Abstract
Reactive gliosis is the universal reaction to brain injury, but the precise origin and subsequent fate of the glial cells reacting to injury are unknown. Astrocytes react to injury by hypertrophy and up-regulation of the glial-fibrillary acidic protein (GFAP). Whereas mature astrocytes do not normally divide, a subpopulation of the reactive GFAP+ cells does so, prompting the question of whether the proliferating GFAP+ cells arise from endogenous glial progenitors or from mature astrocytes that start to proliferate in response to brain injury. Here we show by genetic fate mapping and cell type-specific viral targeting that quiescent astrocytes start to proliferate after stab wound injury and contribute to the reactive gliosis and proliferating GFAP+ cells. These proliferating astrocytes remain within their lineage in vivo, while a more favorable environment in vitro revealed their multipotency and capacity for self-renewal. Conversely, progenitors present in the adult mouse cerebral cortex labeled by NG2 or the receptor for the platelet-derived growth factor (PDGFRα) did not form neurospheres after (or before) brain injury. Taken together, the first fate-mapping analysis of astrocytes in the adult mouse cerebral cortex shows that some astrocytes acquire stem cell properties after injury and hence may provide a promising cell type to initiate repair after brain injury.
Keywords: astrocytes, cell fate, cerebral cortex, stem cells
Reactive gliosis as the response of the CNS to injury is instrumental for sealing off the injured tissue, promoting tissue integrity and restricting inflammation and neuronal death (1, 2). Among glial cells, astrocytes, NG2-expressing glial precursors, and microglia take part in this response (2–4). Astrocytes react to brain injury by hypertrophy of their somata and processes (5), increased synthesis of glial-fibrillary acidic protein (GFAP), or reexpression of the progenitor markers vimentin and nestin (2, 6). Because a subpopulation of these reactive GFAP+ cells also divides (2, 6–8) it has been suggested that these cells arise from endogenous progenitors present in the adult brain (3, 4, 8, 9). Discovering the cellular origin of this reaction to brain injury not only is crucial to design manipulations toward a better repair (10) but also has broader relevance for our concept of mature cell types in the mammalian brain. If the dividing GFAP+ cells in reactive gliosis originate from previously proliferating cells, such as the widespread NG2+ progenitors (3, 4, 9), this implies that mature astrocytes do not resume proliferation and are permanently postmitotic cells, as the oligodendrocytes and neurons. Conversely, if reactive, proliferating GFAP+ cells originate from astroglial cells that resume proliferation after brain injury, this may imply a certain degree of dedifferentiation of astroglial cells toward an immature state. Indeed, proliferating astroglial cells in specific brain regions act as adult neural stem cells (11, 12), and a dedifferentiation of astrocytes toward earlier developmental stages, such as in the postnatal (13) or embryonic brain (14), may have obvious implications for the attempts to reconstitute neurons after brain injury.
Despite the importance of this question, fate mapping analysis has so far been restricted to the developing brain. Here we have used inducible Cre-mediated recombination to target adult astrocytes (15) and examine their progeny after brain injury. As an independent approach we pseudotyped lentiviral vectors to target astrocytes (16, 17). Both of these techniques allow us to permanently label quiescent mature astrocytes in the adult brain before injury and follow their progeny after stab wound (SW) lesion in the adult mouse neocortex.
Results
Fate Mapping of Quiescent Astrocytes upon SW Injury by Tamoxifen-Inducible Recombination in the Glutamate Aspartate Transporter (GLAST) Locus.
