Abstract
Adolescence is a key period for the development of brain circuits underlying affective and behavioral regulation. It remains unclear, however, whether and how adolescent brain structure influences day-to-day affective behavior. Because of significant changes in the nature of family relations that also typically occur during adolescence, parent–child interactions provide a meaningful context where affective behavior and its regulation may be assessed. In a sample of 137 early adolescents, we investigated the relationship between aspects of the adolescents' brain structure and their affective behavior as assessed during observation of parent–child interactions. We found a significant positive association between volume of the amygdala and the duration of adolescent aggressive behavior during these interactions. We also found male-specific associations between the volume of prefrontal structures and affective behavior, with decreased leftward anterior paralimbic cortex volume asymmetry associated with increased duration of aggressive behavior, and decreased leftward orbitofrontal cortex volume asymmetry associated with increased reciprocity of dysphoric behavior. These findings suggest that adolescent brain structure is associated with affective behavior and its regulation in the context of family interactions, and that there may be gender differences in the neural mechanisms underlying affective and behavioral regulation during early adolescence. Particularly as adolescence marks a period of rapid brain maturation, our findings have implications for mental health outcomes that may be revealed later along the developmental trajectory.
Keywords: emotion, family interactions, neuroimaging, structural MRI, gender differences
A growing body of evidence suggests that early adolescence marks entry into a period of substantial neurobiological change, with significant effects on cognitive, social, and emotional development (1). More specifically, it has been proposed that adolescence involves a shift from greater limbic to prefrontal cortical (PFC) control of behavior, with an increase in the inhibitory connections between these two regions (2). These neural changes are believed to underlie a shift from behavior that is driven by affective impulses to more regulated behavior that is guided by consideration of future personal and social consequences (3). Such changes mark adolescence as a critical period during which to examine the neural contributions to affective behavior, particularly emotion regulation.
During adolescence, significant changes also begin to occur in the nature of family relations. One of the primary developmental tasks for families of adolescents is renegotiating the balance between child and parent input with regard to family and personal decision-making and adolescent autonomy (4, 5). Renegotiating this balance often results in disagreement and potential conflict, in part because the adolescent's capacity for affective and behavioral regulation has not reached maturity. Presumably, individual differences in adolescents' propensity for engaging in adaptive behavior in such circumstances is mediated by maturation of neural networks supporting affective regulatory capacities.
Anatomical magnetic resonance imaging (MRI) provides an attractive means for assessing adolescent brain development in vivo and has been applied with great success to demonstrate the dynamic changes in regional brain volumes that occur during this period, and how they relate to cognitive development (6, 7). In this study of a large sample of early adolescents, we explored how individual differences in volumetric estimates of key brain regions involved in affective regulation were related to adolescents' affective behavior as assessed during observation of parent–child interactions.
Specifically, we focused on three key brain regions, the amygdala, anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC), which are known to represent critical nodes in neural networks supporting affective regulation (8). The amygdala has been implicated in the unconscious processing and memory of reactions to affective stimuli, particularly those that are highly arousing or have negative valence (9). Enlarged amygdala volumes have been reported in young patients with first-episode mood and anxiety disorders (10, 11), and it has been suggested that these changes might reflect increased amygdala activation in these patients.
The ACC and OFC have been implicated in the regulatory aspects of affective processing, and it has been suggested that this function may stem from the inhibitory influence of these structures on the amygdala (12). Increased ACC and OFC activity has been observed during effortful regulation of affective states in healthy controls (13–15). Volumetric reductions in these regions have been observed in patients with disorders marked by deficits in the ability to regulate emotion and behavior, including depression (16–18), schizophrenia (19), and borderline personality disorders (20).
There is suggestion of asymmetric involvement of the frontal cortex in affective and behavioral regulation (21), with most evidence pointing toward a greater contribution of left frontal structures, or greater left relative to right frontal functioning, to affect regulation. Although few studies examining brain structure and behavior have tested for asymmetry using appropriate statistical methods, there is some evidence that structural asymmetries in the OFC and ACC favoring the left hemisphere are associated with the regulation of affective behavior (22), and risk for psychopathology (23).
In the current study, adolescent affect regulation was assessed by means of specific measures of affective behavior obtained during parent–child interactions (24). These measures included duration of adolescent aggressive and dysphoric affective behaviors, as well as adolescent reciprocity of parental affective behaviors (e.g., the likelihood of responding to parental aggressive behavior with aggressive behavior). The duration of adolescent negative affective behaviors is considered a behavioral index of affect regulation because the persistence of negative affect reflects an inability to promptly down-regulate or move out of such affective states. Adolescent reciprocity of negative affect during parent–adolescent interactions, on the other hand, reflects the adolescents' ability to regulate their initial reactivity to their parents' negative antecedent behaviors.
