL, but rather STHVNHLHC (SNC9-H8), which was five logs more potent than THVNHLHCL in stimulating BTZ1.4 (SI Fig. 9D). That SNC9-H8 was indeed the naturally processed epitope of TRAMP-C2 cells was confirmed by the fact that the stimulatory activity of the synthetic peptide, and that of naturally processed tumor peptides extracted from TRAMP-C2 cells coeluted upon fractionation by reverse-phase HPLC (SI Fig. 9E).
Antitumor Effect of SPAS-1 Peptide Vaccination.
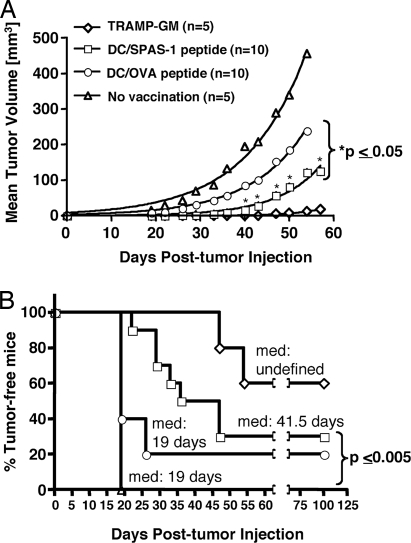
Next, we tested whether prophylactic vaccination with the SNC9-H8 peptide protects against TRAMP-C2 tumor growth. C57BL/6 mice were given four i.v. injections, 3 weeks apart, of bone-marrow-derived dendritic cells (BM-DC) pulsed either with the SNC9-H8 peptide or the irrelevant ovalbumin peptide SIINFEKL (SL8) before challenge with the transplantable TRAMP-C2 cells. As a positive control, mice received four s.c. injections of irradiated TRAMPC2-GM cells, which have been shown to have a protective effect against TRAMP-C2 tumor growth when given prophylactically (unpublished results). As expected, all of the untreated mice developed palpable tumors by day 19 post-tumor challenge, whereas tumor growth in mice that received the TRAMPC2-GM cell vaccine was delayed by at least 28 days (Fig. 3B). Vaccination of mice with SNC9-H8-pulsed BM-DC resulted in a statistically significant delay in tumor growth (medium time of 41.5 days) when compared with untreated mice or mice vaccinated with BM-DC pulsed with the irrelevant peptide SL8 (Fig. 3B). Despite the well documented nonspecific protective effect conferred by vaccination with BM-DC alone (17–20), targeting the response to SPAS-1 (SNC9-H8) led to a significantly greater delay in tumor growth rate compared with vaccination with SL8-pulsed BM-DC (P < 0.05) (Fig. 3A), confirming that SNC9-H8 was indeed a target of the anti-TRAMP tumor T cell response in vivo.
Fig. 3.
Antitumor effect of SPAS-1 peptide vaccination. Male C57BL/6 mice were vaccinated prophylactically four times, 21 days apart, with either SPAS-1 peptide (SNC9-H8), OVA peptide (SL8)-pulsed BM-DC, or with 1.5 × 106 irradiated TRAMPC2-GM tumor cells before TRAMP-C2 tumor challenge. (A) TRAMP-C2 tumor growth is shown for each vaccinated group. Statistics (unpaired Student's t test P value) are given for the SPAS-1 peptide (SNC9-H8) vaccinated group. (B) TRAMP-C2 tumor incidence is shown for each vaccinated group. Median time to tumor detection (med) and P values (Mantel–Haenszel test, GraphPad PRIZM.4) are indicated. Representative results of three independent experiments are shown.
SPAS-1 Epitope Mapping from Different Tissues Reveals a G to A Substitution in Position P8 of the T Cell Epitope.