Targeting the tamoxifen-inducible form of the Cre recombinase (CreERT2) to the locus of the astrocyte-specific glutamate transporter GLAST (Slc1a3) allows inducing recombination in adult astrocytes in vivo (15) that can be monitored in the reporter lines R26R (18) or Z/EG (19). When R26R was used as an indicator of recombination, β-galactosidase (β-gal) was expressed mostly in mature astrocytes positive for the high-affinity glutamate transporter 1 (GLT1), S100β, glutamine synthetase (GS) (Fig. 1 A, A′, and B), and GLAST [knockin of Cre (15)] but negative for nestin, vimentin, and GFAP (Fig. 1B and data not shown). Conversely, no reporter+ cells were detected in control animals treated only with oil lacking tamoxifen even after brain injury [supporting information (SI) Fig. 4 A and B] (15). Although NG2 mostly labels proliferating glial progenitors, it was recently described on a small subset of astrocytes (15, 20). Consistent with these data, β-gal also labeled subpopulations of astrocytes that expressed NG2 or the transcription factor Sox10 (Fig. 1B). No reporter expression was instead detected in PECAM+ endothelial cells (SI Fig. 4 C–D′) or in GSA+ microglia (data not shown). Similar data were also obtained with Z/EG mice (86% of GFP+ cells are S100β). In line with a previous analysis (15), the efficiency of recombination was sufficient to label almost half of all astrocytes in the neocortex (see SI Fig. 5A).
Fig. 1.
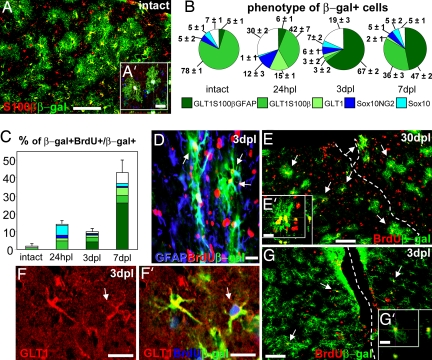
Proliferation and fate of genetically labeled astrocytes in the intact or injured mouse neocortex. (A and A′) β-gal+ cells exhibit an astrocytic morphology and coexpress S100β in the intact cortex (DAPI is blue in A, causing the white color in A′). (B) Identity of β-gal+ cells in the intact cortex and hours postlesion (hpl) or days postlesion (dpl). (C) Percentage and phenotype analysis of β-gal+ cells that incorporated BrdU provided in the drinking water (colors as indicated in B). (D–E′) A large number of genetically labeled β-gal+ astrocytes incorporated BrdU (arrows) 3 dpl (D) or 30 dpl (E) after BrdU in drinking water (dashed line, lesion track). Arrows in D point to BrdU+/β-gal+ cells displaying GFAP up-regulation. (F–G′) β-gal+ astrocytes incorporating BrdU (arrows) 2 h after a single BrdU injection. (Scale bars: 100 μm in A, E, and G and 40 μm in A′, D, E′, F, F′, and G′.)
In contrast to the high proliferation rate of NG2+ or Sox10+ progenitors in the adult cerebral cortex (7, 8), hardly any of the reporter+ cells incorporated the DNA base analogue BrdU, even after application of BrdU for 1 week in the drinking water (Fig. 1C, intact; see also SI Fig. 5B for comparable behavior of reporter− astroglia). These data therefore confirm and extend our previous analysis (15) that CreERT2-mediated recombination in the GLAST locus is targeted primarily to astroglial cells.
Upon SW injury performed in the adult cortex 6–8 days after tamoxifen induction, the number of β-gal+ cells per square millimeter increased by 30–50% within 150 μm from the lesion track compared with the contralateral side (SI Fig. 5C), consistent with the increase in astroglial cells after injury. To monitor whether cell division by the reporter+ cells contributed to this increase in number, BrdU was provided in the drinking water starting at the time of lesion to label all cells dividing upon injury (Fig. 1 C–E′). In contrast to the virtual absence of β-gal/BrdU double-labeled cells in the cortex before injury (Fig. 1C, BrdU for 1 week) or contralateral to the injury site (<2% at all stages), as early as 24 h postlesion >10% of all β-gal+ cells were BrdU+. By 7 days postlesion (dpl) this proportion increased to almost half of all fate-mapped astrocytes (Fig. 1 C–E′). Importantly, the vast majority of the BrdU/β-gal double-labeled cells expressed GLT1, S100β, or GFAP (Fig. 1C), indicating that approximately half of the formerly nonproliferating astrocytes starts to divide after injury. Notably, the proliferative activity of these reporter+ astrocytes closely corresponds to that of reporter− astrocytes (SI Fig. 5B). Astrocytes proliferating after injury could also be labeled by a single pulse of BrdU 2 h before mice were killed (10 ± 2% of β-gal+ cells were BrdU+, and 88 ± 7% of BrdU+/β-gal+ cells were GLT1+ at 3 dpl) (Fig. 1 F–G′), indicating that many of them undergo fast proliferation.