We hypothesized that behaviors indicative of reduced negative affect regulation would be associated with increased amygdala volumes and altered volumetric asymmetries of the OFC and ACC. Given previous research indicating gender differences in adolescent brain development (25), affective behaviors (26), and in the brain correlates of affective processes (27), we also explored gender differences in the associations between these brain structures and adolescent affective behaviors. By examining associations between these structural brain measures and aspects of affective behavior, we were able to provide evidence that, in the context of parent–child interactions, individual differences in affective regulation in adolescence have some neuroanatomical basis.
Results
Raw volumes and gender differences of each region of interest are presented in supporting information (SI) Table 2.
Duration of Adolescent Behaviors.
Gender, left and right amygdala volumes, and OFC, limbic ACC (ACCL), and paralimbic ACC (ACCP) asymmetry indexes were tested as predictors of the duration of adolescent aggressive and dysphoric behaviors. There were no significant main effects of gender on the duration of adolescent behaviors. As summarized in Table 1, the duration of adolescent aggressive behaviors was predicted by the volume of the left and right amygdala, with the latter association being marginally significant (P = 0.057). As post hoc tests revealed that the two models (with left and right amygdala as respective predictors) did not significantly differ from each other, t(133) = 0.320, P = 0.750, it is likely that the effect is bilateral. That is, adolescents who had a larger bilateral amygdala volume maintained their aggressive behaviors for a longer duration.
Table 1.
Summary of hierarchical regressions predicting the duration of adolescent aggressive behaviors and adolescent reciprocity of parental dysphoric behaviors with adolescent brain volume/asymmetry measures
| Predictors and dependent variables | N | ß | t | ΔF | ΔR2 |
|---|---|---|---|---|---|
| DV: duration of aggressive behaviors | |||||
| Left amygdala | 137 | 0.200 | 2.206* | 4.865* | 0.035 |
| Left amygdala × gender | 137 | 0.042 | 0.324 | 0.105 | 0.001 |
| Right amygdala | 137 | 0.168 | 1.916 | 3.673 | 0.027 |
| Right amygdala × gender | 137 | −0.111 | −0.862 | 0.743 | 0.005 |
| OFC asymmetry | 136 | 0.085 | 0.978 | 0.957 | 0.007 |
| OFC asymmetry × gender | 136 | −0.015 | −0.121 | 0.015 | 0.000 |
| ACCL asymmetry | 135 | 0.118 | 1.358 | 1.843 | 0.177 |
| ACCL asymmetry × gender | 135 | 0.232 | 1.871 | 3.500 | 0.026 |
| ACCP asymmetry | 131 | −0.258 | −3.025** | 9.152** | 0.067 |
| ACCP asymmetry × gender | 131 | −0.260 | −2.192* | 4.803* | 0.030 |
| DV: adolescent dysphoric given parent dysphoric | |||||
| Left amygdala | 134 | −0.020 | −0.216 | 0.047 | 0.000 |
| Left amygdala × gender | 134 | 0.002 | 0.012 | 0.000 | 0.000 |
| Right amygdala | 135 | 0.028 | 0.310 | 0.096 | 0.001 |
| Right amygdala × gender | 135 | −0.068 | −0.520 | 0.270 | 0.002 |
| OFC asymmetry | 134 | −0.037 | −0.429 | 0.246 | 0.001 |
| OFC asymmetry × gender | 134 | −0.424 | −3.683*** | 13.563*** | 0.093 |
| ACCL asymmetry | 133 | 0.091 | 1.040 | 1.082 | 0.008 |
| ACCL asymmetry × gender | 133 | 0.138 | 1.110 | 1.232 | 0.009 |
| ACCP asymmetry | 129 | −0.108 | −1.221 | 1.490 | 0.012 |
| ACCP asymmetry × gender | 129 | −0.070 | −0.564 | 0.318 | 0.002 |
These values are based on the model controlling for adolescent gender effects (not significant and therefore not shown here); therefore, change in F and R2 values are displayed. DV, dependent variable; OFC, orbitofrontal cortex; ACCL, anterior cingulate cortex (limbic); ACCP, anterior cingulate cortex (paralimbic). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
ACCP asymmetry also predicted duration of adolescent aggressive behavior although this association was moderated by a gender by ACCP asymmetry interaction. Analysis by gender revealed that ACCP asymmetry predicted longer duration of aggressive behaviors only for males, β = 0.44, t(134) = 4.04, P < 0.001, R2 = 17.8. Males who maintained their aggressive behavior for long periods had reduced leftward asymmetric ACCP volumes, relative to males who maintained their aggressive behavior for shorter periods. Pearson's correlations showed that aggressive duration was significantly correlated with left (r = −0.321, P = 0.006) but not right (r = 0.197, P = 0.103) ACCP volume for males, indicating that the reduction in leftward asymmetry may be best explained by reduced size of the left ACCP rather than increased size of the right ACCP. Adolescent dysphoric behaviors were unrelated to amygdala volume and the asymmetry measures (see SI Table 3).