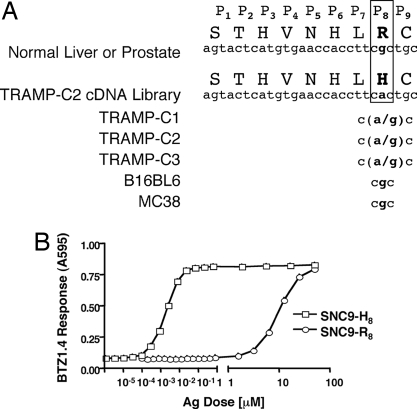
Having determined the genetic region in the Spas-1 gene that codes for the antigenic epitope, we compared the sequence of that region in transcripts isolated from the TRAMP-C2 cells with that of the Spas-1 transcripts obtained by RT-PCR from normal tissues, such as liver and prostate. As shown in Fig. 4A, the Spas-1 sequence obtained through expression cloning of the TRAMP-C2 cDNA library differed from that of normal tissues by a single nucleotide substitution (G to A), a change that translated into a histidine rather than an arginine at the eighth position (P8) of the antigenic epitope. This suggested two possibilities: (i) the antigenic epitope arose as a result of a point mutation in the tumor, or (ii) it resulted from genetic differences between the TRAMP mouse from which the TRAMP-C2 tumor line was derived and the C57BL/6 substrain we vaccinated to generate our TRAMP-specific CD8+ T cell line. The latter was ruled out by the fact that the Spas-1 sequence was identical in normal tissues of both C57BL/6 mice and TRAMP mice. We also ruled out the occurrence of a polymorphism in our C57BL/6 substrain by showing that the Spas-1 sequence was identical in C57BL/6 strains from three independent vendors (data not shown). Sequence analysis of genomic DNA derived from three different TRAMP transplantable tumor sublines revealed in each case a mixed sequence of G and A at the relevant position, whereas sequencing of the transplantable melanoma cell line B16BL6 or of the colon adenocarcinoma MC38 yielded only G at that position (Fig. 4A).
Fig. 4.
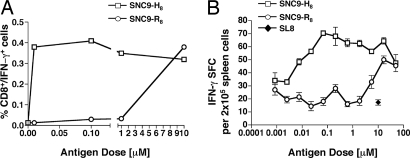
SPAS-1 epitope in normal and tumor tissues. (A) SPAS-1 epitope sequences from different tissues reveal a G to A substitution in position P8 of the T cell epitope: Sequence of the genetic region encoding the amino acid residue P8 in the SPAS-1 T cell epitope is shown for different tissues and tumor cell lines. (B) BTZ1.4 response to titrated amounts of synthetic peptide corresponding to either the wild-type (SNC9-R8) or mutated (SNC9-H8) SPAS-1 T cell epitope pulsed on H-2Db-expressing L cells. Standard deviations of triplicate cultures are shown.
To determine how the wild-type peptide, which contains an arginine at position 8 of the T cell epitope (SNC9-R8) would affect T cell recognition, we pulsed H-2Db-expressing L cells with synthetic peptides corresponding to either the mutated (SNC9-H8) or the wild-type (SNC9-R8) epitopes and determined their ability to stimulate BTZ1.4 (Fig. 4B). Although the T cell hybridoma recognized both the mutated SNC9-H8 and the wild-type SNC9-R8 peptides, the response to the wild-type peptide SNC9-R8 was 4 logarithms less potent than to its mutated counterpart.
TRAMP-Tumor Cell Vaccination in Combination with CTLA-4 Blockade Leads to T Cell Responses Against Both Wild-Type and Mutated SPAS-1 Epitopes in Vivo.
Because the TRAMP tumor cell lines expressed both the wild-type and the mutated form of SPAS-1, we questioned whether mice injected with the TRAMPC2-GM tumor cell vaccine in combination with CTLA-4 blockade could raise T cell responses not only to the mutated but also the wild-type SPAS-1 antigen. C57BL/6 mice were immunized with the TRAMPC2-GM tumor cell vaccine in combination with blocking antibodies to CTLA-4, followed by five vaccination boosts with the TRAMPC2-GM cell vaccine alone. Six days after the last vaccination, spleens were removed and assayed for antigen-specific IFN-γ production in response to SNC9-H8 and SNC9-R8 peptides. As expected, a strong response against the mutated SNC9-H8 peptide was detected, which titrated down into nanomolar peptide concentration. In addition, a significant T cell response was also observed at higher antigen dose in response to the wild-type peptide SNC9-R8 (Fig. 5). This response was 3–4 logarithms weaker compared with its mutated counterpart but showed that vaccination with the TRAMPC2-GM cell vaccine in combination with CTLA-4 blockade is capable of eliciting self-reactive SNC9-R8-specific T cells.