Even 4 weeks after injury, the vast majority (83 ± 3%) of the reporter+, BrdU+ cells were S100β+ and GLT1+, showing that most proliferating astrocytes remain within their lineage. This was the case also for the entire pool of reporter+ cells, of which 80% also contained GLT1 and S100β at 30 dpl (Fig. 1B and SI Fig. 5D). Although some of the reporter+ astroglial cells transiently down-regulated GLT1 and/or S100β because of acute damage (21), the majority of labeled cells up-regulated GFAP. Only 5% of β-gal+ astrocytes expressed GFAP in the uninjured cortex, and this proportion rose to 67% 3 dpl (Fig. 1B), with a subset of these cells also expressing vimentin and nestin. GFAP up-regulation declined at later time points (Fig. 1B) (48 ± 7% GFAP+ cells among reporter+ astrocytes at 30 dpl). Reporter+ astrocytes also participated in glial scar formation and contributed a constant proportion of reactive astrocytes at all stages after injury (SI Fig. 5 A, D, and E). Conversely, no neuroblasts or mature oligodendrocytes were detected among a total of 25,488 reporter+ cells examined after injury, suggesting that astrocytes do not generate these cell types at detectable frequencies (>0.01%). Thus, reporter+ astrocytes remain within the astroglial lineage and take part in reactive gliosis.
Fate Mapping of Quiescent Astrocytes upon SW Injury by Lymphocytic Choriomeningitis Virus (LCMV)-Pseudotyped Lentiviral Vectors.
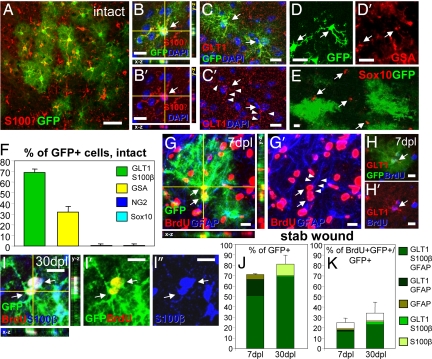
To examine the astroglial lineage exclusively at the site of injury and avoid any possible contribution of cells migrating from the adult stem cell niches or the white matter, we injected lentiviral vectors containing the enhanced GFP pseudotyped with the glycoproteins of the LCMV (16, 17) into the cortical gray matter (GM). This resulted in labeling of astrocytes and some GSA+ microglia, without any Sox10+ or NG2+ cells (Fig. 2 A–F). Not a single labeled cell incorporated BrdU before injury (0 of 220 GFP+ cells) whereas more than one-third had incorporated BrdU after injury (Fig. 2 G–K). The labeled cells that had initiated proliferation were GLT1+, GFAP+, or S100β+ astrocytes, and no GFP+ cells were double-labeled with NG2+ or Sox10+ cells after injury (0 of 147 GFP+ cells 7 dpl; 0 of 172 GFP+ cells 30 dpl) (Fig. 2 J and K). Similarly, no neurons or oligodendrocytes were detected among a total of 1,866 GFP+ cells examined after SW lesion. Thus, using two independent techniques we demonstrate that a considerable subset of previously quiescent astroglial cells initiates proliferation upon injury but does not undergo lineage transitions in vivo.
Fig. 2.
