Abstract
More than 20 years ago, an oxytocin/vasopressin-like peptide, CLITNCPRGamide, was isolated from the locust, Locusta migratoria [Proux JP, et al. (1987) Identification of an arginine vasopressin-like diuretic hormone from Locusta migratoria. Biochem Biophys Res Commun 149:180–186]. However, no similar peptide could be identified in other insects, nor could its prohormone be cloned, or its physiological actions be established. Here, we report that the recently sequenced genome from the red flour beetle Tribolium castaneum contains a gene coding for an oxytocin/vasopressin-like peptide, identical to the locust peptide, which we named inotocin (for insect oxytocin/vasopressin-like peptide) and a gene coding for an inotocin G protein-coupled receptor (GPCR). We cloned the Tribolium inotocin preprohormone and the inotocin GPCR and expressed the GPCR in CHO cells. This GPCR is strongly activated by low concentrations of inotocin (EC50, 5 × 10−9 M), demonstrating that it is the inotocin receptor. Quantitative RT-PCR (qPCR) showed that in adult Tribolium, the receptor is mainly expressed in the head and much less in the hindgut and Malpighian tubules, suggesting that the inotocin/receptor couple does not play a role in water homeostasis. Surprisingly, qPCR also showed that the receptor is 30× more expressed in the first larval stages than in adult animals. The inotocin/receptor couple can also be found in the recently sequenced genome from the parasitic wasp Nasonia vitripennis but not in any other holometabolous insect with a completely sequenced genome (12 Drosophila species, the malaria mosquito Anopheles gambiae, the yellow fever mosquito Aedes aegypti, the silk worm Bombyx mori, and the honey bee Apis mellifera), suggesting that this neuropeptide system is confined to basal holometabolous insects. Furthermore, we identified an oxytocin/vasopressin-like peptide and receptor in the recently sequenced genome from the water flea Daphnia pulex (Crustacea). To our knowledge, this is the first report on the molecular cloning of an oxytocin/vasopressin-like receptor and its ligand from arthropods.
Keywords: inotocin, neuropeptide, GPCR, evolution, reproduction
Historically, vasopressin and oxytocin are the earliest characterized neuropeptides. These peptides were discovered in the beginning of the last century as biological activities from the mammalian posterior pituitary, causing antidiuresis and an increase in blood pressure (vasopressin) or powerful contractions of estrogen-treated uterus preparations and the ejection of milk from the mammary glands (oxytocin). In 1953, they were purified and sequenced by Acher and Chauvet (1) and du Vigneaud et al. (2, 3), i.e., clearly before the sequencing of any other neuropeptides. Both vasopressin and oxytocin are cyclic nonapeptides, containing a cystine bond between the cysteine residues at positions 1 and 6 (Table 1). The essential difference between vasopressin and oxytocin is the presence of a basic amino acid residue (mainly Arg) at position 8 in vasopressin and a neutral residue at position 8 in oxytocin (Table 1).
Table 1.
Structures of vasopressin, oxytocin, and some selected vasopressin- and oxytocin-like peptides
| Name | Peptide structure | Source | Ref. |
|---|---|---|---|
| Vasopressin | CYFQNCPRGamide | Mammals | 1, 3 |
| Lys-Vasopressin | CYFQNCPKGamide | Pig, some marsupials | 49 |
| Phenypressin | CFFQNCPRGamide | Some marsupials | 50 |
| Insect oxytocin/vasopressin-like peptide (inotocin) | CLITNCPRGamide** | Locusta migratoria | 19 |
| CLITNCPRGamide | Tribolium castaneum | Ref. 28, this paper | |
| CLITNCPRGamide* | Nasonia vitripennis | This paper | |
| Crustacean oxytocin/vasopressin-like peptide | CFITNCPPGamide* | Daphnia pulex | This paper |
| Vasotocin | CYIQNCPRGamide | Nonmammalian vertebrates | 51 |
| Arg-conopressin | CIIRNCPRGamide | Conus geographicus | 52 |
| Lys-conopressin | CFIRNCPKGamide | Leech, various mollusks | 53 |
| Oxytocin | CYIQNCPLGamide | Mammals | 2 |
| Isotocin | CYISNCPIGamide | Fish | 54 |
| Annetocin | CFVRNCPTGamide | Annelids | 55 |
| Cephalotocin | CYFRNCPIGamide | Octopus vulgaris | 56 |
| Octopressin | CFWTSCPIGamide | Octopus vulgaris | 57 |
All the above nonapeptides are cyclic and have a cystine bridge between amino acid residues 1 and 6. The amino acid residues in common with vasopressin are highlighted in bold. The vertebrate peptides listed above oxytocin are vasopressin-like (characterized by a basic residue in position 8); those below oxytocin are oxytocin-like (characterized by a neutral residue in position 8). The peptides marked with one asterisk are annotated only, using the recently sequenced genomes from D. pulex and N. vitripennis. The biologically active form of the peptide marked with two asterisks was originally reported to be its antiparallel dimer, whereas the monomer was reported to be inactive (19). This dimer was originally named arginine vasopressin-like insect diuretic hormone (19). Other laboratories, however, could not confirm the diuretic activity of the dimer (20). Our current paper now shows that the monomer is the biologically active form interacting with the receptor (Fig. 3) and, thus, the correct sequence. The monomer, which was originally referred to as fraction 1 (19), was renamed in the current paper as inotocin.