Fig. 5.
TRAMP-tumor cell vaccination in combination with CTLA-4 blockade leads to T cell responses against both wild-type and mutated SPAS-1 epitopes in vivo. Three C57BL/6 mice received the TRAMPC2-GM/anti-CTLA-4 vaccination regimen described in Methods. After the fifth and last boost with the tumor cell vaccine, their splenocytes were tested for specific IFN-γ production in response to titrated doses of either the mutated (SNC9-H8) or the wild-type (SNC9-R8) SPAS-1 peptides in an intracellular cytokine assay (A) and an ELISPOT assay (B). Ten micromolars of the OVA-SL8 peptide was used as irrelevant antigen in the ELISPOT assay. Standard deviations of triplicate cultures are shown. These are representative results of three independent experiments.
In Vitro SPAS-1/SH3GLB2 T Cell Responses in Human Peripheral Blood Lymphocytes.
To assess the immunogenicity of SPAS-1/SH3GLB2 in humans, we examined whether SPAS-1/SH3GLB2-reactive T cells could be elicited from peripheral blood lymphocytes of HLA-A2+ individuals. We used computer algorithms SYFPEITHI, BIMAS, and nHLApred to predict HLA-A2-binding epitopes in the human SPAS-1/SH3GLB2 protein. Five nonamer peptides (P1–P5) were synthesized that had high binding scores according to all three algorithms: P1, (LV-9); P2, (YL-9); P3, (LT-9); P4, (FL-9); and P5, (IL-9). Four of the five peptides bound to HLA-A2, as demonstrated by stabilization of surface HLA-A2 expression in a conventional T2-binding assay (SI Fig. 10).
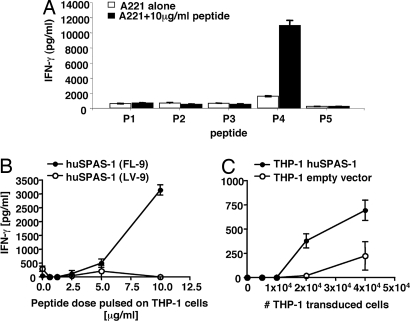
Next, we determined whether these peptides are immunogenic in vitro. T cell priming cultures were set up for each individual peptide with T cells from three healthy HLA-A2+ donors. For each individual HLA-A2+ donor, significant IFN-γ secretion was induced only in the culture restimulated with peptide P4, FLTPLRNFL (FL-9) (Fig. 6A), indicating that T cells specific to this SPAS-1/SH3GLB2 peptide can be induced in humans.
Fig. 6.
SPAS-1/SH3GLB2-derived peptide FL-9 is immunogenic in humans. (A) Mo-DC from HLA-A2+ healthy donors were pulsed with each of the five candidate peptides P1 (LV-9); P2 (YL-9); P3 (LT-9); P4 (FL-9); or P5 (IL-9) and then incubated separately with autologous PBMC for 10 days. After one restimulation, T cell cultures were assessed for their capacity to produce IFN-γ in response to the corresponding peptide, pulsed onto the B lymphoblastoid cell line 721.221 transfected with HLA-A2. (B) Human T cell cultures were assessed for their capacity to produce IFN-γ in response to titrated doses of FL-9, pulsed onto the HLA-A2 expressing cell line THP-1. (C) Responsiveness of the FL-9-reactive CD8+ T cell line to endogenously processed SPAS-1/SH3GLB2 peptides was assessed by coculturing 100,000 CD8+ T cells with titrated numbers of THP-1 cells transduced with either a retroviral vector encoding full-length human SPAS-1/SH3GLB2 DNA or with an empty retroviral vector. Results are representative of T cell cultures from three separate healthy donors. Representative results of four independent experiments and standard deviations of triplicate cultures are shown.