Adolescent Reciprocity of Parent Behaviors.
Gender, OFC, ACCL, and ACCP asymmetry scores, and amygdala volumes were tested as predictors of adolescents' likelihood of reciprocating parental aggressive and dysphoric behaviors. There were no significant main effects of gender on adolescent reciprocity of parental behaviors. As summarized in Table 1, the gender by OFC asymmetry interaction term significantly predicted dysphoric reciprocity.
Analysis by gender revealed that the relationship between OFC asymmetry and dysphoric reciprocity was reversed for males and females. For males, reduced leftward asymmetric OFC volume predicted a greater likelihood of reciprocating parental dysphoric behaviors, β = −0.37, t(72) = −3.33, P = 0.001, R2 = 13.7. For females, greater leftward asymmetric OFC volume predicted a greater likelihood of reciprocating parental dysphoric behaviors; however, this relationship was only marginally significant, β = 0.25, t(62) = 2.00, P = 0.050, R2 = 6.1. For males, Pearson's correlations showed that dysphoric reciprocity was significantly correlated with left (r = −.290, P = 0.014) but not right OFC volume (r = −0.123, P = 0.304), indicating that the rightward asymmetry in males displaying greater reciprocity of dysphoric behaviors may be best explained by reduced size of the left OFC rather than increased size of the right OFC. For females, there were no significant correlations between dysphoric reciprocity and OFC volume in either hemisphere. Excluding an apparent female outlier increased the strength of the gender by OFC volume asymmetry interaction, β = −0.42, t(134) = −3.68, P < 0.001, and reduced the strength of the relationship between OFC volume asymmetry and dysphoric reciprocity for females, β = 0.18, t(61) = 1.37, P = 0.175.
Asymmetry scores did not significantly predict adolescent reciprocity of parental aggressive behaviors. No significant main or interaction effects of amygdala volume on adolescent reciprocity of aggressive or dysphoric behaviors were found (see SI Table 3).
Discussion
The results of this study provide evidence that brain structure is related to observable affective behaviors during ecologically meaningful interpersonal interactions. Increased amygdala volume and decreased leftward asymmetry of the ACCP were associated with increased duration of aggressive behaviors during these interactions, with the latter association being apparent in males but not females. Decreased leftward asymmetry of the OFC was associated with greater reciprocity of dysphoric behaviors, with this association also specific to males. An absence of mean gender differences in affective behaviors suggests that the neural circuits underlying affective behaviors may differ for male and female adolescents during this age period.
Aggressive Affective Behaviors.
Adolescents who maintained their aggressive affective behaviors for a longer duration during a conflict resolution interaction with their parent were found to have larger amygdala volume. This finding is consistent with evidence that the amygdala has a key role in anger and aggressive behavior. Increased baseline amygdala activity has been reported in aggressive adult populations (28), and structural amygdala abnormalities have been reported in adult psychopathologies marked by impulsivity and aggressive behavior, such as borderline personality disorder (20). It has been suggested that the amygdala is part of a frontal cortical-limbic neural circuit whereby an increase in its activity serves to interfere with connected cortical regions involved in cognitive and executive functioning (29, 30). Thus, our finding suggests that increased amygdala volume in adolescence may reflect a predisposition toward sustained experience of negative affect, which may interfere with cognitive and behavioral regulation, and manifest outwardly as aggressive behavioral displays. Longitudinal research is required to establish whether larger than average amygdala volume in early adolescence may engender risk for aggressive behavior and psychopathology later in life.
For males, longer duration of aggressive affective behaviors was also associated with ACCP asymmetry, which seemed to be driven by a reduction in left ACCP volume. This finding is consistent with literature suggesting that a leftward asymmetry of prefrontal activity is associated with a greater ability to regulate negative affect (31). This finding may also be related to individual differences in sulcal morphology in the cingulate region. Previous work has shown that the paracingulate sulcus (PCS), which runs dorsal and parallel to the cingulate sulcus, is present in 30–60% of individuals and more common in the left hemisphere (32, 33). This leftward PCS asymmetry has been associated with better executive functioning (34), and reduced risk for psychopathology (23). We have also previously demonstrated that a leftward PCS asymmetry is associated with increased leftward asymmetry in ACCP gray matter, which seems to be related to the cognitive performance advantage shown by people showing this folding pattern (35). These findings converge on the notion that increased gray matter in the left relative to right ACCP is associated with better cognitive performance and affective regulation, and may be a key determinant of individual differences in these abilities.
The maturation of the prefrontal cortex and its inhibitory connections with the subcortex are thought to be key outcomes of adolescent neurodevelopment, which underlies the successful development of emotional and behavioral regulatory abilities (2). Therefore, the associations of increased amygdala volume and decreased left frontal asymmetries with more negative affective behaviors may represent a delay in brain maturation. Longitudinal research will be important to examine whether these findings have implications for the development of affective and behavioral dysregulation later in life.