In the beginning of the 1980s, the preprohormones of vasopressin and oxytocin were cloned (4–6). Both precursor proteins contained, in addition to their neuropeptide sequences, a larger polypeptide sequence named neurophysin, which initially was thought to be a vasopressin- or oxytocin-carrier protein but nowadays is believed to play a crucial role in the proper folding and sorting of the vasopressin and oxytocin prohormones (7). Subsequently, one G protein-coupled receptor (GPCR) for oxytocin and three GPCRs for vasopressin (V1a, V1b, and V2) have been cloned and characterized from humans and other mammals (8–13).
Vasopressin and oxytocin have a wide range of partially overlapping biological activities. Vasopressin has antidiuretic actions in mammals by stimulating water reabsorption from the renal collecting ducts. It also increases blood pressure and is involved in various aspects of reproduction, such as male/female pair bonding and parental care (14–16). Oxytocin induces contractions of the uterus during birth and milk ejection from the mammary glands during lactation. However, this neuropeptide is also involved in parental care, and in female and male sexual and reproductive behaviors, such as lordosis and penis erection (15–17).
Most animals belong to two evolutionary lineages, the Protostomia (to which most invertebrates belong, among them insects), and Deuterostomia (some invertebrates and all vertebrates). Vasopressin- and oxytocin-like neuropeptides have been identified in representatives from both lineages (Table 1), indicating that the vasopressin/oxytocin hormonal system is evolutionary very old, originating before the split of Proto- and Deuterostomia, 640–760 million years ago (18).
Insects comprise >75% of all animal species. Together with other arthropods, such as crustaceans, they contribute to >80% of all animals. The presence of vasopressin/oxytocin in the phylum Arthropoda has long been unclear. Twenty years ago, using a RIA for vasopressin, two forms of a vasopressin-like peptide have been isolated from the locust Locusta migratoria, CLITNCPRGamide and its antiparallel dimer (19). The dimer was reported to have a diuretic activity in the locust, whereas the monomer was reported to be inactive (19). Other laboratories, however, could not confirm these results (20). The subsequent inability to clone the locust peptide gene and the fact that an oxytocin/vasopressin-like peptide could not be isolated from other insects did cast further doubt on the presence of such peptides in insects. These doubts were strengthened by the absence of oxytocin/vasopressin-like genes in the sequenced genomes from the fruitfly Drosophila melanogaster and 11 other Drosophila species (21), the malaria mosquito Anopheles gambiae (22), the yellow fever mosquito Aedes aegypti (23), the silkworm Bombyx mori (24, 25), and the honey bee Apis mellifera (26). To our great surprise, however, we and others have discovered a gene in the recently sequenced genome from the red flour beetle Tribolium castaneum, coding for an oxytocin/vasopressin-like peptide (27–29). In our present paper, we cloned this peptide gene and also described the cloning and functional characterization of a Tribolium oxytocin/vasopressin-like receptor gene, thereby firmly establishing the presence of this neuropeptide signaling system in the beetle. Furthermore, we have discovered the oxytocin/vasopressin system in the parasitic wasp Nasonia vitripennis and in the water flea Daphnia pulex (Crustacea).
Results
Cloning of the Inotocin Preprohormone.
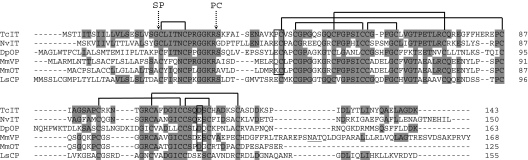
We have recently annotated a gene (GLEAN_06626), coding for a Tribolium preprohormone, containing an oxytocin/vasopressin-like peptide with the structure CLITNCPRGamide (Table 1) (27, 28). We call this monomeric peptide inotocin (insect oxytocin/vasopressin-like peptide; for nomenclature, see also the footnote of Table 1). To confirm that the annotated protein is correct and that it is expressed in Tribolium tissues, we have cloned its cDNA [Fig. 1; supporting information (SI) Fig. 6]. We could also find a similar preprohormone gene in the recently sequenced genomes from the parasitic wasp N. vitripennis and the water flea D. pulex (Table 1, Fig. 1). A comparison with the mouse vasopressin and oxytocin and the Lymnaea stagnalis (mollusk) conopressin preprohormones shows that these proteins have conserved their structural organizations since the split of Proto- and Deuterostomia, ≈700 million years ago (18). The vasopressin- and oxytocin-like peptide sequences are located directly after the signal sequence, followed by dibasic cleavage sites and neurophysin-like sequences (Fig. 1). These neurophysin-like sequences have 14 cysteine residues, suggesting that the prohormones have three rigid cage-like domains: the neuropeptide-containing domain (containing one cystine bridge) and two neurophysin domains (one containing four and the other three cystine bridges; Fig. 1; refs. 30 and 31). All three domains are highly conserved in mammals, insects, and mollusks. Five (of six) preprohormone genes have two introns in common (Fig. 1), confirming that these genes have a common evolutionary origin.