Finally, to determine whether the endogenously processed SPAS-1/SH3GLB2 peptides themselves are immunogenic, we overexpressed the SPAS-1/SH3GLB2 cDNA in the HLA-A2+ human monocytic leukemia-derived cell line THP-1 and asked whether it could activate FL-9-specific T cells. In agreement with our previous findings, specific activation of the FL-9-specific T cells was observed in response to titrated doses of the FL-9 but not the LV-9 peptide pulsed onto THP-1 cells (Fig. 6B). More importantly, FL-9-specific CD8+ T cells also recognized endogenously processed and presented SPAS-1/SH3GLB2 peptide, as demonstrated by the robust T cell activation in response to titrated numbers of THP-1 cells over-expressing SPAS-1/SH3GLB2 (Fig. 6C) compared with THP-1 cells transfected with an empty vector. This higher response was statistically significant over four independent experiments (unpaired Student's t test P value <0.05). The low level expression of endogenous SPAS-1/SH3GLB2 in vector control-transfected THP-1 cells (SI Fig. 11) might have contributed to the background activation of FL-9-specific T cells.
Discussion
We describe in these studies an approach for the functional identification of immunogenic tumor antigens in the TRAMP model of prostate cancer. Because these tumor antigens are defined by their capacity to stimulate T cell responses, our working hypothesis is that their human orthologs will be immunogenic as well and might become appealing candidate targets for future antigen-directed immunotherapies for prostate cancer. We name these immunogenic targets SPAS antigens, for “stimulators of prostatic adenocarcinoma-specific” T cells. By using T cells from mice immunized with a GM-CSF-expressing TRAMP cell vaccine in combination with in vivo CTLA-4 blockade, we have identified the first of these SPAS tumor antigens, SPAS-1, involved in T cell-mediated rejection of TRAMP tumors. The mouse Spas-1 gene, like its human ortholog SH3GLB2, encodes a protein of unknown function. We show that immunization with SPAS-1 peptide-pulsed dendritic cells results in protection against TRAMP-C2 tumor challenge. Because the immunodominant SPAS-1 epitope displays a point mutation that is found in all three available TRAMP-C tumor sublines, it seems likely that the mutation occurred in vivo, because all three sublines were derived from the same mouse but subcultured soon after excision of the primary tumor (12). To determine the frequency by which this mutation occurs in primary tumors, we sequenced the Spas-1 gene from 20 primary TRAMP tumors that were also used for expression analysis (see Fig. 2B) but were unable to detect that particular mutation again (data not shown). It is conceivable that this mutation might have been masked in our sequencing analysis by a larger pool of nonmutated cDNA derived from a heterogeneous cell population in the TRAMP tumor.
Recent published work has documented expression of human SPAS-1/SH3GLB2 in human prostate cancer and possibly in other tumor types. In one study, an extensive expression profiling not only allowed discrimination between normal prostate and tumor tissues, but also delineated three subgroups of prostate cancer that correlate with biological and clinical behavior. Interestingly, SPAS-1/SH3GLB2 appeared to be most highly expressed in the lymph node metastases of the most clinically aggressive subgroup III (21). These findings are corroborated by another published report that shows SPAS-1/SH3GLB2 to be the third most-abundant transcript in androgen-stimulated LNCap cells, a prototypic prostate cancer cell line derived from lymph node metastases (22). Finally, SPAS-1/SH3GLB2 was identified among the 40 most significantly up-regulated genes in malignant granular odontogenic tumors (GCOT) (23). Taken together, these findings are in agreement with our own expression data in mice, where increased expression of Spas-1 is observed in more advanced TRAMP tumors.