The male specificity of this finding adds to a growing body of evidence that the neural mechanisms underlying affective processing differ between males and females (e.g., ref. 27). Males have been found to exhibit structural and functional brain asymmetries to a greater extent than females in a number of prefrontal areas, including the cingulate region (32, 36). It has been suggested that these asymmetries may render males more vulnerable to certain disorders involving dysfunction of the frontal lobes such as ADHD, autism, and dyslexia (37). Although males in the present study did not display more aggressive behavior than females, the more pronounced relationship between ACCP asymmetry and aggressive affective behaviors in males suggests that aggressive affect in male adolescents may function as a mechanism by which their brain asymmetry is implicated in their risk for psychopathology.
Dysphoric Affective Behaviors.
Greater adolescent reciprocity of parental dysphoric behaviors was associated with OFC asymmetry in males, and this association seemed to be driven by a reduction in left OFC volumes. For females, no robust evidence for an association was observed (i.e., a marginally significant result seemed to be driven by a single outlier). Given that reciprocity of negative affect is thought to reflect a deficit in emotion regulation, characterized by greater reactivity to provocative affective behavior, this finding is consistent with an extensive literature implicating the OFC in the regulation of negative emotions and behavior (e.g., ref. 13). Given that dysphoric affective behavior is a particularly prominent feature of mood disorder, our finding is also consistent with evidence that decreased activity and volume in the left OFC are associated with emotion dysregulation in depressed individuals (38, 39), as well as with findings of more generalized left prefrontal hypofunction in this patient population (40).
The male specificity of this finding is of particular interest given that a number of previous studies have reported gender differences in OFC development, structure, and contribution to psychopathology (7, 41). To our knowledge, two studies have investigated gender differences in the relationship between OFC structure and mood disorder. Both of these studies (17, 42), whose samples consisted of major depressive and bipolar patients, respectively, reported deficits in OFC volume only for male patients. Our result suggests that the OFC might be a pertinent site for vulnerability to dysregulation of dysphoria in males.
Although previous work has implicated the OFC in anger and aggression (43, 44), we did not find a relationship between adolescent OFC volume asymmetry and aggressive behavior. Also, although the amygdala has a well established role in fear processing, and there are findings of altered amygdala activity and volume in mood and anxiety disorders (10, 11, 45), we did not find a relationship between amygdala volume and dysphoric behavior. It is possible that other measures of OFC and amygdala function (e.g., resting or task-related activity as indexed by functional imaging techniques) might better capture individual differences in aggressive and dysphoric affective behavior, respectively. It may also be that such brain structure-behavior relationships emerge later in development or only in the context of psychopathology.
Duration Versus Reciprocity.
That different prefrontal regions were associated with the duration versus reciprocity measures of adolescent affective behaviors is potentially informative of the functional heterogeneity of the prefrontal cortex. Although both measures are indices of emotion regulation, duration reflects regulation once the affect has already been instigated, whereas reciprocity reflects regulation of the initial reactivity to a provocative stimulus. From this perspective, our results fit well with some theories specifying the involvement of distinct prefrontal regions in different aspects of emotion regulation. For example, a number of groups have suggested that dorsal and lateral prefrontal regions (including the ACCP) are important for monitoring one's own affective state and the effortful down-regulation of emotional behavior, whereas ventral regions (including the OFC) are more important for reflexive responding to affective stimuli and automatic regulation of this responding (8, 46).
Limitations.
The correlational analysis used in the present study to investigate brain–behavior relationships precludes conclusions regarding causality of relationships. That is, we cannot determine whether differences in regional brain volume result from, or represent early predictors of, interpersonal affective behavior. It is also possible that other developmental processes such as puberty may affect measures of both brain structure and affective behavior in this age group. Direct evidence of such effects in humans, however, is lacking, and there are difficulties involved in discerning pubertal effects from environmental and other perturbations (47). It will be important for future research to explore these potential confounds.
Although we have interpreted our male-specific findings as indicating gender differences in the neural underpinnings of affective behaviors, we stress that this interpretation is speculative. Future studies using whole-brain mapping techniques and functional imaging will be important to provide additional insight into gender differences in the contribution of other brain regions to affective behavior. The marked brain reorganization occurring during adolescence also complicates the interpretation of findings. For example, there is evidence that the female brain matures earlier than the male brain during this period (48), and thus, it is possible that the male-specific brain–behavior associations reflect differing rates of brain development rather than stable gender differences in brain–behavior relationships. Longitudinal assessment of both brain structure and affective behavior is needed to resolve these issues.
Summary and Conclusions.