LCMV-targeted cells in the intact cortex and their progeny after lesion. (A–F) Most LCMV-infected GFP+ cells in the intact cortex have the morphology and antigen profile (S100β or GLT1, red in A–C′, arrows in B–C′) of protoplasmic astrocytes and do not contain Sox10 (red, arrows in E), whereas some are GSA+ microglia (red, arrows in D′). (F) Histogram depicting the proportion of cell types infected by the LCMV virus. (G–K) Progeny of GFP+ cells after injury with examples of GFP+ and GFAP+ (G and G′), GLT1+ (H and H′), and S100β+ (I–I″) astrocytes that incorporated BrdU (arrows point to triple-labeled cells) 7–30 dpl. (J and K) Histograms depict the identity of all GFP+ (J) or BrdU+/GFP+ (K) cells. Arrowheads in C′ and G′ indicate processes positive for GLT1 (C′) or GFAP (G′). (Scale bars: 100 μm in A and 30 μm in B-I″.)
Potential of Astrocytes After SW Injury.
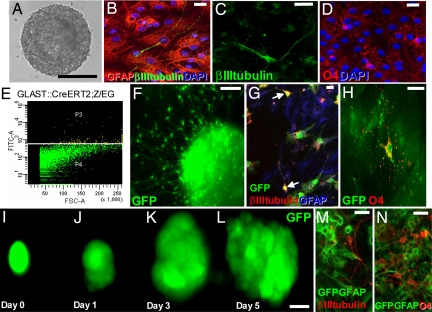
Because the environment in the adult injured brain is known to inhibit neurogenesis (22–24), we examined the potential of the cells reacting to injury 3 dpl using the neurosphere assay. Whereas no neurospheres were generated from cells isolated from the intact cortex contralateral to the SW, cells isolated from the injury site formed neurospheres (1/6,349 cells, n = 800,000 cells, four experiments) (Fig. 3A). These were able to self-renew for more than eight passages (SI Fig. 6A) and generated at each passage, βIII-tubulin+ neurons, GFAP+ astrocytes, and O4+ oligodendrocytes after 7–10 days in differentiation conditions (Fig. 3 B–D). To identify the source of these multipotent progenitors, we isolated astrocytes by FACS on the basis of their green fluorescence from the cortex of the hGFAP-eGFP transgenic mouse line (25) with GFP expressed in reactive astrocytes (data not shown) or the GLAST::CreERT2;Z/EG mice described above (Fig. 3 E–H). GFP+ cells sorted from the lesioned cortex at 3 dpl were all (100%, n = 126) S100β+ and GFAP+ and formed neurospheres with a 10–20 times increased efficiency (1/316 cells hGFAP-eGFP; 1/675 cells GLAST::CreERT2;Z/EG) in contrast to the lack of neurospheres from GFP+ cells isolated from the intact contralateral cortex (0 in 60,000 hGFAP-eGFP; 0 in 19,500 cells GLAST::CreERT2;Z/EG). Also, these neurospheres originating from astrocytes of the injured cortex self-renewed for more than six passages (data not shown) and generated neurons, astrocytes, and oligodendrocytes (Fig. 3 E–N). Notably, the GFP+ cells sorted from the GLAST::CreERT2;Z/EG mice were induced to express the reporter gene before injury as described above. The presence of GFP+ neurospheres therefore clearly demonstrates that astrocytes labeled before injury acquired the capacity to form neurospheres and that the GFP+ neurospheres were not derived from the small proportion of GFP− cells included in the sort (purity of sortings 95%).
Fig. 3.
Neurosphere-forming cells upon injury. (A–D and F–N) Micrographs depicting examples of neurospheres formed by cells isolated from the lesioned cortex (10 days in vitro) (A, F, and I–L) and their progeny after 10 days in differentiating conditions (B–D, G, H, M, and N). (E) The sorting profile of cortical cells isolated 3 dpl from the injury site of GLAST::CreERT2;Z/EG mice (E, sortings for F–H) with the GFP+ signal in yellow. (Scale bars: 50 μm in A, 20 μm in B–D and F–H, and 10 μm in I.)