The broad expression pattern of Spas-1 found in mice raises the question of possible autoimmune side effects in the setting of active immunotherapy. It remains to be determined whether a similar expression pattern in normal tissue will be found in humans. Nonetheless, it has been shown that for many tumor antigens, including the PSMA (24), the antiapoptotic protein ML-IAP (25), the human telomerase reverse transcriptase hTERT (26), the proteinase-3 derived peptide PR1 (27), and the epidermal growth factor receptor HER2/neu (28) an antitumor T cell response can be elicited after vaccination without toxicity toward normal tissues. A proposed mechanism is that the epitopes processed and presented by normal tissues are below the threshold level for T cell recognition, whereas overexpression in tumor cells triggers an anticancer response by breaking previously established tolerance. Therefore, in the event that SPAS-1/SH3GLB2 is measurably overexpressed in cancer compared with normal tissues, it is conceivable that a T cell response can primarily be directed to the site with highest protein expression. Expression studies would need to be done at the protein rather than at the RNA level to evaluate this possibility.
Regarding the issue of immunological tolerance, we provide evidence that tolerance toward the self-antigen SPAS-1/SH3GLB2 is not complete in humans, as demonstrated by our ability to elicit T cells specific for the SPAS-1/SH3GLB2 peptide FL-9 in all healthy individuals tested. Importantly, we also show this reactivity can be directed to an endogenously processed and presented SPAS-1/SH3GLB2 epitope. Similarly, in the mouse, we demonstrate that T cell tolerance to SPAS-1 is not absolute, because vaccination with the TRAMPC2-GM tumor cell vaccine leads to T cell responses to both mutated SNC9-H8 and wild-type SNC9-R8 SPAS-1 epitopes. To fully address the potential usefulness of SPAS-1/SH3GLB2 as either an immunologic target or immune marker, the status of SPAS-1/SH3GLB2-specific T cell responses in prostate cancer patients will need to be determined.
From a preclinical aspect, the SPAS-1 SNC9-H8 epitope represents the first-identified tumor rejection antigen on TRAMP tumor cells. Although several studies have described the expression of other prostate cancer antigens in TRAMP tumor cells, such as PAP, prostate stem cell antigen, and PSMA (29–31), none of these have been shown to be targets for immune rejection in this model. Alternative approaches have been the generation of TRAMP cells expressing artificial antigens such as Flu-HA (32). We believe that the identification of SPAS-1 as the first-described endogenous T cell TRAMP tumor-specific antigen might facilitate the evaluation of vaccines in this mouse model for prostate cancer.
Our experimental strategy for the identification of T cell targets in the TRAMP model was based on the rationale that identified mouse antigens have human orthologs with potential relevance for immunotherapy in human prostate cancer. Our finding that one can elicit specific T cell responses to wild-type SPAS-1/SH3GLB2 in vitro from the blood of healthy individuals, together with the reported overexpression in advanced forms of human prostate cancer, supports the validity of our strategic approach. We propose that the strategy used to clone the SPAS-1 antigen can be broadly applied as a strategy for identifying T cell targets specific to a variety of tumors and, most importantly, with relevance to human cancers.
Methods
For additional details, see SI Methods.
Animals.
C57BL/6 mice were purchased from The Jackson Laboratory. Males from 5 to 7 weeks of age were used.
Tumor Cell Lines.
The TRAMP-C1, TRAMP-C2, TRAMP-C3, GM-CSF-expressing TRAMP-C2 (TRAMPC2-GM), and B7-expressing TRAMP-C2 (B7-TRAMP-C2) cell lines have all been described (12–14).
Immunizations and Generation of T Cell Lines and Hybridomas.
Five-week-old C57BL/6 mice received s.c. injections of 1.5 × 106 irradiated (12 krads) GM-TRAMP-C2 cells, six times, 3 weeks apart, and 3 i.p. injections of 100 μg of anti-CTLA-4 antibody [clone 9H10 (33)] on days 1, 4, and 7 after the first vaccination. CD8+ T cell lines were generated by restimulating splenocytes on mitomycin C-treated B7-TRAMP-C2. T cell hybridomas were generated and tested for specific responses by the production of β-galactosidase activity as described (15).