Fig. 1.
Alignment of the preprohormones for T. castaneum inotocin (TcIT; GenBank accession no. EU156489), N. vitripennis inotocin (NvIT; GenBank accession no. XP_001606547), D. pulex oxytocin/vasopressin-like peptide (DpOP), mouse (Mus musculus) vasopressin (MmVP; GenBank accession no. NP_033862), mouse oxytocin (MmOT; GenBank accession no. NP_035155), and L. stagnalis conopressin (LsCP; GenBank accession no. AAA29289). The amino acid residues identical to TcIT are highlighted. The common intron positions are indicated by vertical boxes. The cleavage sites for the signal peptidases (SP) and prohormone convertases (PC) are indicated by dotted arrows. These proteinases liberate dodecapeptides that are further processed to the amidated oxytocin/vasopressin-like nonapeptides. There are three domains containing cystine bridges: the nonapeptide domain (one cystine bridge), and two neurophysin domains containing four and three cystine bridges, respectively. This overall structure and many amino acid sequences, have been conserved in all six proteins. The N. vitripennis and D. pulex sequences have been annotated, the others have been experimentally determined (cDNA cloning). The introns for the L. stagnalis sequence could not be determined because of the lack of genomic information.
Cloning of the Inotocin Receptor.
The Tribolium genome also contains a gene (GLEAN_16363), coding for a GPCR that is structurally clearly related to the mammalian oxytocin and vasopressin receptors (27, 32). We cloned the cDNA of this GPCR gene, which contained a start codon preceded by several in-frame stop codons in the 5′ untranslated region and a polyadenylation consensus sequence in the 3′ untranslated region (SI Fig. 7). A comparison of the receptor cDNA with the genomic sequence revealed two nucleotide differences, of which one resulted in a changed amino acid residue (SI Table 2). This comparison also revealed five introns (SI Table 3). The cDNA codes for a protein which shows all of the hallmarks of a family-A GPCR (33).
Fig. 2 gives an alignment of the Tribolium receptor protein with the mouse oxytocin and vasopressin (V1a) receptors and the Lymnaea conopressin receptor. Furthermore, we discovered similar oxytocin/vasopressin-like receptors in the recently sequenced genomes from Nasonia and Daphnia (Fig. 2). The Tribolium GPCR and the mouse oxytocin receptor have 46% amino acid residue identities in the transmembrane regions (40% when the complete proteins are compared). Compared with the mouse vasopressin (V1a) receptor, these numbers are 48% and 42%, respectively.
Fig. 2.
Alignment of the T. castaneum inotocin receptor (TcITR; GenBank accession no. EU128495), N. vitripennis inotocin-like receptor (NvITR; GenBank accession no. XP_001600203), D. pulex oxytocin/vasopressin-like receptor (DpOPR), mouse V1a vasopressin receptor (MmVPR; GenBank accession no. NP_058543), mouse oxytocin receptor (MmOTR; GenBank accession no. NP_001074616), and L. stagnalis conopressin receptor (LsCPR; GenBank accession no. AAA91998) proteins. The residues identical to TcITR are highlighted. The transmembrane α-helices are indicated by TMI-TMVII. Introns are indicated by boxes. Potential glycosylation sites, occurring mainly in the extracellular N terminus, are underlined. The N. vitripennis and D. pulex sequences have been annotated, the others have been experimentally determined (cDNA cloning). The introns for the L. stagnalis sequence could not be determined because of the lack of genomic information.
A phylogenetic tree analysis confirms that the cloned Tribolium receptor is about equally related to the mouse oxytocin and vasopressin (V1a) receptors (SI Fig. 8). This analysis also shows that the Tribolium receptor is more closely related to the mammalian oxytocin and vasopressin receptors than to its closest Tribolium receptors, which are Tc 43 and Tc 44 [both orthologues of the Drosophila crustacean cardioactive peptide (CCAP) receptor CG6111] and Tc 46 and Tc 47 [both orthologues of the Drosophila adipokinetic hormone (AKH) receptor, CG11325], suggesting that it is an oxytocin/vasopressin-like receptor (SI Fig. 8).
Functional Expression in Cell Culture and Characterization of the Inotocin Receptor.
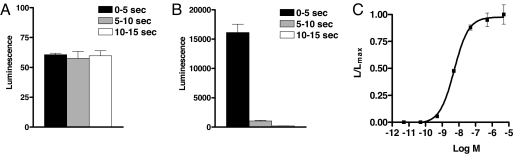
We stably transfected CHO cells in cell culture with DNA coding for the Tribolium oxytocin/vasopressin-like receptor and selected a cloned cell line expressing the receptor most effectively. Low concentrations of inotocin strongly activated the receptor (giving a Ca2+-signal, which is measured as a bioluminescence response) with a signal to noise ratio of >250 (Fig. 3 A and B) and an EC50 value of 5 × 10−9 M (Fig. 3C), demonstrating that the receptor is indeed an inotocin receptor.