To further ensure clonality, we visually inspected cells sorted into separate wells and examined each well containing a single GFP+ cell (n = 329) daily for 7–10 days (Fig. 3 I–L). Strikingly, one of 18 GFP+ cells gave rise to a multipotent neurosphere generating neurons (Fig. 3M), astrocytes, and oligodendrocytes (Fig. 3N). Thus, much of the relatively low efficiency in neurosphere formation seems to be due to death of the cells after sorting. Among the healthy GFP+ astroglial cells (all S100β+ or GFAP+; see above) >5% form neurospheres.
Finally, we aimed to determine whether other glial populations that react to injury may also acquire the capacity to form multipotent neurospheres. However, when we isolated cells from the injury site by NG2 or PDGFRα surface staining (SI Fig. 6 B and C), no multipotent neurospheres were obtained (0 of 35,000 and 25,000, respectively), even upon addition of PDGF to these cultures (26). These data therefore support the idea that mature astrocytes have a rather unique capacity to dedifferentiate and resume multipotency, in keeping with their developmental origin (27).
Viral Tracing of Stem Cell-Derived Lineages Originating in the Subependymal Zone (SEZ) Lining the Lateral Ventricle.
To clarify whether the neurosphere-forming GFP+ cells present in the cortex injury site may originate from the SEZ, one of the regions of continuous adult neurogenesis (11), we injected GFP-containing VSVG-pseudotyped lentiviral vector into the SEZ ipsilateral to the site of SW injury (28). Three days later, most GFP+ cells were detected within the SEZ and the rostral migratory stream with few cells migrating into the white matter (SI Fig. 7 A and B), but none had entered the cortical GM surrounding the site of injury (SI Fig. 7B). To ensure that smaller numbers of GFP+ cells did not escape detection, we used FACS analysis and found that 2.6% of SEZ cells were GFP+ (SI Fig. 7C), whereas no GFP+ cells were detectable in the tissue isolated from the injury site (SI Fig. 7D). This analysis demonstrates that the neurosphere-forming cells that occur with a frequency >5% are not derived from stem cells in the SEZ.
Discussion
The possibility of targeting quiescent astrocytes by either genetic or viral manipulations and following these cells during the reactive response to injury allowed establishment of a direct lineage relation between reactive and quiescent astroglia. Because our fate mapping technique labels a considerable proportion of astrocytes that also exhibit a proliferative behavior similar to reporter− astroglia, we conclude that a considerable proportion of quiescent astrocytes resumes proliferation upon injury and contributes to the generation of reactive astrocytes.
In the adult cerebral cortex mature astrocytes lack expression of GFAP, nestin, vimentin, and tenascin-C and do not proliferate. However, these proteins are contained in some reactive glial cells, some of which also proliferate (2, 8, 29, 30), and in more immature glia such as radial glia and postnatal glial progenitors, which also proliferate (13, 27, 31). Because our fate mapping analysis now reveals that reactive astroglia derive from mature astrocytes, these data suggest that astrocytes exposed to injury may indeed resume properties of glia present at earlier developmental stages. Thus, in contrast to mature oligodendrocytes and neurons, mature astrocytes are not permanently postmitotic but rather retain the capacity to resume proliferation and up-regulate developmental features, a process that we refer to as “dedifferentiation.”
Even more importantly, we demonstrate that these changes occurring in astrocytes are accompanied by the acquisition of stem cell properties, because some of the astrocytes labeled before injury acquire the capacity to form multipotent and self-renewing neurospheres. Notably, astrocytes lose the potential to form neurospheres after the second postnatal week (32), further supporting the concept that reactive astrocytes activate properties of earlier developmental stages. Indeed, we could also show that neither the astrocytes proliferating after injury nor the neurosphere-forming astrocytes are derived from the neurogenic SEZ. These data therefore demonstrate for the first time, to our knowledge, a novel source of multipotent cells in the adult cerebral cortex after brain injury. Strikingly, this is not a general feature of glial cells reacting to injury. Glial progenitors that actively proliferate in the adult cortex even before injury, such as NG2+ or PDGFRα+ cells (3, 4, 8, 33), are not capable of forming multipotent neurospheres. This is notably different from the potential of these cells at postnatal stages (34) and in adult white matter tracts (35). Thus, specifically astrocytes react to brain injury by partial dedifferentiation and acquisition of multipotency.