Mouse T Cell Activation Assays.
Specific T cell responses against peptide/MHC were measured by the production of IFN-γ by either ELISA, ELISPOT, or intracellular cytokine staining using a BD Bioscences kit or through cytolytic activity in a JAM assay according to a published protocol (34). For T cell lines, T cell activation assays were performed 5 days after the last restimulation. For ex vivo T cell responses, assays were performed 6 days after last vaccination.
Real-Time RT-PCR.
Real-time PCR for SPAS-1 and the reference β-actin was performed in an ABI Gene Amp 5700 machine with the SYBR green mastermix kit (Applied Biosystems). A standard curve established using known quantities of PCR template was used for both SPAS-1 and β-actin detection. CT values were set manually above a threshold that reflected linear amplification and converted into arbitrary units by using the known standard curve. SPAS-1 expression data were then normalized relative to β-actin.
BM-DC Vaccinations and Tumor Studies.
BM-DC were generated according to an established protocol (35). BM-DC were harvested on day 8 and pulsed with either 10 μM SPAS-1-SNC9-H8 peptide antigen or 10 μM OVA-SL8 control antigen for 4 h at 37°C. C57BL/6 mice were injected intravenously with 0.1 × 106 total BM-DC cells. Seven days after the last vaccination boost, mice received a s.c. injection of 1.0 × 106 live TRAMP-C2 cells.
Culture of Human DC and T Cell Lines.
Monocyte-derived dendritic cells (Mo-DC) were generated by culturing monocytes isolated from peripheral blood mononuclear cells (PBMCs) of healthy HLA-A2+ donors as described (36). The presence of SPAS-1 peptide-specific CD8+ T cells was determined by coculturing 1 × 105 CD8+ T cells with 5 × 104 HLA-A2-expressing 721.221 B lymphoblastoid cells (A221) cells pulsed with the appropriate peptide. Culture supernatants were collected 24 h later and tested for the presence of IFN-γ by ELISA. All human work was performed under the approved University of California, San Francisco, CHR protocol number H6872-17054-08.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. L. Lanier, D. Brockstedt, and S. Quezada for helpful advice and critical reading of the manuscript. This work was supported by University of California, San Francisco, Prostate SPORE National Institutes of Health Grant P50 CA89520 and by CaPCURE awards. J.P.A. holds the David H. Koch Chair for Immunologic Studies.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. EF676083).
This article contains supporting information online at www.pnas.org/cgi/content/full/0712269105/DC1.
References
- 1.van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 2.Hege KM, Jooss K, Pardoll D. GM-CSF gene-modified cancer cell immunotherapies: of mice and men. Int Rev Immunol. 2006;25:321–352. doi: 10.1080/08830180600992498. [DOI] [PubMed] [Google Scholar]
- 3.Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979;17:159–163. [PubMed] [Google Scholar]
- 4.Vihko P, et al. Molecular cloning and sequence analysis of cDNA encoding human prostatic acid phosphatase. FEBS Lett. 1988;236:275–281. doi: 10.1016/0014-5793(88)80037-1. [DOI] [PubMed] [Google Scholar]
- 5.Israeli RS, Powell CT, Fair WR, Heston WD. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–230. [PubMed] [Google Scholar]
- 6.Fong L, Small EJ. Immunotherapy for prostate cancer. Curr Oncol Rep. 2007;9:226–233. doi: 10.1007/s11912-007-0026-z. [DOI] [PubMed] [Google Scholar]
- 7.Elkord E. Immunology and immunotherapy approaches for prostate cancer. Prostate Cancer Prostatic Dis. 2007;10:224–236. doi: 10.1038/sj.pcan.4500964. [DOI] [PubMed] [Google Scholar]
- 8.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 9.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 10.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Elsas A, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster BA, et al. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- 13.Hurwitz AA, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 14.Kwon ED, et al. Manipulation of T cell Costimulatory and Inhibitory Signals for Immunotherapy of Prostate Cancer. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malarkannan S, Mendoza LM, Shastri N. Generation of antigen-specific, lacZ-inducible T cell hybrids. Methods Mol Biol. 2001;156:265–272. doi: 10.1385/1-59259-062-4:265. [DOI] [PubMed] [Google Scholar]