This study reported significant associations between adolescent affective behaviors during interactions with parents and volumetric measures of the amygdala, OFC, and ACCP, in patterns consistent with what is known about the role of these structures in affective processing and regulation. Although the effect sizes of the reported associations were small (49), they are similar in magnitude to most estimates of regional neuroanatomical contributions to measures of intelligence and other cognitive abilities (50). Gender differences emerged such that the association between the OFC, ACCP and affective behaviors was more pronounced for males. Given the nonsignificant gender differences in the observed behaviors, the results suggest that affective functioning may have different neural bases in males versus females, although the potential impact of sexually dimorphic brain development on these results cannot be discounted. These data provide unique insights into brain–behavior relationships as they occur during a developmentally crucial ecological context. Moreover, they may have important implications for understanding the etiology of a range of psychiatric disorders and, as such, provide an important heuristic for future longitudinal research.
Methods
Participants.
The sample consisted of 137 adolescents (54% male, M age 12.6 yr, SD 0.4 yr, range 11.4–13.7 yr) recruited from schools across metropolitan Melbourne, Australia. Participants were recruited as part of a broader adolescent development study (see SI Methods for further details). Handedness was established by using the Edinburgh Handedness Inventory (51). There were 125 right-handed and 12 left-handed subjects. For no participant was there evidence of current or past case level axis I depressive, substance use, or eating disorder, established using The Schedule for Affective Disorder and Schizophrenia for School-Aged Children: Epidemiologic Version [K-SADS-E (52)]. The vast majority of participants identified their ethnicity as Australian (92%), five identified their ethnicity as mixed Australian, and one each self-identified as Welsh, English, Swiss, Indian, Chinese, and Indonesian. Adolescents participated with either their mothers (n = 114) or fathers (n = 23). Differences according to parent gender were not investigated because of the uneven sampling, and also because such investigation is beyond the scope of this article. Informed consent was obtained for all participants (and their parent or guardian) before their inclusion in the study, in accordance with local ethics committee guidelines.
Family Interactions.
Procedure.
Parents and adolescents participated in 20-min Problem-Solving Interactions (PSI), which were videotaped for coding purposes. Separate cameras videotaped each participant. The PSI is designed specifically to elicit negative affective behaviors. Topics for the PSI were identified based on parent and adolescent responses to the Issues Checklist [IC (53)]. The IC is a list of 44 topics about which adolescents and parents may disagree, such as “[adolescent] lying” and “[adolescent] talking back to parents.” Up to five IC issues that were rated as conflictual (and recent) by parent and adolescent were chosen for dyads to resolve during the PSI.
Observational coding of family interactions.
The affect and verbal content of the videotaped interactions were coded in real time by using the Living in Family Environments [LIFE (54)] coding system. The LIFE is an event-based coding system in which new codes are entered each time the affect or verbal content of the participant changes. The LIFE consists of 10 affect codes (contempt, anger, anxious, dysphoric, pleasant, neutral, happy, caring, whining, and belligerence) and 27 verbal content codes (e.g., validation, approve, provoke). Two composite constructs, derived from the individual affect and content codes, were used in the present study. Aggressive behavior includes all codes with contemptuous, angry, and belligerent affect, as well as disapproving, threatening, or argumentative verbal content with neutral affect. Dysphoric behavior consists of all codes with dysphoric, anxious, or whining affect, as well as complaints and self-derogatory verbal content with neutral affect.
All video recordings were coded by extensively trained observers who were blind to participant characteristics (e.g., symptomatology levels). Approximately 20% of the interactions were coded by a second observer to provide an estimate of observer agreement. Random pairs of observers were assigned to the interactions to minimize drift between any two observers and to ensure that all observers met minimal criteria for acceptable observations. Kappa coefficients for the Aggressive and Dysphoric composite codes were 0.77 and 0.68, respectively, values considered to reflect good to excellent agreement (55). Absence of a particular verbal content or affect code for a participant resulted in a missing value for that code for that participant. Thus, N varied for the different constructs.
For each composite construct, observational indices of adolescent emotion regulation were derived. These indices included the following:
Duration per episode (dpe). Dpe represents the average length of time that specific affective behaviors are maintained each time they are displayed during the course of the interaction task. A longer duration of aggressive and dysphoric behaviors is indicative of emotion dysregulation in that it reflects an inability to promptly down-regulate negative affect.
Sequential z-score. Allison-Liker z-scores (56) for sequential relations indicate the extent to which a specified sequence of behavior occurs more or less often than would be expected as a function of the base rate of each behavior. Z-scores represent whether a particular antecedent behavior increases (positive z-score) or suppresses (negative z-score) the likelihood of a particular consequent behavior. In creating these variables, the parents' antecedent affective behaviors are regarded as provocative stimuli. Greater reciprocity of aggressive and dysphoric behaviors (e.g., positive z-scores of adolescent aggressive responses to parental aggressive behavior) is an index of the adolescents' ability to regulate their affective reaction to the parents' behavior.