Our fate mapping analysis further demonstrates that these reactive astrocytes—despite being multipotent when isolated in vitro—cannot pursue their full potency in vivo and rather remain within their astroglial lineage. This is despite the activation of the same growth factor pathways (FGF2 and EGF) that are used to expand neurosphere cells also after injury in vivo (36, 37). Although these signals can trigger neuronal repair at specific sites in vivo (23), in most other CNS regions the infusion of EGF and FGF2 is not sufficient to elicit a considerable degree of neurogenesis locally (24). This situation can be improved after transduction with potent neurogenic transcription factors (8, 24), but the response is still rather limited. Obviously the antineurogenic environment present in the adult brain parenchyma (38) contributes to the predominant glial fate in vivo, with Notch (39) and BMP signaling (40) acting as potential candidates for this fate restriction. In addition, our results now suggest that the limited neurogenic response even after overexpression of neurogenic factors may also be due to targeting glial cells with less plasticity than the multipotent subset of reactive astrocytes. Indeed, the viral vectors used in previous studies target predominantly NG2+ progenitors (8, 24). Our data now propose reactive astrocytes as a promising source of multipotent cells within the injury site that may be particularly suited to elicit neuronal repair in brain regions far away from zones of adult neurogenesis.
Materials and Methods
Animals, Surgical Procedures, and Tamoxifen Treatment.
Eight- to 12-week-old GLAST::CreERT2 and R26R or Z/EG double heterozygous (GLAST::CreERT2;R26R or GLAST::CreERT2;Z/EG, respectively) mice received tamoxifen dissolved in corn oil (or corn oil only; SI Fig. 4 A and B) to induce Cre activity (15). For SW lesion, animals were anesthetized and injured in the right neocortex (Bregma from −0.9 to −2.7 mm, latero-lateral 1.7 mm, 0.9 mm deep) as described previously (8). Mice were perfused transcardially with 4% paraformaldehyde in phosphate buffer at 24 h postlesion and 3, 7, and 30 dpl.
For viral vector injections, C57BL/6 mice were anesthetized and injected with either VSVG-pseudotyped lentivirus into the SEZ (28) or LCMV-pseudotyped lentiviral vectors (Bregma −1.6 mm, latero-lateral 1.6 mm, 0.5–1 mm deep). SEZ-injected animals underwent SW injury immediately after injection and were killed 3 days later for histology or FACS analysis.
Proliferation Analysis and Immunohistology.
To analyze proliferation of reporter+ or LCMV-infected cells, two protocols were applied. To monitor both fast and slow cell divisions, animals received BrdU in drinking water (1 mg/ml) for 1 week or from the moment of SW until they were perfused. Alternatively, BrdU was injected i.p. (100 mg/kg of body weight) 2 h before mice were killed (single BrdU pulse, labeling fast-proliferating cells in S-phase). Immunostainings were performed according to ref. 8 (see Immunohistology in SI Text for details and a list of antibodies).
Quantitative Analysis.
Quantifications were performed by confocal analysis or by means of NEUROLUCIDA and NEUROEXPLORER software (Microbrightfield). For the GLAST::CreERT2;R26R mice, the analysis was performed on 200,000-μm2 areas in the cortical GM within 150 μm from the lesion track and in corresponding areas of the contralateral hemisphere or intact cortex. Eighty-two to 154 β-gal-expressing cells were counted for each animal per staining. Results are presented as the mean calculated between different animals (at least three animals for each time point unless stated differently), and the variation between animals is depicted as the SEM.
Lentiviral Vector Production.