Fig. 3.
Bioluminescence responses of non-transfected CHO/G-16 cells (A) and CHO/G-16 cells, expressing the inotocin receptor gene (B), 0–5 s (black), 5–10 s (gray) and 10–15 s (white) after addition of 5 × 10−6 M inotocin. Note that the scales in A and B are different. (C) Dose–response curve of the effect of inotocin on CHO/G-16 cells expressing the inotocin receptor. The EC50 of inotocin is 5 × 10−9 M. SEM are given as vertical bars, which are sometimes smaller than the symbols used (squares or lines). In these cases, only the symbols are given. In addition to inotocin, the inotocin receptor is also activated by Arg- and Lys-conopressins, vasotocin, oxytocin, and isotocin (EC50 values, >10−6 M). Vasopressin did not activate the receptor. Thirty-three other insect neuropeptides and eight biogenic amines (SI Text, Materials and Methods) did also not activate the receptor (tested up to 10−5 M).
The receptor was not activated by any other compound from our library of ≈40 insect neuropeptides and biogenic amines. The receptor was also not activated by mammalian vasopressin; however, it could be activated by vasotocin, oxytocin, isotocin, and Arg- and Lys-conopressin, although much higher concentrations were needed than for inotocin (EC50, >10−6 M). These results indicate that the Ile residue in position 3 present in inotocin and the other five neuropeptides, but absent in vasopressin (Table 1) is more important for receptor activation than the Arg residue in position 8, present in inotocin, vasopressin, vasotocin, and Arg-conopressin, but absent in oxytocin, and isotocin. Thus, functionally, the receptor is more oxytocin- than vasopressin-like.
Expression of the Inotocin Receptor and Peptide Genes in Tribolium.
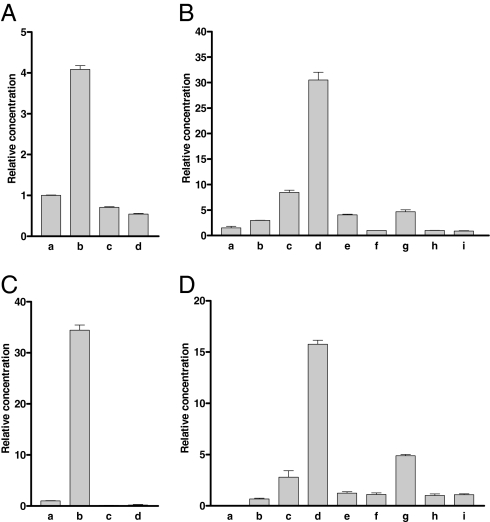
We used qPCR to investigate the expression of the inotocin receptor gene in Tribolium. This gene is mainly expressed in the head of adult animals (≈5× higher concentration of receptor mRNA compared with the rest of the body; Fig. 4A, bars b and c). There is no significant difference between male and female tissues (Fig. 4B, bars h and i). Of all tissues or regions investigated, the hindgut and Malpighian tubules showed the lowest receptor mRNA concentration (Fig. 4A, bar d). These results argue against a role of the inotocin receptor in water reabsorption in the kidneys (Malpighian tubules) or hindgut of the beetle.
Fig. 4.
qPCR of inotocin receptor and preprohormone mRNA in adults or different developmental stages from Tribolium. In each column, at least 60 animals were pooled. The qPCR experiments were run as triplets; the bars (which sometimes are smaller than the lines) represent SEM. Two different ribosomal proteins (rpL32 and rps3) were used as references and gave similar results. Each experiment was repeated at least twice. (A) Inotocin receptor mRNA in adult mixed male and female animals: a, whole bodies; b, heads; c, torsi (body minus head); and d, pooled hindguts and Malpighian tubules. The receptor mRNA concentrations given are relative to bar a (=1). (B) Inotocin receptor mRNA in different developmental stages (the sexes were mixed except for adult animals): a, eggs 0–24 h after egg laying; b, eggs 24–48 h after egg laying; c, eggs 48–72 h after egg laying; d, larvae 96–120 h after egg laying (≈0–1 d after hatching); e, larvae 15–16 d after egg laying; f, larvae 20–21 d after egg laying; g, pupae (24–25 d after egg laying); h, adult female, and i, adult male animals (27 d or more after egg laying). The receptor mRNA concentrations given are relative to bar h (=1). (C) Inotocin preprohormone mRNA in the same tissues as given in A. The mRNA concentrations given are relative to bar a (=1). (D) Inotocin preprohormone mRNA in the same developmental stages as given in B. The mRNA concentrations given are relative to bar h (=1).