Neuroimaging.
Image acquisition.
Magnetic resonance imaging (MRI) scans were performed on a 3 Tesla scanner at the Brain Research Institute, Austin and Repatriation Medical Centre, Melbourne, Australia, using a gradient echo volumetric acquisition sequence (repetition time = 36 ms; echo time = 9 ms; flip angle = 35°, field of view = 20 cm2, pixel matrix = 410 × 410) to obtain 124 T1-weighted contiguous 2-mm-thick slices (voxel dimensions = 0.4883 × 0.4883 × 2.0 mm).
Image preprocessing.
Images were transferred to an SGI/Linux workstation for morphometric analysis. Image preprocessing was carried out by using tools from the FMRIB software library (www.fmrib.ox.ac.uk/fsl). Each 3-dimensional scan was stripped of all non-brain tissue (57) and aligned to the MNI 152 average template (six-parameter rigid body transform with trilinear interpolation) using FLIRT (58). This registration served to align each image axially along the anterior commissure-posterior commissure (AC-PC) plane and sagittally along the interhemispheric fissure without any deformation. Images were resampled to 1 mm3.
Morphometric analysis.
Regions of Interest (ROIs) were defined and quantified based on previous techniques developed and published in the Melbourne Neuropsychiatry Centre. All ROIs were traced by using the software package ANALYZE (Mayo Clinic, Rochester; www.mayo.edu/bir/). Brain tissue was segmented into gray matter, white matter, and cerebrospinal fluid by using an automated algorithm, as implemented in FAST (59). An estimate of whole brain volume (WBV) was obtained by summing gray and white matter pixel counts (i.e., WBV included cerebral gray and white matter, the cerebellum, and brainstem, but not the ventricles, cisterns, or cerebrospinal fluid). ACC and OFC estimates were based on gray matter pixel counts contained within the defined ROIs. Amygdala estimates were based on total voxels within the defined ROI.
Amygdala.
The guidelines for tracing the amygdala were adapted from those described by Velakoulis et al. (60). The posterior boundary of the amygdala was marked by the first appearance of amygdala gray matter above the temporal horn. The lateral border was marked superiorly by the thin strip of white matter separating the amygdala from the claustrum and tail of the caudate, and inferiorly by the temporal stem and extension of the temporal horn. The medial border was marked superiorly by the semilunar gyrus, and inferiorly by subamygdaloid white matter, which separates the amygdala from the entorhinal cortex. The anterior boundary was marked by the joining of the optic chiasm or the point where the lateral sulcus closes to form the endorhinal sulcus (whichever was more posterior). The protocol of Watson et al. (61) was used to separate the amygdala from the hippocampus.
Anterior cingulate cortex (ACC).
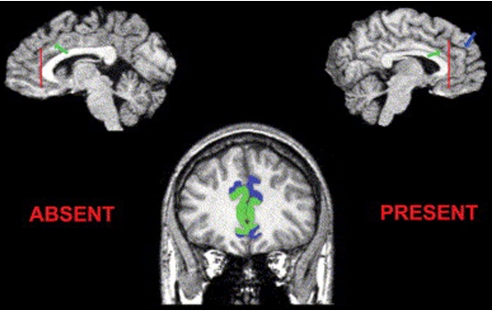
The boundaries of the ACC have been described in detail by Fornito et al. (62). This protocol demarcates limbic and paralimbic portions of the ACC (ACCL and ACCP, respectively) regions by taking into account individual differences in morphology of the cingulated (CS), paracingulate (PCS), and superior rostral sulci (SRS). Briefly, the anterior ACCL contained all gray matter in the gyrus bound by the callosal sulcus and the CS. The ACCP contained all gray matter in the gyrus bound by the CS and PCS, except in cases where the PCS was absent, for which the ACCP contained only the gray matter on the upper bank of the CS. See Fig. 1 for an illustration.
Fig. 1.
Example of changes in the location and extent of the limbic (ACCL; highlighted in green) and paralimbic (ACCP; highlighted in blue) anterior cingulate cortices as a function of variations in the cingulate sulcus (CS; green arrow, Upper row) and paracingulate sulcus (PCS; blue arrow, Upper row). A PCS is absent in the left-hand case and present in the right-hand case. The Upper row presents parasagittal slices through an individual's T1-weighted image. The coronal section illustrates the distinction between absent (left-hand side) and present (right-hand side) cases. Notice that the ACCP is buried in the depths of the CS when the PCS is absent and extends over the paracingulate gyrus when the PCS is present. The same principle applies throughout consecutive coronal sections.
Orbitofrontal cortex (OFC).