Here we used an HIV-based lentiviral eGFP vector pseudotyped with the LCMV glycoprotein (Fig. 2) or VSVG (SI Fig. 7) generated as described (28) or by transient cotransfection of 293T cells with pFUGW (41), where eGFP is encoded under the control of the human ubiquitin C promoter, the packaging plasmid pCMVΔR8.91 (42), and the pseudotyping plasmid pHCMV-GP(WE-HPI) (43) with a stable variant of the LCMV envelope protein (GenBank accession no. AJ318512). Virus particles were produced as in ref. 44.
Isolation of Cortex Cells and Neurosphere Culture.
After removal of the meninges, GM tissue surrounding the SW injury of the neocortex and a similar piece at the same rostrocaudal level in the contralateral hemisphere were dissected at 3 dpl and neurospheres were generated as in ref. 45. For selection of astrocytes the same procedure was performed with cortices (ipsilateral and contralateral to the injury side) of hGFAP-eGFP or GLAST::CreERT2;Z/EG mice, and cells resuspended in culture medium were separated into GFP+ and GFP− fractions by using a BD FACSAria (gating parameters set by side and forward scatter to eliminate debris and dead and aggregated cells) at 530 nm and a flow rate of 1,000 events per second. Ten thousand sorting events (mean of 2,200 cells after counting using a Neubauer chamber) were plated into a single well of a 24-well plate (Nunc) and cultured as above. For single-cell analysis sorted cells were serially diluted into Terasaki wells with 20 μl of medium and cultured for up to 10 days before passage. From hGFAP-eGFP mice, cells expressing NG2 or PDGFRα were isolated from either the GFP+ or GFP− cells and were tested for neurosphere formation according to ref. 45 or in the presence of PDGF (26).
For differentiation each neurosphere was plated onto a glass coverslip coated with polyd-lysine in a single well with 1 ml of neurobasal medium (Invitrogen) supplemented with 1% FCS for 10 days. Differentiation was assessed 6–10 days after plating.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Pavel Osten (Max Planck Institute for Medical Research, Heidelberg, Germany), Dorothee von Laer (Georg-Speyer-Haus, Frankfurt am Main, Germany), and Uwe Maskos (Institut Pasteur, Paris) for plasmids and cell lines; Michael Wegner (University of Erlangen, Erlangen, Germany) and Jack Price (King's College, London) for antibodies; Leanne Godinho, Benedikt Berninger, and Leda Dimou for excellent comments on the manuscript; and Gabi Jäger for excellent technical assistance. This research was supported by grants from the Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, Ministero dell'Universitá e della Ricerca, and Regione Piemonte. A.B., I.R., and A.-P.H. were supported by fellowships from the Humboldt Foundation, European Molecular Biology Organization, and Deutscher Akademischer Austauschdienst, respectively.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709002105/DC1.
References
- 1.Okada S, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 2.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 3.Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: Possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. doi: 10.1002/glia.20121. [DOI] [PubMed] [Google Scholar]
- 4.Magnus T, et al. Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes. J Neurosci Res. 2007;85:2126–2137. doi: 10.1002/jnr.21368. [DOI] [PubMed] [Google Scholar]
- 5.Wilhelmsson U, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci USA. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakakibara S-i, et al. RNA-binding protein Musashi family: Roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci USA. 2002;99:15194–15199. doi: 10.1073/pnas.232087499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyake T, Hattori T, Fukuda M, Kitamura T, Fujita S. Quantitative studies on proliferative changes of reactive astrocytes in mouse cerebral cortex. Brain Res. 1988;451:133–138. doi: 10.1016/0006-8993(88)90757-3. [DOI] [PubMed] [Google Scholar]
- 8.Buffo A, et al. Expression pattern of the transcription factor Olig2 in response to brain injuries: Implications for neuronal repair. Proc Natl Acad Sci USA. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ZJ, Negra M, Levine A, Ughrin Y, Levine JM. Oligodendrocyte precursor cells: Reactive cells that inhibit axon growth and regeneration. J Neurocytol. 2002;31:481–495. doi: 10.1023/a:1025791614468. [DOI] [PubMed] [Google Scholar]