We also investigated the expression of the inotocin receptor gene in various developmental stages (Fig. 4B). Eggs produce moderate amounts of receptor mRNA (depending on the developmental stage of the eggs: 1–8× the concentrations of adult animals) but, surprisingly, early larval stages (0–24 h after hatching) have a 30× higher concentration of receptor mRNA compared with adult beetles (Fig. 4B, bar d). These high concentrations of receptor mRNA decrease in later larval stages (Fig. 4B, bars e and f) but increase again in pupae (5× compared with adult animals; Fig. 4B, bar g). We also measured the expression of the inotocin peptide gene in the same samples as shown in Fig. 4 A and B. These peptide expression patterns paralleled and, thus, supported those from the receptor (Fig. 4 C and D).
Discussion
In terms of numbers, insects (or arthropods) are extremely important animals, but the presence of oxytocin- or vasopressin-like peptides and their receptors in this vast group of animals has long remained uncertain. In our current paper, we describe the cloning of an oxytocin/vasopressin (inotocin) preprohormone and an oxytocin/vasopressin (inotocin) GPCR from the red flour beetle T. castaneum, thus firmly establishing the presence of this neuropeptide system in arthropods.
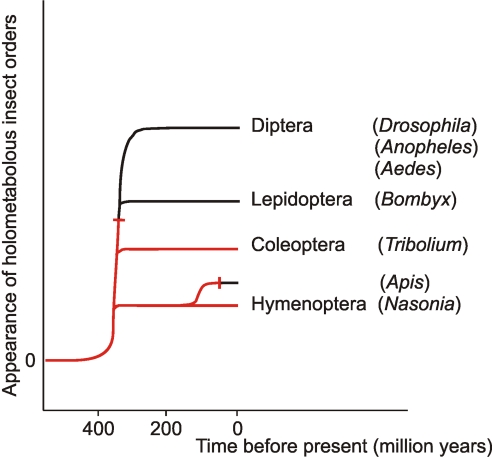
Intriguingly, however, not all arthropods use the oxytocin/vasopressin signaling system, and many insects appear to live perfectly well without it. Insects can be subdivided into Holometabola (insects with a complete metamorphosis: egg, several larval stages, pupa, adult) and Hemimetabola (insects without a clear metamorphosis, where the young animals, also called nymphae, resemble the adults). The major orders belonging to the Holometabola are Diptera (flies), Lepidoptera (butterflies and moths), Coleoptera (beetles), and Hymenoptera (bees, wasps, and ants). So far, all insects with a completely sequenced and published genome belong to the Holometabola, such as the fruitfly D. melanogaster and 11 other Drosophila species, the mosquitoes A. gambiae and A. aegypti (all three genera belonging to the Diptera), the silkworm B. mori (Lepidoptera), the beetle T. castaneum (Coleoptera), and the honey bee A. mellifera (Hymenoptera) (21–27). Bioinformatic screenings by our group showed that genes coding for oxytocin/vasopressin-like peptides and their GPCRs do not occur in any of the above-mentioned Diptera, Lepidoptera, or Hymenoptera (34). Surprisingly, however, the recently sequenced but unpublished genome from the parasitic wasp N. vitripennis does contain an inotocin and a putative inotocin GPCR gene (Figs. 1 and 2), suggesting that this neuropeptide system, within the Holometabola, is confined to Coleoptera and some basal groups of the Hymenoptera. Because the Coleoptera and Hymenoptera are basal orders within the Holometabola (35), the inotocin system appears to be confined to basal holometabolous insects.
The oxytocin/vasopressin system does also occur in Hemimetabola, because the locust L. migratoria, from which inotocin has been isolated (19), belongs to this group of insects. When we go further back in evolution, a special group of freshwater crustaceans, the Branchiopoda, is regarded to be the ancestor group of insects, from which insects evolved ≈420 million years ago (36). The water flea D. pulex is a branchiopod and the first crustacean, for which a genome has been fully sequenced (http://daphnia.cgb.indiana.edu). We have found that also Daphnia has an oxytocin/vasopressin-like peptide and an oxytocin/vasopressin-like GPCR gene (Figs. 1 and 2). Furthermore, other protostomian invertebrates, more basal than arthropods, such as annelids and mollusks, do also have oxytocin/vasopressin-like neuropeptides (Table 1) and their corresponding GPCRs (37–40). From these data, the following picture emerges (Fig. 5). The oxytocin/vasopressin hormonal system is an evolutionary old hormonal system, emerging before the split of Proto- and Deuterostomia. This system has persisted until arthropods, where it is present in crustaceans, and in basal insects. In the holometabolous insects, however, it has been preserved only in the evolutionary lines leading to coleopterans and basal hymenopterans, whereas the other major orders have abandoned it. This loss of the oxytocin/vasopressin system must have occurred at least two times during evolution (see dead-end signs in Fig. 5).
Fig. 5.
Schematic representation of the appearance of the major orders of holometabolous insects and the occurrence of the inotocin hormonal system (highlighted in red). This hormonal system has been conserved only in the evolutionary lines leading to basal holometabolous insects: Coleoptera (beetles) and Hymenoptera (wasps). The inotocin system must have been abandoned at least two times during the evolution of the Holometabola (see dead-end signs).