- 16.Gingrich JR, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 17.Eggert AO, et al. Specific peptide-mediated immunity against established melanoma tumors with dendritic cells requires IL-2 and fetal calf serum-free cell culture. Eur J Immunol. 2002;32:122–127. doi: 10.1002/1521-4141(200201)32:1<122::AID-IMMU122>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Hermans IF, Daish A, Moroni-Rawson P, Ronchese F. Tumor-peptide-pulsed dendritic cells isolated from spleen or cultured in vitro from bone marrow precursors can provide protection against tumor challenge. Cancer Immunol Immunother. 1997;44:341–347. doi: 10.1007/s002620050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salucci V, et al. Adenovirus transduction and culture conditions affect the immunogenicity of murine dendritic cells. Scand J Immunol. 2005;62:206–217. doi: 10.1111/j.1365-3083.2005.01658.x. [DOI] [PubMed] [Google Scholar]
- 20.Toldbod HE, Agger R, Bolund L, Hokland M. Potent influence of bovine serum proteins in experimental dendritic cell-based vaccination protocols. Scand J Immunol. 2003;58:43–50. doi: 10.1046/j.1365-3083.2003.01267.x. [DOI] [PubMed] [Google Scholar]
- 21.Lapointe J, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bainbridge MN, et al. Analysis of the prostate cancer cell line LNCaP transcriptome using a sequencing-by-synthesis approach. BMC Genomics. 2006;7:246. doi: 10.1186/1471-2164-7-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carinci F, et al. Genetic portrait of malignant granular cell odontogenic tumour. Oral Oncol. 2003;39:69–77. doi: 10.1016/s1368-8375(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 24.Renneberg H, et al. Prostate specific membrane antigen (PSM) is expressed in various human tissues: implication for the use of PSM reverse transcription polymerase chain reaction to detect hematogenous prostate cancer spread. Urol Res. 1999;27:23–27. doi: 10.1007/s002400050085. [DOI] [PubMed] [Google Scholar]
- 25.Schmollinger JC, et al. Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc Natl Acad Sci USA. 2003;100:3398–3403. doi: 10.1073/pnas.0530311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair SK, et al. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med. 2000;6:1011–1017. doi: 10.1038/79519. [DOI] [PubMed] [Google Scholar]
- 27.Heslop HE, Stevenson FK, Molldrem JJ. Immunotherapy of hematologic malignancy. Hematology Am Soc Hematol Educ Program. 2003:331–349. doi: 10.1182/asheducation-2003.1.331. [DOI] [PubMed] [Google Scholar]
- 28.Peoples GE, et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23:7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 29.Grossmann ME, Wood M, Celis E. Expression, specificity and immunotherapy potential of prostate-associated genes in murine cell lines. World J Urol. 2001;19:365–370. doi: 10.1007/pl00007104. [DOI] [PubMed] [Google Scholar]
- 30.Kawakami Y, et al. Identification of human tumor antigens and its implications for diagnosis and treatment of cancer. Cancer Sci. 2004;95:784–791. doi: 10.1111/j.1349-7006.2004.tb02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang D, et al. Murine six-transmembrane epithelial antigen of the prostate, prostate stem cell antigen, and prostate-specific membrane antigen: prostate-specific cell-surface antigens highly expressed in prostate cancer of transgenic adenocarcinoma mouse prostate mice. Cancer Res. 2001;61:5857–5860. [PubMed] [Google Scholar]
- 32.Drake CG, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 35.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 36.Fonteneau JF, et al. Generation of high quantities of viral and tumor-specific human CD4+ and CD8+ T-cell clones using peptide pulsed mature dendritic cells. J Immunol Methods. 2001;258:111–126. doi: 10.1016/s0022-1759(01)00477-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.