- 10.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 12.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganat YM, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 15.Mori T, et al. The novel roles of glial cells revisited: The contribution of radial glia and astrocytes to neurogenesis. Glia. 2006;54:21–34. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- 16.Watson DJ, Kobinger GP, Passini MA, Wilson JM, Wolfe JH. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol Ther. 2002;5:528–537. doi: 10.1006/mthe.2002.0584. [DOI] [PubMed] [Google Scholar]
- 17.Stein CS, Martins I, Davidson BL. The lymphocytic choriomeningitis virus envelope glycoprotein targets lentiviral gene transfer vector to neural progenitors in the murine brain. Mol Ther. 2005;11:382–389. doi: 10.1016/j.ymthe.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 19.Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 20.Matthias K, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petito CK. Transformation of postischemic perineuronal glial cells. I. Electron microscopic studies. J Cereb Blood Flow Metab. 1986;6:616–624. doi: 10.1038/jcbfm.1986.109. [DOI] [PubMed] [Google Scholar]
- 22.Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakatomi H, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 24.Ohori Y, et al. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci. 2006;26:11948–11960. doi: 10.1523/JNEUROSCI.3127-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolte C, et al. GFAP promoter-controlled EGFP-expressing transgenic mice: A tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33:72–86. [PubMed] [Google Scholar]
- 26.Chojnacki A, Weiss S. Isolation of a novel platelet-derived growth factor-responsive precursor from the embryonic ventral forebrain. J Neurosci. 2004;24:10888–10899. doi: 10.1523/JNEUROSCI.3302-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto L, Götz M. Radial glial cell heterogeneity—the source of diverse progeny in the CNS. Prog Neurobiol. 2007;83:2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Hack MA, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- 29.Niquet J, Jorquera I, Faissner A, Ben-Ari Y, Represa A. Gliosis and axonal sprouting in the hippocampus of epileptic rats are associated with an increase of tenascin-C immunoreactivity. J Neurocytol. 1995;24:611–624. doi: 10.1007/BF01257376. [DOI] [PubMed] [Google Scholar]
- 30.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 31.Götz M, Bolz J, Joester A, Faissner A. Tenascin-C synthesis and influence on axonal growth during rat cortical development. Eur J Neurosci. 1997;9:496–506. doi: 10.1111/j.1460-9568.1997.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 32.Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horner PJ, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belachew S, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunes MC, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 36.Clarke WE, Berry M, Smith C, Kent A, Logan A. Coordination of fibroblast growth factor receptor 1 (FGFR1) and fibroblast growth factor-2 (FGF-2) trafficking to nuclei of reactive astrocytes around cerebral lesions in adult rats. Mol Cell Neurosci. 2001;17:17–30. doi: 10.1006/mcne.2000.0920. [DOI] [PubMed] [Google Scholar]
- 37.Smith GM, Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52:209–218. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- 38.Seidenfaden R, Desoeuvre A, Bosio A, Virard I, Cremer H. Glial conversion of SVZ-derived committed neuronal precursors after ectopic grafting into the adult brain. Mol Cell Neurosci. 2006;32:187–198. doi: 10.1016/j.mcn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto S-i, et al. Transcription factor expression and Notch-dependent regulation of neural progenitors in the adult rat spinal cord. J Neurosci. 2001;21:9814–9823. doi: 10.1523/JNEUROSCI.21-24-09814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hampton DW, et al. A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo. Eur J Neurosci. 2007;26:3024–3035. doi: 10.1111/j.1460-9568.2007.05940.x. [DOI] [PubMed] [Google Scholar]
- 41.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 42.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 43.Beyer WR, Westphal M, Ostertag W, von Laer D. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: Generation, concentration, and broad host range. J Virol. 2002;76:1488–1495. doi: 10.1128/JVI.76.3.1488-1495.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 45.Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253:733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.