How is it possible that the oxytocin/vasopressin hormonal system has been conserved for several hundred million years in protostomian invertebrates, but that a large portion of the holometabolous insects are able to live without it? The answer to this intriguing question is difficult to give, but it could be that other hormonal systems have overtaken the function of inotocin and its GPCR. The inotocin GPCR is evolutionarily closely related to the insect AKH, CCAP, and corazonin receptors (SI Fig. 8; refs. 32 and 34). This implicates that these receptors have one common origin. It is interesting that also the AKH and corazonin neuropeptides are structurally related, again pointing to a common evolutionary origin of these hormonal systems. This ancestral hormonal system was responsible for the coordination of a certain physiological process. When the need arose for better fine tuning of this coordination, gene duplications of GPCR and ligand genes followed by mutations, created two hormonal systems that, however, still might have retained some overlap. A similar overlap might still persist between the physiological actions of the four (AKH, corazonin, inotocin, and CCAP) neuropeptide receptors shown in SI Fig. 8. This overlap might allow an insect group to abandon one of the hormonal systems, especially when the other receptors have duplicated, as is the case in Tribolium, where there are two CCAP and two AKH receptor genes compared with one of each in Drosophila (SI Fig. 8; refs. 32 and 34). We have recently learned that Tribolium has abandoned the corazonin receptor (SI Fig. 8; ref. 32). Similarly, the Diptera, Lepidoptera, and some groups of the Hymenoptera have abandoned the inotocin hormonal system, perhaps because they have preserved the corazonin system (SI Fig. 8; refs. 32 and 34).
What, then, might be the role of inotocin in insects? If the arguments of the above paragraph hold, the actions of inotocin should overlap with those of AKH (stimulation of carbohydrate and lipid mobilization), CCAP (stimulation of heart beat and induction of ecdysis behavior) or corazonin (induction of ecdysis behavior) (41–44).
The inotocin receptor is mainly expressed in the head (presumably the brain) and there is only low expression in the Malpighian tubules or hindgut (Fig. 4A), suggesting that inotocin does not stimulate water reabsorption as does mammalian vasopressin (14). The very high expression of the inotocin receptor in the early larval stages and in eggs and pupae (Fig. 4B) might point to a role in development of the animal. Future genetic studies, using RNAi, which works very well in Tribolium (45), might clarify the function of the inotocin signaling system in insects.
Materials and Methods
Animals, PCR, qPCR, and software are described in SI Text. Based on the annotated sequences of GLEAN_16363 and GLEAN_06626 (27, 32), PCR and qPCR primers were designed as described in the SI Text. All PCR products were cloned into pCR4-TOPO (Invitrogen) using the TOPO TA cloning kit (Invitrogen) and sequenced. The PCR product of the receptor coding sequence was subcloned into the pIRES2-EGFP expression vector (Clontech) using the Rapid DNA Ligation Kit (Roche Applied Science) and sequenced.
CHO cells stably expressing the human G protein G16 (CHO/G16) were grown as described (46) and transfected by using FuGENE HD transfection reagent (Roche Applied Science). The bioluminescence assay was performed as described (47). We tested our library of 8 biogenic amines and 33 invertebrate neuropeptides (48) and the neuropeptides mentioned in Fig. 3 (synthesized by GeneMed or Bachem).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Ann-Beth Nørholm for typing the manuscript, Thomas Pihl for technical assistance with cloning the inotocin preprohormone, and the Danish Research Agency (Research Council for Nature and Universe) and Novo Nordisk Foundation for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. EU128495, EU156489, XP_001606547, and XP_001600203).
This article contains supporting information online at www.pnas.org/cgi/content/full/0710897105/DC1.