The boundaries of the OFC were based on a previously published method (63). A line through the AC-PC was used to define the superior boundary of the OFC. The posterior boundary was marked by a coronal plane passing through the most posterior aspect of the olfactory sulcus in each hemisphere. All images were manually edited to eliminate subcortical tissue and artifacts related to the eye sockets and nasal bones. The OFC was further parcellated into medial and lateral sectors by using the first prominent sulcus lateral to the olfactory sulcus (which in most cases is the medial orbital sulcus) as a dividing boundary. This sulcus was first identified and marked in the coronal plane; subsequent editing was conducted in the transverse plane.
Statistical Analysis.
Aggressive duration data from one participant was identified as an extreme outlier and therefore was excluded from analysis. Intra- and interrater reliabilities were calculated for each raw ROI volume. Intraclass correlation coefficients (most above 0.9 and none below 0.8) were deemed acceptable for all ROIs. An asymmetry index was calculated for the OFC, ACCL, and ACCP by using the formula (L-R). All brain structural measures were corrected for whole brain size by using a covariance adjustment method (64). Hypotheses were tested by using univariate hierarchical linear regressions where each of the five brain structure measures (i.e., left and right amygdala volume, and OFC, ACCL, ACCP asymmetry indexes) were tested as predictors of affective behaviors. The moderating effects of gender were examined by adding a gender by (centered) brain structural measure interaction term following the main effect terms. Significant interactions were followed up with regression analyses for males and females separately. Because changes in structural brain asymmetry may result from changes in the size of either or both hemispheres (65), to explore this issue, significant main effects or interactions involving asymmetry variables were followed up with Pearson's correlations using left and right hemisphere ROI volumes.
This analysis approach was adopted to reduce the likelihood of type I error. Specifically, we limited the number of analyses by testing for the interactive effects of gender and by using brain asymmetry measures before running follow-up analyses separately within genders or within hemispheres (when significant interaction effects are found).
Supplementary Material
ACKNOWLEDGMENTS.
We thank the Brain Research Institute for support in acquiring the neuroimaging data, and the Oregon Research Institute for its role in the coding of family interaction data. This work was supported by the ORYGEN Research Centre and the Colonial Foundation. Neuroimaging analysis was facilitated by the Neuropsychiatry Imaging Laboratory managed by Ms. Bridget Soulsby at the Melbourne Neuropsychiatry Centre and supported by Neurosciences Victoria. This work was supported by Australian Postgraduate Awards (to S.H. and A.F.), a Melbourne Research Scholarship (to M.B.H.Y.), a Centre for Clinical Research Excellence Postdoctoral Fellowship at ORYGEN Research Centre (to M.B.H.Y.), a J. N. Peters Bequest Fellowship from the University of Melbourne (to A.F.), and National Health and Medical Research Council of Australia Program Grant I.D. 350241 (to M.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709815105/DC1.
References
- 1.Steinberg L. Risk taking in adolescence: What changes and why? Ann NY Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- 2.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 3.Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- 4.Amato PR. Family processes and the competence of adolescents and primary school children. J Youth Adolesc. 1989;18:39–53. doi: 10.1007/BF02139245. [DOI] [PubMed] [Google Scholar]
- 5.Eccles JS, et al. In: Gender Issues in Contemporary Society. Oskamp S, Costanzo M, editors. Thousand Oaks, CA.: Sage Publications; 1993. pp. 59–83. [Google Scholar]
- 6.Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 7.Giedd JN, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 8.Phillips ML, Drevets WC, Rauch SL, Lane RD. Neurobiology of emotion perception. I. The neural basis of normal emotion perception. Biol Psychiatry. 2003;53:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 9.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 10.De Bellis MD, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- 11.Frodl T, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 12.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J Cognit Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 13.Ochsner KN, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Levesque J, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Phan KL, et al. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Bremner JD, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 17.Lacerda ALT, et al. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry. 2004;55:353–358. doi: 10.1016/j.biopsych.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Caetano SC, et al. Smaller cingulate volumes in unipolar depressed patients. Biol Psychiatry. 2006;59:702–706. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Baare WFC, et al. Volumetric analysis of frontal lobe regions in schizophrenia: Relation to cognitive function and symptomatology. Biol Psychiatry. 1999;45:1597–1605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- 20.Tebartz van Elst L, et al. Frontolimbic brain abnormalities in patients with borderline personality disorder: A volumetric magnetic resonance imaging study. Biol Psychiatry. 2003;54:163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- 21.Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- 22.Antonucci AS, et al. Orbitofrontal correlates of aggression and impulsivity in psychiatric patients. Psychiatry Res Neuroimaging. 2006;147:213–220. doi: 10.1016/j.pscychresns.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Yücel M, et al. Morphology of the anterior cingulate cortex in young men at ultra-high risk of developing a psychotic illness. Br J Psychiatry. 2003;182:518–524. doi: 10.1192/bjp.182.6.518. [DOI] [PubMed] [Google Scholar]
- 24.Sheeber L, Allen N, Davis B, Sorensen E. Regulation of negative affect during mother-child problem-solving interactions: Adolescent depressive status and family processes. J Abnormal Child Psychol. 2000;28:467–479. doi: 10.1023/a:1005135706799. [DOI] [PubMed] [Google Scholar]
- 25.Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 26.Else-Quest NM, Hyde JS, Goldsmith HH, Van Hulle CA. Gender differences in temperament: A meta-analysis. Psychol Bull. 2006;132:33–72. doi: 10.1037/0033-2909.132.1.33. [DOI] [PubMed] [Google Scholar]
- 27.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 28.Raine A, et al. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behav Sci Law. 1998;16:319–332. doi: 10.1002/(sici)1099-0798(199822)16:3<319::aid-bsl311>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 30.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 31.Jackson DC, et al. Now you feel it, now you don't: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol Sci. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- 32.Yücel M, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: An MRI morphometric study. Cereb Cortex. 2001;11:17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- 33.Paus T, et al. Human cingulate and paracingulate sulci: Pattern, variability, asymmetry, and probabilistic map. Cereb Cortex. 1996;6:207–214. doi: 10.1093/cercor/6.2.207. [DOI] [PubMed] [Google Scholar]
- 34.Fornito A, et al. Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cereb Cortex. 2004;14:424–431. doi: 10.1093/cercor/bhh004. [DOI] [PubMed] [Google Scholar]
- 35.Fornito A, et al. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: Associations with cortical thickness, surface area, volume, and sulcal depth. Human Brain Mapping. 2008;29:222–236. doi: 10.1002/hbm.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huster RJ, Westerhausen R, Kreuder F, Schweiger E, Wittling W. Morphologic asymmetry of the human anterior cingulate cortex. NeuroImage. 2007;34:888–895. doi: 10.1016/j.neuroimage.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Hier D. Sex differences in hemispheric specialization: Hypothesis for the excess of dyslexia in boys. Ann Dyslexia. 1979;29:74–83. [Google Scholar]
- 38.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 39.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 41.Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb Cortex. 2002;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- 42.Najt P, et al. Anatomical measurements of the orbitofrontal cortex in child and adolescent patients with bipolar disorder. Neurosci Lett. 2007;413:183–186. doi: 10.1016/j.neulet.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dougherty DD, et al. Anger in healthy men: A PET study using script-driven imagery. Biol Psychiatry. 1999;46:466–472. doi: 10.1016/s0006-3223(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 44.Bufkin JL, Luttrell VR. Neuroimaging studies of aggressive and violent behavior: Current findings and implications for criminology and criminal justice. Trauma Violence Abuse. 2005;6:176–191. doi: 10.1177/1524838005275089. [DOI] [PubMed] [Google Scholar]
- 45.Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: The role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- 46.Lieberman MD. Social cognitive neuroscience: A review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- 47.Giedd JN, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 48.Lenroot RK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vacha-Haase T, Thompson B. How to estimate and interpret various effect sizes. J Counseling Psychol. 2004;51:473–481. [Google Scholar]
- 50.Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. NeuroImage. 2004;23:425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 51.Oldfield RC. The assessment and analysis of handedness: The Edinburgh handedness inventory. Neuropsychologia. 1971;9:97–114. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 52.Orvaschel H. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic Version-5. Ft. Lauderdale, FL: Nova Southeastern University; 1995. (K-SADS-E-5) [Google Scholar]
- 53.Prinz RJ, Foster SL, Kent RN, O'Leary KD. Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. J Appl Behav Anal. 1979;12:691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hops H, Davis B, Longoria N. Methodological issues in direct observation: Illustrations with the Living in Familial Environments (LIFE) coding system. J Clin Child Psychol. 1995;24:193–203. [Google Scholar]
- 55.Fleiss JL. Statistical Methods for Rates and Proportions. New York: Wiley; 1981. [Google Scholar]
- 56.Allison PD, Liker JK. Analyzing sequential categorical-data on dyadic interaction: A comment. Psychol Bull. 1982;91:393–403. [Google Scholar]
- 57.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 60.Velakoulis D, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: A high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:133–141. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- 61.Watson C, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic-resonance-imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 62.Fornito A, et al. The influence of sulcal variability on morphometry of the human anterior cingulate and paracingulate cortex. NeuroImage. 2006;33:843–854. doi: 10.1016/j.neuroimage.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 63.Riffkin J, et al. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: Comparison with healthy controls and patients with schizophrenia. Psychiatry Res Neuroimaging. 2005;138:99–113. doi: 10.1016/j.pscychresns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Jack CR, et al. Anterior temporal lobes and hippocampal formations: Normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- 65.Galaburda AM, Corsiglia J, Rosen GD, Sherman GF. Planum temporale asymmetry: Reappraisal since Geschwind and Levitsky. Neuropsychologia. 1987;25:853–868. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.