References
- 1.Acher R, Chauvet J. La structure de la vasopressin de boeuf. Biochim Biophys Acta. 1953;12:487–488. doi: 10.1016/0006-3002(53)90173-5. [DOI] [PubMed] [Google Scholar]
- 2.du Vigneaud V, Ressler C, Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem. 1953;205:949–957. [PubMed] [Google Scholar]
- 3.du Vigneaud V, Lawler HC, Popenoe EA. Enzymatic cleavage of glycinamide from vasopressin and a proposed structure for this pressor-antidiuretic hormone of the posterior pituitary. J Am Chem Soc. 1953;75:4880–4881. [Google Scholar]
- 4.Schmale H, Heinsohn S, Richter D. Structural organization of the rat gene for the arginine vasopressin-neurophysin precursor. EMBO J. 1983;2:763–767. doi: 10.1002/j.1460-2075.1983.tb01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivell R, Schmale H, Richter D. Vasopressin and oxytocin precusors as model preprohormones. Neuroendocrinology. 1983;37:235–240. doi: 10.1159/000123549. [DOI] [PubMed] [Google Scholar]
- 6.Ivell R, Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci USA. 1984;81:2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bree FM, van der Kleij AA, Nijenhuis M, Zalm R, Murphy D, Burbach JP. The hormone domain of the vasopressin prohormone is required for the correct prohormone trafficking through the secretory pathway. J Neuroendocrinol. 2003;15:1156–1163. doi: 10.1111/j.1365-2826.2003.01114.x. [DOI] [PubMed] [Google Scholar]
- 8.Morel A, O'Carroll AM, Brownstein MJ, Lolait SJ. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992;356:523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- 9.Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356:526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaumer M, Seibold A, Gilbert S, Ishido M, Baberis C, Antaramian A, Brabet P, Rosenthal W. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992;357:333–335. doi: 10.1038/357333a0. [DOI] [PubMed] [Google Scholar]
- 11.Lolait SJ, O'Carroll AM, McBride OW, Konig M, Morel A, Brownstein MJ. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992;357:336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto T, Saito M, Mochizuki S, Watanabe Y, Hashimoto S, Kawashima H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J Biol Chem. 1994;269:27088–27092. [PubMed] [Google Scholar]
- 13.Thibonnier M, Auzan C, Madhun Z, Wilkins P, Berti-Mattera L, Clauser E. Molecular cloning, sequencing, and functional expression of a cDNA enconding the human V1a vasopressin receptor. J Biol Chem. 1994;269:3304–3310. [PubMed] [Google Scholar]
- 14.Bankir L. Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res. 2001;51:372–390. doi: 10.1016/s0008-6363(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 15.Debiec J. Peptides of love and fear: vasopressin and oxytocin modulate the integration of information in the amygdala. BioEssays. 2005;27:869–873. doi: 10.1002/bies.20301. [DOI] [PubMed] [Google Scholar]
- 16.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 18.Douzery EJ, Snell EA, Bapteste E, Delsuc F, Philippe H. The timing of eukaryotic evolution: does a relaxed molecular clock reconcile proteins and fossils? Proc Natl Acad Sci USA. 2004;101:15386–15391. doi: 10.1073/pnas.0403984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proux JP, et al. Identification of an arginine vasopressin-like diuretic hormone from Locusta migratoria. Biochem Biophys Res Commun. 1987;149:180–186. doi: 10.1016/0006-291x(87)91621-4. [DOI] [PubMed] [Google Scholar]
- 20.Coast GM, et al. A comparison of the effects of two putative diuretic hormones from Locusta migratoria on isolated locust Malpighian tubules. J Exp Biol. 1993;175:1–14. doi: 10.1242/jeb.175.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Drosophila 12 Genomes Consortium et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450:203–218. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- 22.Holt RA, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- 23.Nene V, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia Q, et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science. 2004;306:1937–1940. doi: 10.1126/science.1102210. [DOI] [PubMed] [Google Scholar]
- 25.Mita K, et al. The genome sequence of silkworm, Bombyx mori. DNA Res. 2004;11:27–35. doi: 10.1093/dnares/11.1.27. [DOI] [PubMed] [Google Scholar]
- 26.Weinstock GM, et al. Insights into social insects from the genome of the honey bee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards S, et al. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008 doi: 10.1038/nature06784. in press. [DOI] [PubMed] [Google Scholar]
- 28.Li B, et al. Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Res. 2008;18:113–122. doi: 10.1101/gr.6714008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amare A, Sweedler JV. Neuropeptide precursors in Tribolium castaneum. Peptides. 2007;28:1282–1291. doi: 10.1016/j.peptides.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen LQ, et al. Crystal structure of a bovine neurophysin II dipeptide complex at 2.8 A determined from the single-wavelength anomalous scattering signal of an incorporated iodine atom. Proc Natl Acad Sci USA. 1991;88:4240–4244. doi: 10.1073/pnas.88.10.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose JP, et al. Crystal structure of the neurophysin-oxytocin complex. Nat Struct Biol. 1996;3:163–169. doi: 10.1038/nsb0296-163. [DOI] [PubMed] [Google Scholar]
- 32.Hauser F, et al. A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum. Front Neuroendocrinol. 2008;29:142–165. doi: 10.1016/j.yfrne.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- 34.Hauser F, et al. A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog Neurobiol. 2006;80:1–19. doi: 10.1016/j.pneurobio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Savard J, et al. Phylogenomic analysis reveals bees and wasps (Hymenoptera) at the base of the radiation of holometabolous insects. Genome Res. 2006;16:1334–1338. doi: 10.1101/gr.5204306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glenner H, Thomsen PF, Hebsgaard MB, Sørensen MV, Willerslev E. Evolution. The origin of insects. Science. 2006;314:1883–1884. doi: 10.1126/science.1129844. [DOI] [PubMed] [Google Scholar]
- 37.Van Kesteren RE, et al. Coevolution of ligand-receptor pairs in the vasopressin/oxytocin superfamily of bioactive peptides. J Biol Chem. 1996;271:3619–3626. doi: 10.1074/jbc.271.7.3619. [DOI] [PubMed] [Google Scholar]
- 38.Kanda A, et al. Cloning of Octopus cephalotocin receptor, a member of the oxytocin/vasopressin superfamily. J Endocrinol. 2003;179:281–291. doi: 10.1677/joe.0.1790281. [DOI] [PubMed] [Google Scholar]
- 39.Kanda A, Satake H, Kawada T, Minakata H. Novel evolutionary lineages of the invertebrate oxytocin/vasopressin superfamily peptides and their receptors in the common octopus (Octopus vulgaris). Biochem J. 2005;387:85–91. doi: 10.1042/BJ20041230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levoye A, et al. Cloning, expression and pharmacological characterization of a vasopressin-related receptor in an annelid, the leech Theromyzon tessulatum. J Endocrinol. 2005;184:277–289. doi: 10.1677/joe.1.05833. [DOI] [PubMed] [Google Scholar]
- 41.Gäde G, Hoffmann KH, Spring JH. Hormonal regulation in insects: facts, gaps, and future directions. Physiol Rev. 1997;77:963–1032. doi: 10.1152/physrev.1997.77.4.963. [DOI] [PubMed] [Google Scholar]
- 42.Dulcis D, Levine RB, Ewer J. Role of the neuropeptide CCAP in Drosophila cardiac function. J Neurobiol. 2005;64:259–274. doi: 10.1002/neu.20136. [DOI] [PubMed] [Google Scholar]
- 43.Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behaviour by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 44.Zitnan D, Kim YJ, Zitnanová I, Roller L, Adams ME. Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen Comp Endocrinol. 2007;153:88–96. doi: 10.1016/j.ygcen.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klingler M. Tribolium. Curr Biol. 2004;14:R639–R640. doi: 10.1016/j.cub.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Stables J, et al. A bioluminescent assay for agonist activity at potentially any G-protein-coupled receptor. Anal Biochem. 1997;252:115–126. doi: 10.1006/abio.1997.2308. [DOI] [PubMed] [Google Scholar]
- 47.Staubli F, et al. Molecular identification of the insect adipokinetic hormone receptors. Proc Natl Acad Sci USA. 2002;99:3446–3451. doi: 10.1073/pnas.052556499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belmont M, Cazzamali G, Williamson M, Hauser F, Grimmelikhuijzen CJP. Identification of four evolutionarily related G protein-coupled receptors from the malaria mosquito Anopheles gambiae. Biochem Biophys Res Commun. 2006;344:160–165. doi: 10.1016/j.bbrc.2006.03.117. [DOI] [PubMed] [Google Scholar]
- 49.Chauvet MT, Colne T, Hurpet D, Chauvet J, Acher R. A multigene family for the vasopressin-like hormones? Identification of mesotocin, lysipressin and phenypressin in Australian macropods. Biochem Biophys Res Commun. 1983;116:258–263. doi: 10.1016/0006-291x(83)90409-6. [DOI] [PubMed] [Google Scholar]
- 50.Chauvet MT, Hurpet D, Chauvet J, Acher R. Phenypressin (Phe2-Arg8-vasopressin), a new neurohypophysial peptide found in marsupials. Nature. 1980;287:640–642. doi: 10.1038/287640a0. [DOI] [PubMed] [Google Scholar]
- 51.Acher R, Chauvet J, Lenci MT, Morel F, Maetz J. Présence d'une vasotocine dans la neurohypophyse de la grenouille (Rana esculenta L.). Biochim Biophys Acta. 1960;42:379–380. doi: 10.1016/0006-3002(60)90814-3. [DOI] [PubMed] [Google Scholar]
- 52.Cruz LJ, et al. Invertebrate vasopressin/oxytocin homologs. Characterization of peptides from Conus geographus and Conus striatus venoms. J Biol Chem. 1987;262:15821–15824. [PubMed] [Google Scholar]
- 53.Salzet M, Bulet P, van Dorsselaer A, Malecha J. Isolation, structural characterization and biological function of a lysine-conopressin in the central nervous system of the pharyngobdellid leech Erpobdella octoculata. Eur J Biochem. 1993;217:897–903. doi: 10.1111/j.1432-1033.1993.tb18319.x. [DOI] [PubMed] [Google Scholar]
- 54.Acher R, Chauvet MT, Crepy D, Chauvet J. Isolement d'une nouvelle hormone neurohypophysaire, l'isotocine, présente chez les poissons osseux. Biochim Biophys Acta. 1962;58:624–625. [Google Scholar]
- 55.Oumi T, et al. Annetocin: an oxytocin-related peptide isolated from the earthworm, Eisenia foetida. Biochem Biophys Res Commun. 1994;198:393–399. doi: 10.1006/bbrc.1994.1055. [DOI] [PubMed] [Google Scholar]
- 56.Reich G. A new peptide of the oxytocin/vasopressin family isolated from nerves of the cephalopod Octopus vulgaris. Neurosci Lett. 1992;134:191–194. doi: 10.1016/0304-3940(92)90514-8. [DOI] [PubMed] [Google Scholar]
- 57.Takuwa-Kuroda K, Iwakoshi-Ukena E, Kanda A, Minakata H. Octopus, which owns the most advanced brain in invertebrates, has two members of vasopressin/oxytocin superfamily as in vertebrates. Regul Pept. 2003;115:139–149. doi: 10.1016/s0167-0115(03)00151-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.